Abstract
Background
For patients with recurrent glioblastoma (rGBM), there are few options following treatment failure with radiotherapy plus temozolomide. Bintrafusp alfa is a first-in-class bifunctional fusion protein composed of the extracellular domain of the TGF-βRII receptor (a TGF-β “trap”) fused to a human IgG1 antibody blocking PD-L1.
Methods
In this phase I, open-label expansion cohort (NCT02517398), patients with rGBM that progressed after radiotherapy plus temozolomide received bintrafusp alfa 1200 mg Q2W until disease progression, unacceptable toxicity, or trial withdrawal. Response was assessed per RANO criteria. The primary endpoint was disease control rate (DCR); secondary endpoints included safety.
Results
As of August 24, 2018, 35 patients received bintrafusp alfa for a median of 1.8 (range, 0.5–20.7) months. Eight patients (22.9%) experienced disease control as assessed by an independent review committee: 2 had a partial response, 4 had stable disease, and 2 had non-complete response/non-progressive disease. Median progression-free survival (PFS) was 1.4 (95% confidence interval [CI], 1.2–1.6) months; 6- and 12-month PFS rates were 15.1% and 11.3%, respectively. Median overall survival (OS) was 5.3 (95% CI, 2.6–9.4) months; 6- and 12-month OS rates were 44.5% and 30.8%, respectively. The DCR (95% CI) was 66.7% (22.3–95.7%) for patients with IDH-mutant GBM (n = 6) and 13.8% (3.9–31.7%) for patients with IDH–wild-type GBM (n = 29). Disease control was seen regardless of PD-L1 expression. Twenty-five patients (71.4%) experienced treatment-related adverse events (grade ≥3; 17.1% [n = 6]).
Conclusions
The percentage of patients achieving disease control and the manageable safety profile may warrant further investigation of bintrafusp alfa in GBM.
Keywords: bintrafusp alfa, glioblastoma, M7824, PD-L1, TGF-β
Key Points.
Of 35 patients with rGBM treated with bintrafusp alfa, 8 (22.9%) had disease control.
Disease control was observed in IDH-mutant GBM (66.7%).
The safety profile was manageable.
Importance of the Study.
Treatment options for patients with rGBM are limited, and historically immunotherapy has shown minimal efficacy. In this expansion cohort of 35 patients with rGBM that progressed after radiotherapy and temozolomide, bintrafusp alfa 1200 mg every 2 weeks demonstrated disease control in 8 patients with rGBM (DCR; 22.9%), with a manageable safety profile. These results support further investigation of bintrafusp alfa in larger studies of patients with rGBM.
Glioblastoma (GBM; grade IV glioma) is the most common and aggressive type of malignant brain tumor, accounting for 45% of all gliomas.1–3 Patients diagnosed with GBM have a poor prognosis; the standard of care for GBM is radiotherapy plus temozolomide, but recurrence rates are approximately 90%, and less than 10% of patients survive 5 years after initial diagnosis.1,3–5 For patients who experience treatment failure with radiotherapy plus temozolomide, there is no established standard of care and participation in a clinical trial is strongly recommended.1,6 Studies evaluating second-line chemotherapy have reported an estimated 12-month overall survival (OS) rate ranging from less than 10% to 34%.6–8
While there are no identified targeted agents with demonstrated efficacy in GBM, molecular testing has been recommended due to potential for treatment options in the context of a clinical trial.1 Mutations in isocitrate dehydrogenase 1 (IDH1) or isocitrate dehydrogenase 2 (IDH2) define a molecular subtype of GBM (IDH mutant) associated with longer survival, and the treatment of some patients with IDH-mutant, low-grade astrocytomas with temozolomide has been associated with recurrence of hypermutated tumors.9–12 Therefore, a few ongoing trials with anti-PD-(L)1 therapies are focused on patients with IDH-mutant recurrent gliomas based upon hypermutated tumors being a promising target for immune checkpoint inhibitors.12–14 There are 2 ongoing phase II studies of nivolumab in patients with IDH-mutant recurrent gliomas (NCT03557359 and NCT03718767), as well as a phase II study investigating avelumab with hypofractionated radiation therapy in patients with IDH-mutant GBM (NCT02968940), which closed due to slow accrual.13–15 The poor prognosis and the lack of available treatments underscore the unmet need for effective therapies for all patients with recurrent GBM (rGBM), including those with IDH mutation.
The transforming growth factor-β (TGF-β) pathway can promote tumor progression and immune evasion in the tumor microenvironment via regulatory effects on immune cells and by impacting processes such as angiogenesis, fibrosis, and epithelial-mesenchymal transition.16,17 The TGF-β pathway is involved in glioma development and progression, and TGF-β has been shown to be overexpressed in malignant glioma tissues.18–20 Thus, inhibiting TGF-β activity in the tumor microenvironment while simultaneously blocking an additional immunosuppressive signaling mechanism, such as the programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) pathway, may provide a potentially effective treatment.
Anti-PD-(L)1 monotherapy has not demonstrated satisfactory clinical benefit in patients with rGBM. In an exploratory cohort from CheckMate 143, objective response rate with nivolumab monotherapy was 11% and with nivolumab + ipilimumab combination therapy was 0–10%.21 Median progression-free survival (PFS) was 1.9 months with nivolumab monotherapy and 1.5 to 2.1 months with nivolumab + ipilimumab combinations; median OS was 10.4 months with nivolumab monotherapy and 7.3–9.2 months with nivolumab + ipilimumab combinations. In a second cohort of CheckMate 143, nivolumab monotherapy did not improve PFS or OS compared with bevacizumab in patients with rGBM (median PFS, 1.5 vs 3.5 months; median OS, 9.8 vs 10.0 months, respectively); objective response rates were 8% versus 23%.22 No checkpoint inhibitors are currently recommended for the treatment of rGBM.1
Bintrafusp alfa is a first-in-class bifunctional fusion protein composed of the extracellular domain of the human TGF-β receptor II (TGF-βRII or TGF-β “trap”) fused via a flexible linker to the C-terminus of each heavy chain of an IgG1 antibody blocking programmed death ligand 1 (anti-PD-L1); it is designed to target tumors via colocalized, simultaneous inhibition of 2 key mechanisms of immunosuppression in the tumor microenvironment.23–25 Bintrafusp alfa has demonstrated antitumor activity and a manageable safety profile in the dose-escalation part and multiple expansion cohorts of this phase I trial.25–29 We report results from a phase I expansion cohort of patients with rGBM who received bintrafusp alfa.
Materials and Methods
NCT02517398 is a phase I, open-label trial investigating bintrafusp alfa in GBM and other solid tumors, including non-small-cell lung cancer, cervical cancer, and triple-negative breast cancer. All enrolled patients provided written informed consent, and the study protocol was approved by the institutional review board or independent ethics committee of each participating institution.
Patients
This expansion cohort includes adult patients (aged ≥18 years) with histologically confirmed grade IV rGBM that progressed after radiotherapy and temozolomide (at first recurrence of disease). Patients must also have a Karnofsky Performance Status of ≥70, life expectancy of ≥12 weeks, no allergy to gadolinium-based contrast media, and received no prior bevacizumab or other anti-vascular endothelial growth factor or antiangiogenic treatments. An interval of ≥12 weeks after the end of prior radiotherapy was required unless there was either histopathologic confirmation of recurrent tumor or new enhancement on magnetic resonance imaging (MRI) outside of the radiotherapy treatment field. Patients were selected regardless of PD-L1 expression level. Relevant exclusions were major surgery or anticancer treatment within 28 days before the start of trial treatment, previous malignant disease, significant acute or chronic infections, active autoimmune disease, and rapidly progressive disease that, in the opinion of the investigator, may predispose to inability to tolerate treatment or trial procedures.
Study Design
Patients received bintrafusp alfa 1200 mg every 2 weeks until confirmed progressive disease (PD), unacceptable toxicity, or trial withdrawal. Dosing modifications, such as changes in infusion rate and dose delays, were permitted; however, dose reductions were not allowed. Premedication with an antihistamine and paracetamol (acetaminophen) approximately 30–60 min before each dose of bintrafusp alfa was mandatory for at least the first 2 infusions and optional afterward.
Outcomes
The primary objective was to assess the disease control rate (DCR) defined as the proportion of patients with best overall response of complete response (CR), partial response (PR), stable disease (SD), or non-CR/non-PD according to the Response Assessment in Neuro-Oncology (RANO) criteria as adjudicated by an independent review committee (IRC). SD was defined as any best response of SD with a minimum duration of 6 weeks from baseline assessment. Non-CR/non-PD was defined as having no target lesion present at baseline with at least one assessment of stable or decreasing disease. Tumor evaluation was performed every 6 weeks up to 12 months and then every 12 weeks until PD. PD was defined as any of the following: ≥25% increase in sum of the products of perpendicular diameters of enhancing lesions compared with the smallest tumor measurement obtained either at baseline (if no decrease) or best response, in patients on stable (including patients not on steroids) or increasing doses of corticosteroids; significant increase in T2/fluid-attenuated inversion recovery (FLAIR) nonenhancing lesion in patients on stable (including patients not on steroids) or increasing doses of corticosteroids compared with baseline scan or best response after initiation of therapy not caused by comorbid events (eg, radiation therapy, demyelination, ischemic injury, infection, seizures, postoperative changes, or other treatment effects); any new lesion; clear clinical deterioration not attributable to other causes apart from the tumor (eg, seizures, medication adverse effects, complications of therapy, cerebrovascular events, infection) or changes in corticosteroid dose; failure to return for evaluation as a result of death or deteriorating condition; or clear progression of nonmeasurable disease. The secondary objectives included safety and tolerability of bintrafusp alfa. Exploratory objectives included best overall response and PFS according to RANO, and OS. Post hoc analyses were completed to assess efficacy based on IDH mutation status, PD-L1 expression, prior steroid use, prior surgery, and the relationship between tumor volume and response.
Adverse events (AEs) and laboratory abnormalities were classified and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v4.03. AEs that had an immune-related cause were identified using a prespecified list of Medical Dictionary for Regulatory Activities terms and must have been treated with corticosteroids, immunosuppressants, or hormonal therapy and have no other clear etiology. Any AE believed to be a potential immune-related or potential TGF-β-related AE30 was considered an AE of special interest.
Statistical Analysis
Efficacy and safety were analyzed in all patients who received ≥1 dose of bintrafusp alfa. DCR was tabulated, and the 2-sided 95% Clopper-Pearson CI was constructed. With 30 patients treated, the study has approximately 97% power to rule out a ≤ 50% DCR (null hypothesis) when the true DCR is 80% at the 5% type I error rate (1-sided). PFS and OS were analyzed using the Kaplan–Meier (KM) method. Follow-up time was calculated using the KM method. Safety and tolerability were analyzed using descriptive statistics.
Biomarker Analysis
In a central laboratory, tumor PD-L1 protein expression was measured by immunohistochemistry staining of formalin-fixed, paraffin-embedded blocks with a proprietary assay (Dako) using the anti-PD-L1 monoclonal antibody clone 73–10. Tumors were categorized based on the proportion of tumor cells expressing PD-L1 according to a threshold of 1% as positive (≥1%) or negative (<1%).27 The methods for evaluating tumor gene expression for immune cells and cytokines are described in the Supplementary Material.
Volumetric Analysis
Two reviewers retrospectively undertook independent 3D volumetric analysis of baseline tumor volumes in baseline MRI scans of patients in this trial. Differences in results were resolved by consensus or adjudication by a third investigator (A.M.S.). Manual segmentation of the MRI brain scans was performed using MIM Maestro (MIM Software Inc.) under the direction of 2 experienced neuro-oncological radiologists. All patient scans were manually segmented on MRIs of 5-mm thickness. Segmentation was performed on postgadolinium T1-weighted images (T1wGd) and FLAIR sequences, with precontrast T1 sequences reviewed but not segmented. All nonartifactual FLAIR abnormalities, including suspected edema, were segmented on the FLAIR sequence, with only enhancing disease segmented on T1wGd. The surgical cavity, cysts, and necrosis were not included in measurements, per RANO criteria.31 In cases of multiple foci of disease, all regions of interest were segmented. For each case, volumetric data on T1wGd and FLAIR images were documented. Enhancing tumor volume was categorized into quartiles and greater than or less than 15 cm3. The baseline tumor cutoff of 15 cm3 was established to align with previously reported data.32–34
Role of the Funding Source
Merck KGaA, Darmstadt, Germany, provided the study drug and worked with investigators on the trial design and plan, collection and analysis of data, and interpretation of results. Funding for a professional medical writer with access to the data was provided by Merck KGaA and GlaxoSmithKline.
Results
Baseline Demographics and Treatment Exposure
From October 4, 2016, to January 23, 2017, 47 patients were screened for this trial, of whom 35 were enrolled and treated with bintrafusp alfa. Baseline characteristics are summarized in Table 1. As of the database cutoff on August 24, 2018, KM median follow-up time was 19.7 months (range, 0.8–20.5). Median duration of treatment was 1.8 months (range, 0.5–20.7). Of 4 patients who remained on treatment at database cutoff (duration of treatment, 19.3–20.7 months), 1 patient was still on treatment at a follow-up cutoff on May 15, 2020 (KM median follow-up time, 39.6 months).
Table 1.
Patient Baseline and Disease Characteristics
| Characteristic | N = 35 |
|---|---|
| Median age, years (range) | 57 (28–75) |
| Sex, n (%) | |
| Male | 24 (68.6) |
| Female | 11 (31.4) |
| ECOG performance status, n (%)a | |
| 0 | 12 (34.3) |
| 1 | 22 (62.9) |
| 2 | 1 (2.9) |
| Prior anticancer regimens, n (%) | |
| 1 | 32 (91.4) |
| 2 | 2 (5.7) |
| 3 | 1 (2.9) |
| Prior systemic steroid use, n (%)b | 18 (51.4) |
| Median time since initial GBM diagnosis, months (range) | 14.5 (4.5–50.2) |
| IDH mutation status, n (%) | |
| Positive | 6 (17.1) |
| Negative | 29 (82.9) |
| PD-L1 tumor expression, n (%) | |
| Positive (≥1% tumor cells) | 24 (68.6) |
| Negative (<1% tumor cells)c | 11 (31.4) |
| Subsequent anticancer treatment, n (%)d | |
| Any | 11 (31.4) |
| Cytotoxic therapy | 8 (22.9) |
| Bevacizumab | 7 (20.0) |
| Tumor volume at baseline, n (%) | |
| <15 cm3 | 12 (34.3) |
| ≥15 cm3 | 20 (57.1) |
| N/A | 3 (8.6) |
| Maximum dexamethasone-equivalent dose, n (%)b | |
| None | 17 (48.6) |
| ≤2 mg | 6 (17.1) |
| >2–4 mg | 4 (11.4) |
| >4 mg | 8 (22.9) |
| Any surgery prior to initiation of study therapy, n (%) | |
| Gross total resection | 15 (42.9) |
| Subtotal resection | 15 (42.9) |
| Biopsy | 2 (5.7) |
| Unknown | 1 (2.9) |
| None | 2 (5.7) |
ECOG, Eastern Cooperative Oncology Group; GBM, glioblastoma; IDH, isocitrate dehydrogenase.
aRecorded on week 1, day 1, and was not used as an inclusion criterion. bReceived between screening and initial dose of bintrafusp alfa. cFour of 6 patients with IDH-mutant tumors had PD-L1-negative tumors. dFive patients received >1 therapy.
Efficacy
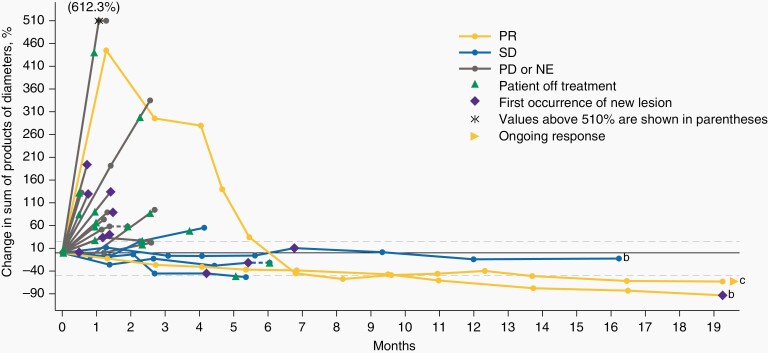
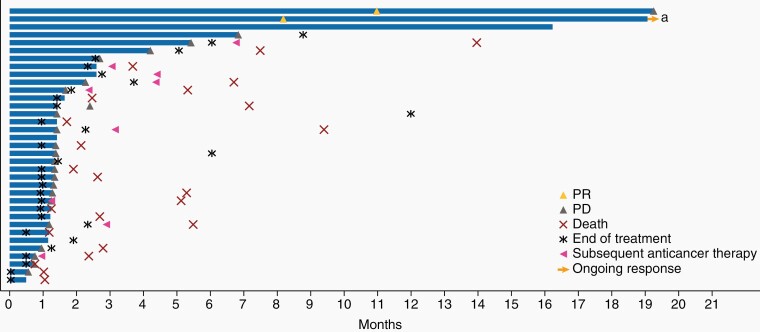
As of August 24, 2018, 8 patients (22.9% [95% confidence interval [CI], 10.4–40.1]) experienced disease control, with response assessed per RANO criteria, as adjudicated by the IRC; among these patients, 2 had a PR, 4 had SD, and 2 had non-CR/non-PD (Figure 1). At data cutoff, 1 patient who experienced a PR was still in response 19.3 months after start of treatment, and the other patient experienced a PD at 19.3 months (Figure 2). Both patients remained on treatment at data cutoff. Per investigator, one of the patients also had a best overall response (BOR) of PR, while the other was assessed to have a BOR of SD (Supplementary Figures 1 and 2). The 4 patients with SD had PFS of 2.3, 4.2, 5.4, and 16.2 (censored) months and duration of SD lasting 1.4, 2.7, 4.4, and 16.2 months from time of first dose, respectively; per investigator, 3 of these patients had BOR of SD and one was assessed to have PD. The 2 patients who had a best response of non-CR/non-PD per IRC were rated as SD by the investigator; both patients had measurable disease per investigator that could not be confirmed by the IRC (Supplementary Figure 1).
Figure 1.
Change in sum of products of diameters assessed by IRCa. a Fifteen of the 35 patients are not displayed in the figure due to reasons including non-evaluable disease (n = 1), non-complete response/non-progressive disease (non-CR/non-PD; n = 2), or absence of post-baseline target lesion measurements (n = 12). b Patients with IDH-mutant glioblastoma (GBM). c Patient with IDH-mutant GBM. This patient was assessed by independent reviewers as having equivocal progressive disease on day 39 prior to attaining a partial response according to RANO criteria at day 249, which permit a partial response after progressive disease that is not unequivocal. The last dose of radiotherapy was given 5.4 months prior to the first dose of bintrafusp alfa. IDH, isocitrate dehydrogenase; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 2.
Duration of response per patient assessed by IRC. a The patient was assessed by independent reviewers as having equivocal progressive disease on day 39 prior to attaining a partial response according to RANO criteria at day 249, which permit a partial response after progressive disease that is not unequivocal. The last dose of radiotherapy was given 5.4 months prior to the first dose of bintrafusp alfa. IRC, independent review committee; PD, progressive disease; PR, partial response.
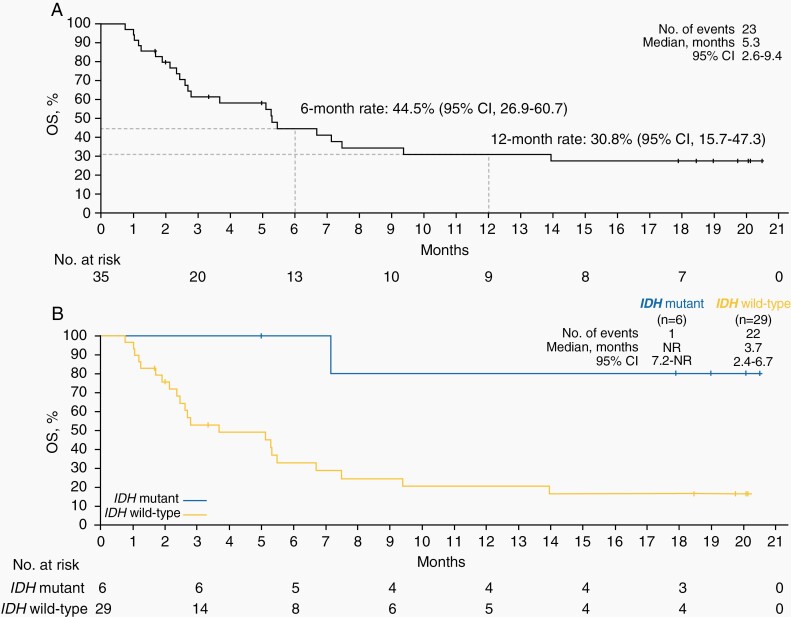
Median PFS by IRC and median OS were 1.4 months (95% CI, 1.2–1.6 months) and 5.3 months (95% CI, 2.6–9.4 months), respectively (Figure 3A; Supplementary Figure 3). The 6-, 12-, and 18-month PFS rates were 15.1%, 11.3%, and 11.3%, respectively. The 6-, 12-, and 18-month OS rates were 44.5%,30.8%, and 27.4%, respectively.
Figure 3.
Overall survival in the overall population (A) and by IDH status (B). IDH, isocitrate dehydrogenase; NR, not reached; OS, overall survival.
A post hoc observation revealed improved outcomes for patients with IDH mutation compared with patients with IDH–wild-type disease. DCR was 66.7% (4 of 6, including 2 PRs, 1 SD, and 1 non-CR/non-PD) for patients with IDH mutation versus 13.8% (4 of 29) for patients with IDH–wild-type disease. Median PFS was 13.0 months (95% CI, 1.3 months to not reached [NR]) versus 1.3 months (95% CI, 1.2–1.4 months). Median OS was NR (95% CI, 7.2 months to NR) versus 3.7 months (95% CI, 2.4–6.7 months; Figure 3B). Disease control was observed irrespective of PD-L1 expression (Table 2). DCR was 16.7% (4 of 24, including 1 PR) for patients with PD-L1-positive tumors versus 36.4% (4 of 11, including 1 PR) for patients with PD-L1-negative tumors. Four of 6 patients with IDH-mutant tumors had PD-L1-negative tumors. Median PFS was 1.3 months (95% CI, 1.2–1.6 months) versus 1.4 months (95% CI, 1.2–19.3 months) for PD-L1-positive versus PD-L1-negative tumors, respectively. Supplementary Table 1 provides treatment outcomes by individual patient baseline characteristics.
Table 2.
Preliminary Efficacy Parameters by IRC and Investigator per RANO
| Outcome (N = 35) | By IRC | By Investigator |
|---|---|---|
| BOR, n (%) | ||
| CR | 0 | 0 |
| PR | 2 (5.7) | 1 (2.9) |
| SDa | 4 (11.4) | 8 (22.9) |
| Non-CR/Non-PD | 2 (5.7) | 0 |
| PD | 25 (71.4) | 22 (62.9) |
| NE | 2 (5.7)b | 4 (11.4)c |
| DCR, n (%) [95% CI] | ||
| All | 8 (22.9) [10.4–40.1] | 9 (25.7) [12.5–43.3] |
| PD-L1 positive (≥1% tumor cells) | 4/24 (16.7) [4.7–37.4] | 4/24 (16.7) [4.7–37.4] |
| PD-L1 negative (<1% tumor cells) | 4/11 (36.4) [10.9–69.2] | 5/11 (45.5) [16.7–76.6] |
| IDH mutant | 4/6 (66.7) [22.3–95.7] | 4/6 (66.7) [22.3–95.7] |
| IDH wild-type | 4/29 (13.8) [3.9–31.7] | 5/29 (17.2) [5.9–35.8] |
| Median PFS, months (95% CI) | ||
| All | 1.4 (1.2–1.6) | 1.4 (1.2–1.6) |
| PD-L1 positive (≥1% tumor cells) | 1.3 (1.2–1.6) | 1.3 (1.2–1.5) |
| PD-L1 negative (<1% tumor cells) | 1.4 (1.2–19.3) | 1.4 (1.2–5.4) |
| IDH mutant | 13.0 (1.3-NR) | 11.3 (1.3-NR) |
| IDH wild-type | 1.3 (1.2–1.4) | 1.3 (1.2–1.4) |
| Median OS, months (95% CI) | ||
| All | 5.3 (2.6–9.4) | |
| IDH mutant | NR (7.2-NR) | |
| IDH wild-type | 3.7 (2.4–6.7) |
BOR, best overall response; CR, complete response; DCR, disease control rate; IDH, isocitrate dehydrogenase; IRC, independent review committee; NE, not evaluable; NR, not reached; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RANO, Response Assessment in Neuro-Oncology; SD, stable disease.
aDefined as a minimum duration of 6 weeks from baseline assessment. bIncludes 1 patient with no IRC review due to no postbaseline assessments and 1 patient classified as having no target lesions. cIncludes the 2 patients with NE per IRC in addition to 2 patients with all postbaseline assessments of NE per investigator, which were classified as PD per IRC.
PFS and OS were also assessed by steroid treatment received between screening and initial dose of bintrafusp alfa (steroids vs no steroids) and prior surgery in a post hoc analysis. The median PFS in patients who received steroids (n = 18) between study screening and first dose of bintrafusp alfa was 1.3 months (95% CI, 1.2–1.6 months) compared with 1.4 months (95% CI, 1.3–4.2 months) in patients who did not receive steroids (n = 17) (Supplementary Table 2). The median OS in these same patients was 2.5 months (95% CI, 1.7–5.5 months) and 9.4 months (95% CI, 5.1 months-NR), respectively. Also, patients who underwent biopsy before trial enrollment had a median PFS of 4.2 months (95% CI, NR-NR) and a median OS of 5.6 months (95% CI, 3.7–7.5 months). Those who had a gross total resection or subtotal resection had a median PFS of 1.4 months (95% CI, 1.0–1.4 months and 1.1–2.7 months, respectively) and a median OS of 7.2 months (95% CI, 1.9 months-NR) and 5.3 months (95% CI, 1.7–14.0 months), respectively.
Post hoc analyses revealed trends between the expression of genes associated with both the immune and the TGF-β pathways and response to bintrafusp alfa treatment; however, none were statistically significant (Supplementary Figure 4A–H).
Post Hoc Volumetric Analysis
IDH mutation status did not correlate with baseline enhancing tumor volume or sum of the products of perpendicular diameters (Supplementary Figure 5). The DCRs in patients with enhancing tumor volume of <15 cm3 and ≥15 cm3 were 25.0% (95% CI, 5.5–57.2%) and 25.0% (95% CI, 8.7–49.1%), respectively. The median OS rates were 14.0 and 2.7 months, respectively, and 12-month OS rates were 50.9% and 17.3%, respectively. Patients were also categorized into quartiles based on sum of products of perpendicular diameters and enhancing tumor volume. The median OS in patients less than or equal to the lower limit of quartile 1 (≤Q1) of the sum of products of perpendicular diameters, >Q1 and ≤Q2, >Q2 and ≤Q3, and >Q3 were 5.3, 5.1, 5.2, and 2.4 months, respectively (Supplementary Figure 6). In terms of enhancing tumor volume, the median OS in patients less than or equal to the lower limit of Q1, >Q1 and ≤Q2, >Q2 and ≤Q3, and >Q3 were not reached, 7.5, 2.5, and 2.0 months, respectively (Supplementary Figure 7); the respective 12-month OS rates were 72.9%, 29.2%, 0%, and 16.7%.
Safety
The most common treatment-related AEs (TRAEs) were gingival bleeding (17.1%), asthenia (14.3%), rash (11.4%), rash maculopapular (11.4%), and pruritus (11.4%) (Table 3). TRAEs of grade ≥ 3 occurred in 6 patients (17.1%). Grade 3 TRAEs were anemia, diarrhea, alanine aminotransferase increased, amylase increased, lipase increased, eczema, papule, and rash papular (observed in 1 patient each [2.9%]). One patient experienced a grade 4 asymptomatic lipase increased. One patient died due to intracranial tumor hemorrhage in the setting of disease progression, which was assessed by the investigator as related to bintrafusp alfa; the patient had entered the trial with disease evidenced by a large tumor and baseline incomplete hemiplegia while receiving prophylactic enoxaparin. Clinical review identified 4 additional patients (total n=5 [14.3%]) who experienced intratumoral or intracranial bleeding events (Supplementary Table 3). All events occurred in the setting of PD, and 4 of 5 were fatal. All cases were reviewed in detail (including radiographic imaging at baseline and after the event) with independent external advisors/neuro-oncologists, who concluded that these events were not unexpected given advanced disease and therefore did not constitute a specific safety signal. Of note, in all cases, the hemorrhage occurred within new lesions attributed to PD. The bleeding events occurred between 2 and 17 days after the last dose of bintrafusp alfa, and 2 of the 5 patients were receiving anticoagulants (for deep vein thrombosis prophylaxis and previous pulmonary embolism). One patient had withdrawn consent following diagnosis of intratumoral hemorrhage in new lesions (PD), and no further information could be obtained. TRAEs leading to treatment discontinuation occurred in 3 patients (8.6%; grade 3 diarrhea and grade 1 rash [n = 1], grade 3 increased alanine aminotransferase levels [n = 1], and death due to intratumoral hemorrhage [described above] [n = 1]). Five patients (14.3%) experienced immune-related AEs (immune-related colitis [n = 1], immune-related endocrinopathies: thyroid disorder [n = 2], and immune-related rash [n = 5]), with 2 patients experiencing grade 3 events (immune-related colitis and immune-related rash) and no grade 4 or 5 events (Supplementary Table 4). Four patients (11.4%) experienced potentially TGF-β-related skin lesions, such as those that have been reported with other TGF-β inhibitors,30 including keratoacanthomas (n = 2), basal cell carcinoma (n = 1), and squamous cell carcinoma of skin (n = 1); these lesions were well managed with emollients and surgical excision as needed and did not require any patient to discontinue treatment.
Table 3.
Treatment-Related AEs Occurring at Any Grade in ≥5% of Patients or at Grade ≥3 and AEs of Special Interest
| N = 35 | Any Grade | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| TRAE | 25 (71.4) | 5 (14.3) | 1 (2.9) | 1 (2.9) |
| Gingival bleeding | 6 (17.1) | 0 | 0 | 0 |
| Asthenia | 5 (14.3) | 0 | 0 | 0 |
| Rash | 4 (11.4) | 0 | 0 | 0 |
| Rash maculopapular | 4 (11.4) | 0 | 0 | 0 |
| Pruritus | 4 (11.4) | 0 | 0 | 0 |
| Rash papular | 3 (8.6) | 1 (2.9) | 0 | 0 |
| Arthralgia | 3 (8.6) | 0 | 0 | 0 |
| Diarrhea | 2 (5.7) | 1 (2.9) | 0 | 0 |
| Keratoacanthoma | 2 (5.7) | 0 | 0 | 0 |
| Eczema | 2 (5.7) | 1 (2.9) | 0 | 0 |
| Anemia | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Alanine aminotransferase increased | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Amylase increased | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Lipase increased | 1 (2.9) | 1 (2.9) | 1 (2.9) | 0 |
| Intracranial tumor hemorrhage | 1 (2.9) | 0 | 0 | 1 (2.9) |
| Papule | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Any AESI | ||||
| Skin lesions | 4 (11.4) | 0 | 0 | 0 |
| Any immune-related adverse event | 5 (14.3) | 2 (5.7) | 0 | 0 |
| Immune-related rash | 5 (14.3) | 1 (2.9) | 0 | 0 |
| Immune-related endocrinopathies: thyroid disorders | 2 (5.7) | 0 | 0 | 0 |
| Immune-related colitis | 1 (2.9) | 1 (2.9) | 0 | 0 |
Data are n (%) in the safety set.
AESI, adverse event of special interest; TRAE, treatment-related adverse event.
Discussion
Bintrafusp alfa demonstrated a manageable safety profile and activity in this cohort of 35 patients with rGBM, a population who, historically, have had limited treatment options following radiotherapy plus temozolomide. The DCR was 22.9% per IRC assessment, with durable responses in 2 patients with confirmed PRs and PFS ranging from 2.3 months to 16.2 months in the 4 patients with SD. In patients with IDH mutation (n = 6), DCR was 66.7% (including the 2 patients with a PR) and 13.8% in patients with IDH–wild-type disease (n = 29). In a study of the TGF-β inhibitor galunisertib, a numerically improved median OS was observed in 8 patients with IDH R132H mutation-positive rGBM compared with those with IDH1–wild-type tumors (n = 108; 10.4 vs 6.9 months); however, this difference was not statistically significant.35 Three of the 7 patients with IDH R132H mutation-positive rGBM treated with galunisertib or galunisertib plus lomustine had SD and one patient had PR; the remaining patient received placebo plus lomustine.36 With bintrafusp alfa, the median OS was 5.3 months, and the 6- and 12-month OS rates were 44.5% and 30.8%, respectively. The 12-month OS rate seen in this phase I study compares favorably to rates reported for second-line chemotherapy in a phase II, phase III, and a retrospective single institution study.6–8 DCR was selected as a prespecified primary endpoint in order to help contextualize the previously reported data with other immunotherapies in GBM. While DCR is used in other ongoing clinical trials in GBM, it is not a validated endpoint per RANO guidelines and is therefore a potential limitation of this study.
The median OS and 12-month OS rates in patients with enhancing tumor volume of <15 cm3 suggest that patients with smaller tumors (ie, those <15 cm3) experience greater benefit with bintrafusp alfa treatment. When analyzed by quartile, patients in the lower quartiles had higher median OS and 12-month OS rates; however, these data warrant further investigation due to the lack of a control arm. Additionally, this small sample contained several patients with high tumor volume. The volumetric analysis used 3D measurements, which have been shown to be more predictive of survival in recurrent gliomas compared with unidimensional and bidimensional measurements.37 Patients with a history of gross total resection experienced longer median OS than patients with a history of biopsy. Conversely, patients with a history of biopsy experienced longer median PFS. A survival benefit was observed among patients who were not on steroids at baseline. This is consistent with another study evaluating steroid use with immune checkpoint inhibitors in glioblastoma.38 Disease control was also observed irrespective of PD-L1 expression. Notably, higher PD-L1 expression (at any cutoff or in any compartment—tumor cell staining, immune cell staining, or both) did not enrich outcomes for responders in this cohort. Potential limitations of PD-L1 immunohistochemistry testing include the changes in PD-L1 expression over time and limited tissue sampling that may not be representative of the entire tumor.39 In this study, the status observed from archival tissue may not accurately reflect the status at tumor progression/study initiation. It is possible that the activity observed in these patients treated with bintrafusp alfa is due to the unique immune microenvironment of IDH-mutant glioma.40 In this study, 17.1% of patients had IDH-mutant GBM; previously reported rates from other studies ranged from 3.7% to 18.8%.6,9,35,41 These results should be viewed as hypothesis generating, and larger studies are needed.
Bintrafusp alfa’s safety profile was manageable and similar to that of anti-PD-(L)1 antibodies,21,22,41 except for potentially TGF-β-related AEs, including skin lesions and gingival bleeding; these types of lesions have been observed with other TGF-β inhibitors (eg, fresolimumab), were readily managed, and did not require treatment discontinuation.42 One event leading to death occurred (intratumoral hemorrhage) and was assessed by the investigator as treatment related in conjunction with disease progression, and 3 patients discontinued treatment due to TRAEs. Five patients (14.3%) experienced intratumoral or intracranial bleeding events in the setting of PD. In all cases, the hemorrhage occurred within new lesions attributed to PD. This rate is similar to rates of intracranial hemorrhage reported in patients with primary brain tumors, such as GBM, receiving various treatment regimens including radiation, surgery, chemotherapy, or antiangiogenic agents; rates ranging from 2.6% to 13.6% have been reported for patients not receiving anticoagulation and from 15.5% to 28.1% for patients receiving anticoagulation treatment.43,44 While the incidence of fatal intratumoral or intracranial bleeding events was higher in this study compared with other clinical trials (0 to <1%) in rGBM, some of the comparator studies only reported adverse events deemed related to treatment,6,21,41,45 whereas this study reported any treatment-emergent bleeding events. Bleeding events in this study occurred in the setting of PD, which is consistently reported as the primary cause of death in rGBM studies.21,41,45
Conclusions
Due to its poor prognosis and the lack of treatment options, rGBM remains an area of unmet need. The previously reported activity of immune checkpoint inhibitors in patients with this disease is modest at best. The conclusions of this study are limited based on the small sample size and lack of control group. Therefore, the safety and preliminary efficacy signals presented herein may warrant further investigation of bintrafusp alfa in GBM, as well as in IDH-mutant gliomas; it further supports the development of biomarkers and novel imaging assessments to better understand which patients will derive clear clinical benefit.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and their families, investigators, co-investigators, and study teams at each of the participating centers and at Merck KGaA, Darmstadt, Germany, and EMD Serono Research & Development Institute, Inc., Billerica, MA, USA; an affiliate of Merck KGaA, Darmstadt, Germany. Tumor volume analysis was supported by the Victorian Government for the Centre for Research Excellence in Brain Cancer, Olivia Newton-John Cancer Research Institute, Heidelberg, VIC, Australia. Medical writing support was provided by Megan Hyde, PharmD, of ClinicalThinking Inc, Hamilton, NJ, USA, which was also funded by Merck KGaA and GlaxoSmithKline in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Funding
This study was funded by Merck KGaA, Darmstadt, Germany, and is part of an alliance between Merck KGaA and GlaxoSmithKline.
Conflicts of interest statement. M.K. has received research funding from AbbVie, Bristol Myers Squibb, and Specialized Therapeutics. MW has received research grants from AbbVie, Adastra, Apogenix, Merck Sharp & Dohme, Merck KGaA, Darmstadt, Germany, Novocure, and Quercis, and honoraria for lectures or advisory board participation or consulting from AbbVie, Adastra, Basilea, Bristol Myers Squibb, Celgene, Medac, Merck Sharp & Dohme, Merck KGaA, Nerviano Medical Sciences, Novartis, Orbus, Philogen, Roche, Tocagen, and Y-mAbs. M.I.dl.F. has advisory affiliations with Puma Biotechnology, Agios Pharmaceuticals, and Forma Therapeutics. D.V. has advisory affiliations with AstraZeneca, Pfizer, Roche, Takeda, and Amgen. D.A.R. received research support (institution) from Acerta; Agenus; Celldex; EMD Serono Research & Development Institute, Inc., Billerica, Massachusetts, USA, an affiliate of Merck KGaA, Darmstadt, Germany; Incyte; Inovio; Midatech; Omniox; and Tragara and received advisory/consulting fees from AbbVie; Advantagene; Agenus; Amgen; Bayer; Bristol Myers Squibb; Celldex; DelMar; EMD Serono Research & Development Institute, Inc.; Genentech/Roche; Inovio; Merck Sharp & Dohme; Merck KGaA; Monteris; Novocure; Oncorus; Oxigene; Regeneron; Stemline; and Taiho Oncology. H.K.G. received consulting fees from Eisai (speaker’s bureau) and advisory board participation and consulting from AbbVie. A.M.S. received research funding from EMD Serono Research & Development Institute, Inc., Billerica, Massachusetts, USA, an affiliate of Merck KGaA, Darmstadt, Germany. I.D. and L.S.O. are employees of EMD Serono Research & Development Institute, Inc., Billerica, Massachusetts, USA, an affiliate of Merck KGaA, Darmstadt, Germany. C.H. is an employee of Merck KGaA, Darmstadt, Germany. All other authors have no conflicts of interest to report.
Authorship Statement. Study design, data collection, data interpretation, and development of the manuscript: M.K., M.W., D.L., K.K., C.K.L., Cr.G., M.I.dl.F., D.V., D.A.R., H.K.G., A.M.S., I.D., C.H., L.S.O., Ca.G., and M.G. Data analysis: sponsor (Merck KGaA, Darmstadt, Germany). Reviewed and approved the final manuscript: M.K., M.W., D.L., K.K., C.K.L., Cr.G., M.I.dl.F., D.V., D.A.R., H.K.G., A.M.S., I.D., C.H., L.S.O., Ca.G., and M.G.
References
- 1. NCCN Clinical Practice Guidelines in Oncology. Central Nervous System Cancers. V2.2020.https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed August 12, 2020.
- 2. Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro Oncol. 2013;15(1):4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 5. Weller M, van den Bent M, Tonn JC, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Gliomas . European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 6. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 7. Carvalho BF, Fernandes AC, Almeida DS, et al. Second-line chemotherapy in recurrent glioblastoma: a 2-cohort study. Oncol Res Treat. 2015;38(7–8):348–354. [DOI] [PubMed] [Google Scholar]
- 8. Van Den Bent M, Eoli M, Sepulveda JM, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol. 2020;22(5):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mandel JJ, Cachia D, Liu D, et al. Impact of IDH1 mutation status on outcome in clinical trials for recurrent glioblastoma. J Neurooncol. 2016;129(1):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shirahata M, Ono T, Stichel D, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136(1):153–166. [DOI] [PubMed] [Google Scholar]
- 11. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi S, Yu Y, Grimmer MR, Wahl M, Chang SM, Costello JF. Temozolomide-associated hypermutation in gliomas. Neuro Oncol. 2018;20(10):1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. ClinicalTrials.gov. Nivolumab in People With IDH-Mutant Gliomas With and Without Hypermutator Phenotype.https://clinicaltrials.gov/ct2/show/NCT03718767. Accessed July 21, 2020.
- 14. ClinicalTrials.gov. Nivolumab for Recurrent or Progressive IDH Mutant Gliomas.https://clinicaltrials.gov/ct2/show/NCT03557359. Accessed July 21, 2020.
- 15. Kurz S, Silverman JS, Hochman T, et al. ATIM-37. Phase II, open-label, single arm, multicenter study of avelumab with hypofractionated radiation (HFRT) for adult patients with secondarily transformed IDH-mutant glioblastoma (GBM). Neuro Oncol. 2019;21(suppl. 6):vi9–vi10. [Google Scholar]
- 16. Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colak S, Ten Dijke P. Targeting TGF-β signaling in cancer. Trends Cancer. 2017;3(1):56–71. [DOI] [PubMed] [Google Scholar]
- 18. Han J, Alvarez-Breckenridge CA, Wang QE, Yu J. TGF-β signaling and its targeting for glioma treatment. Am J Cancer Res. 2015;5(3):945–955. [PMC free article] [PubMed] [Google Scholar]
- 19. Frei K, Gramatzki D, Tritschler I, et al. Transforming growth factor-β pathway activity in glioblastoma. Oncotarget. 2015;6(8):5963–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uhl M, Aulwurm S, Wischhusen J, et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64(21):7954–7961. [DOI] [PubMed] [Google Scholar]
- 21. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10(424):eaan5488. [DOI] [PubMed] [Google Scholar]
- 24. David JM, Dominguez C, McCampbell KK, Gulley JL, Schlom J, Palena C. A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology. 2017;6(10):e1349589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strauss J, Heery CR, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res. 2018;24(6):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strauss J, Gatti-Mays ME, Redman J, et al. Safety and activity of M7824, a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with HPV associated cancers. J Clin Oncol. 2018;36(suppl.): [abstract 3007]. [Google Scholar]
- 27. Paz-Ares L, Kim TM, Vicente D, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J Thorac Oncol. 2020;15(7):1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barlesi F, Isambert N, Felip E, et al. Initial results from phase 1 trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with NSCLC refractory or resistant to prior anti–PD-1/anti–PD-L1 agents. Poster presented at: 32nd Annual Meeting of the Society for Immunotherapy of Cancer; National Harbor, MD; November 8–12, 2017 [abstract O14]. [Google Scholar]
- 29. Kopetz S, Spira AI, Wertheim M, et al. M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with heavily pretreated CRC: preliminary results from a phase 1 trial. Poster presented at: 2018 Gastrointestinal Cancers Symposium; San Francisco, CA; January 18–20, 2018 [abstract 764]. [Google Scholar]
- 30. Lacouture ME, Morris JC, Lawrence DP, et al. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor β by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol Immunother. 2015;64(4):437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 32. Ellingson BM, Harris RJ, Woodworth DC, et al. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol. 2017;19(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ellingson BM, Kim E, Woodworth DC, et al. Diffusion MRI quality control and functional diffusion map results in ACRIN 6677/RTOG 0625: a multicenter, randomized, phase II trial of bevacizumab and chemotherapy in recurrent glioblastoma. Int J Oncol. 2015;46(5):1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cloughesy T, Brenner AJ, Butowski N, Cohen YC, Lowenton-Spier N, Wen P. ATIM-19. Results of the GLOBE study: a phase 3, randomized, controlled, double-arm, open-label, multi-center study of VB-111 combined with bevacizumab vs. bevacizumab monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2018;20(suppl. 6):vi4–vi5. [Google Scholar]
- 35. Capper D, Von Deimling A, Brandes AA, et al. Biomarker and histopathology evaluation of patients with recurrent glioblastoma treated with galunisertib, lomustine, or the combination of galunisertib and lomustine. Int J Mol Sci. 2017;18(5):995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brandes AA, Carpentier AF, Kesari S, et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2016;18(8):1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dempsey MF, Condon BR, Hadley DM. Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D? AJNR Am J Neuroradiol. 2005;26(4):770–776. [PMC free article] [PubMed] [Google Scholar]
- 38. Khasraw M, Reardon DA, Weller M, Sampson JH. PD-1 Inhibitors: do they have a future in the treatment of glioblastoma? Clin Cancer Res. 2020;26(20):5287–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cottrell TR, Taube JM. PD-L1 and Emerging biomarkers in immune checkpoint blockade therapy. Cancer J. 2018;24(1):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Richardson LG, Choi BD, Curry WT. (R)-2-hydroxyglutarate drives immune quiescence in the tumor microenvironment of IDH-mutant gliomas. Transl Cancer Res. 2019;8(suppl 2):S167–S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lukas RV, Rodon J, Becker K, et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J Neurooncol. 2018;140(2):317–328. [DOI] [PubMed] [Google Scholar]
- 42. Morris JC, Tan AR, Olencki TE, et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One. 2014;9(3):e90353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. 2017;129(25):3379–3385. [DOI] [PubMed] [Google Scholar]
- 44. Khoury MN, Missios S, Edwin N, et al. Intracranial hemorrhage in setting of glioblastoma with venous thromboembolism. Neurooncol Pract. 2016;3(2):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.