Abstract
Background
Contemporary primary sclerosing cholangitis (PSC) population-based cohorts describing the epidemiology, natural history, and long-term fluctuations in serum alkaline phosphatase (SAP) and their prognostic relevance are lacking. Therefore, we investigated the incidence and natural history of PSC and quantified SAP fluctuations among those with PSC in Olmsted County, Minnesota over the last 41 years.
Methods
The Rochester Epidemiology Project was used to identify 56 subjects diagnosed with PSC between 1976 and 2017 in Olmsted County. The primary endpoint (n = 19) included liver transplantation, hepatic decompensation, and cholangiocarcinoma.
Results
The age- and sex-adjusted incidence of PSC (per 100,000 person years) nearly doubled from 2001 to 2017 compared to 1976–2000 (1.47; 95% CI 0.99–1.96 versus 0.79; 95% CI 0.42–1.16, p = 0.02). This increase paralleled a rise in patients with markers of a milder phenotype at the time of diagnosis: normal SAP (26.32% versus 0%, p < 0.01) and lower Mayo PSC risk score [0.36 (− 0.57 to 1.55) versus − 0.50 (− 1.25 to 0.35), p = 0.03]. Intra-individual SAP fluctuates with a median coefficient of variation of 36.20%. SAP normalization and dropping below 1.5 × upper limit of normal (ULN) occurs at a rate of 5% and 10% per year, respectively. SAP less than 1.5 × ULN was associated with a lower risk of PSC-related complications (hazard ratio 0.11; 95% CI 0.03–0.42).
Conclusions
The patients with PSC are increasingly being diagnosed with a milder phenotype. While a lower SAP is associated with improved outcomes, the high intra-individual variation of SAP levels calls into question the practice of using a single SAP value as a surrogate endpoint in clinical trials.
Keywords: Primary sclerosing cholangitis, Cirrhosis, Liver function tests, Epidemiology, Cholangiocarcinoma
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease associated with intra and/or extrahepatic biliary strictures which can progress to cirrhosis and which is associated with a heightened risk of malignancies [1]. While PSC is a rare disorder, some studies published nearly a decade ago suggest that its incidence may be increasing [2–4].
The disease course and phenotype of PSC are highly variable and has largely been described in retrospective cohort studies from tertiary centers [5–8]. Historically, a median transplant-free survival of approximately 12 years has been cited [9]. This was challenged by a Dutch population-based study that found a 21-year median transplant free survival [10]. However, this prolonged survival has not been replicated in subsequent population-based studies. Our ability to accurately predict long-term outcomes in PSC is limited due to the lack of accurate surrogate markers in clinical trials. Changes in serum alkaline phosphatase (SAP) constitute the most widely used biomarker in PSC, and a number of studies have reported that a reduction in SAP is associated with improved outcomes [11–18]. Specifically, SAP normalization and reduction below 1.5 × upper limit of normal (ULN) have been associated with improved outcomes [11, 12, 14, 15, 18].
A contemporary update on PSC epidemiology and the natural histories of both clinical outcomes and changes in surrogate markers such as SAP from population-based data is needed for several reasons. First, many prior PSC epidemiology and natural history studies were not population based and, therefore, were subject to referral bias [6, 19]. Second, a North American, population-based, adult PSC epidemiology study has not been published for nearly a decade [20, 21]. Third, the study period examined in population-based studies has typically been limited to approximately 10 years with the longest being 24 years [10, 20, 21]. Lastly, PSC population-based studies often lack important phenotypic details and focus primarily on epidemiology. For example, the long-term intra-individual SAP changes have not been examined in a population-based cohort. This is important as SAP levels are dynamic through the disease course. Moreover, clinical trials often determine their sample size based on anticipated rates of SAP changes. Existing information has largely been obtained from referral cohorts and often does not take into account SAP fluctuations at different time points in the disease course [11–16, 18]. This approach could lead to an underestimation of the true SAP variability and under powering therapeutic trials when a single SAP value is used as an endpoint. The reliability of this approach is in question as SAP levels may spontaneously and transiently become either normal or abnormal at multiple times in the same patient.
This well-defined population-based PSC cohort over a 41-year study period was designed to highlight the contemporary epidemiology of PSC, provide an in-depth description of the natural history of PSC, and to report the long-term rate of SAP fluctuations and its relationship to clinical outcomes.
Methods
Case finding and ascertainment
Population-based epidemiologic studies can be performed in Olmsted County, Minnesota, because medical care is self-contained in this community with two major health care providers including Mayo Clinic and Olmsted Medical Center. These centers provide health care for almost the entire population in Olmsted County. At the patients’ level, data for inpatients, outpatients, and emergency room visits are indexed by Rochester Epidemiologic Project (REP), which is considered a unique data linkage system for population-based epidemiologic studies and has been supported by the National Institute of Health for over fifty years [22].
Following approval from the Institutional Review Board of Mayo Clinic and Olmsted Medical Center, REP was used to identify patients with a diagnosis of cholangitis from Jan 1, 1976 to January 1, 2018. Medical records were reviewed on January 1 2019. Individuals with features of PSC were included based on either typical cholangiographic or histologic features of PSC after excluding other causes of secondary sclerosing cholangitis [23]. To exclude patients who might have been referred to this area for medical care, only patients who resided in Olmsted County for at least 1 year prior to diagnosis of PSC were included. This approach mitigated the possibility of referral bias and enabled us to create a well-defined population-based cohort.
Data collection
Basic demographic data, PSC-related symptoms, PSC phenotype, and associated comorbidities including inflammatory bowel disease (IBD) (established endoscopic and histologic features) were obtained from the time of diagnosis and during subsequent follow-up. Dominant stricture was defined as a stenosis ≤ 1.5 mm in common bile duct or ≤ 1 mm in the intrahepatic ducts according to endoscopic retrograde cholangiopancreatography (ERCP) findings [23]. Cholangitis was determined by the treating clinician and verified by chart review as responsive to medical and/or endoscopic therapy. Refractory pruritus was defined as ongoing itching (in the absence of a stricture amenable to endoscopic or percutaneous therapy) after a trial of at least 2 anti-pruritic therapies (excluding topical therapies and anti-histamines) [24]. Colorectal neoplasia (low-, high-grade dysplasia, or colorectal cancer) was determined by an expert gastrointestinal pathologist. Cholangiocarcinoma (CCA) was defined by either imaging features or cytology or biopsy positive for adenocarcinoma [25]. Beside outcomes of PSC-related neoplasia, non-malignant endpoints of interest included hepatic decompensation (HD) (ascites, variceal hemorrhage or hepatic encephalopathy), liver transplantation (LT), or death. The primary endpoint was death, LT, HD or CCA (whichever came first) while the secondary endpoint was death or LT.
Laboratory testing was abstracted from the time of diagnosis until last follow-up or time of LT (whichever was earlier). For decades, our standard clinical practice is to monitor liver tests every 3–6 months. Indeed, the median number of SAP values was 2 per patient per year. The Mayo PSC risk score, model for end-stage liver disease (MELD) score, and PSC risk estimate tool (PREsTO) were also determined at diagnosis [26, 27].
The reference ranges for serum alkaline phosphatase (SAP) are variable. Consequently, to standardize our reporting of SAP values, we divided the individual SAP value by the corresponding ULN. After abstracting all SAP values (more than 1800 values), we defined 3 SAP groups: (Group A) SAP ≤ 1.0 × ULN; (Group B) SAP > 1.0–1.49; (Group C) SAP ≥ 1.5 × ULN. These cut-offs were chosen based on prior evidence suggesting that individuals who either normalize their SAP or have a value < 1.5 × ULN have an improved prognosis [11, 12, 14, 18]. Individuals were categorized into one of these groups at the time of PSC diagnosis.
SAP changes were examined in 2 ways. First, we analyzed the patients based on the frequency of a stable SAP change as defined by having at least 2 SAP values at least 3 months apart in a different SAP category. For example, if a subject had a SAP value of 0.9 × the ULN at diagnosis they were considered to be in group A. Five years later if that same individual had a SAP value 1.8 × ULN and 3 months after this the SAP value remained 1.8 × the ULN they were considered to have changed to group C. Our second approach involved assessing all SAP values (regardless of the stability of the change or group category) over time to determine the true intra-individual variation.
Statistical analysis
Descriptive statistics were applied for baseline patient characteristics. To determine if the epidemiology of PSC has changed over time, we subdivided the patients into 2 time periods: from Jan 1, 1976 to Dec 31, 2000 and Jan 1, 2001 to Jan 1, 2018. These time periods were selected in part to update the last epidemiologic study of PSC in Olmsted County which studied data from 1976 to 2000 [21]. In addition, our practice changed around 2001 when the use of magnetic resonance cholangiopancreatography (MRCP) to diagnose PSC became routine. The total population of Olmsted County was considered the denominator and was acquired from the Olmsted County census with linear interpolation between census years. Since more than 95% of our cases were Caucasian, the Caucasian population of the United States in 2010 was considered. After excluding individuals who died or left Olmsted County before January 1, 2018, the prevalence of PSC in 2017 was calculated. The change in incidence and the 95% confidence intervals (CI) were calculated by assuming Poisson error distribution of data.
Analysis was performed utilizing SAS version 9.4 (SAS Institute; Cary, NC). All tests were 2-sided with a level of significance of α = 0.05. Categorical data were compared using the Pearson Chi-squared test and continuous variables were compared using the Wilcoxon rank sum tests. Categorical data were expressed as numbers (percentages) while continuous variables were expressed as median, interquartile range (IQR).
Failure plots and the 1 minus Kaplan–Meier were used to illustrate the cumulative incidence of stable SAP group changes after the diagnosis of PSC. This examined individuals who had their first, but stable SAP change measured at least 3 months apart. It did not capture future changes that may have occurred. To do this, we calculated the rate of SAP change over time which captured individuals who had multiple SAP group changes throughout their disease course. In this analysis, individuals were still required to have 2 SAP values at least 3 months apart in the new SAP group. Hence this approach allowed us to quantify stable changes throughout the disease course beyond the initial change. However, SAP can fluctuate unexpectedly and transiently. Consequently, we also considered all SAP values to analyze intra-individual variabilities by assessing the coefficient of variation. Thus the former approach was able to quantify a persistent, clinically meaningful SAP changes while the latter was able to quantify real world SAP aberrancies.
The cumulative incidence of the endpoint of interest was illustrated by failure plots using the Kaplan–Meier method. Cox proportional hazard regression analysis was utilized to examine associations between covariates and the endpoints. Firth’s method for analyzing rare events was used where needed. Persistent changes in the SAP group were treated as time-dependent covariates to analyze its fluctuations. Moreover, the SAP levels at the time of diagnosis were also used. For colorectal neoplasia as an endpoint, colectomy was the censoring event. Unadjusted Cox proportional hazard regression analysis was performed to identify prognostic variables associated with the primary and secondary endpoints. Given the limited number of expected events, only 2 prognostic covariates that were significant in the unadjusted Cox analysis (SAP plus another variable) were included in each multivariate model to determine if SAP was an independent predictor of the primary and secondary endpoints. The discriminative ability of the biochemical variables used in the multivariate models to categorize individuals at risk for developing adverse outcomes was assessed with concordance scores.
For comparing patient survival with the expected survival of the Minnesota Caucasian population of similar age and sex, death or LT, was the end point and the patients were censored at the time of last follow-up. The expected survival was calculated by the method of Ederer et al. on basis of age- and sex-specific conditional probabilities of death in the subsequent year from published census table [28]. The 1-sample log-rank test was applied for assessing the difference between the patients and the expected survival. Standardized mortality ratio (SMR) was calculated by comparing the observed death versus the expected one.
Results
Incidence and prevalence
This population-based cohort included 56 patients who were diagnosed with PSC from 1976 to 2017. The overall age- and sex-adjusted incidence rate of PSC from 1976 to 2017 was 1.11 (95% CI 0.81–1.41). The age-adjusted incidence was more than twofold higher among men compared to women (1.58; 95% CI 1.07–2.08 versus 0.64; 95% CI 0.32–0.96, p < 0.001). In addition, the age- and sex-adjusted incidence nearly doubled from 2001 to 2017 compared to 1976–2000 (1.47; 95% CI 0.99–1.96 versus 0.79; 95% CI 0.42–1.16, p = 0.02), respectively. PSC prevalence in our study was 23.99 (95% CI 16.44–31.55) as of January 1, 2018.
Patient characteristics
The baseline features at the time of diagnosis of PSC are shown in Table 1. The entire cohort (1976–2018) was followed for 9.74 (0.42–40.65) years after their diagnosis. IBD was present among 32 (57.14%) subjects for 3.07 (0.46–10.73) years prior to the diagnosis of PSC. Over half of patients were asymptomatic at diagnosis. Among symptomatic patients, fatigue, and abdominal pain were the most common complaints. The patients with PSC were increasingly being diagnosed with a milder phenotype. For example, individuals diagnosed after 2001 were more likely to have intrahepatic disease only, a normal SAP and a lower Mayo PSC risk score (Table 1).
Table 1.
Clinical features at time of PSC diagnosis
| Baseline feature | Total (n = 56) | 1976–2000 (n = 20) | 2001–2018 (n = 36) | p value |
|---|---|---|---|---|
| Male | 40 (71.43%) | 15 (75.00%) | 25 (69.44%) | 0.65 |
| Age at diagnosis | 33.00 (22.25–47.75) | 37.50 (29.75–51.50) | 29.50 (20.25–47.25) | 0.25 |
| Asymptomatic | 31 (56.36%) | 9 (47.37%) | 22 (61.11%) | 0.32 |
| IBD at diagnosis | 32 (57.14%) | 10 (50.00%) | 22 (61.11%) | |
| Ulcerative colitis | 26 (81.25%) | 8 (80.00%) | 18 (81.82%) | 0.90 |
| Crohn’s disease | 6 (18.75%) | 2 (20.00%) | 4 (18.18%) | |
| PSC-AIH | 3 (5.36%) | 0 | 3 (8.33%) | 0.11 |
| PSC subtype | ||||
| Small duct | 6 (10.71%) | 3 (15.00%) | 3 (8.33%) | 0.44 |
| Intrahepatic | 17 (30.36%) | 3 (15.00%) | 14 (38.89%) | 0.05 |
| Intra and extrahepatic | 33 (58.93%) | 14 (70.00%) | 19 (52.78%) | 0.20 |
| Normal SAP | 10 (17.86%) | 0 | 10 (27.78%) | < 0.01 |
| SAP × ULN | 1.90 (1.16–3.45) | 1.90 (1.36–4.56) | 1.69 (0.92–2.63) | 0.11 |
| Total bilirubin (mg/dL) | 0.70 (0.40–1.60) | 1.20 (0.50–2.30) | 0.60 (0.40–1.30) | 0.07 |
| Albumin (g/dL) | 3.90 (3.5–4.4) | 3.89 (3.27–4.33) | 4.10 (3.60–4.40) | 0.13 |
| Platelet × 103/μL | 280.50 (230.25–333.00) | 281.00 (234.00–336.00) | 279.00 (220.00–331.00) | 0.69 |
| MELD score | 8 (6–10) | 8.5 (7–10) | 7 (6–9) | 0.07 |
| Mayo PSC risk score | − 0.19 (− 1.00 to 0.76) | 0.36 (− 0.57 to 1.55) | − 0.50 (− 1.25 to 0.35) | 0.03 |
| PREsTo | 0.05 (0.03–0.10) | 0.07 (0.03–0.20) | 0.04 (0.03–0.10) | 0.10 |
Continuous variables expressed as median (interquartile range). Categorical variables expressed as number (percentage)
Determined by Chi square testing for categorical variables or Wilcoxon rank sum test for continuous variables
IBD inflammatory bowel disease, PSC-AIH primary sclerosing cholangitis with autoimmune hepatitis, SAP serum alkaline phosphatase, ULN upper limit of normal, MELD model for end-stage liver disease, PRETo PSC risk estimate tool
Non-malignant events
After the diagnosis of PSC was established, IBD developed among 10 (17.85%) additional individuals after a duration of 1.39 (0.28–4.97) years. Hence a total of 42 (75.00%) subjects had IBD (ulcerative colitis n = 36, Crohn’s disease n = 6). Among those with IBD, 42.85% (18/42) underwent a colectomy after 5.14 (1.79–9.26) years following a diagnosis of IBD for either medically refractory disease (n = 12) or colorectal neoplasia (n = 6).
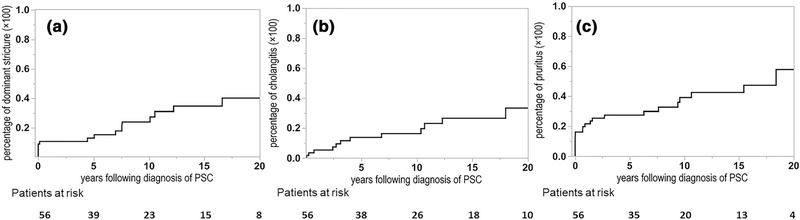
Three out of 6 subjects with small-duct PSC ultimately developed large duct PSC after 10.95 (2.25–16.55) years. All individuals with intrahepatic large duct PSC at diagnosis continued to have the same cholangiographic distribution after 7.24 (2.90–13.21) years of follow-up. Among 17 (30.35%) subjects with a dominant stricture, 11 (64.70%) had symptoms and 6 (35.29%) were malignant. The respective 5- and 10-year cumulative incidence of a dominant stricture was 13.00% (6.29–24.96%) and 23.94% (13.45–38.94%) (Fig. 1a). Cholangitis occurred in 14 individuals with respective 5- and 10-year cumulative incidences of 13.89% (6.73–26.49%) and 16.42% (8.36–29.74%) (Fig. 1b). Pruritus developed in 22 (39.28%) individuals with respective 5- and 10-year cumulative incidences of 27.38% (17.20–40.63%) and 39.03% (25.80–54.09%) (Fig. 1c). Among them, 4 (18.18%) patients experienced refractory itching.
Fig. 1.
Cumulative incidence of PSC-related events. a Dominant stricture; b ascending cholangitis; c pruritus
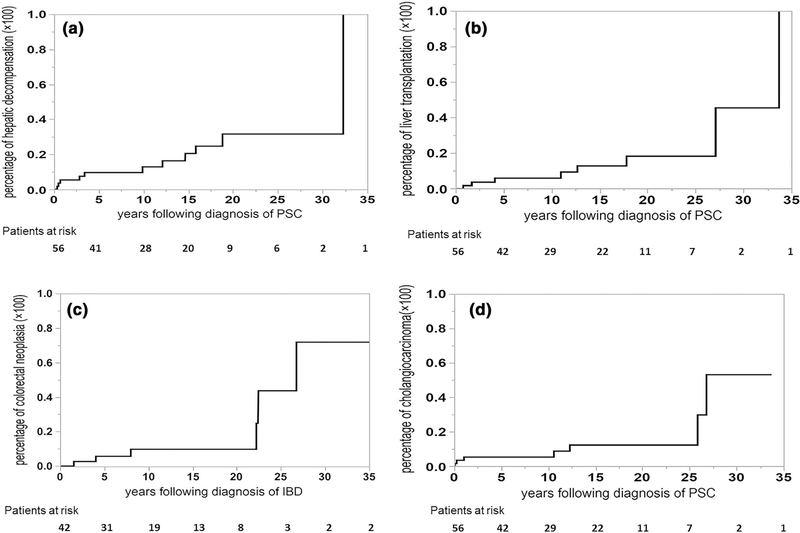
HD occurred in 11 (19.64%) subjects (ascites n = 7, variceal hemorrhage n = 2, hepatic encephalopathy n = 2) within 9.88 (0.68–15.83) years of diagnosis. The respective 5-, 10- and 20-year cumulative incidences of HD were 9.67% (4.06–21.30%), 12.90% (5.74–26.47%) and 31.58% (16.18–52.46%) (Fig. 2a).
Fig. 2.
Cumulative incidence of end-stage liver disease, transplantation, and PSC-related neoplasia. a Hepatic decompensation; b liver transplantation; c colorectal neoplasia; d cholangiocarcinoma
Eight (14.28%) patients underwent LT for the following indications: CCA n = 4; HD n = 2; advanced PSC not associated with malignancy or decompensation n = 2. The respective 10- and 20-year cumulative incidences of LT were 5.96% (1.92–17.00%) and 18.37% (7.80–37.44%) (Fig. 2b). Twelve patients died during the course of this study. CCA was the leading cause of mortality and was responsible for half of patient deaths. Liver failure (25%) and non-liver-related (25%) causes were responsible for the remaining causes of death. The SMR (95% CI) for PSC patients was 2.51 (1.30–4.39) times higher than the age- and sex-adjusted normal population (Supplementary Fig. 1).
PSC-associated malignancy
Colorectal neoplasia occurred in 6 individuals (low-grade dysplasia n = 3, high-grade dysplasia n = 1 or colorectal cancer n = 2). The 5- and 10-year cumulative incidence of colorectal neoplasia following a diagnosis of IBD was 5.67% (1.42–20.12%) and 9.77% (3.10–26.82%), respectively (Fig. 2c).
CCA developed in 7 (12.5%) subjects and was the only hepatobiliary malignancy detected in this cohort. The respective 1-, 10- and 20-year cumulative incidences of CCA were 3.57% (0.89–13.18%), 5.46% (1.77–15.62%) and 12.46% (4.99–27.83%) (Fig. 2d). Among those with CCA, 3 (42.86%) were detected within 1 year of establishing a diagnosis of PSC.
Variability of serum alkaline phosphatase
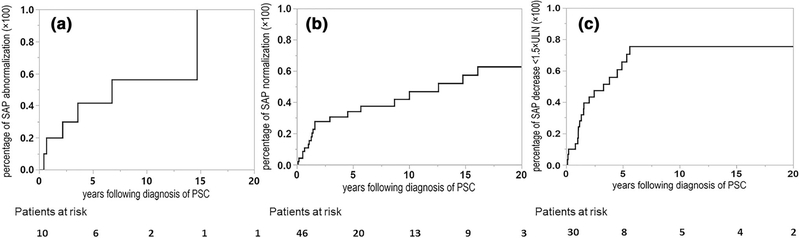
At the time of diagnosis, there were 10 subjects in Group A, 16 in Group B, and 30 in Group C. After a median of 1.38 (0.63–3.56) years, 39 (69.64%) subjects had at least one SAP group change that persisted for a minimum of 3 months (Fig. 3a–c). The respective −1 and 5-year cumulative incidence of developing an abnormal SAP was 20.00% (5.04–54.07%) and 41.66% (16.31–72.35%) (Fig. 3a). Similarly, the respective 1- and 5-year cumulative incidence of normalizing SAP was 10.92% (4.61–23.69%) and 34.06% (21.05–50.02%) (Fig. 3b) while the respective 1- and 5-year cumulative incidence of lowering the SAP threshold below 1.5 × ULN was 13.46% (5.14–30.86%), 65.58% (44.92–81.65%) (Fig. 3c). Developing an abnormal SAP was associated with cholangitis [n = 1/6 (16.66%)], progression of benign strictures seen on cholangiogram [n = 1/6 (16.66%)], or unknown [n = 4/6 (66.66%)]. Among those 4 subjects who developed an abnormal SAP for unknown reasons, 2 reported worsening IBD symptoms around the time of the SAP worsening. None of the patients who developed an abnormal SAP stopped UDCA within the past 1 year. Similarly, the development of a normal SAP could be linked to dilation and/or stenting of biliary stricture [n = 4/20 (20.00%)], initiation of ursodeoxycholic acid [n = 3/20 (15.00%)] or unknown [n = 13/20 (65.00%)]. Hence, most cases of SAP changes were unexplained. We did not find a temporal association with IBD specific medications and SAP normalization.
Fig. 3.
Cumulative incidence of persistent alkaline phosphatase change (initial: The initial persistent SAP group change defined by the first occurrence of 2 or more SAP values measured at least 3 months apart that differed from baseline group). a Individuals with normal alkaline phosphatase at diagnosis who subsequently develop an abnormal alkaline phosphatase; b individuals with an abnormal alkaline phosphatase at diagnosis who subsequently develop a normal alkaline phosphatase; c individuals with an alkaline phosphatase ≥ 1.5 times the upper limit of normal at diagnosis who subsequently develop an alkaline phosphatase value below this threshold
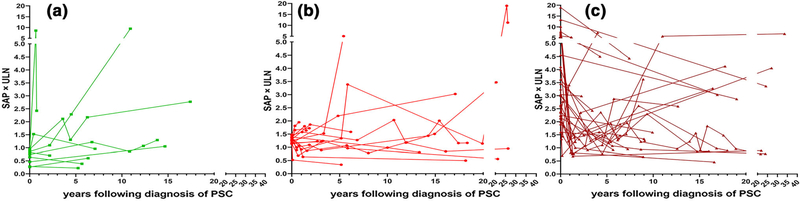
We also examined persistent changes to SAP levels that occurred beyond the initial SAP group change. Indeed, SAP values fluctuated after the index group change (Fig. 4a–c). For example, 24 (42.85%) of subjects had at least two, and 7 (12.50%) had at least 5 group changes over time. Specifically, among the 20 subjects who normalized their SAP, 11 (55.00%) had recurrence of an abnormal SAP after 2.65 (1.37–4.18) years. In addition, of the 19 participants who decreased their SAP to < 1.5 × ULN, 11 (57.89%) had recurrent SAP elevation ≥ 1.5 × ULN threshold after 2.93 (1.10–3.74) years. The rate of developing an abnormal SAP throughout the disease course was 11.25% per year and a total of 52 (92.85%) subjects had an abnormal SAP in their disease course. Moreover, the rate of either normalizing the SAP or lowering the SAP value below the threshold of 1.5 × ULN was 4.86% per year and 10.19% per year, respectively. A total of 30 (53.57%) subjects had a normal SAP and 45 (80.35%) subjects had a SAP < 1.5 × ULN during their disease course.
Fig. 4.
Individual persistent changes of alkaline phosphatase throughout disease course (Each line represents single patient. First and last dot represent SAP at diagnosis and last SAP value, respectively. Any dots in-between these points represent a persistent SAP group change where the individual had 2 or more SAP values measured at least 3 months apart). a Individuals who had normal SAP at the time of diagnosis; b individuals who had SAP of 1–1.49 × ULN at the time of diagnosis; c individuals who had SAP ≥ 1.5 × ULN at the time of diagnosis. Note axes broken in scales change for clarity at 5 × ULN for y-axis and at 20 years for x-axis
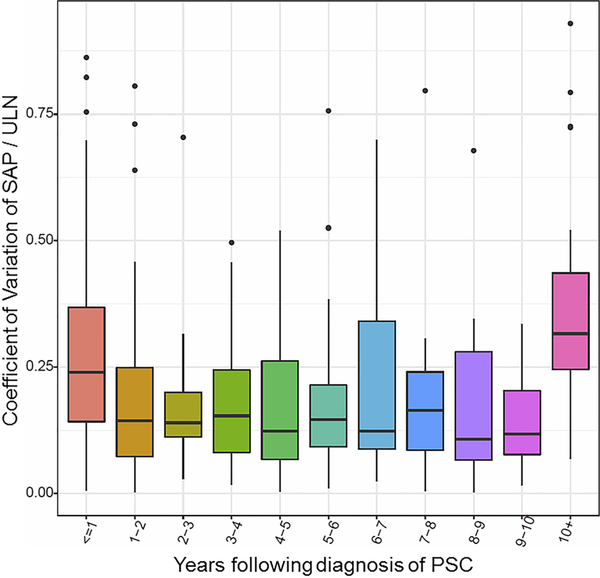
To quantify the true sporadic nature of SAP fluctuations, all SAP values collected during the study period were examined (Fig. 5). The intra-individual SAP values were highly variable throughout the disease course with a median (IQR) coefficient of variation of 36.20% (27.40–48.50%).
Fig. 5.
Intra-individual alkaline phosphatase variability (Examines every SAP value available throughout disease course and all changes that occurred). Each box extends vertically from the first to the third quartiles of coefficient of variation. The second quartile (median) is plotted by a horizontal line in the boxes. Vertical lines extending from boxes, outside the upper and lower quartiles, indicate variability (minimum and maximum) excluding outliers. Outliers are plotted as individual points
Prognostic value of alkaline phosphatase in a population-based cohort
Nineteen subjects developed the primary endpoints (death n = 2, LT n = 1, HD n = 11, CCA n = 5). Among those 19 persons, 6 (31.58%) had at least 2 normal SAP values (minimum at least 3 months apart) within 14.93 (5.22–25.54) years of developing the endpoint. Similarly, 13 (68.42%) patients had at least 2 SAP values < 1.5 × ULN (minimum at least 3 months apart) within 3.28 (0.64–8.56) years of developing the endpoint.
Sixteen subjects developed the secondary endpoints (death n = 8, LT n = 8). Among those 16 persons, 6 (37.50%) had at least 2 normal SAP values (minimum at least 3 months apart) within 15.91 (7.30–26.12) years of developing the endpoint. Similarly 12 (75.00%) had at least 2 SAP values < 1.5 × ULN (minimum at least 3 months apart) within 3.97 (0.44–14.56) years of developing the endpoint.
For the univariate analysis (Table 2), the baseline SAP value was not associated with the primary or secondary endpoint. Rather, individuals who developed a SAP < 1.5 × ULN at any time point (time-dependent covariate) were less likely to develop the primary or secondary endpoint. Consequently, this covariate was included into a series of multivariable models and remained independently associated with the outcomes of interest (Table 2). Similarly, the Mayo PSC risk score, PREsTo, MELD score and age also remained associated with PSC-related complications after adjusting for SAP changes (Table 2). Compared to SAP changes and the MELD score, the Mayo PSC risk score and PREsTo had higher concordance scores and appeared more likely to accurately categorize individuals at high risk for developing adverse outcomes (Supplementary Table 1).
Table 2.
Univariate and multivariate analysis for the primary and secondary endpoints
| Events |
||||
|---|---|---|---|---|
| Primary endpoint |
Secondary endpoint |
|||
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Univariate analysis | ||||
| IBD present | 0.47 (0.17–1.30) | 0.16 | 0.40 (0.12–1.28) | 0.13 |
| UDCA use | 0.60 (0.23–1.55) | 0.28 | 0.41 (0.14–1.22) | 0.10 |
| Mayo risk score at diagnosis | 2.09 (1.55–2.82) | < 0.001 | 2.95 (1.81–4.81) | < 0.001 |
| MELD score at diagnosis | 1.22 (1.11–1.36) | < 0.001 | 1.32 (1.15–1.53) | < 0.001 |
| PREsTo at diagnosis | 1.05 (1.03–1.08) | < 0.001 | 1.03 (1.01–1.05) | < 0.001 |
| Platelets at diagnosis, per 100 | 1.00 (0.99–1.00) | 0.16 | 0.60 (0.35–1.03) | 0.07 |
| Age at diagnosis, per 5 years | 1.40 (1.21–1.62) | < 0.01 | 1.46 (1.23–1.74) | < 0.01 |
| Normal SAP at diagnosis | 0.38 (0.05–2.82) | 0.27 | 0.52 (0.07–4.09) | 0.46 |
| SAP < 1.5 ULN at diagnosis | 0.64 (0.23–1.73) | 0.37 | 0.70 (0.0.24–2.10) | 0.52 |
| Normal SAP (time dependent) | 0.80 (0.24–2.63) | 0.71 | 0.95 (0.29–3.16) | 0.93 |
| SAP < 1.5 × ULN (time dependent) | 0.11 (0.03–0.42) | < 0.01 | 0.03 (0.00–0.56) | 0.02 |
| Multivariate analysis | ||||
| SAP < 1.5 × ULN (time dependent) | 0.10 (0.02–0.45) | 0.01 | 0.05 (0.00–1.01) | 0.05 |
| Mayo PSC risk score | 2.29 (1.59–3.30) | < 0.01 | 2.59 (1.56–4.29) | < 0.01 |
| SAP < 1.5 × ULN (time dependent) | 0.10 (0.02–0.39) | 0.01 | 0.04 (0.00–0.75) | 0.03 |
| MELD score at diagnosis | 1.25 (1.11–1.41) | 0.01 | 1.29 (1.10–1.50) | < 0.01 |
| SAP < 1.5 × ULN (time dependent) | 0.16 (0.04–0.65) | 0.01 | 0.02 (0.001–1.11) | 0.06 |
| PREsTo at diagnosis | 1.05 (1.03–1.07) | < 0.001 | 1.02 (1.00–1.04) | 0.08 |
| SAP < 1.5 × ULN (time dependent) | 0.19 (0.05–0.72) | 0.01 | 0.05 (0.00–0.97) | 0.048 |
| Age at diagnosis | 1.32 (1.14–1.52) | < 0.01 | 1.37 (1.16–1.62) | < 0.01 |
Primary endpoint = death, liver transplantation, hepatic decompensation or cholangiocarcinoma
Secondary endpoint = liver transplantation or death
IBD inflammatory bowel disease, UDCA ursodeoxycholic acid, SAP serum alkaline phosphatase, ULN upper limit of normal, MELD model for end-stage liver disease, PRETo PSC risk estimate tool
Discussion
The Olmsted County PSC cohort assembled herein represents a unique opportunity to highlight PSC epidemiology, natural history, and SAP changes throughout the disease course and over a 41-year time period. Most natural history studies stem from single tertiary centers with limited phenotypic depth. Therefore, using a well-characterized population-based cohort to improve our understanding of PSC is important. We were able to provide an update on PSC epidemiology in North America which suggested that a milder phenotype of PSC was increasingly being detected and might indirectly explain the rise in incidence. Moreover we were able to provide an in-depth assessment of the morbidity and mortality associated with PSC. Last, we were the first to quantify SAP fluctuations and its intra-individual variations throughout the disease course in a population-based cohort and related this to clinically relevant events.
The incidence of PSC in our cohort has increased almost twofold during our study period. There are several explanations for this observation. First, the proportion of individuals with a milder PSC phenotype (normal SAP, lower Mayo PSC risk score and intrahepatic PSC only) has increased. This could suggest detection of PSC at an earlier stage than in previous years. In turn, this may be secondary to more frequent laboratory testing in the general population, heighted awareness of PSC, and more routine use of cross sectional imaging including MRCP. In addition, this increased incidence could mirror the rising incidence of IBD in Olmsted County [29]. Our overall incidence was similar to a population-based study in Sweden (1.3 per 100,000 person years), but higher than the population-based studies from the UK and the Netherlands [4, 10, 30].
To our knowledge, this is the first population-based study that provides an in-depth assessment of disease-related complications including dominant strictures, pruritus, cholangitis, and HD. However, pruritus may be underreported to providers as a recent study from the PSC Partners Patient Registry noted 65.4% of PSC patients in the US reported pruritus [31]. Consistent with prior studies, CCA represents a leading cause of death [32]. Despite examining a population-based cohort, our median LT-free survival was 9.74 years which is consistent with prior studies but lower than the reported 21.30-year LT-free survival in the Netherlands [10, 33].
Individual patients’ SAP values fluctuated widely throughout the disease course for mostly unknown reasons (Figs. 4, 5). Indeed, the rate of normalizing the SAP was 4.86% per year and a total of 30 (53.57%) subjects had 2 or more normal SAP values for at least 3 months during their disease course. Similar to our study, Stanich et al. noted that 40% of patients experienced SAP normalization [11]. In the present study, the rate of lowering the SAP value below 1.5 × ULN was 10.19% per year and 45 (80.35%) subjects had 2 or more SAP values below 1.5 × ULN for at least 3 months. In addition, 68.42% of individuals who developed the primary endpoint had a prior history of decreasing their SAP to below 1.5 × ULN that often occurred within a short time frame before the PSC-related adverse event (median 3.28 years). Despite this, our findings reinforced the association of lowering SAP below 1.5 × ULN and improved outcomes [11–14, 17, 18]. However, the wide intra-individual fluctuations of SAP are a key disadvantage of using SAP as a surrogate endpoint in clinical trials (Figs. 4, 5). If SAP is used as an inclusion metric or an endpoint, it might be advantageous to obtain several SAP measures over a period of time, particularly in single-arm trials without a control group, to help mitigate this limitation. However, even this should be done with caution as our data indicate that SAP can continue to have significant variation after having several stable measurements over the course of at least 3 months (Fig. 4). These observations reinforce the need for improved biomarkers beyond SAP.
Our study has several limitations. First, the number of PSC patients in our community was small. This may be one reason why we did not observe an association with SAP normalization and the primary endpoint. Second, our population is enriched with Caucasians and some findings may not be generalizable to other ethnicities. Last, mild pruritus may not be brought to the attention of clinicians. Therefore, the cumulative incidence of pruritus reported in this study may be an underestimation of its true burden. Similarly, patient compliance with UDCA use is difficult to assess with this study design. Despite these limitations, our key strength was our use of REP, a unique data linkage system, and detailed data collection in a population-based cohort.
In conclusion, the incidence of PSC may be rising because patients are increasingly being diagnosed with a milder phenotype. Our findings quantify PSC-related morbidity and mortality and reaffirm that complications associated with this condition occur commonly. Lastly, while SAP levels that decreased below 1.5 × ULN were associated with improved outcomes, SAP frequently drops below 1.5 × ULN during the disease course, even amongst those who developed adverse outcomes later. The significant intra-individual SAP variations are limitations for SAP’s role as a method to stratify patients or use as a surrogate endpoint in clinical trials. Spontaneous SAP fluctuations should be taken into account when determining sample size for clinical trials with SAP as a surrogate endpoint.
Supplementary Material
Abbreviations
- PSC
Primary sclerosing cholangitis
- IBD
Inflammatory bowel disease
- SAP
Serum alkaline phosphatase
- ULN
Upper limit of normal
- REP
Rochester epidemiologic project
- CCA
Cholangiocarcinoma
- HD
Hepatic decompensation
- MELD
Model for end-stage liver disease
- PREsTo
PSC risk estimate tool
- MRCP
Magnetic resonance cholangiopancreatography
- CI
Confidence interval
- IQR
Interquartile range
- LT
Liver transplant
- HR
Hazard ratio
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00535-020-01663-1) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eaton JE, Talwalkar JA, Lazaridis KN, et al. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponsioen CY. Diagnosis, differential diagnosis, and epidemiology of primary sclerosing cholangitis. Dig Dis. 2015;33(Suppl 2):134–9. [DOI] [PubMed] [Google Scholar]
- 3.Williamson KD, Chapman RW. Primary sclerosing cholangitis: a clinical update. Br Med Bull. 2015;114:53–64. [DOI] [PubMed] [Google Scholar]
- 4.Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: a population-based cohort study. J Hepatol. 2008;48:939–44. [DOI] [PubMed] [Google Scholar]
- 5.Eaton JE, McCauley BM, Atkinson EJ, et al. Variations in primary sclerosing cholangitis across the age spectrum. J Gastroenterol Hepatol. 2017;32:1763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weismuller TJ, Trivedi PJ, Bergquist A, et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology. 2017;152:1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–8. [DOI] [PubMed] [Google Scholar]
- 8.Liu K, Wang RX, Kariyawasam V, et al. Epidemiology and outcomes of primary sclerosing cholangitis with and without inflammatory bowel disease in an Australian cohort. Liver Int. 2017;37:442–8. [DOI] [PubMed] [Google Scholar]
- 9.Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, et al. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51:562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–55. [DOI] [PubMed] [Google Scholar]
- 11.Stanich PP, Bjornsson E, Gossard AA, et al. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Mamari S, Djordjevic J, Halliday JS, et al. Improvement of serum alkaline phosphatase to %3c 1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329–34. [DOI] [PubMed] [Google Scholar]
- 13.Lindstrom L, Hultcrantz R, Boberg KM, et al. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;11:841–6. [DOI] [PubMed] [Google Scholar]
- 14.Rupp C, Rossler A, Halibasic E, et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther. 2014;40:1292–301. [DOI] [PubMed] [Google Scholar]
- 15.de Vries EM, Wang J, Leeflang MM, et al. Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: evaluation of prognostic value. Liver Int. 2016;36:1867–75. [DOI] [PubMed] [Google Scholar]
- 16.Ponsioen CY, Chapman RW, Chazouilleres O, et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology. 2016;63:1357–67. [DOI] [PubMed] [Google Scholar]
- 17.Goode EC, Clark AB, Mells GF, et al. Factors associated with outcomes of patients with primary sclerosing cholangitis and development and validation of a risk scoring system. Hepatology. 2019;69:2120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilscher M, Enders FB, Carey EJ, et al. Alkaline phosphatase normalization is a biomarker of improved survival in primary sclerosing cholangitis. Ann Hepatol. 2016;15:246–53. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen LC, Ersboll AK. When the entire population is the sample: strengths and limitations in register-based epidemiology. Eur J Epidemiol. 2014;29:551–8. [DOI] [PubMed] [Google Scholar]
- 20.Toy E, Balasubramanian S, Selmi C, et al. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 2011;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–9. [DOI] [PubMed] [Google Scholar]
- 22.Melton LJ III. History of the Rochester epidemiology project. Mayo Clin Proc. 1996;3:266–74. [DOI] [PubMed] [Google Scholar]
- 23.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. [DOI] [PubMed] [Google Scholar]
- 24.Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis—a comprehensive review. J Hepatol. 2017;67:1298–323. [DOI] [PubMed] [Google Scholar]
- 25.Rizvi S, Eaton JE, Gores GJ. Primary sclerosing cholangitis as a premalignant biliary tract disease: surveillance and management. Clin Gastroenterol Hepatol. 2015;13:2152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;7:688–94. [DOI] [PubMed] [Google Scholar]
- 27.Eaton JE, Vesterhus M, McCauley BM, et al. Primary sclerosing cholangitis risk estimate tool (PREsTo) predicts outcomes of the disease: a derivation and validation study using machine learning. Hepatology. 2018. 10.1002/hep.30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–21. [PubMed] [Google Scholar]
- 29.Shivashankar R, Tremaine WJ, Harmsen WS, et al. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017;15:857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindkvist B, de Valle MB, Gullberg B, et al. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology. 2010;52:571–7. [DOI] [PubMed] [Google Scholar]
- 31.Kuo A, Gomel R, Safer R, Lindor KD, Everson GT, Bowlus CL. Characteristics and outcomes reported by patients with primary sclerosing cholangitis through an online registry. Clin Gastroenterol Hepatol. 2019;17(7):1372–8. [DOI] [PubMed] [Google Scholar]
- 32.Takakura WR, Tabibian JH, Bowlus CL. The evolution of natural history of primary sclerosing cholangitis. Curr Opin Gastroenterol. 2017;33:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tischendorf JJ, Hecker H, Kruger M, et al. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102:107–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.