Abstract
Fat mass (FM) gain and lean mass (LM) loss are common side effects for patients with prostate cancer receiving androgen deprivation therapy (ADT). Excess FM has been associated with an increased risk of developing obesity-related comorbidities, exacerbating prostate cancer progression, and all-cause and cancer-specific mortality. LM is the predominant contributor to resting metabolic rate, with any loss impacting long-term weight management as well as physical function. Therefore, reducing FM and preserving LM may improve patient-reported outcomes, risk of disease progression, and ameliorate comorbidity development. In ADT-treated patients, exercise and nutrition programs can lead to improvements in quality of life and physical function; however, effects on body composition have been variable. The aim of this review was to provide a descriptive overview and critical appraisal of exercise and nutrition-based interventions in prostate cancer patients on ADT and their effect on FM and LM. Our findings are that FM gain and LM loss are side effects of ADT that could be reduced, prevented, or even reversed with the implementation of a combined exercise and nutrition program. However, the most effective combination of specific exercise and nutrition prescriptions are yet to be determined, and thus should be a focus for future studies.
Keywords: androgen deprivation therapy, prostate cancer, exercise, nutrition, fat mass, lean mass
1. Introduction
Androgen deprivation therapy (ADT) is a mainstay treatment for prostate cancer (PCa), where more than half of patients will receive ADT at some point during their cancer journey [1]. ADT is a pharmaceutical or surgical strategy that deprives the body of androgens, thereby slowing cancer growth [2]. This may be achieved by either reducing testosterone concentrations to castrate levels defined as <50 ng/dL (<1.7 nmol/L) using luteinizing hormone-releasing hormone agonists, antagonists or an orchiectomy procedure, or by blocking the androgen receptors to eliminate testosterone binding using anti-androgens [2]. Given that testosterone plays roles in the activation of lipolysis and hypertrophy of lean mass (LM) [3,4], substantial body composition changes, as well as loss of muscle strength and physical function, can occur [5,6]. Within the first 9 months of treatment initiation, patients have been reported to experience a 13.8% increase in fat mass (FM) and a 2.4% decrease in LM [5]. This change in body composition places patients with PCa at increased risk of obesity-related comorbidities, treatment-related side effects, development of a more aggressive cancer, and PCa-specific mortality [7,8,9,10].
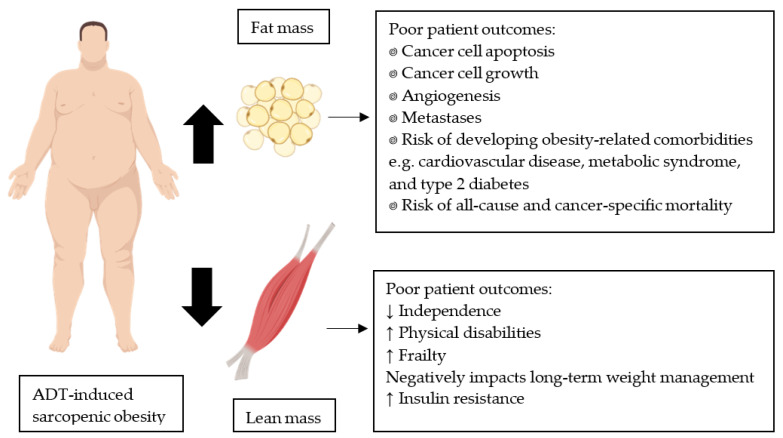
Excess FM upregulates pro-inflammatory cytokines, leading to a state of low-grade chronic inflammation, which is associated with decreased cancer cell apoptosis, increased cancer cell growth, angiogenesis, and metastases, and increased risk of developing cardiovascular disease and type 2 diabetes (Figure 1) [7,11,12]. Post-diagnosis obese prostate cancer patients with non-metastatic disease are more likely to experience cardiovascular disease-related mortality than non-obese patients (hazard ratio of 1.24) [13]. In addition, PCa patients on ADT with greater FM may experience higher fatigue, lower vitality, and higher blood triglyceride concentrations [14,15]. A loss of LM also contributes to poorer patient outcomes [14]. The development of sarcopenic obesity, a progressive loss of LM and gain in FM, has been associated with multiple physical disabilities (Figure 1) [16,17]. Lean mass is also the predominant contributor to resting metabolic rate. Therefore, preserving or increasing LM is important for long-term weight loss maintenance [18]. Promoting LM gain can also increase glucose storage, facilitate glucose clearance from circulation, and reduce the amount of insulin required to maintain normal glucose tolerance [19], which is important as insulin resistance may exacerbate cancer progression [20]. Owing to the association between FM gain or LM loss and worse patient outcomes, strategies to prevent or reverse this process are important to include as adjuvant therapies while on ADT, particularly for those who are obese [9].
Figure 1.
Prostate cancer patients receiving ADT can develop sarcopenic obesity due to a treatment-induced increase in fat mass and decrease in lean mass. These respective body composition changes can lead to poor patient outcomes. Images created with BioRender.com (accessed on 20 November 2020).
Exercise and nutrition interventions are effective strategies to reduce FM and increase LM in non-cancer populations [21]. Researchers conducting clinical studies in the PCa population have reported that exercise interventions result in improved quality of life and reduced ADT-related side effects such as cancer-related fatigue and poorer physical function [22]. Nutrition interventions have been demonstrated to induce weight loss, improve bone health, and in some instances slow PCa progression, although evidence is limited [23,24,25]. Despite these beneficial outcomes, the variety of intervention designs, aims, cohorts, and outcomes, presents variable evidence as to whether exercise and nutrition interventions have a desirable effect on FM and LM for patients undergoing ADT. When examining body composition in ADT-treated patients, exercise has been the preferential intervention utilised. As such, there is a lack of clarity concerning the feasibility and efficacy of combined exercise and nutrition programs and the effect on FM loss, while simultaneously seeking to preserve or enhance LM. Therefore, this review is a descriptive overview and critical appraisal of exercise and nutrition-based interventions in ADT-treated PCa patients and the effect on FM and LM, and to propose possible avenues for further research.
MEDLINE and Scopus databases were searched with published studies included until November 2020. Search terms included various combinations of: prostate cancer; androgen deprivation therapy; exercise; nutrition; body composition; fat mass; lean mass. Secondary searches involved reference lists of eligible articles as well as systematic reviews and meta-analyses assessing interventions given to patients on ADT. The key criterion was to identify studies that included PCa patients receiving ADT at time of intervention, utilising an exercise, nutrition or combined intervention, while including a measure of FM and/or LM.
2. Using Exercise to Decrease Fat Mass and Preserve or Gain Lean Mass
2.1. Aerobic Exercise
Aerobic exercise is an ideal intervention for FM loss as it is familiar to non-exercisers, e.g., walking, easy to implement at home with little to no equipment, promotes higher utilisation of lipids, and includes modes allowing reduced impact on joints, e.g., swimming [26,27]. The aerobic exercise guidelines for prostate cancer patients recommended within clinical practice suggest an accumulation of 150 min/week of moderate-to-vigorous intensity or 300 min/week if weight loss is intended (Table 1) [28]. In this section, we evaluate six studies examining aerobic-based interventions and the effect on FM and LM.
Table 1.
Current prostate cancer-specific exercise and nutrition guidelines, including weight loss guidelines.
| Current Exercise and Nutrition Guidelines | Current Weight Loss Guidelines | |
|---|---|---|
 Aerobic training |
150 min/week of moderate intensity exercise or 75 min/week of vigorous intensity exercise | 300 min/week of moderate intensity exercise or 150 min/week of vigorous intensity exercise |
 Resistance training |
Minimum two strength training sessions/week | |
 Nutritional intake |
Healthy balanced diet with high fruit and vegetables, low saturated fats, and adequate calcium (<1200 mg/d) and vitamin D (>600 IU) | 2100–4200 kJ daily energy deficit |
Images created with BioRender.com (accessed on 5 April 2021).
Hvid et al. [29] compared healthy aged-matched controls with normal testosterone concentrations (10–28 nmol/L), and ADT-treated PCa patients with castrate levels of testosterone (<1.7 nmol/L) completing the same 12 week aerobic-based cycling intervention utilising high-intensity interval training (Table 2). Both groups significantly lost whole-body, trunk, visceral, and subcutaneous FM, while preserving LM, with no between-group differences. The castrate levels of testosterone in ADT-treated patients, therefore, does not appear to inhibit FM loss via high-intensity aerobic exercise. However, the healthy controls exhibited a superior loss of intermuscular FM (−8.5% vs. 0%). The presence of substantial intermuscular FM could interfere with muscle fibre quality and contribute to insulin resistance, reduction in muscle strength, and increased fatigue [27,30,31], although there was no between-group difference for insulin sensitivity; muscle strength and fatigue were not measured. However, this study contained a small sample size and did not include a PCa control group. Therefore, it is unclear whether the intervention prevented further ADT-induced increases in intermuscular FM and if this in turn affects muscle fibre quality. Furthermore, the groups had baseline cardiorespiratory fitness levels of 27.2 mL/kg/min and 25.2 mL/kg/min, respectively, and prostate cancer patients staged T1 a/b to T3 a/b. Therefore, the use of high-intensity aerobic-based exercise is uncertain for patients with poor cardiorespiratory fitness or more advanced disease.
Table 2.
Exercise-only interventions assessing fat and lean mass in prostate cancer patients receiving ADT.
| Study | Study Design | Primary Outcome | Intervention | Body Composition Assessment | Groups (N) | Outcome Variable | Mean Pre-Intervention Values (kg) | Mean Post-Intervention Values (kg) |
|---|---|---|---|---|---|---|---|---|
| Aerobic-based interventions | ||||||||
| Alberga et al. [32] | RCT | Body composition and fitness | 24 weeks 3 ×/week Supervised aerobic exercise at 50–75% HRmax or Supervised resistance exercise at 60–70% 1 RM |
DXA | Aerobic (N = 40) | |||
| ADT | BF% | 31.2% | 33.3% * | |||||
| Lean mass | 65.0 | 63.0 * | ||||||
| No ADT | BF% | 29.9% | 30.5% | |||||
| Lean mass | 66.2 | 65.7 | ||||||
| Resistance (N = 40) | ||||||||
| ADT | BF% | 32.6% | 33.0% §UC | |||||
| Lean mass | 63.7 | 63.4 §UC | ||||||
| No ADT | BF% | 29.7% | 29.2% | |||||
| Lean mass | 66.7 | 67.3 | ||||||
| Usual care (N = 41) | ||||||||
| ADT | BF% | 32.0% | 35.2% §R * | |||||
| Lean mass | 64.2 | 61.1 §R * | ||||||
| No ADT | BF% | 31.2 | 30.6 | |||||
| Lean mass | 65.0 | 65.6 | ||||||
| Hvid et al. [29] | Prospective cohort | Insulin sensitivity and body composition | 12 weeks 3 ×/week 135 min/week Aerobic interval exercise 50–100% VO2max |
DXA and MRI | Prostate cancer exercise (N = 9) | Fat mass | 24.4 | 23.1 # |
| Trunk fat | 14.5 | 13.4 # | ||||||
| Lean mass | 52.3 | 52.3 | ||||||
| BF% | 31.1% | 29.8% # | ||||||
| Visceral a | −8.4% # | |||||||
| Subcutaneous a | −4.9% # | |||||||
| Intermuscular a | 0% § # | |||||||
| Non-cancer exercise (N = 10) |
Fat mass | 20.5 | 19.6 # | |||||
| Trunk fat | 12.4 | 11.8 # | ||||||
| Lean mass | 56.3 | 56.2 | ||||||
| BF% | 25.7% | 25.0% # | ||||||
| Visceral a | −5.8% # | |||||||
| Subcutaneous a | −2.5% # | |||||||
| Intermuscular a | −8.5% # | |||||||
| Santa Mina et al. [33] | RCT | Quality of life | 6 months 3–5 ×/week 90–300 min/week Home-based resistance band/ball/body weight exercise 12–15 RPE Or Home-based aerobic exercise at 60–80% HRmax |
Skinfolds | Aerobic (N = 22) | Chest skinfold | 35.6 mm | 33.5 mm *3 |
| BF% | 28.5% | 27.3% *3 | ||||||
| Resistance (N = 34) | Chest skinfold | 35.3 mm | 33.7 mm | |||||
| BF% | 28.0% | 27.3% | ||||||
| Santa Mina et al. [34] | RCT | Blood biomarkers | See Santa Mina et al. [33] | Skinfolds | Aerobic (N = 13) | BF% | 28.4% | 26.4% |
| Resistance (N = 13) | BF% | 26.5% | 25.3% | |||||
| Uth et al. [35] | RCT | Lean mass | 12 weeks 2 ×/week (1–8 weeks) 3 ×/week (9–12 weeks) 90–180 min/week Supervised football training |
DXA | Football (N= 29) | Fat mass | 27.6 | 26.3 |
| Lean mass | 53.1 | 54.0 § * | ||||||
| BF% | 32.6% | 31.7% | ||||||
| Usual care (N = 28) | Fat mass | 30.0 | 29.7 | |||||
| Lean mass | 56.7 | 56.8 | ||||||
| BF% | 32.9% | 32.9% | ||||||
| Newton et al. [36] | RCT | Bone mineral density | 12 months 2 ×/week Supervised impact exercise at ground reaction force of 3–5 × body weight Resistance exercise 6–12 RM, 2–4 sets 2 ×/week Home-based impact exercise Or 6 months 2 ×/week 150 min/week Supervised aerobic at 65–85% HRmax Resistance exercise 6–12 RM, 2–4 sets Home-based aerobic exercise 6 months Home-based aerobic Resistance (body weight/band) exercise Or 6 months waiting period 6 months 2 ×/week 80 min/week Aerobic exercise at 70% HRmax |
DXA | Resistance/impact (N = 57) |
Fat mass | 24.0 | 25.1 |
| Lean mass | 57.9 | 59.3 | ||||||
| ASM | 25.0 | 25.9 §6DEL | ||||||
| Aerobic/resistance (N = 50) |
Fat mass | 22.8 | 23.7 | |||||
| Lean mass | 58.1 | 58.7 | ||||||
| ASM | 25.2 | 25.6 | ||||||
| Delay/aerobic (N = 47) | Fat mass | 27.1 | 28.3 | |||||
| Lean mass | 59.3 | 60.4 | ||||||
| ASM | 25.3 | 25.9 | ||||||
| Resistance-based interventions | ||||||||
| Galvão et al. [37] | Prospective cohort | Muscle function | 20 weeks 2 ×/week 120 min/week Supervised resistance exercise 6–12 RM, 2–4 sets |
DXA | Resistance (N = 10) | Fat mass | 25.7 | 24.9 |
| Lean mass | 52.2 | 52.0 | ||||||
| BF% | 30.7% | 30.6% | ||||||
| Quadriceps thickness | 2.15 cm | 2.46 cm * | ||||||
| Hamstring thickness | 4.52 cm | 1.53 cm | ||||||
| Biceps thickness | 2.69 cm | 2.91 cm | ||||||
| Triceps thickness | 1.94 cm | 2.33 cm | ||||||
| Alberga et al. [32] | Details in aerobic section | |||||||
| Santa Mina et al. [33] | Details in aerobic section | |||||||
| Santa Mina et al. [34] | Details in aerobic section | |||||||
| Hanson et al. [38] | Prospective cohort | Muscle size and function | 12 weeks 3 ×/week 180 min/week Supervised high-intensity resistance exercise 15 repetitions, first 5 at 5 RM |
DXA and CT | Resistance (N = 17) | Fat mass | 31.2 | 31.1 |
| Subcutaneous | 118 cm2 | 118 cm2 | ||||||
| Intermuscular | 7.9 cm2 | 7.6 cm2 | ||||||
| Lean mass | 62.4 | 64.1 * | ||||||
| BF% | 31.4% | 30.7% * | ||||||
| Nilsen et al. [39] | RCT | Lean mass | 16 weeks 3 ×/week Supervised resistance exercise 6–10 RM, 1–3 sets |
DXA | Resistance (N = 28) | Fat mass | 26.5 | 26.4 |
| Trunk fat | 14.7 | 14.6 | ||||||
| Lean mass | 59.8 | 60.3 | ||||||
| ASM | 25.2 | 25.7 § | ||||||
| BF% | 29.5% | 29.3% | ||||||
| Control (N = 30) | Fat mass | 26.4 | 26.7 | |||||
| Trunk fat | 14.6 | 14.7 | ||||||
| Lean mass | 57.9 | 57.9 | ||||||
| ASM | 24.8 | 24.7 | ||||||
| BF% | 30.0% | 30.2% | ||||||
| Multi-modal interventions | ||||||||
| Galvão et al. [40] | RCT | Lean mass | 12 weeks 2 ×/week Supervised aerobic at 65–80% HRmax Resistance exercise 6–12 RM, 2–4 sets |
DXA | Exercise (N = 29) | Fat mass | 22.5 | 22.3 |
| Trunk fat | 12.2 | 11.9 | ||||||
| Lean mass | 56.1 | 56.8 § | ||||||
| ASM | 23.5 | 24.0 § | ||||||
| BF% | 27.5% | 27.2% | ||||||
| Usual care (N = 28) | Fat mass | 23.2 | 23.5 | |||||
| Trunk fat | 12.4 | 12.2 | ||||||
| Lean mass | 57.8 | 57.8 | ||||||
| ASM | 24.6 | 24.4 | ||||||
| BF% | 27.3% | 27.5% | ||||||
| Galvão et al. [15] | RCT | Various ADT side effects | See Galvão et al. [40] | DXA | Acute ADT (N = 16) | Fat mass | 22.7 | 23.3 § * |
| Trunk fat | 12.2 | 12.4 | ||||||
| Lean mass | 58.5 | 59.1 | ||||||
| ASM | 24.7 | 25.2 | ||||||
| BF% | 26.8% | 27.2% § | ||||||
| Chronic ADT (N = 34) b |
Fat mass | 23.4 | 23.0 * | |||||
| Trunk fat | 12.1 | 11.8 * | ||||||
| Lean mass | 56.5 | 57.4 * | ||||||
| ASM | 23.8 | 24.4 * | ||||||
| BF% | 28.1% | 27.4% * | ||||||
| Cormie et al. [41] | RCT | Lean mass | 12 weeks 2 ×/week 150 min/week Supervised aerobic at 70–85% HRmax Resistance exercise at 60–85% 1 RM Home-based exercise of choice |
DXA | Exercise (N = 32) | Fat mass | 26.9 | 26.3 § |
| Trunk fat | 14.8 | 14.3 § | ||||||
| Visceral fat | 913 g | 874 g * | ||||||
| Lean mass | 56.6 | 56.0 | ||||||
| ASM | 23.7 | 23.5 § | ||||||
| BF% | 30.6% | 30.5% § | ||||||
| Usual care (N = 31) | Fat mass | 26.9 | 27.8 * | |||||
| Trunk fat | 15.2 | 15.5 | ||||||
| Visceral fat | 926 g | 922 g | ||||||
| Lean mass | 58.7 | 57.3 * | ||||||
| ASM | 24.9 | 24.3 * | ||||||
| BF% | 30.3% | 31.4% * | ||||||
| Winters-Stone et al. [42] | RCT | Body composition | 12 months 2 ×/week 165 min/week Supervised resistance at 60–80% 1 RM Impact exercise 1 ×/week Home-based exercise of choice |
DXA | Exercise (N = 29) | Fat mass | 24.3 | 23.9 § |
| Trunk fat | 13.5 | 13.1 | ||||||
| Lean mass | 59.2 | 59.2 | ||||||
| BF% | 28.7% | 28.4% | ||||||
| Flexibility (N = 22) | Fat mass | 28.4 | 29.9 | |||||
| Trunk fat | 15.0 | 15.4 | ||||||
| Lean mass | 57.5 | 57.2 | ||||||
| BF% | 31.6% | 32.4% | ||||||
| Wall et al. [43] | RCT | Cardiorespiratory fitness | 6 months 2 ×/week 150 min/week Supervised aerobic at 70–90% HRmax Resistance exercise 6–12 RM, 1–4 sets 1 ×/week Home-based aerobic exercise |
DXA | Exercise (N = 50) | Fat mass | 24.1 | 24.5 § |
| Trunk fat | 13.2 | 13.0 § | ||||||
| Lean mass | 59.4 | 60.1 § | ||||||
| BF% | 27.2% | 27.2% § | ||||||
| Usual care (N = 47) | Fat mass | 25.7 | 27.2 | |||||
| Trunk fat | 14.2 | 14.9 | ||||||
| Lean mass | 58.7 | 58.6 | ||||||
| BF% | 28.2% | 30.3% | ||||||
| Newton et al. [36] | Details in aerobic section | |||||||
| Ndjavera et al. [44] | RCT | Fat mass | 12 weeks 2 ×/week Supervised aerobic at 55–85% HRmax Resistance exercise 10 RM, 2–4 sets Home-based aerobic exercise |
BIA | Exercise (N = 24) | Fat mass | 24.3 | 21.7 |
| Fat-free mass | 58.2 | 58.9 | ||||||
| Usual care (N = 26) | Fat mass | 23.3 | 22.7 | |||||
| Fat-free mass | 59.1 | 58.2 | ||||||
* = Significant within group change; § = significant between-group change; §UC = significant between-group change with usual care control group; §R = significant between-group change with resistance training group; # = effect of time in the two groups pooled together; §6DEL = significantly different to delayed/aerobic group at 6 months only, not 12 months which is the value reported in the table; *3 = significant loss at 3 months only, but not 6 months which is the value reported in the table. a Only reported mean change; b Acute ADT < 6 months, chronic ADT ≥ 6 months. RCT = randomised controlled trial; ×/week = times per week; HRmax = maximum heart rate; RM = repetition maximum; DXA = dual x-ray absorptiometry; ADT = androgen deprivation therapy; BF% = body fat percent; VO2max = oxygen consumption; MRI = magnetic resonance imaging; RPE = rate of perceived exertion; CT = computed tomography; ASM = appendicular skeletal muscle; BIA = bioimpedance analysis.
Uth et al. [35] utilised an unstructured form of interval-based aerobic training, in the form of football (soccer) game play and skill development (Table 2). Unlike Hvid et al. [29], they recruited patients with bone metastases (19.3%), but similarly assessed an apparently healthy prostate cancer cohort with only 5.3% of patients self-reporting a sedentary lifestyle, with baseline cardiorespiratory fitness of 27.2 and 26.4 mL/kg/min, and mean body mass index of 26.7 and 27.6 kg/m2, respectively. They reported a mean 0.5 kg significant increase in LM and a mean 0.6 kg loss of FM that approached within-group significance. With the improvement in LM and a trend for an effect on FM, sport-orientated activities may be an effective alternative to clinic-based interventions in ameliorating treatment-related body composition changes. Several adverse events were reported in the football group including fracture, tendon tear, and sprain. While no injury was related to bone metastases and most participants recovered and continued with the study, there is uncertainty whether such an intervention would be feasible for high-risk patients, e.g., obese patients with multiple comorbidities. Injury risk is higher within a team sport environment, compared to individual sport or exercise, due to the unpredictable nature of opponents, teammates, and ball. The authors suggested a lead-in period may be required to improve strength, balance, and ball handling to reduce injury risks [35].
In contrast to the previous studies using interval training [29,35], Newton et al. [36] and Alberga et al. [32] utilised clinic-based continuous aerobic exercise (Table 2). Examining a cohort that excluded patients with bone metastases, Newton et al. [36] used a three-arm study design over 12 months comparing impact and resistance exercise, aerobic and resistance exercise, and delayed aerobic exercise after 6 months of usual care. When compared to the aerobic-only exercise group during the 6–12 month period, no differences in FM or LM were noted between groups. Alberga et al. [32] also utilised a three-arm study design comparing aerobic exercise, resistance exercise, and usual care across a 24-week period, in ADT and non-ADT groups, although the two treatment types were not compared. The ADT aerobic group exhibited an undesirable significant increase in body fat percentage (BF%) and 2 kg reduction in LM, although not statistically different to the other ADT groups. The researchers did not report FM, so it is unclear whether a change in FM, in addition to the LM loss, contributed to the modification in BF%. The decline in LM is substantial and concerning, suggesting the prescribed aerobic exercise was insufficient to prevent ADT-related declines in LM, in contrast to a non-significant 0.5 kg loss in LM in the non-ADT aerobic group.
The previously described studies were supervised interventions [29,32,35,36]. However, ongoing supervision is not always viable. Santa Mina et al. [33,34] compared home-based aerobic and resistance exercise over 6 months examining patients with non-metastatic disease. Santa Mina et al. [34] used a smaller non-randomised group of the same cohort to report on blood biomarkers (Table 2). There were significant within-group declines in chest skinfold thickness and BF% at 3 months, but not 6 months [33] and weight change was positively associated with changes in leptin and the leptin:adiponectin ratio, and negatively associated with IGF-1:IGFBP-3 ratio [34], which are proposed markers associated with PCa progression [45]. Although the use of anthropometric measures suggest weight loss may improve risk of cancer progression, the researchers could not confirm if these changes were subject to alterations in FM or LM. Nonetheless, both studies provide valuable insight into the potential of home-based programs, although there is still uncertainty if those with metastatic disease would benefit from a similar program.
2.2. Resistance Exercise
Weight loss can occur through loss of both fat and muscle tissue [46]; however, substantial loss of LM may exacerbate sarcopenia, reduce physical function, and increase risk of falls [47]. Resistance exercise is commonly prescribed for muscle hypertrophy [48]. Within clinical practice prostate cancer patients are recommended to complete resistance training on a minimum of two days each week (Table 1) [28]. This section is an evaluation of six studies examining resistance exercise and the effect on FM and LM.
Galvão et al. [37] and Hanson et al. [38] conducted single-group studies and both excluded patients with metastatic disease (Table 2). Galvão et al. [37] prescribed a traditional periodised resistance training program over 20 weeks and found no change in FM or LM except for a significant increase in quadriceps thickness. In contrast, Hanson et al. [38] utilised drop sets and repetitions to failure over a 12-week program. The exercise set began at five repetition maximum and once volitional fatigue was reached the resistance was reduced until 15 repetitions were achieved. A significant decrease in BF% and increase in LM were reported. The differing results may be explained by the period between the two studies and cohort examined. At the time of the Galvão et al. [37] study, the use of resistance training for PCa patients was somewhat revolutionary and a conservative exercise prescription was implemented with only 10 patients recruited. The Hanson et al. [38] study was completed over a decade later in a cohort of 17 patients of African American ethnicity with higher intensity and sophistication of resistance training design. While these studies demonstrate the feasibility of resistance training in promoting changes to LM, both studies utilised small or non-diverse cohorts, so the generalisability of these results is unclear.
Nilsen et al. [39] examined a 16-week clinic-based high-load periodised resistance training program in which the intervention group significantly improved appendicular skeletal muscle (ASM). However, no changes were found for whole-body LM or FM or for any body composition measure when compared to the usual care controls (Table 2). High-risk patients with medical conditions that could complicate participation were excluded from this study, although cancer stage of included patients was not reported. Nevertheless, three patients withdrew from the intervention group due to pain. Further research is required into the appropriateness of high-load resistance training for high-risk patients and may require a gradual increase in intensity. Furthermore, while the recruitment goal was met in this study, the authors reported to be uncertain whether the effect size selected to calculate sample size was appropriate to detect a change in LM.
Resistance and aerobic exercise are both recommended in the PCa survivorship guidelines [28]. Therefore, it is important to understand how patients respond to each exercise mode. Alberga et al. [32] and Santa Mina et al. [33,34] compared aerobic and resistance exercise (Table 2). Alberga et al. [32] utilised clinic-based periodised resistance training conducted over 24 weeks and reported preservation of BF% and LM, which was significantly different to usual care controls who gained BF% and lost LM. The 2 kg LM loss in the aerobic group although not statistically different to the 0.3 kg loss in the resistance group, is of clinical relevance and highlights the importance of resistance training in preserving LM. Santa Mina et al. [33,34] examined home-based resistance exercise utilising bands, balls, and body weight exercises, and reported no training effect [33,34]. From this work, it appears that resistance training alone is insufficient to induce FM loss. However, it may prevent further ADT-induced body composition changes and specifically alleviate loss of LM.
2.3. Multi-Modal Interventions
The inclusion of multiple exercise modes is important when the intention is to alter both FM and LM. In this section, we evaluate seven studies utilising multi-modal interventions and the effect on FM and LM.
Several authors examined similar cohorts without bone metastases and compared combined aerobic and resistance exercise interventions to usual care controls (Table 2). Galvão et al. [40] reported significant between-group differences in whole-body LM and ASM, but no change in FM over 12 weeks. Cormie et al. [41] found significant between-group differences for whole-body and trunk FM, BF%, and ASM over the 12-week intervention. The intervention group demonstrated a significant within-group loss of visceral FM, while the control group significantly lost LM, ASM, and gained whole-body FM and BF%. Wall et al. [43] reported significant between-group differences for whole-body FM and LM, trunk FM, and BF% but conducted a longer intervention of six months. Ndjavera et al. [44] reported no body composition changes over their 12-week intervention. Cormie et al. [41], Wall et al. [43], and Ndjavera et al. [44] reported greater adjusted group mean differences for FM (−1.4, −1.1, and −1.9 kg, respectively) than Galvão et al. [40] (−0.01 kg), which could be explained by the larger volume of aerobic exercise prescribed in these studies.
Galvão et al. [15] was a secondary analysis of the previously described Galvão et al. [40] study and they compared different durations of ADT: chronic ≥ 6 months, and acute < 6 months, completing the same intervention (Table 2). The authors reported a significant between-group difference in FM with those on chronic ADT experiencing a 0.4 kg loss compared to a 0.6 kg gain in the acute ADT group over 12 weeks. Furthermore, triglyceride concentrations were significantly different between groups, which was associated with the observed changes in FM. Despite these significant findings it resulted in an uneven distribution between acute (n = 16) and chronic (n = 34) ADT-treated patients due to the use of a delayed exercise control group. The smaller number in the acute group may have limited the ability to observe differences between groups. Regardless, it is important to note that body composition declines are greater during the initial 3–6 months of ADT commencement and appear more difficult to ameliorate with exercise therapy.
Aerobic and resistance-based exercise are the most commonly prescribed modes; however, both Newton et al. [36] and Winters-Stone et al. [42] examined the combined effect of impact training, e.g., bounding movements, and resistance training (Table 2). Newton et al. [36] reported that the combined impact/resistance group significantly improved ASM compared to the usual care controls at 6 months. However, no effect on ASM was noted after the same resistance training was undertaken by the aerobic/resistance group. The authors described a potential interference effect when combining aerobic and resistance training within the same session, which may have compromised muscle hypertrophy [49]. Winters-Stone et al. [42] reported that FM was significantly decreased in the impact/resistance group compared to a flexibility control group who continued to gain FM. Additionally, in line with the Santa Mina et al. [34] findings, Winters-Stone et al. [42] reported that the changes in FM mediated differences in insulin, suggesting FM loss induced an insulin-lowering effect.
3. Using Nutrition to Decrease Fat Mass and Preserve or Gain Lean Mass
3.1. Healthy Eating Guidelines and/or Energy Deficit
Healthy eating guidelines are recommended portions of each food group to be consumed daily [50]. Weight loss in its simplest form is achieved through greater energy expenditure over intake creating a daily energy deficit (Figure 2) [51]. Clinical practice guidelines recommend prostate cancer patients to consume a healthy balanced diet high in fruit and vegetables, low in saturated fat, and consume adequate amounts of vitamin D (>600 IU) and calcium (<1200 mg/d), with an energy deficit if weight loss is required (Table 1) [28]. In this section, we review six studies in which healthy eating guidelines and/or an energy deficit were implemented and the effect on FM and LM evaluated.
Figure 2.
Weight loss occurs when energy expenditure is greater than energy intake.
Gilbert et al. [52] and Focht et al. [53] prescribed combined aerobic and resistance-based exercise and conducted small group healthy eating seminars over a 12-week period (Table 3). Gilbert et al. [52] reported a significant difference in LM but no change in FM compared to usual care controls. However, the intervention group reduced their mean FM from 34.5 to 31.6 kg compared to 30.4 to 29.0 kg in the control group. Although the 2.9 kg FM loss for the intervention group is potentially clinically meaningful, no within-group changes were reported. Focht et al. [53] additionally included group-mediated behaviour modification seminars based on social cognitive theory. Compared to usual care controls, the intervention group significantly lost FM and BF%, with no change in LM. Although the exercise and nutrition sessions were well adhered to, only a small subset of the patients provided 3 day weighed food records and, therefore, overall nutritional intake and compliance to nutrition advice were not confirmed. Further, 80% of patients in the intervention group were overweight or obese and prescribed an energy deficit diet. Therefore, the contribution of healthy eating guidelines versus an energy deficit diet to promote FM and LM changes is unclear.
Table 3.
Studies incorporating a nutrition component and assessed fat and lean mass in prostate cancer patients receiving ADT.
| Study | Study Design | Primary Outcome | Intervention | Body Composition Assessment | Groups (N) | Outcome Variable | Mean Pre-Intervention Values (kg) | Mean Post-Intervention Values (kg) |
|---|---|---|---|---|---|---|---|---|
| Healthy eating guidelines and/or energy deficit | ||||||||
| O’Neill et al. [54] | RCT | Fat mass | 6 months ≥5 ×/week 150 min/week Home-based brisk walking UK healthy eating guidelines + energy deficit diet if overweight. |
Skinfolds | Intervention (N = 47) | Fat mass | 28.8 | 26.9 § |
| Lean mass | 58.3 | 59.8 | ||||||
| BF% | 32.6% | 30.8% § | ||||||
| Control (N = 47) | Fat mass | 29.5 | 30.1 | |||||
| Lean mass | 59.8 | 59.1 | ||||||
| BF% | 32.4% | 32.8% | ||||||
| Gilbert et al. [52] | RCT | Brachial artery flow mediated dilatation | 12 weeks 180 min/week 2 ×/week (1–6 weeks) 1 ×/week (7–12 weeks) Supervised aerobic at 55–75% HRmax + resistance exercise at 60% 1 RM 1 ×/week (1–6 weeks) 2 ×/week (7–12 weeks) Home-based exercise of choice Fortnightly healthy eating seminars |
BIA | Intervention (N = 25) | Fat mass | 34.5 | 31.6 |
| Skeletal muscle mass | 31.9 | 32.9 § | ||||||
| Usual care (N = 25) | Fat mass | 30.4 | 29.6 | |||||
| Skeletal muscle mass | 31.2 | 32.3 | ||||||
| Focht et al. [53] | RCT | Mobility | 12 weeks 150 min/week 2 ×/week (1–6 weeks) 1 ×/week (7–8 weeks) Supervised aerobic 3–4 RPE (1–10 scale) + resistance 8–12 RM, 3 sets 1 ×/week (7–8 weeks) 2 ×/week (9–12 weeks) Unsupervised aerobic + resistance Home-based exercise of choice Nutrition counselling sessions—8 as a group and 2 individual phone calls + energy deficit diet if overweight. |
Bod Pod | Intervention (N = 16) | Fat mass b | −1.8 § | |
| Fat-free mass b | −0.06 | |||||||
| BF% b | −1.05% § | |||||||
| Usual care (N = 16) | Fat mass b | 0.9 | ||||||
| Fat-free mass b | −0.5 | |||||||
| BF% b | 0.82% | |||||||
| Freedland et al. [55] | RCT | Insulin resistance | 6 months ≥5 d/week 150 min/week Home-based walking Carbohydrate intake ≤ 20 g/day |
DXA | Intervention (N = 11) | Fat mass | 32.3 | 24.0 § |
| Lean mass | 61.0 | 58.9 § | ||||||
| BF% | 28.3% | 26.6% § | ||||||
| Control (N = 18) | Fat mass | 25.3 | 28.3 | |||||
| Lean mass | 55.9 | 55.4 | ||||||
| BF% | 30.5% | 32.3% | ||||||
| Baguley et al. [56] | RCT | Cancer-related fatigue and quality of life | 12 weeks Individualised consultation with dietician every 2 weeks Mediterranean-style diet |
DXA | Intervention (N = 12) | Fat mass | 29.5 | 27.8 * |
| Lean mass | 53.2 | 52.0 | ||||||
| Usual care (N = 11) | Fat mass | 29.8 | 29.3 | |||||
| Lean mass | 53.4 | 53.4 | ||||||
| Wilson et al. [57] | Prospective cohort | Fat mass | 12 weeks 3 ×/week 300 min/week Supervised resistance exercise at 6–12 RM, 2–4 sets Daily home-based aerobic exercise, RPE 3–8 (1–10 scale) 3 nutrition counselling sessions Calorie deficit diet 40 g protein powder after each supervised exercise session |
DXA | Intervention (N = 14) | Fat mass | 39.8 | 37.0 * |
| Trunk fat | 20.1 | 18.3 * | ||||||
| Visceral fat | 954 g | 866 g * | ||||||
| Lean mass | 55.9 | 55.9 | ||||||
| ASM | 23.3 | 23.3 | ||||||
| BF% | 40.0% | 38.3% * | ||||||
| Protein intake | ||||||||
| Dawson et al. [58] | RCT | Lean mass | 12 weeks 3 ×/week 150 min/week Supervised resistance exercise at 60–83% 1 RM 2 × 25 g protein powder per day |
DXA | Exercise (N = 8) + Exercise/protein (N = 8) |
Fat mass | 30.3 | 31.2 |
| Lean mass | 48.5 | 53.2 § | ||||||
| Fat-free mass | 54.6 | 56.4 § | ||||||
| ASM | 23.5 | 24.8 § | ||||||
| BF% | 36.8% | 35.9% § | ||||||
| Protein (N = 10) + Flexibility control (N = 11) a |
Fat mass | 25.6 | 26.2 | |||||
| Lean mass | 51.5 | 48.6 | ||||||
| Fat-free mass | 51.4 | 51.5 | ||||||
| ASM | 21.5 | 21.6 | ||||||
| BF% | 33.9% | 34.5% | ||||||
* = Significant within group change; § = significant between-group change. a Patients were randomised to 4 groups: exercise, protein and exercise, protein, usual care control; however, for the analysis the two exercising groups and two non-exercising groups were combined as protein had no effect; b only reported mean change. RCT = randomised controlled trial; ×/week = times per week; RM = repetition maximum; DXA = dual x-ray absorptiometry; RPE = rate of perceived exertion; BF% = body fat percent; HRmax = maximum heart rate; BIA = bioimpedance analysis; UK = United Kingdom.
O’Neill et al. [54] prescribed a 6-month home-based walking program, with a dietary booklet encouraging healthy eating habits to patients of all cancer stages (T1–4), although metastatic status was not reported (Table 3). The authors reported a significant reduction in FM and BF%, with no change in LM when compared to usual care controls. While they showed that a home-based intervention can reduce FM, body composition was measured using the less precise technique of skinfold measurement. Similarly, to Focht et al. [53], O’Neill et al. [54] encouraged an energy deficit diet only for patients who were overweight or obese.
Freedland et al. [55] and Wilson et al. [57] targeted overweight or obese patients who did not have symptomatic or bone metastases, respectively (Table 3). Freedland et al. [55] prescribed home-based walking and a low carbohydrate diet over 6 months. Compared to an 11% increase in FM for the usual care controls, the intervention group significantly lost 16.2%. This substantial loss in FM has not been previously achieved in PCa patients on ADT. However, the intervention group also had a significant decline in LM compared to controls. A loss in LM is not uncommon while undergoing weight loss [46], with similar patterns also noted by Baguley et al. [56] in their 12-week nutrition-only intervention (Table 3). Wilson et al. [57] also demonstrated a significant reduction in FM but in contrast, achieved LM preservation. Wilson et al. [57] included supervised resistance training and protein supplementation, which are both considered important for LM preservation [59]. While the intervention designs are different, these studies provide preliminary evidence on the potential for effective FM and LM management for obese ADT-treated PCa patients through diet and exercise, which includes resistance training.
3.2. Protein Intake
The optimisation of protein intake is often incorporated into weight loss nutrition plans to assist the body to mobilise fat and preserve muscle tissue by supporting the upregulation of muscle protein synthesis [59]. Next, we describe a study examining protein supplementation and resistance exercise.
Dawson et al. [58] examined four groups of patients with PCa, including those with metastatic disease (54.3%), over a 12-week period: exercise-only, exercise and protein supplement, protein supplement-only, and usual care control (Table 3). No additional effect was found for protein supplementation and as the study was not powered to detect changes using a four-armed design, results were reported for exercise versus non-exercise groups. In the exercise groups there was a significant increase in LM, ASM, and fat-free mass, a significant reduction in BF%, with no changes in FM. The lack of a synergistic effect of protein supplementation could be attributed to the low adherence of the protein-only group who consumed 1.0 g/kg/day compared to 1.1–1.4 g/kg/dayin the other three groups. Further, the protein supplements were given as 2 × 25 g daily doses. This may not have been sufficient to stimulate muscle protein synthesis as each dose was equivalent to ~0.3 g protein/kg body weight/day, compared to the ~0.4 g protein/kg body weight/daywhich has been shown to be effective in increasing muscle protein synthesis when combined with an acute bout of resistance exercise in ADT-treated PCa patients [59].
4. Discussion
The field of exercise oncology has rapidly developed over the last two decades and we have presented 22 exercise and nutrition interventions conducted in ADT-treated PCa patients between 2006 and 2020. Despite this growth in awareness of the benefits that can be derived from undertaking these practices, most of the studies report only modest changes in FM and LM. In this discussion, we summarise the key conclusions from these studies and propose future research directions to progress the field.
The American Cancer Society weight loss guidelines for PCa patients are no different to that of the general population (Table 1) [28]. Notably, Wilson et al. [57] was the only study to incorporate these guidelines, which are recommended in clinical practice but have not been verified in the ADT-treated population. Although these guidelines have the potential to provide successful body composition changes, the metabolic changes induced by ADT likely require different strategies to induce change compared to the non-ADT population, as alluded to by the results of Alberga et al. [32], although the ADT and non-ADT cohorts were not compared. In this regard, we provide an important initial platform to help identify how these guidelines may be tailored to suit hypogonadal men. Potential questions that would lead to further understanding of how to tailor these weight loss guidelines for ADT-treated patients to maximise FM and LM changes are presented in Table 4.
Table 4.
Potential questions for future research relating to the prescription of exercise and nutrition for prostate cancer patients receiving ADT aiming to lose fat mass and gain lean mass.
| Unanswered Questions for Prostate Cancer Patients on ADT Aiming to Induce Fat Loss and Muscle Gain. | |
|---|---|
 Aerobic training |
1. Will a low-intensity lead-in period designed to build baseline fitness reduce injury risk and improve adherence, particularly for high-risk patients? |
| 2. Is there a minimum intensity/volume for lipolysis and muscle protein synthesis stimulation? | |
 Resistance training |
1. Will a low-intensity familiarisation period designed to build baseline strength reduce injury risk and improve adherence, particularly for high-risk patients? |
| 2. Is there a minimum intensity/volume for muscle protein synthesis stimulation? | |
 Nutritional intake |
1. Who is an energy deficit or healthy eating guideline diet most appropriate for? |
| 2. What is the optimum protein intake to enhance muscle protein synthesis leading to muscle gain? | |
| Other questions inclusive of all elements | 1. Are the benefits gained from a combined exercise and nutrition intervention influenced by length of time on ADT? |
| 2. What is a clinically significant change in fat and lean mass for prostate cancer patients on ADT? |
Images created with BioRender.com (accessed on 5 April 2021).
With body composition changes occurring early in the treatment process [60], it would be preferable to implement an exercise and nutrition intervention at initiation of ADT. However, the magnitude of intervention-induced body composition changes could depend on length of time on ADT, as demonstrated by Galvão et al. [15], where those initiating ADT may experience small or no intervention-induced changes compared to those on chronic ADT. Similarly, Hvid et al. [29] highlighted a patient on ADT for <6 months who did not respond to the exercise intervention and gained 2.6 kg of FM accompanied by a loss in LM of 5.0 kg. Ndjavera et al. [44] also reported no training effect on body composition within the first 3 months of ADT. However, each of these studies were exercise only and it has been established that manipulation of nutrition substantially decreases FM more than exercise alone [61]. Therefore, those initiating ADT may only experience substantial FM loss when nutrition is also addressed, as was demonstrated by Freedland et al. [55]. Regardless of the influence of length of time on ADT on body composition changes, exercise and nutrition should still be recommended from therapy onset as there will be additional health benefits and likely prevention of substantial FM and LM changes, as demonstrated by Cormie et al. [41].
Studies utilising a multi-modal intervention compared to a single-exercise mode showed more consistent beneficial responses in both FM and LM. However, the majority of the multi-modal studies were conducted by the same research group [15,36,40,41,43,57] and, therefore, may not represent the wider PCa population. Capitalising on the unique benefits gained from utilising multiple exercise modes can induce concurrent desired adaptations of FM and LM. However, there is uncertainty of best practice regarding exercise prescription to induce concurrent FM loss and LM preservation or gain. While high-intensity [29,35,38] and high-volume [54,55,57] exercise resulted in the greatest changes in FM or LM, they may not initially be suitable for obese patients who have multiple comorbidities without undergoing a lead-in phase to improve baseline fitness. Moreover, the impact of such interventions on patients with metastatic disease is unclear with only two studies actively recruiting patients of this disease stage [35,58]. Further research is required into the benefits of high-intensity or interval-based interventions, such as high-intensity interval training or team/individual sports, for ADT-treated PCa patients. There may also be a minimum-intensity threshold that stimulates lipolysis and muscle protein synthesis, as demonstrated by Alberga et al. [32], where patients undertaking aerobic exercise continued to gain BF% and lose LM. Furthermore, the use of multiple modes within the same session, as noted by Newton et al. [36], may have an interference effect where physiological pathways involved in manipulating body composition are not stimulated compared to when a single-exercise mode is undertaken.
While bone measurements are not reported in the current review, it is important to highlight that in addition to FM gain and LM loss patients receiving ADT may also experience a loss of bone mass placing them at increased risk of osteopenia or osteoporosis [5]. Newton et al. [36] assessed bone health as their primary outcome and reported preliminary efficacy for the inclusion of impact training in a multi-modal intervention to prevent ADT-induced bone loss. Patients at increased risk of bone loss may also benefit from increased calcium and vitamin D intake, which are included as part of the exercise and nutrition guidelines for prostate cancer patients [28].
The number of interventions measuring body composition that encompassed a nutrition component were less common than those investigating exercise. The employment of an energy deficit was effective at reducing FM as shown in both the O’Neill et al. [54] and Freedland et al. [55] studies. However, preventing LM loss when the body enters a catabolic state requires further clarity. Protein optimisation and the inclusion of resistance training may be important components to promote LM preservation or gain when undergoing weight loss as suggested by Wilson et al. [57] and Dawson et al. [58]. However, as protein supplementation is currently understudied in this population, it is not included in the PCa weight loss guidelines and needs further evaluation. Continued research into optimal diet and exercise prescriptions for prostate cancer patients may further improve the benefits of weight loss and the potential impact on a patient’s prognosis with particular interest in diet and exercise modes that influence microbiome activity. Differences in composition of the gut microbiome have been reported in men with prostate cancer compared to men with benign prostatic conditions and could contribute to prostate cancer pathogenesis and progression [62].
As noted by Nilsen et al. [39], the definition of a clinically significant change in FM and LM needs to be established. A 5% loss of body weight, which should be predominantly FM loss [63], has been shown in the non-cancer population to improve blood pressure, cholesterol, and insulin resistance [64]. While this percentage is also used for cancer patients, the significance is unknown. For example, increases in trunk, visceral, and intermuscular FM are associated with increased insulin resistance, a potential mechanism for the observed association between FM and PCa progression [65,66]. Therefore, a loss of FM in these regional areas, independent of whole-body FM loss, may be more beneficial for PCa patients on ADT than a 5% loss in total body mass [29,63]. Further, it is unknown whether a loss in FM will improve a PCa patient’s risk of disease progression, treatment-related side effects, or comorbidity development. Both Santa Mina et al. [34] and Winters-Stone et al. [42] demonstrated that weight or FM loss was associated with improvements in biomarkers related to cancer progression, which has also been demonstrated in non-ADT PCa patients [67]. Moreover, Galvão et al. [15] reported that a decrease in FM was associated with decreased serum triglyceride levels. These studies provide preliminary evidence that FM loss could improve patient outcomes.
5. Conclusions
Fat mass gain and LM loss are side effects of ADT that might be prevented or reversed with the implementation of an exercise and nutrition intervention. Patients on ADT, particularly those who are obese, require effective strategies to improve their body composition, which in turn may improve general health and cancer-free survival. The implementation of such strategies will be most successful through the effective communication of a multi-disciplinary team including, but not limited to, oncologists, urologists, dietitians, and exercise physiologists. The inclusion of a multi-modal exercise program is needed to stimulate both lipolysis and muscle protein synthesis to ensure FM loss and LM preservation. While exercise should be tailored to the preferences and fitness level of the patient, when FM loss is the objective, energy expenditure should be maximised, which is best achieved through higher volume and intensity with the inclusion of an energy deficit diet. The optimal macronutrient composition of a diet for PCa patients on ADT is unclear but should ultimately follow healthy eating guidelines and optimise protein intake.
Acknowledgments
R.L.W. is supported by a scholarship from Edith Cowan University.
Author Contributions
R.L.W. was responsible for designing the narrative review protocol, conducting the search, screening potentially eligible studies for discussion, interpreting results, and writing the manuscript. D.R.T., R.U.N., N.H.H., P.L.-W. and D.A.G. contributed to the conception and design of the work and provided study selection and manuscript feedback as well as approval of the final version. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Edith Cowan University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rhee H., Gunter J.H., Heathcote P., Ho K., Stricker P., Corcoran N.M., Nelson C.C. Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int. 2014;115:3–13. doi: 10.1111/bju.12964. [DOI] [PubMed] [Google Scholar]
- 2.Labrie F., Dupont A., Bélanger A., St-Arnaud R., Giguère M., Lacourcière Y., Emond J., Monfette G. Treatment of prostate cancer with gonadotropin-releasing hormone agonists. Endocr. Rev. 1986;7:67–74. doi: 10.1210/edrv-7-1-67. [DOI] [PubMed] [Google Scholar]
- 3.Herbst K.L., Bhasin S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Saad F., Aversa A., Isidori A.M., Gooren L.J. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: A review. Curr. Diabetes Rev. 2012;8:131–143. doi: 10.2174/157339912799424573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galvão D.A., Spry N.A., Taaffe D.R., Newton R.U., Stanley J., A Shannon T., Rowling C., Prince R.L. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102:44–47. doi: 10.1111/j.1464-410X.2008.07539.x. [DOI] [PubMed] [Google Scholar]
- 6.Galvao D., Taaffe D., Spry N., Joseph D., Turner D., Newton R. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: A comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2008;12:198–203. doi: 10.1038/pcan.2008.51. [DOI] [PubMed] [Google Scholar]
- 7.Keating N.L., O’Malley A.J., Freedland S.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J. Natl. Cancer Inst. 2009;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y., Ma J. Body Mass Index, Prostate Cancer–Specific Mortality, and Biochemical Recurrence: A Systematic Review and Meta-analysis. Cancer Prev. Res. 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshu C.E., Mondul A.M., Menke A., Meinhold C., Han M., Humphreys E.B., Freedland S.J., Walsh P.C., Platz E.A. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the psa era. Cancer Prev. Res. 2011;4:544–551. doi: 10.1158/1940-6207.CAPR-10-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Cancer Research Fund. American Institute for Cancer Research Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Prostate Cancer. [(accessed on 20 May 2020)]; Available online: https://www.wcrf.org/dietandcancer/prostate-cancer.
- 11.Rhee H., Vela I., Chung E. Metabolic syndrome and prostate cancer: A review of complex interplay amongst various endocrine factors in the pathophysiology and progression of prostate cancer. Horm. Cancer. 2015;7:75–83. doi: 10.1007/s12672-015-0238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng T., Lyon C.J., Bergin S., Caligiuri M.A., Hsueh W.A. Obesity, inflammation, and cancer. Annu. Rev. Pathol. Mech. Dis. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 13.Troeschel A.N., Hartman T.J., Jacobs E.J., Stevens V.L., Gansler T., Flanders W.D., McCullough L.E., Wang Y. Postdiagnosis body mass index, weight change, and mortality from prostate cancer, cardiovascular disease, and all causes among survivors of nonmetastatic prostate cancer. J. Clin. Oncol. 2020;38:2018–2027. doi: 10.1200/JCO.19.02185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton R.U., Jeffery E., Galvão D.A., Peddle-McIntyre C.J., Spry N., Joseph D., Denham J.W., Taaffe D.R., Denham J. Body composition, fatigue and exercise in patients with prostate cancer undergoing androgen-deprivation therapy. BJU Int. 2018;122:986–993. doi: 10.1111/bju.14384. [DOI] [PubMed] [Google Scholar]
- 15.Galvão D.A., Taaffe D.R., Spry N., Joseph D., Newton R.U. Acute versus chronic exposure to androgen suppression for prostate cancer: Impact on the exercise response. J. Urol. 2011;186:1291–1297. doi: 10.1016/j.juro.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner R.N. Body composition in healthy aging. Ann. N. Y. Acad. Sci. 2006;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.-P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hills A.P., Emokhtar N., Byrne N.M. Assessment of physical activity and energy expenditure: An overview of objective measures. Front. Nutr. 2014;1:5. doi: 10.3389/fnut.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieli-Conwright C.M., Courneya K.S., Demark-Wahnefried W., Sami N., Lee K., Buchanan T.A., Spicer D.V., Tripathy D., Bernstein L., Mortimer J.E. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: A randomized controlled trial. J. Clin. Oncol. 2018;36:875–883. doi: 10.1200/JCO.2017.75.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnard R.J., Ngo T.H., Leung P.-S., Aronson W.J., Golding L.A. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate. 2003;56:201–206. doi: 10.1002/pros.10251. [DOI] [PubMed] [Google Scholar]
- 21.Christiansen T., Paulsen S.K., Bruun J.M., Pedersen S.B., Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: A 12-week randomized intervention study. Am. J. Physiol. Metab. 2010;298:E824–E831. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]
- 22.Bourke L., Smith D., Steed L., Hooper R., Carter A., Catto J., Albertsen P.C., Tombal B., Payne H.A., Rosario D.J. Exercise for men with prostate cancer: A systematic review and meta-analysis. Eur. Urol. 2016;69:693–703. doi: 10.1016/j.eururo.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Tsang D.S., Alibhai S.M.H. Bone health care for patients with prostate cancer receiving androgen deprivation therapy. Hosp. Pract. 2014;42:89–102. doi: 10.3810/hp.2014.04.1107. [DOI] [PubMed] [Google Scholar]
- 24.Hackshaw-McGeagh L.E., Perry R.E., Leach V.A., Qandil S., Jeffreys M., Martin R.M., Lane J.A. A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control. 2015;26:1521–1550. doi: 10.1007/s10552-015-0659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes K.A., Ball L.E., Galvao D.A., Newton R.U., Chambers S.K. Nutrition care guidelines for men with prostate cancer undergoing androgen deprivation therapy: Do we have enough evidence? Prostate Cancer Prostatic Dis. 2019;22:221–234. doi: 10.1038/s41391-018-0099-9. [DOI] [PubMed] [Google Scholar]
- 26.Falcone P.H., Tai C.-Y., Carson L.R., Joy J.M., Mosman M.M., McCann T.R., Crona K.P., Kim M.P., Moon J.R. Caloric expenditure of aerobic, resistance, or combined high-intensity interval training using a hydraulic resistance system in healthy men. J. Strength Cond. Res. 2015;29:779–785. doi: 10.1519/JSC.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 27.Collins K.H., Herzog W., Macdonald G.Z., Reimer R.A., Rios J.L., Smith I.C., Zernicke R.F., Hart D.A. Obesity, metabolic syndrome, and musculoskeletal disease: Common inflammatory pathways suggest a central role for loss of muscle integrity. Front. Physiol. 2018;9:112. doi: 10.3389/fphys.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skolarus T.A., Wolf A.M., Erb N.L., Brooks D.D., Rivers B.M., Underwood W., Salner A.L., Zelefsky M.J., Aragon-Ching J.B., Slovin S.F., et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J. Clin. 2014;64:225–249. doi: 10.3322/caac.21234. [DOI] [PubMed] [Google Scholar]
- 29.Hvid T., Winding K., Rinnov A., Dejgaard T., Thomsen C., Iversen P., Brasso K., Mikines K.J., Van Hall G., Lindegaard B., et al. Endurance training improves insulin sensitivity and body composition in prostate cancer patients treated with androgen deprivation therapy. Endocr. Relat. Cancer. 2013;20:621–632. doi: 10.1530/ERC-12-0393. [DOI] [PubMed] [Google Scholar]
- 30.Cheung A.S., Hoermann R., Dupuis P., Joon D.L., Zajac J.D., Grossmann M. Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Eur. J. Endocrinol. 2016;175:229–237. doi: 10.1530/EJE-16-0200. [DOI] [PubMed] [Google Scholar]
- 31.Chang D., Joseph D.J., Ebert M.A., Galvão D.A., Taaffe D.R., Denham J.W., Newton R.U., Spry N.A. Effect of androgen deprivation therapy on muscle attenuation in men with prostate cancer. J. Med. Imaging Radiat. Oncol. 2013;58:223–228. doi: 10.1111/1754-9485.12124. [DOI] [PubMed] [Google Scholar]
- 32.Alberga A.S., Segal R.J., Reid R.D., Scott C.G., Sigal R.J., Khandwala F., Jaffey J., Wells G.A., Kenny G.P. Age and androgen-deprivation therapy on exercise outcomes in men with prostate cancer. Support. Care Cancer. 2012;20:971–981. doi: 10.1007/s00520-011-1169-x. [DOI] [PubMed] [Google Scholar]
- 33.Mina D.S., Alibhai S.M., Matthew A.G., Guglietti C.L., Pirbaglou M., Trachtenberg J., Ritvo P., Alibhai S.M. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. J. Aging Phys. Act. 2013;21:455–478. doi: 10.1123/japa.21.4.455. [DOI] [PubMed] [Google Scholar]
- 34.Mina D.S., Connor M.K., Alibhai S.M., Toren P., Guglietti C., Matthew A.G., Trachtenberg J., Ritvo P. Exercise effects on adipokines and the IGF axis in men with prostate cancer treated with androgen deprivation: A randomized study. Can. Urol. Assoc. J. 2013;7:692–698. doi: 10.5489/cuaj.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uth J., Hornstrup T., Schmidt J.F., Christensen J.F., Frandsen C., Christensen K.B., Helge E.W., Brasso K., Rørth M., Midtgaard J., et al. Football training improves lean body mass in men with prostate cancer undergoing androgen deprivation therapy. Scand. J. Med. Sci. Sports. 2014;24(Suppl. S1):105–112. doi: 10.1111/sms.12260. [DOI] [PubMed] [Google Scholar]
- 36.Newton R.U., Galvão D.A., Spry N., Joseph D., Chambers S.K., Gardiner R.A., Wall B.A., Bolam K.A., Taaffe D.R. Exercise mode specificity for preserving spine and hip BMD in prostate cancer patients. Med. Sci. Sports Exerc. 2018;51:607–614. doi: 10.1249/MSS.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 37.Galvão D.A., Nosaka K., Taaffe D.R., Spry N., Kristjanson L.J., Mcguigan M.R., Suzuki K., Yamaya K., Newton R.U. Resistance training and reduction of treatment side effects in prostate cancer patients. Med. Sci. Sports Exerc. 2006;38:2045–2052. doi: 10.1249/01.mss.0000233803.48691.8b. [DOI] [PubMed] [Google Scholar]
- 38.Hanson E.D., Sheaff A.K., Sood S., Ma L., Francis J.D., Goldberg A.P., Hurley B.F. Strength training induces muscle hypertrophy and functional gains in black prostate cancer patients despite androgen deprivation therapy. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2012;68:490–498. doi: 10.1093/gerona/gls206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsen T.S., Raastad T., Skovlund E., Courneya K.S., Langberg C.W., Lilleby W., Fosså S.D., Thorsen L. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015;54:1805–1813. doi: 10.3109/0284186X.2015.1037008. [DOI] [PubMed] [Google Scholar]
- 40.Galvão D.A., Taaffe D.R., Spry N., Joseph D., Newton R.U. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J. Clin. Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 41.Cormie P., Galvão D.A., Spry N., Joseph D., Chee R., Taaffe D.R., Chambers S.K., Newton R.U. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2015;115:256–266. doi: 10.1111/bju.12646. [DOI] [PubMed] [Google Scholar]
- 42.Winters-Stone K.M., Dieckmann N.F., Maddalozzo G.F., Bennett J.A., Ryan C.W., Beer T.M. Resistance exercise reduces body fat and insulin during androgen-deprivation therapy for prostate cancer. Oncol. Nurs. Forum. 2015;42:348–356. doi: 10.1188/15.ONF.348-356. [DOI] [PubMed] [Google Scholar]
- 43.Wall B., Galvao D., Fatehee N., Taaffe D., Spry N., Joseph D., Hebert J., Newton R. Exercise improves VO2max and body composition in ADT-treated prostate cancer patients. Med. Sci. Sports Exerc. 2017;49:333–334. doi: 10.1249/01.mss.0000517784.17673.03. [DOI] [PubMed] [Google Scholar]
- 44.Ndjavera W., Orange S.T., O’Doherty A.F., Leicht A.S., Rochester M., Mills R., Saxton J.M. Exercise-induced attenuation of treatment side-effects in patients with newly diagnosed prostate cancer beginning androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2020;125:28–37. doi: 10.1111/bju.14922. [DOI] [PubMed] [Google Scholar]
- 45.Allott E.H., Masko E.M., Freedland S.J. Obesity and prostate cancer: Weighing the evidence. Eur. Urol. 2013;63:800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinheimer E.M., Sands L.P., Campbell W.W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 2010;68:375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 47.Batsis J.A., Gill L.E., Bs R.K.M., Adachi-Mejia A.M., Blunt H.B., Bagley P.J., Lopez-Jimenez F., Bartels S.J. Weight loss interventions in older adults with obesity: A systematic review of randomized controlled trials since 2005. J. Am. Geriatr. Soc. 2017;65:257–268. doi: 10.1111/jgs.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam T., Birzniece V., McLean M., Gurney H., Hayden A., Cheema B.S. The adverse effects of androgen deprivation therapy in prostate cancer and the benefits and potential anti-oncogenic mechanisms of progressive resistance training. Sports Med. Open. 2020;6:13–14. doi: 10.1186/s40798-020-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson J.M., Marin P.J., Rhea M.R., Wilson S.M., Loenneke J.P., Anderson J.C. Concurrent training. J. Strength Cond. Res. 2012;26:2293–2307. doi: 10.1519/JSC.0b013e31823a3e2d. [DOI] [PubMed] [Google Scholar]
- 50.National Health Medical Research Council . Australian Dietary Guidelines. National Health and Medical Research Council; Canberra, Australia: 2013. [Google Scholar]
- 51.Gibson A.L., Wagner D., Heyward V. Advanced Fitness Assessment and Exercise Prescription. 8th ed. Human Kinetics; Champaign, IL, USA: 2018. [Google Scholar]
- 52.Gilbert S.E., Tew G.A., Fairhurst C., Bourke L., Saxton J.M., Winter E.M., Rosario D.J. Effects of a lifestyle intervention on endothelial function in men on long-term androgen deprivation therapy for prostate cancer. Br. J. Cancer. 2016;114:401–408. doi: 10.1038/bjc.2015.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Focht B.C., Lucas A.R., Grainger E., Simpson C., Fairman C.M., Thomas-Ahner J.M., Buell J., Monk J.P., Mortazavi A., Clinton S.K. Effects of a group-mediated exercise and dietary intervention in the treatment of prostate cancer patients undergoing androgen deprivation therapy: Results from the IDEA-P trial. Ann. Behav. Med. 2018;52:412–428. doi: 10.1093/abm/kax002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Neill R.F., Haseen F., Murray L.J., O’Sullivan J.M., Cantwell M.M. A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. J. Cancer Surviv. 2015;9:431–440. doi: 10.1007/s11764-014-0417-8. [DOI] [PubMed] [Google Scholar]
- 55.Freedland S.J., Howard L., Allen J., Smith J., Stout J., Aronson W., Inman B.A., Armstrong A.J., George D., Westman E., et al. A lifestyle intervention of weight loss via a low-carbohydrate diet plus walking to reduce metabolic disturbances caused by androgen deprivation therapy among prostate cancer patients: Carbohydrate and prostate study 1 (CAPS1) randomized controlled trial. Prostate Cancer Prostatic Dis. 2019;22:428–437. doi: 10.1038/s41391-019-0126-5. [DOI] [PubMed] [Google Scholar]
- 56.Baguley B.J., Skinner T.L., Jenkins D.G., Wright O.R. Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: A pilot randomised control trial. Clin. Nutr. 2021;40:245–254. doi: 10.1016/j.clnu.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 57.Wilson R.L., Newton R.U., Taaffe D.R., Hart N.H., Lyons-Wall P., Galvão D.A. Weight loss for obese prostate cancer patients on androgen deprivation therapy. Med. Sci. Sports Exerc. 2020;53:470–478. doi: 10.1249/MSS.0000000000002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dawson J.K., Dorff T.B., Schroeder E.T., Lane C.J., Gross M.E., Dieli-Conwright C.M. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: A pilot randomized controlled trial. BMC Cancer. 2018;18:368. doi: 10.1186/s12885-018-4306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanson E.D., Nelson A.R., West D.W.D., Violet J.A., O’Keefe L., Phillips S.M., Hayes A. Attenuation of resting but not load-mediated protein synthesis in prostate cancer patients on androgen deprivation. J. Clin. Endocrinol. Metab. 2017;102:1076–1083. doi: 10.1210/jc.2016-3383. [DOI] [PubMed] [Google Scholar]
- 60.Smith J.C., Bennett S., Evans L.M., Kynaston H.G., Parmar M., Mason M.D., Cockcroft J.R., Scanlon M.F., Davies J.S. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J. Clin. Endocrinol. Metab. 2001;86:4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 61.Mohamad H., McNeill G., Haseen F., N’Dow J., Craig L.C.A., Heys S.D. The effect of dietary and exercise interventions on body weight in prostate cancer patients: A systematic review. Nutr. Cancer. 2015;67:43–60. doi: 10.1080/01635581.2015.976313. [DOI] [PubMed] [Google Scholar]
- 62.Golombos D.M., Ayangbesan A., O’Malley P., Lewicki P., Barlow L., Barbieri C.E., Chan C., DuLong C., Abu-Ali G., Huttenhower C., et al. The role of gut microbiome in the pathogenesis of prostate cancer: A prospective, pilot study. Urology. 2018;111:122–128. doi: 10.1016/j.urology.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 63.Verheggen R.J.H.M., Maessen M.F.H., Green D.J., Hermus A.R.M.M., Hopman M.T.E., Thijssen D.H.T. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: Distinct effects on body weight and visceral adipose tissue. Obes. Rev. 2016;17:664–690. doi: 10.1111/obr.12406. [DOI] [PubMed] [Google Scholar]
- 64.Alamuddin N., Bakizada Z., Wadden T.A. Management of obesity. J. Clin. Oncol. 2016;34:4295–4305. doi: 10.1200/JCO.2016.66.8806. [DOI] [PubMed] [Google Scholar]
- 65.Ferro M., Terracciano D., Buonerba C., Lucarelli G., Bottero D., Perdonà S., Autorino R., Serino A., Cantiello F., Damiano R., et al. The emerging role of obesity, diet and lipid metabolism in prostate cancer. Futur. Oncol. 2017;13:285–293. doi: 10.2217/fon-2016-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foulkes S.J., Daly R.M., Fraser S.F. The clinical importance of quantifying body fat distribution during androgen deprivation therapy for prostate cancer. Endocr. Relat. Cancer. 2017;24:R35–R48. doi: 10.1530/ERC-16-0505. [DOI] [PubMed] [Google Scholar]
- 67.Henning S.M., Galet C., Gollapudi K., Byrd J.B., Liang P., Li Z., Grogan T., Elashoff D., Magyar C.E., Said J., et al. Phase II prospective randomized trial of weight loss prior to radical prostatectomy. Prostate Cancer Prostatic Dis. 2017;21:212–220. doi: 10.1038/s41391-017-0001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.