Abstract
Simple Summary
Benzene is produced by diverse petroleum transformation processes and it is widely employed in industry despite its oncogenic effects. In fact, occupational exposure to benzene may cause hematopoietic malignancy. The leukemogenic action of benzene is particularly complex. Possible processes of onset of hematological malignancies have been recognized as a genotoxic action and the provocation of immunosuppression. However, benzene can induce modifications that do not involve alterations in the DNA sequence, the so-called epigenetics changes. Acquired epigenetic modification may also induce leukemogenesis, as benzene may alter nuclear receptors, and cause changes at the protein level, thereby modifying the function of regulatory proteins, including oncoproteins and tumor suppressor proteins.
Abstract
Benzene carcinogenic ability has been reported, and chronic exposure to benzene can be one of the risk elements for solid cancers and hematological neoplasms. Benzene is acknowledged as a myelotoxin, and it is able to augment the risk for the onset of acute myeloid leukemia, myelodysplastic syndromes, aplastic anemia, and lymphomas. Possible mechanisms of benzene initiation of hematological tumors have been identified, as a genotoxic effect, an action on oxidative stress and inflammation and the provocation of immunosuppression. However, it is becoming evident that genetic alterations and the other causes are insufficient to fully justify several phenomena that influence the onset of hematologic malignancies. Acquired epigenetic alterations may participate with benzene leukemogenesis, as benzene may affect nuclear receptors, and provoke post-translational alterations at the protein level, thereby touching the function of regulatory proteins, comprising oncoproteins and tumor suppressor proteins. DNA hypomethylation correlates with stimulation of oncogenes, while the hypermethylation of CpG islands in promoter regions of specific tumor suppressor genes inhibits their transcription and stimulates the onset of tumors. The discovery of the systems of epigenetic induction of benzene-caused hematological tumors has allowed the possibility to operate with pharmacological interventions able of stopping or overturning the negative effects of benzene.
Keywords: benzene, epigenetic, leukemia, lymphoma, hematological malignancies, gene expression, cancer, air pollution, occupational disease
1. Introduction
1.1. General Considerations on Benzene and Neoplasms
Benzene is a ubiquitous environmental contaminant (air, soil, water), classified in group 1 by the International Agency for research on cancer [1]. It comes from both natural, such as volcanoes and forest fires, and anthropogenic sources that include combustible fuel emissions, vehicles exhaust, hazardous waste sites or industry. Benzene is produced by different petroleum conversion processes and used as an intermediate in the production of a wide number of chemical substances or in the manufacturing of plastics, nylon and synthetic fibres, rubber detergents and pesticide [2].
The main sources of benzene exposure for the general population include vehicle exhaust and cigarette smoke [3,4], while considerably lower exposures to benzene can occur from consumption of food, water and beverages [5]. The limitation of the benzene content in gasoline in 1998 by EU Directive 98/70/EC and the prohibition to smoke in many public places reduced the benzene exposure of the general population significantly. Moreover, in order to improve air quality in Europe, a limit value for the protection of human health of 5 µg/m3 (0.0015 ppm) has been set by the European Directive 2008/50/EC on ambient air quality and cleaner air for Europe of the European Parliament and of the Council.
Occupational exposure to benzene occurs in the petroleum and chemical industries and also as a result of exposure to gasoline engine emissions and combustion products. In occupational settings, in the past, benzene exposure was high with estimated concentrations in the range 10–100 ppm or even higher [6]. Recent studies showed that the occupational exposures to benzene in Europe decreased and are usually below 0.1 ppm, although exposures above 0.1 ppm have been reported for some tasks such as gasoline pump repair and maintenance, or for fuel-tanker drivers or work in refineries [7,8,9,10,11,12].
1.2. Absorption and Metabolism of Benzene
Inhalation is the principal route of exposure to benzene; oral or dermal exposure is also possible although the uptake is small compared to via inhalation [13].
Assessing the cutaneous exposure to benzene, an essential element is percutaneous assimilation, and two factors are critical in absorption evaluations: the rate of benzene absorbed and the permeability coefficient (Kp). The skin has a lipophilic edge, the stratum corneum (SC), while the epidermis and dermis, which are hydrophilic, are beneath. To arrive in the blood, benzene must go by the epidermal cells, the aqueous pores, or the skin appendages. After this, a system of capillaries permits the absorption of the substance. A different modality to pass the SC edge is available intracellularly. Diffusion across this barrier establishes the superior limit of the dermal penetration coefficients for benzene, with a Kp of about 0.1 cm/h [14].
The percutaneous absorption of benzene was evaluated by employing 14 C-benzene at a dosage of 100 μL on the forearm. This gave rise to a benzene dose absorption value of 0.065% ± 0.042%, suggesting a small amount of percutaneous absorption [15]. In fact, with respect to absorption from inhalational uptake, percutaneous uptake is insignificant, with the dermal/inhalational proportion probably being <4 [16].
Analyses of the inhalational absorption of benzene report mean absorption percentages oscillating from about 50 to 80%. Benzene, after absorption, is quickly scattered in all tissues, and the substance has been found in different organs, in biological fluids and in the placenta [17]. Benzene allocation may be governed by the perfusion degree of tissues, with greater amounts in organs such as the lung, brain, kidney, and spleen [17].
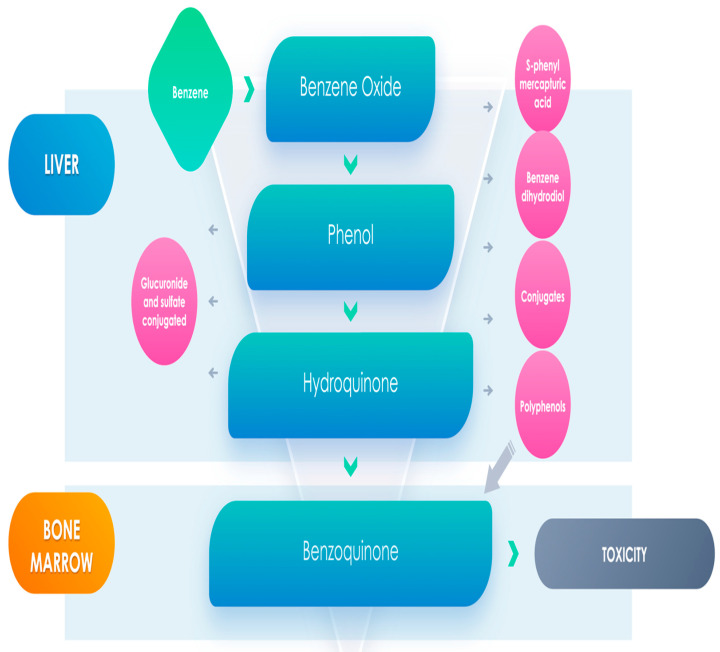
As far the metabolism of benzene, it happens essentially in the liver but also in the lung, with a subsequent metabolism taking place in the bone marrow. It begins with oxidation to benzene oxide principally by cytochrome P450 2E1, and this substance rapidly is transformed to phenol, and then into catechol and hydroquinone metabolites, both of which can be commuted into noxious elements [18]. Otherwise, benzene oxide may be transformed to benzene dihydrodiol, which can be changed to catechol [17,18], and there is also the possibility for the generation of aldehyde metabolites. Benzene metabolites may gather in the bone marrow where heme-protein peroxidases activate phenolic metabolites to semiquinone radicals generating reactive oxygen species, causing further damage to bone marrow cells, which could be essential for the determinism of hematological diseases [19] (Figure 1).
Figure 1.
Benzene metabolites could have a main role in leukemogenesis.
Some analyses have essentially studied the percentage of benzene elimination expired in air and through phenols in the urine. Exhalation is the principal way for elimination of unmetabolized benzene, while benzene is eliminated essentially through urine [20]. The amount of benzene found in expired air drops quickly, but in spite of this, residues are still present after 24 h. Phenol removal via the urine starts about 2 h after exposure, with the phenylsulfate discovered until its urine amount is 400 mg/L, after which phenylglucuronide is also excreted [21].
Moreover, the intricacy of benzene’s metabolic characteristic is augmented by genetic polymorphisms that have been described for enzymes implicated in benzene metabolism such as CYP2E1, glutathione-S-transferases (GST) GSTM1, GSTT1, GSTP1, and NAD(P)H:quinone oxidoreductase 1, which may modify the metabolism of benzene [22]. Finally, a research has demonstrated that variations in polymorphic gene frequencies occur between Africans, Caucasians, and Asians, principally concerning GSTM1, GSTT1, and GSTA1 [23].
1.3. Benzene and Cancer
Several studies have demonstrated that exposure to benzene is capable to cause the onset of solid neoplasms such as breast cancer and urothelial carcinoma. A case-control research by Petralia et al. [24] and other works indicated a correlation between breast cancer and occupational contact with benzene [24,25]. Moreover, Costantini et al. [26] performed an epidemiological cohort study of female workers employing benzene-based glues in a shoe industry in Italy. The analysis confirmed that chronic exposure to benzene can be one of the risk elements for breast cancer [26,27,28], while in a population-based case-referent study of urothelial cancer in Stockholm an exposure to benzene gave an increased relative risk of 2.0 [29,30].
1.4. Benzene and Hematological Malignancies
A larger amount of data is present in the literature to affirm a pathogenetic correlation between exposure to benzene and the onset of hematological neoplasms.
A study, carried out on shoe manufacturers exposed to benzene over a 16-month period, showed that exposure to benzene ≤1 ppm could still provoke injury to the human organism and to the hematopoietic system [31]. The workers were grouped according to their exposure in three groups (<1, 1–10 ppm, >10 ppm. over a 1-month monitoring period). The hematological evaluations conducted on the subjects showed that in the lowest exposure group, leukocyte and platelet counts were significantly decreased relative to the control values (8–15% lower). In the highest exposure group, the decrement was higher. However, a subsequent evaluation of the same data conducted by Lamm & Grunwald [32] concluded that while hematotoxicity was demonstrated at benzene concentrations greater than 10 ppm, it was insubstantial at lower concentrations.
A systematic review recognized 16 reports, which jointly evaluated the occurrence of hematological malignancies across 187,585 residents residing close to a refinery. Inhabitants from the areas less than 5 km from a petrochemical facility presented a 30% higher risk of developing hematological malignancies than habitants from areas with no petrochemical activity [33].
1.5. Benzene and Leukemia
Benzene is acknowledged as a myelotoxin with leukemogenic effects [34]. In fact, it is widely considered as an example of environmental leukemogenic with chronic contact correlated with an augmented risk for acute myeloid leukemia (AML) [35,36,37].
AMLs are a set of hematological tumors that implicate clonal growth of immature myeloid progenitor cells in the bone marrow and peripheral blood. These blast cells are disposed to be extremely proliferative and can alter normal hemopoiesis. Their growth causes a mass of non-functional cells, inducing neutropenia, anemia and thrombocytopenia.
Exposure to elevated levels of benzene (>100 ppm) causes damage in the hemopoietic system, and other forms of leukemia than AML have also been reported [38].
Numerous investigations were performed by the Chinese Academy of Preventive Medicine (CAPM) and the US National Cancer Institute (NCI) on a large number of workers exposed to benzene or benzene-containing combinations. The NCICAPM analyses proved an augmented risk of AML as well as other malignant hematopoietic disorders, such as myelodysplastic syndromes (MDS), a group of clonal hematopoietic stem cell diseases distinguished by ineffective hematopoiesis, peripheral cytopenia, dysplasia in the bone marrow, and an augmented risk of evolving to AML, occurring in about 30% of the patients.
However, in contrast to the above, these results stated an increased risk at concentrations of benzene exposure lower than 10 ppm as average and lower than 40 ppm-years cumulative [39,40,41]. In fact, a nested case-control report was also performed in Australia employing the Australian petroleum industry-monitoring data [42]. The study stated an augmented risk of leukemia correlated with total benzene exposure lower than before reported; for instance, a total exposure above 8 ppm-years augmented the risk for acute nonlymphocytic leukemia by 7-fold [43,44]. Exposure to benzene also provokes diverse damages to the hematopoietic system, comprising variable grades of pancytopenia and aplastic anemia [45,46,47].
1.6. Benzene and Lymphoproliferative Diseases
Other forms of malignancy, such as lymphoproliferative malignancies, were also correlated with chronic exposure to benzene.
Lymphomas are a composite group of tumors—neoplasms of the hematopoietic system, distinguished by the abnormal growth of lymphoid cells or their precursors. Generally, lymphoma is classified into two different groups: non-Hodgkin’s lymphoma (NHL, 90%) and Hodgkin’s lymphoma (HL, 10%). However, the multiplicity of these diseases and the infrequency of some types of the tumors have made it problematic to evaluate the hazard in epidemiological analyses [48]. A report by Glass et al. displayed a correlation between benzene exposure with chronic lymphocytic leukemia (CLL) [42], while the NCI-CAPM analysis identified a 3-fold augment in risk for NHL among the benzene exposed employers, with risk augmenting to 4-fold for employers with 10 or more years of benzene exposure [49]. Meta-analyses of reports on NHL and benzene exposure in refineries and manufacturing other than refineries suggested that both benzene exposure and refinery work were correlated with augmented risks of NHL.
Multiple myeloma (MM) is a plasma neoplasm characterized by an aberrant proliferation of clonal, terminally differentiated B lymphocytes, and a few reports have also correlated MM with benzene exposure [50,51,52].
Acute lymphoblastic leukemia (ALL) is the most frequent type of childhood tumor and accounts for about 20% of all pediatric malignancies. The present survival percentage for pediatric ALL has enhanced (>90%) recently. However, about 20% of children with ALL will finally go through relapse, and the prognosis of relapse is disappointing.
In recent times, a correlation of childhood ALL with air pollution sources, such as gas stations, and automobile repair garages that produce benzene, was proposed [53,54]. The discovery that childhood leukemias are possibly started in utero sustains the idea that exposure of the mother to benzene is also relevant in their onset and progression [55,56]. In fact, experimental animal models demonstrated that in utero exposure to benzene augmented micronuclei occurrence and DNA recombination episodes in fetal and postnatal hematopoietic tissues [57,58].
1.7. Benzene and Stem Cells
As all hematopoietic tumors result from altered stem cells, some authors suggested that different types of myeloid and lymphoid tumors, comprising their pre-stages, can be provoked by occupational exposure to benzene [59]. Benzene and its metabolites cause harmfulness toward hemopoietic stem cells (HSCs), comprising short- and long-lasting injury to HSCs, and determine negative effects on the BM hematopoietic milieu [60]. Benzene contact decreases not only the rate and amount of BM HSCs in animals, but also reduces colony generation, which was also reported in in vivo analyses [61,62].
Yoon et al. described that the HSCs cell cycle was inhibited by p53-regulated increase of p21 in animals subjected to 300 ppm of benzene for 2 weeks [63]. Additionally, permanent DNA injury was reported in Lin- C-kit+ Sca-1+ cells of mouse, 8 months after benzene exposure [64], and benzene metabolites have analogous noxious actions on HSCs. It was also reported that 5 μM of 1,4-benzoquinone extremely blocked the proliferation of single-lineage progenitor colonies such as colony-forming unit-erythroid, -granulocyte or -monocyte, and burst-forming unit-erythroid (BFU-E), while greater dosages of 1,4-benzoquinone (7 and 12 μM) remarkably decreased colony amounts of multi-lineage (progenitor colonies such as colony-forming unit- granulocyte–macrophage and colony-forming unit-multipotential myeloid stem cell) in animal HSCs in vitro [65].
In adjunct to harmfulness toward HSCs, benzene and its metabolites are toxic to the BM niche, which is a specific milieu containing diverse hematopoietic stem cells, stromal cells, immune cells, matrix, and cytokines [66]. Rivedal et al. demonstrated that two metabolites of benzene (trans-muconaldehyde and glutaraldehyde) were the main factors related to the blocking of gap junction intercellular communication [67]. Moreover, the oxidation of HQ into 1,4-benzoquinone causes disproportionate oxidative damage to stromal cells, altering their capacity to produce Nicotinamide adenine dinucleotide phosphate (NADPH) quinone oxidoreductase 1, and decreasing CD34 + cell adhesion [68].
Probably, the effects of benzene on the BM niche could be the main culprits for hematological neoplasms related to exposure to the substance.
2. Mechanisms of Benzene Carcinogenesis
The type of manners by which pollutants participate to carcinogenesis can be multiple. However, these processes can be classified into a restricted number of cases. Guyton et al. reported diverse groups of key events correlated with carcinogens that exemplified several carcinogenic processes [69]. Carcinogens generally exhibit ≥1 of the 10 principal characteristics such as: be genotoxic; cause an alteration of DNA repair systems or provoke genomic instability; operate as an electrophile either directly or after metabolic activation; cause oxidative stress and chronic inflammation; be immunosuppressive; control receptor mediated actions; induce immortalization; modify cell growth, cell death, or nutrient supply; and cause epigenetic alterations [70]. Some of these effects are likely to occur in humans at low exposure levels to benzene (≤1 ppm), in particular genotoxicity (clastogenicity and aneugenicity), immunotoxicity, altered gene expression, and receptor-mediated effects.
Several factors have been implicated to explain the onset of benzene-caused hematological neoplasia, such as activation of benzene in the liver to phenolic metabolites with the subsequent transport of these substances to the bone marrow and transformation to semiquinone radicals and quinones through peroxidase enzymes (Figure 1). Other causes might be the generation of active oxygen species through redox cycling or the alteration of DNA or DNA associated protein, tubulin, or topoisomerase.
From all this, it is clear that the leukemogenic mechanism of benzene is certainly much more complex than what was reported so far. A possible mechanism of initiation of tumors was identified as an effect on oxidative stress and inflammation and the provocation of immunosuppression.
Elements of the oxidative stress and inflammatory pathways, such as Tumor Necrosis Factor (TNF)-α, Interleukin (IL)-6, and superoxide dismutase-1 (SOD1), are modified after benzene exposure, even at concentration in the range 0.5–5 ppm [71]. Moreover, glutathione transferases (GSTs) are important mediators in oxidative stress responses, and the metabolic genotype of GST seems able to modulate the metabolism of benzene [72].
Experimental models have proposed an empowering effect: in double combinations, the toluene–benzene mixture in animals has displayed a synergistic, haemato-toxic action [73]. Analyses of exposure to three-components combination (benzene, toluene, and xylene) in cell lines have demonstrated a direct action on programmed cell death resulting from reactive oxygen species (ROS) generation [74].
Benzene and Immune System
Regarding the benzene immunosuppressive effect, results of a meta-analysis propose a benzene-caused action on the adaptive immune system and stimulation of the innate immune system. Benzene considerably reduces the number of white blood cells, especially CD4+ T-cells, B-cells and natural killer cells [75]. Particularly interesting is the existence of the positive relationship between age and serum levels of IL-10 [76], and a clear involvement of the cytokine network [77]. This agreed with the more potently suppressed production of anti-inflammatory cytokine IL-10 from CD4(+) and CD25(+) T-cells in the elderly individual more than in the young individual [78].
However, it appears that there are other most important mechanisms of the leukemogenic effect of benzene. The actions that commence leukemogenesis seem to be correlated to DNA damage. It is well-known that an approximate 45–55% of AML patients have sign of a chromosomal alteration and 95–96% have demonstrable genomic alterations. Probably, 100% of patients have such modifications, some of which have not yet been recognized [79].
Regarding the mutagenicity of benzene, some studies suggest clastogenic and aneugenic effects at concentrations below 1 ppm in oil refinery workers [80,81]. For concentrations from 0.1 to <1 ppm, the effects are borderline or absent [10,82], while for concentrations below 0.1 ppm, no relevant effects are reported [7,83,84].
The benzene metabolites 1,2,4-benzenetriol and hydroquinone (HQ) are genotoxic and provoke chromosomal alterations which promote the onset of leukemias [85,86,87]. In vitro exposure to HQ at small levels such as 0.2–2.5 μM over 20–72 h provoke monosomy 7 in human bone marrow (BM) and cord blood CD34 + progenitor cells [88]. Selective deletion of chromosome 5q31 at levels as low as 2.5 μM has been reported in human BM CD34 + cells [89].
The DNA damage comprises DNA strand breakage, mitotic recombination, chromosome translocations, and aneuploidy. If these events happen in stem cells, a leukemic clone with specific vantage to proliferate may result, as a consequence of pro-oncogene stimulation, or suppressor gene deactivation [90].
The theory that the genotoxic actions of benzene are subsequent to oxidative stress and inhibition of topoisomerase II was verified by mechanism-oriented transcriptomics studies in p53-knockout and wild-type animals [91]. In vivo and in vitro tests for chromosome abnormalities confirmed the occurrence of these aberrations after benzene exposure. Moreover, experimentations gave essential data to explain the mechanisms implicated in benzene genotoxic capacity, comprising its ability to provoke aneuploidy. However, the findings attained from the in vivo gene mutation tests stated the modest suggestion for benzene’s potential to cause gene mutations. This possibility is very low and is only reported when employing great cumulative dosages for a long time [92].
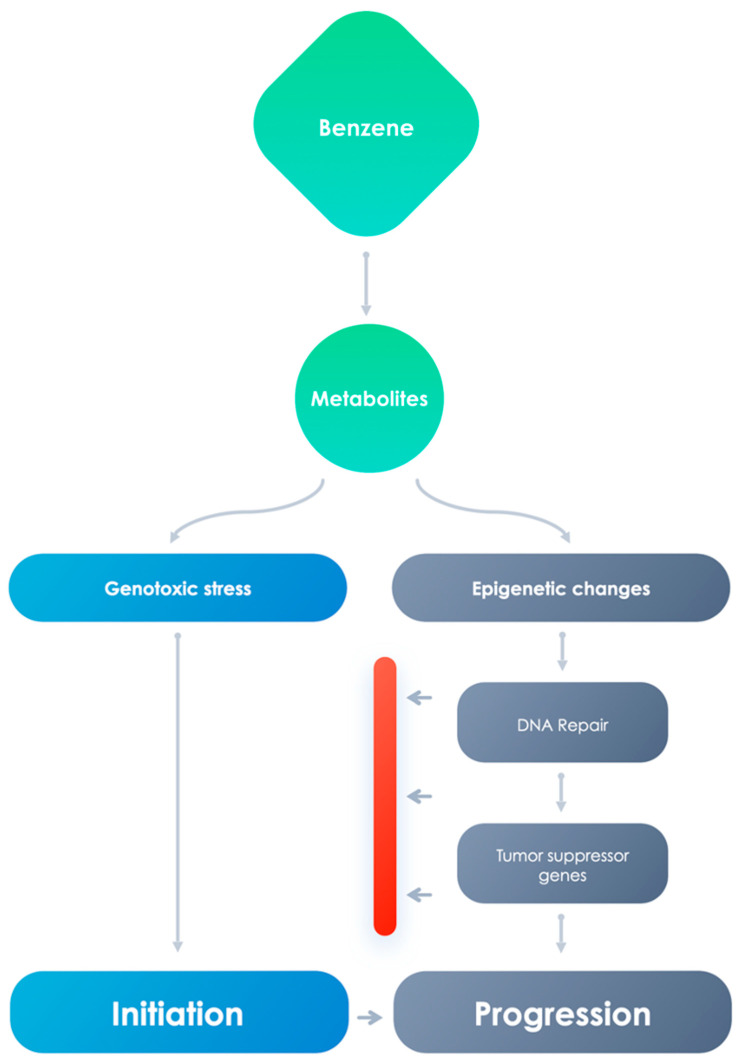
However, it is becoming evident that genetic alterations are insufficient to fully justify several phenomena that influence the onset of leukemias. For example, monozygotic twin pairs, in spite of the presence of indistinguishable DNA sequences, are often different for several characters, suggesting that the identical genotype can have different phenotypes. Although the onset and development of AML after benzene exposure is usually supposed to be analogous to therapy-related AML, in which clonal cytogenetic alterations are believed to be starting events [93,94], nevertheless, this statement is not sustained by quantitative analyses describing cytogenetic studies for actual disease outcomes. One epidemiologic report tried to associate cytogenetic alterations with hematopoietic tumors, the results were not distinctive with respect to pollutant exposure or disease [95]. This indicates the participation of supplementary elements that cannot be clarified exclusively by the genomic and propose the systems via which benzene is able to induce carcinogenesis are many, both genotoxic and non-genotoxic (Figure 2).
Figure 2.
Genotoxic effects and epigenetic changes can influence carcinogenetic development.
3. Epigenetic and Cancer
Human DNA is strictly packed into chromatin by enveloping the core histone octamer of nucleosomes. The N- and C-termini, projecting out from the nucleosome, are expose to a broad assortment of post-translational amino acid alterations comprising methylation, acetylation, phosphorylation, ubiquitinoylation, and SUMOlytion. These alterations of the histones, combined with DNA alterations, act in a synergistic manner to control gene stimulation or silencing in chromatin. The ε-N-acetylation of lysine counterbalances positive charges on histones, diminishing their relations with negatively charged DNA and acts in transmuting chromatin into a relaxed condition and activating the gene transcriptional apparatus for gene activation [96].
In physiological situations, it has been evaluated that methylated cytosines correspond to 2–6% of all the cytosines in normal cells [97]. DNA methylation regulates several biologic activities comprising the gene expression, embryonic growth, X-inactivation, cellular differentiation, silencing of transposable components, and genomic imprinting [98,99].
The word “epigenetic” indicates longstanding alterations in chromatin structure and gene expression that are not provoked by modifications in the DNA sequence itself and can be heritable over cell divisions [100]. Epigenetic events can influence the carcinogenic development by disturbing gene expression and DNA reparation systems [101]. A broad variety of carcinogens have been reported to alter the epigenome, and it has been suggested that their mechanism of action may involve disruption of epigenetic mechanisms [101].
DNA hypomethylation correlates to stimulation of oncogenes [102], while the hypermethylation of 51 cytosine-phospho-guanine (CpG) islands in promoter regions of specific tumor suppressor genes inhibits their transcription and stimulates the onset and the progression of tumors [103].
Chappell et al. [104] performed a systematic analysis of the paper on carcinogens that presented epigenetic endpoints. They evaluated pollutants classified as “carcinogenic to humans” (Group 1), comprising benzene, with convincing suggestion of genotoxic and non-genotoxic mechanisms of carcinogenesis. They evaluated 158 papers that studied epigenetic alterations for 12 carcinogenic elements comprising benzene. 10 or more works stated epigenetic effects [104].
Non-genotoxic carcinogens may affect nuclear receptors, and provoke post-translational alterations at the protein level, so touching the stability or function of regulatory proteins, comprising oncoproteins and tumor suppressor proteins [105]. Changed epigenetic condition provokes genome instability and alteration of controlled proliferation signs, habitually detected in tumor cells [106,107,108].
4. Epigenetics, Hematological Neoplasms, and Benzene
In addition to genotoxic modifications, acquired epigenetic alterations may participate in benzene leukemogenesis [109,110,111], and numerous findings are more coherent with an epigenetic model for onset of benzene-AML in which modified homeostatic control in the BM niche, not cytogenetic damage, prevails in the initial elaboration of the leukemic stem cell phenotype, a system completely different from preceding models of clonal cytogenetic damage [112].
As reported above, a modified DNA and histone methylation process could be implicated in leukemogenesis. Benzene could act modifying histones, globular proteins that undergo posttranslational modifications that modify their correlation with the DNA and other nuclear proteins. H3 and H4 histones have long tails protruding from the nucleosome, which can be covalently modified by acetylation, methylation, ubiquitination, phosphorylation, SUMOylating, citrullination, and ADP-ribosylation, and thus change chromatin structure and gene expression.
DNA methylation is determined by three DNA methyl-transferases (DNMTs) [113]; abnormal concentrations of DNMTs caused by pollutant carcinogens have been already reported all through the leukemogenesis [114].
Histone methylation happens on all basic residues and can occur at diverse residues on the same histone performed by numerous histone methyl-transferases, although certain histone modifications are reciprocally exclusive [115]. The most explored combined methylation is the specific feature that unites the activating H3K4me3 and the repressive H3K27 tri-methylation (me3). This situation was first suggested as a system to silence developmental genes while maintaining them ready for activation and is absent after cell differentiation [116]. Currently, this condition is recognized to also be existing in tumor cells, where it can cause both inhibition of until that time active genes (after loss of H3K4me3), or stimulation of beforehand inhibited genes correlated with tumor progression (after loss of H3K27me3) [117,118].
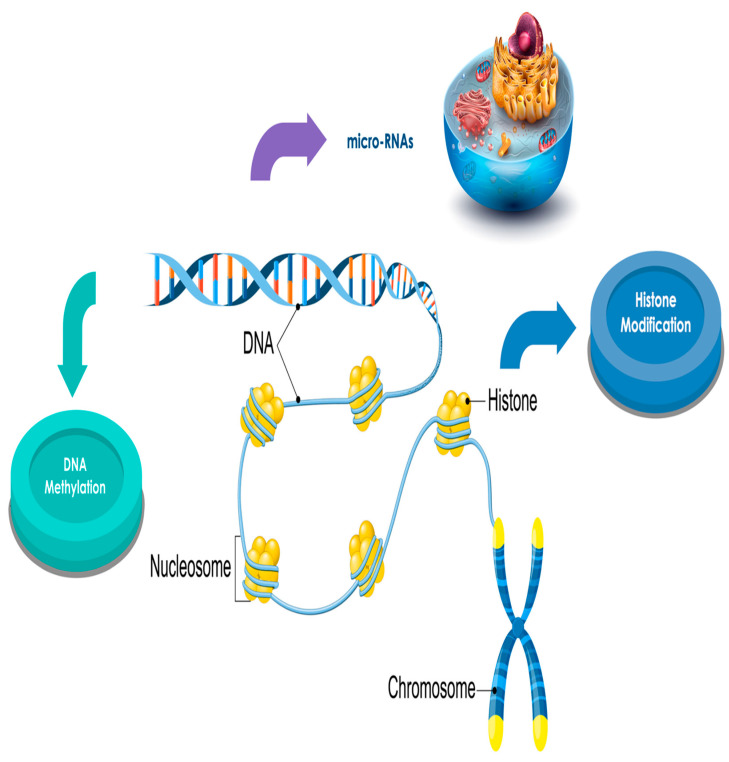
Furthermore, DNA methylation has an action in controlling histone methylation, and vice-versa, with these two conditions sustaining each other to determine the chromatin status [119,120] (Figure 3).
Figure 3.
Benzene acts regulating histone methylation to change chromatin stratus.
Abnormal DNA methylation configurations, comprising gene-specific hypomethylation or hypermethylation, total hypomethylation, and loss of imprinting (LOI), are usual in AML. Total genomic DNA methylation amounts tend to reduce in each phase of the evolution from normal to AML cells [121]. As far the gene-specific hypomethylation, a reduced methylation of melanoma-associated antigens-1 (MAGE-1), a gene hypomethylated in leukemic cells, has been reported [122,123] (Table 1). Moreover, DNA methylation is accountable for imprinting in mammal cells, and this induces certain genes to be expressed either by the paternally or maternally inherited chromosome. LOI is an initial epigenetic occurrence in leukemogenesis that was recognized in blood from patients with AML and MDS but not in peripheral cells or BM hematopoietic progenitor cells from normal subjects [124].
Table 1.
Epigenetics alterations in hematological malignancies.
| Disease | Status | Target | Type of Study | Ref. |
|---|---|---|---|---|
| AML | Hypomethylation | MAGE-1 | In vitro | [122,123] |
| AML (HLA60 cell line) | Hypermethylation | H3K4mc3 | In vitro | [138] |
| ALL (TK6 cells lymphoblastoid cells) | Hypomethylation | DNA | In vitro | [140] |
| ALL (Children) | Hypermethylation | MLL-r | Ex vivo | [132] |
| Experimental model | Target | Type of study | Ref. | |
| Male C76B/6 mice | Leukemia stem cell quiescence and self renewal genes | In vivo | [135] | |
| Exposed subjects | Hypomethylation | p15, MAGE-1, Line-1 | Ex vivo | [136] |
| Exposed subjects | Hypomethylation | P15INK4b | Ex vivo | [157] |
| Exposed subjects | JUN, PF4, CXCL16, ZNF331 | Ex vivo | [158] | |
| Exposed subjects | Hypomethylation Hypomethylation |
PRKG1, PARD3, EPHAS. STAT3, IFNGR1 |
Ex vivo | [162] |
| Exposed subjects | Hypermethylation | p15, p16 | Ex vivo | [163] |
| Bone marrow rat cells, F32 lymphoblast cells | Hypermethylation | PTEN | In vitro and in vivo | [166] |
A particular epigenetic role could be played by benzene in particular forms of leukemia. Children acute leukemia exhibits distinctive clinical and biological characteristics and is generally accompanying to alterations in the mixed-lineage leukemia (MLL) gene (MLL-r), a gene positioned on chromosome 11q23 that controls physiological hematopoietic expansion and differentiation [125]. The MLL-r codes a methyltransferase with action on lysine 4 of histone H3 (H3K4), which, as reported above, provokes modifications in chromatin correlated with epigenetic transcriptional stimulation with activation of gene expression during hematopoiesis [126]; interesting changes to the MLL gene have been described only in animals with alterations in DNA damage response but not in wild-type mice [127]. MLL-r operates as the starting oncogenic occurrence by altering epigenetic activities [102], and genetic and epidemiological analyses have demonstrated that MLL-r may origin from transplacental exposure to benzene metabolites (i.e., benzoquinone) during pregnancy [128,129]. Interestingly, and contrary to the generally accepted dogma of tumor biology, MLL-r children leukemia has been reported to have aberrant hypermethylation in non-enhancer, non-promoter regions [130,131,132,133,134].
Benzene and Hemopoietic System: In Vitro and In Vivo Studies
As it concerns the epigenetic effects exerted by benzene on hematopoietic cells, 1514 differentially expressed genes (DEGs) in BM HSCs and 1703 DEGs in Peripheral BSCs (PBSCs) were evaluated in male C57B/6 animals exposed to benzene. Weighted gene correlation network analysis revealed that transcriptional alterations in hematopoietic cell lineage are essential pathways implicated in benzene-caused damage in BM HSCs. Interestingly, there were 164 common DEGs in both BM and peripheral HSCs, out of which 53 genes were co-controlled in both types of HSCs. Successive pathway investigation of these 53 genes suggested that the most important pathways implicated neutrophil degranulation and CD93 contained in the core of the network of the 53 genes, which are recognized to control leukemia stem cell quiescence and self-renewal [135].
Bollati et al. evaluated if epigenetic alterations occurred in normal subjects by low-level exposure to benzene [123]. Blood DNA samples and exposure information were achieved from traffic police officers, gas station employers, and unexposed subjects (benzene range, <6–478 Mg/m3). Bisulfite-PCR pyrosequencing was employed to quantify DNA methylation in long interspersed nuclear element-1 (LINE-1) and AluI repetitive elements as a substitute of genome-wide methylation and evaluate gene methylation of MAGE-1 and p15. Benzene was correlated with a relevant decrease in LINE-1 and AluI methylation. Increased methylation in p15 and reduced methylation in MAGE-1 were correlated with augmented airborne benzene concentrations. LOI was evidenced only in exposed subjects and not in referents [136].
Analogous findings were reported in a different experimentation in which DNA methylation status after exposure to a VOC mixture comprising benzene and ethylbenzene was evaluated. The analysis was performed on samples from gas station employers (GS) and leather shoe manufacturing workers (LS). SOD1, DNA topoisomerase 2-alpha (TOP2A), and TNF-α promoter methylation condition was augmented in LS. Moreover, in LS, authors also displayed important connection between glutathione S-transferase p1 gene (GSTP1) promoter methylation and both inducible Nitric oxide synthases (iNOS) and cyclooxygenase-2 (COX-2) methylation. In exposed subjects, ethylbenzene exposure amounts demonstrated a relevant connection with TOP2A methylation. These intracellular alterations may be the early system of toxicity causing hematopoietic tumors, perhaps due to a synergistic action of VOC combination [137].
In a different study authors evaluated if long-lasting exposure to low dosages of HQ might be adequate to modify in vitro the epigenetic profile [138]. In HL-60 cell, a human leukemia cell line, investigating the epigenetic alterations happening in chromatin, they demonstrated the temporary occurrence of a specific mark linking the repressive H3Lys27 tri-methylation signature and the stimulating H3Lys4 tri-methylation signature (H3K27me3/H3K4me3), suggesting a trend toward a balanced chromatin conformation. These modifications are missing in time after short-term exposure, while the long-lasting setting displayed a continuing rise in H3K4me3, indicating that prolonged treatment could provoke permanent epigenetic modifications [138]. Benzene also provoked total DNA hypomethylation in human lymphoblastoid TK6 cells at amounts of 1–100 μM [139].
However, diverse results were obtained in other experimentations. No relevant total DNA methylation modifications were reported in a work employing normal hepatic L02 cells or human myeloid HL-60 cells that were treated with benzene for 48 h and which presented alterations in gene expression amounts [140,141], although the exposure levels evaluated were analogous or even greater than those employed in the previous experimentation [139]. Further studies are needed to justify these discrepancies.
Several reports have been performed to investigate the chance that benzene can interfere in the onset of hematological tumors by operating on the tumor suppressor genes. Situated at 9p21 gene cluster region, p14ARF and p15INK4b anti-oncogenes have essential actions in controlling cell growth [142]. This renders this gene cluster an objective for selective block during carcinogenic process [143]. In fact, elevated accumulations of CpG islands on promoter regions of these genes disposes them to frequently be deactivated by promoter methylation [144,145]. Deactivation of p14ARF and p15INK4b genes by changed promoter methylation has been reported in several forms of neoplasms [146].
Jamebozorgi et al. evaluated whether chronic work-related exposure to a small amount of benzene is correlated with the methylation of the p14ARF and p15INK4b promoter CpG islands [147]. Total DNA methylation and promoter-specific methylation of the two tumor suppressor genes, p14ARF and p15INK4b, were performed employing the DNA derived from 40 petrochemical employers subjected to ambient benzene concentrations of <1 ppm, and 31 office employers not subjected to benzene. A rise in total DNA methylation of 5% in p14ARF and 28% in p15INK4b genes was demonstrated in the exposed subjects, while no augmented methylation in the considered genes was detected in the unexposed controls [147].
This result confirms that long lasting work-related exposure to levels inferior to the approved exposure limit of benzene may still provoke DNA methylation of tumor suppressor genes that may finally determine the onset of leukemia.
However, several works performed on police officers and gas station employers stated that low exposure to airborne benzene is correlated with modifications in DNA methylation in blood DNA of normal subjects that are similar to those discovered in hematological malignancies [148,149,150,151,152].
As reported above, exposure to benzene can act on p14 and p15 [147]. Deactivation of p14ARF would cause degradation of the p53 protein, a possible objective for methylation in tumor [153]. Increased methylation of p15INK4b would also provoke a five-fold rise in the risk of p53 methylation [154]. Both genes are elements of the inhibitors of CDK4 (INK4) family of cyclin-dependent kinase (CDK) inhibitors positioned at the same chromosomal region. An augment of methylation abnormalities at their promoter region, which is the usual system for loss of tumor suppressor genes activity, is a possible mechanism able to cause hematologic malignancies [155].
In an in vivo study performed on a Bulgarian group of residents, gene-specific reduction of methylation of p15INK4b is described after exposure to benzene [156]. Moreover, hypermethylation of the p15 promoter, which undoubtedly participates in alteration of cell growth and is linked to AML, was reported in benzene-exposed subjects [157].
Moreover, exposure to benzene seems to be able of changing the expression of several other specific genes. Studies executed employing microarray analysis and real-time PCR demonstrated that the expression of several genes, JUN, PF4, CXCL16, and ZNF331 were remarkably modified in shoe labors [158]. These findings were corroborated by a different research employing Affymetrix and Illumina platforms, and more DEGs were demonstrated to be implicated in programmed cell death [159]. Moreover, the authors successively evaluated total gene expression in peripheral blood mononuclear cells (PBMCs) from employers subjected to <1 ppm to >10 ppm of benzene. In addition to the AML and immune response pathways, a 16-gene expression mark was reported to be correlated with all benzene exposure amounts [160]. Schiffman and McHale evaluated 30 DEGs modified by benzene. They demonstrated that three pairs of genes implicated in immunologic response and inflammation, ACSL1/CLEC5A, PRG2/CLEC5A, and NFKBI/CLEC5A, were predictive of benzene contact at amounts below 1 ppm [161].
Three other hypermethylated genes with contemporaneous mRNA reduction (PRKG1, PARD3, and EPHA8) and two hypomethylated genes with augmented mRNA amount (STAT3, IFNGR1) were also recognized in benzene poisoning subjects [162]. Successive pathway evaluation recognized STAT3 as a main actor in numerous enriched carcinogenesis-relevant gene sets, comprising AML. Promoter DNA hypermethylation of the tumor suppressor genes p15 and p16 was reported in benzene-exposed employers, alongside with a reduction in the mRNA concentration [163]. However, an experimentation performed on gravid animals demonstrated that benzene exposure provoked a reduction of total methylation, but that p15 promoter methylation was unaffected in maternal BM cells and fetal livers, suggesting that this epigenetic effect to benzene exposure could be species-specific [164].
Furthermore, in a study executed employing rat BM cells, genes that regulate programmed cell death were explored [165]. Adding a DNA methyltransferase inhibitor to the benzene-exposed cells augmented the mRNA concentrations of Bcl-2-associated X protein (Bax) and Caspase-3 (Cas3), well-known apoptosis inhibitors, and reduced the amount of cell death in benzene-exposed BM cells. This indicates that benzene-induced cytotoxicity is modulated by epigenetic alteration of programmed cell death-blocking genes.
A reduction in the expression of PTEN, a tumor suppressor gene, and a relevant rise of the PTEN methylation amount was reported in animals exposed to benzene and in F32 human lymphoblast cells treated with benzene in a dose-dependent manner [166]. Expression of the repair gene PARP-1 was also reduced with promoter increased methylation in human lymphoblastoid F32 cells exposed to 10 mM benzene [167].
Finally, a particular epigenetic mechanism exerted by benzene on the induction of hematological neoplasms could be the alteration of the expression of the non-coding genetic material, a group of regulatory RNAs with huge significance in numerous diseases such as allergic, auto immune, and neoplastic disorders [168].
In a report, a total of six miRNAs were increased (let-7d, miR-10b, miR-34a, miR-205, and miR-423-2-5p) and seven decreased (miR-27b, miR-130a, miR-133a, miR-142-5p, miR-185, miR-223, miR-320b, and miR-543) in the blood of subjects with chronic benzene exposure with respect to normal subjects [169]. However, in a different study a correlation between benzene exposure and abnormal miRNA expression was also described in a non-occupational group of subjects. miR-223 expression in gravid women and indoor levels of benzene and toluene were positively correlated and seemed to reduce the number of regulatory T-cells in maternal and cord blood [170]. Animals that were treated with benzene for 4 weeks displayed relevant hematotoxicity, as well as modifications of numerous miRNAs in the BM cells of treated animals. Five miRNAs were augmented, and 45 miRNAs were reduced [171]. The augmented miRNAs were miR-34a-5p, miR-129b-3p, miR-129b-5p, miR-144-5p, and miR-451a, and the most greatly reduced miRNAs were let-7i-3p, miR-33-5p, miR-128-1-5p, miR-188-5p, miR-211-5p, miR-224-5p, miR-504-5p, miR-5107-3p, and miR-5120.
Furthermore, in an experimentation performed on benzene-exposed employers, the expression of two long noncoding (lnc)RNAs (lnc 028291 and lnc 045623) was greater in the samples of exposed employers with respect to controls [172]. These lncRNAs and their correlated mRNAs are implicated in hematopoiesis, chronic myeloid leukemia, immune response, and B cell receptor signaling, indicating their correlation with benzene-caused leukemogenesis.
In conclusion, long-term exposure to benzene appears to be capable of modifying the epigenetic pattern. Genes involved in the inhibition of proliferation, in apoptosis, in the immune response and in inflammatory processes can be differently expressed after exposure to benzene. This could be an essential moment in the carcinogenic process.
5. Future Perspectives
Epigenetic silencing of tumor suppressor genes through DNA increased methylation has been recognized as a common signature of oncogenesis [173]. However, the discovery of the systems of epigenetic induction in the onset of benzene-caused hematological tumors has allowed the possibility to operate with pharmacological interventions able of stopping or overturning the negative effects of benzene.
The methylation of CpG dinucleotides, especially at gene promoters and regulatory regions, has been demonstrated to cause epigenetic gene silencing through the employment of methyl-binding domain (MBD) proteins such as Methyl-CpG Binding Domain Protein 1 (MBD1), MBD2, and Methyl-CpG Binding Protein 2 (MeCP2) and their correlated chromatin remodeling/co-repressor complexes such as Mi2-NuRD [174]. These compounds are able of modifying the chromatin status, provoking a transcriptional repression [175]. Current pharmacological attempts for blocking of epigenetic gene silencing in tumors were directed on blocking DNMTs, and histone deacetylases (HDACs) [176]. Remarkably, such an attempt was conducted according to FDA authorization of two DNMT inhibitors and two HDAC inhibitors for the treatment of MDS and cutaneous T-cell lymphoma [177,178,179].
In the next future, other different therapeutic approaches seem possible. The bromodomain (BrD) operates as the acetyl-lysine binding domain to control gene stimulation in chromatin [180,181]. The human bromodomains are divided into subgroups with different characteristics [182]. One main group is BET (bromodomain and extra-terminal domain) proteins comprised of BRD2, BRD3, BRD4, and BRDT [183]. BRD4, perhaps the most extensively widely studied BET protein has a central role in several pathologies comprising tumors [184,185,186]. Unfortunately, in spite of the discovery of numerous powerful BET inhibitors, there is still no inhibitor able to differentiate between the different bromodomains within any specific BET protein. Discovering such a selective inhibitor is a promising possibility.
However, this is a difficult mission, as is determining the sequence identity of these bromodomains, especially at their acetyl lysine binding pockets [187,188]. Zhang et al. described the structure-guided generation of a novel group of diazobenzene based small molecule blockers for the BET bromodomains. MS436 is the best inhibitor produced employing a structure-activity relationship analysis [189]. They enhanced the affinity of the diazobenzene compounds toward the BRD4 BrD1 by over 100-fold. MS436 has an evaluated Ki of 30–50 nM for the BRD4 BrD1 with a 10-fold selectivity over the BrD2. This could assign to this substance drug-like capacities. Moreover, they verified the effectiveness of four lead diazobenzene BrD inhibitors in blocking BRD4 transcriptional activity in LPS-stimulated, NK-κB-directed production of nitric oxide and IL-6 in murine macrophage RAW264.7 cells [189].
Finally, in an investigation to find DNMT inhibitors from Formosan plants, Weng et al. recognized kazinol Q {4-[6-(1,1-dimethyl-allyl)-7-hydroxy-chroman-2-yl]-3,6-bis-(3-methyl-but-2-enyl)-benzene-1,2-diol} as an inhibitor of recombinant DNMT1 with IC50 of 7 mM [190]. The efficacy of kazinol Q on DNMT block was confirmed by its capacity to restart the expression of a DNA methylation-silenced gene, E-cadherin, in MDA-MB-231 tumoral cells. Furthermore, kazinol Q reduced the growth of tumor cells via the stimulation of programmed cell death. The action of DNMT1 inhibition in determining kazinol Q’s antiproliferative effect was confirmed by the protective action of ectopic expression of DNMT1 on kazinol Q-caused cell death. Molecular modeling evaluation proposes that kazinol Q blocks DNMT action by contending with cytosine binding, a system analogous to that reported for (-)-epigallocatechin-3-gallate (EGCG). However, in comparison to EGCG, kazinol Q shows numerous advantageous characteristics, comprising chemical stability and augmented hydrophobicity, and might have therapeutic application to leukemia treatment [190].
6. Conclusions
It is logical to assume that benzene and its metabolites exert different actions on different elements of the hematopoietic system able to provoke haemato-toxicity and malignancy. The possible mechanisms conducting to the onset and progression of the hematologic malignancies are described as a “multi-hit” model. Cancer stem-like cells may result from tumorigenic modifications commenced in normal, self-renewing hemopoietic stem cells or subsequent progenitors by benzene metabolites, causing the proliferation of the stem cell and progenitor pools and production of preleukemic stem cells. Secondary events may happen in the proliferating cells generating cancer stem like cells. Genomic instability and changes of cellular phenotypes, such as epigenetic alterations, may also happen. Benzene induced epigenetic alterations could operate on nuclear receptors, and provoke post-translational alterations at the protein level, so touching the stability or function of regulatory proteins, comprising oncoproteins and tumor suppressor proteins [191].
A further study of the epigenetic mechanisms of benzene-induced hematological alterations could open the way to new therapeutic possibilities.
Author Contributions
Conceptualization, G.S., M.C., A.A. and S.G.; methodology, G.P.; formal analysis, A.A. and G.P.; data curation, M.C. and G.P.; writing—original draft preparation, A.A.; writing—review and editing, A.A., G.S., M.C. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer (IARC) Monographs on the Identification of Carcinogenic Hazards to Human. Benzene. [(accessed on 10 May 2021)];2018 Available online: https://publications.iarc.fr/576.
- 2.ATSDR Toxicological Profile for Benzene Toxicological Profile. [(accessed on 9 March 2021)];2007 Available online: https://www.atsdr.cdc.gov/toxprofiles/tp3.pdf.
- 3.Arnold S.M., Angerer J., Boogaard P.J., Hughes M.F., O’Lone R.B., Robison S.H., Schnatter A.R. The use of biomonitoring data in exposure and human health risk assessment: Benzene case study. Crit. Rev. Toxicol. 2013;43:119–153. doi: 10.3109/10408444.2012.756455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masiol M., Agostinelli C., Formenton G., Tarabotti E., Pavoni B. Thirteen years of air pollution hourly monitoring in a large city: Potential sources, trends, cycles and effects of car-free days. Sci. Total Environ. 2014;494–495:84–96. doi: 10.1016/j.scitotenv.2014.06.122. [DOI] [PubMed] [Google Scholar]
- 5.Wallace L. Environmental exposure to benzene: An update. Environ. Health Perspect. 1996;104:1129–1136. doi: 10.1289/ehp.961041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams P.R., Paustenbach D.J. Reconstruction of benzene exposure for the Pliofilm cohort (1936–1976) using Monte Carlo techniques. J. Toxicol. Environ. Health A. 2003;66:677–781. doi: 10.1080/15287390306379. [DOI] [PubMed] [Google Scholar]
- 7.Fracasso M.E., Doria D., Bartolucci G.B., Carrieri M., Lovreglio P., Ballini A., Soleo L., Tranfo G., Manno M. Low air levels of benzene: Correlation between biomarkers of exposure and genotoxic effects. Toxicol. Lett. 2010;192:22–28. doi: 10.1016/j.toxlet.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Carrieri M., Tranfo G., Pigini D., Paci E., Salamon F., Scapellato M.L., Fracasso M.E., Manno M., Bartolucci G.B. Correlation between environmental and biological monitoring of exposure to benzene in petrochemical industry operators. Toxicol. Lett. 2010;192:17–21. doi: 10.1016/j.toxlet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Campo L., Rossella F., Mercadante R., Fustinoni S. Exposure to BTEX and Ethers in Petrol Station Attendants and Proposal of Biological Exposure Equivalents for Urinary Benzene and MTBE. Ann. Occup. Hyg. 2016;60:318–333. doi: 10.1093/annhyg/mev083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovreglio P., Maffei F., Carrieri M., D’Errico M.N., Drago I., Hrelia P., Bartolucci G.B., Soleo L. Evaluation of chromosome aberration and micronucleus frequencies in blood lymphocytes of workers exposed to low concentrations of benzene. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014;770:55–60. doi: 10.1016/j.mrgentox.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Periago J.F., Prado C. Evolution of occupational Exposure to Environmental Levels of Aromatic Hydrocarbons in Service Stations. Ann. Occup. Hyg. 2005;49:233–240. doi: 10.1093/annhyg/meh083. [DOI] [PubMed] [Google Scholar]
- 12.Almerud P., Akerstrom M., Andersson E.M., Stranberg B., Sallsten G. Low personal exposure to benzene and 1,3-butadiene in the Swedish petroleum refinery industry. Int. Arch. Occup. Environ. Health. 2017;90:713–724. doi: 10.1007/s00420-017-1234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauma M., Boman A., Johanson G. Predicting the absorption of chemical vapours. Adv. Drug Deliv. Rev. 2013;65:306–314. doi: 10.1016/j.addr.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Hostynek J.J., Lamel S.A., Maibach H.I. Benzene absorption in animals and man: An overview. Rev. Environ. Health. 2012;27:85–101. doi: 10.1515/reveh-2012-0008. [DOI] [PubMed] [Google Scholar]
- 15.Maibach H.I. Percutaneous Penetration of Benzene in Man. American Petroleum Institute; Washington, DC, USA: 1980. Report to the API. [Google Scholar]
- 16.Hanke J., Dutkiewcz T., Pitrowski J. The absorption of benzene through human skin. Int. J. Occup. Health. 2000;6:104–111. doi: 10.1179/oeh.2000.6.2.104. [DOI] [PubMed] [Google Scholar]
- 17.DECOS (Dutch Expert Committee on Occupational Safety) Benzene: Health-Based Recommended Occupational Exposure Limit, No. 2014/03. [(accessed on 22 May 2019)];2014 2014 Feb 21; Available online: https://www.gezondheidsraad.nl/en/task-and-procedure/areas-of-activity/healthy-working-conditions/benzene-health-based-recommended.
- 18.Meek M.E., Klaunig J.E. Proposed mode of action of benzene-induced leukemia: Interpreting available data and identifying critical data gaps for risk assessment. Chem. Biol. Interact. 2010;184:279–285. doi: 10.1016/j.cbi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 19.McHale C.M., Zhang L., Smith M.T. MCurrent understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis. 2012;33:240–252. doi: 10.1093/carcin/bgr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luijten M., Ball N.S., Dearfield K.L., Gollapudi B.B., Johnson G.E., Madia F., Peel L., Pfuhler S., Settivari R.S., Ter Burg W., et al. Utility of a next generation framework for assessment of genomic damage: A case study using the industrial chemical benzene. Environ. Mol. Mutagen. 2020;61:94–113. doi: 10.1002/em.22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rusch G.M., Leong B.K.J., Laskin S. Benzene metabolism. J. Toxicol. Environ. Health. 1997;2:23–36. [PubMed] [Google Scholar]
- 22.ECHA (European Chemicals Agency) Annex 1. Background Document in support of the Committee for Risk Assessment (RAC) Evaluation of Limit Values for Benzene in the Workplace. European Chemicals Agency; Helsinki, Finland: 2018. [(accessed on 12 May 2021)]. Available online: https://echa.europa.eu/documents/10162/13641/benzene_bg_annex1_en.pdf/37b38de4-0e36-6058-eaa4-1ffc56938831. [Google Scholar]
- 23.Carbonari D., Chiarella P., Mansi A., Pigini D., Iavicoli S., Tranfo G. Biomarkers of susceptibility following benzene exposure: Influence of genetic polymorphisms on benzene metabolism and health effects. Biomark. Med. 2016;10:145–163. doi: 10.2217/bmm.15.106. [DOI] [PubMed] [Google Scholar]
- 24.Petralia S.A., Vena J.E., Freudenheim J.L., Dosemeci M., Michalek A., Goldberg M.S., Brasure J., Graham S. Risk of premenopausal breast cancer in association with occupational exposure to polycyclic aromatic hydrocarbons and benzene. Scand. J. Work Environ. Health. 1999;25:215–221. doi: 10.5271/sjweh.426. [DOI] [PubMed] [Google Scholar]
- 25.Wolff M.S., Collman G.W., Barrett J.C., Huff J. Breast cancer and environmental risk factors: Epidemiological and experimental findings. Annu. Rev. Pharmacol. Toxicol. 1996;36:573–596. doi: 10.1146/annurev.pa.36.040196.003041. [DOI] [PubMed] [Google Scholar]
- 26.Costantini A.S., Gorini G., Consonni D., Miligi L., Giovannetti L., Quinn M. Exposure to benzene and risk of breast cancer among shoe factory workers in Italy. Tumori. 2009;95:8–12. doi: 10.1177/030089160909500102. [DOI] [PubMed] [Google Scholar]
- 27.White A.J., Chen J., McCullough L.E., Xu X., Cho Y.H., Teitelbaum S.L., Neugut A.I., Terry M.B., Hibshoosh H., Santella R.M., et al. Polycyclic aromatic hydrocarbon (PAH)-DNA adducts and breast cancer: Modification by gene promoter methylation in a population-based study. Cancer Causes Control. 2015;26:1791–1802. doi: 10.1007/s10552-015-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenga C. Occupational exposure and risk of breast cancer. Biomed. Rep. 2016;4:282–292. doi: 10.3892/br.2016.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steineck G., Plato N., Gerhardsson M., Norell S.E., Hogstedt C. Increased risk of urothelial cancer in Stockholm during 1985–1987 after exposure to benzene and exhausts. Int. J. Cancer. 1990;45:1012–1017. doi: 10.1002/ijc.2910450605. [DOI] [PubMed] [Google Scholar]
- 30.Axelsson G., Barregard L., Holmberg E., Sallsten G. Cancer incidence in a petrochemical industry area in Sweden. Sci. Total Environ. 2010;408:4482–4487. doi: 10.1016/j.scitotenv.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Lan Q., Zhang L., Li G., Vermeulen R., Weinberg R.S., Dosemeci M., Rappaport S.M., Shen M., Alter B.P., Wu Y., et al. Hematotoxicity in workers exposed to low levels of benzene. Science. 2004;306:1774–1776. doi: 10.1126/science.1102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamm S.H., Grunwald H.W. Benzene exposure and hematotoxicity. Science. 2006;312:998. doi: 10.1126/science.312.5776.998b. [DOI] [PubMed] [Google Scholar]
- 33.Jephcote C., Brown D., Verbeek T., Mah A. A systematic review and meta-analysis of haematological malignancies in residents living near petrochemical facilities. Environ. Health. 2020;19:53. doi: 10.1186/s12940-020-00582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalade A., Jaakkola M.S., Pukkala E., Jaakkola J.J. Exposure to benzene at work and the risk of leukemia: A systematic review and meta-analysis. Environ. Health. 2010;9:31. doi: 10.1186/1476-069X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Infante P.F., Rinsky R.A., Wagoner J.K., Young R.J. Leukaemia in benzene workers. Lancet. 1977;310:76–78. doi: 10.1016/S0140-6736(77)90074-5. [DOI] [PubMed] [Google Scholar]
- 36.Hayes R.B., Yin S.N., Dosemeci M., Li G.L., Wacholder S., Travis L.B., Li C.Y., Rothman N., Hoover R.N., Linet M.S. Benzene and the dose-related incidence of hematologic neoplasms in China. J. Natl. Cancer Inst. 1997;89:1065–1071. doi: 10.1093/jnci/89.14.1065. [DOI] [PubMed] [Google Scholar]
- 37.Wong O., Harris F., Armstrong T.W., Fu H. A hospital based case-control study of acute myeloid leukemia in Shanghai: Analysis of environmental and occupational risk factors by subtypes of the WHO classification. Chem. Biol. Interact. 2010;184:112–128. doi: 10.1016/j.cbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 38. Stenehjem JS, Kjærheim K, Bråtveit M, Samuelsen SO, Barone-Adesi F, Rothman N, Lan Q, Grimsrud TK Benzene exposure and risk of lymphohaematopoietic cancers in 25 000 offshore oil industry workers. Br. J. Cancer. 2015;112:1603–1612. doi: 10.1038/bjc.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin S.N., Li G.L., Tain F.D., Fu Z.I., Jin C., Chen Y.J., Luo S.J., Ye P.Z., Zhang J.Z., Wang G.C., et al. Leukaemia in Benzene Workers: A Retrospective Cohort Study. Br. J. Ind. Med. 1987;44:124–128. doi: 10.1136/oem.44.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayes R.B., Yin S.N., Dosemeci M., Li G.L., Wacholder S., Chow W.H., Rothman N., Wang Y.Z., Dai T.R., Chao X.J., et al. Mortality among Benzene-Exposed Workers in China. Environ. Health Perspect. 1996;104:1349–1352. doi: 10.1289/ehp.961041349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin S.N., Hayes R.B., Linet M.S., Li G.L., Dosemeci M., Travis L.B., Zhang Z.N., Li D.G., Chow W.H., Wacholder S., et al. An Expanded Cohort Study of Cancer among Benzene-Exposed Workers in China. Benzene Study Group. Environ. Health Perspect. 1996;104:1339–1341. doi: 10.1289/ehp.961041339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glass D.C., Gray C.N., Jolley D.J., Gibbons C., Sim M.R., Fritschi L., Adams G.G., Bisby J.A., Manuell R. Leukemia Risk Associated with Low-Level Benzene Exposure. Epidemiology. 2003;14:569–577. doi: 10.1097/01.ede.0000082001.05563.e0. [DOI] [PubMed] [Google Scholar]
- 43.Glass D.C., Sim M.R., Fritschi L., Gray C.N., Jolley D.J., Gibbons C. Leukemia Risk and Relevant Benzene Exposure Period-Re: Follow-up Time on Risk Estimates. Am. J. Ind. Med. 2004;42:481–489. doi: 10.1002/ajim.10327. [DOI] [PubMed] [Google Scholar]
- 44.Glass D.C., Gray C.N., Jolley D.J., Gibbons C., Sim M.R. Health Watch Exposure Estimates: Do They Underestimate Benzene Exposure? Chem. Biol. Interact. 2005;153–154:23–32. doi: 10.1016/j.cbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Gross S.A., Paustenbach D.J. Shanghai health study (2001–2009): What was learned about benzene health effects? Crit. Rev. Toxicol. 2018;48:217–251. doi: 10.1080/10408444.2017.1401581. [DOI] [PubMed] [Google Scholar]
- 46.Lu P.C.W., Shahbaz S., Winn L.M. Benzene and its effects on cell signaling pathways related to hematopoiesis and leukemia. J. Appl. Toxicol. 2020;40:1018–1032. doi: 10.1002/jat.3961. [DOI] [PubMed] [Google Scholar]
- 47.Esteller M. Profiling aberrant DNA methylation in hematologic neoplasms: A view from the tip of the iceberg. Clin. Immunol. 2003;109:80–88. doi: 10.1016/S1521-6616(03)00208-0. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein B.D. Benzene as a Cause of Lymphoproliferative Disorders. Chem. Biol. Interact. 2010;184:147–150. doi: 10.1016/j.cbi.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 49.Steinmaus C., Smith A.H., Jones R.M., Smith M.T. Meta-Analysis of Benzene Exposure and Non-Hodgkin Lymphoma: Biases Could Mask an Important Association. Occup. Environ. Med. 2008;65:371–378. doi: 10.1136/oem.2007.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decoufle P., Blattner W.A., Blair A. Mortality among Chemical Workers Exposed to Benzene and Other Agents. Environ. Res. 1983;30:16–25. doi: 10.1016/0013-9351(83)90161-5. [DOI] [PubMed] [Google Scholar]
- 51.Infante P.F. Benzene Exposure and Multiple Myeloma: A Detailed Meta-Analysis of Benzene Cohort Studies. Ann. N. Y. Acad. Sci. 2006;1076:90–109. doi: 10.1196/annals.1371.081. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein B.D. Is Exposure to Benzene a Cause of Human Multiple Myeloma? Ann. N. Y. Acad. Sci. 1990;609:225–230. doi: 10.1111/j.1749-6632.1990.tb32070.x. [DOI] [PubMed] [Google Scholar]
- 53.Brosselin P., Rudant J., Orsi L., Leverger G., Baruchel A., Bertrand Y., Nelken B., Robert A., Michel G., Margueritte G., et al. Acute Childhood Leukaemia and Residence Next to Petrol Stations and Automotive Repair Garages: The Escale Study (Sfce) Occup. Environ. Med. 2009;66:598–606. doi: 10.1136/oem.2008.042432. [DOI] [PubMed] [Google Scholar]
- 54.Whitworth K.W., Symanski E., Coker A.L. Childhood Lymphohematopoietic Cancer Incidence and Hazardous Air Pollutants in Southeast Texas 1995–2004. Environ. Health Perspect. 2008;116:1576–1580. doi: 10.1289/ehp.11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pyatt D., Hays S. A Review of the Potential Association between Childhood Leukemia and Benzene. Chem. Biol. Interact. 2010;184:151–164. doi: 10.1016/j.cbi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Smith M.T., Zhang L., McHale C.M., Skibola C.F., Rappaport S.M. Benzene, the Exposome and Future Investigations of Leukemia Etiology. Chem. Biol. Interact. 2011;192:155–159. doi: 10.1016/j.cbi.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Badham H.J., Winn L.M. In Utero and in Vitro Effects of Benzene and Its Metabolites on Erythroid Differentiation and the Role of Reactive Oxygen Species. Toxicol. Appl. Pharmacol. 2010;244:273–279. doi: 10.1016/j.taap.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Lau A., Belanger C.L., Winn L.M. In Utero and Acute Exposure to Benzene: Investigation of DNA Double-Strand Breaks and DNA Recombination in Mice. Mutat. Res. 2009;676:74–82. doi: 10.1016/j.mrgentox.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Beelte S., Haas R., Germing U., Jansing P.J. Paradigm Change in The Assessment of Myeloid and Lymphoid Neoplasms Associated with Occupational Benzene Exposure. Med. Klin. 2009;104:197–203. doi: 10.1007/s00063-009-1032-8. [DOI] [PubMed] [Google Scholar]
- 60.Wang L., He X., Bi Y., Ma Q. Stem cell and benzene-induced malignancy and hematotoxicity. Chem. Res. Toxicol. 2012;25:1303–1315. doi: 10.1021/tx3001169. [DOI] [PubMed] [Google Scholar]
- 61.Sun R., Zhang J., Wei H., Meng X., Ding Q., Sun F., Cao M., Yin L., Pu Y. Acetyl-l-carnitine partially prevents benzene-induced hematotoxicity and oxidative stress in C3H/He mice. Environ. Toxicol. Pharmacol. 2017;51:108–113. doi: 10.1016/j.etap.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Sun R., Zhang J., Xiong M., Wei H., Tan K., Yin L., Pu Y. Altered expression of genes in signaling pathways regulating proliferation of hematopoietic stem and progenitor cells in mice with subchronic benzene exposure. Int. J. Environ. Res. Public Health. 2015;12:9298–9313. doi: 10.3390/ijerph120809298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon B.I., Hirabayashi Y., Kawasaki Y., Kodama Y., Kaneko T., Kim D.Y., Inoue T. Mechanism of action of benzene toxicity: Cell cycle suppression in hemopoietic progenitor cells (CFU-GM) Exp. Hematol. 2001;29:278–285. doi: 10.1016/s0301-472x(00)00671-8. [DOI] [PubMed] [Google Scholar]
- 64.Giver C.R., Wong R., Moore II D.H., Pallavicini M.G. Persistence of aneuploid immature/primitive hemopoietic sub-populations in mice 8 months after benzene exposure in vivo. Mutat. Res. 2001;491:127–138. doi: 10.1016/S1383-5718(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 65.Chow P.W., Abdul Hamid Z., Chan K.M., Inayat-Hussain S.H., Rajab N.F. Lineage-related cytotoxicity and clonogenic profile of 1,4-benzoquinone-exposed hematopoietic stem and progenitor cells. Toxicol. Appl. Pharmacol. 2015;284:8–15. doi: 10.1016/j.taap.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 66.Zhou H., Kepa J.K., Siegel D., Miura S., Hiraki Y., Ross D. Benzene metabolite hydroquinone up-regulates chondromodulin-I and inhibits tube formation in human bone marrow endothelial cells. Mol. Pharmacol. 2009;76:579–587. doi: 10.1124/mol.109.057323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rivedal E., Witz G., Leithe E. Gap junction intercellular communication and benzene toxicity. Chem. Biol. Interact. 2010;184:229–232. doi: 10.1016/j.cbi.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 68.Zhou H., Dehn D., Kepa J.K., Siegel D., Scott D.E., Tan W., Ross D. NAD(P)H: Quinone oxidoreductase 1-compromised human bone marrow endothelial cells exhibit decreased adhesion molecule expression and CD34+ hematopoietic cell adhesion. J. Pharmacol. Exp. Ther. 2010;334:260–268. doi: 10.1124/jpet.110.167841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guyton K.Z., Kyle A.D., Aubrecht J., Cogliano V.J., Eastmond D.A., Jackson M., Keshava N., Sandy M.S., Sonawane B., Zhang L., et al. Improving prediction of chemical carcinogenicity by considering multiple mechanisms and applying toxico-genomic approaches. Mutat Res. 2009;681:230–240. doi: 10.1016/j.mrrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Smith M.T., Guyton K.Z., Gibbons C.F., Fritz J.M., Portier C.J., Rusyn I., DeMarini D.M., Caldwell J.C., Kavlock R.J., Lambert P.F., et al. et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ. Health Perspect. 2016;124:713–721. doi: 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haro-García L.C., Juárez-Pérez C.A., Aguilar-Madrid G., Vélez-Zamora N.M., Muñoz-Navarro S., Chacón-Salinas R., González-Bonilla C.R., Iturbe-Haro C.R., Estrada-García I., Borja-Aburto V.H. Production of IL-10, TNF and IL-12 by peripheral blood mononuclear cells in Mexican workers exposed to a mixture of benzene-toluene-xylene. Arch. Med. Res. 2012;43:51–57. doi: 10.1016/j.arcmed.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Carrieri M., Spatari G., Tranfo G., Sapienza D., Scapellato M.L., Bartolucci G.B., Manno M. Biological monitoring of low level exposure to benzene in an oil refinery: Effect of modulating factors. Toxicol. Lett. 2018;298:70–75. doi: 10.1016/j.toxlet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Bird M.G., Wetmore B.A., Letinski D.J., Nicolich M., Chen M., Schnatter A.R., Whitman F.T. Influence of toluene co-exposure on the metabolism and genotoxicity of benzene in mice using continuous and intermittent exposures. Chem. Biol. Interact. 2010;184:233–239. doi: 10.1016/j.cbi.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 74.Sarma S.N., Kim Y.J., Song M., Ryu J.C. Induction of apoptosis in human leukemia cells through the production of reactive oxygen species and activation of HMOX1 and Noxa by benzene, toluene, and o-xylene. Toxicology. 2011;280:109–117. doi: 10.1016/j.tox.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 75.Guo H., Ahn S., Zhang L. Benzene-associated immunosuppression and chronic inflammation in humans: A systematic review. Occup. Environ. Med. 2020;78 doi: 10.1136/oemed-2020-106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spatari G., Saitta S., Giorgianni C., Cristani M.T., Quattrocchi P., Abbate A., Carrieri M., Ferraro G., Saija A., Gangemi S. Interleukin-10 involvement in exposure to low dose of benzene. Toxicol. Ind. Health. 2015;31:351–354. doi: 10.1177/0748233713475518. [DOI] [PubMed] [Google Scholar]
- 77. Minciullo PL, Navarra M, Calapai G, Gangemi, S Cytokine network involvement in subjects exposed to benzene. J. Immunol. Res. 2014;2014:937987. doi: 10.1155/2014/937987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hwang K.A., Kim H.R., Kang I. Aging and human CD4(þ) regulatory T cells. Mech. Ageing Dev. 2009;130:509–517. doi: 10.1016/j.mad.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Romer-Seibert Y.J., Meyer S.E. Genetic heterogeneity and clonal evolution in acute myeloid leukemia. Curr. Opin. Hematol. 2021;28:64–70. doi: 10.1097/MOH.0000000000000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim Y.J., Choi J.Y., Paek D., Chung H.W. Association of the NQO1, MPO, and XRCC1 polymorphisms and chromosome damage among workers at a petroleum refinery. J. Toxicol. Environ. Health. 2008;71:333–341. doi: 10.1080/15287390701738558. [DOI] [PubMed] [Google Scholar]
- 81.Kim Y.J., Choi J.Y., Cho Y.H., Woo H.D., Chung H.W. Micronucleus-centromere assay in workers occupationally exposed to low level of benzene. Hum. Exp. Toxicol. 2010;29:343–350. doi: 10.1177/0960327110361500. [DOI] [PubMed] [Google Scholar]
- 82.Pitarque M., Carbonell E., Lapeña N., Marsá M., Torres M., Creus A., Xamena N., Marcos R. No increase in micronuclei frequency in cultured blood lymphocytes from a group of filling station attendants. Mutat. Res. 1996;367:161–167. doi: 10.1016/0165-1218(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 83.Basso E., Cevoli C., Papacchini M., Tranfo G., Mansi A., Testa A. Cytogenetic biomonitoring on a group of petroleum refinery workers. Environ. Mol. Mutagen. 2011;52:440–447. doi: 10.1002/em.20641. [DOI] [PubMed] [Google Scholar]
- 84.Sha Y., Zhou W., Yang Z., Zhu X., Xiang Y., Li T., Zhu D., Yang X. Changes in poly(ADP-ribosyl)ation patterns in workers exposed to BTX. PLoS ONE. 2014;9:e106146. doi: 10.1371/journal.pone.0106146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L., Eastmond D.A., Smith M.T. The nature of chromosomal aberrations detected in humans exposed to benzene. Crit. Rev. Toxicol. 2002;32:1–42. doi: 10.1080/20024091064165. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L., Lan Q., Ji Z., Li G., Shen M., Vermeulen R., Guo W., Hubbard A.E., McHale C.M., Rappaport S.M., et al. Leukemia-related chromosomal loss detected in hematopoietic progenitor cells of benzene-exposed workers. Leukemia. 2012;26:2494–2498. doi: 10.1038/leu.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ji Z., Zhang L., Peng V., Ren X., McHale C.M., Smith M.T. A comparison of the cytogenetic alterations and global DNA hypomethylation induced by the benzene metabolite, hydroquinone, with those induced by melphalan and etoposide. Leukemia. 2010;24:986–991. doi: 10.1038/leu.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith M.T., Zhang L., Jeng M., Wang Y., Guo W., Duramad P., Hubbard A.E., Hofstadler G., Holland N.T. Hydroquinone, a benzene metabolite, increases the level of aneusomy of chromosomes 7 and 8 in human CD34-positive blood progenitor cells. Carcinogenesis. 2000;21:1485–1490. doi: 10.1093/carcin/21.8.1485. [DOI] [PubMed] [Google Scholar]
- 89.Stillman W.S., Varella-Garcia M., Irons R.D. The benzene metabolite, hydroquinone, selectively induces 5q31- and -7 in human CD34+CD19- bone marrow cells. Exp. Hematol. 2000;28:169–176. doi: 10.1016/S0301-472X(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 90.Smith M.T. The mechanism of benzene-induced leukemia: A hypothesis and speculations on the causes of leukemia. Environ. Health Perspect. 1996;104:1219–1225. doi: 10.1289/ehp.961041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faiola B., Fuller E.S., Wong V.A., Recio L. Gene expression profile in bone marrow and hematopoietic stem cells in mice exposed to inhaled benzene. Mutat. Res. 2004;549:195–212. doi: 10.1016/j.mrfmmm.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 92.Wickliffe J.K., Dertinger S.D., Torous D.K., Avlasevich S.L., Simon-Friedt B.R., Wilson M.J. Diet-induced obesity increases the frequency of Pig-a mutant erythrocytes in male C57BL/6J mice. Environ. Mol. Mutagen. 2016;57:668–677. doi: 10.1002/em.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith M.T., Zhang L.P. Biomarkers of leukemia risk: Benzene as a model. Environ. Health Perspect. 1998;106:937–946. doi: 10.1289/ehp.98106s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L., Lan Q., Guo W., Hubbard A.E., Li G., Rappaport S.M., McHale C.M., Shen M., Ji I.Z., Vermeulen R., et al. Chromosome-wide aneuploidy study (CWAS) in workers exposed to an established leukemogen, benzene. Carcinogenesis. 2011;32:605–612. doi: 10.1093/carcin/bgq286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ciccone G., Mirabelli D., Levis A., Gavarotti P., Rege-Cambrin G., Davico L., Vineis P. Myeloid leukemias and myelodysplastic syndromes: Chemical exposure, histologic subtype and cytogenetics in a case-control study. Cancer Genet. Cytogenet. 1993;68:135–139. doi: 10.1016/0165-4608(93)90010-J. [DOI] [PubMed] [Google Scholar]
- 96.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Razin A., Riggs A.D. DNA methylation and gene function. Science. 1980;210:604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- 98.Reik W., Dean W., Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki M.M., Bird A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 100.Herceg Z., Lambert M.P., van Veldhoven K., Demetriou C., Vineis P., Smith M.T., Straif K., Wild C.P. Towards incorporating epigenetic mechanisms into carcinogen identification and evaluation. Carcinogenesis. 2013;34:1955–1967. doi: 10.1093/carcin/bgt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pogribny I.P., Rusyn I. Environmental toxicants, epigenetics, and cancer. Adv. Exp. Med. Biol. 2013;754:215–232. doi: 10.1007/978-1-4419-9967-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sanjuan-Pla A., Bueno C., Prieto C., Acha P., Stam R.W., Marschalek R., Menéndez P. Revisiting the biology of infant t(4;11)/MLL-AF4+ B-cell acute lymphoblastic leukemia. Blood. 2015;126:2676–2685. doi: 10.1182/blood-2015-09-667378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Das P.M., Singal R. DNA methylation and cancer. J. Clin. Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 104.Chappell G., Pogribny I.P., Guyton K.Z., Rusyn I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review. Mutat. Res. Rev. Mutat. Res. 2016;768:27–45. doi: 10.1016/j.mrrev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fukushima S., Kinoshita A., Puatanachokchai R., Kushida M., Wanibuchi H., Morimura K. Hormesis and dose-response-mediated mechanisms in carcinogenesis: Evidence for a threshold in carcinogenicity of non-genotoxic carcinogens. Carcinogenesis. 2005;26:1835–1845. doi: 10.1093/carcin/bgi160. [DOI] [PubMed] [Google Scholar]
- 106.Karpinets T.V., Foy B.D. Tumorigenesis: The adaptation of mammalian cells to sustained stress environment by epigenetic alterations and succeeding matched mutations. Carcinogenesis. 2005;26:1323–1334. doi: 10.1093/carcin/bgi079. [DOI] [PubMed] [Google Scholar]
- 107.Franco R., Schoneveld O., Georgakilas A.G., Panayiotidis M.I. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 108.Iacobuzio-Donahue C.A. Epigenetic changes in cancer. Annu. Rev. Pathol. 2009;4:229–249. doi: 10.1146/annurev.pathol.3.121806.151442. [DOI] [PubMed] [Google Scholar]
- 109.Shlush L.I., Zandi S., Mitchell A., Chen W.C., Brandwein J.M., Gupta V., Kennedy J.A., Schimmer A.D., Schuh A.C., Yee K.W., et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shallis R.M., Ahmad R., Zeidan A.M. The genetic and molecular pathogenesis of myelodysplastic syndromes. Eur. J. Haematol. 2018;101:260–271. doi: 10.1111/ejh.13092. [DOI] [PubMed] [Google Scholar]
- 111.Ren J., Cui J.P., Luo M., Liu H., Hao P., Wang X., Zhang G.H. The prevalence and persistence of aberrant promoter DNA methylation in benzene-exposed Chinese workers. PLoS ONE. 2019;14:e0220500. doi: 10.1371/journal.pone.0220500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Irons R.D., Chen Y., Wang X., Ryder J., Kerzic P.J. Acute myeloid leukemia following exposure to benzene more closely resembles de novo than therapy related-disease. Genes Chromosomes Cancer. 2013;52:887–894. doi: 10.1002/gcc.22084. [DOI] [PubMed] [Google Scholar]
- 113.Portela A., Esteller M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 114.Benbrahim-Tallaa L., Waterland R.A., Dill A.L., Webber M.M., Waalkes M.P. Tumor suppressor gene inactivation during cadmium-induced malignant transformation of human prostate cells correlates with overexpression of de novo DNA methyltransferase. Environ. Health Perspect. 2007;115:1454–1459. doi: 10.1289/ehp.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fischle W., Franz H., Jacobs S.A., Allis C.D., Khorasanizadeh S. Specificity of the chromodomain Y chromosome family of chromodomains for lysine-methylated ARK(S/T) motifs. J. Biol. Chem. 2008;283:19626–19635. doi: 10.1074/jbc.M802655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bernstein B.E., Mikkelsen T.S., Xie X., Kamal M., Huebert D.J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 117.Chapman-Rothe N., Curry E., Zeller C., Liber D., Stronach E., Gabra H., Ghaem-Maghami S., Brown R. Chromatin H3K27me3/H3K4me3 histone marks define gene sets in high grade serous ovarian cancer that distinguish malignant, tumour-sustaining and chemoresistant ovarian tumour cells. Oncogene. 2013;32:4586–4592. doi: 10.1038/onc.2012.477. [DOI] [PubMed] [Google Scholar]
- 118.Hahn M.A., Li A.X., Wu X., Yang R., Drew D.A., Rosenberg D.W., Pfeifer G.P. Loss of the polycomb mark from bivalent promoters leads to activation of cancer-promoting genes in colorectal tumors. Cancer Res. 2014;74:3617–3629. doi: 10.1158/0008-5472.CAN-13-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Greer E.L., Beese-Sims S.E., Brookes E., Spadafora R., Zhu Y., Rothbart S.B., Aristizábal-Corrales D., Chen S., Badeaux A.I., Jin Q., et al. A histone methylation network regulates transgenerational epigenetic memory in C. elegans. Cell Rep. 2014;7:113–126. doi: 10.1016/j.celrep.2014.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bronner C., Krifa M., Mousli M. Increasing role of UHRF1 in the reading and inheritance of the epigenetic code as well as in tumorogenesis. Biochem. Pharmacol. 2013;86:1643–1649. doi: 10.1016/j.bcp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Lubbert M., Oster W., Ludwig W.D., Ganser A., Mertelsmann R., Herrmann F. A switch toward demethylation is associated with the expression of myeloperoxidase in acute myeloblastic and promyelocytic leukemias. Blood. 1992;80:2066–2073. doi: 10.1182/blood.V80.8.2066.2066. [DOI] [PubMed] [Google Scholar]
- 122.Greiner J., Ringhoffer M., Simikopinko O., Szmaragowska A., Huebsch S., Maurer U., Bergmann L., Schmitt M. Simultaneous expression of different immunogenic antigens in acute myeloid leukemia. Exp. Hematol. 2000;28:1413–1422. doi: 10.1016/S0301-472X(00)00550-6. [DOI] [PubMed] [Google Scholar]
- 123.Chambost H., Brasseur F., Coulie P., de Plaen E., Stoppa A.M., Baume D., Mannoni P., Boon T., Maraninchi D., Olive D. A tumour associated antigen expression in human haematological malignancies. Br. J. Haematol. 1993;84:524–526. doi: 10.1111/j.1365-2141.1993.tb03111.x. [DOI] [PubMed] [Google Scholar]
- 124.Zumkeller W. The insulin-like growth factor system in hematopoietic cells. Leuk. Lymphoma. 2002;43:487–491. doi: 10.1080/10428190290011958. [DOI] [PubMed] [Google Scholar]
- 125.Ernst P., Wang J., Korsmeyer S.J. The role of MLL in hematopoiesis and leukemia. Curr. Opin. Hematol. 2002;9:282–287. doi: 10.1097/00062752-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 126.Marchesi F., Girardi K., Avvisati G. Pathogenetic, Clinical, and Prognostic Features of Adult t(4;11)(q21;q23)/MLL-AF4 positive B-cell acute lymphoblastic leukemia. Adv. Hematol. 2011;2011:621627. doi: 10.1155/2011/621627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nanya M., Sato M., Tanimoto K., Tozuka M., Mizutani S., Takagi M. Dysregulation of the DNA damage response and KMT2A rearrangement in fetal liver hematopoietic cells. PLoS ONE. 2015;10:e0144540. doi: 10.1371/journal.pone.0144540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Emerenciano M., Koifman S., Pombo-de-Oliveira M.S. Acute leukemia in early childhood. Braz. J. Med. Biol. Res. 2007;40:749–760. doi: 10.1590/S0100-879X2007000600002. [DOI] [PubMed] [Google Scholar]
- 129.Strick R., Strissel P.L., Borgers S., Smith S.L., Rowley J.D. Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc. Natl. Acad. Sci. USA. 2000;97:4790–4795. doi: 10.1073/pnas.070061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andersson A.K., Ma J., Wang J., Chen X., Gedman A.L., Dang J., Nakitandwe J., Holmfeldt L., Parker M., Easton J., et al. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat. Genet. 2015;47:330–337. doi: 10.1038/ng.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bardini M., Woll P.S., Corral L., Luc S., Wittmann L., Ma Z., Lo Nigro L., Basso G., Biondi A., Cazzaniga G., et al. Clonal variegation and dynamic competition of leukemia-initiating cells in infant acute lymphoblastic leukemia with MLL rearrangement. Leukemia. 2015;29:38–50. doi: 10.1038/leu.2014.154. [DOI] [PubMed] [Google Scholar]
- 132.Stumpel D.J., Schneider P., van Roon E.H., Pieters R., Stam R.W. Absence of global hypomethylation in promoter hypermethylated Mixed Lineage Leukemia-rearranged infant acute lymphoblastic leukemia. Eur. J. Cancer. 2013;49:175–184. doi: 10.1016/j.ejca.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 133.Stam R.W., Schneider P., Hagelstein J.A., van der Linden M.H., Stumpel D.J., de Menezes R.X., de Lorenzo P., Valsecchi M.G., Pieters R. Gene expression profiling-based dissection of MLL translocated and MLL germline acute lymphoblastic leukemia in infants. Blood. 2010;115:2835–2844. doi: 10.1182/blood-2009-07-233049. [DOI] [PubMed] [Google Scholar]
- 134.Bhojwani D., Yang J.J., Pui C.H. Biology of childhood acute lymphoblastic leukemia. Pediatr. Clin. N. Am. 2015;62:47–60. doi: 10.1016/j.pcl.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun R., Xu K., Ji S., Pu Y., Yu L., Yin L., Zhang J., Pu Y. Toxicity in hematopoietic stem cells from bone marrow and peripheral blood in mice after benzene exposure: Single-cell transcriptome sequencing analysis. Ecotoxicol. Environ. Saf. 2021;207:111490. doi: 10.1016/j.ecoenv.2020.111490. [DOI] [PubMed] [Google Scholar]
- 136.Bollati V., Baccarelli A., Hou L., Bonzini M., Fustinoni S., Cavallo D., Byun H.M., Jiang J., Marinelli B., Pesatori A.C., et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67:876–880. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 137.Jiménez-Garza O., Guo L., Byun H.M., Carrieri M., Bartolucci G.B., Barrón-Vivanco B.S., Baccarelli A.A. Aberrant promoter methylation in genes related to hematopoietic malignancy in workers exposed to a VOC mixture. Toxicol. Appl. Pharmacol. 2018;339:65–72. doi: 10.1016/j.taap.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 138.Mancini M., Mandruzzato M., Garzia A.C., Sahnane N., Magnani E., Macchi F., Oulad-Abdelghani M., Oudet P., Bollati V., Fustinoni S., et al. In vitro hydroquinone-induced instauration of histone bivalent mark on human retroelements (LINE-1) in HL60 cells. Toxicol. In Vitro. 2017;40:1–10. doi: 10.1016/j.tiv.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 139.Tabish A.M., Poels K., Hoet P., Godderis L. Epigenetic factors in cancer risk: Effect of chemical carcinogens on global DNA methylation pattern in human TK6 cells. PLoS ONE. 2012;7:e34674. doi: 10.1371/journal.pone.0034674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu J., Ma H., Zhang W., Yu Z., Sheng G., Fu J. Effects of benzene and its metabolites on global DNA methylation in human normal hepatic L02 cells. Environ. Toxicol. 2014;29:108–116. doi: 10.1002/tox.20777. [DOI] [PubMed] [Google Scholar]
- 141.Nishikawa T., Izumo K., Miyahara E., Horiuchi M., Okamoto Y., Kawano Y., Takeuchi T. Benzene induces cytotoxicity without metabolic activation. J. Occup. Health. 2011;53:84–92. doi: 10.1539/joh.10-002-OA. [DOI] [PubMed] [Google Scholar]
- 142.Carnero A., Hannon G.J. The INK4 family of CDK inhibitors. Curr. Top. Microbiol. Immunol. 1998;227:43–55. doi: 10.1007/978-3-642-71941-7_3. [DOI] [PubMed] [Google Scholar]
- 143.Xing E.P., Nie Y., Song Y., Yang G.Y., Cai Y.C., Wang L.D., Yang C.S. Mechanisms of inactivation of p14ARF, p15INK4b, and p16INK4a genes in human esophageal squamous cell carcinoma. Clin. Cancer Res. 1999;5:2704–2713. [PubMed] [Google Scholar]
- 144.Robertson K.D., Jones P.A. The human ARF cell cycle regulatory gene promoter is a CpG island which can be silenced by DNA methylation and down-regulated by wild-type p53. Mol. Cell Biol. 1998;18:6457–6473. doi: 10.1128/MCB.18.11.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Savic S., Franco N., Grilli B., Barascud Ade V., Herzog M., Bode B., Loosli H., Spieler P., Schönegg R., Zlobec I., et al. Fluorescence in situ hybridization in the definitive diagnosis of malignant mesothelioma in effusion cytology. Chest. 2010;138:137–144. doi: 10.1378/chest.09-1951. [DOI] [PubMed] [Google Scholar]
- 146.Jarmalaite S., Kannio A., Anttila S., Lazutka J.R., Husgafvel-Pursiainen K. Aberrant p16 promoter methylation in smokers and former smokers with nonsmall cell lung cancer. Int. J. Cancer. 2003;106:913–918. doi: 10.1002/ijc.11322. [DOI] [PubMed] [Google Scholar]
- 147.Jamebozorgi I., Majidizadeh T., Pouryagoub G., Mahjoubi F. Aberrant DNA Methylation of Two Tumor Suppressor Genes, p14ARF and p15INK4b, after Chronic Occupational Exposure to Low Level of Benzene. Int. J. Occup. Environ. Med. 2018;9:145–151. doi: 10.15171/ijoem.2018.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smith M.T. Advances in understanding benzene health effects and susceptibility. Annu. Rev. Public Health. 2010;31:133–148. doi: 10.1146/annurev.publhealth.012809.103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rinsky R.A., Hornung R.W., Silver S.R., Tseng C.Y. Benzene exposure and hematopoietic mortality: A long-term epidemiologic risk assessment. Am. J. Ind. Med. 2002;42:474–480. doi: 10.1002/ajim.10138. [DOI] [PubMed] [Google Scholar]
- 150.Goldstein B.D. Benzene toxicity. Occup. Med. 1988;3:541–554. [PubMed] [Google Scholar]
- 151.Richardson D.B. Temporal variation in the association between benzene and leukemia mortality. Environ. Health Perspect. 2008;116:370–374. doi: 10.1289/ehp.10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hou L., Zhang X., Wang D., Baccarelli A. Environmental chemical exposure and human epigenetics. Int. J. Epidemiol. 2012;41:79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Esteller M., Tortola S., Toyota M., Capella G., Peinado M.A., Baylin S.B., Herman J.G. Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Res. 2000;60:129–133. [PubMed] [Google Scholar]
- 154.Bodoor K., Haddad Y., Alkhateeb A., Al-Abbadi A., Dowairi M., Magableh A., Bsoul N., Ghabkari A. DNA hypermethylation of cell cycle (p15 and p16) and apoptotic (p14, p53, DAPK and TMS1) genes in peripheral blood of leukemia patients. Asian Pac. J. Cancer Prev. 2014;15:75–84. doi: 10.7314/APJCP.2014.15.1.75. [DOI] [PubMed] [Google Scholar]
- 155.Li J., Bi L., Lin Y., Lu Z., Hou G. Clinicopathological significance and potential drug target of p15INK4B in multiple myeloma. Drug Des. Devel. Ther. 2014;8:2129–2136. doi: 10.2147/DDDT.S71088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seow W.J., Pesatori A.C., Dimont E., Farmer P.B., Albetti B., Ettinger A.S., Bollati V., Bolognesi C., Roggieri P., Panev T.I., et al. Urinary benzene biomarkers and DNA methylation in Bulgarian petrochemical workers: Study findings and comparison of linear and beta regression models. PLoS ONE. 2012;7:e50471. doi: 10.1371/journal.pone.0050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fustinoni S., Rossella F., Polledri E., Bollati V., Campo L., Byun H.M., Agnello L., Consonni D., Pesatori A.C., Baccarelli A., et al. Global DNA methylation and low-level exposure to benzene. Med. Lav. 2012;103:84–95. [PubMed] [Google Scholar]
- 158.Forrest M.S., Lan Q., Hubbard A.E., Zhang L., Vermeulen R., Zhao X., Li G., Wu Y.Y., Shen M., Yin S., et al. Discovery of novel biomarkers by microarray analysis of peripheral blood mononuclear cell gene expression in benzene-exposed workers. Environ. Health Perspect. 2005;113:801–807. doi: 10.1289/ehp.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McHale C.M., Zhang L., Lan Q., Li G., Hubbard A.E., Forrest M.S., Vermeulen R., Chen J., Shen M., Rappaport S.M., et al. Changes in the peripheral blood transcriptome associated with occupational benzene exposure identified by cross-comparison on two microarray platforms. Genomics. 2009;93:343–349. doi: 10.1016/j.ygeno.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McHale C.M., Zhang L., Lan Q., Vermeulen R., Li G., Hubbard A.E., Porter K.E., Thomas R., Portier C.J., Shen M., et al. Global gene expression profiling of a population exposed to a range of benzene levels. Environ. Health Perspect. 2011;119:628–634. doi: 10.1289/ehp.1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schiffman C., McHale C.M. Identification of gene expression predictors of occupational benzene exposure. PLoS ONE. 2018;13:e0205427. doi: 10.1371/journal.pone.0205427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang J., Bai W., Niu P., Tian L., Gao A. Aberrant hypomethylated STAT3 was identified as a biomarker of chronic benzene poisoning through integrating DNA methylation and mRNA expression data. Exp. Mol. Pathol. 2014;96:346–353. doi: 10.1016/j.yexmp.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 163.Xing C., Wang Q.F., Li B., Tian H., Ni Y., Yin S., Li G. Methylation and expression analysis of tumor suppressor genes p15 and p16 in benzene poisoning. Chem. Biol. Interact. 2010;184:306–309. doi: 10.1016/j.cbi.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 164.Philbrook N.A., Winn L.M. Investigating the effects of in utero benzene exposure on epigenetic modifications in maternal and fetal CD-1 mice. Toxicol. Appl. Pharmacol. 2015;289:12–19. doi: 10.1016/j.taap.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 165.Gao A., Zuo X., Song S., Guo W., Tian L. Epigenetic modification involved in benzene-induced apoptosis through regulating apoptosis-related genes expression. Cell Biol. Int. 2011;35:391–396. doi: 10.1042/CBI20100256. [DOI] [PubMed] [Google Scholar]
- 166.Yang J., Zuo X., Bai W., Niu P., Tian L., Gao A. PTEN methylation involved in benzene-induced hematotoxicity. Exp. Mol. Pathol. 2014;96:300–306. doi: 10.1016/j.yexmp.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 167.Gao A., Zuo X., Liu Q., Lu X., Guo W., Tian L. Methylation of PARP-1 promoter involved in the regulation of benzene-induced decrease of PARP-1 mRNA expression. Toxicol. Lett. 2010;195:114–118. doi: 10.1016/j.toxlet.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 168.Heffler E., Allegra A., Pioggia G., Picardi G., Musolino C., Gangemi S. MicroRNA Profiling in Asthma: Potential Biomarkers and Therapeutic Targets. Am. J. Respir. Cell Mol. Biol. 2017;57:642–650. doi: 10.1165/rcmb.2016-0231TR. [DOI] [PubMed] [Google Scholar]
- 169.Bai W., Chen Y., Yang J., Niu P., Tian L., Gao A. Aberrant miRNA profiles associated with chronic benzene poisoning. Exp. Mol. Pathol. 2014;96:426–430. doi: 10.1016/j.yexmp.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 170.Herberth G., Bauer M., Gasch M., Hinz D., Order S., Olek S., Kohajda T., Rolle-Kampczyk U., von Bergen M., Sack U., et al. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J. Allergy Clin. Immunol. 2014;133:543–550. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 171.Wei H., Zhang J., Tan K., Sun R., Yin L., Pu Y. Benzene-Induced Aberrant miRNA Expression Profile in Hematopoietic Progenitor Cells in C57BL/6 Mice. Int. J. Mol. Sci. 2015;16:27058–27071. doi: 10.3390/ijms161126001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bai W., Yang J., Yang G., Niu P., Tian L., Gao A. Long non-coding RNA NR_045623 and NR_028291 involved in benzene hematotoxicity in occupationally benzene-exposed workers. Exp. Mol. Pathol. 2014;96:354–360. doi: 10.1016/j.yexmp.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 173.Baylin S.B., Jones P.A. A decade of exploring the cancer epigenome—Biological and translational implications. Nat. Rev. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lai A.Y., Wade P.A. Cancer biology and NuRD: A multifaceted chromatin remodelling complex. Nat. Rev. 2011;11:588–596. doi: 10.1038/nrc3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cameron E.E., Bachman K.E., Myohanen S., Herman J.G., Baylin S.B. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 176.Foulks J.M., Parnell K.M., Nix R.N., Chau S., Swierczek K., Saunders M., Wright K., Hendrickson T.F., Ho K.K., McCullar M.V., et al. Epigenetic drug discovery: Targeting DNA methyltransferases. J. Biomol. Screen. 2012;17:2–17. doi: 10.1177/1087057111421212. [DOI] [PubMed] [Google Scholar]
- 177.Mahindra A., Laubach J., Raje N., Munshi N., Richardson P.G., Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat. Rev. 2012;9:135–143. doi: 10.1038/nrclinonc.2012.15. [DOI] [PubMed] [Google Scholar]
- 178.Wyhs N., Walker D., Giovinazzo H., Yegnasubramanian S., Nelson W.G. Time-Resolved Fluorescence Resonance Energy Transfer Assay for Discovery of Small-Molecule Inhibitors of Methyl-CpG Binding Domain Protein 2. J. Biomol. Screen. 2014;19:1060–1069. doi: 10.1177/1087057114526433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Musolino C., Sant’antonio E., Penna G., Alonci A., Russo S., Granata A., Allegra A. Epigenetic therapy in myelodysplastic syndromes. Eur. J. Haematol. 2010;84:463–473. doi: 10.1111/j.1600-0609.2010.01433.x. [DOI] [PubMed] [Google Scholar]
- 180.Dhalluin C., Carlson J.E., Zeng L., He C., Aggarwal A.K., Zhou M.M. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 181.Sanchez R., Zhou M.M. The role of human bromodomains in chromatin biology and gene transcription. Curr. Opin. Drug Discov. Dev. 2009;12:659–665. [PMC free article] [PubMed] [Google Scholar]
- 182.Filippakopoulos P., Picaud S., Mangos M., Keates T., Lambert J.P., Barsyte-Lovejoy D., Felletar I., Volkmer R., Muller S., Pawson T., et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vidler L.R., Brown N., Knapp S., Hoelder S. Druggability Analysis and Structural Classification of Bromodomain Acetyl-lysine Binding Sites. J. Med. Chem. 2012;55:7346–7359. doi: 10.1021/jm300346w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W.B., Fedorov O., Morse E.M., Keates T., Hickman T.T., Felletar I., et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nicodeme E., Jeffrey K.L., Schaefer U., Beinke S., Dewell S., Chung C.W., Chandwani R., Marazzi I., Wilson P., Coste H., et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zuber J., Shi J.W., Wang E., Rappaport A.R., Herrmann H., Sison E.A., Magoon D., Qi J., Blatt K., Wunderlich M., et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schroder S., Cho S., Zeng L., Zhang Q., Kaehlcke K., Mak L., Lau J., Bisgrove D., Schnolzer M., Verdin E., et al. Two-pronged binding with bromodomain-containing protein 4 liberates positive transcription elongation factor b from inactive ribonucleoprotein complexes. J. Biol. Chem. 2012;287:1090–1099. doi: 10.1074/jbc.M111.282855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hewings D.S., Fedorov O., Filippakopoulos P., Martin S., Picaud S., Tumber A., Wells C., Olcina M.M., Freeman K., Gill A., et al. Optimization of 3,5-Dimethylisoxazole Derivatives as Potent Bromodomain Ligands. J. Med. Chem. 2013;56:3217–3227. doi: 10.1021/jm301588r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang G., Plotnikov A.N., Rusinova E., Shen T., Morohashi K., Joshua J., Zeng L., Mujtaba S., Ohlmeyer M., Zhou M.M. Structure-guided design of potent diazobenzene inhibitors for the BET bromodomains. J. Med. Chem. 2013;56:9251–9264. doi: 10.1021/jm401334s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Weng J.R., Lai I.L., Yang H.C., Lin C.N., Bai L.Y. Identification of kazinol Q, a natural product from Formosan plants, as an inhibitor of DNA methyltransferase. Phytother. Res. 2014;28:49–54. doi: 10.1002/ptr.4955. [DOI] [PubMed] [Google Scholar]
- 191.Fenga C., Gangemi S., Costa C. Benzene exposure is associated with epigenetic changes (Review) Mol. Med. Rep. 2016;13:3401–3405. doi: 10.3892/mmr.2016.4955. [DOI] [PubMed] [Google Scholar]