Key Points
Question
What is the prevalence of hidradenitis suppurativa?
Findings
In this systematic review and meta-regression analysis of 16 quantitatively assessed studies, the overall prevalence of hidradenitis suppurativa was found to be 0.40%. Studies based on clinical samples revealed a higher pooled prevalence of hidradenitis suppurativa (1.7%) than population-based studies (0.3%).
Meaning
The findings from this study may help in formulating policies, channeling funding and guiding principles for better disease diagnosis using universally valid tools and management.
Abstract
Importance
Hidradenitis suppurativa/acne inversa (HS) is a chronic inflammatory skin disease characterized by occlusion of hair follicles as a primary pathogenic factor. There are scarce data regarding the prevalence of HS.
Objective
To estimate overall HS prevalence.
Data Sources
This review and meta-regression analysis was conducted using the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline. The academic search included PubMed, Cochrane registry, ClinicalTrials.gov, and evidence by NHS UK and Trip databases from inception through May 2020. To analyze HS prevalence, only cross-sectional studies or baseline assessments of longitudinal cohorts using census-based surveys or probabilistic and nonprobabilistic epidemiologic methods were considered. The search terms were (prevalence OR incidence OR epidemiology) AND (hidradenitis suppurativa OR acne inversa OR Verneuil’s disease). No language restriction was applied.
Study Selection
Original investigations that reported HS prevalence were included. After exclusion criteria were applied, 17 studies qualified for qualitative analysis, but only 16 studies were quantitatively assessed.
Data Extraction and Measures
Two reviewers extracted data by age, diagnostic criteria, presence of any comorbidity, sample sizes, continent/location, sex, and other characteristics. Assessment of bias risk used the Joanna Briggs Institute Critical Appraisal Instrument for Studies Reporting Prevalence Data using random-effects models to synthesize available evidence.
Main Outcomes and Measures
Hidradenitis suppurativa prevalence (with 95% CI) among the overall population and among subgroups. Between-study heterogeneity was assessed (Cochran Q statistic) and quantified (I2 statistic).
Results
In 16 quantitatively assessed studies included, prevalence estimates were reported only from Western European and Scandinavian countries, the US, and Australia. Meta-analysis with random effects, after adjusting for publication bias in the prevalence estimates, revealed a 0.40% prevalence (95% CI, 0.26%-0.63%) for HS. Studies based on clinical samples revealed a higher pooled prevalence of HS (1.7%) than population-based studies (0.3%).
Conclusions and Relevance
The findings of this systematic review and meta-regression analysis may help facilitate policy formulation, channeling funding and guiding principles for better disease diagnosis using universal valid tools and management.
This systematic review and meta-analysis estimates worldwide hidradenitis suppurativa prevalence.
Introduction
Hidradenitis suppurativa/acne inversa (HS) is a chronic inflammatory skin disease characterized by occlusion of hair follicles as a primary pathogenic factor associated with a chronic cycle of inflammation, healing, and scarring.1,2 This multifactorial chronic inflammatory condition usually affects intertriginous areas of the body including the axilla, inframammary area, inguinal, perianal, gluteal, and pubic regions. This condition usually begins in the second or third decade of life cohering with substantial psychological and emotional burdens3,4 as well as physical comorbidity.5,6,7
The estimated prevalence of HS shows wide variations, with a point prevalence ranging from 0.3% in Germany to 4.1% in Denmark owing to heterogeneous measurement methods and populations being studied.8,9 According to a study conducted in the UK,4 HS exhibits a 0.77% point prevalence using diagnostic criteria. Point prevalence was reported as 1.19% in the UK and as 3.0% in Germany when potential cases are included.4,8 A validated HS-screening questionnaire with high diagnostic power was used to calculate HS prevalence in Australia, which has been estimated at 0.67%.10 A cross-sectional study in Ireland evaluated HS prevalence using clinician-assessed diagnoses of HS, and estimated prevalence at 1.4%.11 Several studies have been conducted in the US, where HS prevalence has been reported at 0.10%12 based on claims data, which were confounded by diagnostic delay. While several publications have assessed overall HS prevalence in central and Western Europe and North America, representative studies from most Asian and African countries are not available.
There are also scarce data pertaining to HS prevalence, and the included studies report findings among different populations or clinical samples using heterogeneous clinical, screening, and diagnostic systems. This situation suggests the need for systematic review and meta-analytical efforts to estimate HS prevalence, as well as to systematically review and critically appraise the quality and clinical aspects of published prevalence studies. The current effort may help in the formulation of policies and principles for better disease diagnosis and management. Our systematic review and meta-regression analysis aims to estimate overall HS prevalence using rigorous meta-analytical methods.
Methods
Literature Search
This systematic review and meta-regression analysis was conducted following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.7,13,14 A search of academic databases including PubMed, Cochrane registry, ClinicalTrials.gov, and evidence by NHS UK and Trip databases from inception through May 2020 was conducted. A search strategy predefined and adapted for each database included the following search terms: (prevalence OR incidence OR epidemiology) AND (hidradenitis suppurativa OR acne inversa OR Verneuil’s disease). There were no search filters pertaining to age, language, or publication year.
Study Selection
We used Endnote software (Clarivate Analytics) to remove duplicate bibliographic records. Titles and abstracts were then scrutinized by 2 of us (A.J. and D.N.) working independently according to predefined inclusion and exclusion criteria. This process was followed by scrutiny of full texts of eligible studies. Discrepancies were resolved by discussion with a senior investigator (E.O.).
Only original investigations that reported HS prevalence were included. To analyze HS prevalence, only cross-sectional studies or baseline assessments of longitudinal cohort studies (prospective or retrospective) using either census-based surveys or probabilistic and nonprobabilistic epidemiologic methods were deemed suitable. Case-control studies and follow-up assessment of cohort studies were excluded because they are more suitable for exploring disease risk factors. We considered all studies, whether conducted in the context of general population or in clinical settings.
If studies reported findings from overlapping data sets, we chose only those that reported data at a later point. Review articles, case reports, protocols, short communications, personal opinions, letters, posters, conference abstracts, laboratory research, and reports with insufficient data were excluded. We also considered studies that reported HS prevalence among specialized population groups. After eligible studies were identified, their bibliographies were screened for studies suitable for inclusion.
Data Extraction and Quality Assessment
Data pertaining to characteristics of publications and population under study and quantitative data were extracted by 2 of us (A.J. and D.N.) working independently using a predetermined customized extraction form. Characteristics of publications included publication year and affiliation of corresponding authors. Population characteristics included age, diagnostic criteria, inclusion criteria, exclusion criteria, and presence of any comorbidity. Quantitative data included total sample size and proportion of respondents with HS.
Study quality was appraised using the Joanna Briggs Institute Critical Appraisal Instrument for prevalence studies against the following matrices: sample frame suitability, sampling strategy, sample size adequacy, study subjects and setting description, appropriate data analysis performance, reliable and valid diagnosis, and response rate adequacy.15
Statistical Analysis
All data were analyzed using the Comprehensive Meta-analysis Software, version 3 (Bio Stat). Using the random-effects model (inverse variance method), quantitative data from the studies were pooled to report pooled HS prevalence. Random effects were applied throughout the analysis because of expected clinical heterogeneity encountered in different studies. This approach allows heterogeneity in the data to be addressed by considering that differences between studies are random. Between-study heterogeneity was assessed (Cochran Q statistic) and quantified (I2 statistic). Sensitivity analysis using the knockout approach was performed to analyze the proportion of each study to the pooled estimate.16 Publication bias was assessed using the Begg funnel and Egger regression statistic considered significant at P ≤ .10.16 The Duval and Tweedie trim-and-fill method was used to adjust pooled estimate of prevalence for publication bias, using the random-effects model.17
Subgroup analyses were performed to explore differences in the pooled estimate of prevalence among specialized populations, study setting, and region of study sample if they were reported in more than 4 studies.15 Meta-regression was used to check association of age of respondents, year of publication, study quality, and proportion of females with pooled HS prevalence,17 if possible.
Results
Study Selection Process
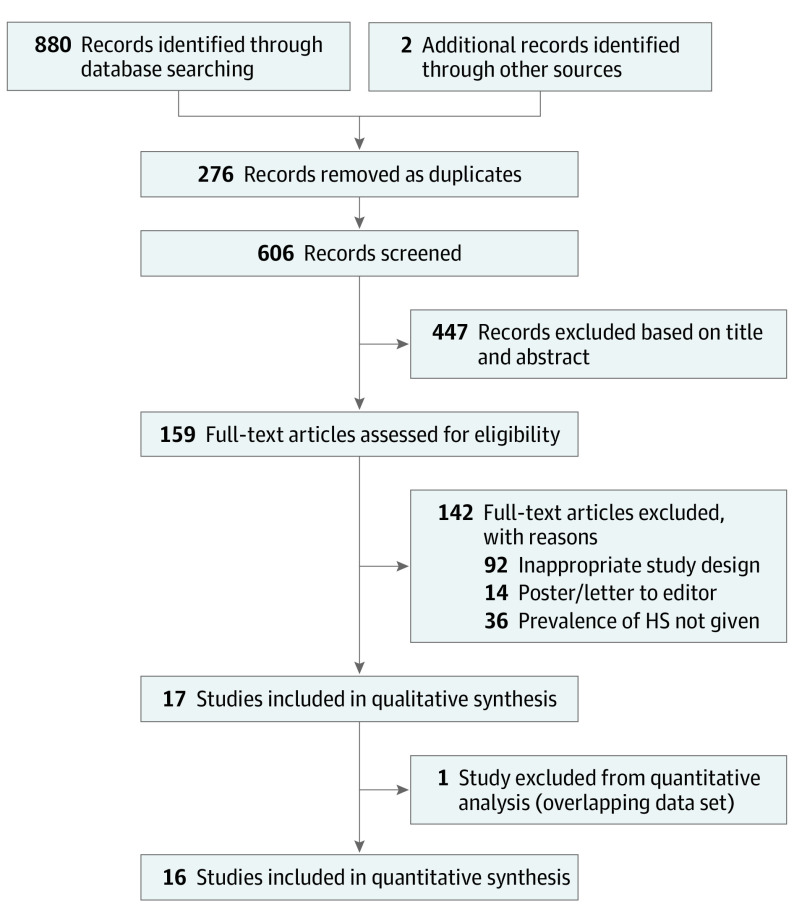
In all, 880 bibliographic records were identified after electronic database searches with 2 additional records identified through other sources. Using Endnote, 276 were excluded, leaving 606 titles and abstracts to be screened. After careful screening and manual search, we included 17 studies in qualitative synthesis and 16 studies in quantitative synthesis (Figure 1). These were mostly in European countries (n = 12), followed by the US (n = 4) and Australia (n = 1). Among European countries, Denmark contributed the most studies (n = 5), followed by 2 each in Germany and France and 1 each in Ireland, Sweden, and the UK (Table 1).4,5,8,9,10,11,12,18,19,20,21,22,23,24,25,26,27
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Flow Diagram.
HS indicates hidradenitis suppurativa.
Table 1. Characteristics of Studies and Populations.
| Source | Country | Setting | Events | Sample size | Prevalence, % | Diagnostic method | Age group or mean age, y | Study period | Population characteristics |
|---|---|---|---|---|---|---|---|---|---|
| Kjaersgaard Andersen et al,5 2020 | Denmark | Clinical sample | 5 | 195 | 2.56 | P | 12.5 | 2007-2015 | Children with overweight and obesity and adolescents presenting at obesity clinic |
| Calao et al,10 2018 | Australia | Clinical sample | 88 | 11 433 | 0.77 | P + Q | 39.4 | 2015-2015 | All adult (≥18 y) Australian residents participating in the Single Source establishment survey run by Roy Morgan Research Ltd |
| Shahi et al,18 2014 | US | Population | 178 | 139 280 | 0.13 | P | 45.2 | 1968-2008 | All individuals at Olmstead County, Minnesota, registered in Rochester Epidemiology Project, mainly white ethnicity |
| Delany et al,11 2018 | Ireland | Clinical sample | 221 | 15 547 | 1.42 | P | 37 | 2015-2015 | All consenting adults presenting at 4 hospitals in Ireland |
| Garg et al,12 2017 | US | Population | 47 620 | 45 855 960 | 0.10 | P | All age groups | 1999-2016 | Population-based sample of more than 48 million unique patients across all US census regions |
| Garg et al,19 2018 | US | Population | 1240 | 4 578 790 | 0.03 | P | 0-17 | 2014-2017 | Children and adolescents registered in population database encompassing 27 participating integrated health care organizations in the US |
| Garg et al,20 2018 | US | Population | 46 860 | 16 813 290 | 0.28 | P | Adults | 2011-2016 | Adult patients registered in population database encompassing 27 participating integrated health care organizations in the US |
| Ingram et al,4 2018 | UK | Population | 33 499 | 4 364 308 | 0.77 | P | NR | 1994-2013 | Patients presenting at primary care practices registered in UK Clinical Practice Research Datalink |
| Jemec et al,21 1996 | Denmark | Population | 6 | 585 | 1.03 | Q | 40.7 | 1992-1995 | Men and women 15 to 69 y of age living in this area |
| Jemec et al,22 1996 | Denmark | Clinical sample | 20 | 507 | 3.94 | P | 27.15 | 1992-1995 | Hospital sample were those came for screening of STDs |
| Killasli et al,23 2020 | Sweden | Population | 13 538 | 9 747 355 | 0.14 | P | 44.2 | 2001-2014 | Individuals registered in National Patient Register and living in Sweden as of December 2014 |
| Kirsten et al,24 2019 | Germany | Population | 791 | 2 319 584 | 0.03 | P | NR | 2010-2015 | Adults registered at German statutory health insurance companies |
| Kirsten et al,8 2021 | Germany | Population | 57 | 20 112 | 0.28 | P | 43.6 | 2014-2017 | Employees in German companies |
| Revuz et al,25 2008 | France | Population | 67 | 6887 | 0.97 | Q | 39 | 2005 | Individuals aged 15 y or older, representative of French population |
| Richard et al,26 2018 | France | Population | 29 | 20 012 | 0.14 | Q | All age groups | 2016 | French general population aged more than 15 y |
| Theut Riis et al,9 2019 | Denmark | Population | 500 | 27 765 | 1.80 | Q | 36.57 | 1995-2015 | Individuals registered as blood donors in nationwide Danish blood donor registry |
| Vinding et al,27 2014 | Denmark | Population | 344 | 16 404 | 2.10 | P | 49 | 2010-2013 | Population in Naestved Municipality registered in Danish General Suburban Population Study |
Abbreviations: P, physician; NR, not reported; Q, questionnaire; STDs, sexually transmitted diseases.
Heterogeneous Diagnostic Methods, Population, and Setting
Studies included in this review posed challenges pertaining to both clinical and statistical heterogeneity. Several important aspects of heterogeneity are highlighted here, including heterogeneity in study designs, source of epidemiologic data, population being considered, and method for screening and diagnosing HS.
Use of different settings for sampling of HS patients was the major source of clinical heterogeneity. Data from clinical sites were heterogeneous because some patients presented to dermatology departments (n = 4) and other patients presented to a Danish obesity clinic serving an adolescent population of overweight and individuals involved with psychiatric facilities (n = 1). All HS diagnoses were assessed by dermatologists.8,11,12,21,28
Population-based prevalence estimates were reported in 13 studies using different strategies for HS diagnosis and screening. Strategies included validated diagnostic questionnaires in which patients self-reported26 and electronic medical records.18,19,20 Swedish population-based registries were used, including the National Patient Register, a longitudinal integration database for health insurance and labor market studies, and the Swedish pregnancy register (Karolinska University Hospital).23 French studies used the population-based survey of the Institut National de la Statistique et des Etudes Economiques.25 German studies obtained HS statistics using German insurance and hospital discharge data.24 One study8 assessed HS prevalence among the working population in Germany through clinical assessments of employees in 343 German companies. Table 1 provides more information on the diagnostic method of each study.
Another source of heterogeneity arose from the method for HS screening and diagnosis used. Calao et al10 used input from physicians and postal questionnaires sent to the general Australian population to recruit clinical samples and deliver diagnoses. Theut Riis et al9 analyzed a nationwide Danish Blood Donor Study that asked participants to complete a Danish Blood Donor Study questionnaire containing diagnostic screening questions for HS. All US-based studies used electronic medical records data (primarily insurance claims data sets) such as the Rochester Epidemiology Project using medical records in Olmstead County, Minnesota18; Garg et al12,19,20 used a platform developed by IBM. The multi-institutional data analytics and research platform integrated 27 participating health care organizations and included more than 55 million unique patients representing approximately 17% of the population across all 4 census regions of the US.
Four studies used a questionnaire to screen respondents for HS.9,21,24,26 The Theut Riis et al9 questionnaire consisted of 2 HS screening questions: (1) “Have you had an outbreak of boils during the last 6 months?” and (2) “Where and how many boils have you had?” This questionnaire yielded 90% sensitivity (95% CI, 73%-98%), 97% specificity (95% CI, 85%-100%), a 96% positive predictive value, and a 92% negative predictive value. Revuz29 and Richard et al26 used a diagnostic questionnaire confirming HS presence based on a dermatologist or physician examination. Jemec et al21 used a 2-item questionnaire confirming whether the patient had painful boils in the armpit or groin during the past 12 months and whether they could empty the boil by pressing it like a pimple.
Prevalence of HS
Meta-analyses were conducted after removal of 1 study12 that provided analyses from an overlapping data set. The 16 studies included comprised 38 082 054 participants and presented substantial statistical heterogeneity (I2 = 99.97%; df = 15; Q = 50 549.44). Meta-regression analysis with random effects revealed an overall HS prevalence of 0.5% (95% CI, 0.3%-0.7%).
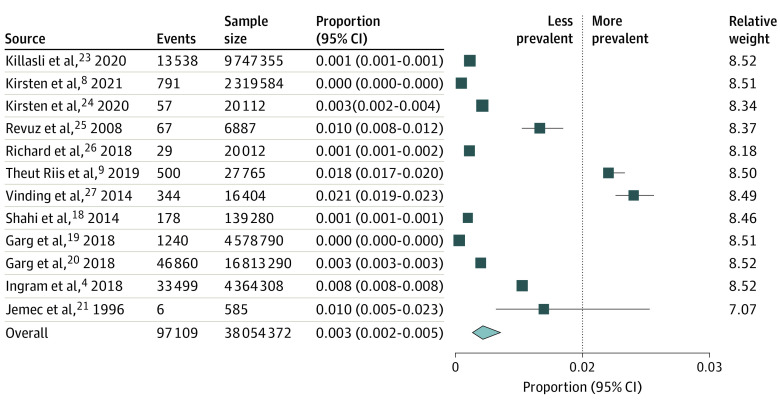
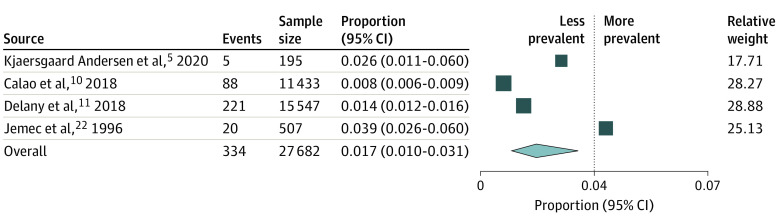
Meta-analyses were then separately conducted for population-based studies4,8,18,19,20,21,23,24,25,26,27 (Figure 2) and clinical sample studies5,10,11,22 ( Figure 3). Clinical sample-based studies yielded a pooled prevalence of 1.7% (95% CI, 1.0%-3.1%) although with substantial heterogeneity (I2 = 94.34%; df = 3; Q = 53.44). Sensitivity analysis did not reveal substantial variation in pooled prevalence when studies were individually removed from the analysis. Among studies with population-based samples, pooled analyses yielded substantial heterogeneity (I2 = 99.99%; df = 12; Q = 96 619.89). They revealed overall HS prevalence to be 0.3% (95% CI, 0.2%-0.5%). Sensitivity analysis noted no significant variations.
Figure 2. Pooled Prevalence of Hidradenitis Suppurativa and Event Rates for Individual Population Studies.
Squares represent mean values, with the size of the squares indicating weight and horizontal lines representing 95% CIs. The diamond represents the pooled mean with the points of the diamond representing 95% CI.
Figure 3. Pooled Prevalence of HS and Event Rates for Individual Clinical Studies.
Squares represent mean values with 95% CIs, with the size of the squares indicating weight. The diamond represents the pooled mean with the points of the diamond representing 95% CI.
Publication Bias
Funnel plot visualization revealed evidence of publication bias with 2 studies found to be missing to left of mean. The Duval and Tweedie trim-and-fill method was used to adjust pooled estimates for publication bias, and it yielded a pooled HS prevalence of 0.40% (95% CI, 0.26%-0.63%) (eFigure 1 in the Supplement).
Subgroup and Meta-regression Analyses
On subgroup analyses (Table 2), significant differences in HS prevalence were noted among different countries, with the highest prevalence noted in Australia 0.8% (95% CI, 0.1%-4.7%), followed by Europe 0.7% (95% CI, 0.4%-1.1%) and the US 0.1% (95% CI, 0%-0.4%). However, this association should be interpreted with caution because of the methodologic substantial heterogeneity among studies conducted in different countries. Among different populations, the highest prevalence was 1.8%, noted among clinical samples (0.7%-4.6%), followed by population-based studies (0.3%, 95% CI, 0.2%-0.5%). No statistically significant differences were noted among studies reporting different methods of diagnosis such as physician, dermatologist-delivered diagnosis, or reporting of HS through questionnaires; however, questionnaire-based studies reported the highest prevalence estimates.
Table 2. Subgroup Analysis Exhibiting Moderators of Prevalence of HS.
| Group | No. of studies | Prevalence, % (95% CI) | z value | Q value | df | P value |
|---|---|---|---|---|---|---|
| Location | ||||||
| Australia | 1 | 0.8 (0.1-4.7) | −3.72 | 12.35 | 2 | .07 |
| Europe | 12 | 0.7 (0.4-1.1) | −13.19 | |||
| US | 3 | 0.1 (0-0.4) | −9.20 | |||
| Type of study sample | ||||||
| Clinical sample | 4 | 1.8 (0.7-4.6) | −8.54 | 11.26 | 2 | .001 |
| Population | 12 | 0.3 (0.2-0.5) | −22.01 | |||
| Diagnostic method | ||||||
| Both | 1 | 0.08 (0.01-0.44) | −5.35 | 1.83 | 2 | .40 |
| Physician | 11 | 0.4 (0.2-0.6) | −20.26 | |||
| Questionnaire | 4 | 0.7 (0.3-0.18) | −10.61 |
Abbreviation: HS, hidradenitis suppurativa.
Meta-regression analysis revealed that prevalence of HS was significantly associated with year of publication of studies (β = −0.08; z = −1.88; P = .06, R2 = 19%). Age of participants was noted in only 11 studies and revealed a statistically significant negative association with HS prevalence (β = −0.07; z = −2.24; P = .02; R2 = 31%). The percentage of female participants in each study sample was noted in 10 studies and was not associated with pooled prevalence of HS (β = −0.01; z = −0.22; P = .83; R2 = 0%). eFigures 2 through 4 in the Supplement present meta-regression plots for these analyses.
Study Quality
Studies were generally of good quality with 2 of 17 studies rated as high quality across all matrices of the Joanna Briggs Institute Critical Appraisal Instrument; 5 studies were rated as poor across 1 domain, 5 across 3 domains, and 2 across 3 domains of the appraisal instrument. Four studies were rated as having a problematic sample frame, inadequate sample size (n = 1), poor details on sample and setting (n = 1), data analysis with insufficient coverage (n = 3), unclear validity (n = 3) and reliability (n = 3) of diagnostic method, and inadequate response rate (n = 3) (eFigure 5 in the Supplement). Meta-regression analysis revealed that with improving quality of the studies, prevalence of HS decreased substantially (β = −0.67; z = −1.89; P = .056). Quality of studies explained 17% of heterogeneity in reporting of HS prevalence (eFigure 6 in the Supplement).
Discussion
The present meta-regression analysis with random effects revealed an overall HS prevalence of 0.40% (95% CI, 0.26%-0.63%) among the populations studied around the world after adjusting for publication bias in the prevalence estimates. This finding is lower than previously suggested. Studies based on clinical samples revealed a higher pooled prevalence of HS (1.7%) than population-based studies (0.3%). The pooled estimates for HS are comparable with other inflammatory diseases. Overall, immune-mediated disease prevalences in Western societies range from 5% to 7%30 with a prevalence for rheumatoid arthritis estimated at 0.5% to 1% in the US and European countries and for psoriasis at 2% in North America. Moreover, our analyses revealed a yearly downward trend in prevalence estimates of HS. Similarly, prevalence estimates seemed to decrease with higher age. No significant association was found between HS prevalence and percentage of females across the studies.
Most of the prevalence estimates for HS were reported from Europe (Western European and Scandinavian countries), the US, and Australia. Because of a paucity of HS studies conducted in other parts of the world, the study results must be interpreted with caution. The results may be expected because HS has previously been associated with the Western societies’ nutrition and metabolic comorbidities.31,32,33 Despite this, future studies from Asia and Africa are particularly welcome to provide prevalence estimates and to channel relevant policy making and funding efforts. Such studies would be especially relevant because of the burden of HS owing to its association with greater odds for psychiatric illnesses such as depression (26.5% vs 6.6%), anxiety (18.1% vs 7.1%), and suicide ideation (0.8% vs 0.3%) compared with heathy counterparts. According to von der Werth and Jemec34 and Matusiak et al,35 HS is particularly debilitating, presenting with more severely affected quality of life than is found in individuals with other dermatologic conditions, such as alopecia and psoriasis. The Global Survey of Impact and Healthcare Needs (VOICE) project by Garg et al36 demonstrated that several critical needs of HS are unmet due to a lack of disease awareness, a delay in diagnosis, a poor control of symptoms and high frequency of comorbidities.
We believe that the finding that HS prevalence apparently diminishes with age may be fully correct. For example, individuals with advancing age may have poor treatment-seeking practices or may experience lower social hinderance in the workplace because of HS. Nevertheless, HS is usually diagnosed during the adult period of life, at a mean age of 21 years (range, 15-33.5 years)10; and few studies have been conducted among children and adolescents, although they report evidence for a mild form of the disease.22 Future studies to explore HS prevalence in children and adolescent populations and the factors associated with lower HS prevalence among populations with advancing age are warranted. However, a low HS prevalence may be true if specialized population groups are considered. The prevalence of HS diminishes in women with menopause. This conflicting data could also result from the nature of meta-regression analyses itself, because this technique essentially considers between-study variance rather than the variance between individual study participants.
As evident in this systematic review, various questionnaires were used to assess HS.27 Our analysis reveals no statistically significant differences in prevalence rates according to different diagnostic or screening methods used among included studies. Several studies4,10,12,18,19,23,24 showed a good sensitivity and specificity for screening questionnaires (>90%). Although we did not evaluate or comment on the methods used for validation of screening questionnaires, research is warranted to ascertain the validation of these screening questionnaires in large samples. We recommend that as resources allow, research studies consider clinician-rendered diagnoses instead of using screening questionnaires.
Strengths and Limitations
The strengths of the present review lie in the search of multiple databases as well as a comprehensive manual search. However, its results should be interpreted with caution. First, this review lacked studies from different regions of the world such as Asia and Africa. This lack may either be due to research deficits on HS in these nations or because regional and national academic databases were not searched. Future reviews may consider this limitation. The study was also limited by a high heterogeneity, both clinical and statistical, across studies. This high heterogeneity can be explained by different populations, heterogeneous clinical samples, and heterogenous diagnostic criteria.
In the present review, pooled prevalence estimates for HS were obtained from studies conducted at community level and highly selected settings such as dermatology clinics. Although subgroup analyses have been provided to acknowledge this, it does overestimate HS prevalence. The results of subgroup analyses based on study settings should be interpreted with caution because of several limitations, including coding biases arising from databases at hospital and insurance companies, small number of eligible studies yielding poor statistical power, and other confounding factors. Owing to a lack of information across studies, analyses pertaining to important factors such as race of participants could not be conducted.
The prevalence estimates given in this investigation should be interpreted with caution owing to clinical and statistical heterogenicity across the studies and lack of high-quality worldwide data. As discussed above, these are major limitations of our study design since the quality of a meta-analytical investigation and the conclusions drawn are dependent on the nature of primary studies.
We identify several important factors associated with this heterogeneity. Noteworthy sources of heterogeneity include data from clinical registries in Scandinavian countries; data collection in dermatology clinics challenged with lack of representativeness; and the use of insurance claims data in the US, which is challenged by coding bias. Use of screening questionnaires and clinician-administered diagnoses are also clinically heterogeneous, despite yielding statistical nonsignificance in subgroup analyses.
Conclusions
This systematic review and meta-regression analysis estimates HS prevalence of 0.40% (95% CI, 0.26%-0.63%), with a higher HS prevalence among the dermatologic care settings than in the general population. Substantial heterogeneity was evident within and across the studies. In addition to yielding HS prevalence estimates, this investigation also provides insights pertaining to use of heterogeneous diagnostic and screening methods for HS. Findings from this study may help in the formulation of policies and guiding principles for better disease diagnosis and management.
eFigure 1. Funnel Plot Exhibiting Publication Bias
eFigure 2. Regression Plot Exhibiting Association Between Percentage of Females in the Study Sample and Prevalence of HS
eFigure 3. Regression Plot Exhibiting Association Between Publication Year in the Study Sample and Prevalence of HS
eFigure 4. Regression Plot Exhibiting Association Between Age of Patients and Prevalence of HS
eFigure 5. Quality of Studies as per Joanna Briggs Institute Tool for Critical Appraisal
eFigure 6. Quality of Studies and Association With Prevalence of HS
References
- 1.Kurzen H, Kurokawa I, Jemec GB, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17(5):455-456. doi: 10.1111/j.1600-0625.2008.00712_1.x [DOI] [PubMed] [Google Scholar]
- 2.Jfri AH, O’Brien EA, Litvinov IV, Alavi A, Netchiporouk E. Hidradenitis suppurativa: comprehensive review of predisposing genetic mutations and changes. J Cutan Med Surg. 2019;23(5):519-527. doi: 10.1177/1203475419852049 [DOI] [PubMed] [Google Scholar]
- 3.Jfri A, Netchiporouk E, Raymond K, Litvinov IV, O’Brien E. Association of clinical severity scores with psychosocial impact in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2020;S0190-9622(20)32410-5. doi: 10.1016/j.jaad.2020.08.022 [DOI] [PubMed] [Google Scholar]
- 4.Ingram JR, Jenkins-Jones S, Knipe DW, Morgan CLI, Cannings-John R, Piguet V. Population-based clinical practice research datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178(4):917-924. doi: 10.1111/bjd.16101 [DOI] [PubMed] [Google Scholar]
- 5.Kjaersgaard Andersen R, Saunte SK, Jemec GBE, Saunte DM. Psoriasis as a comorbidity of hidradenitis suppurativa. Int J Dermatol. 2020;59(2):216-220. doi: 10.1111/ijd.14651 [DOI] [PubMed] [Google Scholar]
- 6.Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol. 2010;2(1):9-16. doi: 10.4161/derm.2.1.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolakis G, Kaleta KP, Vaiopoulos AG, et al. Phenotypes and pathophysiology of syndromic hidradenitis suppurativa: different faces of the same disease? a systematic review. Dermatology.Published online September 17, 2020. doi: 10.1159/000509873 [DOI] [PubMed] [Google Scholar]
- 8.Kirsten N, Zander N, Augustin M. Prevalence and cutaneous comorbidities of hidradenitis suppurativa in the German working population. Arch Dermatol Res. 2021;313(2):95-99. doi: 10.1007/s00403-020-02065-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180(4):774-781. doi: 10.1111/bjd.16998 [DOI] [PubMed] [Google Scholar]
- 10.Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13(7):e0200683. doi: 10.1371/journal.pone.0200683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delany E, Gormley G, Hughes R, et al. A cross-sectional epidemiological study of hidradenitis suppurativa in an Irish population (SHIP). J Eur Acad Dermatol Venereol. 2018;32(3):467-473. doi: 10.1111/jdv.14686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760-764. doi: 10.1001/jamadermatol.2017.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collaboration. 2011. [Google Scholar]
- 15.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group . Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JACEM, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046-1055. doi: 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 17.Borenstein M, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons Ltd; 2009. doi: 10.1002/9780470743386 [DOI] [Google Scholar]
- 18.Shahi V, Alikhan A, Vazquez BG, Weaver AL, Davis MD. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229(2):154-158. doi: 10.1159/000363381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg A, Wertenteil S, Baltz R, Strunk A, Finelt N. Prevalence estimates for hidradenitis suppurativa among children and adolescents in the United States: a gender- and age-adjusted population analysis. J Invest Dermatol. 2018;138(10):2152-2156. doi: 10.1016/j.jid.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 20.Garg A, Papagermanos V, Midura M, Strunk A, Merson J. Opioid, alcohol, and cannabis misuse among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2018;79(3):495-500.e1. doi: 10.1016/j.jaad.2018.02.053 [DOI] [PubMed] [Google Scholar]
- 21.Jemec GB, Heidenheim M, Nielsen NH. Hidradenitis suppurativa: characteristics and consequences. Clin Exp Dermatol. 1996;21(6):419-423. doi: 10.1111/j.1365-2230.1996.tb00145.x [DOI] [PubMed] [Google Scholar]
- 22.Jemec GB, Heidenheim M, Nielsen NH. A case-control study of hidradenitis suppurativa in an STD population. Acta Derm Venereol. 1996;76(6):482-483. [DOI] [PubMed] [Google Scholar]
- 23.Killasli H, Sartorius K, Emtestam L, Svensson Å. Hidradenitis suppurativa in Sweden: a registry-based cross-sectional study of 13538 patients. Dermatology. 2020;236(4):281-288. doi: 10.1159/000505545 [DOI] [PubMed] [Google Scholar]
- 24.Kirsten N, Petersen J, Hagenström K, Augustin M. Epidemiology of hidradenitis suppurativa in Germany - an observational cohort study based on a multisource approach. J Eur Acad Dermatol Venereol. 2020;34(1):174-179. doi: 10.1111/jdv.15940 [DOI] [PubMed] [Google Scholar]
- 25.Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59(4):596-601. doi: 10.1016/j.jaad.2008.06.020 [DOI] [PubMed] [Google Scholar]
- 26.Richard MA, Corgibet F, Beylot-Barry M, et al. Sex- and age-adjusted prevalence estimates of five chronic inflammatory skin diseases in France: results of the “OBJECTIFS PEAU” study. J Eur Acad Dermatol Venereol. 2018;32(11):1967-1971. doi: 10.1111/jdv.14959 [DOI] [PubMed] [Google Scholar]
- 27.Vinding GR, Miller IM, Zarchi K, Ibler KS, Ellervik C, Jemec GB. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol. 2014;170(4):884-889. doi: 10.1111/bjd.12787 [DOI] [PubMed] [Google Scholar]
- 28.Lindsø Andersen P, Kromann C, Fonvig CE, Theut Riis P, Jemec GBE, Holm J-C. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2019;59(1):47-51. doi: 10.1111/ijd.14639 [DOI] [PubMed] [Google Scholar]
- 29.Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2009;23(9):985-998. doi: 10.1111/j.1468-3083.2009.03356.x [DOI] [PubMed] [Google Scholar]
- 30.El-Gabalawy H, Guenther LC, Bernstein CN. Epidemiology of immune-mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J Rheumatol Suppl. 2010;85:2-10. doi: 10.3899/jrheum.091461 [DOI] [PubMed] [Google Scholar]
- 31.Liy-Wong C, Pope E, Lara-Corrales I. Hidradenitis suppurativa in the pediatric population. J Am Acad Dermatol. 2015;73(5)(suppl 1):S36-S41. doi: 10.1016/j.jaad.2015.07.051 [DOI] [PubMed] [Google Scholar]
- 32.Vaiopoulos AG, Nikolakis G, Zouboulis CC. Hidradenitis suppurativa in paediatric patients: a retrospective monocentric study in Germany and review of the literature. J Eur Acad Dermatol Venereol. 2020;34(9):2140-2146. doi: 10.1111/jdv.16520 [DOI] [PubMed] [Google Scholar]
- 33.Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231(2):184-190. doi: 10.1159/000431175 [DOI] [PubMed] [Google Scholar]
- 34.von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144(4):809-813. doi: 10.1046/j.1365-2133.2001.04137.x [DOI] [PubMed] [Google Scholar]
- 35.Matusiak Ł, Bieniek A, Szepietowski JC. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol. 2010;62(4):706-708. doi: 10.1016/j.jaad.2009.09.021 [DOI] [PubMed] [Google Scholar]
- 36.Garg A, Neuren E, Cha D, et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol. 2020;82(2):366-376. doi: 10.1016/j.jaad.2019.06.1301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Funnel Plot Exhibiting Publication Bias
eFigure 2. Regression Plot Exhibiting Association Between Percentage of Females in the Study Sample and Prevalence of HS
eFigure 3. Regression Plot Exhibiting Association Between Publication Year in the Study Sample and Prevalence of HS
eFigure 4. Regression Plot Exhibiting Association Between Age of Patients and Prevalence of HS
eFigure 5. Quality of Studies as per Joanna Briggs Institute Tool for Critical Appraisal
eFigure 6. Quality of Studies and Association With Prevalence of HS