Abstract
Our aims are to assess various colistin dosing regimens against Pseudomonas aeruginosa (P. aeruginosa) infection in critically ill patients and to propose an appropriate regimen based on microbiological data. A Monte Carlo simulation was performed using the published colistin’s pharmacokinetic parameters of critically ill patients, the published pharmacodynamic target from a mouse thigh infection model, and the minimum inhibitory concentration (MIC) results from a Vietnamese hospital. The probability of target attainment (PTA) of 80% and cumulative fraction of response (CFR) of 90% were used to evaluate the efficacy of each regimen. Of 121 P. aeruginosa laboratory datasets, the carbapenem-resistant P. aeruginosa (CRPA) and the colistin-resistant P. aeruginosa rates were 29.8% and 0.8%, respectively. MIC50,90 were both 0.5 mg/L. The simulated results showed that at MIC of 2 mg/L, most regimens could not reach the PTA target, particularly in patients with normal renal function (Creatinine clearance (CrCl) ≥ 80 mL/min). At MIC of 0.5 mg/L and 1 mg/L, current recommendations still worked well. On the basis of these results, aside from lung infection, our study recommends three regimens against P. aeruginosa infection at MIC of 0.5 mg/L, 1 mg/L, and 2 mg/L. In conclusion, higher total daily doses and fractionated colistin dosing regimens could be the strategy for difficult-to-acquire PTA cases, while a less aggressive dose might be appropriate for empirical treatment in settings with low MIC50/90.
Keywords: colistin, Pseudomonas aeruginosa, critically ill patients, PK/PD, Monte Carlo simulation
1. Introduction
Pseudomonas aeruginosa (P. aeruginosa) is one of the most concerning pathogens related to nosocomial infection [1]. Due to the emergence of multi-drug resistant (MDR) and carbapenem-resistant P. aeruginosa (CRPA) isolates, some serious consequences can be named such as reducing treatment of choices, inappropriate initial therapy, and delayed treatment [2,3]. The eventual outcomes are a higher mortality rate, a higher readmission rate, as well as the increase of the length of stay and treatment cost [4,5,6,7]. Given that fact, MDR P. aeruginosa has made it to the list of CDC’s urgent threats since 2013 [8,9].
With the scarcity of new antimicrobial agents in the pipeline, there is room for old antibiotics like colistin to perform against these MDR gram negative strains [10]. However, regarding colistin, the lack of relevant pharmacokinetic (PK) and pharmacodynamic (PD) studies leads to an ununiformed usage among countries. Due to the high prevalence of MDR P. aeruginosa and the potential nephrotoxicity of colistin [11,12,13,14], the scientific base of colistin instruction is worth being considered to improve the effectiveness and to reduce confusion in the clinical practice. It is especially important for critically ill patients who are not only in life-threatening conditions but also vulnerable to medication’s PK alteration and at a high risk of hospital-acquired infections by gram-negative bacteria such as MDR P. aeruginosa. Therefore, in this study, we aim to the combine the concept of the Monte Carlo simulation, the knowledge about PK and PD of colistin, and the local microbiological profile of P. aeruginosa to evaluate current dosing regimens and to propose appropriate ones for the treatment of P. aeruginosa infection in critically ill patients.
2. Materials and Methods
2.1. Microbiology
The microbiological data were obtained from a general hospital in Hochiminh city of Vietnam. The minimum inhibitory concentration (MIC) of colistin and the antimicrobial susceptibility test (AST) result of all P. aeruginosa isolates were extracted from May 2019 to March 2020. The tests were performed by the N240 Card/Vitek2-Biomerieux system. Six groups of antibiotics were used for the AST including antipseudomonal cephalosporin (ceftazidime, cefepime), antipseudomonal penicillin/beta-lactamase inhibitor (ticarcillin/avibactam, piperacillin/tazobactam), antipseudomonal carbapenem (imipenem, meropenem), aminoglycoside (gentamycin, amikacin), antipseudomonal fluoroquinolone (ciprofloxacin, levofloxacin), and polymyxin (colistin). MIC50 and MIC90 of colistin were then calculated from the MIC distribution.
As stated by European Committee on Antimicrobial susceptibility testing (EUCAST), the isolates were susceptible to colistin if the MIC was equal or less than 2 mcg/L [15]. However, it should be kept in mind that Clinical and Laboratory Standards Institute (CLSI) recently issued a new guidance on AST standards, in which colistin efficacy has been reviewed and reclassified as intermediate inhibition against P. aeruginosa at MIC of 2 mg/L or below [16].
2.2. Pharmacokinetic Parameters
According to current findings, concentrations of colistimethate sodium (CMS- colistin’ prodrug) and colistin fit to a two-compartment and a one-compartment pharmacokinetic model, respectively [17,18,19]. In this study, we adapted the published equations and population PK parameters related to critically ill patients from Nation et al. (Table 1 and Equations (1)–(3)) [19].
| (1) |
| (2) |
| (3) |
Table 1.
The population pharmacokinetic (PK) parameters of colistin in critically ill patients.
| Parameters | CMS (SD) | Parameters | Colistin (SD) |
|---|---|---|---|
| V1 (L) | 12.9 (5.2116) | V3/fm (L) | 57.2 (24.882) |
| V2 (L) | 16.1 (11.4149) | CLRCsl-pop/fm (L/h/CrCl) | 0.00834 |
| CLD1 (L/h) | 9.57 (7.66557) | CLNRc/fm (L/h) | 3.11 |
| CLRslope (L/h/CrCl) | 0.034 (0.02557) | fu | 0.49 (0.11) |
| CLNRcms (L/h) | 2.52 (1.00296) |
V1: central volume distribution of CMS, V2: peripheral volume distribution of CMS, CLD1: distributional clearance between central and peripheral compartments of CMS, CLRslope: renal clearance of CMS, CLnrcms: non renal clearance of CMS, V3/fm: apparent volume distribution of colistin, CLRCsl-pop/fm: apparent renal clearance of colistin, CLRNc/fm: apparent renal clearance of colistin, fu: unbound fraction of colistin, CMSc: central concentration of CMS, CMSp: peripheral concentration of CMS, R:, V1: central volume distribution of CMS, V2: peripheral volume distribution of CMS, Col: colistin concentration, CLTCMS: total clearance of CMS, CLNRCMS: non renal clearance of CMS, CLTc: total clearance of colistin, V3: volume distribution of colistin.
2.3. PK/PD Index and PDT
The magnitude of the colistin-related PK/PD index, which is the area of free colistin concentration under the curve over MIC (fAUC/MIC), was extracted from the study of Cheah. et al. [20]. The target of fAUC/MIC chosen for this study is 10, which effectively produces 1 log kill against the MDR strain 19,056 in the mouse thigh infection model.
2.4. Monte Carlo Simulation
The Monte Carlo simulation was run by Crystal Ball ®® software (Oracle Crystal Ball ®® version 11.1.2.4.850) on 10,000 virtual patients. The uniform distribution was used for the protein-binding fraction and creatinine clearance while the log-normal distribution was applied to all other PK parameters [21,22,23]. The fAUC in 24 h (fAUC0-24), probability of target attainment (PTA), and cumulative fraction of response (CFR) were computed from the free colistin concentration, MIC range, and MIC distribution. PTA of 80% and CFR of 90% were the targets of bacteria eradication in our study.
For each regimen, the trough concentration at steady state (Ctrough,ss) was compared with the values of 2.42 mg/L and 3.33 mg/L from the literature [24]. The patients presented with Ctrough,ss above the cut-off value of 2.42 mg/L and 3.33 mg/L were considered to possibly have a higher risk of nephrotoxicity at the end of treatment (EOT) and at day 7 (D7), respectively.
The tested regimens in our study were from the current recommendation of Food and Drug Administration of United State (US-FDA), European Medicines Agency (EMA), Nation et al. [17,25,26], and our suggestions (Table 2). In each regimen, loading doses (LDs) and maintenance doses (MDs) were given in mg of colistin base activity (CBA) and were theoretically administered in 30 min.
Table 2.
Tested dosing regimens.
| Renal Function a | LD b | MD b | Regimen |
|---|---|---|---|
| 101–130 mL/min | 300 mg | 150 mg Q12h | US-FDA, EMA |
| 180 mg Q12h | Nation | ||
| 100 mg Q6h | Proposed | ||
| 120 mg Q6h | Proposed | ||
| 200 mg Q12h | Proposed | ||
| 100 mg Q4h | Proposed | ||
| 150 mg Q6h | Proposed | ||
| 80–100 mL/min | 300 mg | 150 mg Q12h | US-FDA, EMA |
| 180 mg Q12h | Nation | ||
| 150 mg Q8h | Proposed | ||
| 100 mg Q6h | Proposed | ||
| 120 mg Q6h | Proposed | ||
| 100 mg Q8h | Proposed | ||
| 51–79 mL/min | 300 mg | 150 mg Q12h | EMA, Nation |
| 115 mg Q12h | US-FDA | ||
| 180 mg Q12h | Proposed | ||
| 120 mg Q8h | Proposed | ||
| 100 mg Q8h 100 mg Q12h |
Proposed Proposed |
||
| 30–50 mL/min | 300 mg | 75 mg Q12h | US-FDA |
| 125 mg Q12h | EMA | ||
| 110 mg Q12h | Nation | ||
| 250 mg Q24h 200 mg Q24h 100 mg Q12h |
Proposed Proposed Proposed |
||
| 80 mg Q8h | Proposed | ||
| 120 mg Q12h | Proposed | ||
| 100 mg Q24h | Proposed | ||
| 11–29 mL/min | 300 mg | 90 mg Q36h | US-FDA |
| 90 mg Q12h | EMA | ||
| 80 mg Q12h | Nation | ||
| 150 mg Q24h | Proposed | ||
| 200 mg Q36h | Proposed | ||
| 120 mg Q24h 100 mg Q24h |
Proposed Proposed |
||
| 60 mg Q12h | Proposed | ||
| 66 mg Q24h | Proposed | ||
| 1–10 mL/min | 300 mg | 60 mg Q12h | EMA |
| 70 mg Q12h | Nation | ||
| 80 mg Q24h 66 mg Q24h |
Proposed Proposed |
||
| 33 mg Q24h | Proposed | ||
| 40 mg Q12h | Proposed |
a: renal function is stratified by creatinine clearance in mL/min, b: colistin dose is expressed as mg of colistin base activity. LD: loading dose, MD: maintenance dose, US-FDA: Food and Drug Administration of United State, EMA: European Medicines Agency, Q: every.
Our study was approved by the ethical committee of the Faculty of Dentistry/Faculty of Pharmacy, Mahidol University (No: COA. No. Mu-DT/PY-IRB 2020/013.1702), and we received permission to use laboratory results from FV hospital (Hochiminh city, Vietnam).
3. Results
3.1. Microbiology
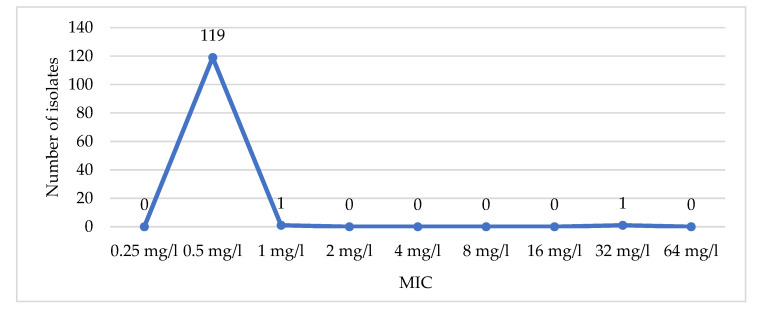
Of 137 datasets of Pseudomonas aeruginosa isolates from all sources (sputum, blood, urine, abscess pus, etc.), 16 sets were excluded (15 duplicates and 1 inadequate information). Tested antibiotics for AST were antipseudomonal cephalosporin (ceftazidime, cefepime), antipseudomonal penicillin/beta-lactamase inhibitor (ticarcillin/clavulanic, piperacillin/tazobactam), antipseudomonal carbapenem (imipenem, meropenem), aminoglycoside (gentamycin, amikacin), antipseudomonal fluoroquinolone (ciprofloxacin, levofloxacin), and polymyxin (colistin). According to the AST in Table 3, 24.8% of isolates (30/121) were MDR (not susceptible to at least 1 agent in at least 3 groups of antibiotics [27]). Among these, 70% of isolates were susceptible to only polymyxin. Colistin remained the most effective agent with only 0.8% of resistance. MIC50/90 of colistin was 0.5 mg/L (Figure 1).
Table 3.
Antimicrobial susceptibility test (AST) results of all P. aeruginosa isolates.
| Total Included Isolates (n = 121) | Number (n) | Percentage (%) |
|---|---|---|
| Source of samples | ||
| Blood | 5 | 4.1 |
| Urine | 22 | 18.2 |
| Respiratory tract | 46 | 38 |
| Others (skin, body fluid, etc.) | 48 | 39.7 |
| Resistance pattern (antibiogram) | ||
| No resistance | 38 | 31.4 |
| Resistant to Ceftazidime | 25 | 20.7 |
| Resistant to Cefepime | 27 | 22.3 |
| Resistant to Ticarcilline/Clavulanic | 68 | 56.2 |
| Resistant to Piperacillin/Tazobactam | 35 | 28.9 |
| Resistant to Carbapenem | 35 | 28.9 |
| Resistant to Aminoglycoside | 30 | 24.8 |
| Resistant to Flouroquinolone | 39 | 32.2 |
| Resistant to Colistin | 1 | 0.8 |
| Multi-drug resistant | 30 | 24.8 |
Figure 1.
Minimum inhibitory concentration (MIC) distribution of colistin among P. aeruginosa isolates.
3.2. PTA of All Regimens in Different Ranges of Creatinine Clearance (CrCl)
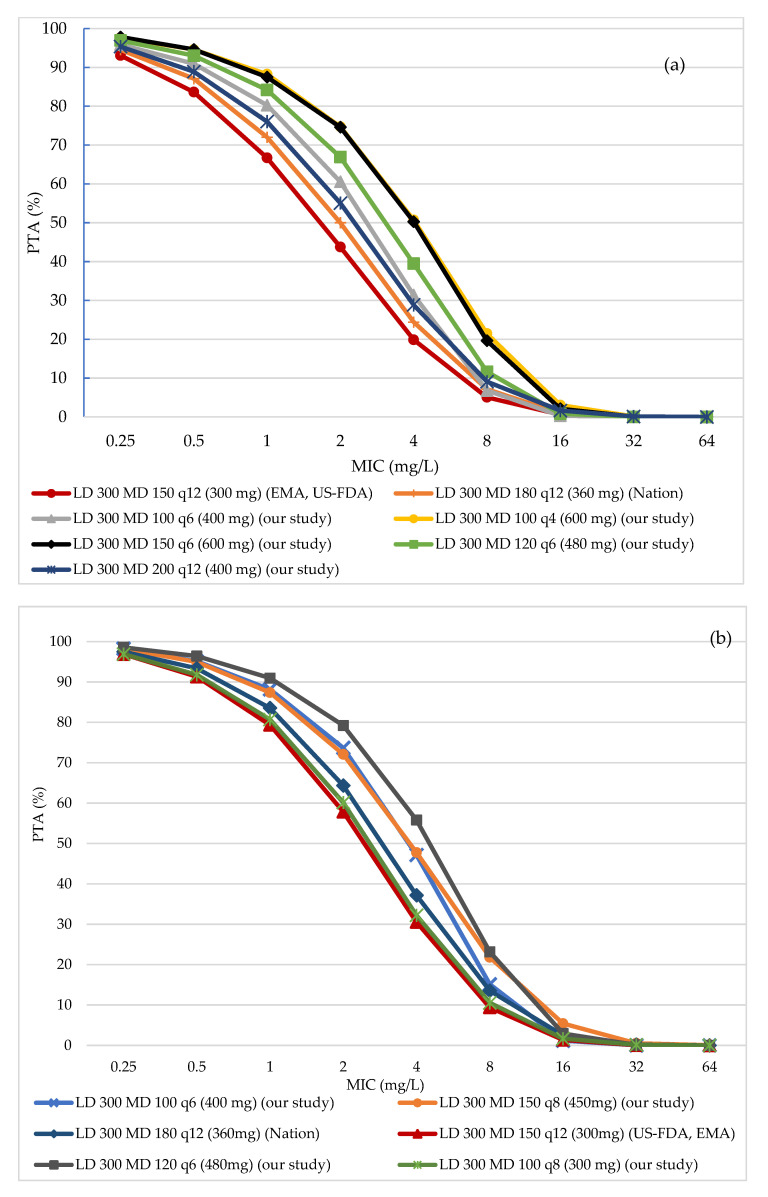
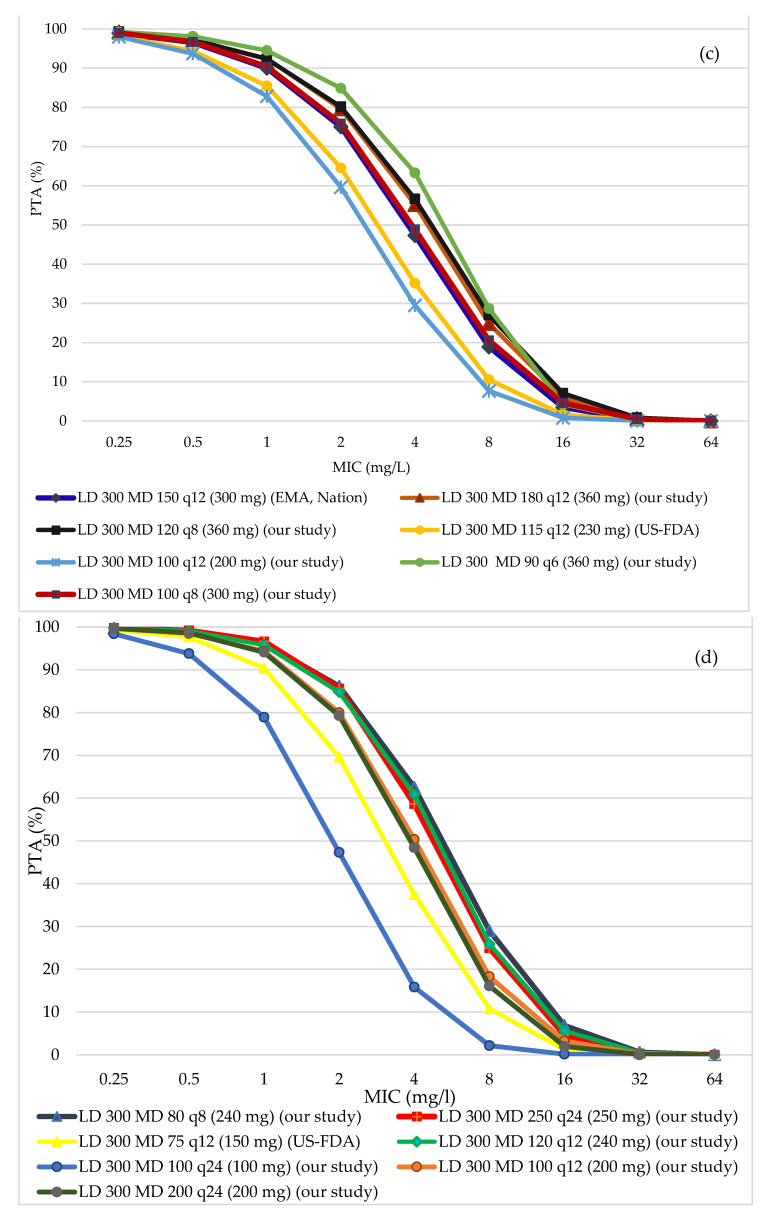
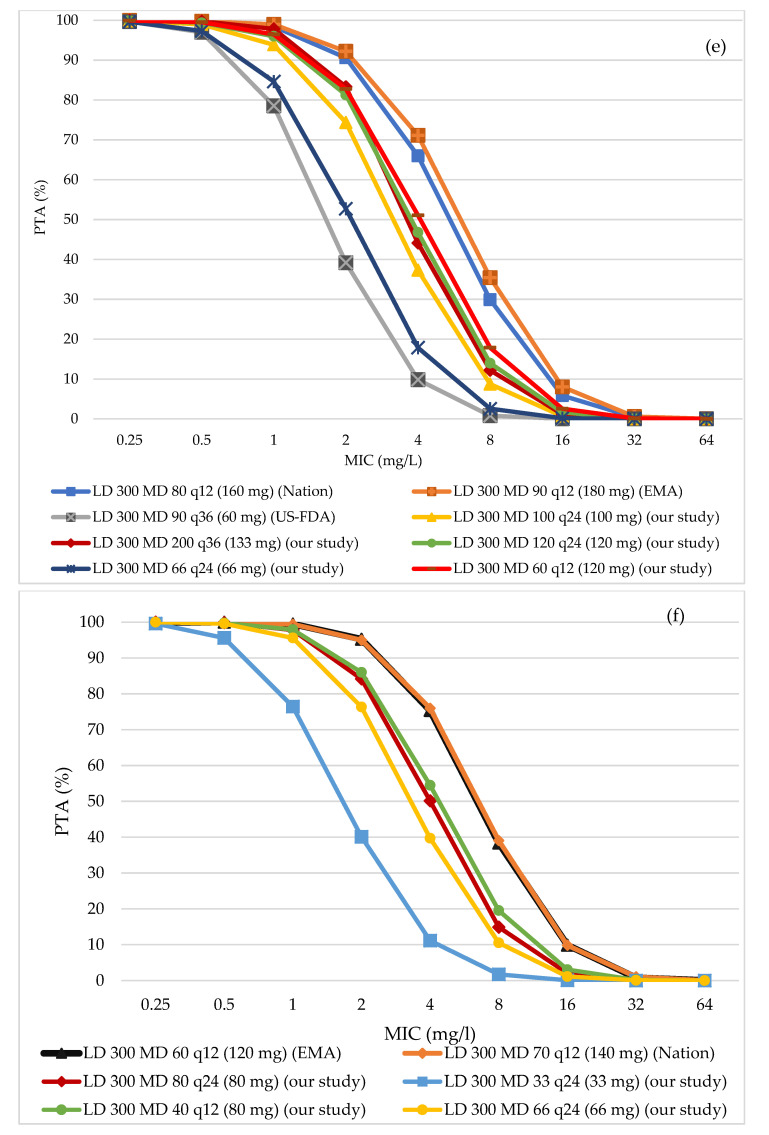
The PTA results of all regimens among various renal function ranges were depicted in Figure 2a–f. At MIC of 2 mg/L, none of the regimens achieved 80% PTA in the group of CrCl 101–130 mL/min. Even at the high dose of 600 mg, the PTA was only around 75%. For those patients with CrCl 80–100 mL/min, only 1 regimen from our study (120 mg every 6 h) was able to reach approximately 80% PTA. At CrCl of 51–79 mL/min, the PTA of approximately 80% and above was seen in three regimens with 360 mg per day. PTA of 80% and beyond was produced by regimens with total daily doses (TDDs) of at least 220 mg, 120 mg, and 80 mg in the three remaining groups of CrCl 30- 50 mL/min, 11–29 mL/min, and 1–10 mL/min, respectively. On the other hand, high PTA results (above 80%) were recorded at MIC of 1 mg/L and 0.5 mg/L with lower TDDs. Especially, at MIC of 0.5 mg/L, 80% PTA was found in all of the tested regimens. With MIC above 2 mg/L, none of the regimens were able to achieve the PTA target regardless of renal function.
Figure 2.
Probability of target attainment (PTA) achievement in the groups of CrCl 101–130 mL/min (a), 80–100 mL/min (b), 51–19 mL/min (c), 30–50 mL/min (d), 11–29 mL/min (e), 1–10 mL/min (f). The detail of PTA results is found in Supplementary material Tables S1–S6. LD: loading dose (in mg colistin base activity), MD: maintenance dose (in colistin base activity), US-FDA: Food and Drug Administration of United State, EMA: European Medicines Agency, q12: every 12 h.
Considerably, in some CrCl clusters, with the same TDD, regimens with different frequencies showed different PTAs. For instance, in the group of CrCl 101–130 mL/min, with the daily dose of 400 mg, the 100 mg every six-hour regimen produced higher PTA compared with the 200 mg every 12-h regimen. The difference was 2.02% (90.93% versus 88.91%) and 5.5% (60.55% versus 55.05%) at MIC of 0.5 mg/L and 2 mg/L, respectively. In the group of 80–100 mL/min CrCl, there was about a 3% difference between two regimens of 150 mg every 12 h and 100 mg every 8 h, at MIC of 2 mg/L. Or for those patients with CrCl 51–79 mL/min, the regimens of 180 mg every 12 h, 120 mg every 8 h, and 90 mg every 6 h produced disparate PTAs with 79.6%, 80.15%, and 84.87%, respectively. For the group of CrCl 1–10 mL/min, less distinguished PTA was found between two regimens, 80 mg every 24 h and 40 mg every 12 h (i.e., 84.16% and 85.99%, respectively).
3.3. CFR and Nephrotoxicity Risk
Both CFR and AKI risk estimation were displayed in Table 4 and Table 5. As stated in those tables, most regimens achieved CFR 90% and above. Only in the group of patients with very good renal function (100–130 mL/min) could we observe some CFR values from 80% to less than 90%. For all tested dosages, the proportion of patients related to higher AKI risk spread out widely from 5% to 90%. The chance of developing AKI at the end of treatment was higher compared to that on day 7. The US-FDA recommendation had almost a lower AKI risk than those from EMA and Nation et al.
Table 4.
PTA, CFR and AKI risk of simulated regimens from US-FDA, EMA, Nation, and our study.
| CrCl (mL/min) | Regimens (TDDs*) | PTA (%) | CFR (%) | AKI Risk (%) | |||
|---|---|---|---|---|---|---|---|
| MIC 0.5 mg/L (MIC90) | MIC 2 mg/L | EOT | D7 | ||||
| 101–130 | 150 mg Q12h (300 mg) | US-FDA, EMA | 83.65 | 43.75 | 82.82 | 24.63 | 16.75 |
| 180 mg Q12h (360 mg) | Nation | 87.1 | 49.99 | 86.26 | 29.23 | 20.17 | |
| 200 mg Q12h (400 mg) | Our study | 88.91 | 55.05 | 88.07 | 33.01 | 23.14 | |
| 100 mg Q6h (400 mg) | Our study | 90.93 | 60.55 | 90.09 | 43.38 | 30.33 | |
| 120 mg Q6h (480 mg) | Our study | 93.68 | 63.27 | 92.82 | 51.31 | 38.08 | |
| 100 mg Q4h (600 mg) | Our study | 94.6 | 74.72 | 93.77 | 62.77 | 50.51 | |
| 150 mg Q6h (600 mg) | Our study | 96.62 | 74.6 | 93.78 | 60.65 | 48.24 | |
| 80–100 | 150 mg Q12h (300 mg) | US-FDA, EMA | 91.32 | 57.85 | 90.47 | 36.59 | 26.24 |
| 180 mg Q12h (360 mg) | Nation | 93.37 | 64.36 | 92.52 | 42.58 | 31.83 | |
| 150 mg Q8h (450 mg) | Our study | 94.96 | 72.07 | 94.12 | 56.45 | 45.01 | |
| 100 mg Q6h (400 mg) | Our study | 95.12 | 73.69 | 94.28 | 59.45 | 46.34 | |
| 120 mg Q6h (480 mg) | Our study | 96.42 | 79.23 | 95.58 | 66.74 | 54.62 | |
| 100 mg Q8h (300 mg) | Our study | 91.79 | 60.15 | 90.94 | 42.57 | 30.66 | |
| 51–79 | 115 mg Q12h (230 mg) | US-FDA | 94.38 | 64.52 | 93.53 | 42.93 | 30.9 |
| 150 mg Q12h (300 mg) | EMA, Nation | 96.52 | 75.02 | 95.67 | 54 | 42 | |
| 180 mg Q12h (360 mg) | Our study | 97.21 | 79.6 | 96.37 | 59.64 | 48.22 | |
| 120 mg Q8h (360 mg) | Our study | 97.3 | 80.15 | 96.46 | 65.75 | 54.22 | |
| 100 mg Q8h (300 mg) | Our study | 96.72 | 75.88 | 95.87 | 59.81 | 46.89 | |
| 100 mg Q12h (200 mg) | Our study | 93.7 | 59.61 | 92.84 | 38.44 | 26 | |
| 30–50 | 75 mg Q12h (150 mg) | US-FDA | 97.66 | 69.6 | 96.79 | 48.2 | 34.7 |
| 125 mg Q12h (250 mg) | EMA | 99.18 | 86.35 | 98.34 | 68.73 | 56.18 | |
| 110 mg Q12h (220 mg)) | Nation | 98.71 | 83.11 | 97.86 | 63.45 | 50.74 | |
| 120 mg Q12h (240 mg) | Our study | 99.07 | 84.77 | 98.23 | 67.98 | 54.81 | |
| 80 mg Q8h (240 mg) | Our study | 98.95 | 86.15 | 98.11 | 73.17 | 61.38 | |
| 100 mg Q12h (200 mg) | Our study | 98.48 | 79.97 | 97.63 | 60.15 | 46.86 | |
| 200 mg Q24h (200 mg) | Our study | 98.62 | 79.26 | 97.77 | 41.24 | 30.2 | |
| 100 mg Q24h (100 mg) | Our study | 93.77 | 47.3 | 92.87 | 19.07 | 11.46 | |
| 250 mg Q24h (250 mg) | Our study | 99.18 | 85.6 | 98.34 | 47.49 | 37.15 | |
| 11–29 | 90 mg Q36h ((60 mg) | US-FDA | 97.01 | 39.17 | 96.06 | 11.51 | 5.79 |
| 90 mg Q12h (180 mg) | EMA | 99.77 | 92.21 | 98.94 | 78.29 | 66.68 | |
| 80 mg Q12h (160 mg) | Nation | 99.71 | 90.59 | 98.88 | 74.87 | 62.55 | |
| 120 mg Q24h (120 mg) | Our study | 99.39 | 81.28 | 98.54 | 45.21 | 33.16 | |
| 60 mg Q12h (120 mg) | Our study | 99.54 | 82.8 | 98.69 | 63.74 | 48.63 | |
| 66 mg Q24h (66 mg) | Our study | 97.33 | 52.72 | 96.42 | 23.17 | 13.08 | |
| 100 mg Q24h (100 mg) | Our study | 98.98 | 74.37 | 98.11 | 39.51 | 26.54 | |
| 150 mg Q24h (150 mg) | Our study | 99.76 | 88.35 | 98.92 | 53.79 | 41.79 | |
| 200 mg Q36h (133 mg) | Our study | 99.78 | 83.31 | 98.94 | 32.3 | 23.16 | |
| 1–10 | 60 mg Q12h (120 mg) | EMA | 99.96 | 95.24 | 99.14 | 83.68 | 72.27 |
| 70 mg Q12h (140 mg) | Nation | 99.97 | 95 | 99.15 | 87.61 | 78.67 | |
| 80 mg Q24h (80 mg) | Our study | 99.85 | 84.16 | 99 | 52.76 | 39.14 | |
| 40 mg Q12h (80 mg) | Our study | 99.77 | 85.99 | 98.93 | 68.95 | 53.11 | |
| 66 mg Q24h (66 mg) | Our study | 99.6 | 76.33 | 98.74 | 45.19 | 32.46 | |
| 33 mg Q24h (33 mg) | Our study | 95.6 | 40.12 | 94.65 | 17.79 | 9.52 | |
TDDs*: total daily doses in mg of colistin base activity, Q: every, PTA: probability of target attainment, CFR: cumulative fraction of response, AKI: acute kidney injury, CrCl: creatinine clearance, MIC90: minimum inhibitory concentration required to inhibit the growth of 90% of organisms, EOT: end of treatment, D7: at day 7, US-FDA: Food and Drug Administration of United State, EMA: European Medicines Agency.
Table 5.
Recommendation for MIC of 0.5 mg/L (MIC90 of our hospital).
| CrCl (mL/min) | Regimens (TDDs*) | PTA (%) | CFR (%) | AKI Risk (%) | Alternative Regimens (TDDs*) | PTA (%) | CFR (%) | AKI Risk (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| EOT | D7 | EOT | D7 | |||||||
| 101–130 | 100 mg Q6h (400 mg) | 90.93 | 90.09 | 43.38 | 30.33 | |||||
| 80–100 | 100 mg Q8h (300 mg) | 91.79 | 90.94 | 42.57 | 30.66 | 150 mg Q12h (300 mg) (US-FDA) | 91.32 | 90.47 | 36.59 | 26.24 |
| 51–79 | 100 mg Q12h (200 mg) | 93.7 | 92.84 | 38.44 | 26 | |||||
| 30–50 | 100 mg Q24h (100 mg) | 93.77 | 92.87 | 19.07 | 11.46 | |||||
| 11–29 | 66 mg Q24h (66 mg) | 97.33 | 96.42 | 23.17 | 13.08 | |||||
| 1–10 | 33 mg Q24h (33 mg) | 95.6 | 94.65 | 17.79 | 9.52 | |||||
TDDs*: total daily doses in mg of colistin base activity, Q: every, PTA: probability of target attainment, CFR: cumulative fraction of response, AKI: acute kidney injury, CrCl: creatinine clearance, MIC90: minimum inhibitory concentration required to inhibit the growth of 90% of organisms, EOT: end of treatment, D7: at day 7, US-FDA: Food and Drug Administration of United State.
3.4. The Recommendation of Our Study
On the basis of the PTA target of 80% and appropriate AKI risk, we made three colistin dosage recommendations according to CrCl ranges at MIC of 0.5 mg/L (Table 5), 1 mg/L (Table 6), and 2 mg/L (Table 7). With CFR of 90% and above, the dosing regimen at MIC of 0.5 mg/L, which was also the MIC90 of our hospital, could be considered as the empirical treatment of colistin in this hospital.
Table 6.
Recommendation for MIC of 1 mg/L.
| CrCl (mL/min) | Regimens (TDDs) | PTA (%) | AKI Risk (%) | Alternative Regimens (TDDs) | PTA (%) | AKI Risk (%) | ||
|---|---|---|---|---|---|---|---|---|
| EOT | D7 | EOT | D7 | |||||
| 101–130 | 120 mg Q6h (480 mg) | 84.19 | 43.09 | 29.18 | ||||
| 80–100 | 100 mg Q6 (400 mg) | 88.16 | 59.45 | 46.34 | ||||
| 51–79 | 100 mg Q8h (300 mg) | 90.4 | 59.81 | 46.89 | 150 mg Q12h (300 mg) | 89.99 | 54 | 42 |
| 30–50 | 100 mg Q12 (200 mg) | 94.35 | 60.15 | 46.86 | 200 mg Q24h (200 mg) | 94.08 | 41.24 | 30.2 |
| 11–29 | 100 mg Q24 (100 mg) | 93.85 | 39.51 | 26.54 | ||||
| 1–10 | 66 mg Q24h (66 mg) | 95.6 | 45.19 | 32.46 | ||||
TDDs*: total daily doses in mg of colistin base activity, Q: every, PTA: probability of target attainment, CFR: cumulative fraction of response, AKI: acute kidney injury, CrCl: creatinine clearance, MIC: minimum inhibitory concentration, EOT: end of treatment, D7: at day 7.
Table 7.
Recommendation for MIC of 2 mg/L.
| CrCl (mL/min) | Regimens (TDDs) | PTA (%) | AKI Risk (%) | Alternative Regimens (TDDs) | PTA (%) | AKI Risk (%) | ||
|---|---|---|---|---|---|---|---|---|
| EOT | D7 | EOT | D7 | |||||
| 101–130 | Not recommend | |||||||
| 80–100 | 120 mg Q6h (480 mg) | 79.23 | 66.74 | 54.62 | ||||
| 51–79 | 120 mg Q8h (360 mg) | 80.15 | 65.75 | 54.22 | 180 mg Q12h (360 mg) | 79.6 | 59.64 | 48.22 |
| 30–50 | 120 mg Q12h (240 mg) | 84.77 | 67.98 | 54.81 | 250 mg Q24h (250 mg) | 85.6 | 47.49 | 37.15 |
| 11–29 | 120 mg Q24h (120 mg) | 81.28 | 45.21 | 33.16 | ||||
| 1–10 | 80 mg Q24h (80 mg) | 84.16 | 52.76 | 39.14 | ||||
TDDs*: total daily doses in mg of colistin base activity, Q: every, PTA: probability of target attainment, CFR: cumulative fraction of response, AKI: acute kidney injury, CrCl: creatinine clearance, MIC: minimum inhibitory concentration, EOT: end of treatment, D7: at day 7.
4. Discussion
In Vietnam, a multicenter observational study by Biedenbach et al. in 2016 reported the carbapenem-resistant P. aeruginosa (CRPA) rate of 66.5% and MIC90 of 0.5 mg/L [11]. According to our small-scale study, the MIC90 was still the same with 0.5 mg/L. However, the resistant rates were lower, with 24.8% and 28.9% for MDR P. aeruginosa and CRPA, respectively. The different rates of CRPA between studies could be attributed to the patients’ characteristics (underlying diseases), carbapenem use, and infection control [28]. The study of Biedenbach et al. focused on hospital-acquired and ventilator-associated pneumonia in five big tertiary hospitals across Vietnam, which suggested that they had many patients with complicated underlying diseases and ICU admission [29]. Meanwhile, our data were from all types of infections in a small general private hospital. The less severe of patients’ condition as well as the small number of patients were assumed to affect the lower usage of all antibiotics in general and carbapenem in specific. Of note, the low MIC90 and low colistin-resistant rate of P. aeruginosa in our study suggested the necessity of colistin against this organism in our population. Therefore, it is prudent to rationalize the dose to increase the efficacy as well as to reduce the resistant incident of colistin.
According to the PTA results, we found some relationship between patients’ renal function and PTA achievement. At the MIC breakpoint of 2 mg/L, PTA fell under the target (80%) in patients with normal renal function (101–130 mL/min). All regimens from US-FDA, EMA, and Nation (300 to 360 mg daily) achieved less than 50% PTA in this CrCl range. Meanwhile, most of those recommendations showed more than 80% PTA in patients with CrCl less than 50 mL/min. At the low MIC of 1 mg/L, the conventional regimens (from US-FDA, EMA, and Nation) similarly failed to gain 80% of PTAs in the group of CrCl 101–130 mL/min. This renal function-related effect was also seen in the study by Jitaree et al. [30]. Their study was about the efficacy of colistin against carbapenem-resistant Klebsiella pneumoniae and Escherichia coli. They reported the low PTA (65–89%) achieved in patients with CrCl > 50 mL/min at MIC ≥ 2 mg/L. This result also confirmed the study by Garonzik et al. [19]. Upon the observation on 115 critically ill patients, they found that patients with good renal function, who were given higher CMS dose, had lower colistin concentration than the others. These findings were supported by the current acknowledgment of CMS’ renal elimination that it was in parallel with CrCl [19,31]. At CrCl of 25 and 120 mL/min, the values of CLrcms were estimated at around 24 to 26 and 92 to 123 mL/min, respectively. Moreover, in a PK study of 73 patients, Gregoire et al. pointed out that the conversion fraction of CMS to colistin depended on renal function [31]. They predicted that at CrCl of 120 mL/min, only 32% of CMS transformed to colistin, but at 10 mL/min of CrCl, this rate increased by up to 80%. Consequently, at high kidney function, the formed colistin concentration was low. This fact, again, cast doubt on the efficacy of colistin against strains with MIC of 2 mg/L and for patients with good renal function.
Furthermore, the US-FDA recommendation was inferior to the other two popular regimens from EMA and Nation. In almost renal function groups, PTAs from US-FDA dosing regimens were lower than those from the other two regimens. The higher the MIC was, the bigger the PTA gap was found between regimens. For example, in the range of CrCl 51–79 mL/min, PTA magnitude from the US-FDA regimen was around 2% lower than the PTA from EMA and Nation at MIC of 0.5 mg/L. However, this difference was five times higher at MIC of 2 mg/L. The PTA difference was up to 50% in the group of CrCl 11–29 mL/min at MIC of 2 mg/L. We found the same results in the study by Jitaree et al. [30]. In which, EMA and Nation regimens showed more than 80% PTA for those with CrCl from 50 mL/min downward. While US-FDA recommendations continued showing much lower PTA. This inferiority was contributed to the low dose found in US-FDA regimens compared to others. Among these three regimens, only US-FDA used the weight-based dosing. When applying on a lower body weight population (60-kg body weight in both our study and Jitaree et al. versus 80-kg typical body weight), the lower doses and lower PTAs were the obvious consequences [32]. As stated in all studies by Garonzik et al., Nation et al., and Gregoire et al., patients’ body weight had little impact on the maintenance dose and was included only in the form of CrCl in the equations developed by Garonzik and Nation [17,19]. Therefore, a weight approach like US-FDA suggestions might result in poor performance, especially when being used for a population with a small body size such as that of the Vietnamese population.
Remarkably, despite the satisfied PTAs, both EMA and Nation recommendations demonstrated a high chance of AKI development in clusters of reduced renal function (CrCl ≤ 50 mL/min). Around 50% to 80% and 63% to 88% of patients had high colistin concentration-related AKI risk at day 7 and at the end of treatment. The lower the CrCl was, the higher the AKI risk was. Although no relationship between the colistin cumulative dose and nephrotoxicity has been found so far [33], those facts suggested the pile-up of colistin toxicity in patients with renal impairment. Forrest et al. [34] also reported that patients with lower kidney function (<80 mL/min) were more vulnerable to AKI, due to colistin exposure. Although many clinical studies observed that AKI happened quite early within 5 to 7 days of colistin initiation [35], our result, on the other hand, revealed that the chance of having AKI risk was higher at the end of treatment than this at day 7. Therefore, we suggested less aggressive approaches than those in EMA and Nation’s recommendation for patients with renal damage.
Given the poor performance of colistin in the upper ranges of CrCl, the questions were whether increasing colistin dose was appropriate and how high was enough. In our study, in the groups of CrCl higher than 50 mL/min, while the maximum approved dose from US-FDA, EMA, and Nation was from 230 to 360 mg CBA/day (EMA recommended 400 mg CBA in some cases), our tested doses were up to 600 mg CBA daily. More than 80% PTA was achieved with 480 mg CBA and 360 mg CBA daily for two groups of 80–100 mL/min and 51–79 mL/min, respectively. However, in the group of CrCl 101–130 mL/min, 74.72% was the maximum PTA achieved with 600 mg CBA daily. Similarly, Jitaree et al. required high doses of 450 to 540 mg CBA for 80% PTAs with CrCl ≥ 80 mL/min [30]. Considerably, the lack of a safety profile for these doses was the limitation. Moreover, at some point, the dose escalation was not directly proportional to the PTA increment. At MIC of 2 mg/L, when we increased the TDD by 33% (from 300 mg to 400 mg), the PTA raised 38.4%. However, when the dose was from 400 mg to 600 mg (50 %increase), only 23.4% of PTA increment was seen. Therefore, weighing between risk and benefit, the use of a high dose should be carefully considered together with the follow-up of renal function and antibiotic combination (if available).
An interesting finding in our study was the effect of the dosing interval. Theoretically, the regimens with the same daily dose would yield similar AUC. However, in our study, different PTAs were found in some regimens with various dosing intervals. For example, in the group of CrCl 101–130 mL/min, at MIC of 2 mg/mL around 5% PTA difference was seen between 100 mg every 6 h and 200 mg every 12 h. Or in the group of 80–100 mL/min, 3% higher PTA was displayed in 100 mg every 8 h compared to 150 mg every 12 h. Even 400 mg daily in a six-hour interval could produce more efficient PTA with 2% higher than that of 450 mg daily in an eight-hour interval. This difference was less visible along with the reduction of CrCl. Around 0.5% to 1.85% differences were seen in other ranges from 1 mL/min to 79 mL/min. Although observed by Jitaree et al. [30], this finding has been firstly reported in our study so far. In order to explain this discrepancy, we tried to look deeper into the PKPD characteristics of CMS and colistin. As a transformed product of CMS, basically, the formed colistin concentration would be increased when we used higher CMS dose. However, when comparing the rate constants k10 and k13 of the two processes of renal elimination and colistin conversion of CMS, respectively, we found that the elimination phase was predominant. From our simulation result, in the group of CrCl 101–130 mL/min, k10 (0.55 h−1, 0.05–8.6) was more than two times higher than k13 (0.22 h−1, 0.02–1.66). Therefore, when we increased the CMS dose, the ratio of CMS renal excretion was much higher than the ratio of colistin conversion. Furthermore, following the first-order kinetic, CMS concentration would be of a half reduction after one half-life time (i.e., 4.5 h). Therefore, the higher the concentration was, the more CMS would be eliminated. When we fractionated the daily dose into small parts with more frequent administration, we were trying to flatten the CMS curve with lower concentration. Consequently, the elimination amount was reduced and more formed colistin was produced. On the other hand, in the group of CrCl 11–29 mL/min, k10 and k13 were similar with 0.29 h−1 (0.03–1.6) and 0.23 h−1 (0.02–1.45), thus, the PTA difference was less significant. Secondly, according to Smith et al. [36], the ratio of half-life time (t1/2) and interval (τ) impacted the steady-state concentration. The bigger the ratio of t1/2/τ was the higher the accumulation was. Hence, accumulated CMS would reduce according to the extension of the dosing interval (using the same TDD). Those reasons somehow made our findings logical and feasible. Notably, those differences were seen mostly in MIC range of 1 to 4 mg/L, where the most uncertainty of colistin efficacy could show up. At MIC ≤ 0.5 mg/L or ≥ 8 mg/L, at which PTA achievement was either too easy or too difficult, respectively, the distinguishment was barely seen. Therefore, utilizing the advantage of a dosing interval was another strategy to improve colistin performance around the MIC breakpoint (i.e., 2 mg/L) for patients with potent renal function.
On the basis of the results of the study, we proposed three dosing schemes at different MICs. The first one was at MIC of 0.5 mg/L which was also the MIC50/90 of our population. At this point, all regimens were chosen according to CFR more than 90%. At MIC of 1 and 2 mg/L, our PTA target was 80% upward. At MIC of 2 mg/L, our study and many others proved that a high PTA was difficult to achieve [30,37]. Any effort to increase PTA might result in arising toxicities rather than benefits. Therefore, weighing the risk and benefit, a PTA target of 80% could be appropriate for MDR P. aeruginosa treatment. Furthermore, the nephrotoxicity rate of colistin was reported from 20–76% in clinical research [33,38,39]. However, this side effect is reversible when discontinuing colistin use. Therefore, balancing the toxicity risk and the severity condition in critically ill patients, at some point, we decided to use high dose colistin with a relatively high chance of AKI development.
When comparing with the recommendations from Jitaree et al., our suggestions were mostly lower. This contributed to the different pharmacodynamics targets. Jitaree et al. studied infection by K. pneumoniae and E. coli with a target of fAUC/MIC ≥ 25. While our target was MDR P. aeruginosa with fAUC/MIC ≥10. Additionally, our PTA target was also lower than the target in Jitaree et al. (80% versus 90%). This difference suggested that the colistin dose was not the same for various GNB treatments, and a common regimen was no longer appropriate. A low dose regimen if possible should be highly recommended for its benefit.
Limitation of Our Study
Our study has some limitations. Firstly, we only assessed the target of 1 log kill from the mouse thigh infection model. Therefore, our result should not be interpreted in the context of lung infection. Secondly, the method to perform microbiologic results (Vitek 2) and the population (all types of infection and patient) might underestimate the real MIC in critically ill patients. Finally, all regimens based on CFR are very specific to a certain health-care setting. As a result, they should not be used widely without considering the various MIC distributions.
5. Conclusions
Our study was successful in comparing the conventional dosing regimens and our own suggestion of colistin against P. aeruginosa in our local microbiological data of the critically ill patient population. The use of fractionated colistin regimens with high doses (and combination therapy, if possible) was recommended, especially for patients with competent renal function. For patients with low colistin MIC or reduced kidney function, less aggressive strategies should be considered to balance the risks and benefits. With further investigation, our regimen at MIC of 0.5 mg/L (MIC90) could be adapted as the empiric therapy for P. aeruginosa treatment (except for the lung infection) in critically ill patients in the studied hospital to avoid unnecessarily high doses.
Supplementary Materials
The following are available at https://www.mdpi.com/article/10.3390/antibiotics10050595/s1, Table S1: PTA of different regimens in CrCl range of 1–10 mL/min, Table S2: PTA of different regimens in CrCl range of 11–29 mL/min, Table S3: PTA of different regimens in CrCl range of 30–50 mL/min, Table S4: PTA of different regimens in CrCl range of 51–79 mL/min, Table S5: PTA of different regimens in CrCl range of 80–100 mL/min, Table S6: PTA of different regimens in CrCl range of 101–130 mL/min.- These are the results of % PTA acquired in different ranges of CrCl.
Author Contributions
Conceptualization and methodology, V.T.K.N., P.M., J.H., and P.T.; software, V.T.K.N., P.M., and J.H.; validation, P.M., and P.T.; formal analysis, V.T.K.N.; investigation, V.T.K.N.; resources, V.T.K.N., P.M., and M.F.S.; data curation, V.T.K.N. and M.F.S.; writing—original draft preparation, V.T.K.N.; writing—review and editing, V.T.K.N. and P.M.; visualization, V.T.K.N.; supervision, P.M. and P.T.; project administration, V.T.K.N., P.M., and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Dentistry/Faculty of Pharmacy, Mahidol University (COA. No. Mu-DT/PY-IRB 2020/013.1702, on 17 February 2020).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Nosocomial Infections Surveillance System Report, Data Summary from January 1992 through June 2004, Issued October 2004. Am. J. Infect. Control. 2004;32:470–485. doi: 10.1016/j.ajic.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Park S.-Y., Park H.J., Moon S.M., Park K.-H., Chong Y.P., Kim M.-N., Kim S.-H., Lee S.-O., Kim Y.S., Woo J.H. Impact of adequate empirical combination therapy on mortality from bacteremic pseudomonas aeruginosa pneumonia. BMC Infect. Dis. 2012;12:308. doi: 10.1186/1471-2334-12-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peña C., Cabot G., Gómez-Zorrilla S., Zamorano L., Ocampo-Sosa A., Murillas J., Almirante B., Pomar V., Aguilar M., Granados A. Influence of virulence genotype and resistance profile in the mortality of pseudomonas aeruginosa bloodstream infections. Clin. Infect. Dis. 2014;60:539–548. doi: 10.1093/cid/ciu866. [DOI] [PubMed] [Google Scholar]
- 4.Lambert M.-L., Suetens C., Savey A., Palomar M., Hiesmayr M., Morales I., Agodi A., Frank U., Mertens K., Schumacher M. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to european intensive-care units: A cohort study. Lancet Infect. Dis. 2011;11:30–38. doi: 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Chen X.-L., Huang A.-W., Liu S.-L., Liu W.-J., Zhang N., Lu X.-Z. Mortality attributable to carbapenem-resistant pseudomonas aeruginosa bacteremia: A meta-analysis of cohort studies. Emerg. Microbes Infect. 2016;5:e27. doi: 10.1038/emi.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matos E.C.O.D., Andriolo R.B., Rodrigues Y.C., Lima P.D.L.D., Carneiro I.C.D.R.S., Lima K.V.B. Mortality in patients with multidrug-resistant pseudomonas aeruginosa infections: A meta-analysis. Rev. Soc. Bras. Med. Trop. 2018;51:415–420. doi: 10.1590/0037-8682-0506-2017. [DOI] [PubMed] [Google Scholar]
- 7.Tabak Y.P., Merchant S., Ye G., Vankeepuram L., Gupta V., Kurtz S.G., Puzniak L.A. Incremental clinical and economic burden of suspected respiratory infections due to multi-drug-resistant pseudomonas aeruginosa in the united states. J. Hosp. Infect. 2019;103:134–141. doi: 10.1016/j.jhin.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Antibiotic Resistance Threats in the United States. [(accessed on 22 May 2020)];2013 Available online: www.cdc.gov/drugresistance/threat-report-2013.
- 9.Centers for Disease Control and Prevention Antibiotic Resistance Threats in the United States. [(accessed on 22 May 2020)];2019 Available online: www.cdc.gov/DrugResistance/Biggest-Threats.html.
- 10.Li J., Nation R.L., Turnidge J.D., Milne R.W., Coulthard K., Rayner C.R., Paterson D.L. Colistin: The re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect. Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 11.Biedenbach D.J., Giao P.T., Hung Van P., Su Minh Tuyet N., Thi Thanh Nga T., Phuong D.M., Vu Trung N., Badal R.E. Antimicrobial-resistant pseudomonas aeruginosa and acinetobacter baumannii from patients with hospital-acquired or ventilator-associated pneumonia in vietnam. CLIN. 2016;38:2098–2105. doi: 10.1016/j.clinthera.2016.07.172. [DOI] [PubMed] [Google Scholar]
- 12.Phu V.D., Wertheim H.F., Larsson M., Nadjm B., Dinh Q.D., Nilsson L.E., Rydell U., Le T.T., Trinh S.H., Pham H.M., et al. Burden of hospital acquired infections and antimicrobial use in vietnamese adult intensive care units. PLoS ONE. 2016;11:e0147544. doi: 10.1371/journal.pone.0147544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran G.M., Ho-Le T.P., Ha D.T., Tran-Nguyen C.H., Nguyen T.S.M., Pham T.T.N., Nguyen T.A., Nguyen D.A., Hoang H.Q., Tran N.V., et al. Patterns of antimicrobial resistance in intensive care unit patients: A study in vietnam. BMC Infect. Dis. 2017;17:429. doi: 10.1186/s12879-017-2529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngoc Van T.T., Quang-Thinh T., Cù T., Nhac Vu H.T. Investigation of the antibiotic resistance: The case of buu dien general hospital in ho chi minh city. Int J Pharm Pharm Sci. 2019;9:116–119. doi: 10.22159/ijpps.2019v11i7.32913. [DOI] [Google Scholar]
- 15.European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of Mics and Zone Diameters. [(accessed on 22 January 2021)];Version 10.0. 2020 Available online: https://www.eucast.org/clinical_breakpoints/
- 16.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. CLSI Supplement m100. [Google Scholar]
- 17.Nation R.L., Garonzik S.M., Thamlikitkul V., Giamarellos-Bourboulis E.J., Forrest A., Paterson D.L., Li J., Silveira F.P. Dosing guidance for intravenous colistin in critically-ill patients. Clin. Infect. Dis. 2017;64:565–571. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plachouras D., Karvanen M., Friberg L.E., Papadomichelakis E., Antoniadou A., Tsangaris I., Karaiskos I., Poulakou G., Kontopidou F., Armaganidis A., et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2009;53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garonzik S., Li J., Thamlikitkul V., Paterson D., Shoham S., Jacob J., Silveira F., Forrest A., Nation R. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011;55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheah S.E., Wang J., Nguyen V.T., Turnidge J.D., Li J., Nation R.L. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against pseudomonas aeruginosa and acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 2015;70:3291–3297. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 21.Jaruratanasirikul S., Thengyai S., Wongpoowarak W., Wattanavijitkul T., Tangkitwanitjaroen K., Sukarnjanaset W., Jullangkoon M., Samaeng M. Population pharmacokinetics and monte carlo dosing simulations of meropenem during the early phase of severe sepsis and septic shock in critically ill patients in intensive care units. Antimicrob. Agents Chemother. 2015;59:2995–3001. doi: 10.1128/AAC.04166-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wicha S.G., Haak T., Zink K., Kees F., Kloft C., Kees M.G. Population pharmacokinetics and target attainment analysis of moxifloxacin in patients with diabetic foot infections. J. Clin. Pharmacol. 2015;55:639–646. doi: 10.1002/jcph.464. [DOI] [PubMed] [Google Scholar]
- 23.Canut A., Isla A., Rodríguez-Gascón A. Pharmacokinetic/pharmacodynamic analysis to evaluate ceftaroline fosamil dosing regimens for the treatment of community-acquired bacterial pneumonia and complicated skin and skin-structure infections in patients with normal and impaired renal function. Int. J. Antimicrob. Agents. 2015;45:399–405. doi: 10.1016/j.ijantimicag.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Sorli L., Luque S., Grau S., Berenguer N., Segura C., Montero M.M., Alvarez-Lerma F., Knobel H., Benito N., Horcajada J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013;13:380. doi: 10.1186/1471-2334-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fda Approved Drug Products Label and Approval History for Coly-Mycin m, nda 050108. [(accessed on 1 May 2021)]; Available online: https://www.Accessdata.Fda.Gov/scripts/cder/daf/index.Cfm?Event=overview.Process&applno=050108.
- 26.European Medicines Agency Polymyxin-containing Medicines. [(accessed on 1 May 2021)]; Available online: https://www.ema.europa.eu/en/medicines/human/referrals/polymyxin-containing-medicines.
- 27.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 28.Palavutitotai N., Jitmuang A., Tongsai S., Kiratisin P., Angkasekwinai N. Epidemiology and risk factors of extensively drug-resistant pseudomonas aeruginosa infections. PLoS ONE. 2018;13:e0193431. doi: 10.1371/journal.pone.0193431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen K.V., Thi Do N.T., Chandna A., Nguyen T.V., Pham C.V., Doan P.M., Nguyen A.Q., Thi Nguyen C.K., Larsson M., Escalante S., et al. Antibiotic use and resistance in emerging economies: A situation analysis for viet nam. BMC Public Health. 2013;13:1158. doi: 10.1186/1471-2458-13-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jitaree K., Sathirakul K., Houngsaitong J., Asuphon O., Saelim W., Thamlikitkul V., Montakantikul P. Pharmacokinetic/pharmacodynamic (pk/pd) simulation for dosage optimization of colistin against carbapenem-resistant klebsiella pneumoniae and carbapenem-resistant escherichia coli. Antibiotics. 2019;8:125. doi: 10.3390/antibiotics8030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregoire N., Mimoz O., Megarbane B., Comets E., Chatelier D., Lasocki S., Gauzit R., Balayn D., Gobin P., Marchand S., et al. New colistin population pharmacokinetic data in critically ill patients suggesting an alternative loading dose rationale. Antimicrob. Agents Chemother. 2014;58:7324–7330. doi: 10.1128/AAC.03508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nation R.L., Garonzik S.M., Li J., Thamlikitkul V., Giamarellos-Bourboulis E.J., Paterson D.L., Turnidge J.D., Forrest A., Silveira F.P. Updated us and european dose recommendations for intravenous colistin: How do they perform? Clin. Infect. Dis. 2016;62:552–558. doi: 10.1093/cid/civ964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y.J., Wi Y.M., Kwon Y.J., Kim S.R., Chang S.H., Cho S. Association between colistin dose and development of nephrotoxicity. Crit. Care Med. 2015;43:1187–1193. doi: 10.1097/CCM.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 34.Forrest A., Garonzik S.M., Thamlikitkul V., Giamarellos-Bourboulis E.J., Paterson D.L., Li J., Silveira F.P., Nation R.L. Pharmacokinetic/toxicodynamic analysis of colistin-associated acute kidney injury in critically ill patients. Antimicrob. Agents Chemother. 2017;61:e01367-17. doi: 10.1128/AAC.01367-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miano T.A., Lautenbach E., Wilson F.P., Guo W., Borovskiy Y., Hennessy S. Attributable risk and time course of colistin-associated acute kidney injury. Clin. J. Am. Soc. Nephrol. 2018;13:542–550. doi: 10.2215/CJN.06980717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith D.A., Beaumont K., Maurer T.S., Di L. Relevance of half-life in drug design. J. Med. Chem. 2018;61:4273–4282. doi: 10.1021/acs.jmedchem.7b00969. [DOI] [PubMed] [Google Scholar]
- 37.Tsuji B.T., Pogue J.M., Zavascki A.P., Paul M., Daikos G.L., Forrest A., Giacobbe D.R., Viscoli C., Giamarellou H., Karaiskos I., et al. International consensus guidelines for the optimal use of the polymyxins: Endorsed by the american college of clinical pharmacy (accp), european society of clinical microbiology and infectious diseases (escmid), infectious diseases society of america (idsa), international society for anti-infective pharmacology (isap), society of critical care medicine (sccm), and society of infectious diseases pharmacists (sidp) Pharmacotherapy. 2019;39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelesidis T., Falagas M.E. The safety of polymyxin antibiotics. Expert Opin. Drug Saf. 2015;14:1687–1701. doi: 10.1517/14740338.2015.1088520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigatto M.H., Behle T.F., Falci D.R., Freitas T., Lopes N.T., Nunes M., Costa L.W., Zavascki A.P. Risk factors for acute kidney injury (aki) in patients treated with polymyxin b and influence of aki on mortality: A multicentre prospective cohort study. J. Antimicrob. Chemother. 2015;70:1552–1557. doi: 10.1093/jac/dku561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.