Abstract
The spread of the emerging pathogen, named as SARS-CoV-2, has led to an unprecedented COVID-19 pandemic since 1918 influenza pandemic. This review first sheds light on the similarity on global transmission, surges of pandemics, and the disparity of prevention between two pandemics. Such a brief comparison also provides an insight into the potential sequelae of COVID-19 based on the inference drawn from the fact that a cascade of successive influenza pandemic occurred after 1918 and also the previous experience on the epidemic of SARS and MERS occurring in 2003 and 2015, respectively. We then propose a systematic framework for elucidating emerging infectious disease (EID) such as COVID-19 with a panorama viewpoint from natural infection and disease process, public health interventions (non-pharmaceutical interventions (NPIs) and vaccine), clinical treatments and therapies (antivirals), until global aspects of health and economic loss, and economic evaluation of interventions with emphasis on mass vaccination. This review not only concisely delves for evidence-based scientific literatures from the origin of outbreak, the spread of SARS-CoV-2 to three surges of pandemic, and NPIs and vaccine uptakes but also provides a new insight into how to apply big data analytics to identify unprecedented discoveries through COVID-19 pandemic scenario embracing from biomedical to economic viewpoints.
Keywords: 1918 influenza pandemic, COVID-19, Containment strategy, Clinical management, Economic evaluation
Historical viewpoint of both 1918 Spain flu and 2019 COVID-19 pandemic
Human history on pandemic caused by the emerging infectious disease has been documented during the period from sixteen to eighteen century mainly afflicted by the Plague.1 In early 1918, there was a pandemic around the world named as “Spain Flu” afflicting the countries worldwide in parallel with the contemporaneous period of World War I. This 1918 influenza pandemic not only led to the imminent squeal of more than 500 million infected cases and at least 50 million deaths but also produced the influence on societal and economic loss due to containment measurements without vaccine and antivirals.1, 2, 3, 4
Most importantly, although the 1918 pandemic has been tentatively controlled by the implementation of nonpharmaceutical interventions (NPIs) at that time it has also shown a series of far-reaching impacts on both health and societal perspectives. The former has been noted by sequences of the following influenza pandemics including H2N3 in 1957 and 1968 and Avian Flu during 1997 and 2004 and H5N2 in 2009.5 , 6 As far as non-health impacts, the 1918 influenza pandemic had been long postulated to be highly associated with 1929 Great Depression worldwide with the implementation of very strict containment measurements such as isolation and lockdown polices.7 , 8
There is a continental transition of influenza pandemic, shifting from the preponderance of the 1918 pandemic in Western countries (the United States and Europe) to the emergence of its viral variants in Asian countries of those subsequent influenza pandemics after 1918 pandemic.2
Of note, avian influenza emerged in several Asian regions, including Thailand, Vietnam, China, and so on.9 It is also very interesting to note that, in addition to influenza pandemic, two large-scale outbreaks regarding the Corona series of virus such as SARS in 2003 and MERS in 2015 also emerged in the Province of Guangdong in south-eastern China and also Korea. In 2003, the emerging SARS has been spread from China, Hong Kong, to Taiwan, resulting in several large-scale community-acquired outbreaks.10, 11, 12
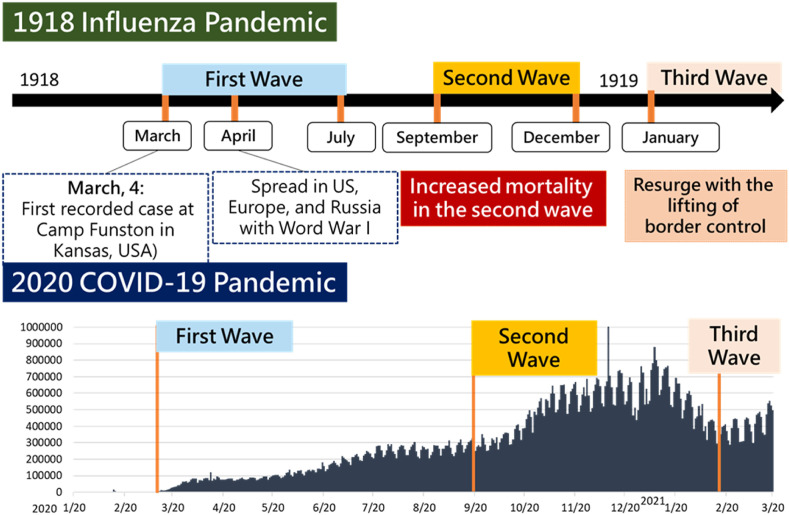
The continental spread of COVID-19 was the opposite of that of 1918 pandemic. From historical viewpoint, there were many similarities between 1918. Fig. 1 shows the chronological order of three waves of pandemic are almost identical. In both years of 1918 and 2020, the declaration of first wave of pandemic was around March, then the second wave resurged probably due to viral variants from September until December, and came the third wave in March of the next year.
Figure 1.
Epidemic curves of the pandemic of 1918 influenza and 2020 COVID-19.
The outbreak of SARS and MERS
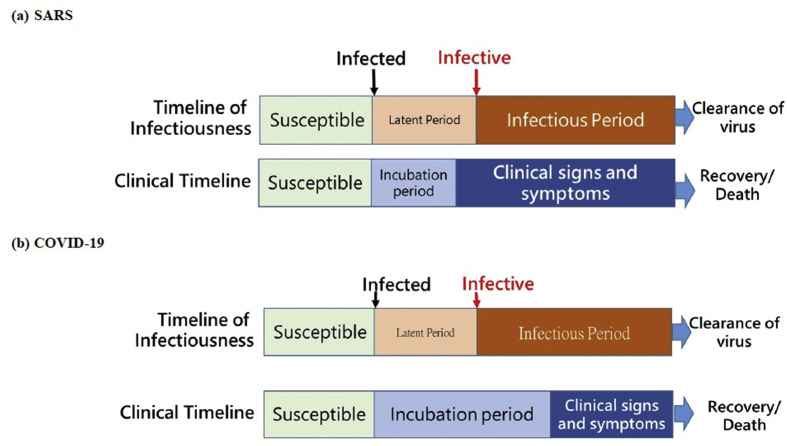
The emergence of SARS in 2003 heralded the potential of coronavirus as a pathogen responsible for large outbreak.10 , 11 , 13 Similar to SARS-CoV-2, the outbreak of SARS in 2003 occurred through a series of clustered transmissions, typically occurred in crowded settings such as hospitals, hotels, and vehicles of public transportation.10 , 11 , 14 Due to the characteristics of being infectious after the presence of fever (Fig. 2 (a) ), the containment strategies focusing on the measurement of body temperature were implemented widely. A typical application in Taiwan was the adoption of fever screening facilities into the working flow of health care services.15 This preventive measure together with the routine implementation of universal precaution including wearing the facial mask and self-hygiene effectively mitigate the risk of large-scale clustered outbreak of SARS in Taiwan in 2003.15 , 16 Even though, the lack of specific tools for early identification of infected subjects and effective antiviral therapies for treating SARS patients resulted in a high case-fatality rate of SARS outbreak in 2003. With the experience of SARS outbreak, infection control measures including the reporting system, syndromic monitoring, procedures and facilities for isolating patients under suspicion, and education programs for health care personnel have been incorporate as part of quality assurance program of health care institutes in Taiwan.16 , 17 The implementation and regulation of border control, isolation, and quarantine from early period of outbreak until the entire COVID-19 pandemic period in Taiwan has rooted in the antecedent experiences on lockdown, isolation, and quarantine polices of SARS outbreaks in 2003.
Figure 2.
Timelines for infectiousness and clinical states of SARS and COVID-19.
A decade after the SARS outbreak, the Middle East respiratory syndrome (MERS) outbreak occurred in South Korea in May, 2015.18 Similar to SARS, a novel coronavirus, MERS-CoV, was identified as the culprit pathogen for a series of clustered events occurred mainly in nosocomial settings in South Korea in 2015.12 , 18 The MERS-CoV was first identified in Saudi Arabia in 2012.19 Although the animal reservoir of camel was proposed as the main source of infection in early phase,20 the clustered events raised serious concerns over human to human transmission.21, 22, 23 This concern was consolidated by the community-acquired outbreak of MERS in South Korea in 2015 following the introduction of MERS-CoV into the hospital caring for a 68 years old patient with travel history to Middle East.18 The comparison between SARS, MERS, and COVID-19 is summarized in Table 1 . The incubation period of SARS-CoV-1 is 4.7 days (95% CI: 4.3–5.1). The incubation periods for MERS (5.8 days (95% CI: 5.0–6.5) and SARS-CoV-2 (5.3 days (95% CI: 4.0–6.9) are longer. The infectious period of SARS-CoV-1 is 8.4 days (95% CI: 3–14) which is shorter for SARS-CoV-2 (7.0 days (95% CI: 4.5–10.4)) and longer for MERS (12.6 days (95% CI: 12.1–13.1)). The reproductive numbers for three highly pathogenic human coronaviruses are close with ranged from 2.6 to 3.0. Compared with the very low case-fatality rate (2.1%), the case-fatality rate over 27% for SARS and MERS are high. A higher 32.1% fatality rate with a significantly higher risk among the elder subjects and those with comorbidities was revealed in a detailed analysis.12 The discovery of antiviral therapy and vaccine for COVID-19 might be the partial reason for lower fatality.
Table 1.
Comparison between SARS, MERS, COVID-19.
| Disease | SARS | MERS | COVID-19 |
|---|---|---|---|
| Year of outbreak | 2002–2003 | 2012–2015 | 2019∼ |
| Pathogen | SARS-CoV-1 | MERS-CoV | SARS-CoV-2 |
| Incubation period(days) | 4.7132 95% CI: 4.3–5.1 |
5.8132 95% CI: 5.0–6.5 |
5.324 95% CI: 4.0–6.9 |
| Infectious period(days) | 8.4133 95% CI: 3-14 |
12.6134 95% CI: 12.1–13.1 |
7.024 95% CI: 4.5–10.4 |
| Infection at incubation period | No | No | Yes |
| Reproductive number | 3.0135 | 2.6136 | 2.844 |
| Case-fatality rate | 27.0%15 | 32.1%12 | 2.1%137 |
| Anti-therapy | No | No | Yes |
| Vaccine | No | No | Yes |
The community-acquired outbreaks of SARS and MERS vividly showed the potential of coronavirus in inducing global pandemic with the serious outcomes of remarkable case-fatality rate. A better understanding for distinct characteristics of latent period and incubation period between SARS and MERS and may give a clue to the transmission and the spread of SARS-CoV-2.
Overall framework of infectious and disease process and evidence-based evaluation of interventions for COVID-19 pandemic
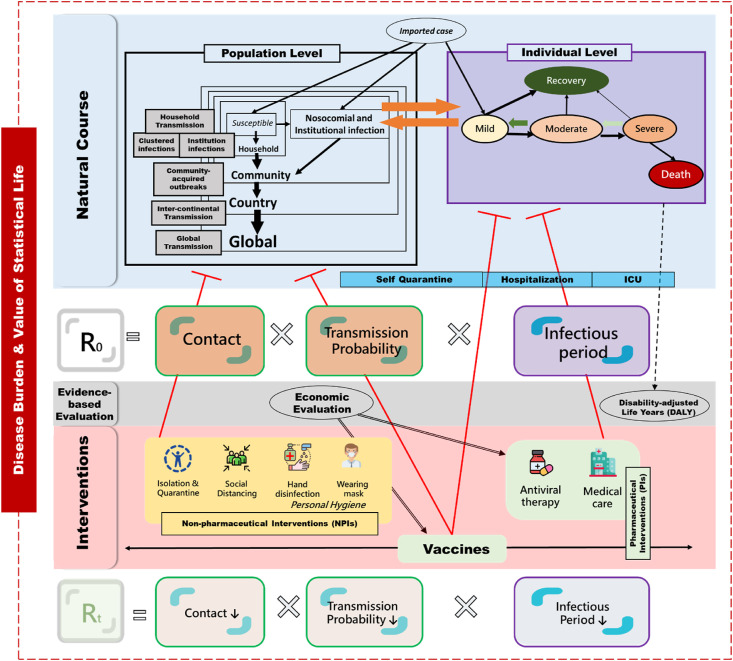
Fig. 3 shows the overall framework for studying emerging infectious disease (EID) with COVID-19 pandemic as an example. It begins from transmission and outbreaks on different levels upheld by the conventional epidemic model (such as susceptible-exposed-infected-recovered (SEIR) model), policies and strategies for NPIs and PIs (pharmaceutical interventions, including vaccine and antiviral therapy), until economic evaluation and estimation for burden of COVID-19 pandemic.
Figure 3.
The framework synthesis of epidemic, containment strategies, clinical management, and economic evaluation of COVID-19 pandemic.
At the population level, it begins with an initial outbreak, household transmission, and early global spread of SARS-CoV-2 through inter-continental transmission before declaration as pandemic, which evolves into global transmission patterns from first to third surge of COVID-19 pandemic. The epidemic models such as the SEIR model are applied to evaluating the basic reproductive number without intervention and effective reproductive number with NPIs or vaccination using different scenarios. The queue model from hospitalization, intensive care unit, until recovery and death is proposed to evaluate the demand and supply of medical capacity during the first and second surge of COVID-19 pandemic. At the individual level, the proposed disease spectrum model has been used to delineate the disease progression from mild to severe COVID-19 and to evaluate antiviral therapy beyond the randomized controlled trial. As vaccine is the most useful means for fighting against COVID-19, economic evaluation of mass vaccination has been provided, making allowance for the value of statistical life and economic production loss. Finally, global burden of COVID-19 has been estimated by using DALYs for health loss and value of statistical life for economic loss associated with COVID-19.
From the origin of China outbreak to intercontinental epidemics before declaration as pandemic
The first outbreak of SARS-CoV-2 occurred in Wuhan, China in December 2019. Beginning with a clustered event in the community associated with a market, the outbreak rapidly swept the entire city with a population of more than 11 million. Li et al. estimated the epidemic growth rate, the epidemic doubling time, and the basic reproductive number by using a transmission model.24 From December 10, 2019 to January 4, 2020, 56% of the first 425 patients with confirmed 2019-nCov were linked to Huanan Seafood Wholesale Market and the patients could have been infected through zoonotic and it is apparent that human-to-human transmission had been occurring and more than half of these cases were older than 45 years old. The mean incubation period was approximately 5.2 days and the basic reproductive number of this outbreak was estimated as 2.2. It is worth mentioning that cases of 2019-nCoV had been doubling in size around one week in Wuhan at this stage. Furthermore, Huang et al. collected 41 admitted hospital patients by January 2, 2020 to figure out the infection of this outbreak.25 Common symptoms at onset of illness included fever, cough, and myalgia or fatigue and there were 6 deaths among reported cases among this cohort. A total of 27 patients had direct exposure to Huanan Seafood Wholesale Market and 9 of them were treated with a higher-level oxygen therapy in the intensive care unit (ICU). Such evidence indicated the origin of the efficient human transmission for 2019-nCoV.
This outbreak not only revealed an important characteristics of SARS-CoV-2 transmission but also heralded the spread of SARS-CoV-2 from hot spot to hot spot over the majority of provinces in China and also inter-continental transmission from the high population density of airport in China to other countries through the trajectory of travelling.26, 27, 28 Based on the comparison of infectious process between SARS and COVID-19, Fig. 2 (b) shows the latent period of SARS-Cov-2 is shorter than the incubation period when the time axis of infection is compared with that of disease onset since the time of infection whereas SARS is not. This means those infected with SARS-CoV-2 become infectious to infect the susceptible subjects before the occurrence of symptoms related to COVID-19. This character accounts for why the epidemic caused by SARS-CoV-2 is more intractable to be prevented than that of SARS as those infectives before symptoms have been proven as pre-symptomatic and asymptomatic cases later during COVID-19 pandemic.
A phenotyping evolution and spatiotemporal clustering phenomenon of the emerging infectious disease, COVID-19, from southern China to countries of the 38° North Latitude via international travel was noted.27, 28, 29, 30, 31 By international mobility after the outbreak in China, several countries had initial outbreak with rapid spreading of SARS-CoV-2 in the early of 2020.26 , 32 In South Korea, the first COVID-19 case, an imported case from China, was confirmed in mid-January. The first outbreak of COVID-19 in South Korea started from the end of January to 19 February, 2020. Due to the church cluster infection, the second outbreak started from 20 February.27 , 33 By 26 February, the government of South Korea implemented the universal testing for COVID-19, and the daily reported cases grew up rapidly.34 The epidemic curve started to decline since 4 March. In Iran, two deaths were reported on 19 February and were confirmed as the first two cases of COVID-19 in the same day in Iran.35 , 36 The outbreak in Iran then occurred on March 24, with more than 1500 daily reported cases up to the end of March. In Rome, Italy, the first two cases of COVID-19 cases who came from Wuhan were confirmed on 31 January.37 , 38 In Lombardy, Italy, the fourth confirmed case was reported on 21 February. After several sporadic cases been reported, extensive tests were performed on subjects with contact history.39 Clustered events associated with a superspreader occurred in Lombardy and started to accumulate COVID-19 cases. After then, the cases numbers in Italy increased dramatically. For other countries in Europe, most of the cases reported from early outbreaks were related to China or Italy. The time series of main outbreak started in other countries surrounding Italy, such as France and Germany. The COVID-19 outbreaks were further spread to the United Kingdom, Scandinavian countries, and other European countries.40 The first confirmed case in the United States was also related to China.40 , 41 The first outbreak not only included imported cases, but also had many cluster infections in different states of the USA. The second outbreak started from 11 March, 2020. On 19 March, the United States government started to increase the capacity of testing. The daily reported case kept increasing until the end of March, 2020.
These transmissions and the effectiveness of containment measurements can be monitored by basic and effective reproductive numbers. The transmission was augmented from hotspot to hotspot with higher reproductive number. By contrast, there was no large community-acquired outbreak in Taiwan during the period. The exempted emerging outbreaks is explained by timely border control and preparedness according to Taiwan experience.42 , 43
Such an evidence-based scientific literatures form the origin of outbreak in Chin to the following links of intercontinental epidemics before the WHO declared COVID-19 pandemic is well documented and proved by the epidemiological data and the epidemic model in the accompanying original article of this special issue.44
Global transmission after pandemic
Following the COVID-19 spreading from the epicenter of Wuhan to the metropolitan cities along 38° North latitude in early months of 2020, SARS-CoV-2 propagated to global countries and regions at accelerated speed. Although World Health Organization (WHO) declared COVID-19 as a public health emergency of international concern (PHEIC) on 30 January, 2020, and announced pandemic on 11 March, 2020,45 underestimating the high SARS-CoV-2 attack and slow reaction in most of public health systems led to the rapid spread of COVID-19 resulting in global pandemic after an initial outbreak was observed.46
Similar to the outbreak occurring in South Korea, Italy, and Iran during the early period, the intrusion of this novel pathogen started from imported cases, followed by the clustered transmission in scenarios of close contact such as household, mass gathering event, and health care facilities including hospitals and long-term care institutes.14 , 31 , 33 , 47, 48, 49 With this augmentation, the COVID-19 outbreak has established its ground and transformed into community-acquired outbreaks lasting for periods before its containment by the implementation of a series of NPIs in the country and region with outbreak. NPIs have been suggested to slow the spread of infection before widespread of effective vaccinations.50
Due to the characteristics of COVID-19 transmission in the pre-symptomatic and asymptomatic state and the high transmissibility as indicated above, several waves of community-acquired outbreaks occurred. The fatal consequences of this long lasting pandemic due to both the high mortality among the elder population and the collapse of health care system in the face of overwhelming cases resulted in an emergent and stringent situation for containing COVID-19 spreading at regional and global scale.51, 52, 53 Given the dilemma of using NPIs as a main strategy for the containment of outbreaks and its tremendous impact of societal and economical activities, the decision on the timing and the extent of lifting social distancing is always scientifically and politically challenging,54 even in the early months of 2021 when effective vaccines are available.
Conventionally, the basic reproductive number (R0) is used to quantify the outbreak and the risk of community-acquired outbreak as indicated in Fig. 3. The outbreak will continue when R0 > 1, whereas the outbreak is controlled while R0 < 1.55 The effective reproductive number (Re) is calculated over time as an indicator together with R0 to explain the effect of containment and mitigation strategies.56 However, relying on reproductive number to manage COVID-19 pandemic is limited.57 This convention of using effective reproductive number (Re) for the identification of community-acquired outbreak based on which the implementation or lifting of NPIs has been confronted by this long lasting and capricious COVID-19 pandemic. No other indicator has been discussed more controversially than the reproduction number.58 There are two novel indicators developed on the basis of learning from COVID-19 pandemic. Chen et al. have developed the index for social distancing based on time-series registry data to assess the balance between COVID-19 disease burden represented by the number of COVID-19 cases and the medical care capacity captured by the number of recovery and case fatality rate of COVID-19,59 and to improve the limited use of reproductive number in COVID-19 pandemic. This novel indicator can be used to get a better understanding of community-acquired outbreaks associated with the containment measurements and new viral variants for global COVID-19 pandemic.
Stemming from the reproductive number and the resources required for containing outbreak and caring infected patients, a new surveillance system is required for monitoring the global and the local community-acquired COVD-19 outbreaks when facing long pandemic period worldwide. To reach the aim of classifying the community-acquired outbreak from different surges of COVID-19 pandemic a series of data-driven models taking into account the duration taken from Rt > 1 to Rt < 1 and case load given each duration was developed. On the basis of this data-driven model, the COVID-19 outbreaks in global countries and regions were categorized into fiver patterns, labelled as “controlled epidemic”, “mutant propagated epidemic”, “propagated epidemic”, “persistent epidemic” and “long persistent epidemic”. Stemming from the classification, the method for the identification of community-acquired outbreak were established and the timing for lifting social index were proposed by using the social distancing index (SDI). The profiles of global transmission and the discoveries on the new indicator of SDI and different patterns of global transmission are detailed in the accompanying original article of this special issue.60
Household transmission of SARS-CoV-2
The patterns of COVID-19 outbreak from first few imported cases to community-acquired outbreak through clustered infections by the enhanced transmission of SARS-CoV-2 in household can be noted since the first pandemic phase.48 , 61 The SARS-CoV-2 is highly transmissible by close contact with infected peopled though fomites or aerosols. Indoor environment with a high likelihood of close contact, such as school or household could be considered as the high-risk settings. Hence, households are the major sources of COVID-19 transmission in the communities. Many clustered infection episodes during periods of outbreaks has been elucidated by household exposure.48 , 61 The elucidation of infection risk among household contact can thus provide valuable information on transmission in population.
The first domestic case was diagnosed on January 28, 2020 in Taiwan, also caused by a family cluster infection because his wife traveled back from Wuhan.62 The higher household attack rate in the early period of COVID-19 pandemic than SARS-CoV and MERS-Cov has been demonstrated in a systematic review study.63 Prompt adoption of containment measurements can reduce the probability from household transmission to community transmission. Studying the household transmission is thus beneficial to reveal the transmission dynamics of a novel respiratory pathogen and to intervene and reduce the viral transmission. By using the empirical data on household clusters in Taiwan, SARS-CoV-2 spreading in such a confined environment is first characterized. Based the estimated results on infection probability in household we further quantify the effectiveness of containment measures implemented in preventing COVID-19 outbreak in Taiwan.
Imported cases in low risk infection area like Taiwan provide a natural opportunity to elucidate household transmission with different transmission mode like Greenwood and Reed-Frost as it is too difficult to have contact tracing of household infection once a large community-acquired outbreak occurred worldwide. Based on a total of 26 households with 39 family clusters in Taiwan, the infected probability was estimated as 44.4% for household transmission in early pandemic period (before 2021). The infected probability was increased to 58.3% in later pandemic period (after 2021). The higher household transmission of SARS-CoV-2 for the period of early 2021 might be due to the emergence of viral variants. This has been addressed in the accompanying original article of this special issue.64
Evaluation of containment and mitigation strategies and vaccine: global and Taiwan perspective
The NPIs from the conventional approaches of quarantine and isolation to the lockdown at different scale have been implement for containing COVID-19 outbreak since first pandemic period. The NPIs of lockdown has been used to contain the outbreak in countries such as Italy.38 , 65 , As part of the border control measures, the effectiveness of NPIs have also been demonstrated in countries such as Taiwan.42 , 66 With the vaccination distribution by the end of year 2020, the NPIs still play a role in containing COVID-19 outbreak due to both the uncertainty of vaccine efficacy in preventing asymptomatic cases and SARS-CoV-2 transmission and the attenuated protection against the emerging variant strains since the third pandemic period.67, 68, 69, 70, 71 Although a series of NPIs have been adopted in countries and regions globally since the early period of first COVID-19 pandemic, its effectiveness in preventing outbreak can hardly been addressed systematically and empirically.72 , 73 This is also true for the implementation of NPIs in conjunction with vaccination distribution.
We thus evaluated the efficacy of NPIs for containing the outbreak of COVID-19 before and after vaccination by using a computer simulation design in conjunction with the susceptible-exposed-infected-recovered (SEIR) type model. With the support from the empirical data on reported COVID-19 cases of Italy and Israel before the implementation of conferment measures, the natural propagation of epidemics was reconstructed. The efficacy for the scenarios of NPIs in flatten the epidemic curve was then assessed on the basis of this nature propagation. On the basis of this simulated study design, the joint effect of confinement measures and vaccination was demonstrated by using Israel scenario. Enlightened by this simulated study design, we further assessed the transitional containment measures of quarantine and isolation using Taiwan scenario.
This systematic approach with computer simulated study design not only provides the backbone for the informed decision making on the implementation of NPIs with the consideration of the effect of vaccination distribution but also explains the successfulness of the border control measured adapted in Taiwan with empirical evidence.
The accompanying original article in this special issue is provided for such a literature review and simulated design on containment measures and vaccination implemented in three scenarios with high infection area such as Italy and Israel as two examples and low risk infection area such as Taiwan as an illustration.74
Social distancing
During the first pandemic period of COVID-19, the NPIs involved with the combination of social distancing, fever screening, and personal hygiene such as wearing mask have been implemented as the mainstay for the prevention and containment of COVID-19 outbreak. The preparedness of NPIs is also crucial for preventing clustered COVID-19 outbreak in mass religious gathering, which have been proved to be highly associated with the acceleration of COVID-19 pandemic in countries such as Korea and Iran.34 , 47 , 75 , 76 Following this rationale, we proposed a framework to assess the preparedness in the context of the classical mass religious gathering of Mazu Pilgrimage in Taiwan.77 This approach was further applied to assessing the NPIs required to prevent the outbreak in the Shincheonji Church of Jesus mass gathering in South Korea.75 On the basis of this assessment, we demonstrated in the accompanying article of this special issue how to quantify the social distancing measures and the scale of implementation required for the containment of COVID-19 transmission in mass religious gathering with two contrasted examples of religious gathering, one in South Korea with the following epidemics and the other in Taiwan demonstrating how the preparedness for containing the outbreak of SARS-Cov-2 was adopted.78
Predicting fatal COVID-19 and medical capacity with the progression rate from pneumonia to ARDS
The elucidation of COVID-19 progression from the mild respiratory disease to pneumonia with the needs of hospitalized care and treatment plays a cardinal role in the triage of COVID-19 cases.79 Strained by the overwhelming number of COVID-19 cases in the first and third pandemic period, the shortage of medical care capacity in terms of facilities, equipment, and personnel have been reported by most of the medical care systems worldwide.53 , 80, 81, 82, 83 The negative association between the overburdened medical care system due to mounting COVID-19 cases and the risk of disease progression and death have been well established.84 The emergence of viral variants with enhanced transmissibility further exacerbates this threat.85 To predict fatal COVID-19 with a surrogate indicator, the medical capacity can be allocated with precision to provide an optimal treatment and to reduce the risk of COVID-19 death.
To address this issue and to provide a real-time monitoring between the balance of medical needs and capacity, Hsu et al. developed an index for predicting the occurrence of fatal COVID-19 by using the progression rate from pneumonia to acute respiratory distress syndrome (ARDS).79 A significant association between this progression index and the reported case fatality rate reported in each county was found (R2 = 95%). In addition, a remarkable variation ranging from 3% to 63% for each county, which was attributed to the corresponding case-fatality rate for each county, was observed. On the basis of this empirical findings, this progression index derived from a disease progression model for COVID-19 can thus be used as a quantitative marker to inform the medical resource allocation to optimize the care for COVID-19 patients.79
Stemming from the established COVID-19 progression model, the indicator by using the progression rate from pneumonia to ARDS was further extended to a global scale to predict the expected fatal COVID-19 and guide the preparedness of medical care capacity in the era of COVID-19 resurgence. Details on this application was elaborated in the accompanying article of this special issue.86
Efficient evaluation for efficacy of antiviral therapy
While the preparedness of medical care capacity is of paramount importance for caring COVID-19 patient, effective treatments and therapies not only can alleviate the risk of disease progression but also accelerate discharge for efficient utilization of medical care resources.87 , 88 Treatment of COVID-19 depends on the course of the infection and the severity of the disease.52 , 89, 90, 91, 92 In the early stage, inhibiting the virus replication by using agents such as remdesivir and antibody therapy is crucial, especially for those who have high risk of disease progression. For COVID-19 patients with severe disease, systemic inflammatory response caused by the immune response to SARS-CoV-2 can lead to cytokine storm with a continuum of inflammatory organ injury. In this stage, anti-inflammatory medications such as dexamethasone thus plays major role.52 , 89, 90, 91, 92 With the elucidation of the pathophysiological effect of SARS-CoV-2 infection, a series of compounds addressing these destructive mechanisms inherited from the clinical progression of COVID-19 have been developed.93 , 94 The timely provision of these antiviral therapies tailored by disease severities can decrease the risk of COVID-19 death and accelerate the rate of recovery and discharge.89 , 95
Following the convention of evidence-based clinical practice, several randomized controlled trials have been conducted to prove the clinical efficacy of these compounds.96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106 Based on the double-blind, randomized, placebo-controlled trial ACTT-1 study, remdesivir therapy showed the efficacy in decreasing the risk of COVID-19 death by 27% with a marginally statistically significance.96 , 97 In order to clarify the inconsistent or underpowered results of remdesivir treatment efficacy, a novel study design and analysis was proposed by Jen et al.107 The study demonstrated that remdesivir treatment can significantly decrease the risk of COVID-19 death by 21%. The number needed to treat of remdesivir treatment required to avoid one death was estimated as 133.3. Following the approach of Jen et al., a Bayesian sequential synthesis design was applied to integrating the information from one-arm compassion study and two-arm ACTT-1 study to evaluate the clinical efficacy of remdesivir with enhanced statistical power. The results showed that remdesivir therapy not only reduces the risk of COVID-19 death (relative risk [RR], 0.69; 95% confidence interval [CI], 0.56 to 0.82) but also increases the odd of discharge (RR, 1.10; 95% CI, 1.01 to 1.18) significantly.
The detailed results and this synthesis design and analysis performed through the combination of one-arm compassion study and two-arm RCT study with the consideration of the dynamic of disease severity refer to the accompanying article of this special issue.108
Assessing medical capacity for COVID-19 patients
Given the severe states of ARDS as a result of clinical progression for COVID-19 cases, the capacity of intensive care unit (ICU) is critical for controlling the mortality of COVID-19. At the beginning of several emerging outbreaks of COVID-19 emerged, a dramatic increase in cases resulted in unaffordable medical capacity and high case fatality rate. If we can determine when and how we should expand the medical capacity such as quarantine beds and ICU capacity, the bed occupancy can be optimized with efficient allocation of medical resources. Several studies have estimated the medical needs for hospital beds in the United States,109 , 110 Mexico,111 Colombia,112 Brazil113 and Italy.114
Allocation of medical capacity has been also affected by SARS-CoV-2 variants of concern (VOCs) that have emerged since late 2020.115 Several studies showed that B.1.1.7 is more likely to transmit to children and the P.1 variant can cause more severe clinical presentations in young adult.116 , 117 A study in Canada demonstrated that the medical needs of hospitalization and ICU care for COVID-19 increased by 21% and 28%, respectively, in March 2021.118 The study further revealed that in Ontario, Canada, the percentage of ICU admission among patients under 60 years have been increasing since December 2020, by when 67% of COVID-19 cases was infected by B.1.1.7 strain.118
Modelling the medical needs for hospitalization, ICU, and home-based setting may resort to a compartment queue model in conjunction with the congestion indices for isolation wards and ICU. The compartment queue model to describe the process from susceptible, self-quarantine and self-isolation at home/hospital, ICU admission to recovery/death, estimating the traffic intensity ratios (TIRs) for the congestion of hospitalization and ICU by taking Lombardy (Italy), France, Spain, Belgium, New York State (United States), South Korea, and Japan for example. These two TIRs can monitor the medical capacity in hospital in response to the COVID-19 pandemic. The higher TIRs the more demand is required to meet increasing cases. TIRs derived from compartment Queue model can be applied to assessing the preparedness of medical capacity during COVID-19 resurgence. The application and the results of these context are provided in the accompanying article of this special issue.119
Economical evaluation for NPIs and vaccines on COVID-19
Non-pharmaceutical interventions were the only effective means to contain the spread of COVID-19 before the roll-out of vaccines in the epoch of COVID-19 pandemic. A simple hand-hygiene was demonstrated as cost-effective with an incremental cost-effectiveness ratio (ICER) of USD $8 only.120 Screening tests and social distancing are also believed to be cost-effective.121 The vaccines with the efficacy in preventing SARS-CoV-2 infection, symptomatic COVID-19, severe COVID-19 state, and COVID-19 death have become available in the beginning of 2021.122, 123, 124 According to the reports of Phase 3 randomized controlled trials of the three major vaccines, BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna) and AZD1222 (Oxford-AstraZeneca), vaccination is anticipated to reduce symptomatic cases by 95%, 94.1%, and 70.4%, respectively. It is also effective in reducing asymptomatic cases by 52.4%, 61.8%, and 27.3% for the three vaccines. With the distribution of these effective vaccines, the containment of COVID-19 outbreaks can be achieved and the progressive lifting of NPIs and border control measures can be expected.
To reach this goal, mass vaccination campaigns have been initiated in many countries and regions of the world. To accelerate the vaccination distribution and reach the goal of population immunity from the level of country and region to global scale, more investment from stakeholders has been urged and propagandized with an expected return of an excessive global benefit. These needs for vaccination distribution at global scale have incurred a global benefit of USD $17.4 trillion, considering the impacts on education and health, with the market design for installing capacity for 3 billion annual vaccine courses, over USD $5800 per course.125
On the perspective of economical evaluation, cost-effectiveness and cost-benefit evaluation are thus of great interest to health decision-makers given such a large scale investment. For an informed decision making, a Markov decision model is often required by taking into account the effectiveness of types of vaccines in preventing the continuum of COVID-19 diseases including asymptomatic, symptomatic, severe, and death and the cost incurred by both mass vaccination program and caring for COVID-19 patients. On the basis of this comprehensive framework, the economical evaluation on the impact of COVID-19 outbreak and the containment measures centered at mass vaccination in terms of cost-effectiveness and cost-benefit were addressed. In the accompanying papers of this issue, vaccination with 70% coverage in areas of epidemic as Israel has, no matter with Pfizer, Moderna, or AstraZeneca, was demonstrated to dominate no vaccination in a cost-utility analysis. The results of cost-benefit analysis suggest that investing USD $1 earlier in vaccine may return ten to thirty USD$ when health and education loss are considered and hundreds of USD$ when value of statistical life (VSL) is taken into account.
The detailed results and the framework of cost-utility analysis and cost-benefit analysis refer to the accompanying original article of this special issue.126
Global burden of COVID-19
The COVID-19 pandemic has caused huge impact on human life in terms of both health and economical outcomes. Following this long-lasting and unprecedented threat to human life, a shorten life expectancy has been foreseen given the high incidence and mortality and prevailed spreading COVID-19.127 For a better understanding of this health and economic impact of COVID-19, the disability-adjusted life years (DALYs) and value of statistical life (VSL) metrics were applied for such a purpose.
DALYs is an important indicator that combines disease incidence and mortality to quantify the burden of a COVID-19. DALYs captures the loss of healthy life year and can be separated in to two parts: years of life lost (YLL) due to premature death and years lost due to disability (YLD) for patients living with the disease.128 While DALYs captures the health outcomes of COVID-19, VSL translates this impact into economic measurement by the methods of Hedonic wage (HWM) and contingent valuation (CVM).129 Using age specific incidence and mortality of COVID-19, the DALYs and VSL for different countries and areas up to the end of April, 2021 can be assessed. Globally, DALYs (in thousands) due to COVID-19 was tallied as 31,930. The estimated VSL were US$591 billion and US$2368 billion based on HWM and CVM, respectively. An increasing trend for global VSL with the propagation of COVID-19 spreading form the first pandemic period in year 2020 to the resurgent phase in 2021 was revealed. It can be noted that a large variation on the impact of COVID-19 across countries and areas under different Human Development Index (HDI) ranging from US$0.001 billion to US$691.4 billion was observed. Big data analytics with machine learning approach to categorize the COVID-19 impact for each country and area into eight categories.
Compared with the global burden of disease (GBD) in 2017, the disease burden of COVID-19 was very close to 45,000 (thousands) for TB and Malaria, 4.61-fold of 6340 for upper respiratory disease, and 4% of 696,000 in the broad category of communicable, maternal, and nutritional deficiency.130
The detailed results on global burden of COVID-19 in terms of DALYs and VSL are provided in the accompanying original article of this special issue.131
In summary, learning from two pandemics spanning over one century, influenza in 1918 and COVID-19 in 2020, and their related successive pandemics and epidemic, this review proposes the study framework of dealing with emerging infectious disease such as COVID-19. The subjects derived from this framework cover a systematic review of contexts from infection and disease process under the theoretical epidemic model, intervention on NPIs and vaccine, and clinical treatment and antiviral therapies, and evidence-based evaluation of these interventions, until global burden of COVID-19. This review also motivates research people involved in COVID-19 to have discoveries on all these contexts in order to provide a panorama and new model after studying the spread and transmission, intervention, and evidence-based evaluation of COVID-19 pandemic.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This study was supported by Ministry of Science and Technology, Taiwan (MOST 109-2327-B-002-009).
References
- 1.Wade L. From Black Death to fatal flu, past pandemics show why people on the margins suffer most. Science. 2020 doi: 10.1126/science.abc7832. [DOI] [Google Scholar]
- 2.Short K.R., Kedzierska K., van de Sandt C.E. Back to the future: lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol. 2018;8:343. doi: 10.3389/fcimb.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belser J.A., Tumpey T.M. The 1918 flu, 100 years later. Science. 2018;359:255. doi: 10.1126/science.aas9565. [DOI] [PubMed] [Google Scholar]
- 4.Taubenberger J.K., Kash J.C., Morens D.M. The 1918 influenza pandemic: 100 years of questions answered and unanswered. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webby R.J., Webster R.G. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 6.Cauchemez S., Ferguson N.M., Wachtel C., Tegnell A., Saour G., Duncan B., et al. Closure of schools during an influenza pandemic. Lancet Infect Dis. 2009;9:473–481. doi: 10.1016/S1473-3099(09)70176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barro R.J., Ursúa J.F., Weng J. The coronavirus and the great influenza pandemic: lessons from the “Spanish flu” for the coronavirus's potential effects on mortality and economic activity. NBER Work Pap. 2020:w26866. http://www.nber.org/papers/w26866 Available at. [Google Scholar]
- 8.Del Angel M., Fohlin C., Weidenmier M.D. Do global pandemics matter for stock prices? NBER Work Pap. 2021:w28356. http://www.nber.org/papers/w28356 Available at. [Google Scholar]
- 9.Webster R., Hulse D. Controlling avian flu at the source. Nature. 2005;435:415–416. doi: 10.1038/435415a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong N.S., Zheng B.J., Li Y.M., Poon, Xie Z.H., Chan K.H., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh Y.H., Chen C.W., Hsu S.B. SARS outbreak, Taiwan, 2003. Emerg Infect Dis. 2004;10:201–206. doi: 10.3201/eid1002.030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y.M., Hsu C.Y., Lai C.C., Yen M.F., Wikramaratna P.S., Chen H.H., et al. Impact of comorbidity on fatality rate of patients with Middle East respiratory syndrome. Sci Rep. 2017;7:11307. doi: 10.1038/s41598-017-10402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q., Gao Y., Wang X., Liu R., Du P., Wang X., et al. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8:629. doi: 10.21037/atm-20-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K.T., Twu S.J., Chang H.L., Wu Y.C., Chen C.T., Lin T.H., et al. SARS in Taiwan: an overview and lessons learned. Int J Infect Dis. 2005;9:77–85. doi: 10.1016/j.ijid.2004.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twu S.J., Chen T.J., Chen C.J., Olsen S.J., Lee L.T., Fisk T., et al. Control measures for severe acute respiratory syndrome (SARS) in Taiwan. Emerg Infect Dis. 2003;9:718–720. doi: 10.3201/eid0906.030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen M.Y., Schwartz J., Shih C.L. Seventeen years after first implementation of traffic control bundling. J Microbiol Immunol Infect. 2021;54:1–3. doi: 10.1016/j.jmii.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui D.S., Perlman S., Zumla A. Spread of MERS to South Korea and China. Lancet Respir Med. 2015;3:509–510. doi: 10.1016/S2213-2600(15)00238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 20.Cotten M., Watson S.J., Kellam P., Al-Rabeeah A.A., Makhdoom H.Q., Assiri A., et al. Transmission and evolution of the Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive genomic study. Lancet. 2013;382:1993–2002. doi: 10.1016/S0140-6736(13)61887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A., et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 23.Memish Z.A., Cotten M., Watson S.J., Kellam P., Zumla A., Alhakeem R.F., et al. Community case clusters of Middle East respiratory syndrome coronavirus in Hafr Al-Batin, Kingdom of Saudi Arabia: a descriptive genomic study. Int J Infect Dis. 2014;23:63–68. doi: 10.1016/j.ijid.2014.03.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinazzi M., Davis J.T., Ajelli M., Gioannini C., Litvinova M., Merler S., et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (2019-nCoV) outbreak. Science. 2020;368:395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S., Jeong Y.D., Byun J.H., Cho G., Park A., Jung J.H., et al. Evaluation of COVID-19 epidemic outbreak caused by temporal contact-increase in South Korea. Int J Infect Dis. 2020;96:454–457. doi: 10.1016/j.ijid.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedford T., Greninger A.L., Roychoudhury P., Starita L.M., Famulare M., Huang M.L., et al. Cryptic transmission of SARS-CoV-2 in Washington state. Science. 2020;370:571–575. doi: 10.1126/science.abc0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J.M., Park S.Y., Lee D., Kim J.S., Park Y., Gwack J., et al. Genomic investigation of the coronavirus disease-2019 outbreak in the Republic of Korea. Sci Rep. 2021;11:6009. doi: 10.1038/s41598-021-85623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J., He W.T., Wang L., Lai A., Ji X., Zhai X., et al. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med. 2020;26:483–495. doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells C.R., Sah P., Moghadas S.M., Pandey A., Shoukat A., Wang Y., et al. Impact of international travel and border control measures on the global spread of the novel 2019 coronavirus outbreak. Proc Natl Acad Sci U S A. 2020;117:7504–7509. doi: 10.1073/pnas.2002616117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J.Y., Hong S.W., Hyun M., Park J.S., Lee J.H., Suh Y.S., et al. Epidemiological and clinical characteristics of coronavirus disease 2019 in Daegu, South Korea. Int J Infect Dis. 2020;98:462–466. doi: 10.1016/j.ijid.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong E., Hagose M., Jung H., Ki M., Flahault A. Understanding South Korea's response to the COVID-19 outbreak: a real-time analysis. Int J Environ Res Publ Health. 2020;17:9571. doi: 10.3390/ijerph17249571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arab-Mazar Z., Sah R., Rabaan A.A., Dhama K., Rodriguez-Morales A.J. Mapping the incidence of the COVID-19 hotspot in Iran - implications for travellers. Trav Med Infect Dis. 2020;34:101630. doi: 10.1016/j.tmaid.2020.101630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadi A., Fadaei Y., Shirani M., Rahmani F. Modeling and forecasting trend of COVID-19 epidemic in Iran until May 13, 2020. Med J Islam Repub Iran. 2020;34:27. doi: 10.34171/mjiri.34.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giovanetti M., Benvenuto D., Angeletti S., Ciccozzi M. The first two cases of 2019-nCoV in Italy: where they come from? J Med Virol. 2020;92:518–521. doi: 10.1002/jmv.25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatto M., Bertuzzo E., Mari L., Miccoli S., Carraro L., Casagrandi R., et al. Spread and dynamics of the COVID-19 epidemic in Italy: effects of emergency containment measures. Proc Natl Acad Sci U S A. 2020;117:10484–10491. doi: 10.1073/pnas.2004978117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 40.Worobey M., Pekar J., Larsen B.B., Nelson M.I., Hill V., Joy J.B., et al. The emergence of SARS-CoV-2 in Europe and North America. Science. 2020;370:564–570. doi: 10.1126/science.abc8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Constantino-Shor C., Rani G., Olin S., Holmes C., Nasenbeny K. Containment of a COVID-19 outbreak in an inpatient geriatric psychiatry unit. J Am Psychiatr Nurses Assoc. 2021;27:77–82. doi: 10.1177/1078390320970653. [DOI] [PubMed] [Google Scholar]
- 42.Cheng H.Y., Chueh Y.N., Chen C.M., Jian S.W., Lai S.K., Liu D.P. Taiwan's COVID-19 response: timely case detection and quarantine, January to June 2020. J Formos Med Assoc. 2021 Jun;120(6):1400–1404. doi: 10.1016/j.jfma.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in taiwan: big data analytics, new Technology, and proactive testing. J Am Med Assoc. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 44.Ku M.S., Huang L.M., Chiu Y.H., Wang W.C., Jeng Y.C., Yen M.Y., et al. Continental transmission of emerging COVID-19 on the 38o North latitude. J Formos Med Assoc. 2021;120:S19–S25. doi: 10.1016/j.jfma.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO . 2020. Rolling updates on coronavirus disease (COVID-19)https://www.who.int/emergencies/diseases/novelcoronavirus-2019/events-as-they-happen Accessed at. [Google Scholar]
- 46.World Health Organization . 2021. COVID-19 weekly epidemiological update, 27 April 2021. [Google Scholar]
- 47.Badshah S.L., Ullah A. Spread of coronavirus disease-19 among devotees during religious congregations. Ann Thorac Med. 2020;15:105–106. doi: 10.4103/atm.ATM_162_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G., et al. Epidemiology of covid-19 in a long-term care facility in king county, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewnard J.A., Lo N.C. Scientific and ethical basis for social-distancing interventions against COVID-19. Lancet Infect Dis. 2020;20:631–633. doi: 10.1016/S1473-3099(20)30190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., et al. Fair allocation of scarce medical resources in the time of covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 52.Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages - the need for ventilators and personal protective equipment during the covid-19 pandemic. N Engl J Med. 2020;382:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 54.Kreps S.E., Kriner D.L. Model uncertainty, political contestation, and public trust in science: evidence from the COVID-19 pandemic. Sci Adv. 2020;6 doi: 10.1126/sciadv.abd4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas J.C., Weber D.J. Oxford University Press; 2001. Epidemiologic methods for the study of infectious diseases. [Google Scholar]
- 56.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adam D. A guide to R - the pandemic's misunderstood metric. Nature. 2020;583:346–348. doi: 10.1038/d41586-020-02009-w. [DOI] [PubMed] [Google Scholar]
- 58.Delamater P.L., Street E.J., Leslie T.F., Yang Y.T., Jacobsen K.H. Complexity of the basic reproduction number (R0) Emerg Infect Dis. 2019;25:1–4. doi: 10.3201/eid2501.171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S.L., Yen A.M., Lai C.C., Hsu C.Y., Chan C.C., Chen T.H. An index for lifting social distancing during the COVID-19 pandemic: algorithm recommendation for lifting social distancing. J Med Internet Res. 2020;22 doi: 10.2196/22469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang W.C., Lin T.Y., Chiu S.Y., Chen C.N., Sarakarne P., Ibrahim M., et al. Global transmission of COVID-19 for classifications of community-acquired outbreaks: machine learning and statistical model analysis. J Formos Med Assoc. 2021;120:S26–S37. doi: 10.1016/j.jfma.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y.C., Liao C.H., Chang C.F., Chou C.C., Lin Y.R. A locally transmitted case of SARS-CoV-2 infection in taiwan. N Engl J Med. 2020;382:1070–1072. doi: 10.1056/NEJMc2001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madewell Z.J., Yang Y., Longini I.M., Jr., Halloran M.E., Dean N.E. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu C.Y., Wang J.T., Huang K.C., Fan A.C.H., Yeh Y.P., Chen S.L.S. Household transmission but without the community-acquired outbreak of COVID-19 in taiwan. JFormos Med Assoc. 2021;120:S38–S45. doi: 10.1016/j.jfma.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertuzzo E., Mari L., Pasetto D., Miccoli S., Casagrandi R., Gatto M., et al. The geography of COVID-19 spread in Italy and implications for the relaxation of confinement measures. Nat Commun. 2020;11:4264. doi: 10.1038/s41467-020-18050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Summers J., Cheng H.Y., Lin H.H., Barnard L.T., Kvalsvig A., Wilson N., et al. Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic. The Lancet Regional Health-Western Pacific. 2020:100044. doi: 10.1016/j.lanwpc.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen R.E., Zhang X., Case J.B., Winkler E.S., Liu Y., VanBlargan L.A., et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27:717–726. doi: 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emary K.R.W., Golubchik T., Aley P.K., Ariani C.V., Angus B., Bibi S., et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A., et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384:1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., et al. Efficacy of the ChAdOx1 nCoV-19 covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu K., Werner A.P., Koch M., Choi A., Narayanan E., Stewart-Jones G.B.E., et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med. 2021;384:1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haushofer J., Metcalf C.J.E. Which interventions work best in a pandemic? Science. 2020;368:1063–1065. doi: 10.1126/science.abb6144. [DOI] [PubMed] [Google Scholar]
- 73.Soltesz K., Gustafsson F., Timpka T., Jaldén J., Jidling C., Heimerson A., et al. The effect of interventions on COVID-19. Nature. 2020;588:E26–E28. doi: 10.1038/s41586-020-3025-y. [DOI] [PubMed] [Google Scholar]
- 74.Lin T.Y., Liao S.H., Lai C.C., Pacie E., Chuang S.Y. Effectiveness of non-pharmaceutical interventions and vaccine for containing the spread of COVID-19: three illustrations before and after vaccination periods. J Formos Med Assoc. 2021;120:S46–S56. doi: 10.1016/j.jfma.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang M.C., Baek J.H., Park D. Lessons from South Korea regarding the early stage of the COVID-19 outbreak. Healthcare. 2020;8:229. doi: 10.3390/healthcare8030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yezli S., Khan A. COVID-19 pandemic: it is time to temporarily close places of worship and to suspend religious gatherings. J Trav Med. 2021;28:taaa065. doi: 10.1093/jtm/taaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang C.L., Chu C.H. The expanding of Mazu belief in Taiwan-A analysis on the 2009 Tachia Mazu go around boundary ceremony. Journal of Data Analysis. 2012;7:107–127. [Google Scholar]
- 78.Hsu C.Y., Chen Y.M., Su C.W., Ku M.S., Kim Y., Jensen T., et al. Preparedness for containing COVID-19 outbreak in mass religious gathering with non-pharmaceutical interventions (NPIs) J Formos Med Assoc. 2021;120:S57–S68. doi: 10.1016/j.jfma.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsu C.Y., Lai C.C., Yeh Y.P., Chan C.C., Chen H.H. Progression from pneumonia to ARDS as a predictor for fatal COVID-19. J Infect Public Health. 2021;14:504–507. doi: 10.1016/j.jiph.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma X., Vervoort D. Critical care capacity during the COVID-19 pandemic: global availability of intensive care beds. J Crit Care. 2020;58:96–97. doi: 10.1016/j.jcrc.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peters A.W., Chawla K.S., Turnbull Z.A. Transforming ORs into ICUs. N Engl J Med. 2020;382:e52. doi: 10.1056/NEJMc2010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosenbaum L. Facing covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic's front line. N Engl J Med. 2020;382:1873–1875. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 83.Truog R.D., Mitchell C., Daley G.Q. The toughest triage - allocating ventilators in a pandemic. N Engl J Med. 2020;382:1973–1975. doi: 10.1056/NEJMp2005689. [DOI] [PubMed] [Google Scholar]
- 84.Bravata D.M., Perkins A.J., Myers L.J., Arling G., Zhang Y., Zillich A.J., et al. Association of intensive care unit patient load and demand with mortality rates in US department of veterans affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.34266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdool Karim S.S., de Oliveira T. New SARS-CoV-2 variants - clinical, public health, and vaccine implications. N Engl J Med. 2021;24 doi: 10.1056/NEJMc2100362. NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang W.J., Chen Y.C., Hsu C.Y., Chen C.D., Chen S.L.S., Chang K.J. Probabilistic forecasts of COVID-19 deaths with the progression rate from pneumonia to ARDS: an open-data-based global study. JFormos Med Assoc. 2021;120:S69–S76. doi: 10.1016/j.jfma.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gupta R. Advancing new tools for infectious diseases. Science. 2020;370:913–914. doi: 10.1126/science.abe0773. [DOI] [PubMed] [Google Scholar]
- 88.Mehta N., Mazer-Amirshahi M., Alkindi N., Pourmand A. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am J Emerg Med. 2020;38:1488–1493. doi: 10.1016/j.ajem.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 90.Lamontagne F., Agoritsas T., Siemieniuk R., Rochwerg B., Bartoszko J., Askie L., et al. A living WHO guideline on drugs to prevent covid-19. BMJ. 2021;372:n526. doi: 10.1136/bmj.n526. [DOI] [PubMed] [Google Scholar]
- 91.National Institute of Health . National Institute of Health; 2020. COVID-19 Treatment guidelines. November 3, 2020.https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/remdesivir/ Available from: [Google Scholar]
- 92.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. Therapeutics and COVID-19: living guideline, 20 November 2020.https://www.who.int/publications/i/item/therapeutics-and-covid-19-living-guideline Available from: [Google Scholar]
- 93.Abdelrahman Z., Liu Q., Jiang S., Li M., Sun Q., Zhang Y., et al. Evaluation of the current therapeutic approaches for COVID-19: a systematic review and a meta-analysis. Front Pharmacol. 2021;12:607408. doi: 10.3389/fphar.2021.607408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu W., Zhou P., Chen K., Ye Z., Liu F., Li X., et al. Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis. CMAJ (Can Med Assoc J) 2020;192:E734–E744. doi: 10.1503/cmaj.200647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goyal A., Cardozo-Ojeda E.F., Schiffer J.T. Potency and timing of antiviral therapy as determinants of duration of SARS-CoV-2 shedding and intensity of inflammatory response. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of covid-19—preliminary report. N Engl J Med. 2020;383:993–994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 97.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen C.P., Lin Y.C., Chen T.C., Tseng T.Y., Wong H.L., Kuo C.Y., et al. A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19) PloS One. 2020;15 doi: 10.1371/journal.pone.0242763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.RECOVERY Collaborative Group. Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., et al. Effect of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.RECOVERY Collaborative Group Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mitjà O., Corbacho-Monné M., Ubals M., Tebe C., Peñafiel J., Tobias A., et al. Hydroxychloroquine for early treatment of adults with mild covid-19: a randomized-controlled trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1009. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Omrani A.S., Pathan S.A., Thomas S.A., Harris T.R.E., Coyle P.V., Thomas C.E., et al. Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19. EClinicalMedicine. 2020;29:100645. doi: 10.1016/j.eclinm.2020.100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jen H.H., Chang W.J., Lin T.Y., Hsu C.Y., Yen A.M., Lai C.C., et al. Evaluating clinical efficacy of antiviral therapy for COVID-19: a surrogate endpoint Approach. Infect Dis Ther. 2021:1–11. doi: 10.1007/s40121-021-00431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liao S.H., Hung C.C., Chen C.N., Yen J.Y., Hsu C.Y., Yen A.M., et al. Assessing efficacy of antiviral therapy for COVID-19 patients: a case study on remdesivir with bayesian synthesis design and multistate analysis. J Formos Med Assoc. 2021;120:S77–S85. doi: 10.1016/j.jfma.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.IHME COVID-19 health service utilization forecasting team, Murray C.J. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator-days and deaths by US state in the next 4 months. MedRxiv. 2020 doi: 10.1101/2020.03.27.20043752. [DOI] [Google Scholar]
- 110.Li R., Rivers C., Tan Q., Murray M.B., Toner E., Lipsitch M. Estimated demand for US hospital inpatient and intensive care unit beds for patients with COVID-19 based on comparisons with wuhan and guangzhou, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Capistran M.A., Capella A., Christen J.A. Forecasting hospital demand in metropolitan areas during the current COVID-19 pandemic and estimates of lockdown-induced 2nd waves. PloS One. 2021;16 doi: 10.1371/journal.pone.0245669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rivera-Rodriguez C., Urdinola B.P. Predicting hospital demand during the COVID-19 outbreak in bogotá, Colombia. Front Public Health. 2020;8:582706. doi: 10.3389/fpubh.2020.582706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Noronha K.V.M.S., Guedes G.R., Turra C.M., Andrade M.V., Botega L., Nogueira D., et al. The COVID-19 pandemic in Brazil: analysis of supply and demand of hospital and ICU beds and mechanical ventilators under different scenarios. Cad Saúde Pública. 2020;36 doi: 10.1590/0102-311X00115320. [DOI] [PubMed] [Google Scholar]
- 114.Giordano G., Colaneri M., Di Filippo A., Blanchini F., Bolzern P., De Nicolao G., et al. Modeling vaccination rollouts, SARS-CoV-2 variants and the requirement for non-pharmaceutical interventions in Italy. Nat Med. 2021 doi: 10.1038/s41591-021-01334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.World Health Organization (WHO) 2019. Coronovirus disease 2019 (COVID-19) situation report-76.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200405-sitrep-76-covid-19.pdf?sfvrsn=6ecf0977_2 [Google Scholar]
- 116.Taylor L. Covid-19: Brazil's spiralling crisis is increasingly affecting young people. BMJ. 2021;373:n879. doi: 10.1136/bmj.n879. [DOI] [PubMed] [Google Scholar]
- 117.Freitas A.R.R., Beckedorff O.A., Cavalcanti L.P.D.G., Siqueira A.M., Castro D.B., Costa C.F., et al. The emergence of novel SARS-CoV-2 variant P.1 in Amazonas (Brazil) was temporally associated with a change in the age and gender profile of COVID-19 mortality. Sci ELO. 2021 doi: 10.1590/SciELOPreprints.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tuite A.R., Fisman D.N., Odutayo A., Bobos P., Allen V., Bogoch I.I., et al. COVID-19 hospitalizations, ICU admissions and deaths associated with the new variants of concern. Sci Briefs Ont COVID-19 Sci Advis Table. 2021:1. [Google Scholar]
- 119.Jen G.H., Chen S.Y., Chang W.J., Chen C.N., Yen A.M.F., Chang R.E. Evaluating medical capacity for hospitalization and intensive care unit of COVID-19: a queue model approach. J Formos Med Assoc. 2021;120:S86–S94. doi: 10.1016/j.jfma.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bagepally B.S., Haridoss M., Natarajan M., Jeyashree K., Ponnaiah M. Cost-effectiveness of surgical mask, N-95 respirator, hand-hygiene and surgical mask with hand hygiene in the prevention of COVID-19: cost effectiveness analysis from Indian context. Clin Epidemiol Glob Health. 2021;10:100702. doi: 10.1016/j.cegh.2021.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rezapour A., Souresrafil A., Peighambari M.M., Heidarali M., Tashakori-Miyanroudi M. Economic evaluation of programs against COVID-19: a systematic review. Int J Surg. 2021;85:10–18. doi: 10.1016/j.ijsu.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Castillo J.C., Ahuja A., Athey S., Baker A., Budish E., Chipty T., et al. Market design to accelerate COVID-19 vaccine supply. Science. 2021;371:1107–1109. doi: 10.1126/science.abg0889. [DOI] [PubMed] [Google Scholar]
- 126.Wang W.C., Liu J.T., Fann J.C., Chang R.E., Jeng Y.C., Hsu C.Y., et al. 2021. Economic evaluation for mass vaccination against COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marois G., Muttarak R., Scherbov S. Assessing the potential impact of COVID-19 on life expectancy. PloS One. 2020;15 doi: 10.1371/journal.pone.0238678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prüss-Üstün A., Mathers C., Corvalán C., Woodward A. 2003. Introduction and methods: assessing the environmental burden of disease at national and local levels. WHO Environmental burden of disease series.https://www.who.int/quantifying_ehimpacts/publications/9241546204/en/ Available online: [Google Scholar]
- 129.Liu J.T., Hammitt J.K., Wang J.D., Tsou M.W. Valuation of the risk of SARS in taiwan. Health Econ. 2005;14:83–91. doi: 10.1002/hec.911. [DOI] [PubMed] [Google Scholar]
- 130.GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan C.Y., Fann J.C.Y., Yang M.C., Lin T.Y., Chen H.H., Liu J.T., et al. Estimating global burden of COVID-19 with disability-adjusted life years and value of statistical life metrics. J Formos Med Assoc. 2021;120:S106–S117. doi: 10.1016/j.jfma.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang X., Rayner S., Luo M.H. Does SARS-CoV-2 has a longer incubation period than SARS and MERS? J Med Virol. 2020;92(5):476–478. doi: 10.1002/jmv.25708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lipsitch M., Cohen T., Cooper B., Robins J.M., Ma S., James L., et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003 Jun 20;300(5627):1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cowling B.J., Park M., Fang V.J., Wu P., Leung G.M., Wu J.T. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 2015 Jun 25;20(25):7–13. doi: 10.2807/1560-7917.es2015.20.25.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bauch C.T., Lloyd-Smith J.O., Coffee M.P., Galvani A.P. Dynamically modeling SARS and other newly emerging respiratory illnesses: past, present, and future. Epidemiology. 2005 Nov;16(6):791–801. doi: 10.1097/01.ede.0000181633.80269.4c. [DOI] [PubMed] [Google Scholar]
- 136.Yang Y.M. National Taiwan University; 2017. Stochastic differential equation model for respiratory infectious disease using bayesian Markov chain Monte Carlo (MCMC) method.https://hdl.handle.net/11296/c24atr Doctoral Dissertation. Available at: [Google Scholar]
- 137.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]