Summary
The H-NS-like proteins MvaT and MvaU act coordinately as global repressors in Pseudomonas aeruginosa by binding to AT-rich regions of the chromosome. Although cells can tolerate loss of either protein, identifying their combined regulatory effects has been challenging because the loss of both proteins is lethal due to induction of prophage Pf4 and subsequent superinfection of the cell. In other bacteria, H-NS promotes cellular fitness by inhibiting intragenic transcription from AT-rich target regions, preventing them from sequestering RNA polymerase; however, it is not known whether MvaT and MvaU function similarly. Here we utilize a parental strain that cannot be infected by Pf4 phage to define the collective MvaT and MvaU regulon and demonstrate that the combined loss of both MvaT and MvaU leads to increased intragenic transcription from loci directly controlled by these proteins. We further show that the loss of MvaT and MvaU leads to a striking redistribution of RNA polymerase containing σ70 to genomic regions vacated by these proteins. Our findings suggest that the ability of H-NS-like proteins to repress intragenic transcription is a universal function of these proteins and point to a second mechanism by which MvaT and MvaU may contribute to the growth of P. aeruginosa.
Keywords: MvaT, MvaU, transcription silencing factors
Graphical Abstract
Introduction
Pseudomonas aeruginosa, a ubiquitous gram-negative rod, is a leading cause of hospital acquired pneumonia, surgical site infections, catheter-associated urinary tract infections, and central line-associated bloodstream infections (Nathwani et al., 2014, Weiner-Lastinger et al., 2020). P. aeruginosa also causes devastating acute infections in immunocompromised individuals and burn victims, and chronic infections in patients with decreased mucociliary clearance, as in cystic fibrosis (Afessa & Green, 2000, Chatzinikolaou et al., 2000, Emerson et al., 2002, Finch et al., 2015, Govan & Deretic, 1996, Santucci et al., 2003). Intrinsic antibiotic resistance (Dotsch et al., 2009, Geisinger & Isberg, 2017, Pang et al., 2019), the ability to form biofilms on living and abiotic surfaces (Costerton et al., 1999, Kurmoo et al., 2020, Vallet et al., 2004, Vallet et al., 2001), and a host of virulence factors (Geisinger & Isberg, 2017), make P. aeruginosa infections difficult to treat (Bassetti et al., 2018, Curran et al., 2018).
In P. aeruginosa the histone nucleoid structuring (H-NS) family-like protein MvaT (Tendeng et al., 2003) has been shown to repress the expression of virulence genes (Castang et al., 2008, Diggle et al., 2002, Westfall et al., 2006), including those of the cupA fimbrial gene cluster required to form biofilms on abiotic surfaces (O'Toole & Kolter, 1998, Vallet et al., 2004, Vallet et al., 2001). MvaT is thought to act coordinately with MvaU, a second H-NS-like protein in P. aeruginosa (Vallet-Gely et al., 2005). Like H-NS and its relatives, MvaT and MvaU are thought to act globally as repressors by specifically binding AT-rich DNA sequences as either homo- or heterodimers (Castang & Dove, 2010, Castang et al., 2008, Ding et al., 2015, Lucchini et al., 2006, Navarre et al., 2006, Oshima et al., 2006, Vallet-Gely et al., 2005), forming oligomers over large stretches of DNA (Castang & Dove, 2010, Winardhi et al., 2012) and bridging adjacent DNA sequences (Dame et al., 2005, Winardhi et al., 2012). MvaT and MvaU likely act in a fashion analogous to H-NS, which is thought to silence transcription by either occluding RNA polymerase (Dorman, 2007, Dorman, 2014, Rimsky et al., 2001), by trapping RNA polymerase at promoters (Dame et al., 2002, Schroder & Wagner, 2000, Shin et al., 2005), or by promoting RNA polymerase pausing and transcription termination (Dole et al., 2002, Kotlajich et al., 2015, Peters et al., 2012, Saxena & Gowrishankar, 2011). However, the mechanism by which MvaT and MvaU globally regulate transcription is not fully understood. Nevertheless, high-order oligomerization appears to be necessary to silence target genes (Castang & Dove, 2010).
That MvaT and MvaU act together to control a common set of genes in P. aeruginosa is supported by several observations. First, these proteins co-purify with one another and can interact with each other directly (Vallet-Gely et al., 2005). Second, both MvaT and MvaU appear to occupy essentially the same AT-rich regions of the P. aeruginosa chromosome, as revealed by chromatin immunoprecipitation (ChIP) and DNA microarray analysis (Castang et al., 2008). Third, MvaT and MvaU have been found to exert synergistic effects on the expression of target genes (Castang et al., 2008, Williams McMackin et al., 2019). Making this latter observation was complicated by the fact that the activities of MvaT and MvaU are essential for cell viability (Castang et al., 2008, Williams McMackin et al., 2019). Indeed, although the loss of either MvaT or MvaU can be tolerated by the cell (Castang et al., 2008, Diggle et al., 2002), the loss of both MvaT and MvaU can only occur in cells that also contain inactivating mutations in genes specifying the endogenous Pf4 prophage or in genes required for production of the type IV pilus, which serves as the receptor for the phage (Castang & Dove, 2012). Despite the essential function of MvaT and MvaU being to limit prophage induction, cells lacking both MvaT and MvaU still exhibit a growth defect even when they are no longer able to make type IV pili or the replicative form of Pf4 (Castang & Dove, 2012). Thus, MvaT and MvaU influence the fitness of P. aeruginosa through mechanisms that appear unrelated to the repression of Pf4 phage induction.
An important role of H-NS in enteric bacteria is to limit transcription that initiates from within target genes (i.e. intragenic transcription) (Lamberte et al., 2017, Singh et al., 2014). Although promoters are typically thought to be located immediately outside of the genes whose expression they drive, it is becoming increasingly apparent that some promoters can be housed within genes and oriented in either the sense or anti-sense direction (Wade & Grainger, 2014). Indeed, the high AT-content of H-NS-bound genes is thought to increase the chance they contain sequences resembling the −10 element of a σ70-dependent promoter (Lamberte et al., 2017, Singh et al., 2014), and recent work indicates that when additional AT-rich sequences are positioned approximately 5 bp upstream of such −10 like-elements, the combined sequence elements can constitute active promoters (Warman et al., 2020). In enteric bacteria, the binding of H-NS to AT-rich DNA sequences is thought to promote cellular fitness by inhibiting intragenic transcription from these sequences and thus preventing them from serving as molecular sinks for the available RNA polymerase in the cell (Lamberte et al., 2017). Like their enteric counterparts, MvaT and MvaU are known to associate with AT-rich regions of DNA (Castang et al., 2008); however, whether MvaT and MvaU act to silence transcription initiation from intragenic promoters in P. aeruginosa was not known. Here we identify the MvaT/MvaU regulon in P. aeruginosa and present evidence that these H-NS-like proteins act to inhibit transcription from both conventionally positioned promoters and from many promoters that reside within MvaT- and MvaU-bound genes. Our findings suggest that limiting the occupancy of AT-rich genes by RNA polymerase may be an important activity of H-NS-like proteins in P. aeruginosa and bolster the argument that the repression of intragenic transcription may be a hallmark of H-NS and H-NS related proteins.
Results
Loss of both MvaT and MvaU causes global changes in gene expression
Previous transcriptomic studies have identified genes that were differentially expressed in cells lacking MvaT or MvaU (Vallet et al., 2004, Westfall et al., 2006). However, understanding the regulatory effects of these proteins is complicated by the fact that they can functionally substitute for one another to some degree, and by the fact that they control the expression of one another’s genes; for example deletion of the mvaU gene leads to compensatory increases in mvaT expression and protein levels (Vallet-Gely et al., 2005, Vallet et al., 2004). Furthermore, evidence suggests that MvaT and MvaU act together to exert their regulatory effects (Castang et al., 2008, Williams McMackin et al., 2019). Thus, to properly define the MvaT/MvaU regulon we sought to compare the transcriptomes of cells encoding both proteins to those that lacked both. Because the combined activities of MvaT and MvaU are essential, we exploited the fact that cells lacking the pilY1 gene, which encodes a regulator and structural component of the type IV pilus, can tolerate the loss of both MvaT and MvaU (Castang & Dove, 2012). We therefore deleted either mvaU or both mvaT and mvaU in a strain lacking pilY1 (referred to henceforth as the parental strain). RNA-sequencing (RNA-seq) was used to identify genes differentially expressed in cells of the ΔmvaU single mutant as well as cells of the ΔmvaT ΔmvaU double mutant compared to those of the parental strain. Only 11 genes are more than 2-fold differentially expressed when mvaU is deleted (Fig. S1A, Table S1). These include lecA and the type III secretion system master regulator encoded by exsA, both of which have been previously reported (Castang et al., 2008, Williams McMackin et al., 2019). In contrast, transcripts arising from 1407 genomic loci—comprised of sense (i.e., coding sequences of annotated genes), antisense, and intergenic regions—are differentially expressed in cells of the ΔmvaT ΔmvaU double mutant strain compared to those of the parental strain (Fig. 1A). These include 535 genes, which represent nearly 10% of all annotated genes in the genome, (Fig. 1B, Table S2). These differentially expressed genes include 431 that are upregulated and 104 that are downregulated in the absence of MvaT and MvaU. The combined regulon of MvaT and MvaU span a multitude of cellular processes (Table S3). Five candidate transcripts found to be upregulated and two found to be downregulated by RNA-seq in the absence of these two proteins were validated by qRT-PCR (Fig. 1C). ΔmvaU mutant cells exhibit only modest increases in transcript abundance in contrast to the marked increases in ΔmvaT ΔmvaU mutant cells (Fig. 1C), consistent with prior observations that MvaT and MvaU appear partially redundant (Castang et al., 2008), though a more predominant role for MvaT in repression of target genes cannot be ruled out (Brencic et al., 2009, Vallet-Gely et al., 2005, Vallet et al., 2004).
Figure 1. Loss of MvaT and MvaU causes global changes in sense and antisense gene expression, including many direct targets of MvaT and MvaU.
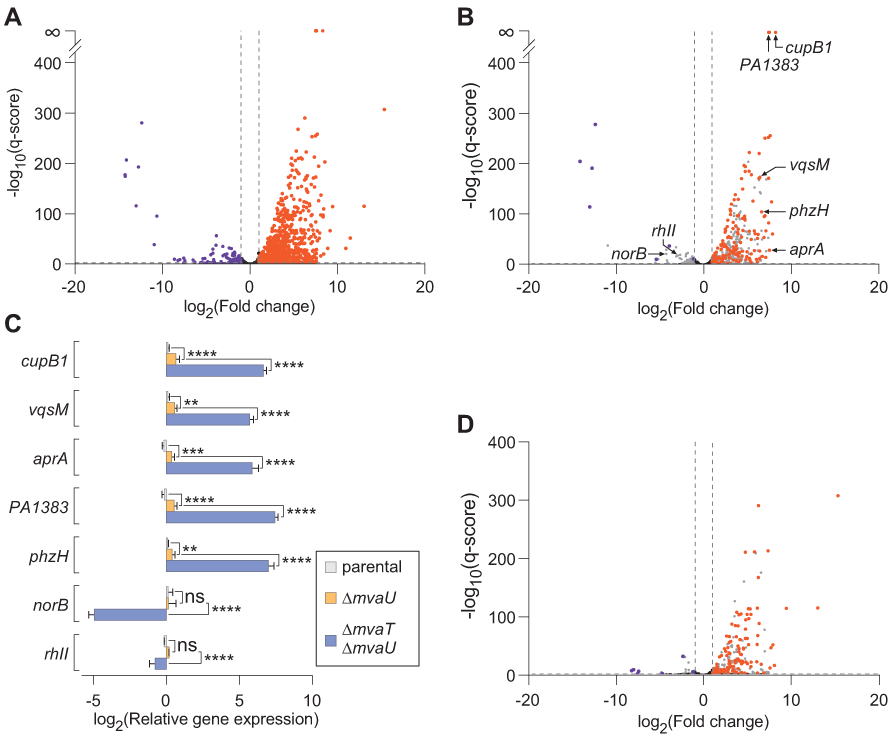
A. RNA-seq was performed in cells lacking pilY1 (parental) and in cells also lacking mvaT and mvaU (ΔmvaT ΔmvaU). A volcano plot demonstrates transcripts with statistically significant differential expression in ΔmvaT ΔmvaU vs parental (q<0.05, horizonal dashed line) and absolute value of fold change ≥2 (lateral of vertical dashed lines). Orange and purple data points indicate transcripts with increased and decreased abundance in ΔmvaT ΔmvaU relative to the parental, respectively. Black data points indicate transcripts whose differential expression does not meet statistical significance or whose fold change was less than 2.
B. Volcano plot of only the coding (sense) transcripts for annotated genes shown in A. Orange and purple data points indicated transcripts with increased and decreased abundance in ΔmvaT ΔmvaU relative to the parental, respectively, and are also direct targets of MvaT or MvaU (as indicated by MvaT-V and MvaU-V ChIP-seq, Table S2). Transcripts that are indirect targets of MvaT/MvaU, did not meet statistical significance, or whose fold change was less than 2 are indicated in grey.
C. Candidate genes (as indicated in the volcano plot in panel B) were validated by qRT-PCR. Bars represent the mean of an experiment performed in biological triplicate and technical duplicate. Error bars represent the standard deviation. Statistical significance was determined by two-way ANOVA: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns: not significant.
D. A volcano plot depicting only transcripts antisense to annotated genes from B. Orange and purple data points indicate transcripts with increased and decreased abundance in ΔmvaT ΔmvaU relative to the parental, respectively, and are also direct targets of MvaT or MvaU (Table S2). Transcripts that are indirect targets of MvaT/MvaU, did not meet statistical significance (q>0.05), or whose fold-change was less than 2 are indicated in grey.
Interestingly, we found that the ΔpilY1 ΔmvaT ΔmvaU strain harbors a deletion of PA0714.1-PA0729.1—a region that encodes multiple genes of the Pf4 prophage, including the excisionase required for initiation of the lytic life cycle (Li et al., 2019) (see Experimental Procedures). This finding suggests that unchecked expression of phage genes may still exert a negative selective pressure even in the absence of the phage receptor (Castang & Dove, 2012). Nevertheless, this mutant continues to have a residual growth defect relative to the parental strain (data not shown).
Loss of MvaT and MvaU increases both sense and antisense transcription
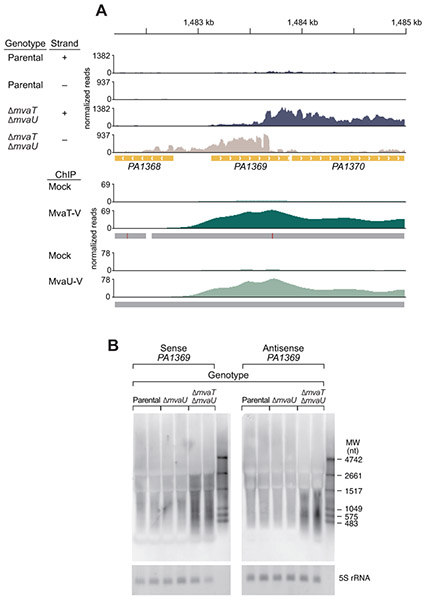
Deletion of both mvaU and mvaT altered transcript abundance for many annotated genes as observed by RNA-seq (Table S2). Importantly, we also observed altered expression of 408 antisense transcripts, comprised of 355 upregulated and 53 downregulated transcripts in cells lacking both H-NS-like family members (Fig. 1D). One representative example is the MvaT- and MvaU-regulated gene PA1369 (Castang et al., 2008, Vallet et al., 2004, Westfall et al., 2006), which encodes a conserved hypothetical protein (Fig. 2A). To evaluate the presence of increased sense and antisense transcripts using an independent technique, a Northern blot was performed using probes designed to detect sense and antisense transcripts arising from PA1369 and RNA from cells of the parental strain, the ΔmvaU single mutant, and the ΔmvaT ΔmvaU double mutant (Fig. 2B). The abundance of the PA1369 sense transcript is increased in cells lacking both H-NS-like proteins compared to that in cells of both the parental strain and the ΔmvaU mutant strain. Notably, antisense PA1369 transcripts are only detectable in the absence of both MvaT and MvaU.
Figure 2. Loss of MvaT and MvaU results in increased sense and antisense transcription.
A. RNA-seq was performed in cells lacking pilY1 (parental) and in cells also lacking mvaT and mvaU (ΔmvaT ΔmvaU). Reads are shown aligned to the plus (blue) and minus (beige) strands, scaled equally. A representative region showing both sense and antisense transcripts only in cells lacking mvaT and mvaU is shown for the genomic region encompassing the PA1369 gene, which encodes an annotated conserved hypothetical protein. ChIP-seq performed in strains that are wild-type with exception of an in-frame epitope tag on either mvaT (MvaT-V) or mvaU (MvaU-V) demonstrate MvaT (dark green) and MvaU (light green) occupancy in the region of increased sense and antisense transcripts in the ΔmvaT ΔmvaU strain. The mock control for MvaT-V and MvaU-V ChIP-seq are wild-type cells (lacking epitope tags used for immunoprecipitation). RNA-seq and both MvaT-V and MvaU-V ChIP-seq were performed in biological triplicate. Representative tracks for a single replicate are shown. The genomic location is indicated at the top of the figure in kilobases (kb). Genes are indicated in gold with arrows indicating the coding strand (right, forward strand; left, reverse strand). Grey bars below ChIP-seq reads indicate the algorithm peak call (≥ 2-fold enrichment over the mock) with the calculated peak maximum indicated as a vertical red line.
B. A Northern blot was performed with RNA from cells with the parental genotype (parental), cells lacking mvaU (ΔmvaU), and cells lacking both mvaT and mvaU (ΔmvaT ΔmvaU). The membrane was hybridized with probes that bind to the coding strand (sense) or non-coding strand (antisense) of PA1369. Increases in both sense and antisense transcripts of apparently different size are seen only when cells lack both mvaT and mvaU. 5S rRNA is shown as a loading control. Two biological replicates for each strain are shown.
Loss of MvaT and MvaU increases intragenic transcription
Examination of RNA-seq reads from cells of the ΔmvaT ΔmvaU double mutant strain relative to those from the parental strain revealed transcripts in both sense and antisense directions that appeared to originate from within genes (for example PA1369, Fig. 2A). We hypothesized that in addition to silencing transcription initiation from canonical promoters positioned outside of genes directly regulated by MvaT and MvaU, these proteins may also play a role in repressing transcription from intragenic promoters that lie within bound genes.
To better correlate the location of MvaT and MvaU across the genome at high resolution with those transcripts whose abundance is altered in cells that lack these two proteins, we performed ChIP-Seq with MvaT and MvaU. To do this, DNA specifying the vesicular stomatitis virus glycoprotein (VSV-G) epitope tag was introduced at the 3’-end of either mvaT (mvaT-v) or mvaU (mvaU-v) at their native loci, respectively, in otherwise WT strains; ChIP-seq was then performed using anti-VSV-G beads to IP MvaT and MvaU together with those regions of DNA to which each was crosslinked. Of the 535 genes whose expression is controlled by MvaT and MvaU, 164 were identified as enriched for MvaT and/or MvaU by ChIP-Seq, the vast majority of which (157) were found to be negatively regulated by these proteins. Moreover, of the 408 antisense transcripts whose abundance was controlled by MvaT and MvaU, 129 of the corresponding genomic regions were identified as enriched for MvaT and/or MvaU by ChIP-Seq. Of these 129, 121 were negatively regulated by MvaT and MvaU. These findings suggest that many of the regulatory effects of MvaT and MvaU are a result of the direct binding of these proteins to target DNA (Figs. 1B and 1D, Tables S4 and S5). Among these direct targets are genomic regions specifying the sense and antisense transcripts arising from PA1369 in cells of the ΔmvaT ΔmvaU double mutant strain (Fig. 2A). Note that genes identified here as enriched for MvaT or MvaU by ChIP-seq correspond well with genes previously identified as associated with these proteins by ChIP-on-Chip (Castang et al., 2008). These include 118 and 119 identified peaks for MvaT and MvaU, respectively, in this study compared to 111 and 102 peaks, respectively, reported previously.
Loss of MvaT and MvaU increases the occupancy of RNA polymerase containing σ70 within target regions
To evaluate whether RNA polymerase is initiating transcription from within MvaT and MvaU target regions in the absence of these two H-NS-like proteins, we performed paired RNA-seq and ChIP-seq using strains bearing C-terminal VSV-G epitope-tagged RpoD (RpoD-V) at the native locus. Notably, addition of the VSV-G epitope tag did not affect RpoD protein abundance (Fig. S3). The rpoD gene encodes the σ70 subunit of RNA polymerase, which is the principal σ factor in the cell. RNA polymerase holoenzyme containing σ70 is the DNA-binding species at σ70-dependent promoters and σ70 is classically thought to dissociate from RNA polymerase following promoter escape (Hansen & McClure, 1980, Nickels et al., 2005), with some noted exceptions (Daube & von Hippel, 1999, Ko et al., 1998, Sengupta et al., 2015, Wade & Struhl, 2004). Thus, the location of σ70 (RpoD) along the DNA has been used as a surrogate to indicate the location of σ70-dependent promoters along the genomes of various bacterial genera (Kroger et al., 2012, Mooney et al., 2009, Ramsey et al., 2015, Singh & Grainger, 2013, Singh et al., 2014).
As expected for targets that are directly regulated by MvaT and MvaU, genes in regions of DNA associated with MvaT-V and MvaU-V show altered expression in cells of the ΔmvaT ΔmvaU double mutant (Table S2). Further, we observed a dramatic increase in RpoD occupancy associated with these regions of DNA in cells lacking MvaT and MvaU (Tables S6 and S7). Indeed, RpoD-V distribution across the genome is markedly altered in ΔmvaT ΔmvaU double mutant cells for genes in the MvaT/MvaU regulon (Fig. 4A, Fig. S2). Moreover, while RpoD-V peak maxima were no more likely to be intragenic at loci directly repressed by both MvaT and MvaU (34.9%, 95% CI 22.4-49.8%) than at non-target loci (36.3%, 95% CI 33.7-38.9%) in the parental strain, maxima were more likely to be intragenic in MvaT/MvaU directly-repressed loci (50.4%, 95% CI 41.4-59.5%) than non-target loci (31.3%, 95% CI 28.6-34.2%) in ΔmvaT ΔmvaU cells (Table S8). A heatmap depiction of RpoD-V occupancy at directly repressed loci, together with their associated upstream and downstream regions, in parental and ΔmvaT ΔmvaU mutant cells, illustrates that MvaT and MvaU directly limit the association of RNA polymerase containing σ70 at both intragenic and extragenic sites (Fig. S4). Furthermore, for many of the putative promoters that are detectable in these directly repressed intragenic regions in the parental strain, their activities appear to increase in cells lacking MvaT and MvaU (Fig. S4).
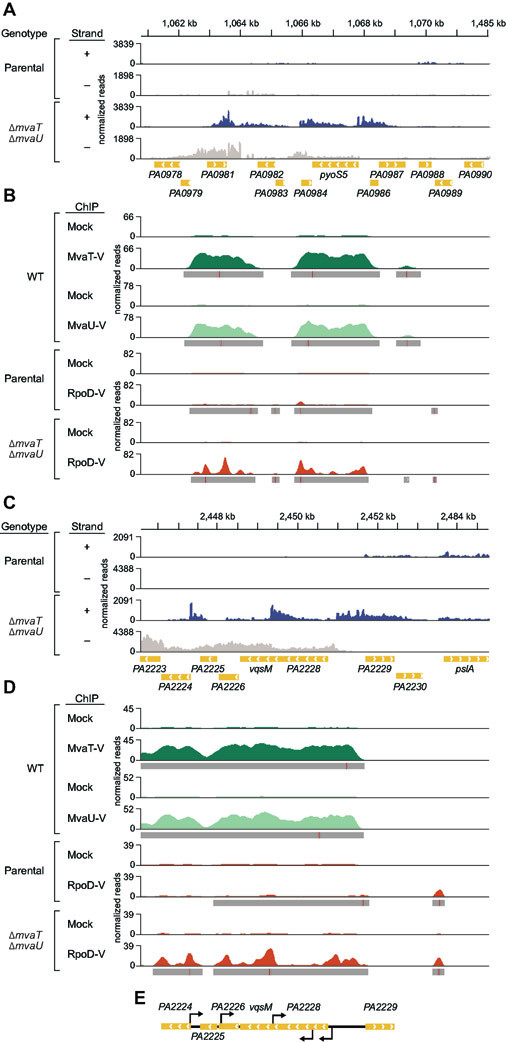
Figure 4. Loss of MvaT and MvaU results in increased intragenic sense and antisense transcription in genomic regions normally occupied by the two proteins.
A. In the locus spanning PA0979-PA0987, both sense and antisense transcripts are upregulated in the strain lacking mvaT and mvaU relative to the parental strain (lacking pilY1) by RNA-seq. Annotated genes in the region are indicated by arrows pointing in the coding direction. RNA-seq reads are aligned to the plus (blue) and minus (beige) strands, scaled equally. RNA-seq was performed in biological triplicate and representative tracks for a single replicate are shown.
B. ChIP-seq performed in strains that are WT with exception of an in-frame epitope tag on either mvaT (MvaT-V, dark green) or mvaU (MvaU-V, light green) demonstrate MvaT and MvaU occupancy in the region of increased sense and antisense transcripts in the ΔmvaT ΔmvaU cells in panel A. Further, epitope-tagged RNA polymerase subunit σ70 (RpoD-V, orange) occupancy, used as a proxy for promoter activity, is increased at loci both upstream of and within genes by ChIP-seq in ΔmvaT ΔmvaU cells compared to parental cells. The mock controls for MvaT-V and MvaU-V ChIP-seq are wild-type cells (lacking epitope tags used for immunoprecipitation) and the mock controls for RpoD-V ChIP-seq are parental and ΔmvaT ΔmvaU cells (containing RpoD without an epitope tag), respectively. ChIP peak calls are indicated by a grey bar under the ChIP read mapping (≥ 2-fold enrichment over the mock) with a red line indicating the calculated peak maximum. The genomic location is indicated at the top of the figure in kilobases (kb). Genes are indicated in gold with arrows indicating the direction of the coding strand (right, forward strand; left, reverse strand). MvaT-V, MvaU-V, and RpoD-V ChIP-seq were performed in biological triplicate. Representative tracks for a single replicate are shown.
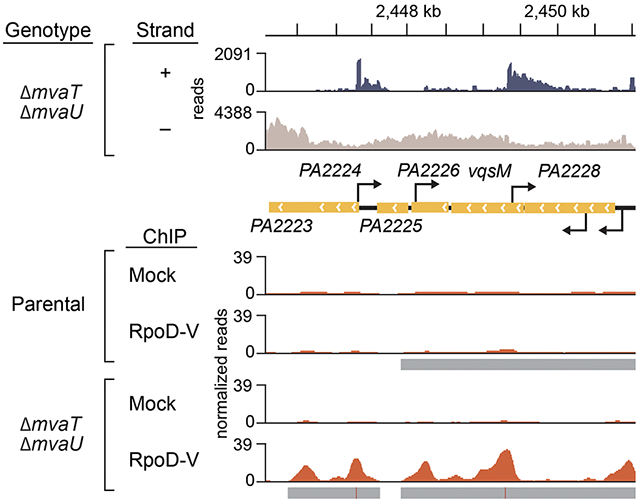
C-D. The region spanning the PA2228-vqsM-PA2226 operon shows increased sense and antisense transcript abundance in the absence of mvaT and mvaU compared to the parental strain. The region of increased transcript abundance is likewise normally associated with MvaT and MvaU in WT cells. Transcripts that appear to arise within genes by RNA-seq correlate with regions of increased RpoD-V enrichment by ChIP-seq in the ΔmvaT ΔmvaU cells compared to the parental.
E. 5’-RACE identifies extragenic, intragenic, and antisense transcription start sites in ΔmvaT ΔmvaU cells that correlate with RpoD-V ChIP-seq peaks identified in D. Antisense start sites are indicated by arrows above the gene and sense start sites below the gene.
To validate the RpoD-V ChIP-seq results, we tested several candidate genes via ChIP and quantitative PCR (ChIP-qPCR) on immunoprecipitated DNA from both the parental and ΔmvaT ΔmvaU strains. Several genes that are directly regulated by MvaT and MvaU as determined by RNA-seq and ChIP-seq (vqsM, PA0981, phzH, PA5087, and cupB1) have increased RpoD-V enrichment by ChIP-qPCR in the mutant relative the parental strain (Fig. 3B). Since RpoD-V protein abundance is constant between the parental and ΔmvaT ΔmvaU strains by quantitative Western blot (Figs. 3C and 3D) and overall RpoD-V occupancy is elevated for many MvaT/MvaU regulated genes in the ΔmvaT ΔmvaU strain, we suspected that RpoD-V might be recruited away from non-MvaT/MvaU-regulated genes and result in decreased transcript abundance for such genes in the ΔmvaT ΔmvaU strain relative to the parental strain. Consistent with this possibility, a small subset of non-MvaT/MvaU regulated genes (including PA1602, Figs. 3A and 3B) showed decreased RpoD-V enrichment in the ΔmvaT ΔmvaU strain. Our data thus raise the possibility that in some instances RNA polymerase may be redistributed in the deletion strain from other sites in the genome, such as PA1602, to MvaT/MvaU-regulated genes.
Figure 3. Loss of MvaT and MvaU results in increased σ70 occupancy in genomic regions normally occupied by the two proteins.
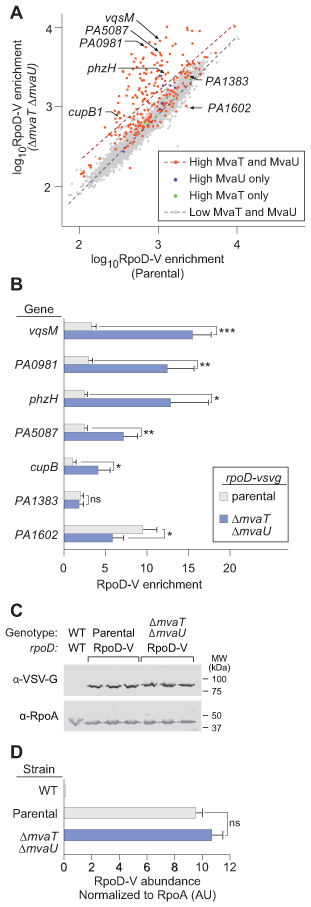
A. RpoD-V (σ70) ChIP-seq enrichment scores were ascertained, summed across each annotated gene for the parental cells and the ΔmvaU ΔmvaT cells. Each data point represents the log10 RpoD-V enrichment for an annotated gene in the parental cells (x-coordinate) and ΔmvaU ΔmvaT cells (y-coordinate). Plotted genes were subdivided by enrichment for MvaT-V and MvaU-V: not enriched for either MvaT-V or MvaU-V (grey), enriched for MvaT-V but not MvaU-V (green), enriched for MvaU-V but not MvaT-V (blue), and enriched for both MvaT-V and MvaU-V (red). Comparison of linear regression for the dual-enriched (high MvaT and MvaU, red dashed line) and dual-unenriched (low MvaT and MvaU, black dashed line) demonstrates a statistically significant difference in slope (p<0.0001, two-tailed ANCOVA) for the two trendlines.
B. Candidate genes were evaluated for enrichment of RpoD-V by ChIP-qPCR in the parental (grey) and ΔmvaT ΔmvaU strains (blue). Error bars represent the standard deviation of a biological triplicate performed in technical triplicate. Significance was determined using multiple t-tests using the two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli, with Q = 1% without the assumption of consistent standard deviation (Benjamini et al., 2006). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
C. Protein was isolated from cells of the parental strain with epitope-tagged RpoD-V, and cells lacking both mvaT and mvaU, each grown in biological triplicate. Quantitative western blot was performed using antibodies against the VSV-G epitope tag on RpoD and a loading control (α subunit of RNA polymerase). Protein isolated from wild-type cells was loaded as a single negative control lane.
D. VSV-G signal is shown normalized to the loading control for the parental cells and ΔmvaT ΔmvaU cells. Bars represent the mean and error bars represent the standard deviation. Normalized RpoD-V signal in the parental and ΔmvaT ΔmvaU cells are not significantly different (two-tailed t-test).
In ΔmvaT ΔmvaU double mutant cells, RpoD associates with many intragenic loci normally associated with MvaT and MvaU, as can be seen at higher resolution for 3 representative regions of the genome (two depicted in Fig. 4; one additional region is shown in Fig. S5). These loci also have RNA-seq signatures consistent with initiation sites that occur at RpoD-V enrichment peaks in ΔmvaT ΔmvaU double mutant cells. The PA2228-vqsM-PA2226 operon, which encodes both the quorum-sensing repressor QsrO (PA2226) (Kohler et al., 2014) and master regulator of quorum-sensing, VqsM (Dong et al., 2005, Liang et al., 2014), was selected for evaluation of intragenic transcription initiation using 5’-rapid amplification of cDNA ends (5’-RACE). Primers spanning the operon and annealing to both sense and antisense transcripts were used to generate cDNA. Subsequent amplification and sequencing allowed the mapping of 5’-ends, which represent possible transcription start sites (TSSs). In addition to an expected TSS upstream of the PA2228-vqsM-PA2226 operon on the sense strand, we identified one intragenic TSS on the sense strand within PA2228 and 3 intragenic TSSs on the antisense strand within vqsM, PA2226, and PA2224, respectively (Fig. 4E). Consistent with the hypothesis that these mapped 5’-ends represent TSSs, each corresponds with a region ofRpoD-V enrichment in the ΔmvaT ΔmvaU mutant cells, as revealed by ChIP-seq. Importantly, RpoD-V enrichment in these regions is less pronounced in the parental strain (i.e., a strain harboring both MvaT and MvaU) (Fig. 4D). Further, predicted promoters for each of the aligned TSSs identified by 5’-RACE each have AT-tracts between the −10 and −35 elements (Fig. S6), as previously described for H-NS.(Warman et al., 2020) Our findings establish that RNA polymerase containing σ70 is associated with sites of antisense and intragenic transcription in ΔmvaT ΔmvaU double mutant cells at loci that are targeted directly by MvaT and MvaU.
Discussion
Here we exploit the finding that pilY1 mutants of P. aeruginosa can tolerate the loss of MvaT and MvaU to define the regulon of these H-NS-like proteins. By using RNA-Seq to compare gene expression profiles of pilY1 parental and ΔmvaT ΔmvaU mutant cells, we identify over 500 genes whose expression is regulated by MvaT and MvaU. We also identify over 400 antisense transcripts that are controlled by these global regulators. ChIP-Seq with MvaT and MvaU in otherwise wild-type cells provides a high-resolution picture of which regulatory targets are direct. Our RNA-Seq analyses suggest that, in addition to controlling the synthesis of sense transcripts that originate from canonical promoters positioned immediately upstream of genes, MvaT and MvaU also control both sense and antisense transcription that originates from within target genes. Moreover, paired σ70 ChIP-seq studies indicate that much of the intragenic transcription that occurs in cells lacking MvaT and MvaU is a result of an increase in σ70-dependent transcription.
MvaT and MvaU act together to repress intragenic and antisense transcription
Prior studies showed that the loss of MvaT and MvaU results in either cell death or growth arrest in P. aeruginosa (Castang & Dove, 2012). This results from the induction of, and superinfection by, the endogenous Pf4 prophage that occurs in the absence of both MvaT and MvaU (Castang & Dove, 2012, Castang et al., 2008). Previous studies examined the effect of depleting MvaT in a strain lacking mvaU on expression of only a subset of MvaT- and MvaU-regulated genes. A synergistic increase in expression was seen for the 9 genes that were tested when cells lacking mvaU experienced 30 minutes of MvaT depletion (Castang et al., 2008). We now demonstrate that MvaT and MvaU together regulate gene expression across much of the genome. The synthesis of over 150 coding transcripts and 120 antisense transcripts are directly regulated by MvaT and MvaU and the indirectly-regulated transcripts include an additional 265 coding transcripts, 283 antisense transcripts, and 872 intergenic transcripts. In the absence of MvaT and MvaU, transcription initiation arises from within genes in both sense and anti-sense directions throughout the genome as revealed by paired RNA-seq and RpoD-V ChIP-seq.
ChIP-seq provides a high-resolution, genome-wide map of σ70-dependent promoters in P. aeruginosa
By performing the first genome-wide mapping of endogenously expressed σ70 in P. aeruginosa using ChIP-seq, this work additionally provides a high-resolution snapshot of likely σ70-dependent promoters, many of which agree well with published TSS mapping performed using RNA-based techniques (Winsor et al., 2016). Further, some of these promoters are revealed only in the absence of both MvaT and MvaU; these include the discussed cryptic intragenic promoters but also may suggest novel alternative promoters and extragenic promoters for previously unidentified genes. Our RpoD-V ChIP-seq in the parental and ΔmvaT ΔmvaU backgrounds comprise a rich dataset of possible σ70-dependent promoter regions for future inquiry.
A role for H-NS-like proteins in repressing intragenic transcription may be conserved
H-NS and H-NS-like proteins are encoded by the genomes of a wide range of bacterial genera (Dillon & Dorman, 2010) and there is mounting evidence for a conserved role in repression of intragenic transcription. Sense and antisense intragenic transcription with loss of H-NS has been observed genome-wide in Escherichia coli (Singh et al., 2014) and at the pilE locus in Neisseria gonorrhoeae (Masters et al., 2016). Actinobacteria harbor Lsr2, a conserved H-NS-like protein (Gehrke et al., 2019, Gordon et al., 2008), and in Streptomyces venezuelae cells lacking Lsr2 experience increased antisense transcription with some transcripts appearing to arise from within genes (Gehrke et al., 2019). Our findings that MvaT and MvaU in P. aeruginosa also repress antisense and intragenic transcription lend further weight to mounting evidence that repression of intragenic transcription is a conserved activity of the H-NS family of proteins.
MvaT and MvaU share many of the activities of H-NS (Castang et al., 2008, Tendeng et al., 2003), including silencing the expression of xenogeneic DNA (Ding et al., 2015). MvaU and MvaT also appear to be partially redundant (Vallet-Gely et al., 2005). Horizontally-acquired AT-rich DNA has the potential to modify MvaT and MvaU activity through several distinct mechanisms. Indeed, phage LUZ24 infects P. aeruginosa (Ceyssens et al., 2008) and encodes genes potentially repressed by MvaT (Wagemans et al., 2015). Interestingly, LUZ24 appears able to circumvent MvaT-mediated regulation by encoding Mip, a protein that inhibits MvaT activity. Although Mip has not been shown to have an inhibitory effect on MvaU (Wagemans et al., 2015), lytic bacteriophages often target multiple levels of host physiology in order to make the cell permissive to viral reproduction (Roucourt, 2020) and other mechanisms may exist for this and other phages to affect MvaU functionality. Acquisition of xenogeneic AT-rich DNA could additionally, at least in theory, directly lower the effective concentration of H-NS-like proteins bound to endogenous targets by acting as a sink.
DNA-binding proteins have been described to act as anti-silencers that displace H-NS and allow transcription of the H-NS target genes. When anti-silencers displace H-NS, they may provide an additional layer of regulation by also relieving repression of intragenic transcription. For example, the response regulator SsrB in Salmonella Typhimurium both displaces H-NS and directly activates transcription of its target genes in the horizontally acquired Salmonella-Pathogenicity-Island-2 (SPI-2) and elsewhere (Tomljenovic-Berube et al., 2010, Walthers et al., 2007, Walthers et al., 2011). SsrB associates with both extragenic and intragenic promoters; further, intragenic promoters account for significant portions of the transcription output for at least some SsrB-regulated genes (Tomljenovic-Berube et al., 2010). Although an effect on intragenic transcription has not yet been described in other organisms, several other anti-silencers that act at least in part by antagonizing H-NS have been identified (Heroven et al., 2004, Kazi et al., 2016, Winardhi et al., 2012, Wyborn et al., 2004, Yu & DiRita, 2002). Recently, Salmonella Typhimurium has been shown to degrade H-NS upon phagocytosis by macrophages in a manner dependent on the DNA-binding of the response regulator PhoP and the Lon protease (Choi & Groisman, 2020). These global decreases in H-NS protein levels lead to increased expression of multiple small RNAs and genes in several pathogenicity islands (Srikumar et al., 2015) that are normally repressed by H-NS (Lucchini et al., 2006). Expression of the SPI6-encoded Type VI secretion system, which is repressed by H-NS (Brunet et al., 2015), however, is not observed within macrophages (Srikumar et al., 2015), suggesting that if H-NS degradation during intramacrophage growth derepresses extragenic and intragenic transcription it may do so in a locus-specific manner. As mentioned, expression of genes comprising the endogenous prophage Pf4 is tightly repressed by either MvaT or MvaU alone. Yet Pf4 phage genes are among the most highly transcribed genes when P. aeruginosa grows in a biofilm (Whiteley et al., 2001). These and other data in P. aeruginosa suggest that repression by MvaT and MvaU is relieved, either locally due to the effect of anti-silencers (Desai et al., 2016) or globally, during the transition from planktonic to biofilm growth (Secor et al., 2015, Vallet et al., 2004, Westfall et al., 2006, Whiteley et al., 2001). But biofilms may not be the only condition in which activity of one or both of these H-NS-like proteins may be modulated.
In E. coli, H-NS limits the detrimental effects of xenogeneic DNA on growth by repressing intragenic transcription from these AT-rich sequences (Lamberte et al., 2017). In P. aeruginosa, MvaT and MvaU are important for growth because they limit the detrimental effects of xenogeneic DNA encoding the Pf4 prophage; they limit phage induction and subsequent superinfection (Castang & Dove, 2012). Nevertheless, cells lacking both MvaT and MvaU still exhibit a growth defect even if they can no longer make the replicative form of Pf4, or lack the receptor for the phage (Castang & Dove, 2012). We posit that akin to the situation in E. coli with H-NS, the derepression of intragenic transcription that occurs in the absence of mvaT and mvaU could negatively impact the growth of P. aeruginosa by redirecting RNA polymerase containing σ70 away from certain promoters whose activities are important for growth. Consistent with this possibility we observe decreases in the occupancy of σ70 at certain promoters in cells lacking MvaT and MvaU; however, we cannot rule out the possibility that loss of MvaT and MvaU results in increased abundance or activity of a repressor that regulates transcription from these σ70-dependent promoters.
In summary, our findings establish that intragenic sense and antisense transcription in P. aeruginosa occurs in the absence of both MvaT and MvaU and bolster the argument that the role for H-NS-like proteins in repressing such transcription is conserved. Whether intragenic and antisense transcripts that are differentially expressed in the absence of both MvaT and MvaU play regulatory roles themselves remains unclear and an area of interest for further research.
Experimental Procedures
Bacterial strains, media, and growth conditions.
Detailed strain construction, plasmid construction, and oligonucleotide information can be found in Tables S9, S10, and S11, respectively.
Strain Construction:
Plasmid pAL22 was used to create strains PAO1 ΔpilY1 and PAO1 ΔmvaU ΔpilY1, containing deletions of pilY1, by allelic exchange (Hmelo et al., 2015). mvaT was subsequently deleted using pEXG2M4315 to generate PAO1 ΔmvaU ΔpilY1 ΔmvaT by allelic exchange. The initial isolates of this strain have a significant growth defect in LB that was partially suppressed after overnight growth (data not shown). Examination of RNA-Seq reads as well as ChIP-seq mock reads suggests that the PAO1 ΔmvaU ΔpilY1 ΔmvaT strain harbors a deletion of PA0714.1- PA0729.1. The junctions of the deletion were confirmed by PCR and Sanger sequencing. An independently constructed isolate of the PAO1 ΔmvaU ΔpilY1 ΔmvaT strain harbors a similar deletion with unique junctions from the strain used for the experiments described herein. These deletions likely arose during deletion of mvaT, as the deletion is absent from the PAO1 ΔmvaU ΔpilY1 strain.
To create strains with in-frame C-terminal epitope tagged rpoD (rpoD-v) at the native locus for ChIP, plasmid pAL34 was used in construction of PAO1 ΔpilY1 rpoD-v and PAO1 ΔmvaU ΔpilY1 rpoD-v. mvaT was subsequently deleted from the latter strain using pEXG2M4315 to generate PAO1 ΔmvaU ΔpilY1 ΔmvaT rpoD-v by allelic exchange. This strain does not carry the deletions present in PAO1 ΔmvaU ΔpilY1 ΔmvaT and continues to have a growth defect (data not shown).
To create strains with in-frame C-terminal epitope tagged mvaT (mvaT-v) and mvaU (mvaU-v) at their native loci, respectively, plasmids pAL32 and pAL23 were used to generate PAO1 mvaT-v and PAO1 mvaU-v by allelic exchange.
Culture Conditions
For routine culture, lysogeny broth (LB) was used. Antibiotic concentrations used with E. coli included Carbenicillin 100 μg mL−1 and Gentamicin 15 μg mL−1. For P. aeruginosa, Carbenicillin 200 μg mL−1 and Gentamicin 30 μg mL−1 were used.
RNA Isolation
Overnight cultures of appropriate strains (AML77, AML85.1.1, AML91, AML121, and AML127.1) in LB were diluted back into 200 mL of LB to a starting OD600 of ~0.01 and grown with aeration at 250 rpm at 37°C to a final OD600 ~0.35. 15 mL of culture was pelleted and resuspended in 1 mL TRI Reagent (Molecular Research Center). RNA was extracted as described previously (Goldman et al., 2011). For samples to be used for paired RNA-seq and ChIP-seq (AML91, AML85.1.1, AML121 and AML127.1), a single culture for each strain in biological triplicate was split for RNA isolation and RpoD ChIP-seq as described in the respective methods sections.
Northern Blot
To generate probes, PCR products containing a T7 promoter sequence (Table S10) were used as templates for the DIG RNA labeling kit (Roche). Agarose gels were loaded with 10 μg of total RNA for each sample or 10 μL of DIG-labeled Blue Color Marker for Small RNA (DiagnoCine); RNA was stained with ethidium bromide and imaged using an Azure Biosystems c600 imager. Ethidium bromide was removed by washes with nuclease-free water and RNA was transferred to positively charged nylon membranes by capillary action overnight. Membranes were crosslinked at 120 J cm−2, prehybridized in a hybridization oven in ULTRAhyb (Ambion) at 65°C for 4 hours, followed by addition of 1.5 μL of probe and overnight hybridization at 65°C. Membranes were washed and blocked with the DIG Wash and Block Buffer Set (Roche), probed with Anti-digoxigenin-AP conjugate Fab fragments (Roche), and visualized using CDP-Star (Roche). Chemiluminescent images were acquired using an Azure Biosystems c600 imager.
RNA-Seq
Illumina cDNA libraries were generated using a modified version of the RNAtag-seq protocol (Shishkin et al., 2015). Briefly, 500 ng-1 μg of total RNA was fragmented, depleted of genomic DNA, dephosphorylated, and ligated to DNA adapters carrying 5’-AN8-3’ barcodes of known sequence with a 5’ phosphate and a 3’ blocking group. Barcoded RNAs were pooled and depleted of rRNA using the RiboZero rRNA depletion kit (Epicentre). Pools of barcoded RNAs were converted to Illumina cDNA libraries in 2 main steps: (i) reverse transcription of the RNA using a primer designed to the constant region of the barcoded adaptor with addition of an adapter to the 3’ end of the cDNA by template switching using SMARTScribe (Clontech) as described (Zhu et al., 2001); (ii) PCR amplification using primers whose 5’ ends target the constant regions of the 3’ or 5’ adaptors and whose 3’ ends contain the full Illumina P5 or P7 sequences. cDNA libraries were sequenced on the Illumina NovaSeqS2 platform to generate paired end reads.
Analysis of RNA-Seq data
Sequencing reads from each sample in a pool were demultiplexed based on their associated barcode sequence using custom scripts. Up to 1 mismatch in the barcode was allowed provided it did not make assignment of the read to a different barcode possible. Barcode sequences were removed from the first read as were terminal G’s from the second read that may have been added by SMARTScribe during template switching. Reads were aligned to the PAO1 genome (NCBI RefSeq NC_002516.2) using BWA(Li & Durbin, 2009) and read counts were assigned to genes and other genomic features using custom scripts. Differential expression analysis was conducted with DESeq2 (Love et al., 2014) and/or EdgeR (Robinson et al., 2010). Visualization of raw sequencing data and coverage plots in the context of genome sequences and gene annotations was conducted using GenomeView (Abeel et al., 2012). Grouping of genes identified by function was performed using KEGG Mapper: Search Pathway (Kanehisa & Sato, 2020).
cDNA synthesis for qRT-PCR
RNA preparations were incubated with a semi-random oligonucleotide with the sequence 5’-NSNSNSNSNS-3’ (NS5, Invitrogen) at 25 ng μl-1 in a 10 μl reaction for 10 minutes at 70 °C and 10 minutes at 25°C. These RNA preparations with the hybridized NS5 oligo were then treated with 500 U of SuperScript III (Invitrogen) in a 20 μl reaction with 1X first strand buffer, 10 mM DTT, 1 mM dNTPs and 20 U RNAseIN (Ambion) for 10 minutes at 25°C, 1 hour at 37°C, 1 hour at 42°C, and finally 10 minutes at 70°C. RNA was removed from these samples by alkaline hydrolysis and cDNAs were purified using a PCR Purification Kit (Qiagen).
Quantitative PCR (qPCR)
qPCR was performed with cDNA (Castang et al., 2008) or ChIP DNA samples (Kambara et al., 2018) essentially as described previously. Statistical analysis was performed using 2-way ANOVA in GraphPad Prism.
5’-Rapid Amplification of cDNA Ends (5’-RACE)
5’-RACE was performed with the 5’ RACE System for Rapid Amplification of cDNA Ends Kit (Invitrogen) according to manufacturer specifications with exception of substitution of Superscript III (Invitrogen) as the reverse transcriptase. When a single PCR product resulted from nested amplification, it was sequenced directly. When multiple products resulted, TA cloning and sequencing of PCR products was performed with the Topo TA Cloning Kit (Invitrogen). cDNA was generated with gene-specific oligo sX-1 or aX-1 (sense, primer location X, first primer) followed by nested amplification using kit primer AUAP and the corresponding second and third nested primers, respectively.
MvaT and MvaU Chromatin Immunoprecipitation Sequencing (ChIP-seq)
ChIP was performed essentially as described previously in biological triplicate (Castang et al., 2008). Briefly, overnight cultures of the appropriate strain (AML87 or AML86) in LB were diluted back into 200 mL of LB to a starting OD600 of ~0.01 and grown with aeration at 250 rpm at 37°C to a final OD600 ~0.35. Samples were crosslinked with formaldehyde at 1% final concentration for 30 minutes, then the reaction was quenched with addition of glycine to a final concentration of 250 mM. Cell pellets were washed 3 times with PBS and frozen at −80°C for further processing. Immunoprecipitation was performed as described (Castang et al., 2008) with the following exceptions: cOmplete, EDTA-free Protease Inhibitor Cocktail (Roche) was used in the lysis buffer; DNA was sheared using a Bioruptor (UCD-200, Diagenode) and circulating 4°C water-bath on the high setting (30 seconds on, 30 seconds off) for 15 minutes to achieve an average fragment size of 500 bp; mouse monoclonal anti-VSV-G agarose beads (Sigma, A1970) were used for IP. ChIP DNA concentration and size distribution were evaluated using a Bioanalyzer high sensitivity DNA chip 50-7000 bp (Agilent).
RpoD ChIP-seq
ChIP was performed in biological triplicate with strains AML91 (mock), AML85.1.1 (mock), AML121, and AML127 similarly to the MvaT and MvaU ChIP samples, with the following exceptions: 15 mL of culture was set aside for RNA isolation and RNA-seq prior to formaldehyde crosslinking; lysis buffer also contained 10 mM EDTA and 6 mg mL−1 lysozyme (EMD Millipore); further, DNA was sheared using a Sonic Dismembrator F60 with a sonicator micro-tip (Fisher) to an average fragment size of ~500 bp.
ChIP-Seq Library Preparation and Sequencing
Library preparation was performed with the NEBNext Ultra II DNA Library Prep Kit for Illumina and NEBNext Multiplex Oligos for Illumina (NEB) according to manufacturer’s instructions. Size selection was performed with AMPure XP beads (Beckman Coulter) to yield insets ~150 bp in length, followed by 9 rounds of PCR amplification. Libraries were sequenced by Elim Biopharmaceuticals (Hayward, CA) on an Illumina HiSeq2500 producing 50 bp paired-end reads.
ChIP-seq Data Analysis
Analysis was performed as described previously (Kambara et al., 2018). The RpoD-V peak maxima locations as part of this analysis was used along with the gene annotation file to determine whether the maxima were intragenic (Table S8) or extragenic in the parental and ΔmvaT ΔmvaU cells, respectively. Extragenic maxima were assigned to the nearest gene. Additionally, to better compare the occupancy of RpoD-V between the parental and ΔmvaT ΔmvaU strains, we calculated summed enrichment values across all annotated genes as well as this same enrichment value normalized by gene length, averaged from three replicates per strain. Because RpoD can associate with a region upstream of a coding sequence, we obtained these enrichment values from 150 bp upstream of the 5’-end of each gene through the 3’-end of the gene as listed in the annotation file. All the summed enrichment values and normalization steps were performed with a custom Python script.
RpoD Enrichment Heat Map for Genes Directly Repressed by MvaT and MvaU.
For the RpoD-V enrichment heat maps in Figure S4, we identified 188 genes found to be both direct targets of MvaT and MvaU and also repressed by MvaT and MvaU (Table S2, Tables S4 and S5). For each of these genes, the average enrichment value (RpoD-V ChIP / mock) at each base pair from 500 bp upstream (−500 bp) of the start codon to 500 bp downstream of the stop codon (+500 bp) was determined for both the parental strain and the ΔmvaT ΔmvaU strain. For each gene, enrichment across the gene body was normalized to a width of 1000 bp using the computeMatrix tool in the deepTools software package (Ramirez et al., 2016). The enrichment values for each region (500 bp upstream to start codon; start codon to stop codon; stop codon to 500 bp downstream of stop codon) were plotted using the heatmaps package in R (Perry, 2020, R Core Team, 2020).
Supplementary Material
Acknowledgements
We wish to thank David Knipe for use of his Bioruptor and Samantha Prezioso for assistance with 5’-RACE. The authors would also like to thank Liang Sun and Shira Rockowitz of the Research Computing Data Science Team in Research Computing at Boston Children’s Hospital for assistance with analyzing the RpoD ChIP-seq enrichment scores and normalization for gene length, Renate Hellmiss for artwork, and Samantha Prezioso and Ann Hochschild for comments on the manuscript. Bioanalyzer analysis was performed in the BCH IDDRC Molecular Genetic Core that is supported by National Institutes of Health award NIH-P30-HD 18655. RNA-Seq libraries were constructed and sequenced at the Broad Institute of MIT and Harvard by the Microbial ‘Omics Core and Genomics Platform, respectively. The Microbial ‘Omics Core also provided guidance on experimental design and conducted preliminary analysis for all RNA-Seq data. This work was supported by National Institutes of Health grants AI105013 (SLD) and K12HD000850 (AML), as well as a postdoctoral fellowship from the Cystic Fibrosis Foundation (MJG).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Data Availability
The sequencing data that support the findings of this study are openly available in the National Center for Biotechnology Information Gene Ex-pression Omnibus (GEO) under accession number GSE155025.
References
- Abeel T, Van Parys T, Saeys Y, Galagan J, and Van de Peer Y (2012) GenomeView: a next-generation genome browser. Nucleic Acids Res 40: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afessa B, and Green B (2000) Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest 117: 1017–1022. [DOI] [PubMed] [Google Scholar]
- Bassetti M, Vena A, Croxatto A, Righi E, and Guery B (2018) How to manage Pseudomonas aeruginosa infections. Drugs Context 7: 212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Krieger AM, and Yekutieli D (2006) Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93: 491–507. [Google Scholar]
- Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, Dove SL, and Lory S (2009) The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol Microbiol 73: 434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet YR, Khodr A, Logger L, Aussel L, Mignot T, Rimsky S, and Cascales E (2015) H-NS Silencing of the Salmonella Pathogenicity Island 6-Encoded Type VI Secretion System Limits Salmonella enterica Serovar Typhimurium Interbacterial Killing. Infect Immun 83: 2738–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castang S, and Dove SL (2010) High-order oligomerization is required for the function of the H-NS family member MvaT in Pseudomonas aeruginosa. Mol Microbiol 78: 916–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castang S, and Dove SL (2012) Basis for the essentiality of H-NS family members in Pseudomonas aeruginosa. J Bacteriol 194: 5101–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castang S, McManus HR, Turner KH, and Dove SL (2008) H-NS family members function coordinately in an opportunistic pathogen. Proc Natl Acad Sci U S A 105: 18947–18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyssens PJ, Hertveldt K, Ackermann HW, Noben JP, Demeke M, Volckaert G, and Lavigne R (2008) The intron-containing genome of the lytic Pseudomonas phage LUZ24 resembles the temperate phage PaP3. Virology 377: 233–238. [DOI] [PubMed] [Google Scholar]
- Chatzinikolaou I, Abi-Said D, Bodey GP, Rolston KV, Tarrand JJ, and Samonis G (2000) Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: Retrospective analysis of 245 episodes. Arch Intern Med 160: 501–509. [DOI] [PubMed] [Google Scholar]
- Choi J, and Groisman EA (2020) Salmonella expresses foreign genes during infection by degrading their silencer. Proc Natl Acad Sci U S A 117: 8074–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, and Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322. [DOI] [PubMed] [Google Scholar]
- Curran CS, Bolig T, and Torabi-Parizi P (2018) Mechanisms and Targeted Therapies for Pseudomonas aeruginosa Lung Infection. Am J Respir Crit Care Med 197: 708–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame RT, Luijsterburg MS, Krin E, Bertin PN, Wagner R, and Wuite GJ (2005) DNA bridging: a property shared among H-NS-like proteins. J Bacteriol 187: 1845–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame RT, Wyman C, Wurm R, Wagner R, and Goosen N (2002) Structural basis for H-NS-mediated trapping of RNA polymerase in the open initiation complex at the rrnB P1. J Biol Chem 277: 2146–2150. [DOI] [PubMed] [Google Scholar]
- Daube SS, and von Hippel PH (1999) Interactions of Escherichia coli sigma(70) within the transcription elongation complex. Proc Natl Acad Sci U S A 96: 8390–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SK, Winardhi RS, Periasamy S, Dykas MM, Jie Y, and Kenney LJ (2016) The horizontally-acquired response regulator SsrB drives a Salmonella lifestyle switch by relieving biofilm silencing. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle SP, Winzer K, Lazdunski A, Williams P, and Camara M (2002) Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J Bacteriol 184: 2576–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SC, and Dorman CJ (2010) Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol 8: 185–195. [DOI] [PubMed] [Google Scholar]
- Ding P, McFarland KA, Jin S, Tong G, Duan B, Yang A, … Xia B (2015) A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT. PLoS Pathog 11: e1004967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole S, Kuhn S, and Schnetz K (2002) Post-transcriptional enhancement of Escherichia coli bgl operon silencing by limitation of BglG-mediated antitermination at low transcription rates. Mol Microbiol 43: 217–226. [DOI] [PubMed] [Google Scholar]
- Dong YH, Zhang XF, Xu JL, Tan AT, and Zhang LH (2005) VqsM, a novel AraC-type global regulator of quorum-sensing signalling and virulence in Pseudomonas aeruginosa. Mol Microbiol 58: 552–564. [DOI] [PubMed] [Google Scholar]
- Dorman CJ (2007) H-NS, the genome sentinel. Nat Rev Microbiol 5: 157–161. [DOI] [PubMed] [Google Scholar]
- Dorman CJ (2014) H-NS-like nucleoid-associated proteins, mobile genetic elements and horizontal gene transfer in bacteria. Plasmid 75: 1–11. [DOI] [PubMed] [Google Scholar]
- Dotsch A, Becker T, Pommerenke C, Magnowska Z, Jansch L, and Haussler S (2009) Genomewide identification of genetic determinants of antimicrobial drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 53: 2522–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson J, Rosenfeld M, McNamara S, Ramsey B, and Gibson RL (2002) Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 34: 91–100. [DOI] [PubMed] [Google Scholar]
- Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, and Chalmers JD (2015) A Comprehensive Analysis of the Impact of Pseudomonas aeruginosa Colonization on Prognosis in Adult Bronchiectasis. Ann Am Thorac Soc 12: 1602–1611. [DOI] [PubMed] [Google Scholar]
- Gehrke EJ, Zhang X, Pimentel-Elardo SM, Johnson AR, Rees CA, Jones SE, … Elliot MA (2019) Silencing cryptic specialized metabolism in Streptomyces by the nucleoid-associated protein Lsr2. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisinger E, and Isberg RR (2017) Interplay Between Antibiotic Resistance and Virulence During Disease Promoted by Multidrug-Resistant Bacteria. J Infect Dis 215: S9–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SR, Sharp JS, Vvedenskaya IO, Livny J, Dove SL, and Nickels BE (2011) NanoRNAs prime transcription initiation in vivo. Mol Cell 42: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BR, Imperial R, Wang L, Navarre WW, and Liu J (2008) Lsr2 of Mycobacterium represents a novel class of H-NS-like proteins. J Bacteriol 190: 7052–7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan JR, and Deretic V (1996) Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60: 539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen UM, and McClure WR (1980) Role of the sigma subunit of Escherichia coli RNA polymerase in initiation. II. Release of sigma from ternary complexes. J Biol Chem 255: 9564–9570. [PubMed] [Google Scholar]
- Heroven AK, Nagel G, Tran HJ, Parr S, and Dersch P (2004) RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis. Mol Microbiol 53: 871–888. [DOI] [PubMed] [Google Scholar]
- Hmelo LR, Borlee BR, Almblad H, Love ME, Randall TE, Tseng BS, … Harrison JJ (2015) Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nat Protoc 10: 1820–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara TK, Ramsey KM, and Dove SL (2018) Pervasive Targeting of Nascent Transcripts by Hfq. Cell Rep 23: 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, and Sato Y (2020) KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci 29: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi MI, Conrado AR, Mey AR, Payne SM, and Davies BW (2016) ToxR Antagonizes H-NS Regulation of Horizontally Acquired Genes to Drive Host Colonization. PLoS Pathog 12: e1005570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko DC, Marr MT, Guo J, and Roberts JW (1998) A surface of Escherichia coli sigma 70 required for promoter function and antitermination by phage lambda Q protein. Genes Dev 12: 3276–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler T, Ouertatani-Sakouhi H, Cosson P, and van Delden C (2014) QsrO a novel regulator of quorum-sensing and virulence in Pseudomonas aeruginosa. PLoS One 9: e87814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlajich MV, Hron DR, Boudreau BA, Sun Z, Lyubchenko YL, and Landick R (2015) Bridged filaments of histone-like nucleoid structuring protein pause RNA polymerase and aid termination in bacteria. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger C, Dillon SC, Cameron AD, Papenfort K, Sivasankaran SK, Hokamp K, … Hinton JC (2012) The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci U S A 109: E1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmoo Y, Hook AL, Harvey D, Dubern JF, Williams P, Morgan SP, … Alexander MR (2020) Real time monitoring of biofilm formation on coated medical devices for the reduction and interception of bacterial infections. Biomater Sci 8: 1464–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberte LE, Baniulyte G, Singh SS, Stringer AM, Bonocora RP, Stracy M, … Grainger DC (2017) Horizontally acquired AT-rich genes in Escherichia coli cause toxicity by sequestering RNA polymerase. Nat Microbiol 2: 16249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, and Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu X, Tang K, Wang P, Zeng Z, Guo Y, and Wang X (2019) Excisionase in Pf filamentous prophage controls lysis-lysogeny decision-making in Pseudomonas aeruginosa. Mol Microbiol 111: 495–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Deng X, Li X, Ye Y, and Wu M (2014) Molecular mechanisms of master regulator VqsM mediating quorum-sensing and antibiotic resistance in Pseudomonas aeruginosa. Nucleic Acids Res 42: 10307–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini S, Rowley G, Goldberg MD, Hurd D, Harrison M, and Hinton JC (2006) H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog 2: e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters TL, Wachter S, Wachter J, and Hill SA (2016) H-NS suppresses pilE intragenic transcription and antigenic variation in Neisseria gonorrhoeae. Microbiology 162: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Davis SE, Peters JM, Rowland JL, Ansari AZ, and Landick R (2009) Regulator trafficking on bacterial transcription units in vivo. Mol Cell 33: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani D, Raman G, Sulham K, Gavaghan M, and Menon V (2014) Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, and Fang FC (2006) Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313: 236–238. [DOI] [PubMed] [Google Scholar]
- Nickels BE, Garrity SJ, Mekler V, Minakhin L, Severinov K, Ebright RH, and Hochschild A (2005) The interaction between sigma70 and the beta-flap of Escherichia coli RNA polymerase inhibits extension of nascent RNA during early elongation. Proc Natl Acad Sci U S A 102: 4488–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole GA, and Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30: 295–304. [DOI] [PubMed] [Google Scholar]
- Oshima T, Ishikawa S, Kurokawa K, Aiba H, and Ogasawara N (2006) Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res 13: 141–153. [DOI] [PubMed] [Google Scholar]
- Pang Z, Raudonis R, Glick BR, Lin TJ, and Cheng Z (2019) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 37: 177–192. [DOI] [PubMed] [Google Scholar]
- Perry M, (2020) heatmaps: Flexible Heatmaps for Functional Genomics and Sequence Features. In.: R package version 1.14.0, pp. [Google Scholar]
- Peters JM, Mooney RA, Grass JA, Jessen ED, Tran F, and Landick R (2012) Rho and NusG suppress pervasive antisense transcription in Escherichia coli. Genes Dev 26: 2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, (2020) R: A language and environment for statistical computing. In. Vienna, Austria: R Foundation for Statistical Computing. http://www.r-project.org/index.html, pp. [Google Scholar]
- Ramirez F, Ryan DP, Gruning B, Bhardwaj V, Kilpert F, Richter AS, … Manke T (2016) deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44: W160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Osborne ML, Vvedenskaya IO, Su C, Nickels BE, and Dove SL (2015) Ubiquitous promoter-localization of essential virulence regulators in Francisella tularensis. PLoS Pathog 11: e1004793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimsky S, Zuber F, Buckle M, and Buc H (2001) A molecular mechanism for the repression of transcription by the H-NS protein. Mol Microbiol 42: 1311–1323. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, and Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roucourt B, (2020) Elucidation of bacteriophage-host interactions between φKMV and Pseudomonas aeruginosa. In: Department of Gene Technology, Department of Biosystems (BIOSYST). Leuven: Katholieke Universiteit Leuven, pp. [Google Scholar]
- Santucci SG, Gobara S, Santos CR, Fontana C, and Levin AS (2003) Infections in a burn intensive care unit: experience of seven years. J Hosp Infect 53: 6–13. [DOI] [PubMed] [Google Scholar]
- Saxena S, and Gowrishankar J (2011) Modulation of Rho-dependent transcription termination in Escherichia coli by the H-NS family of proteins. J Bacteriol 193: 3832–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder O, and Wagner R (2000) The bacterial DNA-binding protein H-NS represses ribosomal RNA transcription by trapping RNA polymerase in the initiation complex. J Mol Biol 298: 737–748. [DOI] [PubMed] [Google Scholar]
- Secor PR, Sweere JM, Michaels LA, Malkovskiy AV, Lazzareschi D, Katznelson E, … Bollyky PL (2015) Filamentous Bacteriophage Promote Biofilm Assembly and Function. Cell Host Microbe 18: 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Prajapati RK, and Mukhopadhyay J (2015) Promoter Escape with Bacterial Two-component sigma Factor Suggests Retention of sigma Region Two in the Elongation Complex. J Biol Chem 290: 28575–28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Song M, Rhee JH, Hong Y, Kim YJ, Seok YJ, … Choy HE (2005) DNA looping-mediated repression by histone-like protein H-NS: specific requirement of Esigma70 as a cofactor for looping. Genes Dev 19: 2388–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishkin AA, Giannoukos G, Kucukural A, Ciulla D, Busby M, Surka C, … Livny J (2015) Simultaneous generation of many RNA-seq libraries in a single reaction. Nat Methods 12: 323–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SS, and Grainger DC (2013) H-NS can facilitate specific DNA-binding by RNA polymerase in AT-rich gene regulatory regions. PLoS Genet 9: e1003589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SS, Singh N, Bonocora RP, Fitzgerald DM, Wade JT, and Grainger DC (2014) Widespread suppression of intragenic transcription initiation by H-NS. Genes Dev 28: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikumar S, Kroger C, Hebrard M, Colgan A, Owen SV, Sivasankaran SK, … Hinton JC (2015) RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Salmonella Typhimurium. PLoS Pathog 11: e1005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendeng C, Soutourina OA, Danchin A, and Bertin PN (2003) MvaT proteins in Pseudomonas spp.: a novel class of H-NS-like proteins. Microbiology 149: 3047–3050. [DOI] [PubMed] [Google Scholar]
- Tomljenovic-Berube AM, Mulder DT, Whiteside MD, Brinkman FS, and Coombes BK (2010) Identification of the regulatory logic controlling Salmonella pathoadaptation by the SsrA-SsrB two-component system. PLoS Genet 6: e1000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet-Gely I, Donovan KE, Fang R, Joung JK, and Dove SL (2005) Repression of phase-variable cup gene expression by H-NS-like proteins in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 102: 11082–11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet I, Diggle SP, Stacey RE, Camara M, Ventre I, Lory S, … Filloux A (2004) Biofilm formation in Pseudomonas aeruginosa: fimbrial cup gene clusters are controlled by the transcriptional regulator MvaT. J Bacteriol 186: 2880–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet I, Olson JW, Lory S, Lazdunski A, and Filloux A (2001) The chaperone/usher pathways of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc Natl Acad Sci U S A 98: 6911–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JT, and Grainger DC (2014) Pervasive transcription: illuminating the dark matter of bacterial transcriptomes. Nat Rev Microbiol 12: 647–653. [DOI] [PubMed] [Google Scholar]
- Wade JT, and Struhl K (2004) Association of RNA polymerase with transcribed regions in Escherichia coli. Proc Natl Acad Sci U S A 101: 17777–17782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagemans J, Delattre AS, Uytterhoeven B, De Smet J, Cenens W, Aertsen A, … Lavigne R (2015) Antibacterial phage ORFans of Pseudomonas aeruginosa phage LUZ24 reveal a novel MvaT inhibiting protein. Front Microbiol 6: 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walthers D, Carroll RK, Navarre WW, Libby SJ, Fang FC, and Kenney LJ (2007) The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol Microbiol 65: 477–493. [DOI] [PubMed] [Google Scholar]
- Walthers D, Li Y, Liu Y, Anand G, Yan J, and Kenney LJ (2011) Salmonella enterica response regulator SsrB relieves H-NS silencing by displacing H-NS bound in polymerization mode and directly activates transcription. J Biol Chem 286: 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warman EA, Singh SS, Gubieda AG, and Grainger DC (2020) A non-canonical promoter element drives spurious transcription of horizontally acquired bacterial genes. Nucleic Acids Res 48: 4891–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, … Dudeck MA (2020) Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect Control Hosp Epidemiol 41: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall LW, Carty NL, Layland N, Kuan P, Colmer-Hamood JA, and Hamood AN (2006) mvaT mutation modifies the expression of the Pseudomonas aeruginosa multidrug efflux operon mexEF-oprN. FEMS Microbiol Lett 255: 247–254. [DOI] [PubMed] [Google Scholar]
- Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, and Greenberg EP (2001) Gene expression in Pseudomonas aeruginosa biofilms. Nature 413: 860–864. [DOI] [PubMed] [Google Scholar]
- Williams McMackin EA, Marsden AE, and Yahr TL (2019) H-NS Family Members MvaT and MvaU Regulate the Pseudomonas aeruginosa Type III Secretion System. J Bacteriol 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winardhi RS, Fu W, Castang S, Li Y, Dove SL, and Yan J (2012) Higher order oligomerization is required for H-NS family member MvaT to form gene-silencing nucleoprotein filament. Nucleic Acids Res 40: 8942–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, and Brinkman FS (2016) Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res 44: D646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyborn NR, Stapleton MR, Norte VA, Roberts RE, Grafton J, and Green J (2004) Regulation of Escherichia coli hemolysin E expression by H-NS and Salmonella SlyA. J Bacteriol 186: 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RR, and DiRita VJ (2002) Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol Microbiol 43: 119–134. [DOI] [PubMed] [Google Scholar]
- Zhu YY, Machleder EM, Chenchik A, Li R, and Siebert PD (2001) Reverse transcriptase template switching: a SMART approach for full-length cDNA library construction. Biotechniques 30: 892–897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data that support the findings of this study are openly available in the National Center for Biotechnology Information Gene Ex-pression Omnibus (GEO) under accession number GSE155025.