Abstract
Introduction: The study of intraepithelial lymphocytes (IEL) by flow cytometry is a useful tool in the diagnosis of coeliac disease (CD). Previous data showed that an increase in %TCRγδ+ and decrease of %CD3− IEL constitute a typical CD cytometric pattern with a specificity of 100%. However, there are no data regarding whether there are differences in the %TCRγδ+ related to sex, age, titers of serology, and degree of histological lesion. Study aims: To confirm the high diagnostic accuracy of the coeliac cytometric patterns. To determine if there are differences between sex, age, serology titers, and histological lesion grade. Results: We selected all patients who fulfilled “4 of 5” rule for CD diagnosis (n = 169). There were no differences in %TCRγδ+ between sexes (p = 0.909), age groups (p = 0.986), serology titers (p = 0.53) and histological lesion grades (p = 0.41). The diagnostic accuracy of complete CD cytometric pattern was: specificity 100%, sensitivity 82%, PPV 100%, NPV 47%. Conclusion: We confirmed, in a validation cohort, the high diagnostic accuracy of complete CD pattern irrespective of sex, age, serology titers, and grade of mucosal lesion.
Keywords: coeliac disease, flow cytometry, age, sex, lesion grade, intraepithelial lymphocytes TCRγδ+
1. Introduction
The diagnosis of coeliac disease (CD) is based on several criteria including positive serology, a spectrum of duodenal damage and clinical symptoms and/or risk conditions, and response to a gluten-free diet (GFD) in patients bearing the HLA-DQ2 or DQ8 genotypes. When some of these criteria are lacking, especially when serology is negative or the duodenal atrophy is not complete, the CD diagnosis is a challenge [1]. In these difficult situations, the study of duodenal intraepithelial lymphocytes (IEL) by flow cytometry is a useful tool for CD diagnosis. It has been shown to be of value in the diagnosis of CD with atrophy [2,3,4,5] and refractory CD [6,7]. An increase in %CD3+ TCRγδ+ IEL (%TCRγδ+) with a decrease in %CD3− IEL (%CD3−) has been described as the typical pattern of CD [8].
The diagnosis of CD in the case of mild histological lesions (Marsh 1) can be difficult due to low sensitivity of serology and low specificity of the lymphocytic enteritis [9,10]. However, the diagnosis of Marsh 1 patients with CD is important because they present with similar clinical symptoms to patients with atrophy that reverse with a gluten-free diet (GFD) [11,12]. Previous ESPGHAN guidelines suggest that both an increase in %TCRγδ+ count assessed by immunohistochemical analysis of biopsies and the presence of IgA anti-tissue transglutaminase (anti-TG2) deposits increase the chances of a diagnosis of CD [7].
The increase of %TCRγδ+ has occasionally been found in some other conditions such as cow’s milk intolerance, food allergy, cryptosporidiosis, Giardiasis, Sjögren syndrome, Olmesartan enteropathy, and IgA deficiency. Nevertheless, the increase in %TCRγδ+ in these diseases tends to be mild and transient [13]. CD is the only disease in which %TCRγδ+ has been found to be systematically and permanently increased, even in patients following a GFD. The concomitant decrease in %CD3− provides increased specificity for the diagnosis [14]. Therefore, this particular cytometric pattern may be used to confirm the CD diagnosis in patients that had already started a GFD before the diagnosis confirmation.
A previous study by our group demonstrated good diagnostic accuracy (sensitivity 85%, specificity 100%, PPV 100%, and NPV 72%) for the typical CD cytometric pattern (increased %TCRγδ+ and decreased %CD3−) in the diagnosis of CD in patients with positive serology, both Marsh 1 and Marsh 3 [8]. However, these findings should be confirmed with a larger validation cohort.
Another important issue is learning whether the cut-off values established for %TCRγδ+ and %CD3− reveal a cytometric CD pattern influenced by age, sex, and degree of histological lesion. In this sense, the information is very limited, but it has been suggested that γδ+ IEL decreases with age [6].
The aims of our study were to determine: (1) whether there are differences in the percentage of TCRγδ+ IEL in CD patients related to sex, age, degree of histological lesion, levels of serology; and (2) the diagnostic accuracy in a large validation cohort of the typical cytometric CD pattern and of the increase in %TCRγδ+ IEL, without the simultaneous decrease in %CD3−.
2. Materials and Methods
2.1. Patients and Controls
For the period of January 2013 to December 2019, we prospectively included all patients who fulfilled CD diagnostic criteria based on the rule of ‘4 of 5’ proposed by Catassi and Fasano [1]: typical symptoms of CD, positivity of serum coeliac disease IgA class autoantibodies, HLA-DQ2 or DQ8 genotypes, coeliac enteropathy at the small intestinal biopsy, response to the GFD (at least 4 of 5 diagnostic criteria or 3 of 4 if the HLA Genotype is not performed). The control group consisted of patients referred to the gastroenterology department for endoscopic assessment including duodenal biopsy (histopathology and flow cytometry) because they had digestive symptoms or/and anemia. Digestive symptoms were defined by the chronic or intermittent presence of either diarrhea, dyspepsia, bloating, and/or abdominal pain. Controls were consecutively included based on the following criteria to rule out CD: (1) negative coeliac serology, (2) negative HLA-DQ2.5 and HLA-DQ8, and (3) normal duodenal biopsy. We excluded patients with intake of NSAIDs and Olmesartan, and patients with Crohn’s disease, autoimmune disease-associated enteropathy, collagenous sprue associated with collagenous colitis, lymphocytic enteritis due to intestinal parasitosis or Helicobacter pylori, and selective IgA deficiency. All CD patients and controls were recorded in a prospective maintained registry.
We performed coeliac serology, HLA genotyping, and duodenal biopsy assessment for histopathology and lymphocyte subpopulations by flow cytometry in all patients and controls.
2.2. Coeliac Serology
Serum IgA-tissue transglutaminase antibody (anti-TG2) and IgA titers were analyzed in serum using a quantitative automated ELISA detection kit (Elia CelikeyTM, Phadia AB, Freiburg, Germany) with recombinant human TG2 as antigen. A value of anti-TG2 ≥8 U/mL was established as the cut-off for normality [15]. Values between 2–8 U/mL were considered as a positive CD serology when titers higher than 1/40 of serum IgA anti-endomisal antibodies (EmA) were also found [16].
2.3. HLA Genotyping
We used a commercial reverse hybridization kit for the determination of CD heterodimers in the HLA genotyping (HLA-DQ2 [A1*0501/0505, B1*0201/*0202], HLA-DQ8 [A1*0301, B1*0301]). HLA-DQ2.5 haplotype is present in 24% of healthy controls and 90% of CD patients in our area [17]. In this study, we considered a positive coeliac genetic when the presence of HLA-DQ2.5, HLA-DQ8 or both was detected [18]. Considering the low frequency of the presence of HLA-DQ2.2 or only one allele of HLA-DQ2 haplotype in CD patients, either DQA1*05 or DQB1*02, the presence of these alleles was allowed in control individuals.
2.4. Duodenal Biopsy Assessment for Histopathology
Four endoscopic biopsies were taken from the second-third portion of the duodenum and one from the duodenal bulb, and these were processed using hematoxylin/eosin staining and CD3 immunophenotyping. Marsh 1 lesion (lymphocytic enteritis) was defined by 25 or more IEL per 100 epithelial nuclei along with normal villous architecture. Two endoscopic biopsies from antrum were also taken to investigate Helicobacter pylori infection in all patients. The lymphocyte count was performed as previously described [19,20]. Control group patients were separated into two subgroups according to the percentage of IEL (≥ or < than 18%) since some authors have suggested that a lower cut-off point should be established to redefine lymphocytic enteritis [21].
2.5. Duodenal Biopsy Assessment by Flow Cytometry
We performed IEL flow cytometry in all patients and controls by taking a duodenal sample from the second-third portion of the duodenum. The sample was obtained using a 2.8 mm biopsy forceps (Radial Jaw 4, Boston Scientific®, Marlborough, MA, USA), and immediately processed as previously described [4,8,12].
Briefly, IELs were isolated by gentle rotation in an orbital shaker at 12 rpm for 90 min in a solution of 1 mM DTT and 1 mM EDTA in 10%FBS HBSS, at room temperature. After two washes with HBSS (10 min, 300 g) IEL mixture was immediately stained for 15 min with the antibody mix described in Table 1. Viability (>90%) was assessed by trypan blue exclusion in Neubauer chamber. IELs were acquired in a four-colour FACSCalibur and analyzed with the Cell-Quest Software (BD Biosciences). PMT voltages and compensation values were manually adjusted using single stained samples. Live IELs were gated on CD45 and low scatter basis, and intraepithelial origin was confirmed with CD103 staining. (>90%).
Table 1.
Antibodies used for flow cytometry staining
| Laser | Fluorochrome | Cell Marker | Antibody Clone | Supplier | Reference | Dilution |
|---|---|---|---|---|---|---|
| 488 | PerCP | CD3 | SK7 | BD 1 | 345,766 | 2.5:100 |
| FITC | CD103 | Ber-ACT8 | BD | 333,155 | 2.5:100 | |
| 633 | PE | TCRγδ | 11F2 | BD | 333,141 | 2.5:100 |
| APC | CD45 | 2D1 | BD | 340,910 | 1.5:100 |
1 BD: BD-Biosciences.
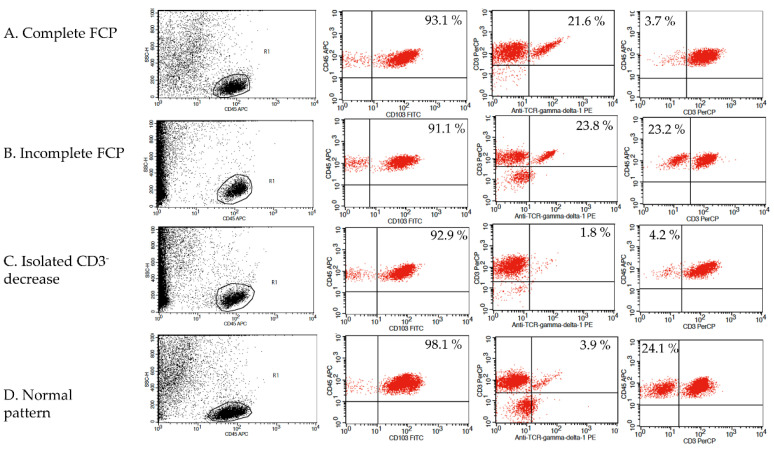
Four cytometric patterns were described using the TCRγδ+ and CD3− IEL percentages: First cytometric pattern was defined by an increase of %TCRγδ+ (>8.5%) and a decrease in %CD3− (<10%) and was labeled as a complete CD IEL flow cytometric pattern (complete FCP). A second cytometric pattern was defined by an isolated increase in %TCRγδ+ and was labeled an incomplete CD IEL flow cytometric pattern (Incomplete FCP). The third and fourth patterns were defined as non-CD patterns: one of them was defined by an isolated decrease in %CD3− and the other, labeled normal cytometric pattern, was defined by a TCRγδ+ ≤ 8.5% plus CD3− > 10%. This corresponds to the normal cut-off established in our laboratory [8,12]. Gating strategy and the four patterns are illustrated in Figure 1.
Figure 1.
Gating strategy and the four patterns cytometric patterns. Complete and incomplete flow cytometric patterns (FCP) are CD related patterns.
2.6. Statistical Analysis
The results were expressed as mean ± SEM or median (Interquartile range, IQR) or as proportions (and their 95% confidence interval -CI- when appropriate). In order to assess the relationship between age and %TCRγδ+ values, the age was classified in 7 groups (0–10 years, 11–20 years, 21–30 years, 31–40 years, 41–50 years, 51–60 years, ≥61 years). To compare %TCRγδ+ related to anti-TG2 serum titers, three groups were stablished: patients with anti-TG2 titers ≥ 30 U/mL, between 8–30 U/mL and between 2–8 U/mL plus EmA higher than 1/40. We used a student t test or ANOVA test for comparing %TCRγδ+ cells related to sex, degree of histological damage, and serology. The non-parametric counterpart (Kruskall–Wallis test) was used to compare the different groups of age because they do not follow a normal distribution assessed by a Kolmogorov–Smirnov test. In addition, we performed a Bonferroni test to assess differences among groups. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) for the complete CD pattern and the isolated increase in %TCRγδ+ were calculated using 2 × 2 tables. Statistical analysis was performed using the SPSS for Windows statistical package (SPSS Inc., Chicago, IL, USA).
2.7. Ethical Statements
The study was conducted according to the guidelines of the Declaration of Helsinki. All participants (or their parents in the case of patients less than 16 years old) provided written informed consent. This study is part of a larger registry that prospectively collects all patients who need to be evaluated to rule out CD. This registry was approved by the Ethics Committee of the Hospital Universitari Mútua Terrassa at the start of the registry in 2010 (Code: EO/1011; date: 25 March 2010). Researchers guaranteed strict measures for preserving patient confidentiality.
3. Results
We included 169 patients who fulfilled CD diagnostic criteria (119 women; mean age 18.8 ± 1.5 years, range 1–83 years). One hundred forty-four patients showed villous atrophy (Marsh 3a type, n = 21; and 3b-c type, n = 123). Twenty-five patients showed architecturally normal small intestinal mucosa with an increase in IEL counts (Marsh type 1 lesion, mean age 36.00 ± 4.48 years, range 4–83 years).
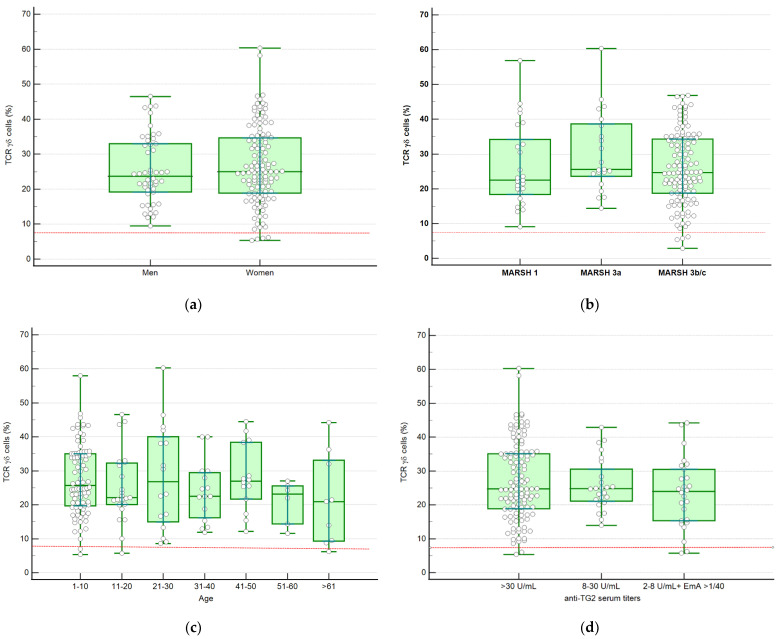
In Table 2 and Figure 2, the percentages of TCRγδ+ in groups of different degrees of histological lesion, sex, age, and anti-TG2 serum titers are shown. No differences were found relative to any of these variables.
Table 2.
Comparison of %TCRγδ+ between different groups of sex, age, and degree of histological lesion
| Variable | Median %TCRγδ+ (IQR) | p | |
|---|---|---|---|
| Sex | Male (n = 50) | 23.70 (18.08–34.00) | 0.909 |
| Female (n = 119) | 25.40 (18.78–35.31) | ||
| Histology | Marsh 1 (n = 25) | 22.51 (16.40–35.62) | 0.41 |
| Marsh 3a (n = 21) | 25.60 (22.85–39.13) | ||
| Marsh 3b-c (n = 123) | 24.70 (18.73–34.48) | ||
| Age | 0–10 (n = 86) | 25.03 (19.32–35.04) | 0.79 |
| 11–20 (n = 23) | 22.13 (20.08–32.31) | ||
| 21–30 (n = 16) | 26.82 (14.98–40.07) | ||
| 31–40 (n = 15) | 22.53 (16.19–36.38) | ||
| 41–50 (n = 14) | 26.98 (21.69–38.44) | ||
| 51–60 (n = 6) | 23.17 (14.38–25.59) | ||
| ≥61 (n = 9) | 21.47 (12.00–38.28) | ||
| Serology | anti-TG2 ≥30 U/mL (n = 119) | 24.75 (19.20–35.31) | 0.53 |
| anti-TG2 8–30 U/mL (n = 24) | 24.81 (20.90–33.60) | ||
| anti-TG2 2–8 U/mL + EmA > 1/40 (n = 26) | 23.98 (15.18–31.70) |
Figure 2.
Scatter plot and box-whisker showing the distribution of patients according to sex (a), degree of histological lesion (b), age (c), and anti-TG2 serum titers (d). Box-plot rectangle spans the interquartile range, the segment inside the rectangle shows median whereas the whiskers above and below plot, the maximum and the minimum. The dotted red line represents the stablished TCRγδ+ cut-off (>8.5%).
The control group included 49 subjects (35 women; median age 40.00 (25.00–51.50) years, range 1–67 years). Median value of IEL% was 16.70 (11.50–20.00). Subjects in the control group with IEL count <18% (n = 27; 20 women, median age 46.00 (35.00–53.00) years, range 1–67 years) had a median %TCRγδ+ of 3.36 (2.63–5.64), whereas controls with an IEL count ranging from 18 to 25% (n = 22) had a median %TCRγδ+ of 3.53 (2.59–7.89). Clinical characteristics of the control group are detailed in Table 3.
Table 3.
Clinical characteristics of the control group.
| Age (years) * | 40.00 (25.00–51.50) |
| Sex (% women) | 71.4% |
| Clinical symptoms 1 | |
| Diarrhea | 19 (36%) |
| Bloating | 10 (20%) |
| Dyspepsia | 10 (20%) |
| Abdominal pain | 4 (8%) |
| Anaemia | 4 (8%) |
| Autoimmune disease | 4 (8%) |
| HLA Genotyping | |
| HLA-DQ2.2 | 16 (32%) |
| HLA-DQA1 * 05 | 14 (29%) |
| HLA-DQB1 * 02 | 9 (19%) |
| Without risk alleles | 10 (20%) |
| IEL count (%) * | 16.70 (11.50–20.00) |
| CD3+ TCRγδ+ IEL (%) * | 3.40 (2.63–5.78) |
| CD3− IEL (%) * | 21.03 (13.79–30.55) |
| Final diagnosis | |
| Irritable bowel syndrome | 25 (51%) |
| Fructose malabsorption | 8 (17%) |
| Gastroesophageal reflux disease | 6 (12%) |
| Lactose malabsorption | 3 (6%) |
| Non-coeliac gluten sensitivity | 2 (4%) |
| Autoimmune pancreatitis | 1 (2%) |
| Chronic pancreatitis and exocrine pancreatic insufficiency | 1 (2%) |
| Factitious diarrhea | 1 (2%) |
| Esophageal dysmotility due to systemic sclerosis | 1 (2%) |
| Control biopsy after Helicobacter pylori eradication | 1 (2%) |
1 If patients reported more than one symptom, the predominant one was selected. * Median (IQR).
In Table 4, the four different FCPs found in CD patients and controls are shown. In CD patients, these patterns are provided depending on the degree of histological damage and in controls taking into account whether they had a percentage of IEL <18% or <25%. The majority of patients in the control group had a normal cytometric pattern; only eight of them showed abnormal patterns. Three of them showed an incomplete FCP (isolated increase of %TCRγδ+ (>8.5%) and the other five showed a selective decrease of %CD3− (non-coeliac pattern). It must be noted that all three patients with the incomplete FCP had an IEL count between 18–25% and there were no controls showing a complete CD pattern. Therefore, none of the controls with IEL count <18% had a coeliac related FCP.
Table 4.
Cytometric patterns in CD patients and control patients.
| CD Patients n = 169 | Controls (n = 49) | ||||
|---|---|---|---|---|---|
| Marsh 1 (n= 25) | Marsh 3a (n= 21) | Marsh 3b-c (n= 123) | IEL < 18 (n = 27) | IEL < 25 (n = 49) | |
| Complete FCP | 19 | 19 | 101 | 0 | 0 |
| Incomplete FCP: Isolated increase of %TCRγδ+ IEL | 4 | 2 | 18 | 0 | 3 |
| Isolated decrease of % CD3− | 2 | 0 | 0 | 2 | 5 |
| Increase of %TCRγδ+ IEL 1 | 23 | 21 | 119 | 0 | 3 |
| Normal pattern | 0 | 0 | 4 | 25 | 41 |
FCP = Flow cytometric pattern. Complete coeliac FCP: CD3+ TCRγδ+ IEL > 8.5% and CD3− < 10%. Incomplete coeliac FCP: isolated increase of CD3+ TCRγδ+ IEL > 8.5%. 1 Total number of patients with increase in %TCRγδ+ (complete + incomplete FCP).
Among CD patients with atrophy (n = 144), 83% had a complete FCP, whereas 13.8% (n = 20) had an incomplete FCP and 2.8% (n = 4) a normal pattern. A similar picture was found for Marsh 1 CD patients (n = 25), with 76% having a complete FCP (76%), 16% (n = 4) an incomplete FCP, and 8% (n = 2) a selective decrease in %CD3−. Thus, more than 90% of CD patients irrespective of the degree of mucosal damage showed CD related FCP.
Sensitivity, specificity, NPV, and PPV of complete FCP and of the increase of %TCRγδ+ were calculated considering both control subjects with IEL under 18% (n = 27) (Table 5) and all patients in the control group with IEL under 25% (n = 49) (Table 6). We found that complete FCP had an 82% sensitivity, 100% specificity, and 100% PPV irrespective of the criteria of IEL normality (below 18% or 25%). By contrast, the more restrictive criteria of IEL normality (<18%) should be adopted only if increased values of %TCRγδ+ are used as a diagnostic tool, reaching in this case an accuracy close to that obtained with the complete FCP. The largest differences in diagnostic accuracy between the two coeliac FCPs, depending on what we consider normal duodenal mucosa (IEL count < 18% or <25%), were in the NPV. In this sense, the highest probability of not having a CD corresponded to individuals having an IEL count < 25% (non-restrictive criteria of normality) and not having an increased %TCRγδ+ (NPV 88%).
Table 5.
Accuracy of coeliac cytometric pattern for the diagnosis of coeliac disease. Control group subjects with IEL count < 18% (n = 27).
| Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) |
NPV % (95% CI) |
|
|---|---|---|---|---|
| Complete FCP | 82 (75–88) | 100 (84–100) | 100 (82–100) | 47 (34–61) |
| Increase of %TCRγδ+ IEL 1 | 96 (92–98) | 100 (84–100) | 100 (97–100) | 81 (64–92) |
FCP = Flow cytometric pattern. Complete coeliac FCP: TCR CD3+ γδ+ IEL > 8.5% and CD3− < 10%. 1 Total number of patients with increase in %TCRγδ+ (complete + incomplete FCP).
Table 6.
Accuracy of the coeliac cytometric pattern for the diagnosis of coeliac disease. Control group subjects under 25% IEL (n = 49).
| Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) |
NPV % (95% CI) |
|
|---|---|---|---|---|
| Complete FCP | 82 (75–88) | 100 (91–100) | 100 (97–100) | 62 (50–73) |
| Increase of %TCRγδ+ IEL 1 | 96 (92–98) | 93 (82–98) | 98 (93–99) | 88 (76–95) |
FCP = Flow cytometric pattern. Complete coeliac FCP: TCR CD3+ γδ+ IEL > 8.5% and CD3− < 10%. 1 Total number of patients with increase in %TCRγδ+ (complete + incomplete FCP).
4. Discussion
The complete IEL cytometric pattern of CD, characterized by an increase of %TCRγδ+ and a concomitant decrease in %CD3−, has been proposed as a in complementary diagnostic tool to reinforce CD diagnosis in doubtful cases, especially when serology is negative [8,22]. This situation may occur in 30% of patients with atrophy and in more than 70% of patients with lymphocytic enteritis or Marsh type 1 CD [23,24].
The most frequent etiology of seronegative duodenal atrophy in Western countries is CD and the percentage increases in patients with positive HLA-DQ2/DQ8 [25]. The diagnosis of CD in cases of lymphocytic enteritis (Marsh 1 lesion) is more challenging since the lesion is much more unspecific than atrophy and other possible etiologies have been proposed [25,26]. As mentioned, only a small percentage of these patients will show a positive coeliac serology and only some patients will progress to villous atrophy after a gluten challenge of eight weeks [27].
The CD diagnosis in seronegative patients is based on the clinical and histological response to a GFD in patients with signs and symptoms of the coeliac spectrum in the presence of a positive HLA-DQ2/-DQ8. This means that the diagnosis of CD is time-consuming and remains uncertain until the effect of a GFD is assessed. In addition, gluten challenge is not well accepted by patients due to the discomfort caused. Nevertheless, it must be considered that this evaluation is sometimes difficult because CD clinical symptoms are quite unspecific and lymphocytic enteritis in non-CD patients may resolve spontaneously [19].
In the present validation cohort, we have confirmed that assessment of the complete FCP is a useful diagnostic tool for CD diagnosis, with a high diagnostic accuracy (82% sensitivity and 100% specificity). In addition, TCRγδ+ IEL subpopulation, which is the main parameter of coeliac lymphogram, is not influenced by age, sex, or the degree of histological damage. Hence, the IEL study through flow cytometry for CD diagnosis can be applied in any situation regardless of the clinical characteristics of the patient. This study also confirms that the normality cut-off previously established for %TCRγδ+ [8] is appropriate in patients bearing the complete coeliac FCP, including elderly patients. However, taking into account that the number of CD patients and controls older than 61 was very small, information focused on this population group is awaited.
Our study was performed in patients with positive serology, to ensure the diagnosis of CD, but it is conceivable that the characteristic behavior of duodenal intraepithelial subpopulations is also maintained in patients with negative serology. In fact, %TCRγδ+ values are not influenced by the levels of serum anti-TG2 titters. Moreover, the results of other studies by our group, showing very high response rates to a GFD in patients with enteropathy of the CD spectrum, negative serology, and coeliac cytometric pattern, lend support to this hypothesis [12,28].
A limitation of studies assessing diagnostic tools in CD is selection of the control group, and this feature of our study merits special mention. The ideal controls should be individuals of the general population who are completely healthy, without digestive symptoms and with negative genetic predisposition and serology. To our knowledge, a study with this type of ‘perfect’ control group has never been performed. In fact, the cut-off of 18 IEL considered ‘normal’ in the duodenal mucosa was established in subjects in whom the duodenal mucosa was microscopically assessed due to digestive symptoms [21]. The CD was ruled out with negative serology and negative HLA-DQ2/DQ8. In our study, we also used the same criteria for control group recruitment, excluding all the individuals with a positive HLA-DQ2.5 and DQ8. The recruitment of these controls was consequently very slow because the percentage of individuals in the general population having either HLA-DQ2 or DQ8 exceeds 60% in our area [17], but this makes the diagnosis of CD almost impossible.
Eight subjects in the whole control group had an abnormal FCP. Three of them had an incomplete FCP and the remaining 5 a selective decrease of %CD3−. By contrast, none of the controls with IEL count < 18% had a coeliac FCP and only two of them had a selective decrease of %CD3−, highlighting how this value should be considered the normal cut-off for histopathological analysis. Consequently, we noted a slight decrease in the diagnostic accuracy when we considered the sub-group that presented an IEL count between 18–25% as controls. All these findings are objective data to redefine the cut-off point <18% for considering duodenal mucosa as normal. Also, it is demonstrated that complete FCP is more accurate than an incomplete CD pattern.
In conclusion, the established normality cut-off for %TCRγδ+ (>8.5%) in IEL flow cytometry study for diagnosis of CD is valid for all age, sex, and histological lesion grade groups. Moreover, we have confirmed the high diagnostic accuracy of the increase in %TCRγδ+ and the complete FCP for CD diagnosis in a large validation cohort.
Acknowledgments
Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas’ (CIBERehd) is an initiative of the Instituto de Salud Carlos III, Madrid, Spain. This institution played no role in the study design, or the acquisition, analysis or interpretation of the data or the report writing.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13051684/s1.
Author Contributions
Guarantor of the article: M.E. Conceptualization, F.F.-B. and M.E.; Data curation, all authors; Formal analysis, P.R.-R., F.F.-B., and M.E.; Investigation, all authors; Methodology, E.T., A.C., C.F., F.F.-B., and M.E.; Project administration, M.E.; Resources, all authors; Supervision, M.E. and F.F.-B.; Validation, M.E.; Visualization, M.E.; Writing—original draft, P.R.-R. and M.E.; Writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The following statement is included on page 4 of the manuscript “The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the Hospital Universitari Mutua Terrassa”. Code: EO/1011; date: 25 March 2010.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study or their parents as noted in the manuscript.
Data Availability Statement
File1.sav, database of the study (supplementary file).
Conflicts of Interest
The authors declare no conflict of interest.
Writing Assistance
Tom Yohannan.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Catassi C., Fasano A. Celiac disease diagnosis: Simple rules are better than complicated algorithms. Am. J. Med. 2010;123:691–693. doi: 10.1016/j.amjmed.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Eiras P., Roldan E., Camarero C., Olivares F., Bootello A., Roy G. Flow cytometry description of a novel CD3−/CD7+ intraepithelial lymphocyte subset in human duodenal biopsies: Potential diagnostic value in celiac disease. Cytometry. 1998;34:95–102. doi: 10.1002/(SICI)1097-0320(19980415)34:2<95::AID-CYTO6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Camarero C., Eiras P., Leon F., Olivares F., Escobar H., Roy G. Intraephitelial lymphocytes and celiac disease: Permanent changes in CD3-/CD7+ and T cell γδ receptor subsets studied by flow cytometry. Acta Paediatr. 2000;89:285–290. [PubMed] [Google Scholar]
- 4.León F. Flow cytometry of intestinal intraepithelial lymphocytes in celiac disease. J. Immunol. Methods. 2011;363:177–186. doi: 10.1016/j.jim.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Calleja S., Vivas S., Santiuste M., Arias L., Hernando M., Nistal E., De Morales JG R. Dynamics of non-conventional intraepithelial lymphocytes-NK, NKT, and γδ T- in celiac disease: Relationship with age, diet, and histopathology. Dig. Dis. Sci. 2011;56:2042–2049. doi: 10.1007/s10620-010-1534-5. [DOI] [PubMed] [Google Scholar]
- 6.Järvinen T.T., Kaukinen K., Laurila K., Kyrönpalo S., Rasmussen M., Mäki M., Korhonen H., Reunala T., Collin P. Intraepithelial lymphocytes in celiac disease. Am. J. Gastroenterol. 2003;98:1332–1337. doi: 10.1111/j.1572-0241.2003.07456.x. [DOI] [PubMed] [Google Scholar]
- 7.Husby S., Koletzko S., Korponay-Szabó I., Mearin M., Phillips A., Shamir R., Troncone R., Giersiepen K., Branski D., Catassi C., et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Bañares F., Carrasco A., García-Puig R., Rosinach M., González C., Alsina M., Loras C., Salas A., Viver J.M., Esteve M. Intestinal Intraepithelial Cytometric pattern is more accurate than subepithelial deposits of anti-tissue transglutaminase Ig A for the diagnosis of Celiac disease in lymphocytic enteritis. PLoS ONE. 2014;9:e101249. doi: 10.1371/journal.pone.0101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivas S., De Morales J.M.R., Fernandez M., Hernando M., Herrero B., Casqueiro J., Gutierrez S. Age-related clinical, serological, and histopathological features of celiac disease. Am. J. Gastroenterol. 2008;103:2360–2365. doi: 10.1111/j.1572-0241.2008.01977.x. [DOI] [PubMed] [Google Scholar]
- 10.Dickey W., Hughes D.F., McMillan S.A. Disappearance of endomysial antibodies in treated celiac disease does not indicate histological recovery. Am. J. Gastroenterol. 2000;95:712–714. doi: 10.1111/j.1572-0241.2000.01838.x. [DOI] [PubMed] [Google Scholar]
- 11.Esteve M., Rosinach M., Fernández-Bañares F., Farré C., Salas A., Alsina M., Vilar P., Abad-Lacruz A., Forné M., Mariné M., et al. Spectrum of gluten-sensitive enteropathy in first-degree relatives of patients with coeliac disease: Clinical relevance of lymphocytic enteritis. Gut. 2006;55:1739–1745. doi: 10.1136/gut.2006.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosinach M., Fernández-Bañares F., Carrasco A., Ibarra M., Temiño R., Salas A., Esteve M. Double-blind randomized clinical trial: Gluten versus placebo rechallenge in patients with lymphocytic enteritis and suspected celiac disease. PLoS ONE. 2016;11:e0157879. doi: 10.1371/journal.pone.0157879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokkonen J., Holm K., Kartunnen T.J., Maki M. Children with untreated food allergy express a relative increment in the density of duodenal gammadelta+ T cells. Scand. J. Gastroenterol. 2000;35:1137. doi: 10.1080/003655200750056592. [DOI] [PubMed] [Google Scholar]
- 14.Palomar P.O., Ruiz A.C., Scapa MA M., Prieto F.L., Ariño G.R., Verge C.R. Adult celiac disease and intraepithelial lymphocytes. New options for diagnosis? Gastroenterol. Hepatol. 2008;31:555–559. doi: 10.1157/13128293. [DOI] [PubMed] [Google Scholar]
- 15.Mariné M., Farre C., Alsina M., Vilar P., Cortijo M., Salas A., Fernández-Bañares F., Rosinach M., Santaolalla R., Loras C., et al. The prevalence of coeliac disease is significantly higher in children compared with adults. Aliment. Pharmacol. Ther. 2011;33:477–486. doi: 10.1111/j.1365-2036.2010.04543.x. [DOI] [PubMed] [Google Scholar]
- 16.Chorzelski T.P., Beutner E.H., Sulej J., Tchorzewska H., Jablonska S., Kumar V., Kapuscinska A. Ig A anti-endomysium antibody. A new immunological marker of dermatitis herpetiformis and coeliac disease. Br. J. Dermatol. 1984;111:395–405. doi: 10.1111/j.1365-2133.1984.tb06601.x. [DOI] [PubMed] [Google Scholar]
- 17.Farré C. Ph.D. Thesis. University of Barcelona; Barcelona, Spain: 2002. Enfermedad Celíaca: Marcadores Serológicos y de Predisposición Genética, Aspectos Clínicos y Poblaciones de Riesgo. [Google Scholar]
- 18.Megiorni F., Pizzuti A. HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: Practical implications of the HLA molecular typing. J. Biomed. Sci. 2012;19:88. doi: 10.1186/1423-0127-19-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosinach M., Esteve M., González C., Temiño R., Mariné M., Monzón H., Sainz E., Loras C., Espinos J.C., Forné M., et al. Lymphocytic duodenosis: Aetiology and long-term response to specific treatment. Dig. Liver Dis. 2012;44:643–648. doi: 10.1016/j.dld.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Walker M.M., Murray J.A. An update in the diagnosis of coeliac disease. Histopathology. 2011;59:166–179. doi: 10.1111/j.1365-2559.2010.03680.x. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrino S., Villanacci V., Sansotta N., Scarfì R., Bassotti G., Vieni G., Princiotta A., Sferlazzas C., Magazzù G., Tuccari G. Redefining the intraepithelial lymphocytes threshold to diagnose gluten sensitivity in patients with architecturally normal duodenal histology. Aliment. Pharmacol. Ther. 2011;33:697–706. doi: 10.1111/j.1365-2036.2011.04578.x. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-Bañares F., Carrasco A., Rosinach M., Arau B., García-Puig R., González C., Tristán E., Zabana Y., Esteve M. A scoring system for identifying patients likely to be diagnosed with low-grade coeliac enteropathy. Nutrients. 2019;11:1050. doi: 10.3390/nu11051050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santaolalla R., Fernández-Bañares F., Rodriguez R., Alsina M., Rosinach M., Marine M., Farre C., Salas A., Forné M., Loras C., et al. Diagnostic value of duodenal antitissue transglutaminase antibodies in gluten-sensitive enteropathy. Aliment. Pharmacol. Ther. 2008;27:820–829. doi: 10.1111/j.1365-2036.2008.03652.x. [DOI] [PubMed] [Google Scholar]
- 24.Rostami K., Kerckhaert J., Tiemessen R., Von Blomberg B.M.E., Meijer J.W., Mulder C.J. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: Disappointing in clinical practice. Am. J. Gastroenterol. 1999;94:888–894. doi: 10.1111/j.1572-0241.1999.983_f.x. [DOI] [PubMed] [Google Scholar]
- 25.Aziz I., Evans K.E., Hooper A.D., Smillie D.M., Sanders D.S. A prospective study into the aetiology of lymphocytic duodenosis. Aliment. Pharmacol. Ther. 2010;32:1392–1397. doi: 10.1111/j.1365-2036.2010.04477.x. [DOI] [PubMed] [Google Scholar]
- 26.Kakar S., Nehra V., Murray J.A., Dayharsh J.A., Burgart R.J. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am. J. Gastroenterol. 2003;98:2027–2033. doi: 10.1111/j.1572-0241.2003.07631.x. [DOI] [PubMed] [Google Scholar]
- 27.Wahab P.J., Crusius B.A., Meijer J.W.R., Mulder C.J. Gluten challenge in borderline gluten-sensitive enteropathy. Am. J. Gastroenterol. 2001;96:1464–1469. doi: 10.1111/j.1572-0241.2001.03812.x. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Bañares F., Crespo L., Núñez C., López-Palacios N., Tristán E., Vivas S., Farrais S., Arau B., Vidal J., Roy G., et al. Gamma delta+ intraepithelial lymphocytes and coeliac lymphogram in a diagnostic approach to coeliac disease in patients with seronegative villous atrophy. Aliment. Pharmacol. Ther. 2020;51:699–705. doi: 10.1111/apt.15663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
File1.sav, database of the study (supplementary file).