Abstract
Exposure to air pollution has been suggested to be associated with an increased risk of women’s health disorders. However, it remains unknown to what extent changes in ambient air pollution affect gynecological cancer. In our case–control study, the logistic regression model was combined with the restricted cubic spline to examine the association of short-term exposure to air pollution with gynecological cancer events using the clinical data of 35,989 women in Beijing from December 2008 to December 2017. We assessed the women’s exposure to air pollutants using the monitor located nearest to each woman’s residence and working places, adjusting for age, occupation, ambient temperature, and ambient humidity. The adjusted odds ratios (ORs) were examined to evaluate gynecologic cancer risk in six time windows (Phase 1–Phase 6) of women’s exposure to air pollutants (PM2.5, CO, O3, and SO2) and the highest ORs were found in Phase 4 (240 days). Then, the higher adjusted ORs were found associated with the increased concentrations of each pollutant (PM2.5, CO, O3, and SO2) in Phase 4. For instance, the adjusted OR of gynecological cancer risk for a 1.0-mg m−3 increase in CO exposures was 1.010 (95% CI: 0.881–1.139) below 0.8 mg m−3, 1.032 (95% CI: 0.871–1.194) at 0.8–1.0 mg m−3, 1.059 (95% CI: 0.973–1.145) at 1.0–1.4 mg m−3, and 1.120 (95% CI: 0.993–1.246) above 1.4 mg m−3. The ORs calculated in different air pollution levels accessed us to identify the nonlinear association between women’s exposure to air pollutants (PM2.5, CO, O3, and SO2) and the gynecological cancer risk. This study supports that the gynecologic risks associated with air pollution should be considered in improved public health preventive measures and policymaking to minimize the dangerous effects of air pollution.
Keywords: air pollutant exposure, gynecologic cancer risk, association
1. Introduction
Air pollution is a serious problem in mainland China. In the East China wide haze events in January 2013, the highest hourly concentration of PM2.5 exceeded 1000 μg/m3 in Beijing [1]. Pollution is the largest environmental cause of disease and death in the world today. Besides causing respiratory diseases [2], air pollution has been suggested to be associated with skin diseases [3,4], pregnancy abortion [5], and cardiovascular disease [6,7]. Moreover, prolonged exposures to hazardous air pollutants may result in cancers [8,9,10] and chronic diseases [11,12]. Concern is growing that exposures associated with air pollution might contribute to women’s health, which is one of the most significant public health issues and a global priority.
In light of the existing studies [13,14], gynecological diseases are threatening the health of women all around the world with more than 100,000 women dying every year. Gynecological tumors bring a heavy burden of disease in many countries [15,16], especially in developing and underdeveloped regions [17]. Over the past two decades, the mortality rates of gynecological cancer in China have been increasing and it has become a major health concern for women [18]. Considerable evidence has testified the certain association between ambient air pollution and gynecological tumors, including breast cancer [19,20], ovarian cancer [21], cervical cancer [22], and uterine fibroid [23]. Several studies [21,22] were only conducted in developed countries with good air quality, which were difficult to indicate the relationship between the gynecologic cancer risk and air pollution in developing countries due to the severe air pollution. A study [24] conducted in China’s Shandong province recently estimated the impacts of PM10, O3 [15], and SO2 [25,26] on the gynecologic cancer. The annual average or multi-year average concentration of air pollutants was utilized in previous experiments [19,27] to examine the association of pollutants and cancer. Some clinical evidence supported the suggestion that the exposure to air pollutants will bring about abnormal DNA methylation [28,29], estrogenic action [9], and inflammation from systemic circulation [28,30], whereas the specific mechanism between air pollutants and these diseases is still unknown.
The relationship and the mechanism need to be further assessed based on the accurate exposure estimate on different exposure levels by using reliable data. To guarantee the reliability and validation of the study, two aspects of data are indispensable: that is, strictly managed data of air pollution from the government and clinical data containing detailed patients’ information. For the former, the reliable and relatively complete air pollutant data was released in China after 2013 [31] and it gave us access to use different possible pollutants for quantitative analysis. For the latter, we collected the clinical records of 35,989 women in Beijing from December 2008 to December 2017. Following the previous studies [5,32,33], the measurements from air monitoring stations nearest to the patient’s residence and working place were used as the main estimate for the short-term exposure concentration of patients.
Based on the air pollution data and clinical data, we quantitatively assessed the relationship between the gynecological cancer risk and the short-term exposure (within one year) concentration of particulate matter with diameter below 2.5 μm (PM2.5), carbon monoxide (CO), ozone (O3) and sulfur dioxide (SO2), respectively. To ensure the robustness of the study results, a series of the following analysis were performed: (1) to identify the characteristics of exposure–response association within different time windows; (2) to obtain the shape of the nonlinear association between the risk of gynecologic cancer and air pollutant exposure through the restricted cubic spline analysis we adopted; and (3) to evaluate the exposure–response relationship of each air pollutant in different concentration ranges.
2. Methods
2.1. Data
2.1.1. Air Pollution Data
Hourly measurements of air pollutants included PM10, PM2.5, SO2, CO, NO2, and O3 from 34 monitoring stations were established in 2013 by the Ministry of Ecology and Environment (MEE, formally the Ministry of Environmental Protection). Since the dataset before June 2014 contained a large number of missing values, we only used the data from June 2014 through December 2017 in this study. The NO2 measurements were not used here due to concerns about contamination by other nitrogen species [34] and we did not get data of other NOx measurements. We excluded the PM10 data, which contained a large number of missing values throughout the years. Daily concentrations of pollutants were computed as the 24-h mean based on hourly measurements. To estimate pollution exposure, pollution measurement stations were selected based on the working and residential addresses of each mother [5].
To extend the PM2.5 data time period previous to June 2014, we made use of the long-term measurements taken by the US Embassy (http://www.stateair.net/web/historical/1/1.html; one site only; accessed on 12 July 2018). The US Embassy data were shown to be consistent with the MEE measurements [35]. For data at each MEE site from June 2014 to December 2017, we established a linear relationship with the US Embassy data on an hourly basis. Measurements at each MEE site were highly correlated with those at the US Embassy site [5]. This allowed us to apply the linear relationship to prior periods when there were no MEE measurements, as done in [5] and here. Using only data from June 2014 through 2017 suggested a similar association between air pollution exposures and gynecologic cancer risks, which supported our use of US Embassy data for earlier times [5].
2.1.2. Meteorological Measurement Data
Three-hourly data over 2009–2017 for air temperature (in °C) and RH (in %) at two meters above the ground were taken from the meteorological measurement station near the southwestern Fourth Ring Road of Beijing. Data at this station are reported to the World Meteorological Organization and maintained at the United States National Oceanic and Atmospheric Administration National Centers for Environment Information (NOAA NCEI) (https://www.ncdc.noaa.gov/isd/data-access; accessed on 14 September 2020). Daily mean air temperature and RH were derived from three-hourly data.
2.1.3. Interpolation of Missing Air Pollutants and Meteorological Data
The air pollution and meteorological data contained missing values at certain times. To fill in the missing meteorological or air pollution data to accurately quantify the exposure level of each participating woman, we interpolated the missing values using the same interpolation methods as our previous study did [36].
2.1.4. Women’s Clinical Data
We collected, processed, and selected clinical data as follows.
Collection of clinical data: We collected clinical data of 54,043 women with gynecological diseases (10,502 women was diagnosed with gynecological cancer) in Beijing, China.
Data screening: Women were asked to provide the address they lived at the longest half a year before diagnosis. Women were excluded from the current study if there were no records of their addresses. We did not account for women’s smoking status, since most Chinese women do not smoke, especially when they feel physically sick.
Basic statistics of finally selected data: After all the aforementioned exclusions, data for a total of 35,989 women in Beijing from 2009 through 2017 were valid for analysis. The selected information includes age, occupation, diagnosis date, and whether the woman was diagnosed with cancer.
Moreover, we divided the whole data into several subgroups based on the selected sociodemographic characteristics from the clinical data, namely age and occupation, which was also considered in some existing studies [37,38].
2.2. Assessment of 35,989 Patients’ Air Pollutant Exposure
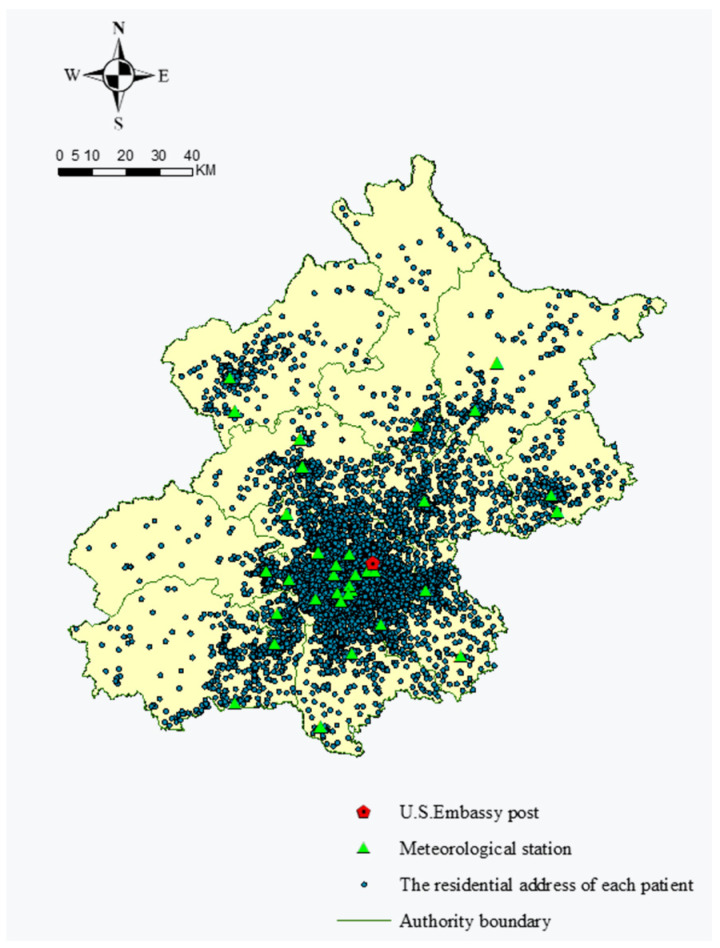
The studied women’s residential and working district addresses and air quality monitoring stations were geocoded to obtain their latitudes and longitudes. We estimated women’s exposure to air pollution by attributing representative concentrations provided by the air quality monitoring stations closest to the women residence and working place after geolocalization. The spatial distributions of 34 air quality monitoring stations and residential places of patients in Beijing are shown in Figure 1.
Figure 1.
Spatial distribution of 35,989 patients’ residence and 34 air quality monitoring stations.
Approximately 86% participating women provided the working addresses. For each participating woman, the exposure concentration of Cd was computed as Cd = Cdw/3 + 2Cdr/3, where Cdw and Cdr denote air pollutant concentrations at the air monitoring stations closest to the participants’ working and residential addresses, respectively. The weights (1/3 and 2/3) approximately accounted for the times a participating woman spent at work and at home. For the other 14% of participating women who did not provide work addresses, we assumed that they did not go to work, and we only used their residence addresses to estimate the pollution exposure.
To define the period of pollution exposure, we examined six time windows of women’s exposure to air pollution (Phase 1–Phase 6), each from 60, 120, 180, 240, 300, and 360 days before diagnosis of gynecologic cancer. We calculated the mean daily concentrations (i.e., the average of 24-h average across multiple days) of the pollutants in different periods (Phase 1, Phase 2, etc.) a woman was exposed.
2.3. Variables Selection
According to several previous studies [39,40], two-sided Student’s t-tests were usually performed to find the possible risk factors of gynecological cancer. Nonetheless, the normally distributed data are the critical condition for t-tests, so we needed to use the Kolmogorov–Smirnov test (K-S test) to identify the distribution of each factor among women. It is noted that the one-year average concentration of PM2.5, CO, O3, SO2, temperature, and relative humidity before the diagnosis date were usually used for the above tests [9]. The p-values of the K-S test were all less than 0.01, which shows that each mentioned factor did not follow the normal distribution. Therefore, we applied a Wilcoxon signed-rank test as an alternative to the Student’s t-test, which is a non-parametric statistical hypothesis test, and the distribution of data cannot be assumed to be normally distributed [41].
2.4. The Model
Firstly, we constructed a logistic regression model to evaluate the risk factors that influence gynecologic cancer. Potential confounding factors including age, occupation, ambient temperature, and ambient humidity were controlled in the final logistic regression model. Model results are reported in the form of odds ratios (ORs) [24,39] and their 95% confidence intervals (CIs). In addition, when associating each pollutant with gynecologic cancer, other pollutants were controlled in the logistic regression model [42]. The logistic regression model was formulated as:
| (1) |
where p represents the probability of gynecological cancer risk, β0 is a constant term. β1, β2, …, β8 represent the regression coefficients of independent variables X1, X2,..., X8. X1 is the ambient air temperature, X2 is the relative humidity, X3 is the patients’ age, X4 is the patients’ occupation, X5 is the concentration of PM2.5, X6 is the concentration of CO, X7 is the concentration of O3, and X8 is the concentration of SO2. The OR value of each independent variable is .
Then, in order to quantify the nonlinear relationship between the gynecological cancer risk and air pollutants, we combined logistic regression with restricted cubic spline as done in some previous studies [5,36]. In the restricted cubic spline, the value range of independent variables are divided, and the “node” is used to define the end of one interval and the beginning of the next interval. For example, [a, b] is the range of the independent variable and k nodes are used to divide the dataset into k + 1 intervals, i.e., . The spline function is a group of piecewise smoothly connected polynomials, and adoption of the spline function makes the final fitting curve smooth and continuous. Based on the regression spline, the restricted cubic spline has a constraint that the function is linear within the intervals of [] and [], and is represented by RSC(X):
| (2) |
with:
| (3) |
| (4) |
| (5) |
| (6) |
where x is the value of the continuous exposure variable X, Si is the spline function, βi is the estimator of Si, and k denotes the number of nodes. Four nodes representing the 25th, 50th, 75th, and 95th percentiles of PM2.5 concentration and three nodes representing the 25th, 50th, and 75th percentiles of CO, SO2, and O3 concentration were selected. The explanation for dividing 5 ranges for PM2.5 and 4 ranges for other pollutants is that the monitoring data shows that PM2.5 was the most serious pollutant in Beijing whose average concentration was 119.5 ± 13.8 μg m−3 in 2013 [43].
The final logistic regression model was combined with the restricted cubic splines to assess the exposure–response relationship, and the relationship between the exposure of each air pollutant and the risk of gynecological cancer was estimated through Equation (3).
| (7) |
Since there was strong collinearity between PM2.5 and CO according to our previous study [5], we did not simultaneously control PM2.5 and CO as confounders in the model. That is, when controlling for PM2.5, we did not consider CO, and when controlling CO, we did not consider PM2.5. As we estimated the association between O3 and the gynecological cancer risk, we controlled for CO and SO2 but not PM2.5, given the strong correlation between PM2.5 and CO. Similarly, as we estimated the association between SO2 (CO) and the gynecological cancer risk, we controlled for CO (SO2) and O3 but not PM2.5.
3. Results
3.1. Linking Air Pollution Exposure to Gynecological Cancer Risks
All participants were grouped by age (6 groups), occupation (2 groups), temperature (5 groups), and relative humidity (5 groups). Table 1 shows the characteristics of gynecological non-cancer and cancer cases (age and occupation) and their environmental factors (like PM2.5, CO, SO2, O3, air temperature, and relative humidity). In addition, the Wilcoxon signed-rank test were performed to find the possible risk factors of gynecological cancer (See Section 2.3). The results showed that the differences of PM2.5, CO, O3, temperature, relative humidity, and occupation between the two groups (cancer and non-cancer) were statistically significant (p < 0.05), indicating that these factors are associated with the risk of gynecological cancer.
Table 1.
Results of Wilcoxon signed-rank tests in different characteristics and environmental factors.
| Figure 2 | Subgroup | Non-Cancer | Cancer | p-Values | PM2.5 | CO | O3 | SO2 |
|---|---|---|---|---|---|---|---|---|
| Age (Years) | <25 | 830 (91.6%) | 76 (8.4%) | 0.011 | 0.039 | 0.040 | 0.005 | 0.003 |
| 25–34 | 7192 (92.6%) | 573 (7.4%) | 0.041 | 0.009 | 0.051 | 0.002 | ||
| 35–44 | 9830 (89.3%) | 1178 (10.7%) | 0.001 | 0.012 | 0.032 | 0.027 | ||
| 45–54 | 9236 (81.1%) | 2155 (18.9%) | 0.000 | 0.042 | 0.002 | 0.017 | ||
| 55–65 | 2294 (56.9%) | 1740 (43.1%) | 0.005 | 0.002 | 0.035 | 0.001 | ||
| >65 | 446 (50.4%) | 439 (49.6%) | 0.002 | 0.006 | 0.047 | 0.003 | ||
| Occupation | White collar | 11,180 (88.7%) | 1428 (11.3%) | 0.007 | 0.000 | 0.000 | 0.032 | 0.004 |
| Blue collar | 2245 (78.3%) | 623 (21.7%) | 0.041 | 0.021 | 0.016 | 0.034 | ||
| Temperature (°C) | <8 | 6010 (82.3%) | 1295 (17.7%) | 0.032 | 0.000 | 0.000 | 0.000 | 0.002 |
| 8–12 | 8366 (82.4%) | 1790 (17.6%) | 0.031 | 0.034 | 0.029 | 0.047 | ||
| 12–16 | 5328 (83.8%) | 1029 (16.2%) | 0.001 | 0.015 | 0.016 | 0.005 | ||
| 16–20 | 8109 (83.4%) | 1614 (16.6%) | 0.017 | 0.020 | 0.048 | 0.006 | ||
| >20 | 2015 (82.3%) | 433 (17.7%) | 0.032 | 0.015 | 0.037 | 0.044 | ||
| Relative Humidity (%) | <40 | 691 (85%) | 122 (15%) | 0.018 | 0.024 | 0.031 | 0.039 | 0.003 |
| 40–50 | 9245 (87.3%) | 1349 (12.7%) | 0.012 | 0.024 | 0.021 | 0.025 | ||
| 50–60 | 18,893 (86.2%) | 3027 (13.8%) | 0.008 | 0.007 | 0.019 | 0.020 | ||
| 60–70 | 1832 (81.4%) | 419 (18.6%) | 0.037 | 0.002 | 0.001 | 0.036 | ||
| >70 | 360 (87.6%) | 51 (12.4%) | 0.005 | 0.002 | 0.043 | 0.010 |
Footnote: The air pollution levels in the table were calculated for the past 1 year from the diagnosis date.
3.2. Associations between Gynecological Cancer Risk and Exposure to PM2.5, SO2, O3, and CO
3.2.1. Correlations between Women’s Exposures in Different Time Periods
To determine if there was a difference on the risk of gynecological cancer in different exposure phases and which phase had the greatest risk of gynecological cancer, we considered six time windows of exposure (Phase 1–Phase 6), and the risks of PM2.5, CO, O3, and SO2 on gynecological cancer were quantified in each phase (see Section 2.2).
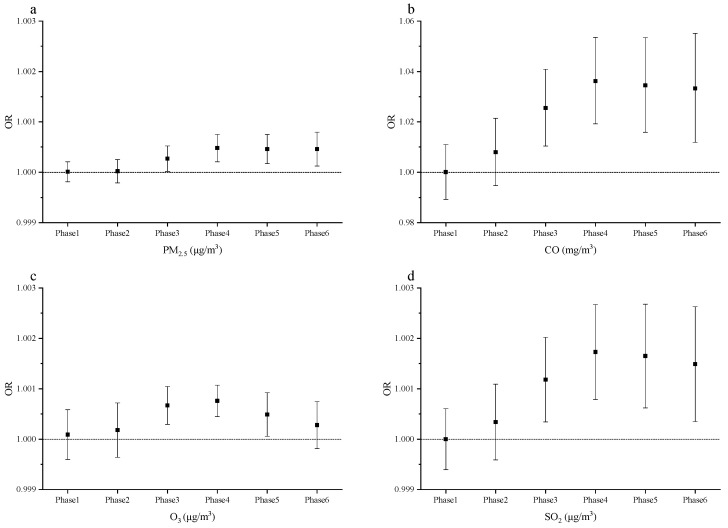
As demonstrated in Figure 2, the association between the risk of gynecological cancer and PM2.5, CO, O3, and SO2 exposures was statistically significant in each phase. Compared with other phases, the risk of PM2.5 exposures in Phase 4 (i.e., the period from 240 days to the participants with diagnosis of gynecological cancer) reaches the peak, and the risks of CO, O3, and SO2 exposures in Phase 4 were higher than the other phases. Thus, the average exposure concentration of each air pollutant in Phase 4 was used for further analysis in the following study.
Figure 2.
The relationship between the concentrations of four air pollutants and the gynecological cancer in six exposure phases. (a) The OR and 95% CI of gynecological cancer with a 1.0 μg/m3 increase in PM2.5 concentration; (b) the OR and 95% CI of gynecological cancer with a 1.0-mg/m3 increase in CO concentration; (c) the OR and 95% CI of gynecological cancer with a 1.0-μg/m3 increase in O3 concentration; (d) the OR and 95% CI of gynecological cancer with a 1.0-μg/m3 increase in SO2 concentration.
3.2.2. Impacts of Air Quality on Gynecological Cancer Risks
The logistic regression model was conducted to analyze four air pollutant exposures in Phase 4, and the relationship between the exposure concentration of four air pollutants and the OR value of gynecological cancer was obtained.
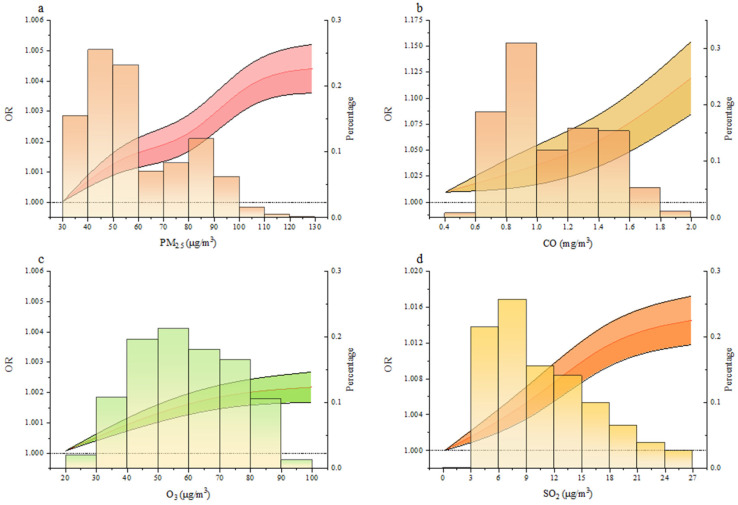
As shown in Figure 3, we combined the logistic regression model and the restricted cubic spline to describe the relationship between each of the air pollutants (PM2.5, SO2, CO, or O3) and the risk of gynecological cancer.
Figure 3.
Exposure–response curve between air pollutant exposure and the risk of gynecological cancer. (a–d), Exposure–response curve of gynecological cancer risk with respect to PM2.5 (a), CO (b), O3 (c), and SO2 (d) exposure. The red line represents the OR value, and the black line denotes the 95% confidence intervals. The histogram shows the distribution of the corresponding pollutant concentration. Here we controlled confounders.
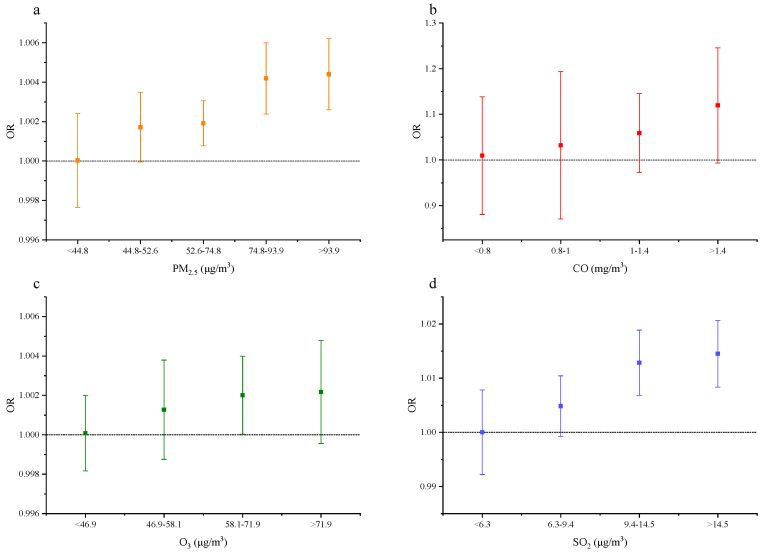
As illustrated in Figure 4, for a 1.0-μg/m3 increase in PM2.5 exposures, the OR of gynecological cancer was 1.000 (95% CI, 0.998–1.002) for PM2.5 of <44.8 μg/m3, 1.002 (95% CI, 1.000–1.003) for PM2.5 of 44.8–52.6 μg/m3, 1.002 (95% CI, 1.001–1.003) for PM2.5 of 52.6–74.8 μg/m3, 1.004 (95% CI, 1.002–1.006) for PM2.5 of 74.8–93.9 μg/m3, and 1.004 (95% CI, 1.003–1.006) for PM2.5 > 93.9 μg/m3.
Figure 4.
Influence of the four air pollutants on the risk of gynecological cancer in different ranges in Phase 4. (a–d), The OR of gynecological cancer with respect to PM2.5 (a), CO (b), O3 (c), and SO2 (d) exposure. The ORs of gynecological cancer with respect to PM2.5, CO, SO2, and O3 exposure. Here we controlled confounders.
For a 1.0 mg/m3 increase in CO exposures, the OR of gynecological cancer is 1.010 (95% CI, 0.881–1.139) for CO <0.8 mg/m3, 1.032 (95% CI, 0.871–1.194) for CO of 0.8–1.0 mg/m3, 1.059 (95% CI, 0.973–1.145) for CO of 1.0–1.4 mg/m3, and 1.120 (95% CI, 0.993–1.246) for CO > 1.4 mg/m3.
For a 1.0-μg/m3 increase in O3 exposures, the OR of gynecological cancer was 1.000 (95% CI, 0.998–1.002) for O3 < 46.9 μg/m3, 1.001 (95% CI, 0.999–1.004) for O3 of 46.9–58.1 μg/m3, 1.002 (95% CI, 1.000–1.004) for O3 of 58.1–71.9 μg/m3, and 1.002 (95% CI, 1.000–1.005) for O3 > 71.9 μg/m3.
For a 1.0-μg/m3 increase in SO2 exposures, the OR of gynecological cancer was 1.000 (95% CI, 0.992–1.008) for SO2 < 6.3 μg/m3, 1.005 (95% CI, 0.999–1.010) for SO2 of 6.3–9.4 μg/m3, 1.013 (95% CI, 1.007–1.019) for SO2 of 9.4–14.5 μg/m3, and 1.015 (95% CI, 1.008–1.021) for SO2 > 14.5 μg/m3.
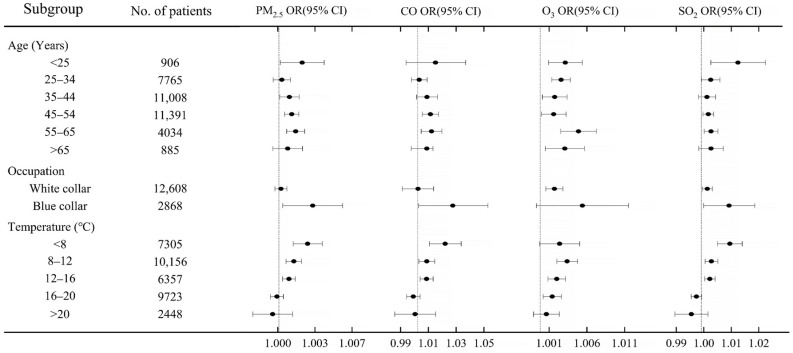
3.3. Associations between Air Pollution and Gynecological Cancer Risks by Sociodemographic Characteristics
Different sociodemographic status might play a crucial role in differentiating air pollution exposure among patients, which result in different risk for gynecological cancers.
As shown in Figure 5, when exposed to air pollution, the elderly patients (>65 years old) and blue-collar patients had a higher risk on gynecological cancer than their counterparts.
Figure 5.
Relationship between air pollution and gynecological cancer with different social and demographic characteristics. The risks of four air pollutants on the risk of gynecological cancers is evaluated in each subgroup with PM2.5, O3, and SO2 increase by 1.0 μg/m3 and CO increase by 1.0 mg/m3.
3.4. Sensitivity Analysis
In the above sections, we assessed the influences of the four air pollutant exposures on the risk of gynecological cancer. Here we estimated the sole effect of each pollutant on gynecological cancer with versus without controlling for other air pollutants.
Considering that we did not have data of SO2, O3, and CO from December 2008 to May 2014, our sensitivity analysis only controlled for PM2.5 for this period. From June 2014 to December 2017, we took other air pollutants (PM2.5, SO2, O3, or CO) as confounders when the relationship between the exposure of one air pollutant and gynecological cancer.
We found that the relationship between each pollutant and gynecological cancer, after controlling other pollutants, were very similar to the relationship without controlling other pollutants as shown in Table 2, Table 3, Table 4 and Table 5, suggesting that the influences of individual factors on gynecological cancer were largely independent.
Table 2.
Influence of PM2.5 on gynecological cancers in Phase 4 with/without controlling other air pollutants.
| PM2.5 (μg/m3) | ORs (Jun. 2014–Dec. 2017, Controlling for SO2 and O3) | ORs (Dec. 2008–Dec. 2017, without Controlling Other Pollutants) | ORs (Jun. 2014–Dec. 2017, without Controlling Other Pollutants) |
|---|---|---|---|
| <44.8 | 1.000 (0.998, 1.002) | 1.000 (0.998, 1.002) | 1.000 (0.998, 1.002) |
| 44.8–52.6 | 1.002 (1.000, 1.003) | 1.002 (1.000, 1.004) | 1.002 (1.000, 1.004) |
| 52.6–74.8 | 1.002 (1.001, 1.003) | 1.004 (1.003, 1.005) | 1.002 (1.001, 1.003) |
| 74.8–93.9 | 1.004 (1.002, 1.006) | 1.005 (1.003, 1.006) | 1.003 (1.001, 1.005) |
| >93.9 | 1.004 (1.003, 1.006) | 1.005 (1.003, 1.007) | 1.004 (1.003, 1.006) |
Table 3.
Influence of SO2 on gynecological cancers in Phase 4 with/without control of other air pollutants.
| SO2 (μg/m3) | ORs (Jun. 2014–Dec. 2017, Controlling for PM2.5, CO, and O3) | ORs (Jun. 2014–Dec. 2017, without Controlling Other Pollutants) |
|---|---|---|
| <6.3 | 1.000 (0.992, 1.008) | 1.001 (0.993, 1.009) |
| 6.3–9.4 | 1.005 (0.999, 1.010) | 1.003 (0.997, 1.008) |
| 9.4–14.5 | 1.013 (1.007, 1.019) | 1.010 (1.004, 1.016) |
| >14.5 | 1.015 (1.008, 1.021) | 1.011 (1.005, 1.018) |
Table 4.
Influence of CO on gynecological cancers in Phase 4 with/without control of other air pollutants.
| CO (mg/m3) | ORs (Jun. 2014–Dec. 2017, Controlling for SO2 and O3) | ORs (Jun. 2014–Dec. 2017, without Controlling Other Pollutants) |
|---|---|---|
| <0.8 | 1.010 (0.881, 1.139) | 1.003 (0.880, 1.125) |
| 0.8–1 | 1.032 (0.871, 1.194) | 1.055 (0.977, 1.133) |
| 1–1.4 | 1.059 (0.973, 1.145) | 1.071 (0.962, 1.180) |
| >1.4 | 1.120 (0.993, 1.246) | 1.122 (0.944, 1.298) |
Table 5.
Influence of O3 on gynecological cancers in Phase 4 with/without control of other air pollutants.
| O3 (μg/m3) | ORs (Jun. 2014–Dec. 2017, Controlling for PM2.5, SO2, and CO) | ORs (Jun. 2014–Dec. 2017, without Controlling Other Pollutants) |
|---|---|---|
| <46.9 | 1.000 (0.998, 1.002) | 1.000 (0.998, 1.002) |
| 46.9–58.1 | 1.001 (0.999, 1.004) | 1.001 (0.999, 1.004) |
| 58.1–71.9 | 1.002 (1.000, 1.004) | 1.002 (1.000, 1.004) |
| >71.9 | 1.002 (1.000, 1.005) | 1.002 (0.999, 1.004) |
4. Discussion
We quantitatively assessed the association between the air pollution exposures and gynecologic cancer risk. The findings indicate the increased risk of gynecologic cancer from exposure to higher concentrations of air pollutants.
Some possible explanations to explain this association is as follows. When exposed to air pollution, the respiratory tract of people is certainly the primary damaged organs, but some studies confirmed that ultrafine particles from air pollutants can migrate through the blood to other organs and cause cancer [44]. Long-term exposure to hazardous environments dominated by air pollutants can induce oxidative stress reaction in cervical cancer cells, consequently damaging DNA and presenting similar symptoms to HPV infection [45], which is similar to the effect of smoking behaviors on the risk of gynecological tumors [22].
Air pollutant such as PM2.5 contain a variety of polycyclic aromatic hydrocarbons (PAHs) and its derivatives which are associated with genetic polymorphisms in the activation of certain carcinogens [25] and steroid hormone metabolism, thereby promoting the proliferation of cancer cells [46]. Substantial studies were in favor of the linkage between the PAHs and breast cancers [46], ovarian cancers [47], cervical epithelial tumors [48], uterine dysplasia [22], reproductive dysfunction, and pathological changes [49,50,51]. What is more, it should be emphasized that the PM2.5 can have the effect of estrogen [52], causing endocrine disorders. When inhaled, toxicants can bypass the liver metabolism and directly enter the systemic circulation [53]. Other air pollutants including CO, O3 were associated with the diseases concerning about fertility such as preeclampsia [54,55,56] and hypertensive disorders of pregnancy [57] in several existing studies. In addition, SO2 can lead to chromosomal aberrations, which can induce reproductive toxicity and carcinogenesis [5].
The findings about disparate exposure risks across different sociodemographic status support our further comparative analysis of these subgroup. In terms of age, it plays an essential role in cancer-related diseases [58,59,60]. The immunity of elderly patients was relatively weaker and might have some underlying diseases [61,62]. Therefore, even exposed to the same concentration of air pollution as young patients, it is likely to bring the elders with higher risk of gynecological cancers. As for occupation, the adverse health effects of air pollution varied depending on the socioeconomic status of patients. In China, compared with white-collar workers who usually work in offices, the female blue-collar workers have a lower socioeconomic status and are more frequently exposed to outdoor work [37], which poses a higher risk of air pollution-induced cancers. This finding is consistent with previous studies that high-income households are less susceptible to the adverse health impacts of exposure to hazardous air pollutants [38,63].
There are a few limitations in our studies. Firstly, there was no study on the effect of indoor air pollution on gynecological cancers due to the lack of indoor pollution data. As a result, we assumed that indoor and outdoor pollution were highly correlated [64]. Since the pollution data from the monitoring stations closest to the women’s residence (2/3 time) and workplace (1/3 time) cannot reflect individual exposure level, the risk of gynecological cancers may be underestimated or overestimated due to the frequency of the window opening and the source of indoor pollution [65]. Although we controlled confounders in the model, the effects of residual confounders were still hard to eliminate, such as smoking behavior. Last but not least, limited by the cognition of biological mechanisms on different gynecological cancer (e.g., breast cancer, ovarian cancer, and cervical cancer) may lead to the possibility of ecological fallacy. Hence, a massive crowd cohort study is necessary and gynecologic cancers in multiple sites on female body can be studied separately to obtain reliable public health evidence. Lastly, there is no consensus on the definition between long-term and short-term exposure to air pollution. Some studies consider the one year as the ‘long term’ [66], and others think it is the ‘short term’ [11,39]. We define the 1-year exposure as the short-term exposure, and our finding supports the association of the risk with short-term air pollution exposures is high. Nevertheless, we fail to conclude the association of the risk with long-term (multi-year) air pollution exposures, which is the limitation of our study.
China and other developing countries are still facing severe air pollution, although some governance policies have been taken in recent years [67]. Integrating the reduction of air pollution into future national policies to improve existing pollution problems is of irreplaceable importance for human health. Our quantitative spatial-temporal studies on the air pollution-induced risk of gynecological cancers contributed to a comprehensive understanding of the relationship between exposure to air pollution and gynecological cancers. Future work will utilize more types of data sources and consider more environmental confounding factors in an effort to characterize the impact of air pollution on human health as accurately as possible.
Author Contributions
Conceptualization, Q.Y.; methodology, Q.Y.; software, Q.Y.; validation, J.L., S.L. and K.H. (Ke Huang); formal analysis, Q.Y.; investigation, K.H. (Kun Hou) and Y.C.; data curation, Q.Y.; writing—original draft preparation, Q.Y.; writing—review and editing, J.L. and S.L.; supervision, L.Z.; project administration, L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China under Grant 2018YFC0213600.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Beijing Obstetrics and Gynecology Hospital (No. 2017-KY-040-01, 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liang Y., Fang L., Pan H., Zhang K., Kan H., Brook J.R., Sun Q. PM2.5 in Beijing-Temporal Pattern and Its Association with Influenza. Environ. Health Glob. Access Sci. Source. 2014;13:102. doi: 10.1186/1476-069X-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel S.P., Cockburn M., Shu Y.-H., Deng H., Lurmann F.W., Liu L., Gilliland F.D. Air Pollution Affects Lung Cancer Survival. Thorax. 2016;71:891–898. doi: 10.1136/thoraxjnl-2015-207927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.English J.S.C., Dawe R.S., Ferguson J. Environmental Effects and Skin Disease. Br. Med. Bull. 2003;68:129–142. doi: 10.1093/bmb/ldg026. [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith L.A. Skin Effects of Air Pollution. Otolaryngol. Neck Surg. 1996;114:217–219. doi: 10.1016/S0194-5998(96)70169-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L., Liu W., Hou K., Lin J., Zhou C., Tong X., Wang Z., Wang Y., Jiang Y., Wang Z., et al. Air Pollution-Induced Missed Abortion Risk for Pregnancies. Nat. Sustain. 2019;2:1011–1017. doi: 10.1038/s41893-019-0387-y. [DOI] [Google Scholar]
- 6.Franklin B.A., Brook R., Pope C.A., III Air Pollution and Cardiovascular Disease. Curr. Probl. Cardiol. 2015;40:207–238. doi: 10.1016/j.cpcardiol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Pun V.C., Kazemiparkouhi F., Manjourides J., Suh H.H. Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. Am. J. Epidemiol. 2017;186:961–969. doi: 10.1093/aje/kwx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raaschou-Nielsen O., Ketzel M., Harbo Poulsen A., Sørensen M. Traffic-related Air Pollution and Risk for Leukaemia of an Adult Population. Int. J. Cancer. 2016;138:1111–1117. doi: 10.1002/ijc.29867. [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Xie P., Xu Y., Kettrup A., Schramm K.-W. Differing Estrogen Activities in the Organic Phase of Air Particulate Matter Collected during Sunny and Foggy Weather in a Chinese City Detected by a Recombinant Yeast Bioassay. Atmos. Environ. 2004;38:6157–6166. doi: 10.1016/j.atmosenv.2004.07.027. [DOI] [Google Scholar]
- 10.Loomis D., Grosse Y., Lauby-Secretan B., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Baan R., Mattock H., Straif K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–1263. doi: 10.1016/S1470-2045(13)70487-X. [DOI] [PubMed] [Google Scholar]
- 11.Chen H., Goldberg M.S., Villeneuve P.J. A Systematic Review of Relation between Long-Term Exposure to Ambient Air Pollution and Chronic Disease: On-Line Appendix. TSP. 2008;1:10–99. doi: 10.1515/reveh.2008.23.4.243. [DOI] [PubMed] [Google Scholar]
- 12.To T., Zhu J., Villeneuve P.J., Simatovic J., Feldman L., Gao C., Williams D., Chen H., Weichenthal S., Wall C., et al. Chronic Disease Prevalence in Women and Air Pollution—A 30-Year Longitudinal Cohort Study. Environ. Int. 2015;80:26–32. doi: 10.1016/j.envint.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer GLOBOCAN 2008: Cancer Incidence and Mortality Worldwide in 2008. [(accessed on 14 May 2021)]; Available online: http://globocan.iarc.fr/
- 14.Adami H.O., Hunter D., Trichopoulos D. Textbook of Cancer Epidemiology. Oxford University Press; New York City, NY, USA: 2009. [DOI] [Google Scholar]
- 15.Parkin D.M., Bray F., Ferlay J., Pisani P. Estimating the World Cancer Burden: Globocan 2000. Int. J. Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 16.Sankaranarayanan R., Ferlay J. Worldwide Burden of Gynecological Cancer. In: Preedy V.R., Watson R.R., editors. Handbook of Disease Burdens and Quality of Life Measures. Springer; New York, NY, USA: 2010. pp. 803–823. [DOI] [Google Scholar]
- 17.Kamangar F., Dores G.M., Anderson W.F. Patterns of Cancer Incidence, Mortality, and Prevalence across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X., Tang H., Chen T. Epidemiology of Gynecologic Cancers in China. J. Gynecol. Oncol. 2018;29:1–7. doi: 10.3802/jgo.2018.29.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh S., Von Euler-Chelpin M., Raaschou-Nielsen O., Hertel O., Tjønneland A., Lynge E., Vejborg I., Andersen Z.J. Long-Term Exposure to Air Pollution and Mammographic Density in the Danish Diet, Cancer and Health Cohort. Environ. Health Glob. Access Sci. Source. 2015;14:1–7. doi: 10.1186/s12940-015-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei Y., Davis J., Bina W.F. Ambient Air Pollution Is Associated with the Increased Incidence of Breast Cancer in US. Int. J. Environ. Health Res. 2012;22:12–21. doi: 10.1080/09603123.2011.588321. [DOI] [PubMed] [Google Scholar]
- 21.Shields T., Gridley G., Moradi T., Adami J., Plato N., Dosemeci M. Occupational Exposures and the Risk of Ovarian Cancer in Sweden. Am. J. Ind. Med. 2002;42:200–213. doi: 10.1002/ajim.10099. [DOI] [PubMed] [Google Scholar]
- 22.Scheurer M.E., Danysh H.E., Follen M., Lupo P.J. Association of Traffic-Related Hazardous Air Pollutants and Cervical Dysplasia in an Urban Multiethnic Population: A Cross-Sectional Study. Environ. Health Glob. Access Sci. Source. 2014;13:1–8. doi: 10.1186/1476-069X-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahalingaiah S., Hart J.E., Laden F., Terry K.L., Boynton-Jarrett R., Aschengrau A., Missmer S.A. Air Pollution and Risk of Uterine Leiomyomata. Epidemiology. 2014;25:682–688. doi: 10.1097/EDE.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo Q., Zhang N., Wang X., Jiang L., Ma T., Yang Q. Effects of Ambient Particulate Matter on Human Breast Cancer: Is Xenogenesis Responsible? PLoS ONE. 2013;8:e76609. doi: 10.1371/journal.pone.0076609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang J., Bae H., Choi S., Yi H., Ko B., Kim N. Impact of Air Pollution on Breast Cancer Incidence and Mortality: A Nationwide Analysis in South Korea. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-62200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jedrychowski W., Maugeri U., Bianchi I. Environmental Pollution in Central and Eastern European Countries: A Basis for Cancer Epidemiology. Rev. Environ. Health. 1997;12:1–24. doi: 10.1515/REVEH.1997.12.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Hystad P., Villeneuve P.J., Goldberg M.S., Crouse D.L., Johnson K. Exposure to Traffic-Related Air Pollution and the Risk of Developing Breast Cancer among Women in Eight Canadian Provinces: A Case-Control Study. Environ. Int. 2015;74:240–248. doi: 10.1016/j.envint.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Heijmans B.T., Tobi E.W., Stein A.D., Putter H., Blauw G.J., Susser E.S., Slagboom P.E., Lumey L.H. Persistent Epigenetic Differences Associated with Prenatal Exposure to Famine in Humans. Proc. Natl. Acad. Sci. USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capello F., Gaddi A.V., editors. Clinical Handbook of Air Pollution-Related Diseases. Springer International Publishing; Cham, Switzerland: 2018. [DOI] [Google Scholar]
- 30.Calderón-Garcidueñas L., Engle R., Mora-Tiscareño A.M.A., Styner M., Gómez-Garza G., Zhu H., Jewells V., Torres-Jardón R., Romero L., Monroy-Acosta M.E., et al. Exposure to Severe Urban Air Pollution Influences Cognitive Outcomes, Brain Volume and Systemic Inflammation in Clinically Healthy Children. Brain Cogn. 2011;77:345–355. doi: 10.1016/j.bandc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Loomis D., Huang W., Chen G. The International Agency for Research on Cancer (IARC) Evaluation of the Carcinogenicity of Outdoor Air Pollution: Focus on China. Chin. J. Cancer. 2014;33:189–196. doi: 10.5732/cjc.014.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowe B., Xie Y., Li T., Yan Y., Xian H., Al-Aly Z. The 2016 Global and National Burden of Diabetes Mellitus Attributable to PM2.5 Air Pollution. Lancet Planet. Health. 2018;2:e301–e312. doi: 10.1016/S2542-5196(18)30140-2. [DOI] [PubMed] [Google Scholar]
- 33.Bonner M.R., Han D., Nie J., Rogerson P., Vena J.E., Muti P., Trevisan M., Edge S.B., Freudenheim J.L. Breast Cancer Risk and Exposure in Early Life to Polycyclic Aromatic Hydrocarbons Using Total Suspended Particulates as a Proxy Measure. Cancer Epidemiol. Prev. Biomark. 2005;14:53–60. [PubMed] [Google Scholar]
- 34.Liu M., Lin J., Wang Y., Sun Y., Zheng B., Shao J., Chen L., Zheng Y., Chen J., Fu T.M., et al. Spatiotemporal Variability of NO2 and PM2.5 over Eastern China: Observational and Model Analyses with a Novel Statistical Method. Atmos. Chem. Phys. 2018;18:12933–12952. doi: 10.5194/acp-18-12933-2018. [DOI] [Google Scholar]
- 35.Liang X., Li S., Zhang S., Huang H., Chen S.X. PM2.5 Data Reliability, Consistency, and Air Quality Assessment in Five Chinese Cities. J. Geophys. Res. 2016;121:10220–10236. doi: 10.1002/2016JD024877. [DOI] [Google Scholar]
- 36.Desquilbet L., Mariotti F. Dose-Response Analyses Using Restricted Cubic Spline Functions in Public Health Research. Stat. Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 37.Ou C.Q., Hedley A.J., Chung R.Y., Thach T.Q., Chau Y.K., Chan K.P., Yang L., Ho S.Y., Wong C.M., Lam T.H. Socioeconomic Disparities in Air Pollution-Associated Mortality. Environ. Res. 2008;107:237–244. doi: 10.1016/j.envres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Forastiere F., Stafoggia M., Tasco C., Picciotto S., Agabiti N., Cesaroni G., Perucci C.A. Socioeconomic Status, Particulate Air Pollution, and Daily Mortality: Differential Exposure or Differential Susceptibility. Am. J. Ind. Med. 2007;50:208–216. doi: 10.1002/ajim.20368. [DOI] [PubMed] [Google Scholar]
- 39.Crouse D.L., Goldberg M.S., Ross N.A., Chen H., Labrèche F. Postmenopausal Breast Cancer Is Associated with Exposure to Traffic-Related Air Pollution in Montreal, Canada: A Case-Control Study. Environ. Health Perspect. 2010;118:1578–1583. doi: 10.1289/ehp.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reding K.W., Young M.T., Szpiro A.A., Han C.J., DeRoo L.A., Weinberg C., Kaufman J.D., Sandler D.P. Breast Cancer Risk in Relation to Ambient Air Pollution Exposure at Residences in the Sister Study Cohort. Cancer Epidemiol. Biomark. Prev. 2015;24:1907–1909. doi: 10.1158/1055-9965.EPI-15-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woolson R.F. Wilcoxon Signed-Rank Test. Wiley Encyclopedia of Clinical Trials. Wiley Encycl. Clin. Trials. 2007:1–3. doi: 10.1002/9780471462422.eoct979. [DOI] [Google Scholar]
- 42.Enkhmaa D., Warburton N., Javzandulam B., Uyanga J., Khishigsuren Y., Lodoysamba S., Enkhtur S., Warburton D. Seasonal Ambient Air Pollution Correlates Strongly with Spontaneous Abortion in Mongolia. BMC Pregnancy Childbirth. 2014;14:1–7. doi: 10.1186/1471-2393-14-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan S., Cao H., Chen Y., Wu C., Hong T., Fan H. Spatial and Temporal Characteristics of Air Quality and Air Pollutants in 2013 in Beijing. Environ. Sci. Pollut. Res. 2016;23:13996–14007. doi: 10.1007/s11356-016-6518-3. [DOI] [PubMed] [Google Scholar]
- 44.Raaschou-Nielsen O., Andersen Z.J., Hvidberg M., Jensen S.S., Ketzel M., Sørensen M., Hansen J., Loft S., Overvad K., Tjønneland A. Air Pollution from Traffic and Cancer Incidence: A Danish Cohort Study. Environ. Health. 2011;10:67. doi: 10.1186/1476-069X-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moktar A., Singh R., Vadhanam M.V., Ravoori S., Lillard J.W., Gairola C.G., Gupta R.C. Cigarette Smoke Condensate-Induced Oxidative DNA Damage and Its Removal in Human Cervical Cancer Cells. Int. J. Oncol. 2011;39:941–947. doi: 10.3892/ijo.2011.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brody J.G., Moysich K.B., Humblet O., Attfield K.R., Beehler G.P., Rudel R.A. Environmental Pollutants and Breast Cancer: Epidemiologic Studies. Cancer. 2007;109(Suppl. S12):2667–2711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- 47.Hung L.J., Tsai S.S., Chen P.S., Yang Y.H., Liou S.H., Wu T.N., Yang C.Y. Traffic Air Pollution and Risk of Death from Breast Cancer in Taiwan: Fine Particulate Matter (PM 2.5) as a Proxy Marker. Aerosol. Air Qual. Res. 2012;12:275–282. doi: 10.4209/aaqr.2011.09.0155. [DOI] [Google Scholar]
- 48.Li X., Ding L., Song L., Gao W., Wang L., Wang J. Effects of Exposure to Polycyclic Aromatic Hydrocarbons Combined with High-Risk Human Papillomavirus Infection on Cervical Intraepithelial Neoplasia: A Population Study in Shanxi Province, China. Int. J. Cancer. 2020;146:2406–2412. doi: 10.1002/ijc.32562. [DOI] [PubMed] [Google Scholar]
- 49.Craig Z.R., Wang W., Flaws J.A. Endocrine-Disrupting Chemicals in Ovarian Function: Effects on Steroidogenesis, Metabolism and Nuclear Receptor Signaling. Reproduction. 2011;142:633–646. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- 50.Petroff B.K., Roby K.F., Gao X., Son D.S., Williams S., Johnson D., Rozman K.K., Terranova P.F. A Review of Mechanisms Controlling Ovulation with Implications for the Anovulatory Effects of Polychlorinated Dibenzo-p-Dioxins in Rodents. Toxicology. 2001;158:91–107. doi: 10.1016/S0300-483X(00)00367-X. [DOI] [PubMed] [Google Scholar]
- 51.Hombach-Klonisch S., Pocar P., Kietz S., Klonisch T. Molecular Actions of Polyhalogenated Arylhydrocarbons (PAHs) in Female Reproduction. Curr. Med. Chem. 2012;12:599–616. doi: 10.2174/0929867310504050599. [DOI] [PubMed] [Google Scholar]
- 52.Wernli K.J., Ray R.M., Gao D.L., Fitzgibbons E.D., Camp J.E., Astrakianakis G., Seixas N., Wong E.Y., Li W., De Roos A.J. Occupational Exposures and Ovarian Cancer in Textile Workers. Epidemiology. 2008;19:244–250. doi: 10.1097/EDE.0b013e31816339f9. [DOI] [PubMed] [Google Scholar]
- 53.Perry N.M., Allgood P.C., Duffy S.W., Mokbel K. Exposure to Traffic Emissions throughout Life and Risk of Breast Cancer. Cancer Causes Control. 2008;19:435. doi: 10.1007/s10552-007-9101-x. [DOI] [PubMed] [Google Scholar]
- 54.Wu J., Ren C., Delfino R.J., Chung J., Wilhelm M., Ritz B. Association between Local Traffic-Generated Air Pollution and Preeclampsia and Preterm Delivery in the South Coast Air Basin of California. Environ. Health Perspect. 2009;117:1773–1779. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinikoor-Imler L.C., Gray S.C., Edwards S.E., Miranda M.L. The Effects of Exposure to Particulate Matter and Neighbourhood Deprivation on Gestational Hypertension. Paediatr. Perinat. Epidemiol. 2012;26:91–100. doi: 10.1111/j.1365-3016.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- 56.Wu M., Ries J.J., Proietti E., Vogt D., Hahn S., Hoesli I. Development of Late-Onset Preeclampsia in Association with Road Densities as a Proxy for Traffic-Related Air Pollution. Fetal Diagn. Ther. 2016;39:21–27. doi: 10.1159/000381802. [DOI] [PubMed] [Google Scholar]
- 57.Lee P.-C., Roberts J.M., Catov J.M., Talbott E.O., Ritz B. First Trimester Exposure to Ambient Air Pollution, Pregnancy Complications and Adverse Birth Outcomes in Allegheny County, PA. Matern. Child Health J. 2013;17:545–555. doi: 10.1007/s10995-012-1028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Y., Zeng H., Zheng R., Li S., Barnett A.G., Zhang S., Zou X., Huxley R., Chen W., Williams G. The Association between Lung Cancer Incidence and Ambient Air Pollution in China: A Spatiotemporal Analysis. Environ. Res. 2016;144:60–65. doi: 10.1016/j.envres.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y., Wang S., Aunan K., Seip H.M., Hao J. Air Pollution and Lung Cancer Risks in China-a Meta-Analysis. Sci. Total Environ. 2006;366:500–513. doi: 10.1016/j.scitotenv.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 60.Cheng I., Tseng C., Wu J., Yang J., Conroy S.M., Shariff-Marco S., Li L., Hertz A., Gomez S.L., Le Marchand L., et al. Association between Ambient Air Pollution and Breast Cancer Risk: The Multiethnic Cohort Study. Int. J. Cancer. 2020;146:699–711. doi: 10.1002/ijc.32308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Derhovanessian E., Solana R., Larbi A., Pawelec G. Immunity, Ageing and Cancer. Immun. Ageing. 2008;5:1–16. doi: 10.1186/1742-4933-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiecolt-Glaser J.K., Glaser R. Stress and Immunity: Age Enhances the Risks. Curr. Dir. Psychol. Sci. 2001;10:18–21. doi: 10.1111/1467-8721.00105. [DOI] [Google Scholar]
- 63.Peterson C.E., Rauscher G.H., Johnson T.P., Kirschner C.V., Barrett R.E., Kim S., Fitzgibbon M.L., Joslin C.E., Davis F.G. The Association between Neighborhood Socioeconomic Status and Ovarian Cancer Tumor Characteristics. Cancer Causes Control. 2014;25:633–637. doi: 10.1007/s10552-014-0357-7. [DOI] [PubMed] [Google Scholar]
- 64.El-Hougeiri N., El Fadel M. Correlation of Indoor-Outdoor Air Quality in Urban Areas. Indoor Built Environ. 2004;13:421–431. doi: 10.1177/1420326X04049344. [DOI] [Google Scholar]
- 65.Wallace L., Williams R. Use of Personal-Indoor-Outdoor Sulfur Concentrations to Estimate the Infiltration Factor and Outdoor Exposure Factor for Individual Homes and Persons. Environ. Sci. Technol. 2005;39:1707–1714. doi: 10.1021/es049547u. [DOI] [PubMed] [Google Scholar]
- 66.Zanobetti A., O’Neill M.S. Longer-Term Outdoor Temperatures and Health Effects: A Review. Curr. Epidemiol. Rep. 2018;5:125–139. doi: 10.1007/s40471-018-0150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin Y., Andersson H., Zhang S. Air Pollution Control Policies in China: A Retrospective and Prospects. Int. J. Environ. Res. Public Health. 2016;13:1219. doi: 10.3390/ijerph13121219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.