Key Points
Question
What is the background rate of venous thromboembolism in patients with vs without a chronic inflammatory skin disease, including psoriasis, atopic dermatitis, alopecia areata, vitiligo, and hidradenitis suppurativa?
Findings
In this cohort study with analysis of claims data on 158 123 patients with a chronic inflammatory skin disease, no indication of an increased incidence of unprovoked clinical venous thromboembolism events was noted in patients with chronic inflammatory skin diseases compared with similar patients without chronic inflammatory skin diseases.
Meaning
In this newer era of advanced systemic medications for treatment of chronic inflammatory skin diseases, having a background rate of venous thromboembolism established may help monitor the safety of newly marketed systemic treatments in dermatology.
Abstract
Importance
Several studies have linked chronic inflammatory skin diseases (CISDs) with venous thromboembolism (VTE) in a range of data sources with mixed conclusions.
Objective
To examine the incidence of VTE in patients with vs without CISD.
Design, Setting, and Participants
A cohort study using commercial insurance claims data from a nationwide US health care database from January 1, 2004, through 2019 was conducted. A total of 158 123 patients with dermatologist-recorded psoriasis, atopic dermatitis, alopecia areata, vitiligo, or hidradenitis suppurativa were included. Risk-set sampling identified patients without a CISD. Patient follow-up lasted until the first of the following occurred: VTE, death, disenrollment, or end of data stream.
Exposures
Patients with vs without CISD.
Main Outcomes and Measures
Venous thromboembolism events were identified with validated algorithms. Incidence rates were computed before and after 1:1 propensity-score matching to account for VTE risk factors. Hazard ratios were estimated to compare the incidence of VTE in the CISD vs non-CISD cohorts.
Results
A total of 158 123 patients were identified with CISD: with psoriasis (n = 96 138), atopic dermatitis (n = 30 418), alopecia areata (n = 17 889), vitiligo (n = 7735), or HS (n = 5934); 9 patients had 2 of these conditions. A total of 1 570 387 patients were without a CISD. The median follow-up time was 1.9 years (interquartile range, 0.8-4.0 years) in patients with CISD. The incidence rate (per 1000 person-years) of outpatient or inpatient VTE was 1.57 in psoriasis, 1.83 in atopic dermatitis, 0.94 in alopecia areata, 0.93 in vitiligo, 1.65 in HS and 1.53 in CISD overall, compared with 1.76 in patients without a CISD. Incidence rates increased in patients aged 50 years or older (2.3 per 1000 person-years) and decreased in those aged 18 to 49 years (0.8 per 1000 person-years). After propensity-score matching to patients without a CISD, the hazard ratio (HR) of VTE was 0.86 (95% CI, 0.75-0.99) in psoriasis, 1.19 (95% CI, 0.95-1.48) in atopic dermatitis, 0.97 (95% CI, 0.65-1.46) in alopecia areata, 0.90 (95% CI, 0.49-1.65) in vitiligo, 1.64 (95% CI, 0.82-3.27) in hidradenitis suppurativa, and 0.94 (95% CI, 0.84-1.05) in CISD overall.
Conclusions and Relevance
In this large-scale cohort study, CISDs were not associated with an increased incidence of VTE after controlling for relevant VTE risk factors in a representative dermatology patient population.
This cohort study examines the incidence of venous thromboembolism in patients with chronic inflammatory skin diseases.
Introduction
Chronic inflammatory conditions, such as rheumatoid arthritis, are known to increase the risk of venous thromboembolism (VTE).1,2,3 Several studies linked chronic inflammatory skin diseases (CISDs) with VTE in a range of data sources. Based on cross-sectional data from the US National Inpatient Sample, a 22% increase in VTE was found among patients with vs without atopic dermatitis (AD), which was slightly higher among adults aged 50 years or older (25%).4 Among patients with severe psoriasis, one study observed a 13% increase in VTE risk in a UK general practice network not excluding patients with cancer, and a Scandinavian claims data analysis found a 2- to 3-fold increase.5,6 In a Taiwan national claims database, patients with dermatomyositis had an 11-fold increase in VTE risk in an analysis that included patients with cancer—a known risk factor for VTE.7
It is important to establish a robust estimate of the background VTE risk in patients with CISDs, including psoriasis (without psoriatic arthritis), AD, alopecia areata (AA), vitiligo, and hidradenitis suppurativa (HS). These CISDs vary in pathogenesis and, as such, the underlying baseline VTE risk may be different among these individual conditions. Some systemic immunomodulatory agents that are used in the treatment of CISDs are suspected or known to modify the VTE risk, including systemic corticosteroids, high-dose Janus kinase inhibitors, and possibly other agents.8,9,10 In addition to treatments, some comorbidities associated with CISD, such as inflammatory bowel disease and rheumatoid arthritis, are also associated with VTE risk. It is important to isolate the CISDs’ effect on VTE from already established VTE risk associated with comorbidities and various treatments. The need to establish this background VTE risk has become more pressing as a number of new systemic immunomodulatory agents for treatment of dermatologic conditions, including Janus kinase inhibitors for AD, AA, and vitiligo, will likely be marketed in the near future.11
There are different approaches to determining the risk for VTE in the CISD population. The variability of findings from previous studies depends on the exclusion criteria. Studies with strict exclusion criteria aim to identify the VTE risk specific to the dermatologic condition of interest independent of patient characteristics, comorbidities, and treatments that may influence the incidence of VTE. Studies with more relaxed exclusion criteria identify the VTE risk of the condition plus its associated comorbidities, and another group of studies identifies the VTE risk of the condition plus the associated comorbidities and their treatments. Although none of these approaches are wrong, they have different utility. To establish a background rate of VTE specific to CISDs and isolated from the noise of comorbidities and various treatments, it is necessary to apply strict exclusions and adjustments. We sought to establish a background incidence of VTE in patients with dermatologist-diagnosed CISDs independent of existing risk factors, comorbidities, and treatments.
Methods
Data Source
We used real-world data from a national commercial insurance claims database (Optum’s deidentified Clinformatics Data Mart Database) from January 1, 2004, through 2019. The database is composed of beneficiaries of commercial and Medicare Advantage health plans. The database contains dated information on plan enrollment, health care use, demographics, and integrated records for inpatient and outpatient encounters and pharmacy dispensing. All patient information was deidentified. The Brigham and Women’s Hospital’s Institutional Review Board approved this study, and a signed data licensing agreement was in place.
Study Cohort
This study sought to compare patients with vs without CISD within the dermatology clinic population. We identified patients of all ages who were diagnosed with a CISD, including psoriasis (without psoriatic arthritis), AD, AA, vitiligo, and HS, according to the following criteria: at least 2 visits to a dermatologist within 1 year and a diagnosis of the same CISD at each visit (eTable 3 in the Supplement provides specific codes). We required that the skin conditions be mutually exclusive, meaning that a patient who had 2 encounters with a dermatologist within a year but received a diagnosis of AD at 1 visit and psoriasis at the next would not qualify. The cohort entry date for the CISD group was the second CISD diagnosis after at least 365 days of continuous enrollment. For the non-CISD group, we risk-set sampled 10 patients without CISD from all plan enrollees who had at least 2 visits to a dermatologist (cohort entry date was the second dermatologist visit) within a year but were not diagnosed with a CISD at any time, ensuring a reliable diagnosis separation of the 2 groups. The patients without CISD were matched on the cohort entry date ±60 days of the patient with CISD and were required to have at least 365 days of continuous enrollment before the matched cohort entry date (eTable 9 and eTable 12 in the Supplement provide more information on the non-CISD group). Patients from either the CISD or non-CISD cohort were excluded if they had any of the following conditions before their cohort entry: VTE, use of an anticoagulant, diagnosis of cancer (excluding nonmelanoma skin cancer),12 prothrombotic condition (factor V Leiden, antiphospholipid antibody syndrome, prothrombin deficiency, and factor C and S deficiency), sickle cell disease, HIV/AIDS, or chronic inflammatory conditions associated with VTE (rheumatoid arthritis, inflammatory bowel disease, and lupus) (eFigure in the Supplement). Including these conditions could raise concern as to whether the VTE risk was due to CISD or to the comorbid inflammatory bowel disease, rheumatoid arthritis, or cancer.
Outcomes
We identified incident VTE, deep venous thrombosis, or pulmonary embolism events during all available follow-up times. Venous thromboembolism was defined as either a hospitalization with a primary discharge diagnosis of VTE or an outpatient visit with a diagnosis of VTE followed by initiation of an anticoagulant within 7 days or hospitalization for VTE within 7 days (positive predictive value, 72%-90%).13,14,15,16 For a more-specific but less-sensitive definition, we limited our secondary outcome to include only inpatient events, defined as hospitalization with a primary discharge diagnosis of deep venous thrombosis or pulmonary embolism (positive predictive value, 83%-90%).13,16 We included a third VTE outcome to capture probable VTE events defined as an inpatient VTE recorded on hospital claims as a discharge diagnosis in any position (positive predictive value, 72%).17 For each definition, the event date was the date on which all criteria were fulfilled. Detailed International Classification of Diseases, Version 9 (ICD-9), and International Statistical Classification of Disease, Version 10 (ICD-10), codes are listed in eTable 3 in the Supplement. Follow-up began the day after the cohort entry day and lasted until the first of the following events occurred: VTE, death, disenrollment, end of enrollment, and end of data stream (eFigure in the Supplement).18
Patient Characteristics
All patient characteristics were assessed during the 365 days before cohort entry (eFigure in the Supplement). We considered age at cohort entry, sex, race, year of cohort entry, history of fracture, surgery, tobacco use, hospitalization, hemiplegia or paraplegia, earlier use of oral or nonoral contraceptives, hormone replacement therapy, systemic glucocorticoid use, cumulative systemic prednisone equivalencies over the previous 180 days, systemic immunomodulatory medication use (nonbiologic immunomodulators: methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, sulfasalazine, and leflunomide; biologic immunomodulators: dupilumab, risankizumab-rzaa, tildrakizumab, ixekizumab, secukinumab, guselkumab, ustekinumab, abatacept, adalimumab, etanercept, golimumab, certolizumab, infliximab, rituximab, anakinra, and tocilizumab; and targeted synthetic immunomodulators: tofacitinib and baricitinib), number of physician visits and unique prescription medications filled, and a range of comorbidities that may be risk factors for VTE (Table 1; eTable 1 in the Supplement).19
Table 1. Baseline Patient Characteristics Comparing Patients With vs Without a CISD, Before 1:1 Propensity-Score Matching.
| Characteristics within 365 d before cohort entry | Unmatched, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Non-CISD (referent) | Psoriasis | Atopic dermatitis | Alopecia areata | Vitiligo | Hidradenitis suppurativa | Overall CISD | |
| No. of patients | 1 570 387 | 96 138 | 30 418 | 17 889 | 7735 | 5934 | 158 123 |
| Age, mean (SD), y | 51.58 (18.42) | 49.81 (15.89) | 47.48 (18.02) | 41.78 (14.35) | 46.43 (16.00) | 37.68 (13.22) | 47.83 (16.40) |
| Sex | |||||||
| Male | 648 232 (41.3) | 47 568 (49.5) | 11 925 (39.2) | 7334 (41.0) | 3607 (46.6) | 1390 (23.4) | 71 832 (45.4) |
| Female | 922 155 (58.7) | 48 570 (50.5) | 18 493 (60.8) | 10 555 (59.0) | 4128 (53.4) | 4544 (76.6) | 86 291 (54.6) |
| Racea | |||||||
| White | 1 308 052 (83.3) | 76 751 (79.8) | 21 433 (70.5) | 10 614 (59.3) | 4546 (58.8) | 3931 (66.2) | 117 283 (74.2) |
| Asian | 51 289 (3.3) | 4370 (4.5) | 2513 (8.3) | 1745 (9.8) | 917 (11.9) | 264 (4.4) | 9810 (6.2) |
| Black | 95 035 (6.1) | 6264 (6.5) | 3850 (12.7) | 2293 (12.8) | 811 (10.5) | 1062 (17.9) | 14 280 (9.0) |
| Hispanic | 116 011 (7.4) | 8753 (9.1) | 2622 (8.6) | 3237 (18.1) | 1461 (18.9) | 677 (11.4) | 16 750 (10.6) |
| Comorbid conditions that may increase VTE risk | |||||||
| Obesity or weight gain | 128 263 (8.2) | 11 179 (11.6) | 2406 (7.9) | 1528 (8.5) | 644 (8.3) | 1462 (24.6) | 17 219 (10.9) |
| Tobacco use | 73 151 (4.7) | 6744 (7.0) | 1411 (4.6) | 697 (3.9) | 215 (2.8) | 694 (11.7) | 9761 (6.2) |
| Pregnancy | 36 817 (2.3) | 1699 (1.8) | 906 (3.0) | 366 (2.0) | 156 (2.0) | 225 (3.8) | 3352 (2.1) |
| Miscarriage | 5176 (0.3) | 213 (0.2) | 120 (0.4) | 53 (0.3) | 27 (0.3) | 25 (0.4) | 438 (0.3) |
| Stroke (excluding TIA) | 1098 (0.1) | 46 (0.0) | 21 (0.1) | 1 (0) | 2 (0.0) | 1 (0) | 71 (0) |
| Transient ischemic attack | 10 800 (0.7) | 526 (0.5) | 156 (0.5) | 51 (0.3) | 33 (0.4) | 9 (0.2) | 776 (0.5) |
| Nephrotic syndrome | 780 (0) | 40 (0) | 12 (0) | 5 (0) | 3 (0) | 1 (0) | 61 (0) |
| Varicose veins | 26 668 (1.7) | 1275 (1.3) | 577 (1.9) | 190 (1.1) | 92 (1.2) | 56 (0.9) | 2190 (1.4) |
| Dyslipidemia | 540 837 (34.4) | 33 361 (34.7) | 9204 (30.3) | 4027 (22.5) | 2262 (29.2) | 1316 (22.2) | 50 171 (31.7) |
| Endocarditis | 13 915 (0.9) | 1064 (1.1) | 412 (1.4) | 109 (0.6) | 49 (0.6) | 191 (3.2) | 1825 (1.2) |
| Atrial fibrillation | 2875 (0.2) | 105 (0.1) | 42 (0.1) | 4 (0.0) | 0 (0.0) | 1 (0) | 152 (0.1) |
| Syncope | 27 179 (1.7) | 1332 (1.4) | 495 (1.6) | 219 (1.2) | 105 (1.4) | 92 (1.6) | 2243 (1.4) |
| Peripheral vascular disease | 63 062 (4.0) | 3299 (3.4) | 963 (3.2) | 246 (1.4) | 156 (2.0) | 97 (1.6) | 4761 (3.0) |
| Hypertension | 351 067 (22.4) | 22 959 (23.9) | 7011 (23.0) | 2395 (13.4) | 1247 (16.1) | 835 (14.1) | 34 448 (21.8) |
| Liver disease | 24 719 (1.6) | 2674 (2.8) | 495 (1.6) | 276 (1.5) | 144 (1.9) | 154 (2.6) | 3743 (2.4) |
| Dementia or other neurologic disorders | 18 571 (1.2) | 765 (0.8) | 270 (0.9) | 70 (0.4) | 45 (0.6) | 39 (0.7) | 1189 (0.8) |
| Diabetes, severe or complicated | 60 503 (3.9) | 4657 (4.8) | 975 (3.2) | 314 (1.8) | 258 (3.3) | 268 (4.5) | 6472 (4.1) |
| Congestive heart failure | 38 978 (2.5) | 2175 (2.3) | 679 (2.2) | 147 (0.8) | 88 (1.1) | 88 (1.5) | 3177 (2.0) |
| Chronic obstructive pulmonary disease | 78 996 (5.0) | 5449 (5.7) | 1559 (5.1) | 578 (3.2) | 249 (3.2) | 376 (6.3) | 8212 (5.2) |
| Alcohol abuse | 12 019 (0.8) | 1099 (1.1) | 263 (0.9) | 136 (0.8) | 35 (0.5) | 76 (1.3) | 1609 (1.0) |
| Kidney disease or failure | 54 303 (3.5) | 2467 (2.6) | 821 (2.7) | 194 (1.1) | 131 (1.7) | 94 (1.6) | 3708 (2.3) |
| Hemiplegia or paraplegia | 4010 (0.3) | 183 (0.2) | 63 (0.2) | 16 (0.1) | 10 (0.1) | 15 (0.3) | 287 (0.2) |
| Gagne chronic comorbidity score, mean (SD)b | 0.18 (0.92) | 0.15 (0.86) | 0.13 (0.83) | 0.08 (0.60) | 0.11 (0.68) | 0.20 (0.78) | 0.14 (0.82) |
| Fracture | |||||||
| Upper and lower extremities | 39 351 (2.5) | 2138 (2.2) | 637 (2.1) | 362 (2.0) | 180 (2.3) | 130 (2.2) | 3447 (2.2) |
| Neck of femur, hip | 2723 (0.2) | 84 (0.1) | 30 (0.1) | 9 (0.1) | 5 (0.1) | 1 (0) | 129 (0.1) |
| Surgery | |||||||
| Gynecologic | 4111 (0.3) | 237 (0.2) | 75 (0.2) | 51 (0.3) | 24 (0.3) | 40 (0.7) | 427 (0.3) |
| Abdominal | 10 007 (0.6) | 586 (0.6) | 165 (0.5) | 92 (0.5) | 40 (0.5) | 55 (0.9) | 938 (0.6) |
| Cardiovascular | 10 180 (0.6) | 614 (0.6) | 165 (0.5) | 41 (0.2) | 25 (0.3) | 21 (0.4) | 866 (0.5) |
| Musculoskeletal | 13 491 (0.9) | 702 (0.7) | 181 (0.6) | 79 (0.4) | 41 (0.5) | 43 (0.7) | 1046 (0.7) |
| Systemic glucocorticoid use | |||||||
| Past 180 d | 218 484 (13.9) | 16 471 (17.1) | 8019 (26.4) | 2404 (13.4) | 983 (12.7) | 1080 (18.2) | 28 958 (18.3) |
| Past 60 d | 69 419 (4.4) | 5296 (5.5) | 3415 (11.2) | 711 (4.0) | 289 (3.7) | 1080 (18.2) | 10 040 (6.3) |
| Sum of daily dose of systemic glucocorticoids, mean (SD), prednisone milligram equivalencies | 53.19 (303.54) | 70.21 (321.60) | 133.20 (450.27) | 55.58 (293.82) | 45.25 (257.80) | 75.04 (357.05) | 79.63 (347.01) |
| Use of systemic immunomodulators | |||||||
| Nonbiologicc | 19 765 (1.3) | 7136 (7.4) | 829 (2.7) | 277 (1.5) | 99 (1.3) | 61 (1.0) | 8404 (5.3) |
| Biologicd | 1116 (0.1) | 15 028 (15.6) | 164 (0.5) | 32 (0.2) | 11 (0.1) | 353 (5.9) | 15 589 (9.9) |
| TNF inhibitore | 588 (0) | 10 990 (11.4) | 35 (0.1) | 13 (0.1) | 9 (0.1) | 329 (5.5) | 11 377 (7.2) |
| Non-TNF inhibitorf | 436 (0) | 3715 (3.9) | 126 (0.4) | 16 (0.1) | 2 (0) | 6 (0.1) | 3865 (2.4) |
| Infliximab | 92 (0) | 323 (0.3) | 3 (0) | 3 (0) | 0 | 18 (0.3) | 347 (0.2) |
| Targeted synthetic immunomodulatorsg | 1 (0) | 5 (0) | 0 | 15 (0.1) | 0 | 1 (0) | 21 (0) |
| Other medications | |||||||
| Antiarrhythmics | 75 112 (4.8) | 4482 (4.7) | 1483 (4.9) | 646 (3.6) | 269 (3.5) | 291 (4.9) | 7173 (4.5) |
| Isotretinoin | 24 507 (1.6) | 138 (0.1) | 314 (1.0) | 42 (0.2) | 21 (0.3) | 237 (4.0) | 752 (0.5) |
| Antiplatelets | 35 882 (2.3) | 2238 (2.3) | 607 (2.0) | 150 (0.8) | 94 (1.2) | 59 (1.0) | 3148 (2.0) |
| Statins | 328 843 (20.9) | 20 598 (21.4) | 5206 (17.1) | 1958 (10.9) | 1209 (15.6) | 683 (11.5) | 29 655 (18.8) |
| Tamoxifen | 653 (0) | 31 (0) | 4 (0) | 6 (0) | 5 (0.1) | 2 (0) | 48 (0) |
| Nonoral contraceptives | 15 087 (1.0) | 822 (0.9) | 347 (1.1) | 200 (1.1) | 69 (0.9) | 201 (3.4) | 1639 (1.0) |
| Oral contraceptives | 211 249 (13.5) | 9024 (9.4) | 4740 (15.6) | 2454 (13.7) | 910 (11.8) | 1168 (19.7) | 18 296 (11.6) |
| Hormone replacement therapy | 190 847 (12.2) | 8490 (8.8) | 4039 (13.3) | 2088 (11.7) | 784 (10.1) | 880 (14.8) | 16 281 (10.3) |
| Health care use | |||||||
| No. of prescription medications, mean (SD) | 17.72 (23.14) | 22.27 (26.15) | 20.64 (24.96) | 13.10 (18.12) | 15.05 (20.61) | 22.07 (25.96) | 20.56 (25.07) |
| No. of office visits, mean (SD) | 3.93 (3.10) | 4.40 (3.41) | 4.82 (3.51) | 4.04 (3.12) | 4.19 (3.53) | 4.83 (3.54) | 4.45 (3.42) |
Abbreviations: CISD, chronic inflammatory skin disease; TIA, transient ischemic attack; TNF, tumor necrosis factor; VTE, venous thromboembolism.
Optum uses proprietary algorithms to define race; race is a derived ethnicity. The member’s ethnicity is derived by using the member’s name and geographic location. Once the ethnicity is determined, the member is mapped to 1 of 4 race categories (Asian, Black, Hispanic, and White).
A Gagne chronic comorbidity score of −2 indicates the lowest risk of dying in the next year and a score of 26 indicates the highest risk of dying in the next year.
Nonbiologic immunomodulators were defined as methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, sulfasalazine, leflunomide.
Biologic immunomodulators were defined as adalimumab, etanercept, golimumab, certolizumab, infliximab, dupilumab, risankizumab-rzaa, tildrakizumab, ixekizumab, secukinumab, guselkumab, ustekinumab, abatacept, rituximab, anakinra, tocilizumab.
TNF inhibitor biologic immunomodulators were defined as adalimumab, etanercept, golimumab, certolizumab, infliximab.
Non-TNF inhibitor biologic immunomodulators were defined as dupilumab, risankizumab-rzaa, tildrakizumab, ixekizumab, secukinumab, guselkumab, ustekinumab, abatacept, rituximab, anakinra, tocilizumab.
Targeted synthetic immunomodulators were defined as tofacitinib or baricitinib.
Statistical Analysis
We tabulated baseline patient characteristics for patients with and without CISD and separately for each CISD component condition: psoriasis, AD, AA, vitiligo, and HS. We recorded the duration of follow-up, including reasons for censoring, and computed incidence rates with 95% CIs.
To account for potential differences in VTE risk factors, we used propensity score (PS) matching.20,21 We included all pre-exposure patient characteristics without further variable selection as independent variables for PS matching. The PS predicting the presence or absence of CISD was estimated with a multivariable logistic regression for each CISD component condition. Propensity score matching was performed using 1:1 nearest neighbor matching with a maximum caliper of 0.02 on the PS scale.22 We computed standardized differences in the covariates between the 2 groups after PS matching to quantify the balance achieved at baseline.23,24 Kaplan-Meier curves were plotted for CISD and each component condition after PS matching. Hazard ratios (HRs) comparing the incidence of VTE in patients with vs without CISD were estimated by fitting Cox proportional hazards regression models.25
To study effect modification, we separately analyzed 3 subgroups of patients: those (1) with no previous use of systemic immunomodulators, (2) aged 18 to 49 years, and (3) aged 50 years or older. To demonstrate the robustness of our approach, we included a positive control (rheumatoid arthritis vs non–rheumatoid arthritis) and a negative tracer outcome (skull or face fracture). In a sensitivity analysis, we included additional adjustment for nonmelanoma skin cancer requiring excision. All analyses were conducted using the Aetion Evidence Platform, version 4.12,26 which has been validated.27,28,29
Results
We identified 158 123 qualifying patients with a diagnosis of a CISD, which comprised patients with psoriasis (n = 96 138), AD (n = 30 418), AA (n = 17 889), vitiligo (n = 7735), and HS (n = 5934). We identified 1 570 387 patients without a CISD through 10:1 risk-set sampling. The median follow-up time was 1.9 years (interquartile range, 0.8-4.0 years) in patients with CISD and 2.0 years (interquartile range, 0.8-4.2) in those without CISD; 25% had 4.2 years of follow-up or longer. The reasons for censoring were similar between groups (eTable 4 in the Supplement).
Patients with a CISD were overall younger than those without CISD (mean [SD], 47.83 [16.40] vs 51.58 [18.42] years) (Table 1). The youngest patients were those with HS (mean [SD], 37.68 [13.22] years) and alopecia (mean [SD], 41.78 [14.35] years). There was a slight female predominance in the non-CISD (922 155 [58.7%]), AD (18 493 [60.8%]), and alopecia (10 555 [59.0%]) cohorts and a female predominance in the HS (4544 [76.6%]). Across all patients, White race was most common, with a slightly lower percentage in the vitiligo (4546 [58.8%]), alopecia (10 614 [59.3%]), and HS (3931 [66.2%]) cohorts. Use of systemic immunomodulators was more prevalent in patients with a CISD (nonbiologic immunomodulator, 8404 [5.3%] and biologic immunomodulator, 15 589 [9.9%]) compared with those without a CISD (nonbiologic immunomodulator, 99 [1.3%] and biologic immunomodulator, 11 [0.1%]). During follow-up, use of systemic immunomodulators increased in patients with CISD (eTable 5 in the Supplement). Across CISDs, we observed that, before matching, the demographics, comorbidity patterns, and systemic immunomodulatory treatments were aligned with the known epidemiologic factors and treatment guidelines.30,31,32,33,34,35,36,37,38,39 As an example, patients with HS were younger (37.68 vs 51.58 years in patients with non-CISD), more commonly female (76.6% vs 58.7%), Black (1062 [17.9%] vs 95 035 [6.1%]), and obese (1462 [24.6%] vs 128 263 [8.2%]), with increased use of tobacco (694 [11.7%] vs 73 151 [4.7%]), isotretinoin (237 [4.0%] vs 24 507 [1.6%]), oral contraceptives (1168 [19.7%] vs 211 249 [13.5%]) and tumor necrosis factor inhibitors (329 [5.5%] vs 588 [0%) as anticipated (Table 1).34 After 1:1 PS matching, all exposure patients (ie, those with CISDs) were matched with 1 of 10 non-CISD candidates. The distribution of all patient characteristics was well balanced between groups after 1:1 PS matching (eTable 2 in the Supplement).
Incidence of VTE
Before PS matching, the incidence rate per 1000 person-years of inpatient or outpatient VTE with evidence of treatment was 1.57 in psoriasis, 1.83 in AD, 0.94 in AA, 0.93 in vitiligo, and 1.65 in HS compared with 1.76 in patients without CISD (Table 2). Overall, the incidence rate of VTE was 1.53 per 1000 person-years in CISD vs 1.76 in non-CISD. The incidence rate was almost unchanged when excluding patients with systemic immunomodulator use (1.5) (Figure 1).
Table 2. Incidence Rate and Hazard Ratio of Venous Thromboembolism Before and After 1:1 Propensity-Score Matching.
| Outcome/comparison | Unmatched | 1:1 PS-matched | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Person-time per 1000 person-years | No. of events | Incidence rate per 1000 person-years | HR (95% CI) | No. of patients | Person-time per 1000 person-years | No. of events | Incidence rate per 1000 person-years | HR (95% CI) | |
| VTE, inpatient or outpatienta,b | ||||||||||
| Psoriasis | 96 138 | 272 791 | 428 | 1.57 | 0.89 (0.81-0.98) | 82 887 | 239 793 | 383 | 1.6 | 0.86 (0.75-0.99) |
| Non-CISD | 1 570 387 | 4 597 465 | 8110 | 1.76 | 1 [Reference] | 82 887 | 239 292 | 442 | 1.85 | 1 [Reference] |
| Atopic dermatitis | 30 418 | 93 360 | 171 | 1.83 | 1.04 (0.89-1.21) | 30 418 | 93 360 | 171 | 1.83 | 1.19 (0.95-1.48) |
| Non-CISD | 1 570 387 | 4 597 465 | 8110 | 1.76 | 1 [Reference] | 30 418 | 89 940 | 139 | 1.55 | 1 [Reference] |
| Alopecia areata | 17 889 | 48 908 | 46 | 0.94 | 0.53 (0.40-0.71) | 17 877 | 48 896 | 46 | 0.94 | 0.97 (0.65-1.46) |
| Non-CISD | 1 570 387 | 4 597 465 | 8110 | 1.76 | 1 [Reference] | 17 877 | 48 419 | 47 | 0.97 | 1 [Reference] |
| Vitiligo | 7735 | 21 528 | 20 | 0.93 | 0.53 (0.34-0.82) | 7735 | 21 528 | 20 | 0.93 | 0.90 (0.49-1.65) |
| Non-CISD | 1 570 387 | 4 597 465 | 8110 | 1.76 | 1 [Reference] | 7735 | 21 313 | 22 | 1.03 | 1 [Reference] |
| Hidradenitis suppurativa | 5934 | 13 309 | 22 | 1.65 | 0.94 (0.62-1.42) | 5777 | 13 120 | 21 | 1.6 | 1.64 (0.82-3.27) |
| Non-CISD | 1 570 387 | 4 597 465 | 8110 | 1.76 | 1 [Reference] | 5777 | 13 296 | 13 | 0.98 | 1 [Reference] |
| Overall CISDc | 158 123 | 449 934 | 687 | 1.53 | 0.87 (0.80-0.94) | 144 381 | 416 203 | 638 | 1.53 | 0.94 (0.84-1.05) |
| Non-CISD | 1 570 387 | 4 597 465 | 8110 | 1.76 | 1 [Reference] | 144 381 | 410 208 | 669 | 1.63 | 1 [Reference] |
| VTE, inpatient (primary position)a | ||||||||||
| Psoriasis | 96 138 | 273 541 | 162 | 0.59 | 0.79 (0.67-0.92) | 82 887 | 240 483 | 144 | 0.6 | 0.73 (0.59-0.91) |
| Non-CISD | 1 570 387 | 4 610 640 | 3476 | 0.75 | 1 [Reference] | 82 887 | 239 941 | 196 | 0.82 | 1 [Reference] |
| Atopic dermatitis | 30 418 | 93 643 | 65 | 0.69 | 0.92 (0.72-1.18) | 30 418 | 93 643 | 65 | 0.69 | 1.04 (0.73-1.48) |
| Non-CISD | 1 570 387 | 4 610 640 | 3476 | 0.75 | 1 [Reference] | 30 418 | 90 160 | 60 | 0.67 | 1 [Reference] |
| Alopecia areata | 17 889 | 48 986 | 17 | 0.35 | 0.46 (0.29-0.74) | 17 877 | 48 974 | 17 | 0.35 | 0.89 (0.46-1.70) |
| Non-CISD | 1 570 387 | 4 610 640 | 3476 | 0.75 | 1 [Reference] | 17 877 | 48 508 | 19 | 0.39 | 1 [Reference] |
| Vitiligo | 7735 | 21 567 | 9 | 0.42 | 0.55 (0.29-1.06) | 7735 | 21 567 | 9 | 0.42 | 1.48 (0.53-4.17) |
| Non-CISD | 1 570 387 | 4 610 640 | 3476 | 0.75 | 1 [Reference] | 7735 | 21 345 | 6 | 0.28 | 1 [Reference] |
| Hidradenitis suppurativa | 5934 | 13 337 | 8 | 0.6 | 0.80 (0.40-1.59) | 5777 | 13 148 | 7 | 0.53 | 0.79 (0.29-2.11) |
| Non-CISD | 1 570 387 | 4 610 640 | 3476 | 0.75 | 1 [Reference] | 5777 | 13 314 | 9 | 0.68 | 1 [Reference] |
| Overall CISD | 158 123 | 451 110 | 261 | 0.58 | 0.77 (0.68-0.87) | 144 381 | 417 314 | 241 | 0.58 | 0.85 (0.72-1.01) |
| Non-CISD | 1 570 387 | 4 610 640 | 3476 | 0.75 | 1 [Reference] | 144 381 | 411 333 | 279 | 0.68 | 1 [Reference] |
| Negative tracer (skull or face fracture) | ||||||||||
| Psoriasis | 96 138 | 272 717 | 460 | 1.69 | 0.91 (0.82-0.99) | 82 887 | 239 741 | 400 | 1.67 | 0.89 (0.78-1.02) |
| Non-CISD | 1 570 387 | 4 596 773 | 8567 | 1.86 | 1 [Reference] | 82 887 | 239 189 | 447 | 1.87 | 1 [Reference] |
| Atopic dermatitis | 30 418 | 93 286 | 184 | 1.97 | 1.06 (0.91-1.22) | 30 418 | 93 286 | 184 | 1.97 | 1.12 (0.91-1.39) |
| Non-CISD | 1 570 387 | 4 596 773 | 8567 | 1.86 | 1 [Reference] | 30 418 | 89 882 | 158 | 1.76 | 1 [Reference] |
| Alopecia areata | 17 889 | 48 872 | 66 | 1.35 | 0.72 (0.57-0.92) | 17 877 | 48 861 | 66 | 1.35 | 0.93 (0.67-1.31) |
| Non-CISD | 1 570 387 | 4 596 773 | 8567 | 1.86 | 1 [Reference] | 17 877 | 48 371 | 70 | 1.45 | 1 [Reference] |
| Vitiligo | 7735 | 21 510 | 30 | 1.39 | 0.75 (0.52-1.07) | 7735 | 21 510 | 30 | 1.39 | 1.02 (0.61-1.71) |
| Non-CISD | 1 570 387 | 4 596 773 | 8567 | 1.86 | 1 [Reference] | 7735 | 21 294 | 29 | 1.36 | 1 [Reference] |
| Hidradenitis suppurativa | 5934 | 13 327 | 11 | 0.83 | 0.44 (0.25-0.80) | 5777 | 13 138 | 11 | 0.84 | 1.01 (0.44-2.34) |
| Non-CISD | 1 570 387 | 4 596 773 | 8567 | 1.86 | 1 [Reference] | 5777 | 13 316 | 11 | 0.83 | 1 [Reference] |
| Overall CISD | 158 123 | 449 750 | 751 | 1.67 | 0.90 (0.83-0.97) | 144 381 | 416 043 | 688 | 1.65 | 0.96 (0.86-1.07) |
| Non-CISD | 1 570 387 | 4 596 773 | 8567 | 1.86 | 1 [Reference] | 144 381 | 410 116 | 707 | 1.72 | 1 [Reference] |
Abbreviations: CISD, chronic inflammatory skin disease; HR, hazard ratio; PS, propensity score; VTE, venous thromboembolism.
Inpatient VTE was defined as a hospitalization with a primary discharge diagnosis of VTE (deep vein thrombosis or pulmonary embolism).
Outpatient VTE was defined as an outpatient visit with a diagnosis of VTE followed by a prescription filled for an anticoagulant within 7 days or hospitalization for VTE within 7 days.
Overall chronic inflammatory skin disease was defined as a composite of the individual conditions: psoriasis, atopic dermatitis, alopecia areata, vitiligo, and hidradenitis suppurativa.
Figure 1. Estimates of Developing Venous Thromboembolism (VTE) in Patients With vs Without a Chronic Inflammatory Skin Disease (CISD).
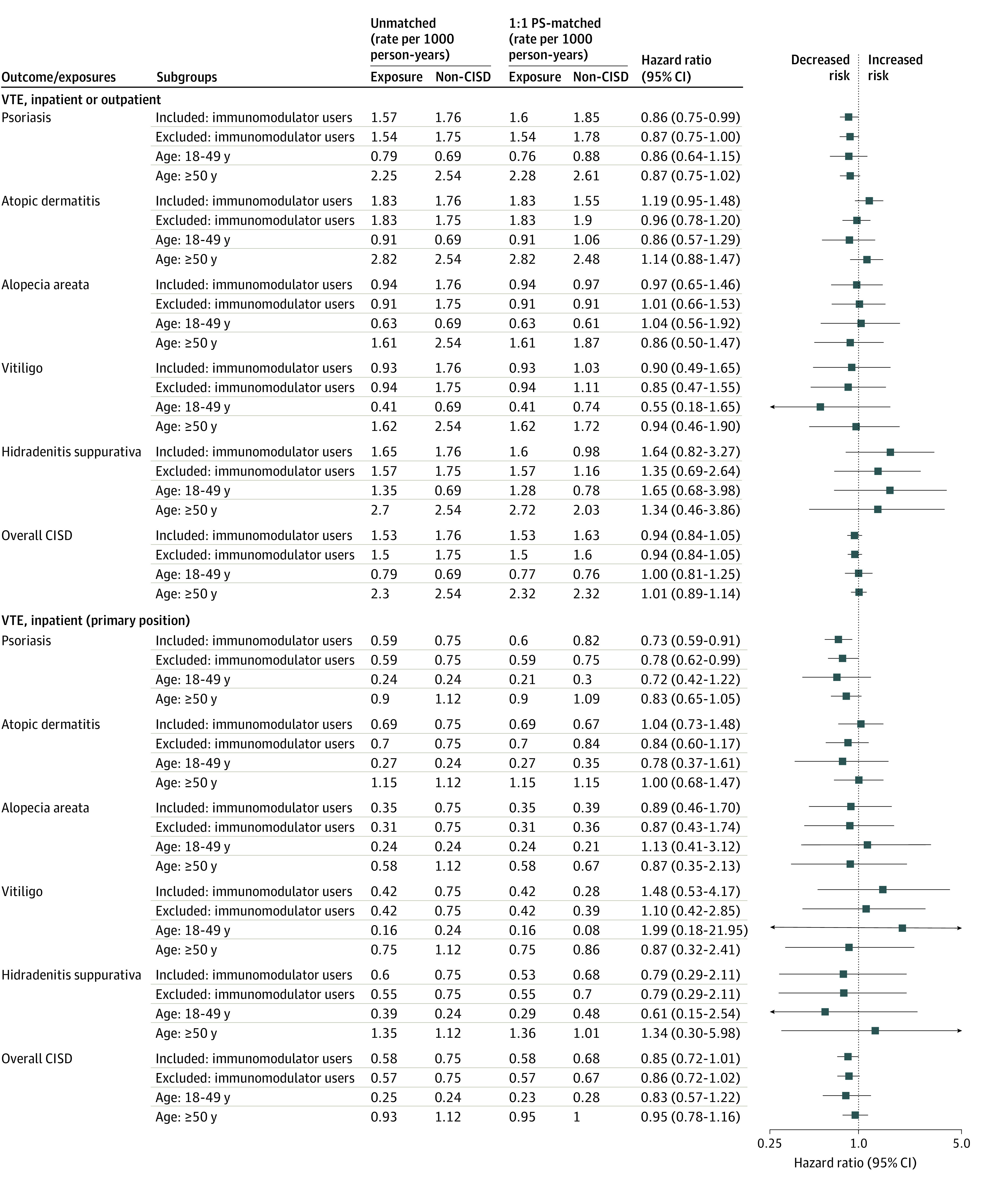
Chronic inflammatory skin disease was defined as a composite of the individual conditions: psoriasis, atopic dermatitis, alopecia areata, vitiligo, and hidradenitis suppurativa. Non-CISD was defined as 2 dermatologist visits with no diagnosis of a CISD and matched to the exposure (CISD) patients’ cohort entry date ±60 days. Inpatient VTE was defined as a hospitalization with a primary discharge diagnosis of VTE. Outpatient VTE was defined as an outpatient visit with a diagnosis of VTE followed by initiation of anticoagulant therapy within 7 days or hospitalization for VTE within 7 days. Immunomodulator users included patients receiving treatment with nonbiologic immunomodulatory agents (methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, sulfasalazine, and leflunomide), biologic immunomodulatory agents (dupilumab, risankizumab-rzaa, tildrakizumab, ixekizumab, secukinumab, guselkumab, ustekinumab, abatacept, adalimumab, etanercept, golimumab, certolizumab, infliximab, rituximab, anakinra, and tocilizumab), and targeted synthetic immunomodulatory agents (tofacitinib and baricitinib). PS indicates propensity score.
After PS matching, the risk of inpatient or outpatient VTE was not significantly increased in patients with psoriasis (HR, 0.86; 95% CI, 0.75-0.99), AD (HR, 1.19; 95% CI, 0.95-1.48), AA (HR, 0.97; 95% CI, 0.65-1.46), vitiligo (HR, 0.90; 95% CI, 0.49-1.65), or CISD overall (HR, 0.94; 95% CI, 0.84-1.05), when compared with those without a CISD. We observed a numerically increased risk in patients with HS (HR, 1.64; 95% CI, 0.82-3.27), which was based on 34 events among 13 120 patient pairs resulting in wide 95% CIs (Figure 1). Kaplan-Meier plots reflected these results, showing no increase in VTE risk for the CISD vs non-CISD groups over up to 13 years of follow-up (Figure 2).
Figure 2. Time to Venous Thromboembolism (VTE) in Patients With vs Without a Chronic Inflammatory Skin Disease (CISD).
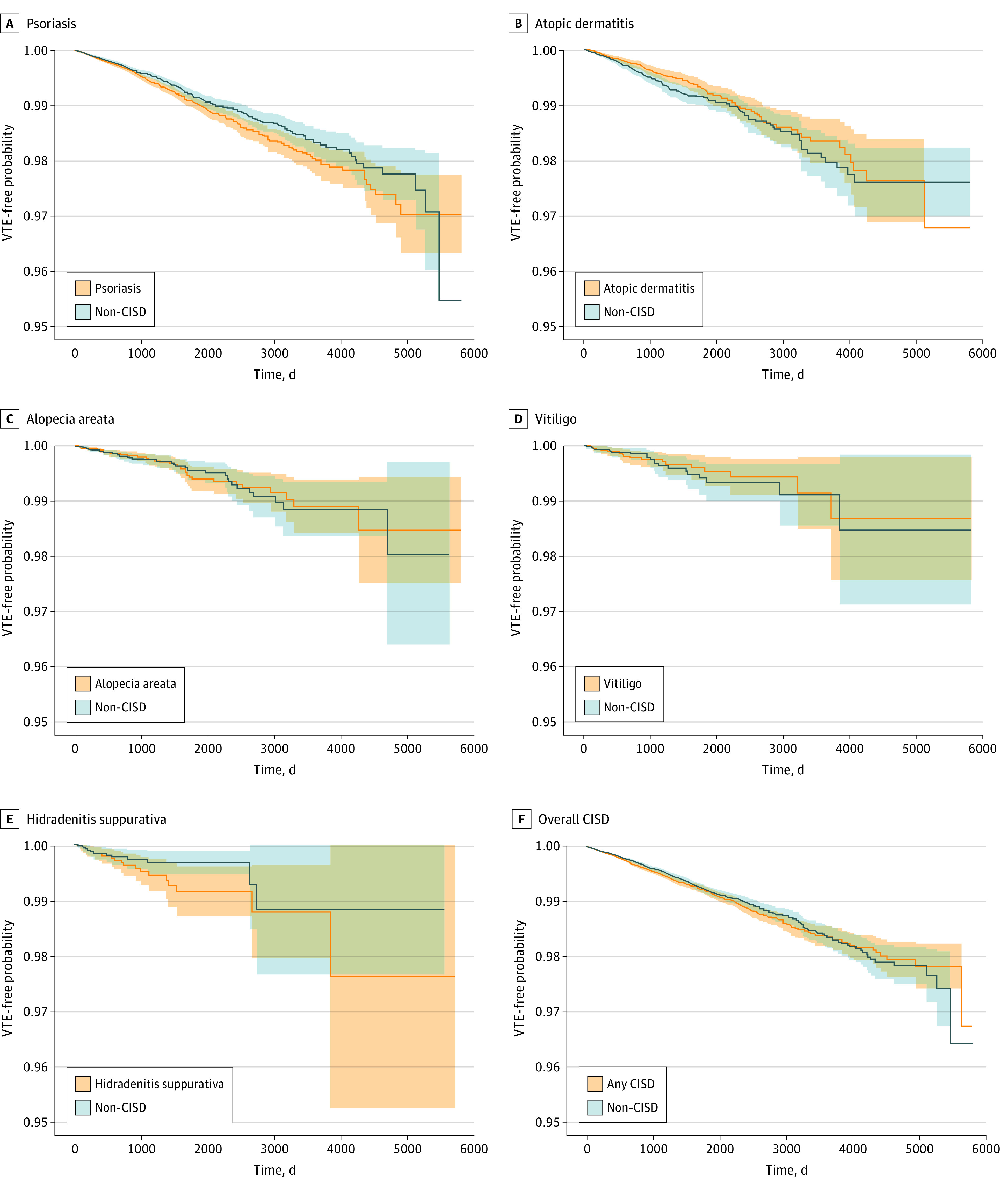
The VTE event in patients with psoriasis (A), atopic dermatitis (B), alopecia areata (C), vitiligo (D), hidradenitis suppurativa (E), and overall CISD (F) was defined as inpatient- or outpatient-diagnosed VTE with evidence of treatment after 1:1 propensity-score matching. Dark lines within the graphs represent 95% CIs.
Incidence of Inpatient VTE Only
When limiting our outcome definition to inpatient VTE, the incidence rate (per 1000 person-years) was 0.59 in psoriasis, 0.69 in AD, 0.35 in AA, 0.42 in vitiligo, and 0.60 in HS compared with 0.75 in non-CISD (Table 2). Overall, the incidence rate of inpatient VTE was 0.58 in CISD vs 0.75 in non-CISD. After PS matching, the risk of inpatient VTE was not increased in patients with psoriasis (HR, 0.73; 95% CI, 0.59-0.91), AD (HR, 1.04; 95% CI, 0.73-1.48), AA (HR, 0.89; 95% CI, 0.46-1.70), HS (HR, 0.79; 95% CI, 0.29-2.11), or CISD overall (HR, 0.85; 95% CI, 0.72-1.01), compared with patients without a CISD. We observed a numerically increased risk in patients with vitiligo (HR, 1.48; 95% CI, 0.53-4.17), based on only 16 inpatient events (Table 2).
Sensitivity Analyses
The incidence rate remained almost unchanged when excluding patients using systemic immunomodulators in CISD vs non-CISD. This was also observed for the component conditions (Figure 2). Another sensitivity analysis that included eczema not otherwise specified in our definition of AD did not change the HR estimate meaningfully. Additional adjustment for nonmelanoma skin cancer with excision also did not change the effect estimates meaningfully (eTable 11 in the Supplement).
Stratified by age, the incidence (per 1000 person-years) of inpatient and outpatient VTE events in patients aged 18 to 49 years was 0.8 vs 0.7 in patients without a CISD and, in those aged 50 years or older, was 2.3 vs 2.5 in patients without a CISD. The increased incidence among patients aged 50 years or older was consistently observed for the component conditions as well (Figure 2; eTable 6, eTable 7, and eTable 8 in the Supplement provide more details). After PS matching, there was no significant difference in VTE risk in patients with vs without CISD who were otherwise comparable in those aged 18 to 49 years (HR, 1.00; 95% CI, 0.81-1.25) or 50 years or older (HR, 1.01; 95% CI, 0.88-1.14) (Figure 2).
To examine the sensitivity of our approach to detect elevated VTE risk, we replicated earlier findings of Kim et al3 of a 2-fold increase in VTE risk among patients with rheumatoid arthritis (HR, 2.01; 95% CI, 1.83-2.21) (eTable 10 in the Supplement). To further test the robustness of our study approach, we estimated the incidence of skull or face fractures as a negative tracer outcome that is not possibly associated with CISD. We found no association with our negative tracer for CISD (HR, 0.96; 95% CI, 0.86-1.07) or across all component conditions (Table 2).
Discussion
In this large-scale study in a commercially insured US population, patients with CISD diagnosed by a dermatologist and without known risk factors for VTE had an incidence rate of VTE of 1.53 per 1000 person-years, in line with those reported by population-representative surveys.40,41,42,43 None of the 5 component CISD conditions (psoriasis, AD, AA, vitiligo, and HS) showed elevated VTE risks compared with risks in individuals without CISD. The VTE incidence is mostly associated with patients aged 50 years or older as expected, given older age is a risk factor for VTE.40 Findings may generalize to the entire spectrum of patients treated by dermatologists capturing the patients seen in routine practice, but may have limited generalizability to underinsured patients.
Active systemic inflammation modulates thrombosis and can affect hypercoagulability.44 Inflammatory mediators and cytokines associated with VTE, including tumor necrosis factor and interleukin-6, are elevated in chronic inflammatory diseases, such as inflammatory bowel disease and rheumatoid arthritis.45 Although these and other inflammatory mediators are involved in some CISDs, the overall lower inflammatory burden in patients with such dermatologic conditions may explain our study findings of no increased background risk of VTE in CISDs.
To our knowledge, this is the first large-scale study to examine the background VTE risk in CISD that specifically compared patients with CISD against those who are similar in all characteristics except they do not have a CISD. The study further separated 5 CISD conditions from each other to avoid contamination by patients with multiple conditions and estimate an interpretable background rate. For example, patients with psoriatic arthritis were excluded from the psoriasis group.
Establishing a background rate of VTE is overdue for 2 reasons. First, nonskin chronic inflammatory diseases, such as rheumatoid arthritis, have long been linked to an elevated VTE risk owing to their substantially increased inflammatory state3,44,46; therefore, ruling out an association between CISDs and an increased VTE risk is useful. Second, several systemic immunomodulatory agents that have quickly gained popularity in dermatology, in particular, Janus kinase inhibitors, were associated with an increased risk for VTE in high doses among a cohort of patients with rheumatoid arthritis3 and a cohort of those with inflammatory bowel disease.47 Some of these Janus kinase inhibitors have recently received a secondary indication for the treatment of dermatologic diseases, and many new Janus kinase inhibitors that are currently in clinical trials have shown efficacy in treating dermatologic conditions including, but not limited to, psoriasis, AA, vitiligo, and AD.48 In this new era of effective systemic medications for CISD, having a background VTE risk established will help monitor the safety of newly marketed systemic treatments in dermatology.
Limitations
Establishing population-based incidence rates of serious medical events, such as VTE, in dermatologic conditions is subject to several considerations and limitations. The first challenge was a clear separation of homogeneous patient groups. A diagnosis of a CISD by a dermatologist is reliable, particularly if this diagnosis is confirmed over more than 1 visit.49,50,51,52 Although, to our knowledge, no ICD-10 code validation study has been conducted for AD, a US validation study investigating ICD-9 codes for AD found that 1 code for AD by a dermatologist had a positive predictive value of 67.7% (for definite or probable AD).53 We additionally required a second dermatologist visit with an AD code, which should have further increased the positive predictive value; however, we cannot completely rule out any misclassification of the underlying condition. Establishing a group of patients who have no CISD with high certainty is more difficult because subtle disease presentation or lack of access to a specialist may leave CISD unrecorded. We therefore required that our non-CISD comparison group equally have 2 dermatologist visits, but that did not result in a CISD recording. Assuming that a dermatologist would, with highest likelihood, diagnose and record an existing CISD may result in a group free of CISD. Patients with past CISD (eg, childhood AD), although sometimes recorded, may have not been detected through our screening; the VTE risk in those with lower inflammatory burden associated with nonactive disease is likely similar to those without CISD. Similarly, we used the fact that the CISD group had dermatologist visits to categorize patients into 5 mutually exclusive groups of well-characterized CISDs. The patient characteristics in each of the 5 conditions were as expected from clinical practice, which supports that we were able to capture the intended populations in claims data.54
The second consideration was outcome surveillance. We used a broad outcome definition to include inpatient and outpatient VTE events, resulting in a representative incidence rate estimate; consequently, the incidence rate of VTE in our non-CISD population (0.75-1.76 per 1000 person-years) is in line with other population-based surveys (1.04-1.83 per 1000 person-years).40,41,42,43 For comparative analyses of incidence rates, it is desirable to have high-specificity end point definitions to result in unbiased HR estimates.55 For this reason, we included a secondary outcome limited to inpatient VTE events recorded as the primary discharge diagnosis.
Separating the disease from its treatment and comorbidities presented the third important consideration of this study. To be able to draw conclusions on the VTE risk attributable to the underlying skin disease rather than its treatment or comorbidities, we balanced all patients with pertinent VTE risk factors; treatments, including use of systemic immunomodulators; and comorbidities via exclusions or 1:1 PS matching. This allowed us to make comparative statements on the VTE risk attributable to CISD or its component disease relative to patients who have equal risk factor distributions but not the skin disease of interest. Differences in results between our work and others are attributable to the above, particularly, excluding patients with cancer from our analysis, adding confounder adjustment with 1:1 PS matching including matching on systemic immunomodulator use, not limiting the population to an inpatient setting, and using a cohort study design rather than a cross-sectional design.
Insensitive methods may lead to null findings, which is the fourth consideration in our study. Other studies had shown an increase in VTE risk, for example, in patients with severe psoriasis, which we cannot reproduce. This is explained by our focus on the disease independent of its treatment, comorbidities, and VTE risk factors (eg, cancer). Nevertheless, negative findings need special scrutiny of the study methods to avoid false-negative results. First, we used the same study method to replicate the known finding of an increased VTE risk in patients with rheumatoid arthritis. Second, we used a negative tracer outcome (skull or face fractures that are unrelated to skin diseases) and, as expected, found no association.
The fifth consideration involved residual confounding. Claims data are known to underrecord several risk factors for VTE, such as smoking and obesity. Although these are weaker risk factors than hormone therapy, trauma, or coagulopathy, we attempted to indirectly identify those condition via diagnoses related to smoking (eg, chronic obstructive pulmonary disease and chronic bronchitis), and obesity (diabetes, varicose veins, statin use, and hyperlipidemia).
Effect modification by CISD severity was the sixth consideration. Disease severity and inflammatory burden may modify the risk of VTE but are difficult to assess in claims data. This study population captured patients who sought specialty care (2 dermatologist visits in the past year), of whom 25% used systemic immunomodulators or systemic corticosteroids; these characteristics may suggest that the population comprises more patients with moderate to severe disease. The subgroup analysis without systemic immunomodulator use may represent patients with milder disease.
Conclusions
In this large-scale analysis of claims data, we found no indication of an increased background rate of clinical VTE events in a representative population of patients with dermatologist-diagnosed CISD.
eFigure. Cohort Study Design Diagram
eTable 1. All Baseline Characteristics, Comparing Patients With vs Without a CISD, Before 1:1 Propensity-Score Matching
eTable 2. Baseline Patients Characteristics and Standardized Differences, Comparing Patients With vs Without a CISD, Before and After 1:1 Propensity-Score Matching
eTable 3. International Classification of Diseases Codes Used to Identify CISD and Venous Thromboembolism
eTable 4. Follow-up Time and Reasons for Censoring, After Propensity-Score Matching
eTable 5. Exploratory Analysis of Systemic Immunomodulator Use During Follow-up, After 1:1 Propensity-Score Matching
eTable 6. Incidence Rate and Hazard Ratio of Inpatient or Outpatient VTE, Stratified by Subgroups, Before and After 1:1 Propensity-Score Matching
eTable 7. Incidence Rate and Hazard Ratio of Inpatient VTE (Primary Position), Stratified by Subgroups, Before and After 1:1 Propensity-Score Matching
eTable 8. Incidence Rate and Hazard Ratio of Inpatient VTE (Any Position), Stratified by Subgroups, Before and After 1:1 Propensity-Score Matching
eTable 9. Exploratory Analysis of Dermatologic Patient Characteristics on the Visit Day That Defined Cohort Entry
eTable 10. Incidence of VTE in Patients With Rheumatoid Arthritis Versus Without Rheumatoid Arthritis, Before 1:1 Propensity-Score Matching
eTable 11. Incidence Rate and Hazard Ratio of Venous Thromboembolism, With Additional Adjustment for Non-Melanoma Skin Cancer, Before and After 1:1 Propensity-Score Matching
eTable 12. Exploratory Analysis to Confirm Referent Patients Had No CISD Diagnosis Code Recorded by Any Physician Before Cohort Entry or on the Day of Cohort Entry
References
- 1.Silvestri E, Scalera A, Emmi G, et al. Thrombosis in autoimmune diseases: a role for immunosuppressive treatments? Semin Thromb Hemost. 2016;42(6):650-661. doi: 10.1055/s-0036-1579642 [DOI] [PubMed] [Google Scholar]
- 2.Johannesdottir SA, Schmidt M, Horváth-Puhó E, Sørensen HT. Autoimmune skin and connective tissue diseases and risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost. 2012;10(5):815-821. doi: 10.1111/j.1538-7836.2012.04666.x [DOI] [PubMed] [Google Scholar]
- 3.Kim SC, Schneeweiss S, Liu J, Solomon DH. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(10):1600-1607. doi: 10.1002/acr.22039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaheen MS, Silverberg JI. Association of inflammatory skin diseases with venous thromboembolism in US adults. Arch Dermatol Res. 2021;313(4):281-289. [DOI] [PubMed] [Google Scholar]
- 5.Ogdie A, Kay McGill N, Shin DB, et al. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a general population-based cohort study. Eur Heart J. 2018;39(39):3608-3614. doi: 10.1093/eurheartj/ehx145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlehoff O, Gislason GH, Lindhardsen J, et al. Psoriasis carries an increased risk of venous thromboembolism: a Danish nationwide cohort study. PLoS One. 2011;6(3):e18125. doi: 10.1371/journal.pone.0018125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung WS, Lin CL, Sung FC, Lu CC, Kao CH. Increased risk of venous thromboembolism in patients with dermatomyositis/polymyositis: a nationwide cohort study. Thromb Res. 2014;134(3):622-626. doi: 10.1016/j.thromres.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 8.Rogers MAM, Lin P, Nallamothu BK, Kim C, Waljee AK. Longitudinal study of short-term corticosteroid use by working-age adults with diabetes mellitus: risks and mitigating factors. J Diabetes. 2018;10(7):546-555. doi: 10.1111/1753-0407.12631 [DOI] [PubMed] [Google Scholar]
- 9.Coelho MC, Santos CV, Vieira Neto L, Gadelha MR. Adverse effects of glucocorticoids: coagulopathy. Eur J Endocrinol. 2015;173(4):M11-M21. doi: 10.1530/EJE-15-0198 [DOI] [PubMed] [Google Scholar]
- 10.Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis. 2020;79(11):1400-1413. doi: 10.1136/annrheumdis-2019-216761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847. doi: 10.3389/fimmu.2019.02847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudy SF, Li K, Moubayed SP, Most SP. Risk of venous thromboembolism in patients with keratinocyte carcinoma. JAMA Facial Plast Surg. 2018;20(6):453-459. doi: 10.1001/jamafacial.2018.0331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. The validity of ICD codes coupled with imaging procedure codes for identifying acute venous thromboembolism using administrative data. Vasc Med. 2015;20(4):364-368. doi: 10.1177/1358863X15573839 [DOI] [PubMed] [Google Scholar]
- 14.Walker RF, Zakai NA, MacLehose RF, et al. Association of testosterone therapy with risk of venous thromboembolism among men with and without hypogonadism. JAMA Intern Med. 2020;180(2):190-197. doi: 10.1001/jamainternmed.2019.5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanfilippo KM, Wang TF, Gage BF, Liu W, Carson KR. Improving accuracy of International Classification of Diseases codes for venous thromboembolism in administrative data. Thromb Res. 2015;135(4):616-620. doi: 10.1016/j.thromres.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ammann EM, Cuker A, Carnahan RM, et al. Chart validation of inpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) administrative diagnosis codes for venous thromboembolism (VTE) among intravenous immune globulin (IGIV) users in the Sentinel Distributed Database. Medicine (Baltimore). 2018;97(8):e9960. doi: 10.1097/MD.0000000000009960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):154-162. doi: 10.1002/pds.2341 [DOI] [PubMed] [Google Scholar]
- 18.Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf. 2010;19(8):858-868. doi: 10.1002/pds.1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23)(suppl 1):I9-I16. [DOI] [PubMed] [Google Scholar]
- 20.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156. doi: 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 22.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):69-80. doi: 10.1002/pds.3263 [DOI] [PubMed] [Google Scholar]
- 23.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Stat Med. 2014;33(10):1685-1699. doi: 10.1002/sim.6058 [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox DR. Regression models and life tables. J Royal Stat Society Series B. 1972;34(2):187-220. [Google Scholar]
- 26.Aetion Inc . Aetion evidence platform. Software for real-world data analysis. 2020. Accessed February 1, 2021. http://aetion.com
- 27.Wang SV, Verpillat P, Rassen JA, Patrick A, Garry EM, Bartels DB. Transparency and reproducibility of observational cohort studies using large healthcare Databases. Clin Pharmacol Ther. 2016;99(3):325-332. doi: 10.1002/cpt.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SC, Solomon DH, Rogers JR, et al. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi-database cohort study. Arthritis Rheumatol. 2017;69(6):1154-1164. doi: 10.1002/art.40084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patorno E, Schneeweiss S, Gopalakrishnan C, Martin D, Franklin JM. Using real-world data to predict findings of an ongoing phase IV cardiovascular outcome trial: Cardiovascular Safety of Linagliptin Versus Glimepiride. Diabetes Care. 2019;42(12):2204-2210. doi: 10.2337/dc19-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidbury R, Davis DM, Cohen DE, et al. ; American Academy of Dermatology . Guidelines of care for the management of atopic dermatitis: section 3—management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327-349. doi: 10.1016/j.jaad.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072. doi: 10.1016/j.jaad.2018.11.057 [DOI] [PubMed] [Google Scholar]
- 32.Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II—topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1):91-101. doi: 10.1016/j.jaad.2019.02.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists’ guidelines for the management of alopecia areata: 2012. Br J Dermatol. 2012;166(5):916-926. doi: 10.1111/j.1365-2133.2012.10955.x [DOI] [PubMed] [Google Scholar]
- 34.Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol. 2020;183(6):990-998. doi: 10.1111/bjd.19435 [DOI] [PubMed] [Google Scholar]
- 35.Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82(3):675-682. doi: 10.1016/j.jaad.2019.08.032 [DOI] [PubMed] [Google Scholar]
- 36.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345-360. doi: 10.1016/S0140-6736(20)31286-1 [DOI] [PubMed] [Google Scholar]
- 37.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945-1960. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE; Vitiligo Working Group . Current and emerging treatments for vitiligo. J Am Acad Dermatol. 2017;77(1):17-29. doi: 10.1016/j.jaad.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 39.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74-84. doi: 10.1016/S0140-6736(14)60763-7 [DOI] [PubMed] [Google Scholar]
- 40.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi: 10.1038/nrcardio.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ III. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585-593. doi: 10.1001/archinte.158.6.585 [DOI] [PubMed] [Google Scholar]
- 42.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19-25. doi: 10.1016/j.amjmed.2004.01.018 [DOI] [PubMed] [Google Scholar]
- 43.Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126(9):832.e13-832.e21. doi: 10.1016/j.amjmed.2013.02.024 [DOI] [PubMed] [Google Scholar]
- 44.Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis: a systematic review of clinical studies. Thromb Haemost. 2005;94(2):362-365. doi: 10.1160/TH05-04-0266 [DOI] [PubMed] [Google Scholar]
- 45.Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142. doi: 10.3389/fped.2018.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Poll T, Büller HR, ten Cate H, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990;322(23):1622-1627. doi: 10.1056/NEJM199006073222302 [DOI] [PubMed] [Google Scholar]
- 47.Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther. 2019;50(10):1068-1076. doi: 10.1111/apt.15514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newsom M, Bashyam AM, Balogh EA, Feldman SR, Strowd LC. New and emerging systemic treatments for atopic dermatitis. Drugs. 2020;80(11):1041-1052. doi: 10.1007/s40265-020-01335-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asgari MM, Wu JJ, Gelfand JM, et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996-2009. Pharmacoepidemiol Drug Saf. 2013;22(8):842-849. doi: 10.1002/pds.3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Löfvendahl S, Theander E, Svensson Å, Carlsson KS, Englund M, Petersson IF. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden—a population-based register study. PLoS One. 2014;9(5):e98024. doi: 10.1371/journal.pone.0098024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim GE, Shlyankevich J, Kimball AB. The validity of the diagnostic code for hidradenitis suppurativa in an electronic database. Br J Dermatol. 2014;171(2):338-342. doi: 10.1111/bjd.13041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavian J, Li SJ, Lee EY, et al. Validation of case identification for alopecia areata using International Classification of Diseases coding. Int J Trichology. 2020;12(5):234-237. doi: 10.4103/ijt.ijt_67_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu DY, Dalal P, Sable KA, et al. Validation of International Classification of Disease Ninth Revision codes for atopic dermatitis. Allergy. 2017;72(7):1091-1095. doi: 10.1111/all.13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Elsevier; 2018. [Google Scholar]
- 55.Schneeweiss S, Glynn RJ, Tsai EH, Avorn J, Solomon DH. Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology. 2005;16(1):17-24. doi: 10.1097/01.ede.0000147164.11879.b5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Cohort Study Design Diagram
eTable 1. All Baseline Characteristics, Comparing Patients With vs Without a CISD, Before 1:1 Propensity-Score Matching
eTable 2. Baseline Patients Characteristics and Standardized Differences, Comparing Patients With vs Without a CISD, Before and After 1:1 Propensity-Score Matching
eTable 3. International Classification of Diseases Codes Used to Identify CISD and Venous Thromboembolism
eTable 4. Follow-up Time and Reasons for Censoring, After Propensity-Score Matching
eTable 5. Exploratory Analysis of Systemic Immunomodulator Use During Follow-up, After 1:1 Propensity-Score Matching
eTable 6. Incidence Rate and Hazard Ratio of Inpatient or Outpatient VTE, Stratified by Subgroups, Before and After 1:1 Propensity-Score Matching
eTable 7. Incidence Rate and Hazard Ratio of Inpatient VTE (Primary Position), Stratified by Subgroups, Before and After 1:1 Propensity-Score Matching
eTable 8. Incidence Rate and Hazard Ratio of Inpatient VTE (Any Position), Stratified by Subgroups, Before and After 1:1 Propensity-Score Matching
eTable 9. Exploratory Analysis of Dermatologic Patient Characteristics on the Visit Day That Defined Cohort Entry
eTable 10. Incidence of VTE in Patients With Rheumatoid Arthritis Versus Without Rheumatoid Arthritis, Before 1:1 Propensity-Score Matching
eTable 11. Incidence Rate and Hazard Ratio of Venous Thromboembolism, With Additional Adjustment for Non-Melanoma Skin Cancer, Before and After 1:1 Propensity-Score Matching
eTable 12. Exploratory Analysis to Confirm Referent Patients Had No CISD Diagnosis Code Recorded by Any Physician Before Cohort Entry or on the Day of Cohort Entry


