Abstract
Purpose
Both undertreatment and overtreatment of hypothyroidism with thyroid hormone are associated with adverse clinical outcomes. Disparities in the treatment of hypothyroidism may lead to a higher risk of adverse outcomes for certain sociodemographic groups. Our objectives were to identify sociodemographic disparities between those with treated and untreated hypothyroidism, and between those who were adequately and inadequately treated.
Methods
This is a cross-sectional study of a representative sample of US adults aged 20 years and older with hypothyroidism (n = 698). The main measures were age, gender, race/ethnicity, education, income, and health care access differences among those with treated and untreated hypothyroidism.
Results
Of those with hypothyroidism, women were more likely than men to be taking thyroid hormone (odds ratio [OR] 2.66 [95% confidence interval (CI) 1.42–4.99]), as were older participants (45–69 years old vs 20–44 years old: OR 7.25 [95% CI 4.15–12.67]; 70 years of age and older: OR 11.00 [95% CI 5.30–22.79]). Health care access was strongly associated with thyroid hormone use (OR 14.32, 95% CI 3.63–56.58). Hispanic race/ethnicity was associated with inadequate treatment compared with non-Hispanic whites (OR 2.42, 95% CI: 1.14–5.14).
Main Conclusions
Male gender, younger age, and lack of health care access were associated with untreated hypothyroidism, and Hispanic race was associated with inadequate treatment of hypothyroidism. Clinicians should be aware of these sociodemographic disparities in the hypothyroid population and consider strategies to improve treatment of hypothyroidism in men, younger adults, Hispanics, and those without routine health care access.
Keywords: hypothyroidism, disparities, thyroid hormone, sociodemographic
While thyroid hormone replacement was established as the treatment for the clinical syndrome of hypothyroidism over a century ago, the debate remains regarding which patients should receive therapy [1]. In 2004, a consensus statement from the American Association of Clinical Endocrinologists and the American Thyroid Association recommended that most patients with serum thyroid stimulating hormone (TSH) levels between 4.5 and 10mIU/L should be treated with thyroid hormone [2]. Conversely, a systematic review published in the same year argued against the treatment of subclinical disease in most patients [3]. Since 2004, additional literature has not resolved this debate; an important consequence of these conflicting clinical practice recommendations is potentially heterogenous treatment patterns.
A major result of the 2004 consensus statement has been a decline in the threshold for initiation of thyroid hormone and increasing thyroid hormone prescriptions. For example, a large UK study identified that between 2001 and 2009, the median TSH level at the time of thyroid hormone initiation declined from 8.7 to 7.9 mIU/L [4]. Similar results were seen in a Danish national registry [5]. In addition, the proportion of the US population reporting thyroid hormone use increased from 4.1% to 8.0% between 1997 and 2016 [6]. Sociodemographic trends of thyroid hormone use during this time period suggest larger increases in use amongst those ages 65 years and older and of non-Hispanic white race/ethnicity, although no corresponding laboratory data was available to assess clinical thyroid status. Prior work has identified disparities in thyroid screening and medication use by race and health care access, which may provide some explanation for these findings [7].
The clinical significance of disparities in thyroid hormone prescribing is that some groups may be at risk of overtreatment, while other groups with hypothyroidism may remain undertreated. Overtreatment with thyroid hormone leading to suppression of the TSH level is a relatively common occurrence [8] and has been associated with significant adverse outcomes: decreased bone mineralization, increased incidence of atrial fibrillation, decreased quality of life, and increased overall mortality [9–12]. Untreated and undertreated clinical and subclinical hypothyroidism with markedly elevated TSH levels (defined by a TSH level ≥10mIU/L) have been associated with several adverse health outcomes, including: risk of progression to clinical hypothyroidism [13, 14], coronary heart disease and heart failure [9, 15], and cognitive dysfunction [16, 17]. Using a nationally representative dataset with available thyroid function data, we examined the sociodemographic characteristics between those with untreated and treated hypothyroidism to (1) identify sociodemographic differences between those populations, and (2) identify sociodemographic risk factors for inadequate treatment of hypothyroidism in those on thyroid hormone.
Materials and Methods
In this cross-sectional study, we used data collected from multiple survey cycles from the National Health and Nutrition Examination Survey (NHANES) 2007–2012. The NHANES is a nationally representative survey of the US civilian, noninstitutionalized population. Participants are identified through a stratified, multistage probability sampling design. These survey years were unique because thyroid function testing was included in standard laboratory testing, allowing for the determination of clinical and subclinical hypothyroid status. This study was deemed to have exempt status by the University of Chicago Institutional Review Board.
Study participants
We included all adult participants ages 20 years and older who underwent thyroid function testing as part of the laboratory data collection of NHANES between 2007 and 2012. In the 2007–2008 survey years, all participants aged 12 years and above who had no history of hemophilia and no recent chemotherapy administration were eligible for thyroid function testing. In the 2009–2012 survey years, a randomly selected one-third subsample underwent thyroid function testing. Participants who reported being pregnant at the time of being surveyed or had a history of thyroid cancer were excluded.
Subgroup definitions
All participants with hypothyroidism were initially classified into 1 of 3 hypothyroidism treatment statuses: (1) presumed treated hypothyroidism (ie, a history of thyroid disease and taking thyroid hormone), (2) untreated subclinical hypothyroidism (ie, not taking thyroid hormone with thyroid function consistent with subclinical hypothyroidism), and (3) untreated clinical hypothyroidism (ie, not taking thyroid hormone with thyroid function consistent with clinical hypothyroidism). Untreated subclinical hypothyroidism was defined as having a TSH level between 5.6 and 9.9 mIU/L and a free thyroxine (FT4) level ≥0.6 ng/dL. Untreated clinical hypothyroidism was defined as a TSH level >5.6 mIU/L and an FT4 level <0.6 ng/dL, or having a TSH level ≥10 mIU/L and any FT4 level. The cutoff levels were based on the reference ranges provided in the NHANES lab methods for each corresponding year [18–20]. A subject with a TSH level ≥10 mIU/L, even with an FT4 level within normal limits, was considered to have clinical hypothyroidism because of the current general consensus to treat most patients with a TSH level of 10 mIU/L regardless of the FT4 level [21]. Participants were determined to have a history of thyroid disease if answering “yes” to the question: “Has a doctor ever or other health professional ever told you that you had a thyroid problem?” All forms of thyroid hormone were included, including levothyroxine, liothyronine, desiccated thyroid hormone extract, and thyroid hormone not otherwise specified.
Furthermore, participants with treated hypothyroidism were divided into 3 subgroups: (1) undertreated, (2) overtreated, and (3) adequately treated. Undertreated hypothyroidism was defined as having a TSH level >5.6 mIU/L, and overtreated hypothyroidism was defined as having a TSH level <0.3 mIU/L. All other participants taking thyroid hormone were defined as being adequately treated.
Variables of interest
Gender, age (20–44 years, 45–69 years, and 70 years and over), race/ethnicity (non-Hispanic white, Hispanic, non-Hispanic black, and other race/ethnicity), education level (did not complete high school [HS], completed HS, and completed college), and household income ($0–44 999, $45 000–99 999, and $100 000 and above) were selected as primary variables of interest in this study. Those classified in the NHANES dataset as Mexican-American and other Hispanic were combined into 1 “Hispanic” race/ethnicity group. Access to routine health care, included in the health care access section of the NHANES questionnaire, was included as a potential mediator of observed sociodemographic differences in hypothyroidism treatment.
Statistical methods
To approximate the total number of people in the US population for the study, each study participant was ascribed a corresponding sample weight [22]. The participant sample weights corresponding to those who underwent thyroid function testing (labeled WTMEC2YR for the 2007–2008 survey years and WTSA2YR for the 2009–2012 survey years) were extracted from the NHANES dataset and then divided by 3 to give equal weight to each of the survey cycles included in the analysis, per the NHANES analytical guidelines [22]. All subsequent analyses were performed using the “svy” R package for complex survey analysis. All subgroup characteristics were calculated as weighted proportions. Differences in categorical variables were compared using the Chi-square test with the Rao-Scott second-order correction and the adjusted Wald test, as appropriate. Multivariable logistic regression modeling was used to calculate odds ratios with confidence intervals for each sociodemographic category. Regression model results adjusted for access to routine care were similar to unadjusted results, thus only the adjusted model results are presented. In the multivariable regression analysis “don’t know” responses to individual survey questions were excluded. Data manipulation and analyses were completed using RStudio statistical software (version 1.2.5033).
Results
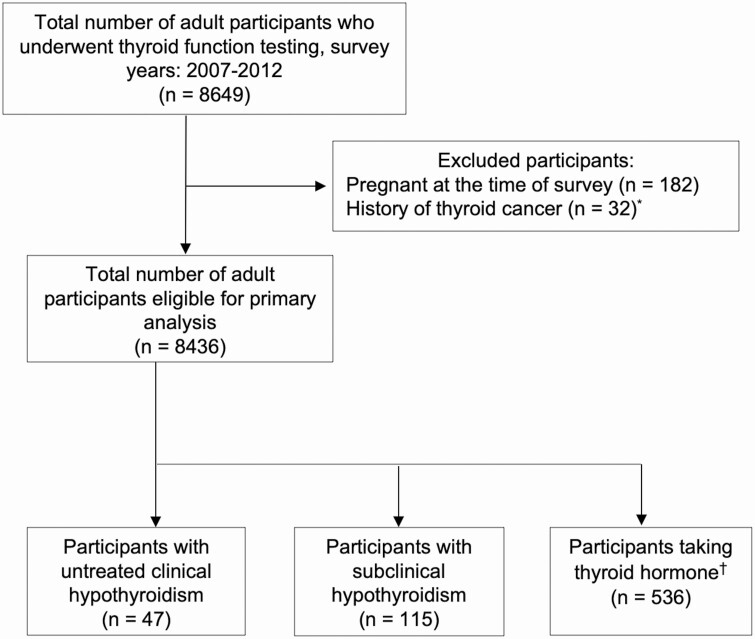
A total of 8436 participants who underwent thyroid function testing were included in the primary analysis, after excluding those who were pregnant at the time of being surveyed and had a history of thyroid cancer. Of those eligible, 536 participants had treated hypothyroidism, 115 had untreated subclinical hypothyroidism, and 47 had untreated clinical hypothyroidism (Fig. 1). Thyroid disease history was reported in 14.9% of participants with untreated subclinical hypothyroidism and 30.0% of participants with untreated clinical hypothyroidism.
Figure 1.
Flow diagram of NHANES participants included in the primary analysis. Treated hypothyroidism was defined as taking thyroid hormone and reporting a history of thyroid disease. Untreated subclinical hypothyroidism was defined as TSH level between 5.6 and 9.9 mIU/L with an FT4 ≥0.6 ng/dL. Untreated clinical hypothyroidism was defined as a TSH level >5.6 mIU/L and an FT4 <0.6 ng/mL, or a TSH level ≥10.0 mIU/L and any FT4 level. *One participant was pregnant and had a history of thyroid cancer. †Of 565 participants taking thyroid hormone, 29 did not report a history of thyroid disease. Abbreviations: FT4, free thyroxine; NHANES, National Health and Nutrition Examination Survey; TSH, thyroid-stimulating hormone.
Sociodemographic differences in treated versus untreated hypothyroidism
In bivariate analyses, hypothyroidism treatment status differed by gender, age, and access to routine care (Table 1). There were a greater proportion of men within the untreated subclinical hypothyroid group compared with the group with treated hypothyroidism (45.4% vs 18.1%, P <0.0001). In addition, the age group 20–44 years made up a greater proportion of those with untreated subclinical (29.0%) and clinical hypothyroidism (45.6%) compared with the group with treated hypothyroidism (6.7%; P <0.0001). Access to routine care also differed: 22.8% of those with untreated clinical hypothyroidism reported not having access to routine care vs 9.2% (P <0.0001) of those with treated hypothyroidism. Race/ethnicity, education level, and household income were not associated with differences in the hypothyroidism treatment status.
Table 1.
Sociodemographic characteristics and health care access of NHANES participants with hypothyroidism, stratified by treatment status and degree of hypothyroidism
| Treated Hypothyroidism | Untreated Subclinical Hypothyroidism | Untreated Clinical Hypothyroidism | |||
|---|---|---|---|---|---|
| Variables | (n = 536) | (n = 115) | P-value | (n = 47) | P-value |
| Reports thyroid disease | (%) | (%) | <0.0001 | (%) | <0.0001 |
| No | 0.0 | 85.1 | 70.0 | ||
| Yes | 100.0 | 14.9 | 30.0 | ||
| Gender | <0.0001 | 0.46 | |||
| Men | 18.1 | 45.4 | 25.6 | ||
| Women | 81.9 | 54.6 | 74.4 | ||
| Age, years | <0.0001 | <0.0001 | |||
| 20–44 | 6.7 | 29.0 | 45.6 | ||
| 45–69 | 60.9 | 53.4 | 39.7 | ||
| 70 and over | 32.4 | 17.7 | 14.7 | ||
| Race/ethnicity | 0.084 | 0.36 | |||
| Non-Hispanic white | 87.9 | 84.3 | 86.3 | ||
| Hispanic | 5.2 | 10.0 | 10.1 | ||
| Non-Hispanic black | 2.9 | 1.3 | 3.5 | ||
| Other | 4.0 | 4.5 | 0.0 | ||
| Education | 0.70 | 0.051 | |||
| Did not complete HS | 12.2 | 16.0 | 17.5 | ||
| Completed HS | 54.8 | 51.9 | 58.4 | ||
| Completed college | 33.0 | 32.2 | 21.6 | ||
| Don’t know | 0.0 | 0.0 | 2.4 | ||
| Household income ($) | 0.60 | 0.20 | |||
| 0–45 000 | 37.5 | 32.1 | 46.2 | ||
| 45 000–10 0000 | 28.8 | 32.0 | 43.5 | ||
| Above 100 000 | 26.2 | 23.7 | 7.6 | ||
| Don’t know | 7.5 | 12.2 | 2.7 | ||
| Access to routine care | 0.25 | <0.0001 | |||
| No | 0.9 | 9.2 | 22.8 | ||
| Yes | 99.1 | 90.8 | 77.2 | ||
Treated hypothyroidism was defined as taking thyroid hormone and reporting a history of thyroid disease. Untreated subclinical hypothyroidism was defined as a TSH level between 5.6 and 9.9 mIU/L with an FT4 ≥0.6 ng/dL. Untreated clinical hypothyroidism was defined as a TSH level >5.6 mIU/L and an FT4 <0.6 ng/mL, or TSH level ≥ 10.0mIU/L and any FT4 level. P-values refer to the comparisons between those with treated hypothyroidism and those with untreated subclinical or clinical hypothyroidism. Abbreviations: FT4, free thyroxine; HS, high school; NHANES: National Health and Nutrition Examination Survey; TSH: thyroid-stimulating hormone.
In multivariate analyses, results were similar. When considering all participants meeting the criteria for hypothyroidism, women were estimated to be more likely to be taking thyroid hormone compared with men (OR 2.66 [95% CI 1.42–4.99]). Older age groups were associated with a higher likelihood of taking thyroid hormone (45–69 years old vs 20–44 years old: OR 7.25 [95% CI 4.15–12.67]; 70 years of age and older: OR 11.00 [95% CI 5.30–22.79]) (Table 2). In addition, access to routine health care was a strong predictor of thyroid hormone use (OR 14.32, 95% CI 3.63–56.58). Race/ethnicity, education level, and household income were not significant predictors of taking thyroid hormone.
Table 2.
Multivariate regression analyses of sociodemographic characteristics and health care access as predictors of thyroid hormone use in NHANES participants with hypothyroidism
| Variables | All Participants (n = 698) | Excluding Those with Untreated Subclinical Hypothyroidism (n = 583) | ||
|---|---|---|---|---|
| Gender | OR | 95% CI | OR | 95% CI |
| Men | Ref | Ref | Ref | |
| Women | 2.66 | 1.42–4.99 | 1.17 | 0.35–3.84 |
| Age group | ||||
| 20–44 | Ref | Ref | Ref | |
| 45–69 | 7.25 | 4.15–12.67 | 8.62 | 3.58–20.76 |
| 70 years and over | 11.00 | 5.30–22.79 | 15.56 | 5.79–41.79 |
| Race/ethnicity | ||||
| Non-Hispanic white | Ref | Ref | Ref | |
| Hispanic | 0.96 | 0.41–2.22 | 0.92 | 0.21–4.08 |
| Non-Hispanic black | 2.21 | 0.55–8.92 | 1.30 | 0.21–8.01 |
| Education | ||||
| Did not complete HS | Ref | Ref | Ref | |
| Completed HS | 1.82 | 0.78–4.20 | 1.69 | 0.50–5.72 |
| Completed college | 1.91 | 0.76–4.76 | 2.11 | 0.53–8.44 |
| Household income ($) | ||||
| 0–45 000 | Ref | Ref | Ref | |
| 45 000–10 0000 | 0.67 | 0.34–1.32 | 0.87 | 0.26–2.86 |
| Above 100 000 | 0.99 | 0.46–2.12 | 3.34 | 0.76–14.70 |
| Access to routine care | ||||
| No | Ref | Ref | Ref | |
| Yes | 14.32 | 3.63–56.58 | 20.13 | 3.34–121.26 |
Subclinical hypothyroidism was defined as a TSH level between 5.6 and 9.9 mIU/L with an FT4 ≥0.6ng/mL.
Abbreviations: FT4, free thyroxine; HS, high school; NHANES, National Health and Nutrition Examination Survey; Ref, reference; TSH, thyroid-stimulating hormone.
When those with untreated subclinical hypothyroidism (TSH level 5.6–9.9 mIU/L) were excluded from the analysis, older age and routine access remained significant predictors of thyroid hormone use (45–69 years old: OR 8.62 [95% CI 3.58–20.76]; 70 years of age and older: OR 15.56 [95% CI 5.79–41.79]; access to routine health care: OR 20.13 [95% CI 3.34–121.26]). However, gender was no longer a predictor of thyroid hormone use (OR 1.17 [95% CI 0.35–3.84]). Again, race/ethnicity, education level, and household income were not associated with taking thyroid hormone.
Treatment status in those on thyroid hormone
Of the 536 participants with treated hypothyroidism, 56 were undertreated at the time of thyroid function testing (10.4%, unweighted) and 68 were overtreated (12.7%, unweighted) (Table 3). Of note, an estimated 15.2% of the undertreated group was Hispanic. Among those with normal thyroid function on thyroid hormone, 99.6% had access to routine care.
Table 3.
Sociodemographic characteristics and health care access of NHANES participants with hypothyroidism on thyroid hormone, stratified by treatment status
| Variables | Adequate Treatment (n = 412) | Undertreated (n = 56) | P-value | Overtreated (n = 68) | P-value |
|---|---|---|---|---|---|
| Gender | (%) | (%) | 0.84 | (%) | 0.82 |
| Men | 18.5 | 17.2 | 16.4 | ||
| Women | 81.5 | 82.8 | 83.6 | ||
| Age group | 0.19 | 0.37 | |||
| 20–44 | 6.2 | 15.4 | 3.1 | ||
| 45–69 | 59.7 | 58.5 | 70.3 | ||
| 70 years and over | 34.0 | 26.1 | 26.6 | ||
| Race/ethnicity | 0.015 | 0.63 | |||
| Non-Hispanic white | 88.9 | 81.3 | 86.2 | ||
| Hispanic | 3.9 | 15.2 | 6.1 | ||
| Non-Hispanic black | 2.7 | 3.6 | 4.0 | ||
| Other | 4.5 | 0.0 | 3.7 | ||
| Education | 0.83 | 0.74 | |||
| Did not complete HS | 11.7 | 12.7 | 14.9 | ||
| Completed HS | 56.2 | 49.9 | 49.4 | ||
| Completed college | 32.1 | 37.5 | 35.7 | ||
| Household income ($) | 0.27 | 0.99 | |||
| 0–45 000 | 36.9 | 46.8 | 34.4 | ||
| 45 000–10 0000 | 29.4 | 22.1 | 29.6 | ||
| Above 100 000 | 25.5 | 28.7 | 28.8 | ||
| Don’t know | 8.1 | 2.5 | 7.2 | ||
| Access to routine care | 0.45 | 0.26 | |||
| No | 0.4 | 0.9 | 4.2 | ||
| Yes | 99.6 | 99.1 | 95.8 |
Undertreated hypothyroidism was defined as TSH > 5.6mIU/L. Overtreated hypothyroidism was defined as TSH < 0.3mIU/L. Adequate treatment was defined as a TSH between 0.3 and 5.6 mIU/L. P-values refer to the comparisons between those with adequate treatment of hypothyroidism and those with undertreated or overtreated hypothyroidism.
Abbreviations: HS, high school; NHANES, National Health and Nutrition Examination Survey; TSH, thyroid-stimulating hormone.
In the multivariate analyses, the overtreated and undertreated groups were combined in order to have sufficient group sizes. Of note, Hispanic race was associated with an increased risk of inadequate treatment compared to non-Hispanic whites (OR 2.42, 95% CI: 1.14–5.14) (Table 4). Gender, age, education level, household income, and access to routine care were not associated with inadequate treatment.
Table 4.
Multivariate regression analysis of sociodemographic characteristics and health care access as predictors of inadequate treatment with thyroid hormone in NHANES participants with hypothyroidism
| Variables | OR | 95% CI |
|---|---|---|
| Gender | ||
| Male | Ref | |
| Female | 1.15 | 0.44–3.02 |
| Age group | ||
| 20–44 | Ref | |
| 45–69 | 0.97 | 0.40–2.38 |
| 70 years and over | 0.70 | 0.28–1.75 |
| Race/Ethnicity | ||
| Non-Hispanic white | Ref | |
| Hispanic | 2.42 | 1.14–5.14 |
| Non-Hispanic black | 1.37 | 0.33–5.73 |
| Other | 0.48 | 0.15–1.48 |
| Education | ||
| Did not complete HS | Ref | |
| Completed HS | 0.75 | 0.35–1.61 |
| Completed college | 1.07 | 0.42–2.76 |
| Household income ($) | ||
| 0–45 000 | Ref | |
| 45 000–10 0000 | 0.82 | 0.43–1.59 |
| Above 100 000 | 0.97 | 0.35–2.69 |
| Access to routine care | ||
| No | Ref | |
| Yes | 0.12 | 0.01–1.13 |
Inadequate treatment was defined as a TSH > 5.6 mIU/L or TSH < 0.3 mIU/L.
Abbreviations: HS, high school; NHANES, National Health and Nutrition Examination Survey; Ref, reference; TSH, thyroid-stimulating hormone.
Discussion
We found multiple sociodemographic disparities in the treatment of hypothyroidism in this nationally representative US study population. Amongst those meeting the criteria for hypothyroidism, male gender, age less than 45 years, and lack of access to routine health care were associated with the lack of treatment with thyroid hormone. Amongst those taking thyroid hormone, Hispanics were more likely to be inadequately treated based on thyroid function testing.
The majority of participants with untreated clinical hypothyroidism did not self-report having thyroid disease, suggesting that most cases were undiagnosed. The public health challenge of undiagnosed clinical hypothyroidism has persisted since the publication of large cross-sectional studies highlighting the issue in the early 2000s [23, 24]. The proportionately lower treatment rate in younger people with hypothyroidism may be the result of lower case finding due to less exposure to the health care system. Because young adults less frequently have hypothyroidism, there is often a lower clinical suspicion of hypothyroidism given the nonspecific nature of many hypothyroid symptoms [25, 26]. Early case identification and treatment of hypothyroidism is important to avoid long-term cardiovascular, cognitive, and reproductive health complications [27]. However, it is important to note that the apparent association between younger age and lack of treatment is also likely the result of relatively higher rates of case detection and treatment in older individuals. The HUNT Study in Norway found significant reductions in the prevalence of untreated hypothyroidism in people over 60 years of age from 1995 to 2008, with much smaller differences in those under 40 years of age, although the prevalence of hypothyroidism in the younger population had remained low [28]. Together, these findings and the results of this study suggest that the population with untreated hypothyroidism has become smaller and younger in the setting of increased thyroid hormone prescribing.
Taking into consideration the higher prevalence of subclinical hypothyroidism and median TSH threshold for the initiation of thyroid hormone below 10 mIU/L, it is likely that many of the study participants were being treated for subclinical hypothyroidism, particularly older participants [4, 5, 23, 24]. It is noteworthy that the female gender bias towards taking thyroid hormone was no longer observed when excluding those with subclinical hypothyroidism. This observation, along with the greater-than-expected proportion of men in the subclinical hypothyroid population, suggests that women were more likely than men to be treated for subclinical hypothyroidism with TSH levels < 10mIU/L. This is despite prior data suggesting men with elevated TSH levels are at an increased risk of developing clinical hypothyroidism compared with women [29]. Higher rates of thyroid hormone use amongst women with subclinical hypothyroidism may be the result of increased case finding due to higher clinical suspicion for thyroid disease, as well as patient preference for treatment.
The health implications of higher rates of thyroid hormone therapy for subclinical hypothyroidism are not entirely clear. In 2 large observational studies of patients with subclinical hypothyroidism, thyroid hormone use was associated with fewer cardiovascular events and reduced all-cause mortality only in those 65–70 years of age and younger [30, 31]. A recent randomized trial of levothyroxine for the treatment of hypothyroidism in older adults could find no improvements in hypothyroid symptoms or in systolic/diastolic heart function associated with treatment after 18 months [32, 33]. Meanwhile, there are clear health risks associated with thyroid hormone use due to overtreatment, in addition to increased costs of prescriptions and thyroid function monitoring. Several studies have linked suppressed TSH levels (<0.03 mIU/L) with an increased risk of cardiovascular events, fracture, and excess mortality [10, 12, 34].
Following years of increased thyroid hormone prescribing, there is now increasing interest in the appropriate use of thyroid hormone [35, 36]. Recent clinical guidelines are more specific regarding age and TSH thresholds for treatment of subclinical hypothyroidism, and a trend towards lower prescription rates has been observed in 1 health system [21, 37]. Our results point to a disproportionate rate of women and older patients receiving thyroid hormone, many of whom likely have mild subclinical hypothyroidism. Systemic efforts to encourage judicious prescribing of thyroid hormone, especially in the setting of subclinical hypothyroidism where the benefit with thyroid hormone is less clear, should be considered for these patient populations to avoid overtreatment and minimize low-value care.
Amongst those taking thyroid hormone, 23% were receiving either too little or too much thyroid hormone replacement. Being Hispanic was a significant risk factor for inadequate treatment. Our findings add to the existing literature describing the prevalence of inadequate thyroid hormone replacement in those with hypothyroidism [8, 38, 39], although the proportion of thyroid hormone users with inadequate thyroid function was lower in our study than others. It is unclear why Hispanics on thyroid hormone were less likely to be adequately treated, although greater TSH and antithyroid antibody levels have been previously seen in Mexican Americans compared with non-Hispanic blacks [23]. Very little literature exists on the racial/ethnic differences in thyroid functioning. Our results suggest that Hispanic ethnicity may be important to consider as a reason for more frequent thyroid hormone monitoring, in addition to other clinical factors, including weight changes, new medications, and aging in general [40]. Further study into the specific causes and implications of inadequate hypothyroid treatment within the Hispanic population is warranted.
There are several limitations to this study. No data are provided in NHANES on the specific type of thyroid disorder and thyroid function testing at the time of thyroid hormone initiation, thus the proportion of participants on thyroid hormone who had clinical vs subclinical hypothyroidism at the time of diagnosis is unknown. Due to the limited number of participants with abnormal thyroid function, it was possible that differences by sociodemographics were undetectable, leading to the possibility of type II errors. Another important consideration is the timeframe of the study (2007–2012), with most of the data being collected over 10 years ago. These years represent the most recent time period in which thyroid function data were available in the NHANES dataset. While it is possible that a significant change in the pattern of thyroid hormone prescribing has occurred since that time, most indications are that thyroid hormone prescribing has increased at a steady rate during and after the study period [6, 36]. Therefore, we believe the findings of the study remain relevant as the identified disparities are likely still present (or have worsened) since the end of the study period.
It is important to recognize that other medications (eg, corticosteroids, lithium, amiodarone) and supplements (eg, biotin) can affect thyroid hormone absorption, metabolism, and laboratory testing, leading to falsely high or low TSH or FT4 levels [41, 42]. We found the proportions of participants on corticosteroids, lithium, and amiodarone in the study population to be quite small (2%, <1%, and 1%, respectively). Similarly, no participant in the study population was taking a dedicated biotin supplement. Therefore, it is unlikely that these drugs or supplements significantly affected our results. An individual patient’s medications and supplement list should be carefully considered before diagnosing and treating a thyroid disease. An additional consideration is that different forms of thyroid hormone replacement may affect thyroid hormone levels differently [43]. In this study population, less than 5% of participants taking thyroid hormone replacement were taking desiccated thyroid extract, limiting this issue’s effect on the overall results. Finally, we recognize that some participants may be taking thyroid hormone but do not carry a diagnosis of hypothyroidism. Those participants taking thyroid hormone but who did not report a history of thyroid disease were excluded from the analysis.
In conclusion, there are multiple sociodemographic disparities in the treatment of hypothyroidism. Male gender, younger age, and lack of routine access to health care were risk factors for lack of treatment for hypothyroidism, putting those groups at a greater risk of the adverse effects of untreated hypothyroidism. Additionally, the findings suggest women were more likely than men to receive thyroid hormone for mild subclinical hypothyroidism. Hispanics taking thyroid hormone were more likely to be inadequately treated compared with non-Hispanic whites. In an effort to minimize these disparities in the future and reduce adverse events from undertreatment and overtreatment of hypothyroidism, strategies may be developed to improve the quality of treatment in men, younger adults, Hispanics, and those that lack routine health care access, and more judicious prescribing of thyroid hormone for mild subclinical hypothyroidism should be considered.
Additional Information
Disclosures: Dr Ettleson, Ms Zhu, and Dr Laiteerapong have nothing to disclose. Dr Bianco reports personal fees from Synthonics, Allergan, and BLA Technology. These are not relevant to the content of this manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in the References.
References and Notes
- 1. McAninch EA, Bianco AC. The history and future of treatment of hypothyroidism. Ann Intern Med. 2016;164(1):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT; American Association of Clinical Endocrinologists/American Thyroid Association/Endocrine Society . Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. Endocr Pract. 2004;10(6):497–501. [DOI] [PubMed] [Google Scholar]
- 3. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228–238. [DOI] [PubMed] [Google Scholar]
- 4. Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med. 2014;174(1):32–39. [DOI] [PubMed] [Google Scholar]
- 5. Medici BB, Nygaard B, la Cour JL, et al. Changes in prescription routines for treating hypothyroidism between 2001 and 2015: an observational study of 929 684 primary care patients in Copenhagen. Thyroid. 2019;29(7):910–919. [DOI] [PubMed] [Google Scholar]
- 6. Johansen ME, Marcinek JP, Doo Young Yun J. Thyroid hormone use in the United States, 1997-2016. J Am Board Fam Med. 2020;33(2):284–288. [DOI] [PubMed] [Google Scholar]
- 7. Stoll K. Disparities in thyroid screening and medication use in Quebec, Canada. Health Equity. 2019;3(1):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okosieme OE, Belludi G, Spittle K, Kadiyala R, Richards J. Adequacy of thyroid hormone replacement in a general population. Qjm. 2011;104(5):395–401. [DOI] [PubMed] [Google Scholar]
- 9. Rodondi N, den Elzen WP, Bauer DC, et al. ; Thyroid Studies Collaboration . Subclinical hypothyroidism and the risk of coronary heart disease and mortality. Jama. 2010;304(12):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid. 2018;28(5):566–574. [DOI] [PubMed] [Google Scholar]
- 11. Chu JW, Crapo LM. The treatment of subclinical hypothyroidism is seldom necessary. J Clin Endocrinol Metab. 2001;86(10):4591–4599. [DOI] [PubMed] [Google Scholar]
- 12. Thayakaran R, Adderley NJ, Sainsbury C, et al. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ. 2019;366(8212):l4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87(7):3221–3226. [DOI] [PubMed] [Google Scholar]
- 14. Díez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab. 2004;89(10):4890–4897. [DOI] [PubMed] [Google Scholar]
- 15. Gencer B, Collet TH, Virgini V, et al. ; Thyroid Studies Collaboration . Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rieben C, Segna D, da Costa BR, et al. Subclinical thyroid dysfunction and the risk of cognitive decline: a meta-analysis of prospective cohort studies. J Clin Endocrinol Metab. 2016;101(12):4945–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(11):4240–4248. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. NHANES 2007–2008 Laboratory Methods. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. NHANES 2009–2010 Laboratory Methods. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. NHANES 2011–2012 Laboratory Methods. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 21. Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: a review. JAMA. 2019;322(2):153–160. [DOI] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 23. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 24. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. [DOI] [PubMed] [Google Scholar]
- 25. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mendes D, Alves C, Silverio N, Batel Marques F. Prevalence of undiagnosed hypothyroidism in Europe: a systematic review and meta-analysis. Eur Thyroid J. 2019;8(3):130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garber JR, Cobin RH, Gharib H, et al. ; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults . Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200–1235. [DOI] [PubMed] [Google Scholar]
- 28. Asvold BO, Vatten LJ, Bjøro T. Changes in the prevalence of hypothyroidism: the HUNT Study in Norway. Eur J Endocrinol. 2013;169(5):613–620. [DOI] [PubMed] [Google Scholar]
- 29. Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43(1):55–68. [DOI] [PubMed] [Google Scholar]
- 30. Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172(10):811–817. [DOI] [PubMed] [Google Scholar]
- 31. Andersen MN, Olsen AS, Madsen JC, et al. Long-term outcome in levothyroxine treated patients with subclinical hypothyroidism and concomitant heart disease. J Clin Endocrinol Metab. 2016;101(11):4170–4177. [DOI] [PubMed] [Google Scholar]
- 32. Stott DJ, Rodondi N, Bauer DC; TRUST Study Group . Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;377(14):2534–2544. [DOI] [PubMed] [Google Scholar]
- 33. Villar HC, Saconato H, Valente O, Atallah AN. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Syst Rev. 2007;2007(3):CD003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab. 2010;95(1):186–193. [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez-Gutierrez R, Maraka S, Ospina NS, Montori VM, Brito JP. Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol. 2017;5(4):246–248. [DOI] [PubMed] [Google Scholar]
- 36. Pearce EN. Thyroid hormone use doubled in the United States from 1997 to 2016. Clin Thyroidol. 2020;32(5):208–210. [Google Scholar]
- 37. Jonklaas J, DeSale S. Levothyroxine prescriptions trends may indicate a downtrend in prescribing. Ther Adv Endocrinol Metab. 2020;11:2042018820920551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vaisman F, Coeli CM, Ward LS, et al. How good is the levothyroxine replacement in primary hypothyroidism patients in Brazil? Data of a multicentre study. J Endocrinol Invest. 2013;36(7):485–488. [DOI] [PubMed] [Google Scholar]
- 39. Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab. 2009;94(4):1342–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duntas LH, Jonklaas J. Levothyroxine dose adjustment to optimise therapy throughout a patient’s lifetime. Adv Ther. 2019;36(Suppl 2):30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barbesino G. Drugs affecting thyroid function. Thyroid. 2010;20(7):763–770. [DOI] [PubMed] [Google Scholar]
- 42. Samarasinghe S, Meah F, Singh V, et al. Biotin interference with routine clinical immunoassays: understand the causes and mitigate the risks. Endocr Pract. 2017;23(8):989–998. [DOI] [PubMed] [Google Scholar]
- 43. Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MK. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98(5):1982–1990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in the References.