Abstract
Ticks are important vectors of various pathogens that result in clinical illnesses in humans and domestic and wild animals. Information regarding tick infestations and pathogens transmitted by ticks is important for the identification and prevention of disease. This study was a large-scale investigation of ticks collected from dogs and their associated environments in the Republic of Korea (ROK). It included detecting six prevalent tick-borne pathogens (Anaplasma spp., A. platys, Borrelia spp., Babesia gibsoni, Ehrlichia canis, and E. chaffeensis). A total of 2293 ticks (1110 pools) were collected. Haemaphysalis longicornis (98.60%) was the most frequently collected tick species, followed by Ixodes nipponensis (0.96%) and H. flava (0.44%). Anaplasma spp. (24/1110 tick pools; 2.16%) and Borrelia spp. (4/1110 tick pools; 0.36%) were detected. The phylogenetic analyses using 16S rRNA genes revealed that the Anaplasma spp. detected in this study were closely associated with A. phagocytophilum reported in humans and rodents in the ROK. Borrelia spp. showed phylogenetic relationships with B. theileri and B. miyamotoi in ticks and humans in Mali and Russia. These results demonstrate the importance of tick-borne disease surveillance and control in dogs in the ROK.
Keywords: dog ticks, Haemaphysalis longicornis, Ixodes nipponensis, Haemaphysalis flava, anaplasmosis, Lyme borreliosis, Korea
1. Introduction
Ticks are obligate blood-feeding parasites that transmit zoonotic tick-borne pathogens, including protozoa, viruses, and bacteria, to animal and human hosts [1,2]. Approximately 10% of known tick species are vectors of pathogens of medical and veterinary importance [3]. Some tick species are known vectors of one or several tick-borne diseases (TBDs), such as borreliosis (Lyme disease and Borrelia relapsing fever and other Borrelia spp. transmitted by Ixodes ticks), babesiosis (Babesia spp. transmitted by Haemaphysalis spp., Rhipicephalus spp., and Dermacentor spp.), ehrlichiosis (Ehrlichia canis genogroup transmitted by Rhipicephalus spp., Amblyomma americanum [4,5,6], Ixodes persulcatus, I. ovatus, and I. silvarum), and anaplasmosis (Anaplasma phagocytophilum transmitted by I. scapularis and I. pacificus [7]). An understanding of the specific tick hosts and associated pathogens is important to identify the risks of TBDs for domestic animals and humans.
Ticks are common parasites of domestic animals, including dogs, and have a high risk of transmitting tick-borne pathogens [8,9,10]. Dogs are reservoirs of some tick-borne pathogens [6]. They are the most common animal bred for various purposes, including pets and military dogs. Close contact with dogs may result in the transfer of ticks and TBDs to humans. Therefore, dogs may be considered sentinel animals for TBDs impacting human health [11,12,13,14]. Identifying the prevalence of dog ticks and associated pathogens provides an understanding of the distribution of tick-borne pathogens. It raises awareness of TBDs among pet owners and other people who contact dogs [15].
In the ROK, TBDs, such as Lyme disease, anaplasmosis, ehrlichiosis, tularemia, bartonellosis, and babesiosis, are of medical importance. In particular, the number of Lyme disease cases has rapidly increased since 2012 [16,17,18]. Molecular and serological detection methods have revealed that over 40% of dogs in the ROK are infected with pathogens that cause TBDs, including A. phagocytophilum, E. canis, Borrelia burgdorferi, Babesia gibsoni, Dirofilaria immitis, and Mycoplasma haemocanis [19,20]. The identification of reservoir hosts and potential vectors of the pathogenic agents is of interest. A previous study using molecular detection methods reported I. nipponensis ticks infected with B. garinii in dogs in the Gyeongsangbuk province, ROK [21]. However, the prevalence of ticks on dogs and tick-borne pathogens harbored by ticks collected from dogs in the ROK remains poorly investigated.
This study was part of a large-scale tick surveillance program of domestic pets, military working dogs, and stray dogs from shelter-associated environments in the ROK. Assays to detect six common tick-borne pathogens (A. phagocytophilum, A. platys, Borrelia spp., Babesia gibsoni, E. canis, and E. chaffeensis) were conducted. This study highlights the importance of the prevention of TBDs in dogs and humans in the ROK.
2. Results
2.1. Distribution of Dog Ticks in the ROK
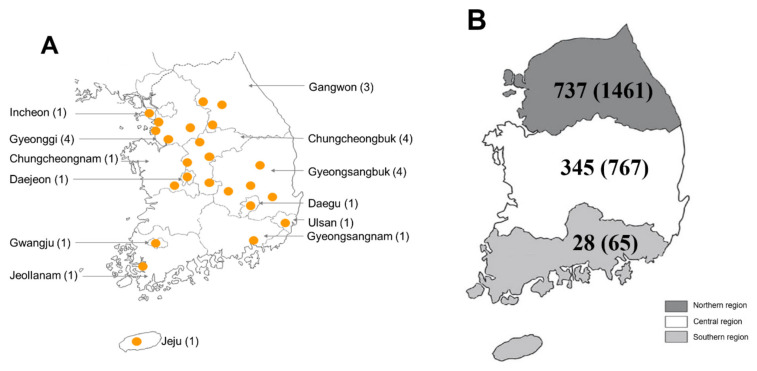
A total of 2293 ticks categorized into 1110 tick pools were collected from 24 sites in 13 provinces or metropolitan cities in the ROK (Figure 1). Overall, 807 ticks (35.2%) were found on pet dogs, 624 (27.2%) on military dogs, 572 (24.95%) on stray dogs, and 290 (12.65%) in stray dog shelter environments (Table 1).
Figure 1.
Collection of ticks from dogs and vegetation at dog shelters in the ROK. Ticks were collected at 24 sites in 13 provinces or metropolitan areas. The number of sites per province or metropolitan area is shown in parentheses (A). The numbers of tick pools collected from the northern, central, and southern regions are shown, with the number of collected ticks shown in parentheses (B).
Table 1.
Identification of dog ticks in the ROK.
| Species | Stage | Tick Pool (Number of Ticks) | Total | |||
|---|---|---|---|---|---|---|
| Pet Dogs | Stray Dogs | Dog Shelters 1 | Military Working Dogs | |||
| Haemaphysalis longicornis | Larvae | 4 (49) | 26 (126) | 4 (166) | 0 | 34 (341) |
| Nymph | 73 (606) | 51 (149) | 9 (93) | 15 (110) | 148 (958) | |
| Adult | 111 (129) | 282 (295) | 29 (29) | 478 (509) | 900 (962) | |
| Sub total | 188 (784) | 359 (570) | 42 (288) | 493 (619) | 1082 (2261) | |
| H. flava | Larvae | 0 | 0 | 0 | 0 | 0 |
| Nymph | 1 (3) | 0 | 1 (1) | 1 (3) | 3 (7) | |
| Adult | 2 (2) | 0 | 0 | 1 (1) | 3 (3) | |
| Sub total | 3 (5) | 0 (0) | 1 (1) | 2 (4) | 6 (10) | |
| Ixodes nipponensis | Larvae | 0 | 0 | 0 | 0 | 0 |
| Nymph | 0 | 0 | 0 | 0 | 0 | |
| Adult | 18 (18) | 2 (2) | 1 (1) | 1 (1) | 22 (22) | |
| Sub total | 18 (18) | 2 (2) | 1 (1) | 1 (1) | 22 (22) | |
| Total | 209 (807) | 361 (572) | 44 (290) | 496 (624) | 1110 (2293) | |
1 Ticks were collected from vegetation bordering stray dog shelter pens. Data are presented as numbers.
The highest number of ticks collected from dogs/dog shelters was observed in the northern region (1461 ticks, 63.72%), followed by the central region (767 ticks, 33.45%), and the southern region (65 ticks, 2.83%) (Table 2 and Figure 1).
Table 2.
Numbers of ticks collected from dogs and dog shelters by latitudinal region.
| Region | Site | Species | Total (%) | ||
|---|---|---|---|---|---|
| Haemaphysalis longicornis | H. flava | Ixodes nipponensis | |||
| Northern | Pet dogs | 552 | 4 | 12 | 568 |
| Stray dogs | 189 | 0 | 1 | 190 | |
| Dog shelters 1 | 100 | 0 | 0 | 100 | |
| Military working dogs | 599 | 3 | 1 | 603 | |
| Subtotal | 1440 (62.80) | 7 (0.31) | 14 (0.61) | 1461 (63.72) | |
| Central | Pet dogs | 228 | 1 | 6 | 235 |
| Stray dogs | 321 | 0 | 1 | 322 | |
| Dog shelters | 188 | 1 | 1 | 190 | |
| Military working dogs | 20 | 0 | 0 | 20 | |
| Subtotal | 757 (33.01) | 2 (0.09) | 8 (0.35) | 767 (33.45) | |
| Southern | Pet dogs | 4 | 0 | 0 | 4 |
| Stray dogs | 60 | 0 | 0 | 60 | |
| Dog shelters | 0 | 0 | 0 | 0 | |
| Military working dogs | 0 | 1 | 0 | 1 | |
| Subtotal | 64 (2.79) | 1 (0.04) | 0 | 65 (2.83) | |
| Total (%) | 2261 (98.60) | 10 (0.44) | 22 (0.96) | 2293 (100) | |
1 Ticks were collected from vegetation bordering stray dog shelter pens. Data are presented as numbers (percentage).
2.2. Identification of Tick Species
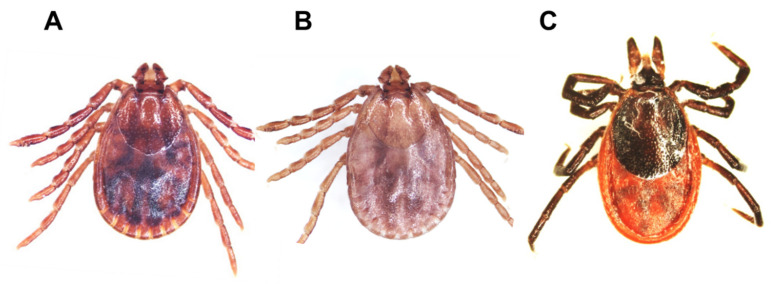
The most commonly collected tick species was H. longicornis (98.60%; 2261/2293), followed by I. nipponensis (0.96%; 22/2293) and H. flava (0.44%; 10/2293) (Table 2 and Figure 2).
Figure 2.
Morphological identification of three tick species collected from dogs in the ROK. Haemaphysalis longicornis female (A), H. flava female (B), and Ixodes nipponensis female (C) are shown.
2.3. Detection of Tick-Borne Pathogens in Dog Ticks
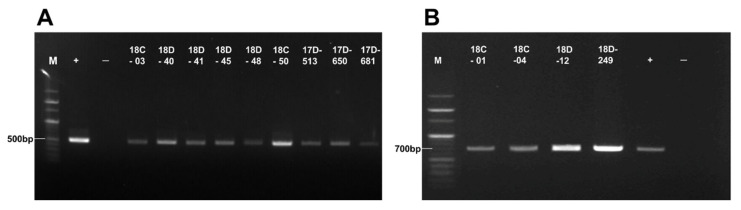
Anaplasma spp. and Borrelia spp. were detected in the collected ticks using polymerase chain reaction (PCR). Detection of these pathogens was confirmed using 16S rRNA gene fragments of amplicons of 511 bp (Anaplasma spp.) and 714 bp (Borrelia spp.) (Figure 3).
Figure 3.
Detection of Anaplasma spp. and Borrelia spp. from dog ticks collected in the ROK. Anaplasma spp. were detected by the amplification of a 16S rRNA gene with a band of 511 bp. Nine of 24 tick pools positive for Anaplasma spp. are shown (A). Amplification of the Borrelia spp. 16S rRNA gene (714 bp) was observed in four tick pools (B). A positive control using recombinant DNA (+) and negative control (-) using no DNA template are shown. M represents the 100 bp DNA marker.
Overall, 2.16% of the tick pools (24/1110 tick pools) contained Anaplasma spp. and 0.36% (4/1110 tick pools) contained Borrelia spp. Anaplasma spp. was detected in 22/1082 H. longicornis tick pools (2.03%) and 2/22 (9.09%) I. nipponensis tick pools (Table 3). Anaplasma spp. was detected in all stages (larvae, nymphs, and adults) of H. longicornis, but only in adults of I. nipponensis. Borrelia spp. was detected in 3/22 (13.64%) adult I. nipponensis tick pools and 1/1082 (0.09%) adult H. longicornis tick pools (Table 3).
Table 3.
Pathogens detected by molecular (PCR).
| Scheme | Stage | Number of Tick Pools | Species | |||||
|---|---|---|---|---|---|---|---|---|
| Anaplasma spp. | A. platys | E. canis | E. chaffeensis | Borrelia spp. | B. gibsoni | |||
| Haemaphysalis longicornis | Larvae | 34 | 3 | - | - | - | - | - |
| Nymph | 148 | 5 | - | - | - | - | - | |
| Adult | 900 | 14 | - | - | - | 1 | - | |
| Subtotal | 1082 | 22 (2.03) | - | - | - | 1 (0.09) | - | |
| H. flava | Larvae | 0 | - | - | - | - | - | - |
| Nymph | 3 | - | - | - | - | - | - | |
| Adult | 3 | - | - | - | - | - | - | |
| Subtotal | 6 | - | - | - | - | - | - | |
| Ixodes nipponensis | Larvae | 0 | - | - | - | - | - | - |
| Nymph | 0 | - | - | - | - | - | - | |
| Adult | 22 | 2 | - | - | - | 3 | - | |
| Subtotal | 22 | 2 (9.09) | - | - | - | 3 (13.64) | - | |
| Total | 1110 | 24 (2.16) | 0 | 0 | 0 | 4 (0.36) | 0 | |
Data are presented as numbers (percentage).
Half of the tick pools found to be positive for Anaplasma spp. (12/24 tick pools) were collected from the northern region of the ROK, including 11 tick pools collected from military dogs and one tick pool collected from a pet. In the central region, eight tick pools were positive for Anaplasma spp., including five collected from stray dogs and three collected from pets. All four tick pools from the southern region that were positive for Anaplasma spp. were collected from stray dogs (Table 4).
Table 4.
Distribution of tick-borne pathogens in dog ticks collected in the ROK.
| Region | Site | Number of Tick Pools | Species | |||||
|---|---|---|---|---|---|---|---|---|
| Anaplasma spp. | A. platys | E. canis | E. chaffeensis | Borrelia spp. | B. gibsoni | |||
| Northern | Pet dogs | 84 | 1 (1.19) | - | - | - | 1 (1.19) | - |
| Stray dogs | 169 | - | - | - | - | 1 (0.59) | - | |
| Dog shelters 1 | 2 | - | - | - | - | - | - | |
| Military working dogs | 482 | 11 (2.28) | - | - | - | - | - | |
| Subtotal | 737 | 12 (1.63) | - | - | - | 2 (0.27) | - | |
| Central | Pet dogs | 121 | 3 (2.48) | - | - | - | 2 (1.65) | - |
| Stray dogs | 169 | 5 (2.96) | - | - | - | - | - | |
| Dog shelters | 42 | - | - | - | - | - | - | |
| Military working dogs | 13 | - | - | - | - | - | - | |
| Subtotal | 345 | 8 (2.32) | - | - | - | 2 (0.58) | - | |
| Southern | Pet dogs | 4 | - | - | - | - | - | - |
| Stray dogs | 23 | 4 (17.39) | - | - | - | - | - | |
| Dog shelters | 0 | - | - | - | - | - | - | |
| Military working dogs | 1 | - | - | - | - | - | - | |
| Subtotal | 28 | 4 (14.29) | - | - | - | 0 | - | |
| Total (%) | 1110 | 24 (2.16) | 0 | 0 | 0 | 4 (0.36) | 0 | |
1 Ticks were collected from vegetation bordering stray dog shelter pens. Data are presented as numbers (percentage).
Borrelia spp. was only detected in ticks collected in the northern and central regions and was predominantly detected in ticks collected from pet dogs (75%, 3/4). However, one positive pool (25%, 1/4) was collected from a stray dog (Table 4).
2.4. Sequencing and Phylogenetic Analysis of Tick-Borne Pathogens
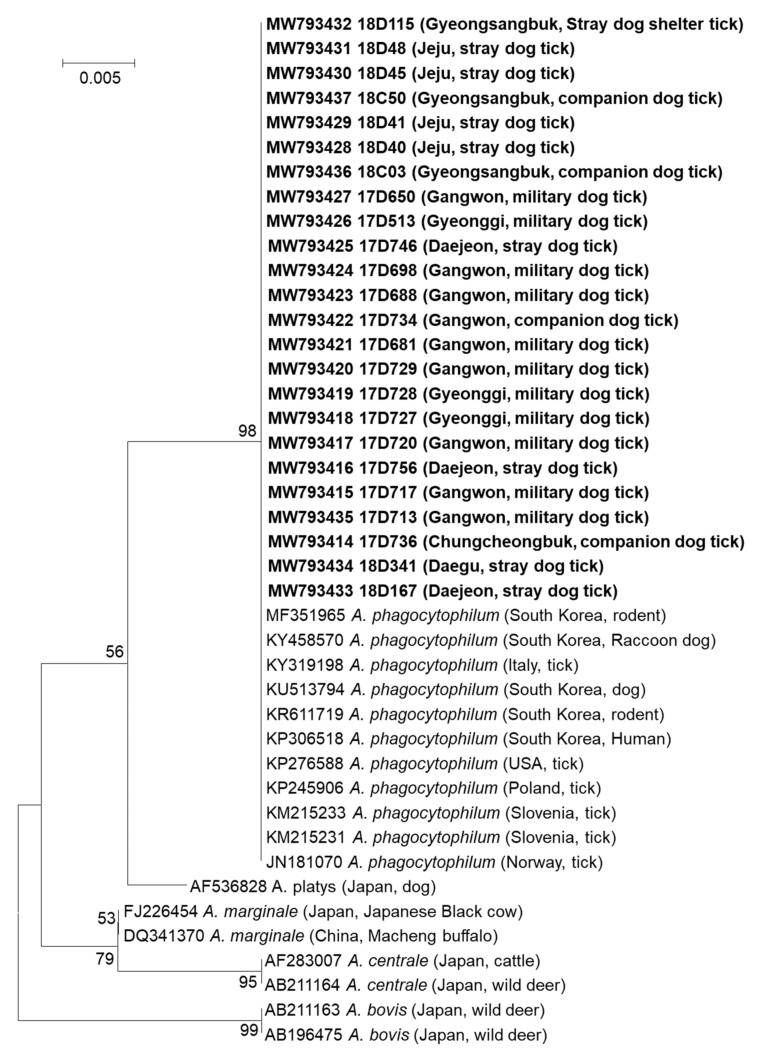
Anaplasma spp. detection was confirmed using sequencing analyses. The sequence of Anaplasma spp. detected in each of the 24 positive pools was 98.01–100% identical to previously deposited sequences of A. phagocytophilum in the National Center for Biotechnology Information (NCBI) GenBank database. In addition, phylogenetic analyses revealed a close relationship between the Anaplasma spp. detected in this study with previously reported A. phagocytophilum found in rodents, raccoon dogs, domestic/stray/military working dogs, and humans in the ROK, USA, Poland, Slovenia, and Norway (Figure 4).
Figure 4.
The phylogenetic analysis of Anaplasma spp. detected in ticks collected from dogs in the ROK. The maximum-likelihood tree was created based on the nucleotide sequences of 16S RNA (511 bp) of Anaplasma spp. detected in this study and other countries. MEGA7 software with 1000 bootstrap replications was used to create the phylogenetic tree. The NCBI accession numbers and names of the Anaplasma spp. positive pools are shown in bold. The names of the province or metropolitan city and host of the detected Anaplasma spp. are shown in parentheses. Reference strains of Anaplasma spp. with NCBI accession numbers and country of detection and host are also shown.
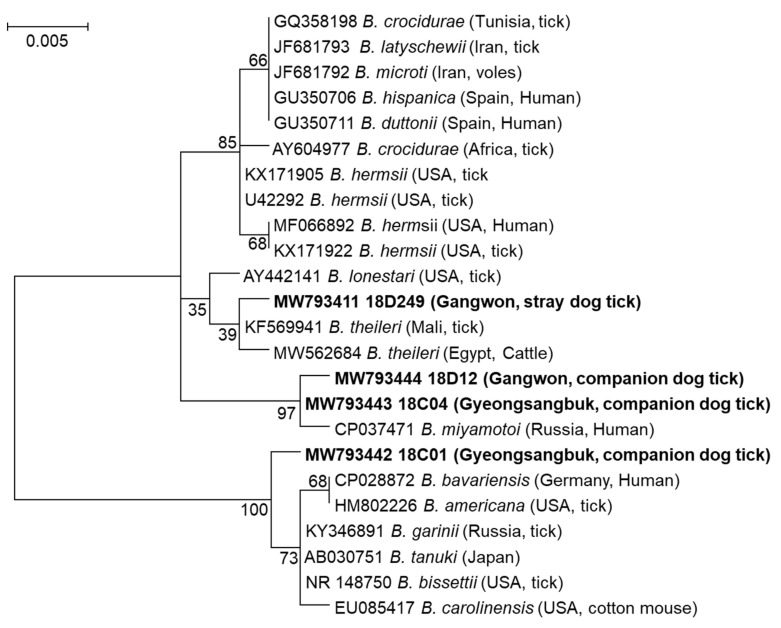
The sequences of the Borrelia spp. detected in four tick pools in this study were 98.62–100% identical to previously reported sequences of Borrelia spp. listed in the NCBI database. Phylogenetic analysis revealed that the Borrelia strain detected in tick pool 18D249 demonstrated a close relationship with B. theileri previously reported in ticks and cattle from Mali and Egypt, respectively. The Borrelia strains detected in the 18D12 and 18C04 tick pools were closely related to B. miyamotoi (Borrelia relapsing fever) detected in a human in Russia. The 18C01 tick pool strain was similar to B. garinii, B. tanuki, and B. bissettii strains from Russia, Japan, and the USA, respectively (Figure 5).
Figure 5.
Phylogenetic analysis of Borrelia spp. detected in ticks collected from dogs in the ROK. The maximum-likelihood tree presenting the phylogenetic relationship between detected Borrelia spp. and previously reported strains was generated based on the nucleotide sequences of 16S rRNA (714 bp) of Borrelia spp. detected in this study. MEGA7 software with 1000 bootstrap replications was used. The NCBI accession numbers and names of positive Borrelia spp. tick pools are in bold. The country, province, or metropolitan city and host of the detected Borrelia spp. are shown in parentheses. Reference strains of Borrelia spp. with NCBI accession numbers and country of detection and host are also shown.
3. Discussion
This study determined the distribution of tick-borne pathogens detected in ticks collected from pet, stray, and military working dogs and dog shelter environments in the ROK. Three tick species (H. longicornis, I. nipponensis, and H. flava) were identified. H. longicornis, which is commonly associated with grass/herbaceous vegetation habitats, was the most commonly collected species. H. flava, which is commonly found in forested habitats, was notably less prevalent. I. nipponensis, which is associated with both grass/herbaceous vegetation and forested habitats, was also less prevalent [22]. As dogs are more likely to enter grass/herbaceous vegetation than forests, they are exposed to H. longicornis ticks more frequently in the ROK [22,23,24]. However, a previous study reported that only I. nipponensis ticks were collected from dogs in the Gyeongsangbuk province [21].
While A. phagocytophilum, Borrelia spp., Ehrlichia spp., and Babesia spp. are present in the ROK, only Anaplasma spp. and Borrelia spp. were detected in this study. Few cases of A. phagocytophilum in humans in the ROK have been reported [25]. The composition of tick-borne pathogens in dog ticks varies worldwide. Rickettsia spp., Borrelia spp., A. phagocytophilum, and Babesia sp. have been reported in Latvia [15]. A. phagocytophilum, Ehrlichia canis, and Babesia gibsoni have been reported in Taiwan [26]. E. canis, Hepatozoon canis, Rickettsia spp., Candidatus Neoehrlichia mikurensis and A. platys have been reported in Nigeria [27]. Five genera of pathogens (Anaplasma spp., Babesia spp., Borrelia spp., Ehrlichia spp., and Theileria cervi) have been reported in dog ticks in Russia [28]. The pathogens detected in this study were consistent with previously reported TBDs in dogs [19,20]. These results are useful for the surveillance of TBDs present in dogs that may impact the transmission of these pathogens to dog owners or handlers throughout the ROK.
A. phagocytophilum has been found in various tick species worldwide [29,30,31]. In this study, only H. longicornis and I. nipponensis ticks were positive for Anaplasma spp., with a predominance in H. longicornis ticks, which may be due to the lower numbers of I. nipponensis and H. flava that were collected in this study. These findings are consistent with a previous study conducted in the ROK [32]. H. longicornis ticks carrying Anaplasma spp. were collected from pets, military working dogs, and stray dogs, but not from vegetation surrounding dog shelters. The phylogenic analyses demonstrated a close relationship between the Anaplasma spp. detected in this study and previously reported A. phagocytophilum strains from dogs and humans in the ROK. Therefore, the transmission of A. phagocytophilum to humans may result from exposure to ticks on pets. Therefore, pet owners, dog shelter workers, and handlers of military working dogs should be educated regarding the potential of transmitting anaplasmosis.
The primary vectors of Borrelia spp. are Ixodes spp. [33,34]. In this study, H. longicornis ticks were also found to be vectors of Borrelia spp. H. longicornis ticks in this study may have fed on a Borrelia-positive animal, resulting in the detection of the pathogen. However, whether H. longicornis ticks are a vector of Borrelia spp. has not been determined. The sequence analyses demonstrated that there are at least three species or strains of Borrelia spp. in the ROK, including B. theileri, B. miyamotoi, and an unidentified Borrelia sp. Additional analyses using other genes (such as the flagellin gene and PCR-restriction fragment length polymorphism) are necessary to determine the specific phylogenetic identification of Borrelia spp. [35].
In this study, nationwide surveillance of dog ticks and tick-borne pathogens was conducted, and three tick species were collected. H. longicornis was the most prevalent tick species detected in this study, followed by I. nipponensis and H. flava. Anaplasma spp. and Borrelia spp. were detected on H. longicornis and I. nipponensis ticks only. Phylogenetic analyses suggested that at least two species of Borrelia (B. theileri and B. miyamotoi) were present. In contrast, a third species of Borrelia detected in this study remains unidentified. This study demonstrates that dogs and dog owners/handlers in the ROK have a relatively high risk of becoming infected with Anaplasma spp. or Borrelia spp. Therefore, disease screening is important not only to determine the distribution and prevalence of dog TBDs but also to understand the potential impact on veterinary and human health.
4. Materials and Methods
4.1. Collection of Ticks
Ticks were collected from pet dogs, military working dogs, stray dogs, and vegetation surrounding dog shelters in 13 provinces and metropolitan cities in the ROK from 2017 to 2018 (Figure 1). Ticks were removed using fine forceps to secure the tick mouthparts at the point of attachment and gently pulling the tick out to avoid breaking off the mouthparts. After removal, the ticks were transferred to a 50 mL conical tube and placed in a cooler to be transferred to the Parasitic and Honeybee Disease Laboratory, Animal and Plant Quarantine Agency for species identification and pathogen detection.
4.2. Identification of Tick Species
Ticks were identified using morphological keys [36,37,38] then placed in 1.5 mL cryovials according to species, host, date, and stage of development. The samples were preserved in 70% ethanol and stored at −80 °C until they were used to detect tick-borne pathogens. The tick pools each included 1–5 adult ticks, 1–30 nymphs, or 1–50 larvae.
4.3. Isolation of Tick Nucleic Acids
Total nucleic acid extraction was performed with the Maxwell RSC viral total nucleic acid purification kit (Promega, Madison, WI, USA) for each tick pool. Briefly, 330 µL of lysis buffer and six stainless steel beads with diameters of 2.381 mm (SNC, Hanam, Korea) were used to homogenize the ticks with a Precellys 24 tissue homogenizer (Bertin Instruments, Montigny-le-Bretonux, France). The tick homogenate was placed in a Maxwell RSC instrument (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The purification of the total nucleic acids was conducted automatically. Finally, 50 µL of total nucleic acids were acquired from each pool and used to detect tick-borne pathogens.
4.4. Detection of Tick-Borne Pathogens
Conventional PCR was performed to detect six tick-borne pathogens: Anaplasma spp., A. platys, E. canis, E. chaffeensis, Borrelia spp., and Babesia gibsoni. Specific primers of each target agent (Table 5) and the AccuPower ProFi Taq PCR PreMix (Bioneer, Daejeon, Korea) were utilized. Each 20 µL reaction mix included 1 µL (10 pmol) of each primer, 5 µL of total nucleic acids, and 13 µL of double-distilled water (ddH2O). The PCR conditions for the detection of each pathogen are shown in Table 5.
Table 5.
Primers used for the detection of tick-borne pathogens.
| Pathogens | Primers | Sequences (5′-3′) | Target Gene (bp) | PCR Conditions | References |
|---|---|---|---|---|---|
| Anaplasma spp. | PITA-F | GTCGAACGGATTATTCTTTA | 16S rRNA (511) | 95 °C (5 min); 37 cycles of 95 °C (30 s), 50 °C (30 s), and 72 °C (40 s); 72 °C (7 min) | [39] |
| PITA-R | TTCACCTTTAACTTACCGAA | ||||
| A. platys | EPLAT5 | TTTGTCGTAGCTTGCTATGAT | 16S rRNA (359) | 95 °C (5 min); 37 cycles of 95 °C (30 s), 53 °C (30 s), and 72 °C (30 s); 72 °C (7 min) | [40] |
| EPLAT3 | CTTCTGTGGGTACCGTC | ||||
| Ehrlichia canis | ECAN5 | GCAAATTATTTATAGCCTCTGGCTATAG | 16S rRNA (365) | 95 °C (5 min); 37 cycles of 95 °C (30 s), 56 °C (30 s), and 72 °C (30 s); 72 °C (7 min) | [41,42] |
| HE3 | TTATAGGTACCGTCATTATCTTCCCTA | ||||
| E. chaffeensis | HE1 | ACAATATTGCTTATAACCTTTTGGTTATA | 16S rRNA (390) | 95 °C (5 min); 37 cycles of 95 °C (30 s), 56 °C (30 s), and 72 °C (30 s); 72 °C (7 min) | |
| HE3 | TTATAGGTACCGTCATTATCTTCCCTA | ||||
| Borrelia spp. | B3 | GCAGCTAAGAATCTTCCGCA | 16S rRNA (714) | 95 °C (5 min); 37 cycles of 95 °C (30 s), 58 °C (30 s), and 72 °C (1 min); 72 °C (7 min) | [43] |
| B6 | CAACCATGCAGCACCTGTATAT | ||||
| Babesia gibsoni | PIRO-F | AGTCATATGCTTGTCTTA | 18S rRNA (500) | 95 °C (5 min); 37 cycles of 95 °C (30 s), 47 °C (30 s), and 72 °C (40 s); 72 °C (7 min) | [44] |
| PIRO-R | CCATCATTCCAATTACAA |
4.5. Sequencing and Phylogenetic Analysis
The products of conventional PCR were analyzed using agarose gel electrophoresis. After electrophoresis, the PCR products were purified using a QIA quick purification kit (Qiagen, Hilden, Germany) and sequenced by Macrogen (Seoul, Korea). The sequences of the Anaplasma spp. and Borrelia spp. detected in this study were deposited in the NCBI database with accession numbers of MW793414-MW793437 (Anaplasma spp.) and MW793441-MW793444 (Borrelia spp.). The generated sequences were compared to previously reported sequences in the NCBI GenBank database. Identical sequences of the Anaplasma spp. and Borrelia spp. were aligned using Clustal X version 2.0 [45]. Maximum-likelihood phylogenetic trees were created using the Kimura 2-parameter model, gamma distribution, and bootstrapping 1000 times with MEGA7 software [46].
Author Contributions
Conceptualization, Y.S.C. and J.N.; methodology, J.N. and Y.P.; software, A.-T.T. and H.-J.S.; validation, A-T.T. and J.N.; formal analysis, Y.S.C.; investigation, J.N., Y.P, H.-J.S., K.-H.K., S.M., J.L., H.L., and M.-S.Y.; resources, S.M., J.L., and H.L.; data curation, A.-T.T., J.N., and Y.S.C.; writing—original draft preparation, A.-T.T., H.-C.K., T.A.K.; writing—review and editing, Y.S.C.; visualization, A.-T.T.; supervision, Y.S.C.; project administration, S.-S.Y.; funding acquisition, Y.S.C. and T.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Animal and Plant Quarantine Agency in the Republic of Korea, grant number B-1543081-2020-22-03, and partial funding was provided by the Armed Forces Health Surveillance Branch, Global Emerging Infections Surveillance and Response System (AFHSB-GEIS), Silver Spring, MD (ProMIS ID #P0131_20_ME_03) and the 65th Medical Brigade, Camp Humphreys, Republic of Korea.
Institutional Review Board Statement
This study was approved by the Animal and Plant Ethics Committee of Animal and Plant Quarantine Agency as complying with the Korean Legislation for Animal Protection (Law No. 15502, articles 23 and 24). The Animal Ethics Committee approval number was 2017-347.
Informed Consent Statement
All participating veterinary hospitals, shelters, and military forces provided their consent to participate in the study.
Data Availability Statement
The data presented in this study are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; collection, analyses, or interpretation of data; writing of the manuscript; or decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De la Fuente J., Estrada-Pena A., Venzal J.M., Kocan K.M., Sonenshine D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008;13:6938–6946. doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 2.Ogbu K.I., Olaolu O.S., Ochai S.O., Tion M.T. A review of some tick-borne pathogens of dogs. JASVM. 2018;3:140–153. doi: 10.31248/JASVM2018.106. [DOI] [Google Scholar]
- 3.Jongejan F., Uilenberg G. The global importance of ticks. Parasitology. 2004;129:3–14. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- 4.Cao W., Zhan L., He J., Foley J.E., de Vlas S.J., Wu X., Yang H., Richardus J.H., Habbema J.D.F. Natural Anaplasma phagocytophilum infection of ticks and rodents from a forest area of Jilin province, China. Am. J. Trop. Med. Hyg. 2006;75:664–668. doi: 10.4269/ajtmh.2006.75.664. [DOI] [PubMed] [Google Scholar]
- 5.Ohashi N., Inayoshi M., Kitamura K., Kawamori F., Kawaguchi D., Nishimura Y., Naitou H., Hiroi M., Masuzawa T. Anaplasma phagocytophilum-infected ticks, Japan. Emerg. Infect. Dis. 2005;11:1780–1783. doi: 10.3201/eid1111.050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw S.E., Day M.J., Birtles R.J., Breitschwerdt E.B. Tick-borne infectious diseases of dogs. Trends Parasitol. 2001;17:74–80. doi: 10.1016/S1471-4922(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 7.Biggs H.M., Behravesh C.B., Bradley K.K., Dahlgren F.S., Drexler N.A., Dumler J.S., Folk S.M., Kato C.Y., Lash R.R., Levin M.L., et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis-United States. MMWR Recomm. Rep. 2016;65:1–44. doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- 8.Abdullah S., Helps C., Tasker S., Newbury H., Wall R. Ticks infesting domestic dogs in the UK: A large-scale surveillance programme. Parasites Vectors. 2016;9:391. doi: 10.1186/s13071-016-1673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck S., Schreiber C., Schein E., Krücken J., Baldermann C., Pachnicke S., Samson-Himmelstjerna G.V., Kohn B. Tick infestation and prophylaxis of dogs in northeastern Germany: A prospective study. Ticks Tick Borne Dis. 2014;5:336–342. doi: 10.1016/j.ttbdis.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Földvári G., Farkas R. Ixodid tick species attaching to dogs in Hungary. Vet. Parasitol. 2005;129:125–131. doi: 10.1016/j.vetpar.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Abdullah S., Helps C., Tasker S., Newbury H., Wall R. Prevalence and distribution of Borrelia and Babesia species in ticks feeding on dogs in the UK. Med. Vet. Entomol. 2018;32:14–22. doi: 10.1111/mve.12257. [DOI] [PubMed] [Google Scholar]
- 12.Duncan A.W., Correa M.T., Levine J.F., Breitschwerdt E.B. The dog as a sentinel for human infection: Prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic states. Vector Borne Zoonotic Dis. 2004;4:221–229. doi: 10.1089/vbz.2004.4.221. [DOI] [PubMed] [Google Scholar]
- 13.Irwin P.J., Robertson I.D., Westman M.E., Perkins M., Straubinger R.K. Searching for Lyme borreliosis in Australia: Results of a canine sentinel study. Parasites Vectors. 2017;10:114. doi: 10.1186/s13071-017-2058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith F.D., Ballantyne R., Morgan E.R., Wall R. Estimating Lyme disease risk using pet dogs as sentinels. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:163–167. doi: 10.1016/j.cimid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Namina A., Capligina V., Seleznova M., Krumins R., Aleinikova D., Kivrane A., Akopjana S., Lazovska M., Berzina I., Ranka R. Tick-borne pathogens in ticks collected from dogs, Latvia, 2011–2016. BMC Vet. Res. 2019;15:398. doi: 10.1186/s12917-019-2149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi W.Y. Diagnosis and epidemiology of toxoplasmosis in Korea. Korean J. Parasitol. 1990;28:41–44. doi: 10.3347/kjp.1990.28.Suppl.41. [DOI] [PubMed] [Google Scholar]
- 17.Im J.H., Baek J., Durey A., Kwon H.Y., Chung M.H., Lee J.S. Current status of tick-borne diseases in South Korea. Vector Borne Zoonotic Dis. 2019;19:4. doi: 10.1089/vbz.2018.2298. [DOI] [PubMed] [Google Scholar]
- 18.Korea Centers for Disease Control and Prevention (KCDC) Infectious Diseases Surveillance Yearbook 2018. Korea Centers for Disease Control and Prevention (KCDC); Chungbuk, Korea: 2019. [Google Scholar]
- 19.Seo M.G., Kwon O.D., Kwak D. Molecular detection and phylogenetic analysis of canine tick-borne pathogens from Korea. Ticks Tick Borne Dis. 2020;11:101357. doi: 10.1016/j.ttbdis.2019.101357. [DOI] [PubMed] [Google Scholar]
- 20.Suh G.H., Ahn K.S., Ahn J.H., Kim H.J., Leutenegger C., Shin S. Serological and molecular prevalence of canine vector-borne diseases (CVBDs) in Korea. Parasites Vectors. 2017;10:146. doi: 10.1186/s13071-017-2076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S.H., Goo Y.K., Geraldino P.J.L., Kwon O.D., Kwak D. Molecular detection and characterization of Borrelia garinii (Spirochaetales: Borreliaceae) in Ixodes nipponensis (Ixodida: Ixodidae) parasitizing a dog in Korea. Pathogens. 2019;8:289. doi: 10.3390/pathogens8040289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J.L., Kim H.C., Coburn J.M., Chong S.T., Chang N.W., Robbins R.G., Klein T.A. Tick surveillance in two southeastern provinces, including three metropolitan areas, of the Republic of Korea during 2014. Syst. Appl. Acarol. 2017;22:271–288. doi: 10.11158/saa.22.2.10. [DOI] [Google Scholar]
- 23.Kim-Jeon M.D., Jegal S., Jun H., Jung H., Park S.H., Ahn S.K., Lee J., Gong Y.W., Joo K., Kwon M.J., et al. Four year surveillance of the vector hard ticks for SFTS, Ganghwa-do, Republic of Korea. Korean J. Parasitol. 2019;57:691–698. doi: 10.3347/kjp.2019.57.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J.W., Lee S.H., Lee G.S., Seo J.J., Chung J.K. Epidemiological characteristics of field tick-borne pathogens in Gwang-ju metropolitan area, South Korea, from 2014 to 2018. Osong Public Health Res. Perspect. 2020;11:177–184. doi: 10.24171/j.phrp.2020.11.4.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim K.H., Yi J.Y., Oh W.S., Kim N.H., Choi S.J., Choe N.J., Lee J.K., Oh M.D. Human granuylocytic anaplasmosis, South Korea, 2013. Emerg. Infect. Dis. 2014;20:1708–1711. doi: 10.3201/eid2010.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu T.J., Sun H.J., Wu Y.C., Huang H.P. Prevalence and risk factors of canine ticks and tick-borne diseases in Taipei, Taiwan. J. Vet. Clin. Sci. 2009;2:75–78. [Google Scholar]
- 27.Kamani J., Baneth G., Mumcuoglu K.Y., Waziri N.E., Eyal O., Guthmann Y., Harrus S. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria. PLoS Negl. Trop. Dis. 2013;7:e2108. doi: 10.1371/journal.pntd.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livanova N.N., Fomenko N.V., Akimov I.A., Ivanov M.J., Tikunova N.V., Armstrong R., Konyaev S.V. Dog survey in Russian veterinary hospitals: Tick identification and molecular detection of tick-borne pathogens. Parasites Vectors. 2018;11:591. doi: 10.1186/s13071-018-3161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palomar A.M., Portillo A., Santibáñez P., Mazuelas D., Roncero L., García-Álvarez L., Santibáñez S., Gutiérrez Ó., Oteo J.A. Detection of tick-borne Anaplasma bovis, Anaplasma phagocytophilum and Anaplasma centrale in Spain. Med. Vet. Entomol. 2015;29:349–353. doi: 10.1111/mve.12124. [DOI] [PubMed] [Google Scholar]
- 30.Polin H., Hufnagl P., Haunschmid R., Gruber F., Ladurner G. Molecular evidence of Anaplasma phagocytophilum in Ixodes ricinus ticks and wild animals in Austria. J. Clin. Microbiol. 2004;42:2285–2286. doi: 10.1128/JCM.42.5.2285-2286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuen S. Anaplasma Phagocytophilum-the most widespread tick-borne infection in animals in Europe. Vet. Res. Commun. 2007;31:79–84. doi: 10.1007/s11259-007-0071-y. [DOI] [PubMed] [Google Scholar]
- 32.Kim C.M., Kim M.S., Park M.S., Park J.H., Chae J.S. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A. bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vector Borne Zoonotic Dis. 2003;3:17–26. doi: 10.1089/153036603765627424. [DOI] [PubMed] [Google Scholar]
- 33.Fraenkel C.J., Garpmo U., Berglund J. Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J. Clin. Microbiol. 2002;40:3308–3312. doi: 10.1128/JCM.40.9.3308-3312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurokawa C., Lynn G.E., Pedra J.H.F., Pal U., Narasimhan S., Fikrig E. Interactions between Borrelia burgdorferi and ticks. Nat. Rev. Microbiol. 2020;18:587–600. doi: 10.1038/s41579-020-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukunaga M., Okada K., Nakao M., Konishi T., Sato Y. Phylogenetic analysis of Borrelia species based on flagellin gene sequences and its application for molecular typing of Lyme disease borreliae. Int. J. Syst. Bacteriol. 1996;46:898–905. doi: 10.1099/00207713-46-4-898. [DOI] [PubMed] [Google Scholar]
- 36.Hoogstraal H., Roberts F.H., Kohls G.M., Tipton V.J. Review of Haemaphysalis (kaiserinana) longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Norteastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae) J. Parasitol. 1968;54:1197–1213. doi: 10.2307/3276992. [DOI] [PubMed] [Google Scholar]
- 37.Hoogstraal H., Wassef H.Y. The Haemaphysalis ticks (Ixodoidea: Ixodidae) of birds. 3. H. (ornithophysalis) subgen. n.: Definition, species, hosts, and distribution in the Oriental, Palearctic, Malagasy, and Ethiopian faunal regions. J. Parasitol. 1973;59:1099–1117. [PubMed] [Google Scholar]
- 38.Yamaguti N., Tipton V.J., Keegan H.L., Toshioka S. Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young Univ. Sci. Bull. Biol. Ser. 1971;15:1–226. [Google Scholar]
- 39.Hancock S.I., Breitschwerdt E.B., Pitulle C. Differentiation of Ehrlichia platys and E. equi infections in dogs by using 16S ribosomal DNA-based PCR. J. Clin. Microbiol. 2001;39:4577–4578. doi: 10.1128/JCM.39.12.4577-4578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathew J.S., Ewing S.A., Murphy G.L., Kocan K.M., Corstvet R.E., Fox J.C. Characterization of a new isolate of Ehrlichia platys (Order Rickettsiales) using electron microscopy and polymerase chain reaction. Vet. Parasitol. 1997;68:1–10. doi: 10.1016/S0304-4017(96)01052-7. [DOI] [PubMed] [Google Scholar]
- 41.Anderson B.E., Sumner J.W., Dawson J.E., Tzianabos T., Greene C.R., Olson J.G., Fishbein D.B., Olsen-Rasmussen M., Holloway B.P., George E.H. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J. Clin. Microbiol. 1992;30:775–780. doi: 10.1128/JCM.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy G.L., Ewing S.A., Whitworth L.C., Fox J.C., Kocan A.A. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet. Parasitol. 1998;79:325–339. doi: 10.1016/S0304-4017(98)00179-4. [DOI] [PubMed] [Google Scholar]
- 43.Kang J.G., Ko S., Smith W.B., Kim H.C., Lee I.Y., Chae J.S. Prevalence of Anaplasma, Bartonella and Borrelia species in Haemaphysalis longicornis collected from goats in North Korea. J. Vet. Sci. 2016;17:207–216. doi: 10.4142/jvs.2016.17.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ano H., Makimura S., Harasawa R. Detection of Babesia species from infected dog blood by polymerase chain reaction. J. Vet. Med. Sci. 2001;63:111–113. doi: 10.1292/jvms.63.111. [DOI] [PubMed] [Google Scholar]
- 45.Larkin M., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the manuscript.