Abstract
Background:
Serum kynurenic acid is associated with poor outcomes after infant cardiopulmonary bypass (CPB), but comprehensive mapping of the kynurenine pathway (KP) after CPB has yet to be performed.
Aims:
To map changes in KP induced by infant CPB.
Methods:
Compared changes in serum KP metabolites through 48hrs post-op with liquid-chromatography-tandem mass spectrometry.
Results:
Infant CPB results in marked increase in proximal, but not distal metabolites of the KP.
Conclusions:
Infant CPB leads to accumulation of circulating KP metabolites, which have important neurologic & immunologic activities. Thus, further exploration of the KP is warranted in these high-risk infants.
Keywords: Targeted Metabolomics, Kynurenic Acid, Quinolinic Acid, Congenital Heart Disease, Single Ventricle Palliation, Bidirectional Glenn
1. Introduction
Congenital heart disease (CHD) is the most common birth defect in the United States, with an incidence of 4 – 14 per 1,000 live births (Hoffman and Kaplan 2002). Single ventricle heart disease (SVHD) is one of the most severe types of CHD, in which there is one functional pumping chamber, rendering it uniformly fatal without intervention. While there is no cure, SVHD patients have the potential to survive into adulthood with surgical palliation in early childhood. Like other forms of severe CHD, this cohort of patients is particularly vulnerable given their chronic heart failure, cyanosis, and need for multiple surgeries with cardiopulmonary bypass (CPB).
Children undergoing cardiothoracic surgery with CPB endure significant physiologic stress as a result of surgical trauma, activation of inflammatory cascades, and ischemia-reperfusion injury (Kozik and Tweddell 2006). The resulting physiologic derangements increase patients’ risk of prolonged critical illness, organ injury and mortality (Blinder et al. 2012; Shi et al. 2008). Thus, a better understanding of the complex pathophysiology and pathways involved in CHD patients undergoing CPB would enhance our ability to perform perioperative monitoring, prognostication, and targeted interventions.
Metabolomics is a unique tool by which to analyze a dynamic, pathophysiologic state, such as that induced by cardiothoracic surgery (Mussap et al. 2013). In an untargeted, pilot study, Correia et al.(2015) demonstrated substantial shifts in the metabolic profile of infants after cardiac surgery, highlighting the potential of metabolomics in illness stratification and intervention. It follows then that targeted studies could help us further understand disease pathology and prognosis by facilitating the analysis of compounds specific to a pathway of interest. Our group previously sought to expand on Correia’s work using targeted metabolomic fingerprinting to examine global metabolic shifts and pathway derangements in neonates and young infants undergoing cardiac surgery. Among our findings was that kynurenic acid, a key metabolite of the kynurenine pathway (KP), was associated with increased ICU length of stay and mortality (Davidson et al. 2018). Broadly, the KP intermediate metabolites are known to have immunomodulatory, vasoactive, and neuroactive properties, and have been implicated in the pathogenesis of cardiac arrest, inflammatory disorders, pulmonary hypertension, and neurodegenerative diseases (Ristagno et al. 2014; Wirthgen et al. 2017).
A recent study by Simonato et al.(2019) performed untargeted metabolomic analysis of pre- and postoperative urine from 14 infants undergoing the arterial switch operation for D-transposition of the great arteries. They demonstrated prominent changes in urinary tryptophan, kynurenic acid, kynurenine, and 3-OH-kynurenine in immediate post-operative urine (prior to separation from CPB) compared to preoperative urine. Comprehensive assessment of circulating tryptophan metabolites after infant cardiac surgery, however, has not been previously performed. Furthermore, longitudinal changes in this metabolic pathway through the early postoperative course remain to be defined.
Given the possible association between the KP and the stress response to CPB, as well as the known biologic activity of pathway intermediates, further investigation of the KP in pediatric cardiac surgery patients is warranted. In this study we performed serial, targeted metabolic assessment of the complete kynurenine arm of tryptophan metabolism in a cohort of infants undergoing stage 2 palliation for SVHD (Glenn or hemi-Fontan surgeries), as well as in healthy controls. We hypothesized that preoperatively, infants with SVHD would demonstrate differences in tryptophan/KP metabolism compared to healthy controls. During the postoperative inflammatory state, we hypothesized there would be increased metabolism of tryptophan via the KP leading to accumulation of biologically active, circulating pathway intermediates.
2. Methods
2.1. Study Design
We analyzed serum samples from a prospective, observational cohort study of infants with SVHD undergoing superior cavo-pulmonary anastomosis (SCPA-stage 2 palliation, Glenn or hemi-Fontan surgery) with CPB. The Colorado Multiple Institution Review Board approved the study, and informed written consent was obtained from subjects’ families before enrollment. We enrolled infants aged 31 days to 2 years, undergoing catheterization for pre-SCPA evaluation or undergoing SCPA without plans for cardiac catheterization. Exclusion criteria were weight < 4kg, and patients with a pulsatile source of pulmonary blood flow in addition to the cavo-pulmonary anastomosis. Healthy controls were infants aged 3–12 months undergoing anesthesia for elective, non-cardiac procedures without cardio-pulmonary pathology, acute illness/infection, or known genetic abnormalities.
2.2. Metabolite Analysis
Serum samples were drawn pre-operatively and at 2, 24, and 48 hours after surgery, and then stored at -80°C for batch analysis. Sample analysis was performed using liquid-chromatography-tandem mass spectrometry as previously validated, and complete methods have previously been published (Davidson et al. 2018). Briefly, the serum sample was combined with cooled methanol, incubated for protein precipitation overnight, and dried in a SpeedVac concentrator centrifuge. After reconstitution in a water/methanol solution, a targeted metabolomics analysis for kynurenine pathway metabolites was performed (Davidson et al. 2018). Metabolites were identified with metabolomics standards initiative (MSI) Level 1. Once the data were acquired, MultiQuant software was used for data processing and relative quantitation of KP metabolites in serum.
2.3. Statistical Analysis
The main outcome variables were metabolites in the KP pathway. Relative intensities for all metabolites were log base 2 transformed and standardized before analysis to comply with statistical model assumptions and make comparisons more meaningful. Mean case and control metabolites were compared using Student’s t-tests. Kynurenine/tryptophan ratio (K/T) was calculated as an indirect measure of indolamine 2,3-dioxygenase (IDO) activity (Chen and Guillemin 2009). Mean metabolite changes over time in the CPB patients were compared using repeated measures ANOVA in Proc Mixed using an unstructured covariance matrix, which was used to model the correlation within patients. Estimate statements were developed to examine the comparisons of interest. Statistical significance level was established as p<0.05. All statistical analyses were performed in SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Figures were developed in R version 3.6.1 (The R Foundation for Statistical Computing).
3. Results
3.1. Demographics
A total of 17 cases and 34 controls were enrolled. Cases had a median age of 4.4 months (IQR 3.8–5.4), and median weight of 5.5 kg (IQR 5.2–5.9). Further clinical characteristics are shown in Supplemental Table 1.
3.2. Pre-op & Early Post-op Period
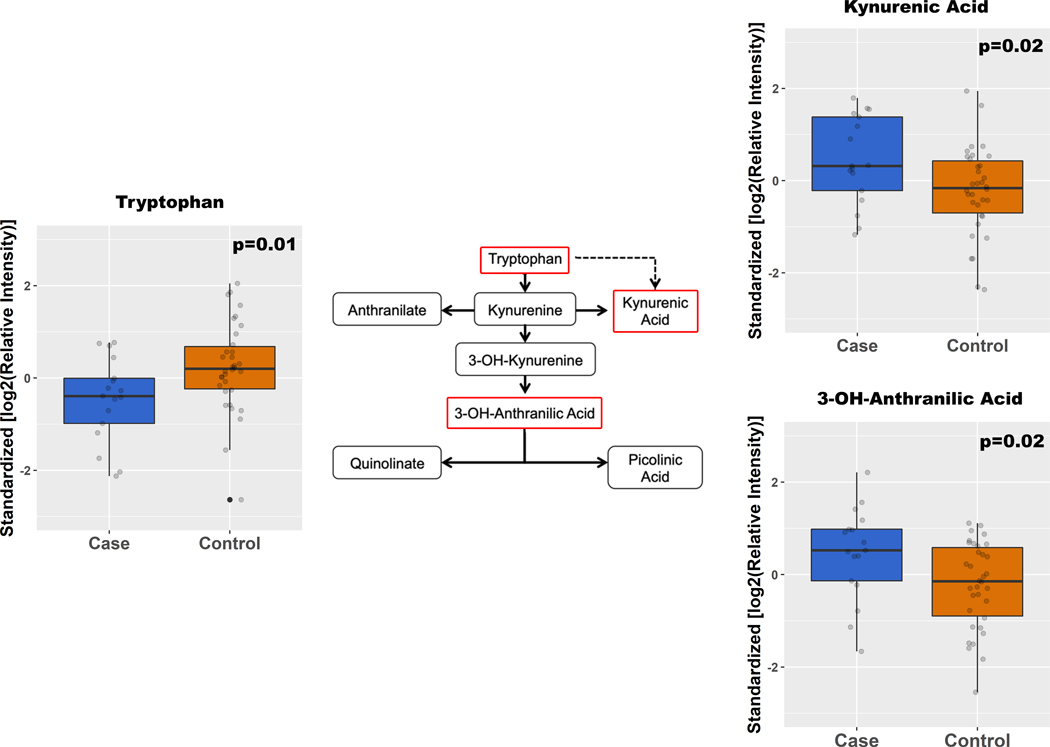
The full dataset of relative metabolite levels is presented in Supplemental Table 2. Figure 1 compares KP metabolites in preoperative cases to controls. Notably, cases demonstrated a relative depression of tryptophan by 1.2x (p=0.01), but had an elevation of several KP metabolites, including: kynurenic acid (KYNA) by 1.3x (p=0.02), and 3-OH-anthranillic acid (3HAA) by 1.9x (p=0.01). Cases also exhibited a higher K/T ratio compared to controls (0.044 [IQR 0.030, 0.065] vs 0.037 [IQR 0.024, 0.045]; p=0.017). We then analyzed KP metabolite changes over time, shown in Figure 2. Two hours after surgery, there was an increase in all proximal pathway metabolites. Specifically, the following had significant fold increases: KYNA 1.8x (p=0.0064); 3-OH-kynurenine (3HK) 10x, (p<1×10−4); anthranilic acid (AA) 8.7x (p=4×10−4). Serotonin levels were not significantly altered (p=0.29), suggesting that these changes to tryptophan metabolism were specific to the KP. In contrast, metabolites beyond 3HAA showed no significant change, or decreased in relative quantities two hours after surgery. Relative levels of circulating nicotinamide were also significantly decreased postoperatively [p<1×10−9], suggesting that the nicotinamide/NAD pathway may also be affected downstream of the KP.
Fig. 1.
Median metabolites with respective IQR in preoperative cases versus controls. Displayed here are metabolites with statistically significant differences (p < 0.05), and their corresponding positions on the kynurenine pathway outlined in red.
Fig. 2.
Median metabolites in cases over time (pre-op, and 2, 24, 48 h post-op), depicted in their respective positions in the kynurenine pathway. Time points that had a statistically significant difference compared to pre-op baseline (p < 0.05) are identified by an asterisk (*).
3.3. Late Post-op Period
We noted two metabolite patterns 24–48 hours after surgery. First, there was sustained elevation of some metabolites in this late, postoperative period. Kynurenine (KYN) remained 1.4x elevated from baseline at post-op 24 and 48 hours (p=0.01 and p=0.01, respectively). Similarly, KYNA was 2.1x higher (p=0.001) and 1.9x higher (p=0.004) than baseline post-op 24 and 48 hours, respectively. In contrast, the remaining metabolites began to recover toward preoperative baseline by post-op 24 hours. Namely, both 3HK and AA were down to a 3x increase from baseline (p=0.01 and p=0.003, respectively) at this time. A similar trend is seen with distal metabolites, exemplified by picolinic acid (PA) which is only 1.3x down from baseline at post-op 48 hours (p=0.007).
3.4. KP Metabolites and Renal Function
All subjects had daily creatinine levels measured as part of routine postoperative care. Acute kidney injury by AKIN criteria occurred in 3/15 patients (stage 1 in two patients and stage 2 in one patient). Most circulating KP metabolites (KYN, AA, 3HK, 3HAA, PA, and QA), showed no correlation with creatinine clearance. Relative tryptophan levels showed a strong positive linear correlation with creatinine clearance on both postoperative days 1 (r=0.74; p=0.0009) and 2 (r=0.71; p=0.003), with higher creatinine clearance associated with higher relative tryptophan levels. KA levels demonstrated moderate inverse correlation with creatinine clearance on postoperative day 1 (r=0.57; p=0.02) with a similar trend on postoperative day 2 (r=0.48; p=0.08).
3.5. Discussion
This study demonstrates metabolite shifts in the KP in our SVHD patients prior to and following stage 2 palliation. Key findings include: 1) distinct metabolic patterns when comparing cases to controls; 2) an elevation of proximal metabolites in the early postoperative period, of which KYN and KYNA were sustained 48 hours after surgery; 3) and an early depression of distal metabolites with subsequent trend towards baseline levels.
Preoperatively, SVHD infants demonstrated small, but significant differences in tryptophan metabolism compared to healthy controls. The etiology of this pattern is likely multifactorial. For one, literature supports that inflammatory cytokines induce IDO, one of the rate-limiting enzymes that induces the KP (Mandi and Vecsei 2012). The kynurenine to tryptophan (K/T) ratio is an index of IDO activity, in which higher ratios reflect increased enzyme activity and thus, increased tryptophan catabolism via the KP. This elevated K/T ratio in our subjects suggests a possible upregulation of IDO activity in SVHD infants who endure marked physiologic stress and could account for their lower tryptophan levels, although other factors such as decreased degradation of kynurenine through decreased kynurenine monooxygenase could also contribute to the elevated K/T ratio (Badawy and Guillemin 2019). Patient nutritional status is also worth considering as a potential cause, since tryptophan is an essential amino acid, and abnormal levels have been linked with cardiovascular disease (Song et al. 2017). While studies quantifying serum amino acids in critical illness are limited, malnutrition in CHD, due to feeding difficulties and a hypercatabolic state, are well documented and could further contribute to tryptophan dysregulation in our cases (Gielen et al. 2014). Ultimately, the pattern of KP alteration even before CPB supports the hypothesis that inter-stage SVHD infants may experience a baseline level of metabolic stress, the clinical implications of which warrant further study.
Postoperatively, our findings show an early activation of the KP, as evidenced by increased circulating proximal metabolites two hours after surgery. This activation is likely secondary to upregulation of IDO caused by a combination of increased cytokines and oxidative stress known to accompany infant cardiac surgery. While these findings have not previously been reported after infant CPB, Ristagno et al.(2013) demonstrated similar increases in KP metabolites within one hour of cardiac arrest resuscitation. At 48 hours postoperatively, we noted an accumulation of proximal metabolites compared to preoperative levels, specifically KYN and KYNA, which are known regulators of vascular tone and immune response, respectively (Chen and Guillemin 2009). Elevations of these metabolites have been correlated with severity of post-cardiac arrest outcomes (Ristagno et al. 2013). Notably we did not find an association between KP metabolites and renal function, with the exception of tryptophan and KA. Zhang, et al previously described a similar decrease in circulating tryptophan in renal transplant patients with acute kidney injury (Zhang et al. 2018). The authors attributed this finding to increased flux through the KP, although alternative mechanisms such as urinary loss were not evaluated. KA has previously been associated with outcomes in acute and chronic renal failure (Dabrowski et al. 2014). It is unclear, however, if these differences are due to altered clearance or increased renal/systemic production.
Though proximal metabolites were elevated in our patients postoperatively, we found that distal metabolites were unchanged or depressed. This shift, seen distal to 3HK in the KP, suggests a potential rate-limiting step at the kynureninase enzyme. While stable or decreased levels of distal, neurotoxic metabolites seems desirable a priori, this pattern could also impair de novo nicotinamide adenosine dinucleotide (NAD) synthesis downstream of quinolate. NAD is a key electron carrier in multiple redox reactions, most notably glycolysis and mitochondrial oxidative phosphorylation, and is also required for multiple NAD-dependent enzymes that regulate DNA repair, posttranslational modifications, and calcium handling. Decreased NAD is seen in both cardiovascular and renal disease, while increased levels may be protective (Poyan Mehr et al. 2018; Matasic et al. 2018). While comprehensive evaluation of the complex NAD pathway is beyond the scope of the current study, depressed levels of nicotinamide suggest that this critical pathway may be altered in the postoperative period and study of the interplay with the KP is warranted.
Collectively, our findings underscore the potential importance of KP regulation in SVHD both before and immediately after stage 2 palliation. These changes likely result from complex interplays between the abnormal cardiovascular physiology seen in SVHD and the response to surgery, including systemic inflammation and oxidative stress. As such, there is need for continued study of the interplay between KP changes, SVHD, and cardiac surgery, and it is important to consider the potential for accumulating, bioactive intermediates from this pathway as drivers of postoperative pathology.
While this study highlights the potential for targeted metabolomics to inform patient-specific phenotypes in vulnerable populations receiving critical care, we acknowledge a few limitations. In studying a homogenous, infant cohort with distinct physiology, our findings may not be fully generalizable to neonates or other CHD populations. Given our small sample size, our conclusions will need to be validated in larger studies. Our sample size also precludes us from evaluating the relationship between metabolite concentrations and clinical outcomes within the cohort. Thus, future studies to quantify circulating KP metabolites and perform outcome analyses are anticipated. Expanding on this, studies to quantify KP intermediates in specific tissues are also needed to supplement our understanding of metabolite production and bioactivity.
Supplementary Material
Acknowledgments
Funding: This study was funded by the American Heart Association (Grant number: 18IPA34170070).
Footnotes
Compliance with Ethical Standards: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest: All authors declare no conflicts of interest.
References
- Badawy AA, & Guillemin G. (2019). The Plasma [Kynurenine]/[Tryptophan] Ratio and Indoleamine 2,3-Dioxygenase: Time for Appraisal. Int J Tryptophan Res, 12, 1178646919868978, doi: 10.1177/1178646919868978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinder JJ, Goldstein SL, Lee VV, Baycroft A, Fraser CD, Nelson D, et al. (2012). Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg, 143(2), 368–374, doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Chen Y, & Guillemin GJ (2009). Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res, 2, 1–19, doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia GD, Wooi Ng K, Wijeyesekera A, Gala-Peralta S, Williams R, MacCarthy-Morrogh S, et al. (2015). Metabolic Profiling of Children Undergoing Surgery for Congenital Heart Disease. Crit Care Med, 43(7), 1467–1476, doi: 10.1097/CCM.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowski W, Kocki T, Pilat J, Parada-Turska J, & Malbrain ML (2014). Changes in plasma kynurenic acid concentration in septic shock patients undergoing continuous veno-venous haemofiltration. Inflammation, 37(1), 223–234, doi: 10.1007/s10753-013-9733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JA, Pfeifer Z, Frank B, Tong S, Urban TT, Wischmeyer PA, et al. (2018). Metabolomic Fingerprinting of Infants Undergoing Cardiopulmonary Bypass: Changes in Metabolic Pathways and Association With Mortality and Cardiac Intensive Care Unit Length of Stay. J Am Heart Assoc, 7(24), e010711, doi: 10.1161/JAHA.118.010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen M, Vanhorebeek I, Wouters PJ, Mesotten D, Wernerman J, Van den Berghe G, et al. (2014). Amino acid concentrations in critically ill children following cardiac surgery*. Pediatr Crit Care Med, 15(4), 314–328, doi: 10.1097/PCC.0000000000000075. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, & Kaplan S. (2002). The incidence of congenital heart disease. J Am Coll Cardiol, 39(12), 1890–1900, doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Kozik DJ, & Tweddell JS (2006). Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg, 81(6), S2347–2354, doi: 10.1016/j.athoracsur.2006.02.073. [DOI] [PubMed] [Google Scholar]
- Mandi Y, & Vecsei L. (2012). The kynurenine system and immunoregulation. J Neural Transm (Vienna), 119(2), 197–209, doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- Matasic DS, Brenner C, & London B. (2018). Emerging potential benefits of modulating NAD(+) metabolism in cardiovascular disease. Am J Physiol Heart Circ Physiol, 314(4), H839–H852, doi: 10.1152/ajpheart.00409.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussap M, Antonucci R, Noto A, & Fanos V. (2013). The role of metabolomics in neonatal and pediatric laboratory medicine. Clin Chim Acta, 426, 127–138, doi: 10.1016/j.cca.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, et al. (2018). De novo NAD(+) biosynthetic impairment in acute kidney injury in humans. Nat Med, 24(9), 1351–1359, doi: 10.1038/s41591-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristagno G, Fries M, Brunelli L, Fumagalli F, Bagnati R, Russo I, et al. (2013). Early kynurenine pathway activation following cardiac arrest in rats, pigs, and humans. Resuscitation, 84(11), 1604–1610, doi: 10.1016/j.resuscitation.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Ristagno G, Latini R, Vaahersalo J, Masson S, Kurola J, Varpula T, et al. (2014). Early activation of the kynurenine pathway predicts early death and long-term outcome in patients resuscitated from out-of-hospital cardiac arrest. J Am Heart Assoc, 3(4), doi: 10.1161/JAHA.114.001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Zhao Z, Liu X, Shu Q, Tan L, Lin R, et al. (2008). Perioperative risk factors for prolonged mechanical ventilation following cardiac surgery in neonates and young infants. Chest, 134(4), 768–774, doi: 10.1378/chest.07-2573. [DOI] [PubMed] [Google Scholar]
- Simonato M, Fochi I, Vedovelli L, Giambelluca S, Carollo C, Padalino M, et al. (2019). Urinary metabolomics reveals kynurenine pathway perturbation in newborns with transposition of great arteries after surgical repair. Metabolomics, 15(11), 145, doi: 10.1007/s11306-019-1605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Ramprasath T, Wang H, & Zou MH (2017). Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol Life Sci, 74(16), 2899–2916, doi: 10.1007/s00018-017-2504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirthgen E, Hoeflich A, Rebl A, & Gunther J. (2017). Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front Immunol, 8, 1957, doi: 10.3389/fimmu.2017.01957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang Q, Xia T, Fu S, Tao X, Wen Y, et al. (2018). Diagnostic value of plasma tryptophan and symmetric dimethylarginine levels for acute kidney injury among tacrolimus-treated kidney transplant patients by targeted metabolomics analysis. Sci Rep, 8(1), 14688, doi: 10.1038/s41598-018-32958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.