Key Points
Question
Does a tendency toward sleeping and waking earlier have a potential causal role in reducing the risk of major depressive disorder?
Findings
This 2-sample mendelian randomization analysis of data from nearly 840 000 adults of European ancestry found an association between earlier sleep timing patterns and lower risk of major depressive disorder.
Meaning
These data suggest that sleep timing patterns are a risk factor for major depressive disorder, and they should be examined further in randomized clinical trials of sleep interventions.
This cohort study examines the association of genetically proxied morning diurnal preference with risk of major depressive disorder using mendelian randomization.
Abstract
Importance
Morning diurnal preference is associated with reduced risk of major depressive disorder (MDD); however, causality in this association is uncertain.
Objective
To examine the association of genetically proxied morning diurnal preference with depression risk using mendelian randomization.
Design, Setting, and Participants
This 2-sample mendelian randomization study used summary-level genetic associations with diurnal preference and MDD. Up to 340 genetic loci associated with diurnal preference in a meta-analysis of the UK Biobank and 23andMe cohorts were considered as genetic proxies for diurnal preference. The effect size of these variants was scaled using genetic associations with accelerometer-based measurement of sleep midpoint. Genetic associations with MDD were obtained from a meta-analysis of genome-wide association studies data from the Psychiatric Genomics Consortium and UK Biobank. The inverse-variance weighted method was used to estimate the association of genetically proxied morning diurnal preference, corresponding to a 1-hour earlier sleep midpoint, with MDD risk.
Exposures
Morning diurnal preference scaled to a 1-hour earlier, objectively measured sleep midpoint.
Main Outcomes and Measures
Risk of MDD, including self-reported and clinically diagnosed cases, as ascertained in meta-analyses of genome-wide association studies.
Results
A total of 697 828 individuals (all of European ancestry) were in the UK Biobank and 23andMe cohorts; 85 502 in the UK Biobank had measurements of the sleep midpoint. A further 170 756 individuals with MDD and 329 443 control participants (all of European ancestry) were in the Psychiatric Genomics Consortium and UK Biobank data. Genetically proxied earlier diurnal preference was associated with a 23% lower risk of depression (odds ratio [OR] per 1-hour earlier sleep midpoint, 0.77 [95% CI, 0.63-0.94]; P = .01). This association was similar when restricting analysis to individuals with MDD as stringently defined by the Psychiatric Genomics Consortium (OR, 0.73 [95% CI, 0.54-1.00]; P = .05) but not statistically significant when defined by hospital-based billing codes in the UK Biobank (OR, 0.64 [95% CI, 0.39-1.06]; P = .08). Sensitivity analyses examining potential bias due to pleiotropy or reverse causality showed similar findings (eg, intercept [SE], 0.00 [0.001]; P = .66 by Egger intercept test).
Conclusions and Relevance
The results of this mendelian randomization study support a protective association of earlier diurnal preference with risk of MDD and provide estimates contextualized to an objective sleep timing measure. Further investigation in the form of randomized clinical trials may be warranted.
Introduction
Morning diurnal preference (ie, a preference for early sleep timing), a correlate of circadian rhythms and homeostatic sleep-wake regulation, has emerged as a potentially modifiable1 protective factor for major depressive disorder (MDD) risk,2 with supporting evidence from observational3,4,5,6 and small interventional studies.7 Plausible mechanisms for such an association include better alignment with typical work-rest schedules, higher daily light exposure,8 and increased sensitivity to rhythmic features of the motivational reward system and affect.9 However, it remains unclear whether these epidemiologic associations of morning diurnal preference with MDD risk are causal, given potential biases from reverse causality or residual confounding. Furthermore, epidemiological studies typically measure diurnal preference on a binary or ordinal scale,10 which impedes interpretation of association estimates.
Causal inference from observational studies may be improved by the use of mendelian randomization (MR), a technique that leverages randomly allocated genetic variants associated with a risk factor (eg, morning diurnal preference) to test for potential causal effects on disease outcomes (eg, MDD).11,12 Since genetic variants are randomly allocated at gametogenesis, they are less confounded than conventionally measured risk factors.13 Furthermore, germline genetic variation cannot be modified after conception, rendering MR robust to reverse causality. Several hundred variants associated with morning diurnal preference have been identified in populations of European ancestry,10 and this permits the application of this MR paradigm to explore the potential effects of morning diurnal preference on disease risk. While prior MR analyses found evidence for a protective association between morning diurnal preference with subjective well-being and schizophrenia, estimates for MDD were null.10 However, inference from this analysis is limited by the small number of individuals with MDD (n = 9240) and the use of exposure units without a readily interpretable effect (log-odds increase in morning diurnal preference).14
Substantially larger MDD cohorts15,16 are now available, permitting well-powered MR investigations into the potential effect of diurnal preference on MDD risk. Furthermore, recent genetic analyses using objective sleep timing measures permit contextualization of the diurnal preference findings to clinically interpretable units.17 In this analysis, we leverage these genetic data in the MR framework to test the hypothesis that earlier diurnal preference is associated with lower MDD risk.
Methods
Overview
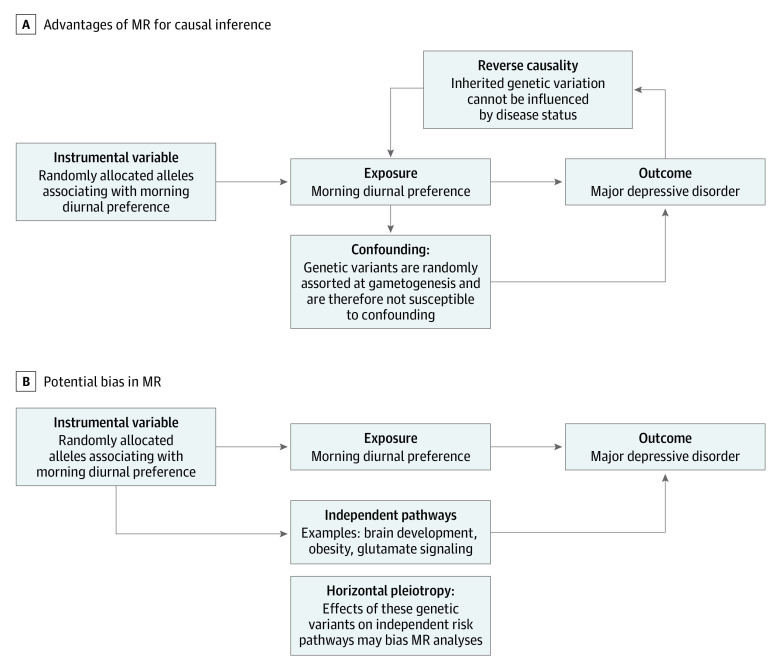
We conducted a 2-stage, 2-sample MR analysis, using results from the first stage of analysis to scale estimates to represent a change in diurnal preference corresponding to a 1-hour advance in sleep midpoint (eFigure 1 in the Supplement). In the first stage, we tested the association of earlier diurnal preference with sleep midpoint, a behavioral marker for diurnal preference.18 In the second stage, we tested the association of earlier diurnal preference with MDD risk. There is no sample overlap in the MR analysis of diurnal preference on MDD or diurnal preference on sleep midpoint, so these analyses are not biased by sample overlap.19 This 2-sample MR study design leverages summary statistics for which individual-level data are not available. Three assumptions must be met for valid potential causal inference in this MR analysis: (1) variants used in MR must strongly associate with diurnal preference, (2) associations of variants with diurnal preference or MDD must not be confounded, and (3) the variants must have a mean association with MDD only through the effect associated with diurnal preference (ie, no unbalanced horizontal pleiotropy).12 These assumptions are addressed below and graphically displayed in Figure 1.
Figure 1. Directed Acyclic Graphs Demonstrating Advantages of Mendelian Randomization (MR).
A, Advantages with respect to potential causal inference. B, Potential bias in mendelian randomization due to pleiotropy. Some genetic variants associated with diurnal preference have also been associated with the independent pathways listed in this section.
Ethical Approval
All contributing GWAS studies sought informed consent from their study participants.10,16,17 We used publicly available, deidentified, summary-level genetic association data, which, per the US code of federal regulations (45 CFR 46.102), does not constitute human subjects research and therefore did not require institutional review board approval.
Genetic Associations With Diurnal Preference and Sleep Midpoint
As genetic proxies for morning diurnal preference (also referred to as an early chronotype), we selected variants associated wifth morning diurnal preference from a meta-analysis of genome-wide association studies (GWAS) conducted using data from the UK Biobank20 (UKB) and 23andMe cohorts.10 Detailed GWAS phenotype definitions are provided in the eMethods in the Supplement. Both the UKB and 23andMe GWAS controlled for population stratification through adjustment for principal components of ancestry in the genetic association analyses10 (addressing the second MR assumption that genetic associations are unconfounded). Meta-analysis of these GWAS identified 351 loci (P < 5 × 10−8), with 340 independent genetic loci present in both the UKB and 23andMe (eTable 1 in the Supplement, adapted from Jones et al10). The UKB was also included in the MDD GWAS, and this sample overlap may bias MR estimates away from the null.19 In concordance with previous analyses,21 we mitigated this bias by using genetic associations for the 340 loci from the 23andMe contribution to the meta-analysis.
The unit of the genetic associations with diurnal preference was a log-odds increase in morning diurnal preference. To increase the interpretability of this association, we leveraged a GWAS of accelerometer-derived sleep midpoint (hours)17 conducted using up to 7 days of accelerometer data in the UKB. In this study, sleep midpoint was defined as the midpoint between the start and end of a given sleep period and reported as hours since the previous midnight (mean [SD], 27 [0.91] hours, corresponding to 3:00 am).17 Participants sleeping fewer than 3 or more than 12 hours per night (approximately 5 SDs from the mean) were excluded. We used genetic associations with sleep midpoint measured on the per-hour scale. Details regarding the scaling procedure are provided in the MR Analyses section. All genetic associations were adjusted for age and sex, and population stratification was controlled for through the use of linear mixed models and statistical adjustment for principal components of ancestry.10,17
Genetic Associations With MDD
For the primary MDD outcome, we used a meta-analysis of GWAS from the Psychiatric Genomics Consortium (PGC) and the UKB. The PGC data set included a meta-analysis of 33 studies that was described in detail elsewhere.15 Cases were ascertained by trained interviewers, clinician-administered checklists, and medical record reviews.15 The UKB data set used a broader MDD definition of participants who had answered yes to either of the following questions: “Have you ever seen a general practitioner (GP) for nerves, anxiety, tension or depression?” and “Have you ever seen a psychiatrist for nerves, anxiety, tension or depression?”16 Despite potential case misclassification with anxiety disorders, this phenotype has a strong genetic correlation (>0.85) with MDD defined by International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) codes.16 For concordance with other analyses, we refer to this phenotype as MDD but recognize that this case definition does not meet stringent diagnostic criteria for MDD. The PGC and UKB GWAS data sets have been meta-analyzed and made publicly available (https://www.med.unc.edu/pgc/download-results/).
We analyzed 3 additional MDD GWAS data sets to assess generalizability of results across varying MDD case definitions. Because the UKB GWAS used a less stringent case definition than the PGC, we separately analyzed MDD in the UKB and PGC data sets. We then tested whether associations were consistent when cases were identified in the UKB using hospital-based ICD-9 and ICD-10 codes.16 All MDD GWAS controlled for age, sex, and population stratification through adjustment for principal components of ancestry (addressing the second MR assumption).15,16
Data Harmonization and Instrument Strength
Data harmonization (detailed in the eMethods and eTable 2 in the Supplement) yielded 332 genetic variants that were also present in the MDD GWAS data set (eTable 3 in the Supplement). These variants were then extracted from the sleep midpoint GWAS (eTable 4 in the Supplement). We tested the MR assumption that the variants strongly associate with diurnal preference by calculating the mean F statistic across all 332 variants.22 An F statistic greater than 10 is an accepted threshold for considering a genetic variant to be strong enough for MR analyses.23
MR Analyses
In the first stage of analysis, we used the random-effects inverse-variance weighted method24 to estimate the association of a log-odds increase in morning diurnal preference with an earlier sleep midpoint. The resulting β coefficient, reported in hours of sleep midpoint per log-odds of morningness, is referred to as the scaling factor.
In the second stage of analysis, we used the inverse-variance weighted method24 to estimate the association of a genetically proxied log-odds increase in morning diurnal preference on MDD risk. We divided the resulting estimate by the scaling factor to represent the effect size of a change in diurnal preference that would correspond to a 1-hour earlier sleep midpoint on MDD risk (eFigure 2 in the Supplement). The standard error was identically transformed, so the P values were unchanged. To extend applicability of our findings and provide estimates for smaller-scale shifts resulting from behavioral interventions, we also provide estimates of MDD risk as a function of a 0.5-hour advance in sleep midpoint. We repeated this analysis procedure to estimate the association of genetically proxied morning diurnal preference with MDD in the PGC, UKB, and UKB ICD-9 and ICD-10–coded data sets.
MR Sensitivity Analyses
Mendelian randomization estimates may be biased by horizontal pleiotropy if the included genetic variants affect the outcome (MDD) through pathways independent of the instrumented risk factor (diurnal preference)12 (Figure 1). For example, some variants associated with diurnal preference are independently associated with obesity (eg, variants near the FTO gene),10 which is a potentially causal risk factor for MDD.25 We therefore explored the robustness of the MR findings using a range of sensitivity analyses, including (1) model-based sensitivity analyses, (2) outlier detection methods, (3) assessment for bias because of pleiotropic effects of the genetic variants on other sleep traits, and (4) alternative variant selection and weighting strategies. Many of these approaches are less powerful than the inverse-variance weighted method, and we therefore primarily assessed consistency of point estimates and overlap of 95% CIs rather than P values.26 An α threshold of .05 was used to declare statistical significance. We also assessed for bias because of reverse causality by testing the association of MDD genetic liability (using the PGC-UKB data set to identify independent variants at r2 < 0.001) on diurnal preference in UK Biobank (n = 449 734). These analyses are further detailed in the eMethods in the Supplement. All analyses were performed using R version 3.5.3 (R Foundation for Statistical Computing) and the TwoSampleMR software version 0.4.22 (Wellcome Open). Data were accessed and analyzed from February 2020 to January 2021.
Results
The meta-analysis of a GWAS of diurnal preference from the UKB and 23andMe cohorts included 697 828 individuals (all of European ancestry), of which the 23andMe contribution was 248 098 individuals. A total of 85 502 individuals (all of European ancestry) from the UKB had accelerometer data.
For the primary analysis, 45 369 individuals with MDD and 97 250 control participants (all of European ancestry) were included from the PGC data set. Additionally, 113 769 individuals with MDD and 208 811 control participants from the UKB (all of European ancestry; mean [SD] age, 57 [8.1] years; 172 677 women [53.5%]) were included; data on MDD per ICD-9 and ICD-10 codes were included for 8276 affected individuals and 209 308 control participants in the UKB. A meta-analysis of these samples included 170 756 affected individuals and 329 443 control participants after removal of overlapping samples.
Primary Analysis
The mean F statistic of the genetic variants proxying morning diurnal preference was 25, supporting the strength of the genetic variants for use in MR analyses. In the first stage of analysis, to obtain a scaling factor, we tested the association of genetically proxied morning diurnal preference with sleep midpoint. A log-odds increase in morning diurnal preference was associated with sleep midpoint earlier by 0.125 hours (95% CI, −0.135 to –0.115 hours; P = 1.20 × 10−145). We therefore divided effect estimates for the effect size of morning diurnal preference genetic instrument on MDD by 0.125, to reflect the consequence of morning diurnal preference corresponding to a 1-hour earlier sleep midpoint.
In the second stage of analysis, we tested the association of genetically proxied morning diurnal preference with MDD using the combined PGC-UKB meta-analysis. We found that genetically proxied morning diurnal preference was associated with reduced risk of MDD (odds ratio [OR] per 1-hour earlier sleep midpoint, 0.77 [95% CI, 0.63-0.94]; P = .01; Figure 2). The OR of MDD for a 0.5-hour sleep midpoint advance was 0.88 (95% CI, 0.79-0.97; P = .01).
Figure 2. Mendelian Randomization Estimates for the Association of Morning Diurnal Preference With Major Depressive Disorder.
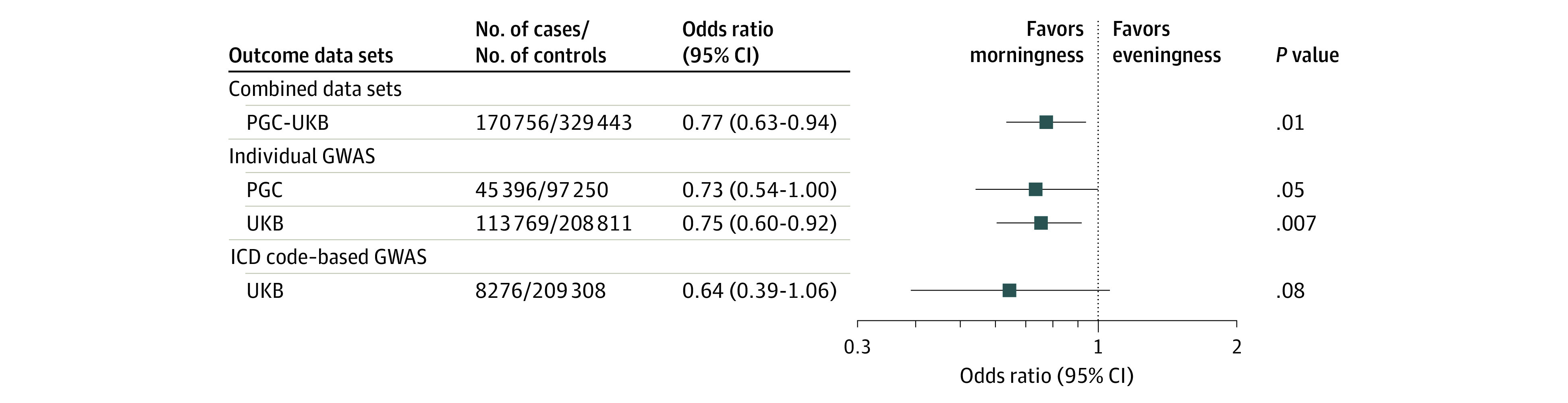
The x-axis shows the odds ratio for the association of genetically proxied morning diurnal preference with depression, scaled to a 1-hour earlier sleep midpoint. Bars represent 95% CIs. GWAS indicates genome-wide association studies; ICD, International Classification of Diseases, Ninth and Tenth Revisions; PGC, Psychiatric Genomics Consortium; UKB, UK Biobank.
Sensitivity Analyses
We tested whether the association of genetically proxied diurnal preference with MDD varied across different outcome definitions. Results were similar when considering the subset of MDD cases in the PGC meta-analysis (n = 45 369 affected individuals and 97 250 control participants; OR, 0.73 [95% CI, 0.54-1.00]; P = .05) and the UKB alone (n = 133 769 affected individuals and 208 111 control participants; OR, 0.75 [95% CI, 0.60-0.92]; P = .007; Figure 3). Similarly, using hospital record–based MDD diagnoses in the UKB resulted in similar point estimates, despite lower case numbers (n = 8276 affected individuals and 209 308 control participants; OR, 0.64 [95% CI, 0.39-1.06]; Figure 2).
Figure 3. Mendelian Randomization (MR) Sensitivity Analyses for the Association of Morning Diurnal Preference With Major Depressive Disorder.
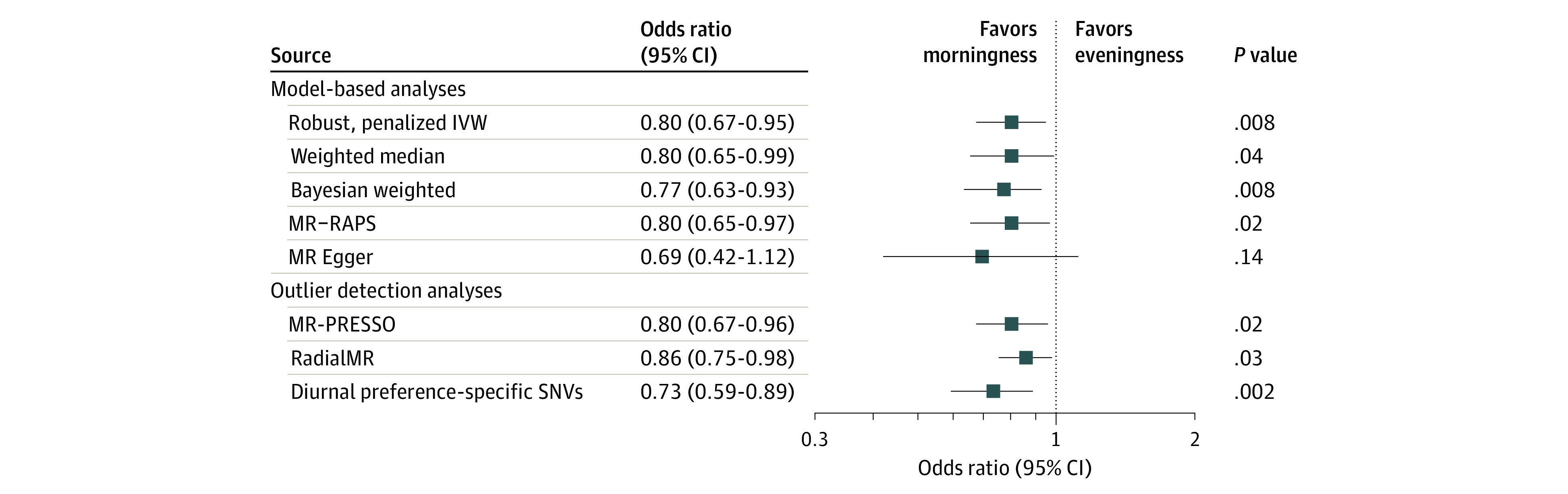
The x-axis shows the odds ratio for the association of genetically proxied morning diurnal preference with depression, scaled to a 1-hour earlier sleep midpoint. Bars represent 95% CIs. All displayed effect estimates used the outcome of major depressive disorder from the Psychiatric Genomics Consortium–UK Biobank meta-analysis. The diurnal preference–specific single-nucleotide variants were not associated at genome-wide significance (P < 5 × 10−8) with any other sleep traits in the UK Biobank (sleep duration, napping, daytime sleepiness, and insomnia symptoms). IVW indicates inverse-variance weighted; MR-PRESSO, MR pleiotropy residual sum and outlier, MR-RAPSB, MR–robust adjusted profile score; SNV, single-nucleotide variant.
We performed sensitivity analyses to assess the plausibility of the assumption that the genetic variants influenced MDD risk through their association with morning diurnal preference. Model-based sensitivity analyses, including the Egger intercept test (intercept [SE], 0.00 [0.001]; P = .66), provided similar results that were inconsistent with major bias attributable to pleiotropy (Figure 3). Removing outlier variants or variants associated with other sleep traits resulted in estimates consistent with the main analyses (Figure 3; eTables 4 and 5 in the Supplement). Diagnostic plots did not suggest that results were influenced by single outliers or systematic bias across multiple variants (eFigure 3 and eTable 6 in the Supplement). One of the variants used to proxy diurnal preference was strongly associated with MDD (P = 2.36 × 10−15; OR for A allele of rs77960, 1.04 [95% CI, 1.03-1.05]) and was therefore likely influencing MDD through pleiotropic mechanisms independent of diurnal preference. However, leave-one-out analysis indicated that this single-nucleotide variant did not drive the effect size estimates (OR, 0.76 [95% CI, 0.62-0.92]; P = .005; eTable 7 in the Supplement).
To assess for bias due to residual linkage-disequilibrium of variants modeled as genetic instruments, we used a stricter clumping threshold of r2 < 0.001 and found similar results (217 single-nucleotide variants; OR, 0.78 [95% CI, 0.63-0.98]; P = .03). Findings were similar when limiting analyses to variants reaching genome-wide significance in the 23andMe sample alone (252 single-nucleotide variants; OR, 0.80 [95% CI, 0.63-1.02]; P = .07). Finally, to rule out the possibility that results may be influenced by biases specific to the 23andMe sample, we performed MR analyses using instrument weights from UKB as the exposure and MDD in the PGC as the outcome (eMethods in the Supplement). We found similar effect size estimates when using variant effect size estimates from the entire UKB sample and the PGC data set as the outcome (OR, 0.77 [95% CI, 0.57-1.04]; P = .09) and UKB participants who did not report relevant comorbidities, medication use, or participation in shift work (OR, 0.78 [95% CI, 0.57-1.07]; P = .12). Finally, we found no association between genetic liability to MDD and diurnal preference (0.003 [95% CI, −0.09 to 0.10] increase in log-odds of morning diurnal preference per log-odds increase of MDD; P = .95).
Discussion
In this 2-sample MR study, we found that genetically proxied morning diurnal preference corresponding to a 1-hour earlier sleep midpoint was associated with a 23% lower risk of MDD. Results were consistent across sensitivity analyses. This study addresses several gaps in the evidence supporting the association of morning diurnal preference with MDD risk, each of which are outlined here.
Most of the data supporting a protective association between morning diurnal preference and MDD risk are from population-based observational studies.5,6 There is also evidence for an association of delayed sleep phase syndrome—which may be considered an extreme evening preference—with increased MDD risk and depressive symptoms, particularly in those with a circadian form of the disorder.9,27,28 Although these studies adjust for confounders and remove participants with MDD ascertained at baseline, residual confounding and reverse causality through subclinical MDD remain alternative explanations for these associations. Specifically, MDD alters sleep and circadian rhythms6,29 and may therefore bias observational associations of diurnal preference with mood disorders. Furthermore, diurnal preference and MDD have shared potential psychosocial and environmental influences, such as exposure to electronic screens30,31 and levels of physical activity.32,33 Such shared potential causes may confound and induce a spurious association between diurnal preference and MDD. An additional limitation is that epidemiologic studies do not typically measure diurnal preference using a quantitative and trackable measure, such as sleep midpoint, which precludes clinical interpretation of effect sizes and hampers the design of interventions. The present analysis addresses these limitations of confounding through the use of randomly assorted genetic variation and addresses limitations of interpreting effect size estimates by scaling diurnal preference to sleep midpoint. We emphasize that the use of sleep midpoint for scaling of estimates is not to imply that the association with diurnal preference is entirely mediated through sleep midpoint, but rather to contextualize effect sizes to a more interpretable and actionable parameter. Indeed, any parameter reliably measuring an aspect of diurnal preference may have been used for scaling the association if there were corresponding GWAS summary statistics.
Our data are consistent with results from a recent randomized clinical trial7 that shifted the sleep timing of individuals with an evening diurnal preference. The multifaceted intervention targeted sleep timing, meal timing, light exposure, caffeine intake, napping behavior, and exercise behavior. This intervention resulted in a mean 2-hour advance in sleep-wake timing and advanced the dim light melatonin onset, a circadian rhythm timing marker. There was no effect on sleep duration or the time between sleep onset and dim light melatonin onset timing, which suggests the absence of sleep deprivation or worsening of circadian misalignment as a consequence of this intervention.7 Participants allocated to the intervention arm had improvements in the overall Depression, Anxiety, and Stress Scale score, as well as the depression and stress subscale scores.7 This trial provides evidence for the feasibility of interventions to produce clinically relevant shifts in sleep and circadian timing without shortening sleep or causing misalignment between sleep and melatonin timing. While this trial also lends support to a causal protective effect of morningness on mood, the study was small (n = 22) and did not assess hard clinical end points. In contrast, our analysis tested the association with lifelong modification of diurnal preference and assessed the clinical end point of MDD diagnosis. Taken together, our results suggest that interventions such as those implemented in this trial7 may be worth scaling to larger clinical trials.
Strengths
Key strengths of this study include the use of MR, which may reduce bias due to confounding and reverse causality. Additional strengths are the use of large sample sizes for precise estimation of MR effect sizes and the use of a clinically relevant scale for effect sizes.
Limitations
There are also limitations to consider. First, despite consistency in sensitivity analyses, it remains possible that our results are biased by horizontal pleiotropy. Second, these summary-level MR analyses test the linear association of modifying diurnal preference around the population mean, and the effect sizes may not generalize to extremes of diurnal preference (eg, delayed sleep phase syndrome34). Future work with larger samples may leverage nonlinear MR methods to investigate a dose-response curve for the association between diurnal preference and MDD risk. Similarly, these summary-level analyses cannot test whether the magnitude of the effect size for diurnal preference and MDD risk varies according to sleep duration. Third, the effect sizes estimated in this MR analysis correspond to a lifelong difference in diurnal preference and may not reflect the magnitude of effect size from a shorter-term intervention.12 Fourth, sleep and wake times used to calculate sleep midpoint were not confirmed using a sleep diary. Any consequent systematic error may bias our results, although the direction of any such bias cannot be anticipated. Fifth, although MDD is highly heterogeneous,35 we did not investigate MDD subtypes (ie, typical vs atypical), because no well-powered GWAS were available for these phenotypes, to our knowledge. Large differences in the effect magnitude of diurnal preference on MDD subtypes may attenuate the overall effect size estimates for the unstratified MDD analysis. Sixth, although the UKB MDD definition may include misclassified cases of anxiety disorders, the consistency of results across multiple different populations and more stringent phenotype definitions suggests that this is unlikely to explain our findings. Nevertheless, similar genetic investigations should be performed against the outcome of anxiety disorders as well-powered GWAS data become more available. Finally, our results may not generalize to populations that are not of European ancestry or very young or very old individuals. Similarly, since the UK Biobank and 23andMe participants are healthier than the general population, our results may not generalize to patients with a high burden of comorbidities.36
Conclusions
This 2-sample MR analysis found robust genetic evidence for a protective association between earlier diurnal preference with lower MDD risk, and contextualized this association using an objective sleep timing measure. The findings support further investigation in the form of randomized clinical trials of sleep timing interventions for the prevention of MDD.
eMethods.
eReferences.
eFigure 1. Workflow of Mendelian randomization analyses
eFigure 2. Graphical portrayal of Mendelian randomization scaling method
eFigure 3. Graphical assessment of bias in the primary Mendelian randomization analysis.
eTable 1. Genetic associations of all 340 genetic variants with morning diurnal preference in the 23andMe sample.
eTable 2. Lead variants associated with diurnal preference that were not available in the PGC-UKB MDD GWAS ('Original SNP') and identified proxy SNP.
eTable 3. Associations of available diurnal preference genetic variants with major depressive disorder in the PGC-UKB meta-analysis.
eTable 4. Associations of morning diurnal preference genetic variants with midpoint of sleep.
eTable 5. Variants identified as outliers by MR-PRESSO and RadialMR.
eTable 6. Random-effects inverse-variance weighted MR estimates for the effect of genetically proxied morning diurnal preference on depression (PGC-UKB) after progressive filtering of variants associated with other sleep phenotypes (sleep duration, short sleep duration, long sleep duration, daytime sleepiness, insomnia symptoms).
eTable 7. Leave-one-out MR estimates for the effect of genetically proxied morning diurnal preference on major depressive disorder (using the PGC-UKB dataset).
References
- 1.Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. Chronotype and social jetlag: a (self-) critical review. Biology (Basel). 2019;8(3):1-19. doi: 10.3390/biology8030054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kivelä L, Papadopoulos MR, Antypa N. Chronotype and psychiatric disorders. Curr Sleep Med Rep. 2018;4(2):94-103. doi: 10.1007/s40675-018-0113-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merikanto I, Lahti T, Kronholm E, et al. Evening types are prone to depression. Chronobiol Int. 2013;30(5):719-725. doi: 10.3109/07420528.2013.784770 [DOI] [PubMed] [Google Scholar]
- 4.Basnet S, Merikanto I, Lahti T, et al. Associations of common noncommunicable medical conditions and chronic diseases with chronotype in a population-based health examination study. Chronobiol Int. 2017;34(4):462-470. doi: 10.1080/07420528.2017.1295050 [DOI] [PubMed] [Google Scholar]
- 5.Vetter C, Chang SC, Devore EE, Rohrer F, Okereke OI, Schernhammer ES. Prospective study of chronotype and incident depression among middle- and older-aged women in the Nurses’ Health Study II. J Psychiatr Res. 2018;103:156-160. doi: 10.1016/j.jpsychires.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haraden DA, Mullin BC, Hankin BL. The relationship between depression and chronotype: a longitudinal assessment during childhood and adolescence. Depress Anxiety. 2017;34(10):967-976. doi: 10.1002/da.22682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facer-Childs ER, Middleton B, Skene DJ, Bagshaw AP. Resetting the late timing of ‘night owls’ has a positive impact on mental health and performance. Sleep Med. 2019;60:236-247. doi: 10.1016/j.sleep.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 8.LeGates TA, Altimus CM, Wang H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594-598. doi: 10.1038/nature11673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasler BP, Allen JJB, Sbarra DA, Bootzin RR, Bernert RA. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. 2017;176(2-3):166-173. doi: 10.1093/sleep/zsw002. [DOI] [PubMed] [Google Scholar]
- 10.Jones SE, Lane JM, Wood AR, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10(1):343. doi: 10.1038/s41467-018-08259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1-22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 12.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith GD, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4(12):e352. doi: 10.1371/journal.pmed.0040352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ripke S, Wray NR, Lewis CM, et al. ; Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium . A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497-511. doi: 10.1038/mp.2012.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wray NR, Ripke S, Mattheisen M, et al. ; eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668-681. doi: 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard DM, Adams MJ, Shirali M, et al. ; 23andMe Research Team . Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun. 2018;9(1):1470. doi: 10.1038/s41467-018-03819-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SE, van Hees VT, Mazzotti DR, et al. Genetic studies of accelerometer-based sleep measures yield new insights into human sleep behaviour. Nat Commun. 2019;10(1):1585. doi: 10.1038/s41467-019-09576-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehto JE, Aho O, Eklund M, et al. Circadian preferences and sleep in 15- to 20-year old Finnish students. Sleep Sci. 2016;9(2):78-83. doi: 10.1016/j.slsci.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample mendelian randomization. Genet Epidemiol. 2016;40(7):597-608. doi: 10.1002/gepi.21998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry A, Katsoulis M, Masi S, et al. The relationship between sleep duration, cognition and dementia: a mendelian randomization study. Int J Epidemiol. 2019;48(3):849-860. doi: 10.1093/ije/dyz071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao JV, Schooling CM. Thyroid function and ischemic heart disease: a mendelian randomization study. Sci Rep. 2017;7(1):8515. doi: 10.1038/s41598-017-07592-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133-1163. doi: 10.1002/sim.3034 [DOI] [PubMed] [Google Scholar]
- 24.Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyrrell J, Mulugeta A, Wood AR, et al. Using genetics to understand the causal influence of higher BMI on depression. Int J Epidemiol. 2019;48(3):834-848. doi: 10.1093/ije/dyy223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regestein QR, Monk TH. Delayed sleep phase syndrome: a review of its clinical aspects. Am J Psychiatry. 1995;152(4):602-608. doi: 10.1176/ajp.152.4.602 [DOI] [PubMed] [Google Scholar]
- 28.Shirayama M, Shirayama Y, Iida H, et al. The psychological aspects of patients with delayed sleep phase syndrome (DSPS). Sleep Med. 2003;4(5):427-433. doi: 10.1016/S1389-9457(03)00101-1 [DOI] [PubMed] [Google Scholar]
- 29.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10(4):473-481. doi: 10.31887/DCNS.2008.10.4/plfranzen [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting ereaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112(4):1232-1237. doi: 10.1073/pnas.1418490112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grøntved A, Singhammer J, Froberg K, et al. A prospective study of screen time in adolescence and depression symptoms in young adulthood. Prev Med. 2015;81:108-113. doi: 10.1016/j.ypmed.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 32.Lewis P, Korf HW, Kuffer L, Groß JV, Erren TC. Exercise time cues (zeitgebers) for human circadian systems can foster health and improve performance: a systematic review. BMJ Open Sport Exerc Med. 2018;4(1):e000443. doi: 10.1136/bmjsem-2018-000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi KW, Chen CY, Stein MB, et al. ; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample mendelian randomization study. JAMA Psychiatry. 2019;76(4):399-408. doi: 10.1001/jamapsychiatry.2018.4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtis BJ, Ashbrook LH, Young T, et al. Extreme morning chronotypes are often familial and not exceedingly rare: the estimated prevalence of advanced sleep phase, familial advanced sleep phase, and advanced sleep-wake phase disorder in a sleep clinic population. Sleep. 2019;42(10):1-9. doi: 10.1093/sleep/zsz148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai N, Choi KW, Fried EI. Reviewing the genetics of heterogeneity in depression: operationalizations, manifestations and etiologies. Hum Mol Genet. 2020;29(R1):R10-R18. doi: 10.1093/hmg/ddaa115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026-1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eReferences.
eFigure 1. Workflow of Mendelian randomization analyses
eFigure 2. Graphical portrayal of Mendelian randomization scaling method
eFigure 3. Graphical assessment of bias in the primary Mendelian randomization analysis.
eTable 1. Genetic associations of all 340 genetic variants with morning diurnal preference in the 23andMe sample.
eTable 2. Lead variants associated with diurnal preference that were not available in the PGC-UKB MDD GWAS ('Original SNP') and identified proxy SNP.
eTable 3. Associations of available diurnal preference genetic variants with major depressive disorder in the PGC-UKB meta-analysis.
eTable 4. Associations of morning diurnal preference genetic variants with midpoint of sleep.
eTable 5. Variants identified as outliers by MR-PRESSO and RadialMR.
eTable 6. Random-effects inverse-variance weighted MR estimates for the effect of genetically proxied morning diurnal preference on depression (PGC-UKB) after progressive filtering of variants associated with other sleep phenotypes (sleep duration, short sleep duration, long sleep duration, daytime sleepiness, insomnia symptoms).
eTable 7. Leave-one-out MR estimates for the effect of genetically proxied morning diurnal preference on major depressive disorder (using the PGC-UKB dataset).