Abstract
Various bi-directional associations exist between oral health and gastro-intestinal diseases. The oral microbiome plays a role in the gastro-intestinal carcinogenesis and fusobacteria are the most investigated bacteria involved. This paper aims to review the current knowledge and report the preliminary data on salivary levels of Fusobacterium nucleatum, Porphyromonas gingivalis and Candida albicans in subjects with different gastro-intestinal conditions or pathologies, in order to determine any differences. The null hypothesis was “subjects with different gastro-intestinal diseases do not show significant differences in the composition of the oral microbiota”. Twenty-one subjects undergoing esophagastroduodenoscopy or colonscopy were recruited. For each subject, a salivary sample was collected before the endoscopy procedure, immediately stored at −20 °C and subsequently used for genomic bacterial DNA extraction by real-time PCR. Low levels of F. nucleatum and P. gingivalis were peculiar in the oral microbiota in subjects affected by Helicobater pylori-negative chronic gastritis without cancerization and future studies will elucidate this association. The level of C. albicans did not statistically differ among groups. This preliminary study could be used in the future, following further investigation, as a non-invasive method for the search of gastrointestinal diseases and associated markers.
Keywords: oral microbiota, oral dysbiosis, chronic gastritis, microbiome, Fusobacterium nucleatum, Porphyromonas gingivalis, Candida albicans, salivary markers, RT-PCR
1. Introduction
1.1. Oral Microbiota: An Overview
The mouth is the opening tract of the digestive system, and its unhealthy state has been bi-directionally associated with various systemic and gastro-intestinal diseases [1,2,3,4,5]. For example, atrophic glossitis and angular cheilitis may underlie Plummer–Vinson syndrome—a sideropenic dysphagia secondary to iron deficiency associated with gastric ulcerations [6] as well as pernicious anemia, which is due to the failure of the gastric cells to produce the intrinsic factor responsible for the absorption of vitamin B12 in the intestine [7]. Additionally, the oral manifestations of a number of chronic bowel diseases, such as Crohn’s and coeliac diseases, are well documented and oral signs and/or symptoms can be assumed to be related to these diseases [8,9,10].
The existence of an oral–gut axis has also been confirmed by the discovery of an association between intestinal bowel diseases (IBD) and pathogens of oral origin [11,12,13,14] as well as recent evidence that the administration of gut-derived probiotics can be useful in the prevention of dental caries [15]. This suggests that improving the condition of the gut microbiota may lead to a simultaneous improvement in the operational taxonomic units (OTUs) of bacteria residing in the oral cavity.
As defined by Berg et al. [16], the human microbiota is made up of all types of microorganisms (archaea, eukaryotes, bacteria and viruses), that live on and in the human body, each housed in specific ecological niches, including oral ones [17]. Instead, the term “microbiome” refers to all of their genomic material and products [18]. Ever since research into the oral microbiome began [19], new associations have been continuously identified between alterations in the composition of the oral microbiome and various gastrointestinal diseases. This is possible due to the development and use of high-throughput culture-independent technologies, such as reverse transcriptase-polymerase chain reaction (RT-PCR) [20] and next generation sequencing (NSG) [21], both capable of identifying microorganisms and their genes, even when they are not cultivable [22]. The “microbiome project” aims to identify the microbiome components of the entire human body [19] and the effects of various dysbiosis on human health. Approximately 700 species of prokaryotes have been identified in the oral microbiome. These species belong to 185 genera and 12 phyla, of which approximately 54% are officially named, 14% are unnamed (but cultivated) and 32% are known only as uncultivated phylotypes [9]. The 12 phlya are Firmicutes, Fusobacteria, Proteobacteria, Actinobacteria, Bacteroidetes, Chlamydiae, Chloroflexi, Spirochaetes, SR1, Synergistetes, Saccharibacteria (TM7) and Gracilibacteria (GN02) [23]. In addition, the oral cavity also contains diverse forms of microbes such as protozoa, fungi and viruses. Entamoeba gingivalis and Trichomonas tenax are the most commonly found protozoa and are mainly saprophytic. The Candida species is the most prevalent fungi seen associated with the oral cavity. Ghannoum et al. [24] carried out culture-independent studies on 20 healthy hosts and reported 85 fungal genera. The main species observed were those belonging to Candida, Cladosporium, Aureobasidium, Saccharomycetales, Aspergillus, Fusarium and Cryptococcus [25]. Of particular interest, Candida albicans is a dimorphic yeast that is occasionally found in healthy mouths as a saprophyte [26] but can infect oral mucosa after dysregulations of the normal oral flora, under local [27] and systemic circumstances, both para-physiological (pregnancies, elderly, early childhood) and iatrogenic (prolonged steroids and/or antibiotics therapies) [28] as well as in dysmetabolic/dysimmune pathologies (diabetes, obesity and/or immunodeficiencies) [29,30,31]. In all these situations, the antagonistic bacterial–fungal relationship favors the switch of C. albicans to its infectious phenotype, with carcinogenic potential, as has been reported for other microbial species such as papillomaviruses [32] in particular conditions [33].
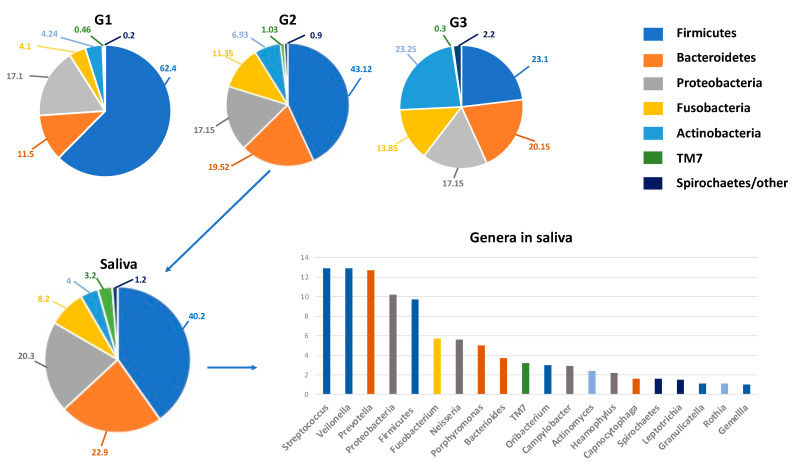
Under physiological conditions, the microorganisms of the core microbiota are qualitatively and quantitatively arranged in three different ecological niches/intraoral habitats as follows: Group 1 includes microorganisms at the level of the keratinized gingiva, the hard palate and the buccal mucosa; Group 2 comprises those on the tongue, tonsils, throat (posterior wall of the oropharynx) and in the saliva; and Group 3 comprises those in sub- and supra-gingival plaque [34,35] (Figure 1).
Figure 1.
The oral microbiota of a healthy mouth. On top, the percentage composition of phyla in the various niches (G1–G3) of the oral microbiota. G1, Group 1: keratinized gingiva, hard palate and the buccal mucosa; G2, Group 2, tongue, tonsils, throat and saliva; G3, Group 3, sub- and supra-gingival plaque. On the bottom, details on the percentage composition of phyla (left) and genera mainly present in salivary microbiota (right). The genera are represented in descending percentages, and color bars reflect the phyla they belong to. The organisms inhabiting saliva account for 99.9% of all bacteria in the oral cavity and are usually planktonic organisms.
Oral dysbiosis consists of a qualitative and quantitative imbalance of the composition of the oral microbiota. The specific predominance of some pathogenic microorganisms over others is associated with specific oral and systemic diseases. Fusobacterium nucleatum and Porphyromonas gingivalis are the bacteria most frequently and variably investigated and are mainly known to be responsible for periodontal diseases, although they can also be found in subjects without periodontitis [36,37]. Scientific literature reports that their presence is associated with the onset and/or worsening of a wide range of systemic diseases [38], such as osteoporosis [5], cardiovascular diseases [39,40], rheumatoid arthritis [41] and neurodegenerative diseases such as Alzheimer’s and Parkinson’s [42,43].
1.2. Oral Dysbiosis and Gastrointestinal Diseases
The term “gastritis” refers to a series of acute or chronic inflammations of the stomach, secondary to endogenous or exogenous irritants, which determine a reparative and/or reactive response of the gastric mucosa. The etiology of acute gastritis (AC) recognizes numerous causes, such as drugs, caustic agents, radiations and traumas, while chronic gastritis (CG) is mainly sustained by Helicobacter pylori infections or autoimmunological triggers, and is dichotomized in H. pylori-related and H. pylori-unrelated gastritis [44]. The severity and persistence of inflammation in CG or the onset of ulcers, mainly related to H. pylori infections and non-steroidal anti-inflammatory drugs (NSAIDs), are associated with the risk of developing dysplasia of varying degrees and gastric cancers [45].
Furthermore, Cui et al. [46] analyzed the diversity of the oral microbiome of subjects with and without gastritis and found that the levels of 11 species decreased and 10 increased in patients with gastritis.
With regards to the gastro-intestinal system, F. nucleatum and P. gingivalis can reach the stomach directly during swallowing, but they and their toxins have also been found in the colon and in the fecal microbiome, because they pass gastric juices alive and by systemic dissemination from the ulcerated gingival pockets through the hematogenous route [47,48].
For these reasons, recent literature has focused on the hypothesis that gastric and colorectal cancers (CRC) and their precursors, such as gastritis and inflammatory bowel diseases (IBDs), may also be associated with oral dysbiosis [49,50]; furthermore, oral bacteria can colonize the gut microbiome, thus influencing intestinal and extra-intestinal health by altering the permeability of the intestinal mucosa and perpetuating chronic inflammatory states, both locally and systemically, with the hematogenous dissemination of lipopolysaccharides (LPSs) and other toxins responsible for distant consequences [51] as well as CRC and pancreatic cancers [49] (Figure 2).
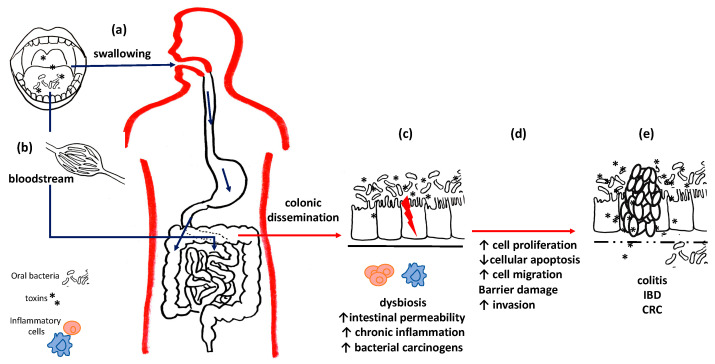
Figure 2.
Oral bacteria and intestinal diseases. Oral bacteria can reach the intestines and stomach both through swallowing (a) and through the bloodstream (b). (c) At the level of the colon mucosa, they can compete with the local flora and establish intestinal dysbiosis. Pathogenic bacteria and their toxins alter the permeability of the basement membrane, perpetuate chronic inflammation and promote carcinogenesis. (d) Uncontrolled cell cycles reduced apoptotic efficiency and barrier damage results, thus leading to (e) chronic inflammatory diseases and the onset of cancers.
Zhang et al. [52] suggested three mechanisms of action of oral microbiota in the pathogenesis of cancer. The first is related to the state of chronic inflammation induced, especially by anaerobic species in particular, such as Porphyromonas, Prevotella and Fusobacterium. These pathogens are, in fact, able to stimulate the production of important mediators of the inflammatory process, such as the cytokines interleukin (IL)-1β, IL-6, IL-17, IL-23, and Tumor Necrosis Factor α (TNF-α). The consequent harmful effects develop mainly on fibroblasts, epithelial and endothelial cells, and components of the extracellular matrix, with an increase in the expression of the metalloproteases MMP-8 and MMP-9 and a consequent increase in cell proliferation, mutagenesis, initiation of angiogenic processes and oncogenesis [53,54].
A second mechanism of action involves the ability of oral bacteria to influence cell proliferation, rearrangement of the cytoskeleton, activation of the transcription factor NF-kB and inhibition of apoptosis. For example, it has been shown that P. gingivalis can inhibit apoptosis by influencing different pathways, reducing the expression levels of proapoptotic molecules such as p53 [54,55] and Bad [54,56], inhibiting the activation of caspase-9 [55] and increasing the production of anti-apoptotic factors, such as Bcl-2 [54,57].
The third mechanism involves the oral pathogens’ production of many carcinogenic substances. These include reactive oxygen (ROS) and nitrogen (RNS) species [58,59], mainly produced by species such as Streptococcus oralis, S. mitis, S. sanguinis, S. gordonii, S. oligofermentans [60], Lactobacillus fermentum, L. jensenii, L. acidophilus, L. minutus and Bifidobacterium adolescentis [61]. ROS and RNS production induces NADPH oxidase and nitric oxide synthase (NOS), respectively, along with their reactive oxygen and nitrogen species, which have been identified in various tumor types [62,63].
Other species, such as Porphyromonas gingivalis, Prevotella intermedia, Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum, produce volatile sulphur compounds (VSC), including hydrogen sulphide (H2S), methyl mercaptan (CH3SH), dimethyl sulphide ((CH3) 2S), (CH3SS), (CH3SS), and (CH3SS), whose presence is often associated with the onset of cancer [64,65,66].
Some species are able to produce more acids (e.g., Peptostreptococcus stomatis aciduric produces acetic, butyric, isobutyric, isovaleric, and isocaproic acids) [67] that can add to the acidic and hypoxic microenvironment of tumors, thereby increasing metastatic efficiency [68,69]. Furthermore, various oral microbial species, such as S. gordonii, S. mitis, S. oralis, S. salivarius and S. sanguinis streptococci [70], metabolize alcohol to acetaldehyde, which is indisputably carcinogenic.
Cordero et al. [71] reported that the relationship between oral hygiene and intestinal inflammation, which are mutually involved through signaling pathways, are linked to tumor-promoting inflammation. In fact, during the inflammatory process, the massive presence of pathogens or the simple imbalance of the oral microbiota play an important role in the onset of CRC from a chronic inflamed bowel, such as in cases of IBD. The authors based this hypothesis on the evidence that part of the gut microbiota comes from the oral one. Thus, as stated by Flemer et al. [49], “the oral microbiota in colorectal cancer is distinctive and predictive”. After profiling the microbiota from oral swabs, colonic mucosae and stools in individuals with CRC, colorectal polyps and healthy controls, they concluded that (i) oral bacteria were more abundant in CRC than in healthy controls, (ii) that the oral microbiome of healthy controls was different from those with CRC, and (iii) that F. nucleatum was most abundant in CRCs.
Numerous studies have also reported a significant increase in F. nucleatum in the gastric microbiome of subjects with gastric cancers and gastritis.
In 2013, Salazar et al. [72] conducted a clinical study to measure the levels of periodontal pathogenic bacteria in dental plaque and salivary samples from subjects with gastric precancerous lesions via quantitative RT-PCR. They found a high but not statistically significant increase in P. gingivalis and therefore, they hypothesized that high levels of periodontal colonization by pathogens may be associated with an increased risk of precancerous gastric lesions. In 2017, Coker et al. [73] identified differences in microbial diversity and richness between gastric cancers and various types of gastritis, thus indicating the presence of microbial dysbiosis in gastric carcinogenesis. Specifically, Prevotella intermedia and F. nucleatum, together with Prevotella oris and Catonella morbi, were significantly enriched in the gastric cancer microbiome compared to precancerous stages and they formed an increasingly strong co-occurrence network with disease progression.
The recent works of Yamamura et al. [74] and Hsieh et al. [75] have reinforced these correlations. Yamamura et al. [74] detected a significant increase in the mount of F. nucleatum DNA in oesophageal and gastric cancers as well as CRCs. Hsieh et al. [75] profiled gastric bacterial species in patients with gastritis and gastric cancer and found that F. nucleatum, along with Clostridium colicans, was frequently abundant in gastric cancer patients, supporting a specific gastric cancer signature.
Regarding C. albicans, the scientific literature has so far paid little attention to the study of its relationship with systemic health, limited to the role of candidiasis in a few systemic diseases, and has not yet considered its possible gastro-intestinal implications [76,77]. The main findings on oral bacteria associated with gastrointestinal disease are reported in Table 1.
Table 1.
Oral bacteria associated with gastrointestinal diseases.
| G-i Diseases | Oral Bacteria/Fungi | Main Findings | Ref. |
|---|---|---|---|
| Gastritis | Streptococci, Fusobacteria | significantly higher in gastritis vs. healthy controls | [46] |
| Veillonella parvula, Corynebacterium matruchotii, Kingella oralis, Atopobium rimae, Aggregatibacter aphrophilus, Streptococcus sanguinis, Acinetobacter lwoffii, Prevotella amnii, Prevotella bivia, Cardiobacterium hominis and Oribacterium sinus | decreased in gastritis patients vs. healthy control | [46] | |
| Streptococcus infantis, Treponema vincentii, Leptotrichia unclassified, Campylobacter rectus, Campylobacter showae, Capnocytophaga gingivalis, Leptotrichia buccalis, Campylobacter concisus, Selenomonas flueggei and Leptotrichia hofstadii | increased in gastritis patients vs. healthy control (mainly Campylobacter spp.) | [46] | |
| Gastric Precancerous lesions | Campylobacter concisus | positively associated with the precancerous cascade of gastritis | [46] |
| Porphyromonas gingivalis, Treponema denticola | increased in dental plaque of subjects with gastric precancerous lesions | [72] | |
| Actinobacillus actinomycetemcomitans, Treponema denticola | significantly associated with gastric precancerous lesions | [72] | |
| Tannerella forsythia | significantly inversely associated with gastric precancerous lesions | [72] | |
| Oesophageal and Gastric Cancers | Tannerella forsythia, Porphyromonas gingivalis | associated with higher risk of oesophageal cancers | [52] |
| Streptococcus anginosus | higher in oesophageal cancer tissues than in oral cancer tissues | [52] | |
| Fusobacterium nucleatum | higher in oesophageal cancer tissues than matched normal mucosa; significantly associated with tumor stage and cancer-specific survival | [52] | |
| Neisseria spp., Candida glabrata | potential role in alcohol-related carcinogenesis |
[52] | |
|
Parvimonas micra, Peptostreptococcus stomatis, Prevotella intermedia, Fusobacterium nucleatum, Prevotella oris, Gemella and Catonella morbi, Streptococcus anginosus, Dialister pneumosintes, Slackia exigua |
significantly increased in gastric cancer compared with precancerous stages |
[73] | |
| Inflammatory bowel diseases (IBDs) | Bacteroidetes | significantly increased in IBDs | [51] |
| Proteobacteria and Actinobacteria | increased in IBDs | [51] | |
| Campylobacter concisus | increases the mucosal permeability by affecting the tight junctions in IBDs | [51] | |
| Fusobacterium nucleatum | overrepresented in IBDs | [51] | |
| Candida albicans | isolated from the intestine more frequently in IBD patients | [51] | |
| CRC |
Haemophilus spp., Prevotella spp., Alloprevotella Lachnoanaerobaculum, Neisseria and Streptococcus spp. |
less abundant in CRC than healthy controls | [49] |
| Fusobacterium nucleatum, Parvimonas micra, Peptostreptococcus stomatis, Dialister pneumosintes | tumor-associated bacteria | [49] | |
| Peptostreptococcus, Parvimonas, Fusobacterium | more abundant in CRC than in healthy controls | [49] | |
| Fusobacterium nucleatum | induces inflammatory response and promotes CRC development |
[52] | |
| Treponema denticola, Prevotella intermedia | increases the CRC risk | [52] | |
| Porphyromonas gingivalis | causes inflammation and promotes tissue degenerative processes | [52] | |
| Fusobacterium nucleatum | associated with CRC regional lymph node metastases | [55] | |
| Fusobacterium nucleatum, Selenomonas, Prevotella, Parvimonas micra, Peptostreptococcus stomatis | increased in CRC; induces colon cancer growth and progression | [71] | |
| Lachnospiraceae | can protect against CRC | [71] | |
| Fusobacterium nucleatum | sustains both the biofilm and the CRC tumorigenesis |
[47] |
In the light of the above review of the literature, the aim of this study was to establish a possible association of the salivary levels of F. nucleatum, P. gingivalis and C. albicans with various gastro-intestinal conditions and/or pathologies, in order to highlight any differences and their possible clinical significance and correlations. The null hypothesis was “subjects with different gastro-intestinal diseases do not show significant differences in the composition of the oral microbiota”.
2. Materials and Methods
2.1. Patients
All the procedures in the present study involving human participants were performed after approval from the Internal Ethics Committee (protocol number #68/2020, Comitato Etico Università della Campania “Luigi Vanvitelli”—Azienda Ospedaliera Universitaria “Luigi Vanvitelli”—AORN “Ospedale dei Colli”), and in accordance with the 1964 Helsinki declaration and its later amendments.
A series of consecutive subjects referred to the Digestive Endoscopy Unit of the University of Campania “Luigi Vanvitelli”, Naples, Italy, were considered. The exclusion criteria were as follows: recent antimicrobial therapy and/or use of oral antiseptic (less than two weeks prior the enrollment) and presence of chronic and/or acute confounding infections, such as HCV, HBV and HIV, established by serological tests exhibited by each patient invited to participate. All the subjects who agreed to participate, gave their informed written consent for their anamnestic data and a salivary sample to be collected before the scheduled endoscopic procedure, which was performed in accordance with the standards protocols.
For subjects with clinical suspicion of gastric diseases, a complete esophagastroduodenoscopy (EGDS) was performed, while patients with suspected CRC or a past history of CRC underwent colonoscopy (CS) for post-cancer follow-up. When necessary, one or more biopsies were performed simultaneously with the endoscopic procedure to analyze suspicious lesions with a conventional histology.
Two healthy subjects, who underwent CS for hemorrhoids and had no pathological findings or other gastrointestinal symptoms, were considered as the control group.
Saliva was collected by asking the patient to spit once per minute into a sterile Eppendorf, mainly two hours after the last brushing of teeth in the morning and prior to the endoscopic procedure, until the appropriate amount (5 mL) was obtained. The study was double-blinded—both the examiner collecting the saliva and microbiologist dealing with the samples were blinded to the gastro-intestinal conditions of the patients.
2.2. Saliva Analysis for Microbiota Evaluation
Salivary samples frozen at −20 degree Celsius were used for genomic bacterial DNA extraction with the Qiaamp DNA mini kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions.
Real-time PCR was carried out to detect the presence of periodontal pathogens with the LC FastStart DNA Master SYBR Green kit (Roche Diagnostics, Penzberg, Germany) in a 20 μL final volume using 2 μL of DNA, 3 mM MgCl2, and 0.5 mM sense and antisense primers (Table 2).
Table 2.
Primer sequence and amplification conditions.
| Gene | Primers Sequence | Conditions | ProductSize (bp) |
|---|---|---|---|
| Fusobacterium nucleatum | 5′-AGAGTTTGATCCTGGCTCAG-3′ 5′-GTCATCGTGCACACAGAATTGCTG-3′ |
5″ at 95 °C, 16″ at 55 °C, 8″ at 72 °C for 40 cycles | 407 |
| Porphyromonas gingivalis | 5′-TGTAGATGACTGATGGTGAAAACC-3′ 5′-ACGTCATCCCCACCTTCCTC-3′ |
5″ at 95 °C, 5” at 52 °C, 4″ at 72 °C for 40 cycles | 198 |
| Candida albicans | 5′-TTTATCAACTTGTCACACCAGA-3′ 5′-GGTCAAAGTTTGAAGATATACGT-3′ |
10″ at 95 °C, 10″ at 58 °C, 15″ at 72 °C for 30 cycles | 354 |
After amplification, the melting curve analysis was performed by heating to 95 °C for 15 s with a temperature transition rate of 20 °C s−1, cooling to 60 °C for 15 s with a temperature transition rate of 20 °C s−1, and then heating the sample at 0.1 °C s−1 to 95 °C. The results were then analyzed using the LightCycler software (Roche Diagnostics, Penzberg, Germany) [78,79].
The standard curve of each primer pair was established with serial dilutions of the DNA; all PCR reactions were run in triplicate.
2.3. Statistical Analysis
Significant differences among the groups were assessed using the t-student test and Excel (ver.16.16® 2018 Microsoft). The data were expressed as means ± standard deviation (SD) of three independent experiments.
3. Results
Overall, 21 subjects were considered (13 women, eight men; mean age 58.86 ± 13.49 years). All seven subjects with CG were negative for H. pylori; the six ex-CRC subjects and the four healthy controls did not report any pathological findings. Four subjects reported histological findings of CRC.
The mean levels of F. nucleatum, P. gingivalis and C. albicans in each patient and in each group are detailed in Table 3. With regard to C. albicans, it was discontinuously found and statistical differences were not reported, neither between groups (Table 3), nor between subjects with (n = 5; mean levels of C. albicans: 82.9 ± 156.86) and without removable dentures (n = 16; mean levels of C. albicans: 20.00 ± 51.00); hence, its analysis was excluded from further examinations. Moreover, no differences were found in F. nucleatum and P. gingivalis amounts between denture wearers and non-wearers.
Table 3.
Datasets of the subjects enrolled.
| Id. Patient | Age (Years) | Sex | F.n. (ng/dL) | P.g. (pg/mL) | C.a. (pg/mL) | F.n. per Group (Mean ± SD) | P.g. per Group (Mean ± SD) | C.a. per Group * (Mean ± SD) | |
|---|---|---|---|---|---|---|---|---|---|
| CG Group | 2 | 49 | F | 0.10 | 0.03 | 156 | 1.10 ± 1.62 | 0.57 ± 1.17 | 39.36 ± 57.80 |
| 3 | 50 | F | 0.02 | 0.05 | 0 | ||||
| 6 | 68 | F | 2.40 | 0.04 | 0 | ||||
| 8 | 23 | F | 4.20 | 3.20 | 71.5 | ||||
| 10 | 47 | F | 0.00 | 0.15 | 12 | ||||
| 30 | 55 | F | 0.93 | 0.08 | 0 | ||||
| 44 | 46 | F | 0.08 | 0.44 | 36 | ||||
| Ex-CRC Group | 17 | 67 | M | 40.50 | 29.75 | 0 | 31.62 ± 34.40 | 7.78 ± 11.37 | 14.08 ± 15.76 |
| 22 | 58 | M | 3.85 | 1.4 | 0 | ||||
| 24 | 66 | F | 1.37 | 8.75 | 0 | ||||
| 27 | 63 | M | 95.00 | 0.01 | 32.5 | ||||
| 48 | 71 | F | 28.50 | 6.75 | 22.5 | ||||
| 50 | 62 | M | 20.50 | 0.000 | 29.50 | ||||
| CRC Group | 20 | 80 | M | 9.50 | 0.05 | 365.5 | 9.13 ± 6.03 | 2.88 ± 3.68 | 91.25 ± 182.50 |
| 29 | 63 | M | 1.50 | 0.03 | 0 | ||||
| 31 | 87 | F | 9.25 | 3.70 | 0 | ||||
| 39 | 63 | M | 16.25 | 7.75 | 0 | ||||
| Healthy control | 9 | 49 | F | 56.50 | 296.50 | 0 | 65.06 ± 14.92 | 110.19 ± 127.37 | 0 |
| 42 | 54 | F | 85.00 | 10.25 | 0 | ||||
| 45 | 51 | M | 67.50 | 78.00 | 0 | ||||
| 34 | 64 | F | 51.25 | 56.5 | 0 |
F.n. Fusobacterium nucleatum; P.g. Porphyromonas gingivalis; C.a. Candida albicans. * t-student test revealed no significant differences between any paired groups.
The levels of F. nucleatum were statistically the lowest in the CG group compared to any other group, while, unexpectedly, they were significantly higher in the control group than in the CRC group, and no significant differences were reported between the healthy subjects and the ex-CRC patients (Table 4).
Table 4.
Correlations between groups: F. nucleatum levels.
| CG Group | Ex-CRC Group | CRC Group | Healthy Group | |
|---|---|---|---|---|
| Sample size | 7 | 6 | 4 | 4 |
| Mean F.n. values | 1.10 | 31.62 | 9.13 | 65.06 |
| Standard Deviation | 1.62 | 34.40 | 6.03 | 14.92 |
| t-student test | CG vs. ex-CRC * p < 0.05 CG vs. CRC * p < 0.05 CG vs. Healthy * p < 0.05 Healthy vs. CRC * p < 0.05 Healthy vs. Ex-CRC n.s. (p = 0.11) CRC vs. ex-CRC n.s. (p = 0.24) |
|||
* Statistically significant at p < 0.05; n.s.: Not Significant.
The lowest levels of P. gingivalis were found in the CG group with a statistically significant difference as compared to the healthy controls. The latter group showed higher but not statistically relevant amounts of P. gingivalis compared to the CRC and ex-CRC subjects, and the CRC group showed higher mean amounts than the CRC group (Table 5).
Table 5.
Correlations between groups: P. gingivalis levels.
| CG Group | Ex-CRC Group | CRC Group | Healthy Control | |
|---|---|---|---|---|
| Sample size | 7 | 6 | 4 | 4 |
| Mean P.g. values | 0.57 | 7.78 | 2.88 | 110.19 |
| Standard Deviation | 1.17 | 11.37 | 3.68 | 127.37 |
| t-student test | CG vs. ex-CRC n.s. (p = 0.12) CG vs. CRC n.s. (p = 0.15) CG vs. Healthy * p < 0.05 Healthy vs. CRC n.s. (p = 0.14) Healthy vs. Ex-CRC n.s. (p = 0.07) CRC vs. ex-CRC n.s. (p = 0.44) |
|||
* Statistically significant at p < 0.05; n.s.: Not Significant.
4. Discussion and Conclusions
Periodontitis is a biofilm-induced chronic condition which involves inflammation and destruction of periodontal tissue [80] by oral bacteria and is considered a global disease burden [81], being sixth among the most prevalent human diseases [82]. Many studies in the last 20 years have shown the existence of a clear association between periodontitis and the onset of other chronic systemic inflammatory diseases [83] due to the inflammatory state and activation of the immune response triggered by periodontal pathogens, following the onset of oral microbiota dysbiosis [84].
Diseases associated with periodontitis include diabetes [85], head and neck cancer [86], pulmonary disease [87], survival of dental implants [88] and cardiovascular diseases [89]. In recent years, there has been a growing interest in the existence of an oral–gut axis and its related pathologies. Yu et al. reported a significantly positive association between peptic ulcer and periodontal disease [90]. The presence of periodontal disease has often been detected in patients with IBD [91], and it has also been shown, conversely, that patients with IBD suffer from a more severe degree of periodontal disease [92]. Wei et al. [93] reported that chronic periodontitis (CP) was potentially correlated with oral H. pylori in adults, and that it may be a possible risk factor for CP. Boylan et al. and Byun et al. showed an increased risk of gastric and duodenal ulcer among patients with periodontal disease [94,95], while Umeda et al. suggested that patients with periodontitis who harbor H. pylori in the oral cavity should be closely monitored [96]. However, the pathway underlying the correlation between periodontitis and H. pylori-related chronic gastritis/peptic ulcer is not completely understood and needs to be studied more thoroughly.
Another study stated that the salivary microbiota can affect the development of the intestinal microbiota, as saliva flows through the gastrointestinal tract, allowing the bacteria present in it to easily reach the intestine. It has in fact been shown, through a study aimed at assessing the metatranscriptome and metagenome of the human gut microbiota, that the DNA of bacteria belonging to the salivary microbiota is detectable in the gut even in low concentrations [97].
This review provides preliminary data on the assessment of salivary levels of F. nucleatum, P. gingivalis and C. albicans in patients with CG and with a history of CRC.
Twenty-one subjects were enrolled: nine had undergone EGDS and 12 CS. In each subject the levels of F. nucleatum, P. gingivalis and C. albicans were measured by RT-PCR and correlated with their endoscopic and histologic diagnosis, to establish any differences between the groups.
C. albicans was found intermittently, and no statistical differences were reported either between groups or between subjects with and without removable dentures (as well as the amounts of F. nucleatum and P. gingivalis). The finding of C. albicans was not associated with clinical signs of oral candidiasis, thus suggesting a carrier state of some subjects, without any correlation with their gastrointestinal conditions.
Levels of F. nucleatum were the lowest in the CG group and the highest in the control group. The ex-CRC patients showed relatively, but not significantly, higher levels than those of CRC group. This last finding contradicted the literature reporting the increase of F. nucleatum in stool samples from CRC subjects and its association with colon carcinogenesis and chronic cancer-related inflammation [48]. If we hypothesize that these marked differences may be related to the source of the sample (saliva instead of feces), it would be reasonable to exclude salivary tests for CRC screening, but further comparative studies should clarify this point better.
The lowest levels of F. nucleatum and P. gingivalis were found in the CG group, with statistically significant differences in F. nucleatum compared to each group, and P. gingivalis, as compared to healthy subjects. In contrast, subjects with ex-CRC revealed a different profile in which there were relative high concentrations of F. nucleatum and low P. gingivalis, as compared to patients with CRC.
Another key feature of the CG group was that all subjects were H. pylori-negative. Literature reports that H. pylori-positive individuals have a significant increase in the amount of F. nucleatum in the oral cavity, as H. pylori selectively adheres and co-aggregates with Fusobacteria [98]. Therefore, it is reasonable to speculate that low levels of F. nucleatum can influence or be influenced by the lack of H. pylori. What is still unclear is whether H. pylori-negativity should be considered a consequence of low levels of F. nucleatum, or whether the low levels of F. nucleatum are due to a lack of H. pylori.
In the first case, the expression of H. pylori could be considered to be directly correlated to the quantity of F. nucleatum and, therefore, it could be hypothesized as indirectly reducing H. pylori by acting on the salivary reduction of F. nucleatum with oral hygiene protocols to directly rebalance the composition of the oral microbiota and, indirectly, the gastric one. This intervention could reduce the need for antibiotic therapies for the eradication of H. pylori by abolishing their adverse effects and drug-resistances. Conversely, if the low levels of F. nucleatum were a consequence of the lack of H. pylori, it would be possible to indirectly estimate the presence/absence of H. pylori in CG by measuring the amount of F. nucleatum in the oral cavity. In any case, although these doubts are still to be clarified, and these hypotheses require confirmation through larger clinical studies, the hypothesis of considering the measurement of F. nucleatum as a predictor of the presence of H. pylori in CGs and their cancerization has been corroborated by various studies [98,99,100,101].
Furthermore, the microbial diversity in subjects with gastritis H. pylori-negative compared with those with gastritis H. pylori-positive, was proven by several studies [101] as well the fact that saliva and stomach aspirates share similar bacterial composition and significantly highest abundance of Fusobacteria, compared to other gastro-intestinal sites. Particularly, Fusobacteria were found to be more abundant in the stomach than saliva in a series of subjects with gastritis H. pylori-negative [102]. On this basis, it is reasonable and follows Zhao et al. [102] that, if the saliva is the main source for the gastric microbiome, a correlation between H. pylori and oral bacterial species may exist and may influence and/or be influenced by each other [102].
The consistency of this study was strongly affected by the small sample size; thus, it must be considered as just “exploratory” and in need of improvement. Unfortunately, this was due to the interruption of recruitment after the outbreak of the COVID-19 pandemic, in March 2020.
The intestinal and oral environments are infinitely complex, and the microbiota of these environments is a key element in maintaining homeostasis, so saliva should be considered as a means of monitoring the intestine for future research in gastrointestinal tract diseases.
However, the preliminary results encourage and recommend further cohort studies on patients suffering from CG, in order to establish whether the salivary quantification of F. nucleatum and P. gingivalis can actually serve as a non-invasive marker for monitoring the onset of H. pylori or cancerization.
Acknowledgments
This work was supported by Campania Regional Government Lotta alle patologie oncologiche, iCURE (CUP B21C17000030007).
Author Contributions
Conceptualization, A.I. (Angelo Itro), R.S., G.D. (Giovanna Donnarumma), P.S., A.F. (Alessandro Federico); methodology, M.C., A.F. (Alessandra Fusco) and A.G.G.; formal analysis and investigation, M.C., A.F. (Alessandra Fusco), A.G.G., G.D. (Gianna Dipalma) and A.I. (Annalisa Itro); data curation, M.C., F.I. and S.L.; writing—original draft preparation, M.C., A.F. (Alessandra Fusco), writing—review and editing, M.C., A.F. (Alessandra Fusco), and G.D. (Gianna Dipalma); supervision R.S., G.D. (Giovanna Donnarumma) and F.I. All authors have read and agreed to the published version of the manuscript.
Funding
The research activity of Maria Contaldo and Stefania Lama was supported by Campania Regional Government Lotta alle patologie oncologiche, iCURE (CUP B21C17000030007). This work was supported by Campania Regional Government Lotta alle patologie oncologiche, iCURE (CUP B21C17000030007).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Internal Ethics Committee—Comitato Etico Università della Campania “Luigi Vanvitelli”—Azienda Ospedaliera Universitaria “Luigi Vanvitelli”—AORN “Ospedale dei Colli”, (protocol number 68, date of approval 30/01/2020).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study to publish this paper.
Conflicts of Interest
All the authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Benedittis M., Petruzzi M., Favia G., Serpico R. Oro-dental manifestations in Hallopeau-Siemens-type recessive dystrophic epidermolysis bullosa. Clin. Exp. Dermatol. 2004;29:128–132. doi: 10.1111/j.1365-2230.2004.01485.x. [DOI] [PubMed] [Google Scholar]
- 2.Della Vella F., Lauritano D., Lajolo C., Lucchese A., Di Stasio D., Contaldo M., Serpico R., Petruzzi M. The Pseudolesions of the Oral Mucosa: Differential Diagnosis and Related Systemic Conditions. Appl. Sci. 2019;9:2412. doi: 10.3390/app9122412. [DOI] [Google Scholar]
- 3.Della Vella F., Contaldo M., Fucile R., Panza F., Dibello V., Kalemaj Z., Ninivaggi R., Petruzzi M., Serpico R. ORO-Dental Manifestations in West Syndrome. Curr. Top. Med. Chem. 2019;19:2824–2828. doi: 10.2174/1568026619666191114122732. [DOI] [PubMed] [Google Scholar]
- 4.Contaldo M., Luzzi V., Ierardo G., Raimondo E., Boccellino M., Ferati K., Bexheti-Ferati A., Inchingolo F., Di Domenico M., Serpico R. Bisphosphonate-related osteonecrosis of the jaws and dental surgery procedures in children and young people with osteogenesis imperfecta: A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2020;121:556–562. doi: 10.1016/j.jormas.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Contaldo M., Itro A., Lajolo C., Gioco G., Inchingolo F., Serpico R. Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl. Sci. 2020;10:6000. doi: 10.3390/app10176000. [DOI] [Google Scholar]
- 6.Adeyemo T.A., Adeyemo W.L., Adediran A., Akinbami A.J., Akanmu A.S. Orofacial manifestations of hematological disorders: Anemia and hemostatic disorders. Indian J. Dent. Res. 2011;22:454–461. doi: 10.4103/0970-9290.87070. [DOI] [PubMed] [Google Scholar]
- 7.Chiang C.P., Chang J.Y., Wang Y.P., Wu Y.H., Wu Y.C., Sun A. Atrophic glossitis: Etiology, serum autoantibodies, anemia, hematinic deficiencies, hyperhomocysteinemia, and management. J. Formos. Med. Assoc. 2020;119:774–780. doi: 10.1016/j.jfma.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Pastore L., Lo Muzio L., Serpico R. Atrophic glossitis leading to the diagnosis of celiac disease. N. Engl. J. Med. 2007;356:2547. doi: 10.1056/NEJMc070200. [DOI] [PubMed] [Google Scholar]
- 9.Pastore L., Carroccio A., Compilato D., Panzarella V., Serpico R., Lo Muzio L. Oral manifestations of celiac disease. J. Clin. Gastroenterol. 2008;42:224–232. doi: 10.1097/MCG.0b013e318074dd98. [DOI] [PubMed] [Google Scholar]
- 10.Onitake T., Ueno Y., Tanaka S., Hanaoka R., Yoshioka K., Hatakeyama T., Oka S., Yoshida S., Hiyama T., Ito M., et al. Cheilitis granulomatosa as an early manifestation of Crohn’s disease. Clin. J. Gastroenterol. 2009;2:190–193. doi: 10.1007/s12328-009-0084-x. [DOI] [PubMed] [Google Scholar]
- 11.Umar S. Intestinal Stem Cells. Curr. Gastroenterol. Rep. 2010;12:340–348. doi: 10.1007/s11894-010-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrens M. Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier Inc.; Amsterdam, The Netherlands: 2020. Chapter: Extraoral Taste Receptors. [Google Scholar]
- 13.Troll J.V., Hamilton M.K., Abel M.L., Ganz J., Bates J.M., Stephens W.Z., Melancon E., Van Der Vaart M., Meijer A.H., Distel M., et al. Microbiota promote secretory cell determination in the intestinal epithelium by modulating host Notch signaling. Development. 2018;145:dev155317. doi: 10.1242/dev.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee A., McKinley E.T., von Moltke J., Coey R.J., Lau K.S. Interpreting heterogeneity in intestinal tuft cell structure and function. J. Clin. Investig. 2018;128:1711–1719. doi: 10.1172/JCI120330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peck B.C.E., Shanahan M.T., Singh A.P., Sethupathy P. Gut Microbial Influences on the Mammalian Intestinal Stem Cell Niche. Stem Cells Int. 2017;2017:1–17. doi: 10.1155/2017/5604727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg G., Rybakova D., Fischer D., Cernava T., Vergès M.C., Charles T., Chen X., Cocolin L., Eversole K., Corral G.H., et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome. 2020;8:103. doi: 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baquero F., Nombela C. The microbiome as a human organ. Clin. Microbiol. Infect. 2012;18:2–4. doi: 10.1111/j.1469-0691.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 18.Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C., Yu W.H., Lakshmanan A., Wade W.G. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pannone G., Sanguedolce F., De Maria S., Farina E., Lo Muzio L., Serpico R., Emanuelli M., Rubini C., De Rosa G., Staibano S., et al. Cyclooxygenase isozymes in oral squamous cell carcinoma: A real-time RT-PCR study with clinic pathological correlations. Int. J. Immunopathol. Pharmacol. 2007;20:317–324. doi: 10.1177/039463200702000211. [DOI] [PubMed] [Google Scholar]
- 21.Sala-Comorera L., Caudet-Segarra L., Galofré B., Lucena F., Blanch A.R., García-Aljaro C. Unravelling the composition of tap and mineral water microbiota: Divergences between next-generation sequencing techniques and culture-based methods. Int. J. Food Microbiol. 2020;334:108850. doi: 10.1016/j.ijfoodmicro.2020.108850. [DOI] [PubMed] [Google Scholar]
- 22.Appanna V.D. Human Microbes—The Power Within. Springer; Singapore: 2018. The Human Microbiome: The Origin; pp. 1–36. [Google Scholar]
- 23.Perera M., Al-Hebshi N.N., Speicher D.J., Perera I., Johnson N.W. Emerging role of bacteria in oral carcinogenesis: A review with special reference to perio-pathogenic bacteria. J. Oral Microbiol. 2016;8:32762. doi: 10.3402/jom.v8.32762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghannoum M.A., Jurevic R.J., Mukherjee P.K., Cui F., Sikaroodi M., Naqvi A., Gillevet P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma N., Bhatia S., Sodhi A.S., Batra N. Oral microbiome and health. AIMS Microbiol. 2018;4:42–66. doi: 10.3934/microbiol.2018.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petruzzi M., Della Vella F., Cassandro A., Mosca A., Di Comite M., Contaldo M., Grassi F.R., Lauritano D. Dorsal tongue porphyrin autofluorescence and Candida saprophytism: A prospective observational study. PLoS ONE. 2019;14:e0223072. doi: 10.1371/journal.pone.0223072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milillo L., Lo Muzio L., Carlino P., Serpico R., Coccia E., Scully C. Candida-related denture stomatitis: A pilot study of the efficacy of an amorolfine antifungal varnish. Int. J. Prosthodont. 2005;18:55–59. [PubMed] [Google Scholar]
- 28.Sardaro N., Della Vella F., Incalza M.A., Di Stasio D., Lucchese A., Contaldo M., Laudadio C., Petruzzi M. Oxidative Stress and Oral Mucosal Diseases: An Overview. In Vivo. 2019;33:289–296. doi: 10.21873/invivo.11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paoletti I., Fusco A., Grimaldi E., Perillo L., Coretti L., Di Domenico M., Cozza V., Contaldo M., Serpico R., Guida A., et al. Assessment of host defence mechanisms induced by Candida species. Int. J. Immunopathol. Pharmacol. 2013;26:663–672. doi: 10.1177/039463201302600309. [DOI] [PubMed] [Google Scholar]
- 30.Contaldo M., Romano A., Mascitti M., Fiori F., Della Vella F., Serpico R., Santarelli A. Association between denture stomatitis, candida species and diabetic status. J. Biol. Regul. Homeost. Agents. 2019;33:35–41. [PubMed] [Google Scholar]
- 31.Di Domenico M., Pinto F., Quagliuolo L., Contaldo M., Settembre G., Romano A., Coppola M., Ferati K., Bexheti-Ferati A., Sciarra A., et al. The Role of Oxidative Stress and Hormones in Controlling Obesity. Front. Endocrinol. 2019;1310:540. doi: 10.3389/fendo.2019.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pannone G., Santoro A., Carinci F., Bufo P., Papagerakis S.M., Rubini C., Campisi G., Giovannelli L., Contaldo M., Serpico R., et al. Double demonstration of oncogenic high risk human papilloma virus DNA and HPV-E7 protein in oral cancers. Int. J. Immunopathol. Pharmacol. 2011;24:95–101. doi: 10.1177/03946320110240S217. [DOI] [PubMed] [Google Scholar]
- 33.Castillo G.D.V., Blanc S.L., Sotomayor C.E., Azcurra A.I. Study of virulence factor of Candida species in oral lesions and its association with potentially malignant and malignant lesions. Arch. Oral Biol. 2018;91:35–41. doi: 10.1016/j.archoralbio.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Segata N., Haake S.K., Mannon P., Lemon K.P., Waldron L., Gevers D., Huttenhower C., Izard J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13:R42. doi: 10.1186/gb-2012-13-6-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wade W.G., Prosdocimi E.M. Profiling of Oral Bacterial Communities. J. Dent. Res. 2020;99:621–629. doi: 10.1177/0022034520914594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teles R., Teles F., Frias-Lopez J., Paster B., Haffajee A. Lessons learned and unlearned in periodontal microbiology. Periodontol. 2000. 2013;62:95–162. doi: 10.1111/prd.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contaldo M., Lucchese A., Lajolo C., Rupe C., Di Stasio D., Romano A., Petruzzi M., Serpico R. The Oral Microbiota Changes in Orthodontic Patients and Effects on Oral Health: An Overview. J. Clin. Med. 2021;10:780. doi: 10.3390/jcm10040780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmstrup P., Damgaard C., Olsen I., Klinge B., Flyvbjerg A., Nielsen C.H., Hansen P.R. Comorbidity of periodontal disease: Two sides of the same coin? An introduction for the clinician. J. Oral Microbiol. 2017;9:1332710. doi: 10.1080/20002297.2017.1332710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rydén L., Buhlin K., Ekstrand E., de Faire U., Gustafsson A., Holmer J., Kjellström B., Lindahl B., Norhammar A., Nygren A., et al. Periodontitis Increases the Risk of a First Myocardial Infarction: A Report from the PAROKRANK Study. Circulation. 2016;133:576–583. doi: 10.1161/CIRCULATIONAHA.115.020324. [DOI] [PubMed] [Google Scholar]
- 40.Carinci F., Martinelli M., Contaldo M., Santoro R., Pezzetti F., Lauritano D., Candotto V., Mucchi D., Palmieri A., Tagliabue A., et al. Focus on periodontal disease and development of endocarditis. J. Biol. Regul. Homeost. Agents. 2018;32:143–147. [PubMed] [Google Scholar]
- 41.Potempa J., Mydel P., Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 2007;13:606–620. doi: 10.1038/nrrheum.2017.132. [DOI] [PubMed] [Google Scholar]
- 42.Pritchard A.B., Crean S., Olsen I., Singhrao S.K. Periodontitis, Microbiomes and their Role in Alzheimer’s Disease. Front. Aging Neurosci. 2017;9:336. doi: 10.3389/fnagi.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singhrao S.K., Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J. Oral Microbiol. 2019;11:1563405. doi: 10.1080/20002297.2018.1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kayaçetin S., Güreşçi S. What is gastritis? What is gastropathy? How is it classified? Turk. J. Gastroenterol. 2014;25:233–247. doi: 10.5152/tjg.2014.7906. [DOI] [PubMed] [Google Scholar]
- 45.Cheng X.J., Lin J.C., Tu S.P. Etiology and Prevention of Gastric Cancer. Gastrointest. Tumors. 2016;3:25–36. doi: 10.1159/000443995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui J., Cui H., Yang M., Du S., Li J., Li Y., Liu L., Zhang X., Li S. Tongue coating microbiome as a potential biomarker for gastritis including precancerous cascade. Protein Cell. 2019;10:496–509. doi: 10.1007/s13238-018-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flynn K.J., Baxter N.T., Schloss P.D. Metabolic and Community Synergy of Oral Bacteria in Colorectal Cancer. mSphere. 2016;1:e00102–e00116. doi: 10.1128/mSphere.00102-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abed J., Maalouf N., Manson A.L., Earl A.M., Parhi L., Emgård J.E.M., Klutstein M., Tayeb S., Almogy G., Atlan K.A., et al. Colon Cancer-Associated Fusobacterium nucleatum May Originate from the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell Infect. Microbiol. 2020;10:400. doi: 10.3389/fcimb.2020.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flemer B., Warren R.D., Barrett M.P., Cisek K., Das A., Jeffery I.B., Hurley E., O’Riordain M., Shanahan F., O’Toole P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2018;67:1454–1463. doi: 10.1136/gutjnl-2017-314814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y., Baba Y., Ishimoto T., Iwatsuki M., Hiyoshi Y., Miyamoto Y., Yoshida N., Wu R., Baba H. Progress in characterizing the linkage between Fusobacterium nucleatum and gastrointestinal cancer. J. Gastroenterol. 2019;54:33–41. doi: 10.1007/s00535-018-1512-9. [DOI] [PubMed] [Google Scholar]
- 51.López R.L., Burgos M.J.G., Gálvez A., Pulido R.P. The human gastrointestinal tract and oral microbiota in inflammatory bowel disease: A state of the science review. APMIS. 2017;125:3–10. doi: 10.1111/apm.12609. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y., Wang X., Li H., Ni C., Du Z., Yan F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018;99:883–893. doi: 10.1016/j.biopha.2018.01.146. [DOI] [PubMed] [Google Scholar]
- 53.Szkaradkiewicz A.K., Karpiński T.M. Microbiology of chronic periodontitis. J. Biol. Earth Sci. 2013;3:M14–M20. [Google Scholar]
- 54.Karpiński T.M. Role of Oral Microbiota in Cancer Development. Microorganisms. 2019;7:20. doi: 10.3390/microorganisms7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitmore S.E., Lamont R.J. Oral bacteria and cancer. PLoS Pathog. 2014;10:e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao L., Jermanus C., Barbetta B., Choi C., Verbeke P., Ojcius D.M., Yilmaz Ö. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 2010;25:89–101. doi: 10.1111/j.2041-1014.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakhjiri S.F., Park Y., Yilmaz O., Chung W.O., Watanabe K., El-Sabaeny A., Park K., Lamont R.J. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol. Lett. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- 58.Landskron G., De la Fuente M., Thuwajit P., Thuwajit C., Hermoso M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abranches J., Zeng L., Kajfasz J.K., Palmer S.R., Chakraborty B., Wen Z.T., Richards V.P., Brady L.J., Lemos J.A. Biology of oral streptococci. Microbiol. Spectr. 2018;6:6. doi: 10.1128/microbiolspec.GPP3-0042-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brauncajs M., Sakowska D., Krzemiński Z. Production of hydrogen peroxide by lactobacilli colonising the human oral cavity. Med. Dośw. Mikrobiol. 2001;53:331–336. [Google Scholar]
- 62.Hussain S.P., Hofseth L.J., Harris C.C. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 63.Piao J.Y., Lee H.G., Kim S.J., Kim D.H., Han H.J., Ngo H.K., Park S.A., Woo J.H., Lee J.S., Na H.K., et al. Helicobacter pylori activates IL-6-STAT3 signaling in human gastric cancer cells: Potential roles for reactive oxygen species. Helicobacter. 2016;21:405–416. doi: 10.1111/hel.12298. [DOI] [PubMed] [Google Scholar]
- 64.Attene-Ramos M.S., Wagner E.D., Plewa M.J., Gaskins H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- 65.Hellmich M.R., Szabo C. Hydrogen sulfide and cancer. Handb. Exp. Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh S.B., Lin H.C. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms. 2015;3:866–889. doi: 10.3390/microorganisms3040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Downes J., Wade W.G. Peptostreptococcus stomatis sp. nov., isolated from the human oral cavity. Int. J. Syst. Evol. Microb. 2006;56:751–754. doi: 10.1099/ijs.0.64041-0. [DOI] [PubMed] [Google Scholar]
- 68.Lunt S.J., Chaudary N., Hill R.P. The tumor microenvironment and metastatic disease. Clin. Exp. Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 69.Mazzio E., Smith B., Soliman K. Evaluation of endogenous acidic metabolic products associated with carbohydrate metabolism in tumor cells. Cell Biol. Toxicol. 2010;26:177–188. doi: 10.1007/s10565-009-9138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pavlova S.I., Jin L., Gasparovich S.R., Tao L. Multiple alcohol dehydrogenases but no functional acetaldehyde dehydrogenase causing excessive acetaldehyde production from ethanol by oral streptococci. Microbiology. 2013;159:1437–1446. doi: 10.1099/mic.0.066258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cordero O.J., Varela-Calviño R. Oral hygiene might prevent cancer. Heliyon. 2018;4:e00879. doi: 10.1016/j.heliyon.2018.e00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salazar C.R., Sun J., Li Y., Francois F., Corby P., Perez-Perez G., Dasanayake A., Pei Z., Chen Y. Association between selected oral pathogens and gastric precancerous lesions. PLoS ONE. 2013;8:e51604. doi: 10.1371/journal.pone.0051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coker O.O., Dai Z., Nie Y., Zhao G., Cao L., Nakatsu G., Wu W.K., Wong S.H., Chen Z., Sung J.J.Y., et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. 2018;67:1024–1032. doi: 10.1136/gutjnl-2017-314281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamamura K., Baba Y., Miyake K., Nakamura K., Shigaki H., Mima K., Kurashige J., Ishimoto T., Iwatsuki M., Sakamoto Y., et al. Fusobacterium nucleatum in gastroenterological cancer: Evaluation of measurement methods using quantitative polymerase chain reaction and a literature review. Oncol. Lett. 2017;14:6373–6378. doi: 10.3892/ol.2017.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsieh Y.Y., Tung S.Y., Pan H.Y., Yen C.W., Xu H.W., Lin Y.J., Deng Y.F., Hsu W.T., Wu C.S., Li C. Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan. Sci. Rep. 2018;8:158. doi: 10.1038/s41598-017-18596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klimesova K., Zakostelska Z.J., Tlaskalova-Hogenova H. Oral Bacterial and Fungal Microbiome Impacts Colorectal Carcinogenesis. Front. Microbiol. 2018;9:774. doi: 10.3389/fmicb.2018.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bandara H.M.H.N., Panduwawala C.P., Samaranayake L.P. Biodiversity of the human oral mycobiome in health and disease. Oral Dis. 2019;25:363–371. doi: 10.1111/odi.12899. [DOI] [PubMed] [Google Scholar]
- 78.Fusco A., Savio V., Cammarota M., Alfano A., Schiraldi C., Donnarumma G. Beta-Defensin-2 and Beta-Defensin-3 Reduce Intestinal Damage Caused by Salmonella typhimurium Modulating the Expression of Cytokines and Enhancing the Probiotic Activity of Enterococcus faecium. J. Immunol. Res. 2017;2017:6976935. doi: 10.1155/2017/6976935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martu I., Goriuc A., Martu M.A., Vata I., Baciu R., Mocanu R., Surdu A.E., Popa C., Luchian I. Identification of Bacteria Involved in Periodontal Disease Using Molecular Biology Techniques. Rev. Chim. 2017;68:2407–2412. doi: 10.37358/RC.17.10.5895. [DOI] [Google Scholar]
- 80.Garlet G.P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 81.Petersen P.E., Ogawa H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontol. 2000. 2012;60:15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 82.Kassebaum N.J., Smith A.G.C., Bernabe E., Fleming T.D., Reynolds A.E., Vos T., Murray C.J.L., Marcenes W. GBD Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017;96:380–387. doi: 10.1177/0022034517693566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loos B.G. Systemic effects of periodontitis. Ann. R. Australas. Coll. Dent. Surg. 2006;18:27–29. doi: 10.1111/j.1601-5037.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 84.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor G.W. Bidirectional interrelationships between diabetes and periodontal diseases: An epidemiologic perspective. Ann Periodontol. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- 86.Zeng X.T., Deng A.P., Li C., Xia L.Y., Niu Y.M., Leng W.D. Periodontal disease and risk of head and neck cancer: A meta-analysis of observational studies. PLoS ONE. 2013;8:e79017. doi: 10.1371/journal.pone.0079017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng X.T., Tu M.L., Liu D.Y., Zheng D., Zhang J., Leng W. Periodontal disease and risk of chronic obstructive pulmonary disease: A meta-analysis of observational studies. PLoS ONE. 2012;7:e46508. doi: 10.1371/journal.pone.0046508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wen X., Liu R., Li G., Deng M., Liu L., Zeng X.T., Nie X. History of periodontitis as a risk factor for long-term survival of dental implants: A meta-analysis. Int. J. Oral Maxillofac. Implants. 2014;29:1271–1280. doi: 10.11607/jomi.3544. [DOI] [PubMed] [Google Scholar]
- 89.Kelly J.T., Avila-Ortiz G., Allareddy V., Johnson G.K., Elangovan S. The association between periodontitis and coronary heart disease: A quality assessment of systematic reviews. J. Am. Dent. Assoc. 2013;144:371–379. doi: 10.14219/jada.archive.2013.0130. [DOI] [PubMed] [Google Scholar]
- 90.Yu H.C., Chen T.P., Wei C.Y., Chang Y.C. Association between Peptic Ulcer Disease and Periodontitis: A Nationwide Population-Based Case-Control Study in Taiwan. Int. J. Environ. Res. Public Health. 2018;15:912. doi: 10.3390/ijerph15050912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brito F., de Barros F.C., Zaltman C., Pugas-Carvalho A.T., de Vasconcellos-Carneiro A.J., Guimarães-Fischer R., Gustafsson A., de Silva-Figueredo C.M. Prevalence of periodontitis and DMFT index in patients with Crohn’s disease and ulcerative colitis. J. Clin. Periodontol. 2008;35:555–560. doi: 10.1111/j.1600-051X.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- 92.Habashneh R.A., Khader Y.S., Alhumouz M.K., Jadallah K., Aylouni Y. The association between inflammatory bowel disease and periodontitis among Jordanians: A case-control study. J. Periodontal. Res. 2012;47:293–298. doi: 10.1111/j.1600-0765.2011.01431.x. [DOI] [PubMed] [Google Scholar]
- 93.Wei X., Zhao H.Q., Ma C., Zhang A.B., Feng H., Zhang D., Liu C. The association between chronic periodontitis and oral Helicobacter pylori: A meta-analysis. PLoS ONE. 2019;14:e0225247. doi: 10.1371/journal.pone.0225247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boylan M.R., Khalili H., Huang E.S., Michaud D.S., Izard J., Joshipura K.J., Chan A.T. A prospective study of periodontal disease and risk of gastric and duodenal ulcer in male health professionals. Clin. Transl. Gastroenterol. 2014;5:e49. doi: 10.1038/ctg.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Byun S.H., Min C., Hong S.J., Choi H.G., Koh D.H. Analysis of the Relation between Periodontitis and Chronic Gastritis/Peptic Ulcer: A Cross-Sectional Study Using KoGES HEXA Data. Int. J. Environ. Res. Public Health. 2020;17:4387. doi: 10.3390/ijerph17124387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Umeda M., Kobayashi H., Takeuchi Y., Hayashi J., Morotome-Hayashi Y., Yano K., Aoki A., Ohkusa T., Ishikawa I. High prevalence of Helicobacter pylori detected by PCR in the oral cavities of periodontitis patients. J. Periodontol. 2003;74:129–134. doi: 10.1902/jop.2003.74.1.129. [DOI] [PubMed] [Google Scholar]
- 97.Franzosa E.A., Morgan X.C., Segata N., Waldron L., Reyes J., Earl A.M., Giannoukos G., Boylan M.R., Ciulla D., Gevers D., et al. Relating the metatranscriptome and metagenome of the human gut. Proc. Natl. Acad. Sci. USA. 2014;111:E2329–E2338. doi: 10.1073/pnas.1319284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andersen R.N., Ganeshkumar N., Kolenbrander P.E. Helicobacter pylori adheres selectively to Fusobacterium spp. Oral Microbiol. Immunol. 1998;13:51–54. doi: 10.1111/j.1399-302X.1998.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 99.Anand P.S., Kamath K.P., Anil S. Role of dental plaque, saliva and periodontal disease in Helicobacter pylori infection. World J. Gastroenterol. 2014;20:5639–5653. doi: 10.3748/wjg.v20.i19.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teoman I., Ozmeriç N., Ozcan G., Alaaddinoğlu E., Dumlu S., Akyön Y., Baloş K. Comparison of different methods to detect Helicobacter pylori in the dental plaque of dyspeptic patients. Clin. Oral Investig. 2007;11:201–205. doi: 10.1007/s00784-007-0104-5. [DOI] [PubMed] [Google Scholar]
- 101.Chua E.G., Chong J.Y., Lamichhane B., Webberley K.M., Marshall B.J., Wise M.J., Tay C.J. Gastric Helicobacter pylori infection perturbs human oral microbiota. PeerJ. 2019;7:e6336. doi: 10.7717/peerj.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Y., Gao X., Guo J., Yu D., Xiao Y., Wang H., Li Y. Helicobacter pylori infection alters gastric and tongue coating microbial communities. Helicobacter. 2019;24:e12567. doi: 10.1111/hel.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]