Abstract
BACKGROUND: During early life, dynamic gut colonization and brain development co-occur with potential cross-talk mechanisms affecting behaviour. METHODS: We used 16S rRNA gene sequencing to examine the associations between gut microbiota and neurodevelopmental outcomes assessed by the Bayley Scales of Infant Development III in 71 full-term healthy infants at 18 months of age. We hypothesized that children would differ in gut microbial diversity, enterotypes obtained by Dirichlet multinomial mixture analysis and specific taxa based on their behavioural characteristics. RESULTS: In children dichotomized by behavioural trait performance in above- and below-median groups, weighted Unifrac b-diversity exhibited significant differences in fine motor (FM) activity. Dirichlet multinomial mixture modelling identified two enterotypes strongly associated with FM outcomes. When controlling for maternal pre-gestational BMI and breastfeeding for up to 3 months, the examination of signature taxa in FM groups showed that Turicibacter and Parabacteroides were highly abundant in the below-median FM group, while Collinsella, Coprococcus, Enterococcus, Fusobacterium, Holdemanella, Propionibacterium, Roseburia, Veillonella, an unassigned genus within Veillonellaceae and, interestingly, probiotic Bifidobacterium and Lactobacillus were more abundant in the above-median FM group. CONCLUSIONS: Our results suggest an association between enterotypes and specific genera with FM activity and may represent an opportunity for probiotic interventions relevant to treatment for motor disorders.
Keywords: microbiota, neurodevelopment, gut–brain axis, fine motricity, probiotics
1. Introduction
In an infant, two concurrent biological processes take place during the first years of life—the foundational period of gut microbial colonization and the formation and refinement of neural networks responsible for a vast repertoire of behaviours characteristic of early life [1,2,3]. The assembly of the gut microbiota starts from birth, characterized by a rapid rate of colonization and expansion of gut bacteria dominated by Actinobacteria and Proteobacteria shifting towards a community dominated by Firmicutes and Bacteroidetes, a maturation process that coincides in time with the intense synaptogenesis and pruning in the cerebral cortex, ending in adolescence [4,5]. Experiments in animal models have shown that these two processes are physiologically connected. Experimental manipulations that alter the intestinal microbiota impact anxiety- and depression-related behaviours [5,6,7], and cognitive effects have also been reported [8,9]. Early gut establishment appears to be of critical importance because bacterial colonization of germ-free mice does not normalize anxiety phenotypes at 10 weeks of age [10], but does normalize anxiety phenotypes when the microbiota is introduced earlier in life, at 3 weeks of age [11]. Behavioural changes are accompanied by changes in neurochemistry, gene expression, dendritic morphology, and subcortical brain volumes [7,11,12,13,14,15,16]. Germ-free mice also display an increased rate of turnover of noradrenaline, dopamine, and 5-HT in the brain [7], possibly responsible for their well-documented increased motor activity, since these neurotransmitters have roles in increasing blood flow to muscle and central motor control, respectively.
In infants, this mechanistic relationship has not been empirically demonstrated but several associations between gut microbiota and behaviour have been reported. Altered microbial composition of the gut has been reported in children with autism [17,18,19], and it is also linked to childhood temperament at 18–27 months of age using the Early Childhood Behavior Questionnaire [20], to cognition at 2 years of age using the Mullen Scales of Early Learning [21], and to communication, motor, personal and social skills at 3 years of age using the Ages and Stages Questionnaire, Third Edition [22]. In this study, we examined whether the gut microbiota was associated with infant neurodevelopment in 71 full-term healthy infants aged 18 months. We used 16S rRNA gene sequencing for the analysis of the infants’ gut microbiota. The global neurodevelopment was assessed with the Bayley Scales of Infant and Toddler Development®, Third Edition (Bayley®-III) [23], the most common assessment tool used for global neurodevelopment before 2 years of age, that explores mental, motor and socioemotional development. We hypothesized that underlying community types or enterotypes defined by Dirichlet multinomial mixture modelling and taxa assessed by differential abundance analysis would vary between infants according to neurodevelopmental characteristics.
2. Materials and Methods
2.1. Subjects, Experimental Design and Ethical Guidelines
In the present study, 71 full-term healthy infants aged 18 months, who did not present any intestinal disorders and had not taken antibiotics, were chosen from the panel of infants that belonged to PREOBE observational study cohort [24]. In this period of life, the transition from weaning to solid food consumption occurs. General characteristics of the study population are shown in Table S1. In this project, pregnant women were recruited between 2007 and 2012 at the San Cecilio and Mother-Infant University Hospitals in Granada, Spain. The study exclusion criteria for mothers were: simultaneous participation in any other research study, any kind of drug treatment, diagnosed diseases (e.g., hypertension or preeclampsia, intrauterine growth retardation, maternal infection, hypo/hyper- thyroidism, hepatic or renal disease), and vegan diet. Fresh stools were collected at 18 months after delivery and were immediately stored at −80 °C until processing. The study included anthropometric measurements, health questionnaires and medical assessments of the child. This project followed the ethical standards recognized by the Declaration of Helsinki (reviewed in Hong-Kong 1989 and in Edinburgh 2000) and the EEC Good Clinical Practice recommendations (document 111/3976/88 1990), and current Spanish legislation regulating clinical research in humans (Royal Decree 561/1993). The study was explained to the participants before starting, and the parents signed an informed consent form for themselves and provided written consent on behalf of their children.
2.2. DNA Extraction from Stool Samples
Genomic DNA was extracted from faecal bacteria of 18-month-old (n = 71) infants as previously described [25]. Briefly, faecal samples were resuspended in 1 mL of TN150 buffer (10 mM Tris-HCl pH 8.0 and 150 mM NaCl). Zirconium glass beads (0.3 g) and 150 mL of buffered phenol were added, and bacteria were disrupted with a mini bead beater set to 5000 rpm at 4 °C for 15 s (Biospec Products, Bartlesville, OK, USA). After centrifugation, genomic DNA was purified from the supernatant using phenol-chloroform extraction. Quality was checked by agarose gel electrophoresis and quantified with Quant-iT PicoGreen dsDNA assay kit (Invitrogen, Darmstadt, Germany).
2.3. 16S rRNA Gene Sequencing and Data Processing
Genomic DNA from faecal bacteria was used as a template for 16S rRNA gene amplification using 27F and 338R universal primers and two consecutive PCR reactions to integrate Illumina multiplexing sequences as previously described [26]. The library was prepared by pooling equimolar ratios of amplicons and was sequenced using an Illumina MiSeq platform (Genetic Service, University of Granada, Granada, Spain). Reads were demultiplexed and sorted, and paired ends were matched to give 240 nt reads. The dataset was filtered and OTUs were defined at 99% similarity with MOTHUR programs unique.seqs and pre.cluster [27]. Taxonomic classifications of OTUs were assigned using the naïve Bayesian algorithm CLASSIFIER of Ribosomal Database Project [28]. OTUs were considered unassigned when the confidence value score was lower than 0.8, and were annotated using upper taxonomic ranks.
2.4. Assessment of Infant Cognitive Development
Infant cognitive development was assessed at 18 months of age using the Bayley Scales of Infant and Toddler Development®, Third Edition [23], by trained psychologists in the presence of the mother of the child. These scales measure the level of motor, language and cognitive or mental development.
2.5. Statistical and Data Analysis
Statistical analyses were carried out using R statistical package [29]. a-diversity was measured at the OTU level using phyloseq package in R [30]. b-diversity for compositional data was calculated as Unifrac distance with GUnifrac package [31]. Quantifications of variances were calculated using PERMANOVA with the adonis function in the R package Vegan [32]. Dirichlet multinomial mixture (DMM) clustering is an unsupervised clustering method that uses Laplace approximation to identify groups of community assemblies or enterotypes at genus level [33]. The number of Dirichlet components was initially set to k = 4. Taxa unclassified at the genus level or present in less than 20% of the samples were excluded for DMM clustering. Fisher’s exact test was used to determine if infant FM groups were associated with enterotypes. Significant differential phylotype abundance at several different taxonomy levels was constructed from non-normalized raw count tables with the DESeq2 package using a two-sided Wald test with adjustment for multiple comparisons by the Benjamini–Hochberg method [34]. For all determinations, the significance cut-off was set at p ≤ 0.05 or false discovery rate (FDR) ≤ 0.05 when multiple test correction was applied.
3. Results
3.1. Study Population Characteristics and Bayley®-III Scores
This study included 71 mother–infant pairs (45 boys and 26 girls) that were recruited in the PREOBE study cohort (Table 1). Bayley®-III scales of infant development were recorded in these full-term healthy infants at the age of 18 months (Table S2). Children included in this study met the threshold for typical neurodevelopment and were dichotomized into two groups, above and below the median (50th percentile), according to their scores in three individual Bayley®-III domains: composite cognition, language (receptive language, expressive language and composite language) and motor (gross motor and fine motor). The medians (ranges) for Bayley®-III scores were as follows: composite cognition (125 (90–140)), receptive language (12 (8–15)), expressive language (11 (6–15)), composite language (106 (83–124)), gross motor (12 (7–12)) and fine motor (13 (8–19)).
Table 1.
Characteristics of the population according to the dichotomization between Fine Motricity Scores from the Bayley®-III.
| Characteristic | Below the Median | Above the Median | p |
|---|---|---|---|
| Maternal characteristics | |||
| Age (years) | 32.87 (5.37) | 33.57 (2.97) | 0.485 |
| Pre-pregnancy BMI | 0.034 * | ||
| Normal-Weight | 12 (38.71%) | 9 (22.5%) | |
| Overweight | 9 (29.03%) | 24 (60.0%) | |
| Obesity | 10 (32.26%) | 7 (17.5%) | |
| Pre-pregnancy weight (kg) | 72.12 (14.3) | 72.38 (15.17) | 0.966 |
| Weight gain (kg) | 8.71 (4.23) | 8.35 (7.62) | 0.832 |
| Diabetes | 0.515 | ||
| No | 17 (54.84%) | 25 (62.5%) | |
| Yes | 14 (45.16%) | 15 (37.5%) | |
| Education | 0.154 | ||
| Primary/Secondary | 20 (64.52%) | 18 (45.0%) | |
| University | 11 (35.48%) | 20 (50.0%) | |
| Smoking during pregnancy | 0.744 | ||
| No | 28 (90.32%) | 37 (92.5%) | |
| Yes | 3 (9.68%) | 3 (7.5%) | |
| Drinking alcohol during pregnancy | 0.855 | ||
| No | 30 (96.77%) | 39 (97.5%) | |
| Yes | 1 (3.23%) | 1 (2.5%) | |
| Type of delivery | 0.644 | ||
| Eutocic | 20 (64.52%) | 22 (55.0%) | |
| Dystocic | 4 (12.90%) | 5 (12.5%) | |
| Cesarean | 7 (22.58%) | 13 (32.5%) | |
| Infant characteristics | |||
| Sex | |||
| Male | 22 (70.97%) | 23 (57.5%) | 0.243 |
| Female | 9 (29.03%) | 17 (42.5%) | |
| Birth weight (g) | 3253.87 (467.99) | 3287.25 (578.51) | 0.794 |
| Birth length (cm) | 50.68 (1.96) | 49.83 (2.02) | 0.098 |
| Birth head Circumference (cm) | 34.75 (1.29) | 34.66 (1.47) | 0.803 |
| Placenta (g) | 498.52 (108.69) | 532.06 (127.56) | 0.281 |
| Breastfeeding † | 0.032 * | ||
| Exclusive | 14 (45.16%) | 29 (72.5%) | |
| Mixed | 4 (12.90%) | 5 (12.5%) | |
| Artificial | 13 (41.94%) | 6 (15.0%) |
Values listed are the total for the variable (percent of total value n) or means (SD). Differences between Bayley®-III Fine Motricity groups according to T-Student’s test. Chi-square test was applied to qualitative variables. * p-values ≤ 0.05. † Breastfeeding practice information was collected at 3 months of age of the child.
3.2. Taxonomic Profiling of Infants’ Gut Microbiota
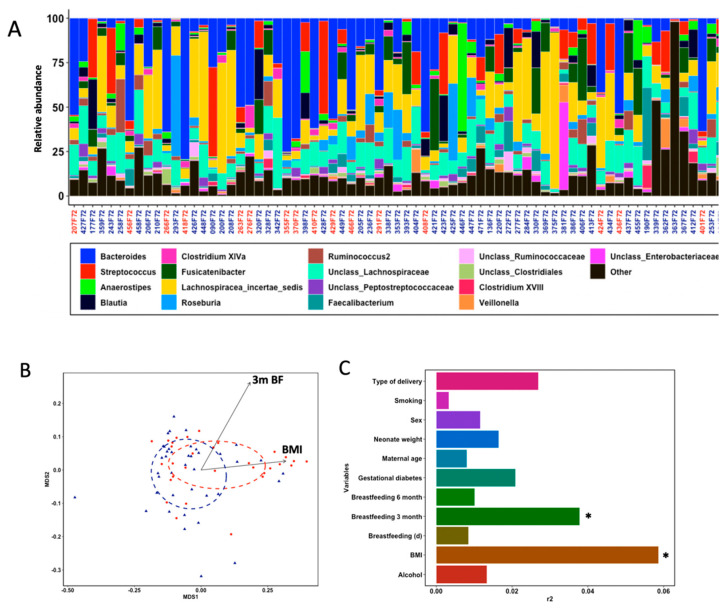
We collected faecal samples from healthy infants of 18 months of age to characterize the gut microbial composition by amplicon sequencing of the 16S rRNA hypervariable V1-V2 gene region. After quality filtering, 3,354,210 read sequences (mean per sample reads = 47,242; SD = 29,220) rendered a gut microbial profile consisting of 679 species-level bacterial operational taxonomic units (OTUs) that narrowed to 87 distinct genera belonging to 38 families after high confidence phylogenetic annotation. The phylogenetic composition and categorical breakdown of identified OTUs in our samples are presented in the supporting material (Table S3). Taxonomic classification of OTUs was performed against RDP taxonomic database, resulting in a community membership dominated by taxa within Firmicutes (516), followed by Bacteroidetes (104), Proteobacteria (32), Actinobacteria (23) and Fusobacteria (4). Analysis of core microbes of all infants revealed twenty-one highly abundant OTUs (>1% of all sequence reads), but only six of them were detected in all samples. These prevalent and highly abundant OTUs belonged to the Lachnospiracea_incertae_sedis, Streptococcus, Fusicatenibacter, Anaerostipes and Faecalibacterium genera. At genus level, nineteen genera were highly abundant (>1% of all sequence reads) and accounted for 89.6% of total reads, with dominance of Bacteroides, Lachnospiracea_incertae_sedis, unclass_Lachnospiraceae, Fusicatenibacter and Streptococcus (each providing more than 5% of all sequence reads) (Figure 1A).
Figure 1.
Gut microbial composition and structure in 71 full-term healthy infants at 18 months of age. (A) Phylogeny of infants’ gut microbiota at the genus level of 17 core genera detected in ≥98% of study subjects. Sample names are coloured according to enterotype: Firm in blue, Bact in red. Gut microbial composition and structure in 71 full-term healthy infants at 18 months of age. (B) Scatterplot from principal coordinate analysis using weighted Unifrac metrics in above-median (blue) and below-median (red) fine motor groups. Additional significant explanatory variables are represented by black arrows originating from the coordinate system origin. (C) Horizontal bars show the influence of anthropometric, maternal and nutritional variables (r2) on gut microbiota composition. * p < 0.05 for PERMANOVA test with 999 permutations.
3.3. Gut Microbial Structure Is Different Regarding Fine Motor Skills
We initially examined whether measures of gut microbial community structure and diversity differed between infants categorized as above and below the median in the six individual Bayley®-III scores. Microbial a-diversity (intra-sample diversity), assessed by the number of taxa (richness), a phylogenetic diversity measurement (Faith’s phylogenetic diversity) and a non-phylogenetic measurement of bacterial abundance and evenness (Shannon’s diversity indexes), was not significantly different between above- and below-median groups for each Bayley®-III scale (Table S4). Additionally, principal coordinate analyses based on UniFrac distance metrics was used to compare b-diversity (inter-sample diversity) between above- and below-median groups for each Bayley®-III scale. UniFrac measures the distance between microbial communities based on their abundance (weighted) and occurrence (unweighted) coupled to their phylogenetic relatedness. Considering all OTUs, the only Bayley®-III score with a strong association with gut microbial community structure and composition was FM skills, explaining 4% of microbiota variation (Figure 1B and Table S5). Infants below-median clustered away from infants above-median using weighted (p = 0.021) but not unweighted (p = 0.552) UniFrac distance metrics (Tables S5 and S6). We also tested whether the gut microbiota b-diversity was associated with anthropometric, maternal and nutritional variables (Table S7). The distance-based PERMANOVA test showed a significant explaining effect of maternal pregestational BMI (categorized as normoweight, overweight or obese) and type of breastmilk feeding up to the third month (formula, mixed or exclusive breastfeeding) on b-diversity using the weighted UniFrac distance matrix (Figure 1B,C). Interestingly, breastmilk feeding up to the third month (d) showed no effect on microbial community dissimilarities, suggesting that consumption of any breast milk impacted gut microbial assemblages in our cohort rather than the frequency of breastmilk feeding [35]. Together, FM, maternal pregestational BMI and type of breastmilk feeding up to the third month explained 13.7% of the variation in our dataset. No significant associations with maternal age, smoking during pregnancy (Yes/No), alcohol drinking during pregnancy (Yes/No), neonate weight, sex, type of delivery (C-section, vaginal) or gestational diabetes (Yes/No) were observed. These significant associations were considered in downstream statistical analyses to avoid covariate effects.
3.4. Enterotypes Are Associated with Fine Motor Activity in Infants
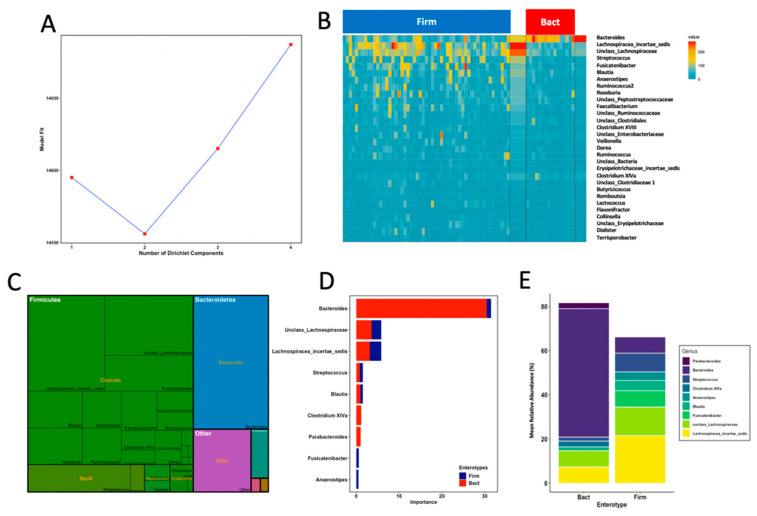
To investigate the potential association of FM scores with the prevalence of gut microbial community profiles, we identified community types in infant’s samples by using Dirichlet multinomial mixtures (DMM) modelling that assigned them into clusters based on the contribution of taxa at the genus level. Model fitting rendered an optimum number of two DMM community types, here referred to as enterotypes (Figure 2A). The two gut microbial enterotypes had weights p = 0.78 and 0.22 and variability Θ = 15.45 and 51.36. Thus, the highly abundant enterotype (78% of samples) comprised samples with more variable community profiles, while the less prevalent enterotype (22% of samples) comprised samples with more similar community assemblages (Figure 2B). We observed that enterotypes were classified by thirty genera that accounted for 85.4% of total reads in our dataset, with mean total reads ranging from 0.07% (Flavonifractor) to 21.3% (Bacteroides) (Figure 2C). Highly abundant genera (>1% of total reads) such as unclass_Clostridiales, unclass_Ruminococcaceae, Ruminococcus, unclass_Peptostreptococcaceae, unclass_Enterobacteriaceae, Enterococcus, Faecalibacterium, Ruminococcus2 and Roseburia did not drive enterotype classification. A core set of nine genera were the most important drivers in model prediction (Figure 2D). The first enterotype contained samples with mixed microbial composition whose most important drivers were a group of genera within Firmicutes such as Lachnospiracea_incertae_sedis, unclass_Lachnospiraceae, Streptococcus and Blautia, and specific contribution by Fusicatenibacter and Anaerostipes (Figure 2D). The second enterotype was mostly driven by Bacteroides with specific contribution of Clostridium XIVa and Parabacteroides to enterotype assignment. On the basis of their respective genus-level dominance profiles, we referred to enterotypes as Firmicutes dominant type (Firm) and Bacteroides dominant type (Bact). Signature genera of each enterotype classification accounted for 81.7% (Bact; SD = 7.2) and 68.8% (Firm; SD = 18.5) of the mean total abundances in their corresponding samples (Figure 2E). These results show that signature genera accounted for most reads in the Bact enterotype, with Bacteroides as the main contributor to overall enterotype assemblage, whereas signature Lachnospiracea_incertae_sedis and unclass_Lachnospiraceae were the most abundant genera in the more variable Firm enterotype. Finally, to test for associations between enterotypes and FM, we performed a Fisher’s exact two-tailed test. Belonging to an enterotype was significantly associated with an infant’s FM group, where above-median samples belonged to the Firm enterotype and below-median group mostly belonged to the Bact enterotype (p = 0.04, odds ratio = 0.27).
Figure 2.
Probabilistic modelling with Dirichlet multinomial mixtures of faecal samples revealed 2 enterotypes in 71 full-term healthy infants at 18 months of age. (A) The model was fit to k = 4 and rendered the lowest model fit value of Laplace approximation for four components which was taken as evidence for the presence of the two main community types. (B) Heatmap showing the relative abundance of the 30 most dominant genera per DMM enterotype. Abundance represents square root sequence reads. (C) Contribution of the 30 most dominant genera to overall community composition. (D) Contribution to model prediction of microbial core genera to each enterotype. (E) Contribution of core signature genera (mean relative abundances) to microbial profiles in each DMM enterotype.
3.5. Thirteen Genera including Bifidobacterium and Lactobacillus Differentiated Fine Motricity Groups
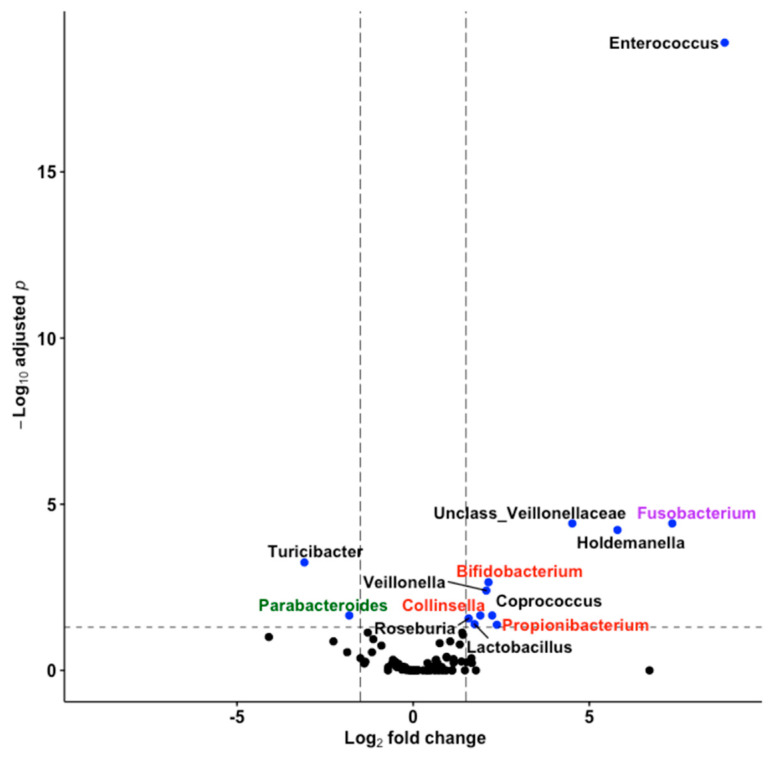
To further investigate the association of fine motricity with microbiota composition, we used the statistical software DESeq2 and adjusted the model for covariates to identify taxa at the genus level with differential abundances between below- and above-median FM groups. We identified 11 genera overabundant in the above-median group and 2 genera in the below-median group (Figure 3). These overabundant genera were phylogenetically different. Discriminating genera belonged to Actinobacteria, Firmicutes and Fusobacteria in above-median FM infants, and to Bacteroidetes and Firmicutes in below-median FM infants. The gut microbiota of above-median FM infants showed a high prevalence of Bifidobacterium, Collinsella, Coprococcus, Enterococcus, Fusobacterium, Holdemanella, Lactobacillus, Propionibacterium, Roseburia, Veillonella and an unassigned genus within Veillonellaceae. In contrast, the gut microbiota of below-median FM infants was enriched in Parabacteroides and Turicibacter. Being probiotic Bifidobacterium and Lactobacillus enriched in above-median FM infants, we conducted a more detailed analysis on the association of microbial species belonging to Bifidobacterium (7 OTUs) and Lactobacillus (11 OTUs) genera with FM. The analysis revealed that only Bifidobacterium OTU-242 tended to be enriched in infants with above-median FM (Log2FC 2.51, FDR < 0.05). Both BLASTN and EzTaxon nucleotide sequence comparisons showed that OUT-242 had 100% sequence identity with the 16S rRNA of B. bifidum ATCC 29521.
Figure 3.
Differential abundance of gut microbiota between infants with above- and below-median fine motricity groups. Volcano plot showing shrunken log2 fold changes in mean abundance of genera versus −log10 of FDR. Blue colours depict significance and label names denote the taxonomy of genera at phylum level: Firmicutes (black), Actinobacteria (red), Bacteroidetes (green), Fusobacteria (purple). Dashed lines denote thresholds of significance (FDR < 0.05).
4. Discussion
There is increasing agreement among human and animal studies that the gut microbiota impacts brain development, neurological outcomes and disorders, resulting in long-term changes in behaviour that we are only beginning to appreciate. Seminal studies in germ-free mice emphasized that early colonization with a complete specific-pathogen-free microbiota ameliorated brain development and behavioural abnormalities [11,14,36]. Cross-sectional clinical studies associated altered microbial composition with the pathophysiology of Alzheimer’s disease, autism spectrum disorder, multiple sclerosis, Parkinson’s disease, and stroke [37]. Critically important is understanding the behavioural effects of gut microbial colonization during early life, the most rapid and dynamic phase of postnatal brain development [38]. Moreover, bacterial composition begins to converge toward an adult-like microbiota by the end of the first year of life and fully resembles the adult microbiota by 2.5 years of age. A mid-time point where microbiota and neurodevelopment are still converging would probably be better to observe differences between infants. Data on the direct connection between gut microbiota and infant neurodevelopment are lacking. In the current study, we studied the association of gut microbiota structure assessed by a-diversity (within subject) and b-diversity (between subjects) metrics with neurodevelopment at the cognitive, language and motor levels in a cohort of healthy developing 18-month-old infants. We further sought to identify specific signature taxa within microbial profiles or enterotypes. This is the first study to describe associations between gut microbiota enterotypes with fine motor skills in infants and to identify significantly different signature taxa. Importantly, associations between enterotypes and fine motor scores were present when controlling for breastfeeding and maternal pregestational BMI as covariates.
Almost certainly considered by the field as the gold standard, the Bayley®-III is currently the most widespread individually administered test for behavioural assessment of children and young children aged 0 to 42 months, used in both clinical and research settings [23]. Conducted by an expert examiner, the structure of the Bayley®-III, which is divided into distinct composite scores (Cognitive, Language and Motor), allows a more precise assessment of specific developmental domains, identifying children with developmental delays and to provide information for intervention planning. In our study, children with above or below median values for each Bayley®-III subscale did not differ substantially in a-diversity, as indicated by community richness, evenness, phylogenetic similarity and species abundances. Our results contrast what was found by Carlson et al. [21], where higher a-diversity was associated with lower scores on the overall composite score, visual reception scale, and expressive language scale at 2 years of age using the Mullen Scales of Early Learning. However, our findings agree with the study of Sordillo et al. in 3 year old children [22]. The authors used the Ages and Stages Questionnaire, Third Edition, and reported no associations between gut microbiota a-diversity and developmental outcomes. Consistent associations of microbiota b-diversity with infant gut microbiota were observed for FM subscale in our study. FM abilities refer to the control of small muscles that is highly related to cognitive functions that process visual information [39]. Despite this connection, examination of Bayley®-III scales showed no significant associations of cognitive and language subscales with gut microbiota in our cohort. The significant association between microbiota b-diversity and FM groups was observed using weighted UniFrac distances and were not paralleled with unweighted UniFrac distances. This result indicates that differences in OTU abundances rather than in the presence and absence of OTUs are associated with the overall community structure in FM groups. Differences in b-diversity in healthy infants have only been reported by Christian et al. [20], who observed significant associations between the gut microbiota and temperament in boys. In our study, child sex had no impact on gut microbiota structure.
In order to reveal plausible underlying microbial assemblages in FM groups, we enterotyped our study cohort using Dirichlet multinomial mixtures on genus-level OTU profiles. Based on the genus-level proportional abundances, two distinct enterotypes were identified and termed Bact and Firm due to the dominant contribution of Bacteroides and Firmicutes in each enterotype. Our enterotype analysis aligned with previous reports of microbiota variation centred around Bifidobacterium, Bacteroides, Prevotella and Ruminococcaceae [40,41]. The first 2 years of life are characterized by a succession of bacterial taxa in which a facultative aerobic Enterobacteriaceae-rich community evolves into a strict anaerobe-rich community containing Bacteroides that is progressively enriched in a diverse mixture of Ruminococcaceae, Faecalibacterium, and Lachnospira within Firmicutes [42]. Of note, a statistically significant association between the prevalence of Bact and Firm enterotypes with fine motricity skills was observed. This is the first published prospective infant study linking enterotypes and infant neurodevelopment, showing poorer performances of infants on FM with a Bacteroides-dominant community. Our results confirm the observations by Sordillo et al. on the association of Bacteroides-dominated co-abundance grouping with poorer FM scores [22]. In addition, we identified taxa whose abundance was significantly different between FM groups. The motricity of below-median FM group was associated with increased abundances of Turicibacter and Parabacteroides. This is the first report of an association between these taxa and host behaviour in healthy infants. Finegold et al. established a list of significant genera among severely autistic vs. non-sibling control samples, where Turicibacter and Parabacteroides were significantly increased in autistic children [17]. Motor coordination impairment is a common condition in children with neurodevelopmental disorders such as autism [43]. In our cohort, better fine motor skills were associated with increased abundances of eleven genera, including Coprococcus and an unassigned genus within Veillonellaceae. Kang et al. [44] reported that Coprococcus and unclass_ Veillonellaceae were less abundant in autistic individuals compared with neurotypical controls between the ages of 3 and 16 years. An interesting finding in the above-median FM group was an over-abundance of Lactobacillus and Bifidobacterium, the most important and widely used probiotic genera. Several studies explored the role of these probiotics in the gut–brain axis. Bravo et al. observed that the ingestion of L. rhamnosus (JB-1) regulated emotional behaviour and central GABA receptor expression in mice via the vagus nerve [45]. In another study, Davari et al. reported that a supplementation of L. acidophilus, B. lactis and L. fermentum improved diabetes-induced impairment of synaptic activity and cognitive function in rats [46]. Still, more studies on rodent–human translation are required. A well-known study from Messaoudi et al. revealed a reduced self-reported anxiety in humans treated with L. helveticus R0052 and B. longum R0175 [47]. Similar effects have been observed in other investigations of mood. For instance, in a recent randomised controlled trial [48], healthy male and female participants consumed either a placebo product or a mixture of several probiotics (B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, and L. lactis W19 and W58). Relative to placebo, probiotic-treated participants exhibited substantially reduced reactivity to a sad mood (assessed by the Leiden Index of Depression Sensitivity Scale). Therefore, administration of probiotics independently or in combination may positively affect motor skills in early childhood [49].
5. Conclusions
We observed a significant association between gut microbiota and fine motricity skills in 18-month-old full-term healthy infants, robust to adjustment for infant, mother, nutritional and perinatal variables. Our study adds to the mounting evidence connecting the gut microbiota with the gut–brain axis, where the initial stages of gut colonization and assemblage may be linked with neurodevelopmental outcomes with potential long-term associations. These results suggest targeting for negatively associated species such as Turicibacter and Parabacteroides in appropriate mice models, and invite further interventional studies using Lactobacillus and Bifidobacterium strains to influence motricity outcomes in infants.
Acknowledgments
The authors want to acknowledge the women and children who participated in the study and also the obstetricians, pediatricians and technicians of the EURISTIKOS team at the Department of Pediatrics at the University of Granada, Spain. I.A. participated in the PhD Program in Nutrition and Food Science of the University of Granada. The authors are grateful to the Spanish Ministry of Education, Culture and Sports for the pre-doctoral fellowship granted to Inmaculada Acuña (FPU16/04587). The present manuscript will be part of a Ph.D. thesis accomplished by I.A.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13051673/s1, Table S1: General characteristics of the studied population, Table S2: Distribution of Bayley®-III scores of infant development in 71 full-term healthy infants at the age of 18 months Table S3: List of the operational taxonomic units (OTUs) and genera characterized in infant gut microbiota, Table S4: Association between a-diversity indexes and each Bayley®-III scale. T-Student’s test. Table S5: Associations between gut microbiota composition (16S rDNA gene sequences) and Bayley®-III scales according to the weighted UniFrac, Table S6: Associations between gut microbiota composition (16S rDNA gene sequences) and Bayley®-III scales according to the unweighted UniFrac, Table S7: Associations between gut microbiota composition (16S rDNA gene sequences) and other variables of interest according to the weighted UniFrac.
Author Contributions
Conceptualization and study design, C.C.; methodology, C.C., A.S.; software, A.S.; validation, A.S., C.C.; gut microbiota analysis, I.A., T.C., A.R., M.A., A.L.-M.; investigation, F.J.T.-E., T.C.; resources, C.C.; data curation, I.A., T.C., A.R., A.S.; writing—original draft preparation, A.S.; writing—review and editing, I.A., T.C., A.R., A.S., C.C.; supervision, A.S., C.C.; project administration, C.C.; funding acquisition, C.C. The authors wish to acknowledge that I.A., T.C. and A.R. contributed equally to the present work. All authors have read and agreed to the published version of the manuscript.
Funding
This study was granted by Spanish Ministry of Innovation and Science. Junta de Andalucía: Excellence Projects (P06-CTS-02341); Spanish Ministry of Economy and Competitiveness (BFU2012-40254-C03-02) and partially funded by the European Commission MyNewGut FP7 EU Project (Grant agreement n° 613979). Research post-doctoral fellowship from the Alfonso Martín Escudero Foundation; MyNewGut FP7 EU Project (Grant agreement n° 613979); DynaHEALTH EU Project HORIZON 2020 (Grant agreement n°: 633595-2); Marie Curie post-doctoral fellowship (FP7, no. 329812, NutriOmics); The first author received Pre-Doc scholarships from the Spanish Ministry of Education, Culture and Sports (FPU16/04587). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki (reviewed in Hong-Kong 1989 and in Edinburgh 2000) and the EEC Good Clinical Practice recommendations (document 111/3976/88 1990), and current Spanish legislation regulating clinical research in humans (Royal Decree 561/ 1993).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tau G.Z., Peterson B.S. Normal Development of Brain Circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roca-Saavedra P., Mendez-Vilabrille V., Miranda J.M., Nebot C., Cardelle-Cobas A., Franco C.M., Cepeda A. Food Additives, Contaminants and Other Minor Components: Effects on Human Gut Microbiota—A Review. J. Physiol. Biochem. 2018;74:69–83. doi: 10.1007/s13105-017-0564-2. [DOI] [PubMed] [Google Scholar]
- 4.Jost T., Lacroix C., Braegger C.P., Rochat F., Chassard C. Vertical Mother-Neonate Transfer of Maternal Gut Bacteria via Breastfeeding. Environ. Microbiol. 2014;16:2891–2904. doi: 10.1111/1462-2920.12238. [DOI] [PubMed] [Google Scholar]
- 5.Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., Zeng L., Chen J., Fan S., Du X., et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 6.De Palma G., Blennerhassett P., Lu J., Deng Y., Park A.J., Green W., Denou E., Silva M.A., Santacruz A., Sanz Y., et al. Microbiota and Host Determinants of Behavioural Phenotype in Maternally Separated Mice. Nat. Commun. 2015;6:7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 7.Diaz Heijtz R., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fröhlich E.E., Farzi A., Mayerhofer R., Reichmann F., Jac A., Wagner B., Zinser E., Bordag N., Magnes C., Fröhlich E., et al. Cognitive Impairment by Antibiotic-Induced Gut Dysbiosis: Analysis of Gut Microbiota-Brain Communication. Brain Behav. Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savignac H.M., Tramullas M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria Modulate Cognitive Processes in an Anxious Mouse Strain. Behav. Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 10.Neufeld K.M., Kang N., Bienenstock J., Foster J.A., Kang N., Bienenstock J., Foster J.A. Effects of Intestinal Microbiota on Anxiety-like Behavior. Commun. Integr. Biol. 2011;4:492–494. doi: 10.4161/cib.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F. The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 12.Desbonnet L., Garrett L., Clarke G., Bienenstock J., Dinan T.G. The Probiotic Bifidobacteria Infantis: An Assessment of Potential Antidepressant Properties in the Rat. J. Psychiatr. Res. 2009;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced Anxiety-like Behavior and Central Neurochemical Change in Germ-Free Mice. Neurogastroenterol. Motil. 2011;23:255.e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 14.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X., Kubo C., Koga Y. Postnatal Microbial Colonization Programs the Hypothalamic—Pituitary—Adrenal System for Stress Response in Mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J., Deng Y., Blennerhassett P., Macri J., McCoy K.D., et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 16.Luczynski P., Whelan S.O., O’Sullivan C., Clarke G., Shanahan F., Dinan T.G., Cryan J.F. Adult Microbiota-Deficient Mice Have Distinct Dendritic Morphological Changes: Differential Effects in the Amygdala and Hippocampus. Eur. J. Neurosci. 2016;44:2654–2666. doi: 10.1111/ejn.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finegold S.M., Dowd S.E., Gontcharova V., Liu C., Henley K.E., Wolcott R.D., Youn E., Summanen P.H., Granpeesheh D., Dixon D., et al. Pyrosequencing Study of Fecal Microflora of Autistic and Control Children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., Conlon M.A. Increased Abundance of Sutterella Spp. and Ruminococcus Torques in Feces of Children with Autism Spectrum Disorder. Mol. Autism. 2013;4:42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomova A., Husarova V., Lakatosova S., Bakos J., Vlkova B., Babinska K., Ostatnikova D. Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Christian L.M., Galley J.D., Hade E.M., Schoppe-Sullivan S., Kamp Dush C., Bailey M.T. Gut Microbiome Composition Is Associated with Temperament during Early Childhood. Brain. Behav. Immun. 2015;45:118–127. doi: 10.1016/j.bbi.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson A.L., Xia K., Azcarate-Peril M.A., Goldman B.D., Ahn M., Styner M.A., Thompson A.L., Geng X., Gilmore J.H., Knickmeyer R.C. Infant Gut Microbiome Associated With Cognitive Development. Biol. Psychiatry. 2018;83:148–159. doi: 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sordillo J.E., Korrick S., Laranjo N., Carey V., Weinstock G.M., Gold D.R., Connor G.O. Association of the Infant Gut Microbiome With Early Childhood Neurodevelopmental Outcomes An Ancillary Study to the VDAART Randomized Clinical Trial. JAMA Netw Open. 2020;2:1–13. doi: 10.1001/jamanetworkopen.2019.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. Harcourt Assessment, Inc.; San Antonio, TX, USA: 2006. [Google Scholar]
- 24.Berglund S.K., García-Valdés L., Torres-Espinola F.J., Segura M.T., Martínez-Zaldívar C., Aguilar M.J., Agil A., Lorente J.A., Florido J., Padilla C., et al. Maternal, Fetal and Perinatal Alterations Associated with Obesity, Overweight and Gestational Diabetes: An Observational Cohort Study (PREOBE) BMC Public Health. 2016;16:207. doi: 10.1186/s12889-016-2809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrer M., Ruiz A., Lanza F., Haange S., Oberbach A., Till H., Bargiela R., Campoy C., Segura M.T., Richter M., et al. Microbiota from the Distal Guts of Lean and Obese Adolescents Exhibit Partial Functional Redundancy besides Clear Differences in Community Structure. Environ. Microbiol. 2013;15:211–226. doi: 10.1111/j.1462-2920.2012.02845.x. [DOI] [PubMed] [Google Scholar]
- 26.Camarinha-Silva A., Jáuregui R., Chaves-Moreno D., Oxley A.P.A., Schaumburg F., Becker K., Wos-Oxley M.L., Pieper D.H. Comparing the Anterior Nare Bacterial Community of Two Discrete Human Populations Using Illumina Amplicon Sequencing. Environ. Microbiol. 2014;16:2939–2952. doi: 10.1111/1462-2920.12362. [DOI] [PubMed] [Google Scholar]
- 27.Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Team R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 30.McMurdie P.J., Holmes S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J., Bittinger K., Charlson E.S., Hoffmann C., Lewis J., Wu G.D., Collman R.G., Bushman F.D., Li H. Associating Microbiome Composition with Environmental Covariates Using Generalized UniFrac Distances. Bioinformatics. 2012;28:2106–2113. doi: 10.1093/bioinformatics/bts342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oksanen J., Blanchet F.G., Kindt R., Legendre P., Minchin P.R., O’Hara R.B., Simpson G.L., Solymos P., Stevens M.H.H., Wagner H. Vegan: Community Ecology Package. R Package Version 2.5-6. [(accessed on 27 October 2020)];2019 Available online: http://CRAN.Rproject.org/package=vegan.
- 33.Holmes I., Harris K., Quince C. Dirichlet Multinomial Mixtures: Generative Models for Microbial Metagenomics. PLoS ONE. 2012;7:30126. doi: 10.1371/journal.pone.0030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anders S., Huber W. Differential Expression Analysis for Sequence Count Data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He X., Parenti M., Grip T., Lönnerdal B., Timby N., Domellöf M., Hernell O., Slupsky C.M. Fecal Microbiome and Metabolome of Infants Fed Bovine MFGM Supplemented Formula or Standard Formula with Breast-Fed Infants as Reference: A Randomized Controlled Trial. Sci. Rep. 2019;9:11589. doi: 10.1038/s41598-019-47953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J. Effects of Intestinal Microbiota on Brain Development in Humanized Gnotobiotic Mice. Sci. Rep. 2018;16 doi: 10.1038/s41598-018-23692-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cryan J.F., O’Riordan K.J., Sandhu K., Peterson V., Dinan T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 38.Cerdó T., García-Valdés L., Altmäe S., Ruíz A., Suárez A., Campoy C. Role of Microbiota Function during Early Life on Child’s Neurodevelopment. Trends Food Sci. Technol. 2016;57:273–288. doi: 10.1016/j.tifs.2016.08.007. [DOI] [Google Scholar]
- 39.Zuccarini M., Guarini A., Savini S., Faldella G., Sansavini A. Do 6-Month Motor Skills Have Cascading Effects on 12-Month Motor and Cognitive Development in Extremely Preterm and Full-Term Infants? Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bokulich N.A., Chung J., Battaglia T., Henderson N., Jay M., Li H., Lieber A.D., Wu F., Perez-Perez G.I., Chen Y., et al. Antibiotics, Birth Mode, and Diet Shape Microbiome Maturation during Early Life. Sci. Transl. Med. 2016;8:1–13. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Planer J.D., Peng Y., Kau A.L., Blanton L.V., Ndao I.M., Tarr P.I., Warner B.B., Gordon J.I. Development of the Gut Microbiota and Mucosal IgA Responses in Twins and Gnotobiotic Mice. Nature. 2016;534:263–266. doi: 10.1038/nature17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz A., Cerdó T., Jáuregui R., Pieper D.H., Marcos A., Clemente A., García F., Margolles A., Ferrer M., Campoy C., et al. One-Year Calorie Restriction Impacts Gut Microbial Composition but Not Its Metabolic Performance in Obese Adolescents. Environ. Microbiol. 2017;19:1536–1551. doi: 10.1111/1462-2920.13713. [DOI] [PubMed] [Google Scholar]
- 43.Macdonald M., Lord C., Ulrich D.A. Motor Skills and Calibrated Autism Severity in Young Children with Autism Spectrum Disorder. Adapt. Phys. Activ. Q. 2014;31:95–105. doi: 10.1123/apaq.2013-0068. [DOI] [PubMed] [Google Scholar]
- 44.Kang D.-W., Park J.G., Ilhan Z.E., Wallstrom G., LaBaer J., Adams J.B., Krajmalnik-Brown R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davari S., Talaei S.A., Alaei H., Salami M. Probiotics Treatment Improves Diabetes-Induced Impairment of Synaptic Activity and Cognitive Function: Behavioral and Electrophysiological Proofs for Microbiome-Gut-Brain Axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 47.Messaoudi M., Violle N., Bisson J.F., Desor D., Javelot H., Rougeot C. Beneficial Psychological Effects of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Healthy Human Volunteers. Gut Microbes. 2011;2 doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 48.Steenbergen L., Sellaro R., van Hemert S., Bosch J.A., Colzato L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain Behav. Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Cerdó T., Ruíz A., Suárez A., Campoy C. Probiotic, Prebiotic, and Brain Development. Nutrients. 2017;9:1247. doi: 10.3390/nu9111247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.