Abstract
Background
Candida auris is an emerging, multidrug-resistant yeast that spreads in healthcare settings. People colonized with C. auris can transmit this pathogen and are at risk for invasive infections. New York State (NYS) has the largest US burden (>500 colonized and infected people); many colonized individuals are mechanically ventilated or have tracheostomy, and are residents of ventilator-capable skilled nursing facilities (vSNF). We evaluated the factors associated with C. auris colonization among vSNF residents to inform prevention interventions.
Methods
During 2016–2018, the NYS Department of Health conducted point prevalence surveys (PPS) to detect C. auris colonization among residents of vSNFs. In a case-control investigation, we defined a case as C. auris colonization in a resident, and identified up to 4 residents with negative swabs during the same PPS as controls. We abstracted data from medical records on patient facility transfers, antimicrobial use, and medical history.
Results
We included 60 cases and 218 controls identified from 6 vSNFs. After controlling for potential confounders, the following characteristics were associated with C. auris colonization: being on a ventilator (adjusted odds ratio [aOR], 5.9; 95% confidence interval [CI], 2.3–15.4), receiving carbapenem antibiotics in the prior 90 days (aOR, 3.5; 95% CI, 1.6–7.6), having ≥1 acute care hospital visit in the prior 6 months (aOR, 4.2; 95% CI, 1.9–9.6), and receiving systemic fluconazole in the prior 90 days (aOR, 6.0; 95% CI, 1.6–22.6).
Conclusions
Targeted screening of patients in vSNFs with the above risk factors for C. auris can help identify colonized patients and facilitate the implementation of infection control measures. Antimicrobial stewardship may be an important factor in the prevention of C. auris colonization.
Keywords: Candida auris, colonization, mechanical ventilation, skilled nursing facilities, multidrug-resistant organisms
Candida auris colonization among ventilator-capable skilled nursing facility residents is associated with recent hospitalization and antibiotic use. Odds of colonization were similar for colonized patients’ roommates and others on the same ward. Entire units should be considered at risk.
Candida auris is an emerging, multidrug-resistant yeast that causes invasive infections associated with high mortality [1]. Candida auris was first reported in 2009 [2] and has since been identified in over 30 countries on 6 continents [3]. It is listed as an urgent threat in the Center for Disease Control and Prevention’s 2019 Antibiotic Resistance Threat Report [4], because it tends to be resistant to multiple classes of antifungals and can spread in healthcare settings, causing large, multifacility outbreaks. Candida auris appears to be able to spread readily in healthcare settings for 2 main reasons: it can colonize a patient’s skin and other body sites for prolonged periods, and it can contaminate and persist in the healthcare environment [5–7]. Colonization puts patients at risk for invasive infection; ~5–10% of known colonized patients develop invasive infections [8, 9]. Colonization, especially with the high organism burden found in many patients colonized with C. auris, also allows for shedding into the environment and transmission to others [10, 11]. Furthermore, quaternary ammonium compounds, a commonly used class of disinfectants, do not yield the target log reduction in C. auris for effective disinfection [12].
In June 2016, the Centers for Disease Control and Prevention released a clinical alert regarding C. auris, and the first US cases were reported within 1 month [13, 14]. By September 2016, 14 cases were identified in New York. By September 2018, 2 years later, the New York State Department of Health (NYSDOH) had identified 515 people in New York who were infected or colonized with C. auris [15]. Many of those infected or colonized were residents on ventilator units in skilled nursing facilities (vSNFs) and had tracheostomies, often requiring mechanical ventilation and percutaneous gastrostomy tubes [16, 17]. Contact tracing and epidemiologic investigations surrounding cases earlier in the epidemic in New York revealed that the prevalence of C. auris colonization in vSNF units was nearly 10 times higher than the prevalence in skilled nursing facilities that did not provide care for ventilated residents [16]. Although C. auris cases are commonly detected in acute care hospitals and transmission has been documented to occur in those facilities, epidemiologic evidence—including the fact that most patients had resided in a high-acuity postacute care facility, such as a vSNF or long-term acute care hospital (LTACH) in the months before diagnosis with C. auris—suggests that the potential for transmission is considerable in these facilities. Infection control efforts to prevent transmission of multidrug-resistant organisms (MDROs) have historically focused primarily on acute care hospitals, but emerging evidence suggests that high-acuity postacute care facilities like vSNFs and LTACHs are important sources of MDRO transmission, in part because of the concentration of patients with severe underlying conditions, the prolonged lengths of stay, and fewer resources being allocated for infection control than in hospitals. LTACHs provide hospital-level care for patients requiring more than short-term hospitalization, including those with ongoing ventilator dependence; vSNFs provide nursing-level care, especially for those unable to be weaned off a ventilator even after an LTACH stay. Residents often transfer across an interconnected network of postacute care facilities and acute care hospitals, allowing for interfacility transmission of MDROs to shared populations; vSNFs and LTACHs can serve as amplifiers for MDRO spread throughout a region, as was seen in Chicago for carbapenem-resistant Enterobacteriaceae [18]. Because available evidence suggested that a substantial proportion of patients with C. auris in New York had received care in vSNFs (notably, New York has only 3 LTACHs), and information about C. auris in this population remains limited, we aimed to identify the factors associated with C. auris colonization in New York vSNFs to inform infection prevention measures.
METHODS
Settings and Study Design
Following detection of the first C. auris cases in 2016 and through 2018, the NYSDOH intensively tracked cases of C. auris infection identified during clinical care in New York, and conducted contact tracing at facilities where case residents resided in the 90 days before C. auris infection. To identify asymptomatic colonized people at affected facilities, given their contribution to transmission, the NYSDOH conducted point prevalence surveys (PPS), in which residents were swabbed in the nares, axilla, and groin. These PPS were conducted on units where the index person currently resided or had resided in the previous 90 days. Swabs were sent to the New York State (NYS) Wadsworth Center Laboratory for C. auris polymerase chain reaction testing and culture, as previously described [11, 16]. The same swab was used for both tests. People with culture-positive swabs were considered screening cases. During August 2016–September 2018, a total of 104 PPS were conducted at 64 facilities, including 22 vSNFs.
Considering the ongoing transmission in NYS, we conducted a case-control investigation to assess the factors associated with C. auris colonization in vSNFs that met all of the following 3 criteria: at least 1 PPS conducted during August 2016–September 2018, at least 5 people with C. auris colonization identified on any of the surveys performed at the facility, and willingness to participate in the investigation. We chose facilities with at least 5 colonized people for ease of execution of the investigation. A case was defined as a resident of a selected New York vSNF who had C. auris colonization (ie, screening case; not active infection), as determined by a positive culture for C. auris from a PPS swab collected during August 2016–September 2018. The control group included residents of the same vSNFs, screened during the same PPS, who were not culture positive for C. auris. We included every case identified during a PPS and used a random number generator to select controls sampled on the same date until we had up to 4 times as many controls as cases for each PPS.
We reviewed medical records for cases and controls and abstracted data using a standardized case report form. We collected information on resident demographic characteristics, underlying conditions, functional status, use of antibacterial and antifungal medications 90 days before screening, devices (including mechanical ventilation and tracheostomy), history of MDRO infection or colonization, and room location history. We also collected data on healthcare facility transfers, including site of hospitalization, dates of admission and discharge at each healthcare facility, and total number of facility transfers in the 6 months before C. auris screening. All facilities were provided with infection control guidance that included recommendations for personal protective equipment, hand hygiene, and proper environmental disinfection. The degree to which the facilities implemented these recommendations varied, but we were unable to measure this on a numerical scale.
Variables and Definitions
A history of MDRO colonization was defined by a history of contact precautions in the previous 90 days for Clostridioides difficile, carbapenem-resistant Enterobacteriaceae, carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Acinetobacter baumannii, vancomycin-resistant Enterococcus spp., methicillin-resistant Staphylococcus aureus (MRSA), or extended-spectrum beta-lactamase producing bacteria. We used an age-adjusted Charlson comorbidity index to evaluate the severity of underlying conditions [19]. To assess functional status, we used a modified Barthel index for activities of daily living (ADLs) [20]. We included personal hygiene, bathing, feeding, toileting, bowel control, bladder control, and chair/bed transfers, but did not include ambulation, stair climbing, and dressing, which are included in the original Barthel index, as these data were unavailable in medical records. Each of the ADLs was scored as fully independent, needs assistance, or unable to perform task. All-cause mortality was determined based on information abstracted from the electronic and facility records at 30 and 90 days.
We also collected data on roommate colonization status and proximity to other colonized residents. When a room occupancy history was available, we recorded which rooms were previously occupied by a colonized resident. We also recorded whether cases and controls resided in a room adjacent to a colonized resident’s room. Adjacent was defined as sharing a wall with another room, and these data were only collected when facility floor maps were available to confirm room locations. Lastly, we recorded whether the resident resided in a single-, double-, triple-, or quadruple-occupancy room at the time of C. auris screening.
Statistical Analysis
Variables were assessed for associations using univariate and multivariable logistic regression. Risk factors and potential confounders included in the multivariable logistic regressions were selected using a priori information based on the use of directed acyclic graphs. These graphs were used to build estimated causal structures to determine potential confounders. Unadjusted odds ratios (ORs) and adjusted odds ratios (aORs) were calculated with 95% confidence intervals (CIs). All data were analyzed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Of the 45 vSNFs in the C. auris–affected areas of New York, 22 vSNFs had cared for C. auris cases within 90 days of diagnosis (48.9%); 8 of these vSNFs (17.8%) had ≥5 cases identified on PPS during August 2016–September 2018, but only 6 fit all criteria. All 6 were included in our investigation, encompassing 81% of surveillance cases identified by PPS in vSNFs where ≥5 cases were identified. The 6 included vSNFs had a median of 271 residential beds (range, 200–320) and 21 ventilator beds (range, 10–80). These 6 vSNFs included 11 units that could provide care for residents with advanced respiratory needs (range, 1–4 per facility).
Across these 6 vSNFs, 12 PPS were conducted, with 2 vSNFs having 3 PPS, 2 having 2 PPS, and 2 having a single PPS. Multiple PPS were prompted by the burden of colonization found on prior PPS or the detection of a clinical or surveillance case at an acute care facility with known passthrough to a given vSNF, as well as the facility’s willingness to participate. The median prevalence of C. auris colonization on PPS was 12% (range, 3–33%). These PPS identified 60 cases, allowing for the selection of 218 controls for the investigation (4 controls were not available for all cases). Residents in the case and control groups did not differ by age (median, 68.0 years [range, 26–97] for cases vs 69.5 years [range, 20–98] for controls; P = .771), sex (50.0% male for cases vs 47.2% male for controls; P = .702), or race/ethnicity (Table 1).
Table 1.
Comparison of Clinical Characteristics Between Patients Colonized With Candida auris and Patients With Negative C. auris Screening Swabs
| Clinical characteristics | Case, n = 60, n (%) | Control, n = 218, n (%) | Odds ratioa (95% CI) |
|---|---|---|---|
| Demographics | |||
| Male, ncase = 60; ncontrol = 214 | 30 (50.0) | 101 (47.2) | 1.12 (.62–2.02) |
| Age, median (range) | 68.0 (26–97) | 69.5 (20–98) | 1.00 (.98–1.02) |
| Race/ethnicity, ncase = 58; ncontrol = 192 | |||
| White, non-Hispanic | 25 (43.1) | 72 (37.5) | Ref |
| Black, non-Hispanic | 17 (29.3) | 62 (32.3) | .78 (.38–1.60) |
| Asian, non-Hispanic | 9 (15.5) | 34 (17.7) | 1.81 (.31–2.13) |
| Hispanic | 6 (10.3) | 23 (12.0) | .83 (.27–2.52) |
| Other, non-Hispanic | 1 (1.7) | 1 (.5) | 2.97 (.17–50.64) |
| BMI, ncase = 55; ncontrol = 197 | |||
| Underweight | 5 (9.1) | 16 (8.1) | 1.08 (.33–3.54) |
| Normal | 23 (41.8) | 73 (37.1) | Ref |
| Overweight | 13 (23.6) | 62 (31.5) | .65 (.30–1.43) |
| Obese | 14 (25.5) | 46 (23.4) | .98 (.44–2.18) |
| Clinical characteristics | |||
| Diabetes | 25 (41.7) | 87 (39.9) | 1.05 (.58–1.91) |
| Neurologic diseaseb | 49 (81.7) | 160 (73.4) | 1.61 (.77–3.38) |
| Dementia | 21 (35.0) | 63 (28.9) | 1.35 (.72–2.55) |
| Cardiovascular diseasec | 30 (50.0) | 126 (57.8) | .69 (.38–1.27) |
| Myocardial infarction | 2 (3.3) | 12 (5.5) | .56 (.12–2.73) |
| Congestive heart failure | 18 (30.0) | 50 (22.9) | 1.46 (.74–2.91) |
| Peripheral vascular disease | 5 (8.3) | 10 (4.6) | 2.10 (.64–6.84) |
| Cerebrovascular attack | 17 (28.3) | 88 (40.4) | .55 (.29–1.05) |
| Chronic kidney disease | 7 (11.7) | 26 (11.9) | .95 (.38–2.37) |
| Mild liver diseased | 1 (1.7) | 5 (2.3) | .71 (.08–6.33) |
| Hemiplegia/paraplegia/quadriplegia | 5 (8.3) | 40 (18.3) | .36 (.13–1.02) |
| CCI with age, median (IQR) | 5.33 (4–7) | 5.56 (4–7) | .95 (.84–1.08) |
| Modified Barthel index, median (range), ncase = 47; ncontrol = 184 | 0 (0–10) | 0 (0–60) | .84 (.70–1.01) |
| Medical devices present | 60 (100.0) | 194 (89.0) | N/A |
| Tracheostomy | 58 (96.7) | 179 (82.1) | 11.33 (2.11–60.77) |
| Mechanical ventilation | 53 (88.3) | 138 (63.3) | 5.62 (2.22–14.23) |
| Surgically placed feeding tube | 56 (93.3) | 180 (82.6) | 3.04 (1.01–9.19) |
| Urinary catheter | 24 (40.0) | 41 (18.8) | 3.43 (1.75–6.70) |
| Central venous catheter | 5 (8.3) | 6 (2.8) | 3.31 (.93–11.83) |
| Any MDRO in the 90 days prior to PPSe | 26 (43.3) | 53 (24.3) | 2.58 (1.36–4.89) |
| Clostridiodes difficile | 10 (16.7) | 18 (8.3) | 2.29 (.98–5.40) |
| Methicillin-resistant Staphylococcus aureus | 8 (13.3) | 16 (7.3) | 1.93 (.76–4.89) |
| Carbapenem-resistant Enterobacteriaceae | 5 (8.3) | 6 (2.8) | 3.52 (.99–12.52) |
| ESBL-producing bacteria | 4 (6.7) | 10 (4.6) | 1.58 (.46–5.37) |
| Vancomycin-resistant Enterococcus spp. | 4 (6.7) | 3 (1.4) | 5.06 (1.06–24.21) |
| Carbapenem-resistant Pseudomonas aeruginosa | 3 (5.0) | 6 (2.8) | 1.86 (.42–8.15) |
| Carbapenem-resistant Acinetobacter baumannii | 1 (1.7) | 4 (1.8) | .95 (.10–9.27) |
| Mortality | |||
| 90-day all-cause mortality | 10 (16.7) | 9 (4.1) | 4.80 (1.79–12.89) |
| 30-day all-cause mortality | 3 (5.0) | 5 (2.3) | 2.21 (.49–9.96) |
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; CI, confidence interval; ESBL, extended-spectrum beta-lactamase; IQR, interquartile range; MDRO, multidrug-resistant organism; PPS, point prevalence survey.
aUnivariate models used to calculate odds ratios included PPS in the models.
bNeurologic disease includes cerebral palsy, chronic cognitive deficit, dementia, epilepsy, seizure disorders, multiple sclerosis, neuropathy, Parkinson’s disease, and others.
cCardiovascular disease includes cerebrovascular attack, stroke, transient ischemic attack, congenital heart disease, congestive heart failure, myocardial infarction, and others.
dMild liver disease includes cirrhosis without portal hypertension and chronic hepatitis.
eAny MDRO includes Clostridioides difficile, carbapenem-resistant Enterobacteriaceae, carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Acinetobacter baumannii, vancomycin-resistant Enterococcus spp., methicillin-resistant Staphylococcus aureus, or ESBL-producing bacteria, as well as coinfections with multiple MDROs. Due to coinfections, the sum of specific MDROs exceeds the number of individuals with any MDRO.
The underlying conditions did not differ between cases and controls, although multiple conditions were common in both groups. Most had neurologic disease (81.7% in cases vs 73.4% in controls; P = .190), and about half had cardiovascular disease (50.0% vs 57.8%, respectively; P = .283) and diabetes (41.7% vs 39.9%, respectively; P = .807). Similarly, Charlson comorbidity index scores did not differ between groups (mean, 5.33 [interquartile range {IQR}, 4–7] in cases vs 5.56 [IQR, 4–7] in controls; P = .543). Overall, both groups had minimal ability to perform ADLs, with median Barthel index scores of 0 [range, 0–10 in cases vs 0–60 in controls].
Both cases and controls often had medical devices (100% of cases vs 89.0% of controls), but the presence of certain devices was associated with C. auris colonization, including tracheostomy (96.7% vs 82.1%, respectively; OR, 11.33; 95% CI, 2.1–60.8), mechanical ventilation (88.3% vs 63.3%, respectively; OR, 5.62; 95% CI, 2.2–14.2), percutaneous gastrostomy or jejunostomy tubes (93.3% vs 82.6%, respectively; OR, 3.04; 95% CI, 1.0–9.2), and indwelling urinary catheters (40.0% vs 18.8%, respectively; OR, 3.43; 95% CI, 1.8–6.7). A history of MDRO colonization was also more common among cases than controls (43.3% vs 24.3%, respectively; OR, 2.58; 95% CI, 1.4–4.9). The 90-day all-cause mortality rate was higher among cases (16.7%) than controls (4.1%).
Antibacterial use in the 90 days before C. auris screening was more common among cases than controls, with cases receiving a median of 3 (IQR, 1–5) courses of systemic antibacterial drugs, compared with 1 course (IQR, 0–3) among controls (OR, 1.34; 95% CI, 1.2–1.5; Table 2). The most commonly prescribed antibacterial drugs were carbapenems (43.3% of cases vs 16.1% of controls; P < .01), vancomycin (41.7% vs 21.6%, respectively; P < .01), levofloxacin (15.0% vs 17.9%, respectively; P = .602), cefepime (15.0% vs 10.6%, respectively; P = .341), and piperacillin/tazobactam (13.3% vs 10.6%, respectively; P = .546). Receipt of carbapenems (OR, 4.68; 95% CI, 2.4–9.2) and receipt of vancomycin (OR, 2.71; 95% CI, 1.4–5.1) in the 90 days before PPS were associated with C. auris colonization. Receipt of systemic antifungals was uncommon in both cases and controls (11.7% vs 6.4%, respectively; P = .175). In both cases and controls, the most commonly prescribed antifungals were fluconazole (11.7% vs 3.2%, respectively; P = .008) and nystatin (0% vs 4.6%, respectively; P = .092). Receipt of fluconazole in the 90 days before PPS was associated with C. auris colonization (OR, 4.21; 95% CI, 1.4–12.9).
Table 2.
Antibacterial Use, Antifungal Use, Acute Care Hospitalizations Prior to Point Prevalence Survey, and Roommate Analyses in Candida auris Colonized Cases and Controls, New York, 2016–2018
| Drug or hospital exposure | Case, n = 60 | Control, n = 218 | Odds ratioa (95% CI) |
|---|---|---|---|
| Antibiotic use, median (IQR) | |||
| Total antibiotic courses in the 90 days before PPS | 3 (1–5) | 1 (0–3) | 1.31 (1.15–1.49) |
| Systemic antibiotic courses in the 90 days before PPS | 3 (1–5) | 1 (0–3) | 1.34 (1.16–1.54) |
| Received any antibiotic in the 90 days before PPS, n (%) | 51 (85.0) | 152 (69.7) | 2.75 (1.24–6.11) |
| ≥1 systemic antibiotic in the 90 days before PPS, n (%) | 48 (80.0) | 139 (63.8) | 2.51 (1.22–5.17) |
| Specific antibacterial drugs used in the 90 days before PPS, n (%) | |||
| Received vancomycin or a carbapenem | 36 (60.0) | 64 (29.4) | 4.30 (2.24–8.27) |
| Carbapenems | 26 (43.3) | 35 (16.1) | 4.68 (2.38–9.21) |
| Vancomycin | 25 (41.7) | 47 (21.6) | 2.71 (1.44–5.10) |
| Levofloxacin | 9 (15.0) | 39 (17.9) | .82 (.36–1.84) |
| Cefepime | 9 (15.0) | 23 (10.6) | 1.58 (.66–3.75) |
| Piperacillin/tazobactam | 8 (13.3) | 23 (10.6) | 1.31 (.55–3.15) |
| Antifungal use, median (IQR) | |||
| Total antifungal courses in the 90 days before PPS | 0 (0–1) | 0 (0–1) | .79 (.52–1.19) |
| Systemic antifungal courses in the 90 days before PPS | 0 (0–0) | 0 (0–0) | 1.48 (.81–2.71) |
| ≥1 systemic antifungal in the 90 days before PPS, n (%) | 7 (11.7) | 14 (6.4) | 2.11 (.77–5.80) |
| Specific antifungal drugs used in the 90 days before PPS, n (%) | |||
| Fluconazole | 7 (11.7) | 7 (3.2) | 4.21 (1.37–12.94) |
| Nystatin | 0 (0) | 10 (4.6) | .37 (.02–2.29)b |
| ACH visits, median (IQR) | |||
| Total ACH visits in the 6 months before PPS | 1 (1–3) | 1 (0–2) | 1.29 (1.07–1.56) |
| Multi-day ACH visits in the 6 months before PPS | 1 (1–2) | 1 (0–1) | 1.48 (1.18–1.85) |
| ≥1 ACH visits in the 6 months before PPS, n (%) | 47 (81.0) | 112 (56.3) | 3.53 (1.69–7.41) |
| Roommate colonization status, ncase = 59; ncontrol = 200 | |||
| Room with a colonized roommate | 4 (6.8) | 27 (13.5) | .44 (.14–1.33) |
| Room type at time of screening,c ncase = 57; ncontrol = 198 | |||
| In a room with 1 bed | 8 (14.0) | 28 (14.1) | Ref |
| In a room with 2 beds | 42 (73.7) | 151 (76.3) | 1.00 (.41–2.43) |
| In a room with 4 beds | 7 (12.3) | 19 (9.6) | 1.46 (.42–5.01) |
Abbreviations: ACH, acute care hospital; CI, confidence interval; IQR, interquartile range; PPS, point prevalence survey.
aUnivariate models used to calculate odds ratios included PPS in the models.
bCalculated with a Haldane-Anscombe correction.
cThere were no rooms containing 3 beds.
Cases more frequently had ≥1 transfer to an acute care hospital in the 6 months before C. auris screening than did controls (81.0% vs 56.3%, respectively; OR, 3.53; 95% CI, 1.7–7.4).
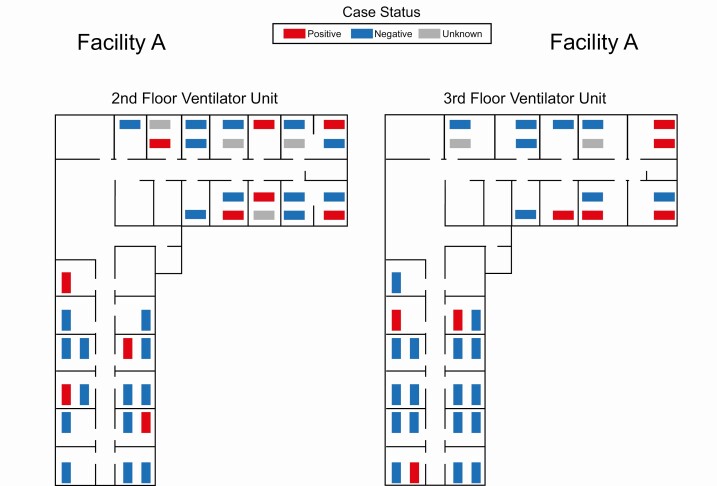
Lastly, we analyzed whether roommates or room location were associated with C. auris colonization. Among residents with room location data available (ncase = 59; ncontrol = 200), the odds of having a roommate colonized with C. auris at the time of the PPS were similar among cases and controls (OR, 0.44; 95% CI, .1–1.3). We only found 2 rooms that housed multiple residents colonized with C. auris concurrently. Among residents with room type data available (ncase = 57; ncontrol = 198), the odds of C. auris colonization were similar between residents staying in single-occupancy rooms and residents staying in double-occupancy (OR, 1.00; 95% CI, .4–2.4) or quadruple-occupancy rooms (OR, 1.46; 95% CI, .4–5.0). None of the facilities had triple-occupancy rooms. Examples of the maps used to generate the data for these analyses are depicted in the maps in Figure 1 for Facility A, for which the most complete data were available for analyses.
Figure 1.
Facility maps depicting the Candida auris colonization status and location of patients at the time of a single point prevalence survey in the ventilator unit of a skilled nursing facility, New York, 2016–2018.
In multivariable logistic regression analyses, compared with controls, cases were more likely to be mechanically ventilated (aOR, 5.88; 95% CI, 2.3–15.4), to have been hospitalized in the 6 months before screening (aOR, 4.23; 95% CI, 1.9–9.6), to have received carbapenems in the 90 days before screening (aOR, 3.52; 95% CI, 1.6–7.6), and to have received fluconazole in the 90 days before screening (aOR, 6.0; 95% CI, 1.6–22.6; Table 3).
Table 3.
Multivariable Logistic Regression Models for Assessing Factors for Association With Candida auris Colonization, New York, 2016–2018
| Factors | aOR | 95% Confidence Interval | |
|---|---|---|---|
| Lower | Upper | ||
| Mechanically ventilateda | 5.88 | 2.25 | 15.37 |
| Any ACH visit in the 6 months prior to PPSb | 4.23 | 1.87 | 9.60 |
| Received a carbapenem in the 90 days prior to PPSc | 3.52 | 1.62 | 7.63 |
| Received systemic fluconazole in the 90 days prior to PPSd | 5.98 | 1.58 | 22.64 |
| Received vancomycin in the 90 days prior to PPSe | 1.65 | .75 | 3.67 |
| Any MDRO in the 90 days prior to PPSf | 1.25 | .56 | 2.76 |
| Room with a colonized roommateg | .37 | .12 | 1.16 |
| Room type at time of screeningh | |||
| In a room with 1 bed | Ref | Ref | Ref |
| In a room with 2 beds | 1.44 | .55 | 3.80 |
| In a room with 4 beds | 2.04 | .54 | 7.70 |
Abbreviations: ACH, acute care hospital; aOR, adjusted odds ratio; CCI, Charlson comorbidity index; MDRO, multidrug-resistant organism; PPS, point prevalence survey.
aThe model for mechanical ventilation includes race/ethnicity, sex, age, and CCI.
bThe model for ACH visits includes race/ethnicity, sex, age, CCI, and body mass index.
cThe model for receipt of a carbapenem includes race/ethnicity, sex, age, CCI, ACH visits, and presence of a urinary catheter.
dThe model for receipt of systemic fluconazole includes race/ethnicity, sex, age, CCI, and ACH visits.
eThe model for receipt of vancomycin includes race/ethnicity, sex, age, CCI, ACH visits, and presence of a urinary catheter.
fThe model for MDROs includes race/ethnicity, sex, age, CCI, ACH visits, and receipt of broad-spectrum antibiotics.
gThe model for rooming with a colonized roommate includes presence of an MDRO in the 90 days prior to PPS, sex, and mechanical ventilation.
hThe models for room type include the presence of an MDRO in the 90 days prior to PPS, sex, and mechanical ventilation.
DISCUSSION
In NYS and other states with C. auris outbreaks, high-acuity postacute care facilities, like vSNFs, care for a disproportionate number of C. auris cases, and in this population of vulnerable individuals, the risk of transmission is high. This investigation identified specific invasive medical devices, recent antimicrobial use, recent hospitalization, and colonization with other MDROs as resident-level risk factors associated with C. auris colonization in vSNFs. The risk factors for C. auris colonization closely resemble many of those for other MDROs, like carbapenemase-producing organisms, which are also a major problem in high-acuity postacute care facilities. For example, in Orange County, California, LTACHs were found to have an 80% prevalence of MDROs [21]. Similarly, in Chicago, 40% of vSNF residents were colonized with carbapenemase-producing organisms, and these facilities likely served as reservoirs of transmission to other facilities [22]. Like these organisms, the prevalence of C. auris has exceeded 50% in certain Chicago vSNFs and has reached 33% in some New York vSNF units [17, 23].
Our study underscores the importance of targeting infection prevention measures to facilities that care for chronically ventilated residents as part of a regional approach for containment of novel, resistant pathogens, particularly given the high level of MDRO co-colonization and frequent hospitalizations. The early identification of people with C. auris infection or colonization is key to controlling spread, and this investigation and others suggest that vSNF and LTACH populations are often high-yield sites for early-detection interventions. Admission and discharge screening at acute care hospitals could be considered for patients being admitted from or discharged to vSNFs or LTACHs in areas with known C. auris cases. Admission screening in vSNFs may be challenging because of limited staff to perform screenings and lower capacities for packaging and shipping of specimens, while admission screening in LTACHs may be more feasible. Early C. auris detection can allow for the targeted implementation of basic infection control measures, such as hand hygiene, transmission-based precautions, effective environmental cleaning and disinfection, and the development of cohorted areas with designated staff to care for affected patients in order to limit transmission. Communication between facilities about residents’ C. auris colonization status is also essential in ensuring receiving facilities implement proper precautions.
Just as antibiotic use is a risk factor for MDRO colonization and other Candida infections, we observed an association between broad-spectrum antibacterial use and C. auris colonization [24–27]. While vancomycin was found to be associated with colonization, it is worth noting that this drug is often given in combination with antibiotics with Gram-negative coverage, which have been proven to disrupt the gut microbiome [28–30]. Systemic antibacterial use can lead to intestinal Candida overgrowth [31]; similarly, broad-spectrum antibiotic use may disrupt the skin microbiome, and the potential selection pressure exerted by use of fluconazole, a drug most C. auris isolates are resistant to, may facilitate C. auris colonization. Further study of the skin microbiome is needed, including of the role of the fungal mycobiome and impacts of antimicrobial use. Antimicrobial stewardship, an important part of any medical care, may be useful in reducing the risk of C. auris colonization.
A notable finding from this investigation was the lack of association between a resident’s C. auris colonization status and the number of beds in a room or the colonization status of roommates. These findings are similar to those for MRSA transmission, in which roommates accounted for only a small portion of the risk of MRSA acquisition [32]. Unlike influenza or group A Streptococcus, for which roommates are at the greatest risk, MRSA acquisition risk is not limited to roommates [33, 34]. While a ventilator-dependent resident who recently returned from a hospital after receiving broad-spectrum antibiotics and who shares a room with a resident colonized with C. auris is likely at risk for acquiring C. auris colonization due to proximity to another colonized resident and having the risk factors for colonization, the risk of colonization is not limited to these roommates alone. The finding that cases may be distributed in an entire unit underscores the need to look at a unit level when defining the potential group at risk when facilities attempt to control this pathogen. Potential explanations for these findings include contamination of mobile medical equipment and inadequate environmental disinfection [6, 7, 11, 35, 36]. Using dedicated medical equipment for each resident, conducting meticulous cleaning and disinfection with appropriate products, and adhering to proper hand hygiene and personal protective equipment use when indicated may be important in preventing transmission on these wards [8, 14].
There were several additional limitations to this study. First, because PPS occurred at specific points in time, we did not know how long before positive tests residents became colonized with C. auris. Therefore, we may have misattributed some exposures as occurring before colonization when they in fact occurred after. Colonization may also have occurred at other facilities, such as hospitals. Medical records rarely contained start and end dates for antimicrobials, and we were unable to determine days of therapy nor examine a dose-response relationship between use and colonization. We acknowledge that longitudinal data on the colonization status and location of roommates were limited. Our results were based on the roommate’s colonization status at the time of the PPS because longitudinal roommate colonization data were rarely available, so cases may have shared a room with other colonized residents whose colonization status was not yet known or whose room location history was not available. In addition, quadruple-occupancy rooms on the ventilator-capable units were less common than double and single rooms in these facilities, which might be different in other postacute care facilities, including those outside NYS. This investigation focused primarily on resident-level factors, whereas facility-level factors, including infection prevention and control measures, likely also play a strong role in the transmission of C. auris and other MDROs. Facility-level factors are more difficult to assess from medical records, and likely changed over time as interventions were implemented in response to C. auris cases.
Residents in vSNFs and other high-acuity postacute care settings are at increased risk for colonization with C. auris and other MDROs, and this can allow for amplification through a healthcare network. In our investigation, the odds of C. auris colonization differed with the prevalence of invasive devices, exposure to broad-spectrum antibiotics, receipt of fluconazole, and recent hospitalizations, but not with being a roommate of a resident with C. auris. As such, contact tracing for C. auris should potentially consider the entire unit at risk, and assessment for colonization should extend beyond roommates. Interventions that may mitigate spread include admission and discharge screening in facilities where it is feasible, discharge screening from acute care hospitals, interfacility communication about MDRO status upon transfer, and robust infection control efforts. Given the elevated prevalence of MDROs, including C. auris, in some high-acuity postacute care facilities, a better understanding of practical ways to prevent transmission, including bolstering the basics of infection prevention and novel strategies beyond the basics in these settings, is urgently needed to reduce the spread of these MDROs through the broader healthcare system.
Notes
Study group. Additional members of the New York Candida auris Investigation Workgroup are Karen Southwick, Ronald Jean Denis, Richard Erazo, and Rafael Fernandez (New York State Department of Health, MARO, New Rochelle, New York); Coralie Bucher (New York State Department of Health, Albany, New York); Lynn Leach and Yan Zhu (Wadsworth Laboratory, Albany, New York); and Emily Lutterloh (New York State Department of Health and Albany School of Public Health, Albany, New York).
Acknowledgments. The authors thank the staff and leadership of the affected ventilator-capable skilled nursing facilities in New York State who participated in this project.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the US Centers for Disease Control and Prevention Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases Cooperative Agreement Notice of Funding Opportunity number CK14-1401 (grant number NU50CK000423 to the New York State Department of Health) and the New York State Department of Health.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
New York Candida auris Investigation Workgroup:
Karen Southwick, Ronald Jean Denis, Richard Erazo, Rafael Fernandez, Coralie Bucher, Lynn Leach, Yan Zhu, and Emily Lutterloh
References
- 1. Chowdhary A, Sharma C, Meis JF. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 2017; 13:e1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 2009; 53:41–4. [DOI] [PubMed] [Google Scholar]
- 3. Lone SA, Ahmad A. Candida auris—the growing menace to global health. Mycoses 0. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/myc.12904. Accessed 17 April 2019. [DOI] [PubMed]
- 4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2019. Available at: www.cdc.gov/DrugResistance/Biggest-Threats.html. Accessed 19 November 2019.
- 5. Bergeron G, Bloch D, Murray K, et al. Candida auris colonization in the community setting-New York City (NYC), 2017—2018. In: 2019. CSTE Annual Conference. Raleigh, North Carolina. 2019. Available at: https://cste.confex.com/cste/2019/meetingapp.cgi/Paper/11146. Accessed 22 June 2020. [Google Scholar]
- 6. Adams E, Quinn M, Tsay S, et al. Candida auris in healthcare facilities, New York, USA, 2013–2017. Emerg Infect Dis 2018; 24:1816–24. Available at: https://wwwnc.cdc.gov/eid/article/24/10/18-0649_article. Accessed 5 June 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eyre DW, Sheppard AE, Madder H, et al. A Candida auris outbreak and its control in an intensive care setting. N Engl J Med 2018; 379:1322–31. [DOI] [PubMed] [Google Scholar]
- 8. Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 2016; 5:35. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5069812/. Accessed 17 April 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Southwick K, Adams EH, Greenko J, et al. New York State 2016–2018: progression from Candida auris colonization to bloodstream infection. Open Forum Infect Dis 2018; 5:S594–5. [Google Scholar]
- 10. Sexton J, Bentz ML, Black S, et al. Mechanisms of Candida auris transmission within the healthcare environment. 2019. Available at: https://eventpilotadmin.com/web/page.php?page=IntHtml&project=ASM19&id=485. Accessed 18 November 2019.
- 11. Zhu Y, O’Brien B, Leach L, et al. Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: impact and lessons learned. J Clin Microbiol 2020; 58:e01503–19. Available at: https://jcm.asm.org/content/58/4/e01503-19. Accessed 22 June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cadnum JL, Shaikh AA, Piedrahita CT, et al. Effectiveness of disinfectants against Candida auris and other candida species. Infect Control Hosp Epidemiol 2017; 38:1240–3. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Clinical alert to US healthcare facilities—June 2016. Candida auris, fungal diseases. 2019. Available at: https://www.cdc.gov/fungal/candida-auris/candida-auris-alert.html. Accessed 17 April 2019.
- 14. Vallabhaneni S, Kallen A, Tsay S, et al. ; MSD . Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013-August 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1234–7. [DOI] [PubMed] [Google Scholar]
- 15. Leach L, Zhu Y, Chaturvedi S. Development and validation of a real-time PCR assay for rapid detection of Candida auris from surveillance samples. J Clin Microbiol 2018; 56:e01223–17. Available at: https://jcm.asm.org/content/56/2/e01223-17. Accessed 14 February 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adams EH, Quinn M, Ostrowsky B, et al. The value added from Candida auris point prevalence and environmental studies in New York State. 2018. Available at: https://idsa.confex.com/idsa/2018/webprogram/Paper72423.html. Accessed 21 May 2019.
- 17. Kerins JL, Tang AS, Forsberg K, et al. Rapid emergence of Candida auris in the Chicago region. 2018. Available at: https://idsa.confex.com/idsa/2018/webprogram/Paper73072.html. Accessed 21 May 2019.
- 18. Hayden MK, Lin MY, Lolans K, et al. ; Centers for Disease Control and Prevention Epicenters Program . Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 2015; 60:1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 20. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965; 14:61–5. [PubMed] [Google Scholar]
- 21. McKinnell JA, Singh RD, Miller LG, et al. The SHIELD Orange County project: multidrug-resistant organism prevalence in 21 nursing homes and long-term acute care facilities in Southern California. Clin Infect Dis 2019; 69:1566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin MY, Froilan MC, Lolans K, et al. The importance of ventilator skilled nursing facilities (vSNFs) in the regional epidemiology of carbapenemase-producing organisms (CPOs). Open Forum Infect Dis 2017; 4:S137–8. [Google Scholar]
- 23. Pacilli M, Kerins J, Clegg W, et al. Repeated prevalence surveys for Candida auris and carbapenemase-producing organisms in a ventilator-capable skilled nursing facility, Chicago 2018. In: SHEA 2019. Boston, MA: April 2019.
- 24. Russo A, Falcone M, Fantoni M, et al. Risk factors and clinical outcomes of candidaemia in patients treated for Clostridium difficile infection. Clin Microbiol Infect 2015; 21:493.e1–e4. [DOI] [PubMed] [Google Scholar]
- 25. Falcone M, Venditti M, Sanguinetti M, Posteraro B. Management of candidemia in patients with Clostridium difficile infection. Expert Rev Anti Infect Ther 2016; 14:679–85. [DOI] [PubMed] [Google Scholar]
- 26. Seelig MS. Mechanisms by which antibiotics increase the incidence and severity of candidiasis and alter the immunological defenses. Bacteriol Rev 1966; 30:442–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang J, Cassone M, Gibson K, et al. Gut microbiota features on nursing home admission are associated with subsequent acquisition of antibiotic resistant organism colonization. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa662/5849515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pérez-Cobas AE, Gosalbes MJ, Friedrichs A, et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 2013; 62:1591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Connelly S, Subramanian P, Hasan NA, Colwell RR, Kaleko M. Distinct consequences of amoxicillin and ertapenem exposure in the porcine gut microbiome. Anaerobe 2018; 53:82–93. [DOI] [PubMed] [Google Scholar]
- 30. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 2011; 108:4554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol 2013; 21:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stone ND, Lewis DR, Johnson TM 2nd, et al. ; Southeast Veterans Affairs Long-Term Care Methicillin-Resistant Staphylococcus aureus Cooperative . Methicillin-resistant Staphylococcus aureus (MRSA) nasal carriage in residents of Veterans Affairs long-term care facilities: role of antimicrobial exposure and MRSA acquisition. Infect Control Hosp Epidemiol 2012; 33:551–7. [DOI] [PubMed] [Google Scholar]
- 33. Furuno JP, Shurland SM, Zhan M, et al. Comparison of the methicillin-resistant Staphylococcus aureus acquisition among rehabilitation and nursing home residents. Infect Control Hosp Epidemiol 2011; 32:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen B, Cohen CC, Løyland B, Larson EL. Transmission of health care-associated infections from roommates and prior room occupants: a systematic review. Clin Epidemiol 2017; 9:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Escandón P, Chow NA, Caceres DH, et al. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin Infect Dis 2019; 68:15–21. [DOI] [PubMed] [Google Scholar]
- 36. Welsh RM, Bentz ML, Shams A, et al. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol 2017; 55:2996–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]