Abstract
The treatment of Type 2 Diabetes Mellitus (DM2) comprises physical activity (PA), diet, and medication. PA provides important benefits for people with diabetes. However, the majority of patients with DM2 do not attain the recommended levels of PA. Despite the evidence of the benefits to health of engaging in PA, the recommendations have not been fully translated into clinical improvements. Using a scoping review, this study aimed to identify the factors that influence levels of physical activity in adults with DM2. Eighteen studies published from 2009–2020 were identified by a search of relevant systematic databases between March 2019 and December 2020. The scoping review was carried out in accordance with the model defined by Arksey and O’Malley. The synthesis revelated sociodemographic characteristics, and six components—personal, motivation, social, mental, clinical, and self-efficacy—were identified as factors. Those that were most frequently identified were motivation and social support. In conclusion, these results should be considered to implement strategies to encourage people with DM2 to engage in physical exercise and thus improve the management of their condition.
Keywords: Diabetes Mellitus type 2, exercise, motivation, patient compliance, healthy lifestyle, scoping review
1. Introduction
Due to its increasing prevalence and high burden of disease, Type 2 Diabetes Mellitus (DM2) is a significant global health problem. It is a major cause of blindness, kidney failure, heart attack, stroke, and lower limb amputation [1].
The International Diabetes Federation has reported that 463 million people had diabetes in 2019, of whom 90% to 95% were diagnosed with DM2. It is estimated that by 2045, 700 million will be diagnosed as diabetic [2].
The rise in the incidence of diabetes is due to the growing epidemic of overweight and obesity, sedentary lifestyles, and population aging [3]. DM2 carries a high economic burden [4].
It has been shown that the adoption of healthy life habits and adherence to medication reduce mortality and morbidity in chronic diseases such as DM2. However, the adherence to pharmacological treatments and the observance of healthy lifestyle habits in chronic diseases is currently estimated to be 50% [5], and previous studies have shown that long-term maintenance of weight loss and complete adherence to diet and recommendations to engage in physical exercise are rare in patients with DM2 [6,7,8].
The onset of DM2 forces patients to make a series of lifestyle modifications that have to be maintained permanently. Given the considerable effort required to modify deeply ingrained habits, it is clear that treatment is a challenge, particularly because adults are more resistant to change than younger age groups [5].
The treatment of DM2 comprises physical activity (PA), diet, and medication. PA provides benefits such as the improvement of insulin sensitivity and a reduction in body weight [9,10,11].
Physical inactivity is a major modifiable lifestyle risk factor associated with cardiovascular disease [12]. Among patients with DM2, however, the perceived need to increase PA is low (compared, for example, with improving dietary habits); with regard to PA, patients with DM2 present low levels of determination, action, maintenance, and stabilization-of-change [13,14]. Thus, the design of DM2 treatment protocols should seek to raise awareness of the importance of PA.
Most of the interventions aimed at promoting PA are focused on educational, informative, and directed aspects of nursing but do not consider aspects such as self-determination, motivation and social support that have been determined by the correct adherence to a new lifestyle [15,16,17].
Self-determination theory (SDT) is a general theory of human motivation that has been increasingly used to explain the internalization process that leads to autonomous motivation for permanent behavior change. In the most autonomous forms of motivation, exercise is performed because (a) it provides pleasure and satisfaction (intrinsic motivation); (b) the person integrates exercise as a central value in his/her value system (integrated regulation); or (c) the person simply values exercise as important for his/her health (identified regulation). Autonomous motivation is the strongest predictor of success in increasing PA. Physician–patient interactions and lifestyle interventions should focus on promoting self-motivated reasons for change and the integration of change within the personality [18].
The level of motivation for any change in behavior is known to be closely related to the probability of action. Once the motivation factor has been addressed, strategies can be introduced to manage competing priorities. This temporal ordering of intervention strategies is supported by the theory that people who lack motivation will be less receptive to discussing strategies for behavior change [19].
Autonomous or self-determined motivation is one of the factors that best predicts compliance with PA and is linked to factors of well-being. The concept of well-being comprises physical fitness and functional capacity, mental health and resources, and a positive attitude and quality of life [20].
Social support, defined as the structure and quality of social relationships, can improve health outcomes by improving adherence to healthy behaviors and by impacting emotions and mood [21]. Social support from both family and health personnel has been described as a medium-term facilitator: “Social support from family did not mediate short-term physical activity changes but was the most consistent mediator of intermediate-term changes of physical activity” [22].
There is growing evidence that patients’ motivation for effective self-management may be enhanced by an autonomous supportive health care climate [23]. Studies have found that autonomous or self-determined motivation for diabetes care predicts increased physical exercise [20,24].
Perceived autonomy support was associated with autonomous motivation, which increases the effect of perceived autonomy support on PA. Thus, autonomy support was associated with patients’ PA through autonomous motivation. Self-care competence did not mediate the effect of autonomous motivation on PA. Koponen [25] supports the idea of SDT that internalization of the value of good health behavior is necessary for engagement in a physically active lifestyle. Health care practitioners can promote patients’ PA by supporting their autonomous motivation. Awareness of the benefits of exercise does not predict action change because the physiological changes that individuals with diabetes can experience after exercise do not promote, directly, such a change. For this reason, interventions in this area should not only aim to raise awareness of the benefits of the new behavior, but also involve self-efficacy and social support in order to establish programs that allow control and maintenance of future health behaviours [26].
Despite the evidence of the benefits to health of engaging in PA, the recommendations have not been fully translated into clinical improvements. Adherence of patients with DM2 to PA remains low, despite the many attempts to increase it. Few studies published to date have sought to understand what drives patients with DM2 to adopt prevention behaviors and specifically PA.
2. Aim
This study aimed to systematically examine and synthetize published studies on the facilitators of, and barriers to, engaging in PA in adults with DM2. The identification of the main facilitators and barriers may inform the development of effective programs to increase and maintain PA levels of adults with DM2.
3. Methods Design
We performed a scoping review (SR) following the methodological model of Arksey and O’Malley [27] and the later adaptation by Levac et al. [28].
3.1. Identifying the Research Question
What are the factors that influence levels of physical activity in adults with Type 2 Diabetes Mellitus?
3.2. Identifying Relevant Studies
A search was carried out in the main bibliographic databases in the health sciences: SCOPUS, CINAHL, Cochrane, Web of Science (WOS), and PUBMED; and other secondary databases such as Open Access Theses and Dissertations (OATD). Additional sources were identified using other databases such as Google Scholar or free Internet searches.
The MeSH terms used were “Diabetes Mellitus Type 2, Exercise, physical activity, fitness, habits change, habits modification, habits choice, lifestyle change, compliance, motivation, barrier, facilitator, adherence, compliance, non-adherence, non-compliance”.
Table 1 describes the final search strategy, which was adapted to the selected databases according to the specific language used in each one (See Table 1).
Table 1.
Database search strategy.
| 1. exercise | 11. barrier |
| 2. physical activity | 12. facilitator |
| 3. fitness | 13. adherence |
| 4. 1 or 2 or 3 | 14. compliance |
| 5. diabetes mellitus type 2 | 15. nonadherence |
| 6. habits change | 16. noncompliance |
| 7. habits modification | 17. motivation |
| 8. habits choice | 18. 11 or 12 or 13 or 14 or 15 or 16 or 17 |
| 9. lifestyle change | 19. 4 and 5 and 10 and 18 |
| 10. 6 or 7 or 8 or 9 |
Only papers published between 2009 and 2020 in Spanish or English were considered. The search period lasted from March 2019 to December 2020.
3.3. Study Selection
Quantitative, qualitative, and mixed designs were selected, plus different types of review (conceptual, narrative, or systematic). Participants were patients with DM2 who carried out some type of physical activity. Patients with Gestational Diabetes, Type 1 Diabetes Mellitus, and patients with prediabetes were excluded. Studies published between 2009 and 2020 in English or Spanish were admitted.
The results were exported to the Mendeley bibliographic manager database, version 1.19.3 (https.//www.mendeley.com, access on 22 January 2020). Duplicates were excluded. Subsequently, a peer review of title and abstract was performed, and then a peer review of the full text. Studies were excluded when they did not meet the inclusion criteria or did not focus sufficiently on the topic. MVC and GTN each selected a number of studies independently. Disagreements were resolved with the help of the third member of the research team (ERA).
The Excel 16.24 computer program (Microsoft, Washington, DC, USA) was used as the database and for the classification of the information. At this stage, the recommendations of Levac et al. [28] were followed to aid decision making among researchers. The entire research team held four meetings (one at the start, two during the course of the study, and one at the end), although the three reviewers involved maintained continuous contact throughout. This iterative process refined the search strategy and the inclusion of articles.
3.4. Charting the Data
Applying an adaptation of the proposal of Sánchez-Meca [29], extrinsic data (author and year), methodological data (population and sample, design, evaluation and instruments, results) and substantive data (intervention or tolls, motivation or conditioning factors) in Table 1 were analyzed (see Table 2).
Table 2.
Characteristics of the studies’ design: quantitative, qualitative, and mixed.
| Author(s), Year of Publication |
Study Location | Study Population | Methodology | Theory | Intervention/Tolls (Web, Interview, Primary Care) | Motivation (Email, Motivational Interview) |
|---|---|---|---|---|---|---|
| [30] Alharbi, M., Gallagher, R., Neubeck, L., Bauman, A., et al. 2016 | Australia | n = 134 | Quantitative (randomized controlled trial) and qualitative (semi-structured interviews) | Bandura | 1 h group-based supervised structured exercise twice a week and four 90 min group-based information sessions. Then, 3 telephone follow-up calls over the following 8 months | NO |
| [31] Balducci, S., Sacchetti, M., Haxhi, J., Orlando, G., Zanuso, S., et al. 2015 | Italy | n = 300 | Randomized controlled trial | Social cognitive theory and health belief model | Intervention in the INT group consisted of aggregated behavioral-change techniques once-a-year for 3 years. Theoretical, individual, face-to-face counseling sessions and practical exercise | Efforts are designed to convince the patient that regular PA is the pre-eminent cure for DM2 and to understand the positive expectations the individual patients had of this change in behavior |
| [32] Bekele, H., Asefa, A., Getachew, B., Belete, A.M. | Africa | - | Systematic Review | PICO. The levels of evidence and the quality guides of articles and research papers were evaluated based on the Johns Hopkins Method of Research Evidence Appraisal Tool. | No | No |
| [13] Centis, E., Trento, M., Dei Cas, A., et al. 2014 | Italy | n = 1353 consecutive outpatients with DM2 | Not specified. Use of questionnaires. Descriptive analysis | Prochaska’s model | Face-to-face questionnaire | Motivation to change was tested by the EMME-3 questionnaire for diet and PA |
| [30] Collins, T., Lunos, S., Ahluwalia, J. 2010 | USA | n = 145 subjects with chronic disease (DM1 or DM2 or peripheral arterial disease) | Randomized clinical trial, cross-sectional study | Social Cognitive Theory | Questionnaires, treadmill walking, six-minute walk test | Walking intervention to improve distance at 6 months in individuals with DM2 and peripheral arterial disease |
| [33] Gallé, F., Di Onofrio, V., Cirella, A., et al. 2017 | Italy | n = 130 Overweight and inactive patients with DM2 | Neither experimental nor controlled. Pre-post, prospective | No | 1-h training group sessions performed two times per week and short-form 12 questionnaire | Motivational program, a nutrition program, and an exercise program |
| [26] Gómez-Zúñiga, B., Pousada, M., Hernandez, M., et al. 2015 | USA | n = 3916 people with diabetes completed the BBT (BIG BLUE TEST) | Not specified. RCT | No | Web site (shares his or her experience by collecting own data and answering some questions through the Web) | No |
| [18] Koponen, A.M., Simonsen, N., Suominen, S. 2018 | Finland | n = 256 | Observational, cross-sectional mail survey | Self-determination theory perspective | Interviews with successful and unsuccessful participants | Studied autonomous motivation but did not apply any interventions to increase it |
| [34] Laranjo, L., Neves, A., Costa, A., et al. 2015 | Portugal | n = 16 Patients with type 2 DM were recruited at the Portuguese Diabetes Association outpatient clinic | Qualitative | No | Three video-recorded focus groups. Pre-tested interview guide | No |
| [35] Liebreich, T., Plotnikoff, R.C., Courneya, K.S., et al. 2009 | Canada | n = 49 | Prospective. 2-arm randomized controlled trial. Control/intervention groups | Social Cognitive Theory (SCT) | Web site | Individualized emails were sent on a weekly basis, providing general feedback on the specific topic of the week, progress, and motivation. |
| [19] Miller, S., Marolen, K. 2012 | USA | n = 22 African women with DM2. Two focus groups of 11 participants | Qualitative | Trans-theoretical Model of Behavior Change | Focus group | No |
| [36] Patel, N., Ferrer Harriet, B., Tyrer, F., et al. 2017 | UK | - | Narrative review | Emergent (‘berry picking’) model of information retrieval | No | No |
| [37] Schmidt, S.K., Hemmestad, L., Macdonald, C.S., Langberg, H., Valentiner, L.S. | Denmark | n = 6 (qualitative) | Longitudinal Qualitative Study | Health belief model (HBM), self-determination theory (SDT) and relevant research on the topic | Two rounds of in-depth, semi-structured interviews, conducted in August 2016 and February 2017 | No |
| [38] Richardson, C.R., Buis, L.R., Janney, A.W., et al. 2010 | USA | n = 324 | 2-arm randomized controlled trial | Bandura’s social-cognitive theory and social influence theories including social learning theory | Web page | Individually tailored motivational messages |
| [39] Schneider, K., Panza, E., Handschin, B., Ma, Y., et al. 2016 | USA | n = 20 | Randomization. | Linear mixed models: Littell, Stroup, Milliken, Wolfinger, and Schabenberger | Orientation session and 38 group exercise classes over 24 weeks | No |
| [40] Soderlund, P.D. 2018 | USA | - | Review: type not specified | No | PRISMA | MI proficient counselors who emphasize that PA self-management may help foster PA behavior change |
| [22] Van Dyck, D., De Greef, K., Deforche, B., et al. 2011 | Belgium | n = 143 | Randomized controlled trial | Social cognitive theory of Bandura + Prochaska’s trans-theoretical model de and self-determination theory | Baseline questionnaire administered in patients’ homes by a psychologist (IPAQ). The subject was provided with a pedometer. Follow-up by telephone | There was no increase in autonomous motivation towards physical activity in this study group, although our intervention also incorporated self-determination theory constructs |
| [41] Wycherley, T., Mohr, P., Noakes, M., et al. 2012 | Australia | n = 106 participants commenced and 84 completed the initial 16-week research based supervised lifestyle intervention program. Of the 81 participants invited, 3(37%) completed the 1-year follow-up | Not specified, but it was a non-randomized controlled trial and qualitative study | Standardized open-ended telephone interview | Getting involved in a structured exercise program may lead to improvements that may intrinsically motivate and facilitate exercise participation in the longer term | No |
In this table the variables under study were recorded in two dimensions: either as barriers to PA, or as facilitators. These dimensions emerged inductively through the content analysis, which was first carried out independently by each researcher and later jointly displayed the main characteristics.
3.5. Collating, Summarizing, and Reporting Results
The researchers followed the three steps recommended by Levac [28]: analysis, reporting the results, and considering the impact of the findings. A qualitative analysis was carried out, consisting of a report of the results according to the main characteristics of each study and type of review.
4. Results
4.1. Identification and Selection of Relevant Papers
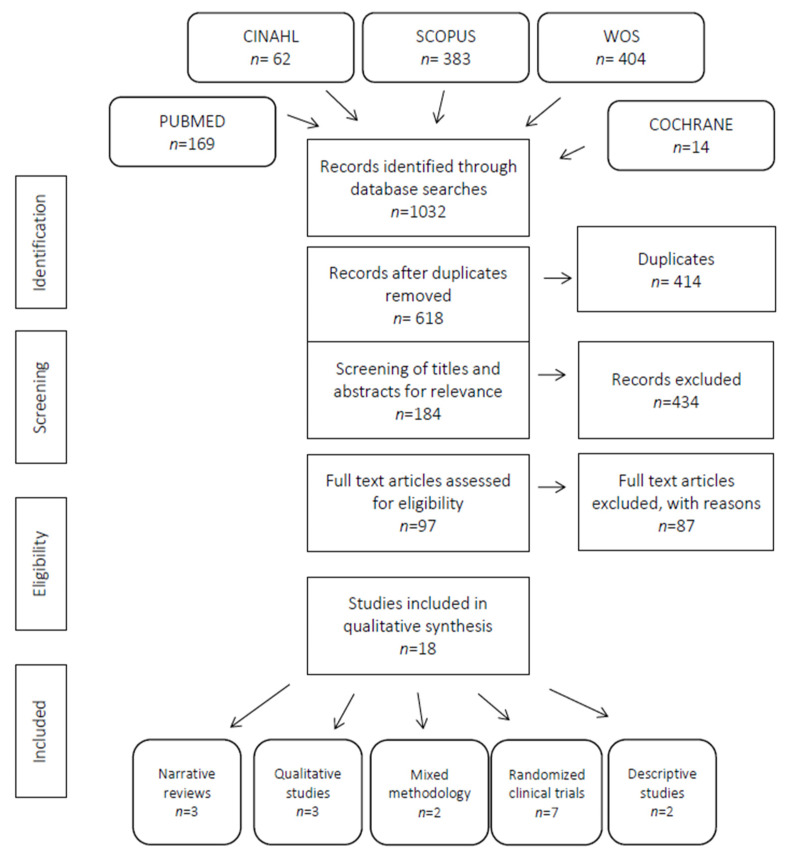
A total of 1032 articles were obtained from the electronic databases. After removing 414 duplicates and excluding 434 articles after reviewing their titles and abstracts, the number of potentially eligible articles was reduced to 97. After the second phase of full-text screening, 87 further articles were excluded, leaving a final total of 18 articles. See Figure 1.
Figure 1.
PRISMA flow-diagram of screening process for review.
Of the 18 selected articles, six were from the USA [19,26,38,39,40,42], three from Italy [13,31,33], two from Australia [30,41], one from Portugal [33], one from the UK [30], one from Belgium [22], one from Canada [31], one from Denmark [41], one from Africa [40], and one from Finland [18]. All articles were published in English. Seven had a quantitative design, three a qualitative design, and three a descriptive design; there were three narrative reviews and also two mixed-method reviews.
4.2. Risk of Bias, Validity, and Methodological Quality
The methodological quality of the included studies was assessed using CASPE [43] (see Table S1). The total scores on this scale range from 0 (best) to 10 (worst).
The articles analyzed here had scores ranging from 0 to 5. There were two very high-quality papers with scores of 0, indicating a high level of quality: [22] and [39]. There were also six very high-quality articles with scores of 1 [30,31,40,41,42] and [13] or 2 [18,31] and [32] and the quantitative part of the study by [30]. Studies with scores of 3 or 4 were classed as medium quality [39], the quantitative and qualitative parts of the mixed-methods review by [19,34,36,41]. Only two papers had scores of 5 and were therefore of medium–low quality: one observational [26] and the other the qualitative part of the mixed-methods review by [30]. The qualitative and quantitative parts of the two mixed-methods studies were analyzed separately.
4.3. Factors That Influence the Performance of Physical Activity in Adults with Type 2 Diabetes Mellitus
The synthesis shows the factors that were identified as facilitators of or barriers to the performance of physical activity in adults with DM2 in Table 2. The factors were classified according to socio-demographic characteristics and into six components: personal, motivation, social, mental, clinical, and self-efficacy (see Table 3).
Table 3.
Barriers to, and facilitators of, the performance of PA.
| Author(s), Year of Publication |
Conditioning Factors: Barriers | Conditioning Factors: Facilitators |
|---|---|---|
| [30] Alharbi, M., Gallagher, R., Neubeck, L., Bauman, A., et al. 2016 | The most common barriers were lack of motivation (40.3%), lack of time overall (30.6%), and lack of time due to family commitments (17.2%). Baseline self-efficacy, depressive symptoms, being female, overweight, and having coronary heart disease | No |
| [31] Balducci, S., Sacchetti, M., Haxhi, J., Orlando, G., Zanuso, S., et al. 2015 | Barriers that are outside the patient’s own control include lack of specific knowledge on the part of both physicians and exercise trainers and lack of dedicated facilities | No |
| [32] Bekele, H., Asefa, A., Getachew, B., Belete, A.M. 2020 | Barriers included the poor knowledge, the perception that exercise potentially exacerbates illness, lack of an exercise partner, specific locations away from home, the rainy season in Africa, criticism by others, and lack of support from the partner, health professionals, family members, and friends. Lack of knowledge and education, poverty and cost, population changes, and lack of access to healthcare |
No |
| [13] Centis, E., Trento, M., Dei Cas, A., et al. 2014 | Older age and longer disease duration, Higher motivation to change was recorded in the area of diet compared to that of AF | Higher educational level, self-efficacy was higher in males |
| [42] Collins, T., Lunos, S., Ahluwalia, J. 2010 | Walking alone or in rainy or cold weather | Self-efficacy, motivation |
| [33] Gallé, F., Di Onofrio, V., Cirella, A., et al. 2017 | Physical barriers (disorders, excessive weight, hypoglycemic crisis), psychophysical barriers (laziness, lack of companions, of physician recommendations, low importance attributed to physical activity, feeling unable to exercise) and environmental barriers (lack of time, of green areas, of a gym close by, of equipment at home) | Personal trainer, higher educational level, women |
| [26] Gómez-Zúñiga, B., Pousada, M., Hernandez, M., et al. 2015 | Less social support, DM1 fear of hypoglycemia | Social norms, self-efficacy |
| [18] Koponen, A.M., Simonsen, N., Suominen, S. 2018 | Poor health, stress, and insulin medication slightly, higher age, poor health, and social support | Autonomous motivation, self-care competence and perceived autonomy support correlated |
| [34] Laranjo, L., Neves, A., Costa, A., et al. 2015 | Lack of motivation and willpower, and not having created the habit of exercising. To a lesser degree: fatigue, muscle and joint pain, lack of information regarding the specific types of physical activities, lack of family or friend support. Themes PA: Decisional, Fatigue, Pain and Co-morbidities | Information and knowledge translation, as well as family and social ties |
| [35] Liebreich, T., Plotnikoff, R.C., Courneya, K.S., et al. 2009 | No | Information accessed through the virtual library, and therefore increased their physical activity as well |
| [19] Miller, S., Marolen, K. 2012 | Lack of motivation, laziness, competing priorities | Social support, motivation |
| [36] Patel, N., Ferrer Harriet, B., Tyrer, F., et al. 2017 | Car travel, racial harassment, or abuse when exercising and, for women, expectations to remain in the home, fear for personal safety, lack of same gender venues and concerns over the acceptability of wearing ‘western’ exercise clothing | Weight gain might compromise family/carer responsibilities, desire to be healthy, DM2 diagnosis, and exercise classes held in ‘safe’ environments such as places of worship |
| [37] Schmidt, S.K., Hemmestad, L., Macdonald, C.S., Langberg, H., Valentiner, L.S. 2020 | No | Five motivating factors were identified: achievement of results (reduce their daily medicine intake and live an overall healthier life), social support and relatedness (with help from the coaches, they developed skill and confidence in exercising), support from health care professionals and identification with acceptance of lifestyle (displayed signs of the new lifestyle being part of their lives and self-image) |
| [38] Richardson, C.R., Buis, L.R., Janney, A. W, et al. 2010 | Participants with low baseline social support for physical activity used the online community features more than participants with high baseline social support | Participants in both arms who reported having social support at the end of the study were more likely to increase their step counts. More posts written, and pages viewed correlated with greater reported motivation to increase walking |
| [39] Schneider, K., Panza, E., Handschin, B., Ma, Y., et al. 2016 | Pain, general exercise barriers and symptoms of major depression, comorbid depression and inadequately controlled diabetes | Family, social support |
| [40] Soderlund, P.D. 2018 | Time within the healthcare setting | (1) counselors focused on a minimal number of DM2 self-management behaviors (2) MI counselors should emphasize either the frequency or duration of MI sessions (3) MI proficient counselors MI may be more effective when counselors prioritize a minimal number of target behaviors over the course of a few sessions |
| [22] Van Dyck, D., De Greef, K., Deforche, B., et al. 2011 | Self-efficacy towards physical activity barriers was not a mediator during the intervention period (short-term), but only after the intervention ended (intermediate-term) | Positive social norms and modeling from family. Coping with relapse, defined as the ability to avoid and cope with relapse-inducing situations. Sport partner. Social support from family did not mediate short-term physical activity changes but was the most consistent mediator of intermediate-term changes of physical activity |
| [41] Wycherley, T., Mohr, P., Noakes, M., et al. 2012 | Undertaking moderate to high intensity exercise and overcoming the initial challenge of doing exercise | Supervised exercise training during the program indicated access to appropriate programs/facilities, more affordable gym membership and having a personal trainer /motivator. the motivation derived from the general improvements they experienced during the program, encouragement and troubleshooting efforts of the staff, personal persistence and, less commonly, the motivating effects of having lost weight and achieved improvements in diabetes control. Support from staff |
Regarding socio-demographic characteristics, the key obstacles were older age coupled with longer disease duration [13,18], excess weight [30,33,41], and female sex. One study identified female sex as a facilitator because healthy behaviors were recorded more frequently among women [39] but another study identified being a woman as an obstacle, because opinions about women’s physical abilities may affect their self-efficacy to perform PA [30].
Regarding the personal component, the key factors identified as facilitators were having information about PA [35,36], higher educational level [13,33], wanting to be healthy [36], identification with acceptance of lifestyle [41], and achieving improvements in the control of DM2 [37,41]. Obstacles included poor knowledge and the perception that exercise potentially exacerbates illness [40], lack of time [30,33], fear of hypoglycemia [26,33], having to do moderate to high intensity exercise and having to overcome the initial challenge of exercising [41], lack of willpower and not being in the habit of exercising [33], and the low importance attributed to PA and the feeling of an inability to exercise [39].
Regarding the motivation component, the main facilitator was the motivation factor itself [18,19,30,31,35,41]. Other studies reported a lack of motivation as an obstacle [19,30,34].
With regard to the social component, the key factors identified as facilitators were social support from family and health professionals [19,22,31,34,37,39] and having a personal trainer [34,37]. Obstacles were the absence of social support [18,26,32,34,38], lack of recommendations or medical information [31,32,33,34], lack of information given by health professionals regarding the specific types of suitable PA, not knowing how often they should exercise or how to implement a plan for regular PA [33], having to do PA alone [30,32,33], and the lack of parks, gymnasiums, and other facilities nearby for carrying out PA [31,32,33].
Regarding the mental component, the only factor identified was depression, which was reported to be a barrier [30,39].
Regarding the clinical component, the key factors identified as obstacles were muscle and joint fatigue and pain [34,39].
With respect to the self-efficacy component, the self-efficacy factor itself was identified as a facilitator [18,26,30,42].
5. Discussion
5.1. Socio-Demographic Characteristics
The results of the study suggest that older subjects and women were less likely to engage in PA. The older the subjects, the less likely they were to perform PA, due to the possible associated complications, the physiological changes involved in the aging process, and the high prevalence of non-communicable chronic diseases, or a feeling of being unable to exercise [44,45,46]. In one study of patients with DM2, only 21% engaged in PA; 6% were normal weight individuals, 12% were overweight, and 3% were obese. Thus, obesity was found to reduce the likelihood of performing PA [44]. Women reported having less energy and less ability than men [47], and indicated they need to increase their confidence and overcome barriers in order to perform PA [48]. No studies identified female gender as a facilitator of PA with the exception of the study by [33].
5.2. Personal Component
Feelings of obligation, internal pressure, discomfort, and guilt do not favor adherence to PA. On the contrary, self-esteem, enjoyment, and a sense of challenge are the main incentives for discovering the benefits of PA [49]. The adoption of PA must be a gradual incremental process so as to allow adaptations at metabolic and functional levels. Individuals need encouragement and accompaniment; they need to be presented with new challenges that enable them to go beyond the current limits of their physical abilities [50]. For a change in behavior (such as an increase in PA) to have consistent, long-term effects, it is important that patients internalize it, identify with it, and acknowledge its importance [49].
5.3. Motivation Component
Motivation was the factor most frequently identified as a facilitator of physical activity, and lack of motivation was described by several authors as a barrier. Motivation is a key factor in maintaining PA in the long-term [37]. Neel [50] described the syndrome of the emergence of genetic homeostasis due to prolonged periods of physical inactivity. This prolonged inaction can lead to a loss of physical fitness due to genetic dysfunction: not due to a gene deficiency, but to a lack of motivation to perform activities, which fails to stimulate proper functioning. Papín et al. [51] found a positive relationship between the motivation to perform PA and the time since the diagnosis of the disease, basic psychological needs (autonomy, competence, and social support), and respondents’ resilience. Furthermore, an inverse relationship was found between HbA1c and the motivation to perform PA and basic psychological needs [52].
5.4. Social Component
Social influence (exerted by sedentary friends or family members) also represents a disincentive for physical activity. Only 15% of the study population identified the lack of resources as an important limitation for carrying out PA [47]. This finding is consistent with previous research that highlights the importance of social support, but also its complex role in the management of diabetes self-care. For example, social support encompasses many behaviors such as giving advice, assistance, and listening [19]. According to the study by Alarcón-Moraa [53], patients with longer disease duration perceive less social support in general, and less emotional and affective support in particular. These results corroborate those of Kadirvelu [54], who mention that, although in most cases family support tends to be given freely, it tends to be more forthcoming when the diagnosis of diabetes is recent, and diminishes when the disease continues for a longer period. Regarding the lack of information, the peer training carried out at some centers has a positive impact on physical exercise, the use of health resources, and self-efficacy in care. It fosters a positive relationship between patients and health staff, generates group support and self-confidence, and facilitates emotional management [55].
5.5. Mental Component
The only mental component identified was depression. Twenty percent of adults with DM2 had depression, and women with DM2 were two or three times more likely to have depression than men with DM2 [56]. Depression in DM2 is associated with poorer glycemic control, poor self-control of diabetes, and a high risk of mortality [33]. Additionally, research has shown that antidepressant medication may have direct effects on glycemic control that are independent of its effects on weight and mood [57].
5.6. Clinical Component
Fatigue and muscle and joint pain were identified as barriers. Moderate to extreme pain was present in 57.8% of diabetic patients [58]. Pain was strongly associated with poor mental health and physical functioning, but not with poorer glycemic control.
5.7. Self-Efficacy Component
Bandura conceptualizes self-efficacy as the confidence in one’s ability to carry out a specific behavior. Related to physical activity, self-efficacy refers to one’s confidence in being physically active in certain situations [59]. Those with higher self-efficacy were less likely to smoke and more likely to adherence to diet and engage in exercise [60]. Factors that predicted lower self-efficacy for exercise were lack of motivation, higher BMI, a diagnosis of coronary heart disease, more depressive symptoms, and female sex [30].
6. Strengths and Limitations of the Review
This scoping review compiled information from studies with a wide range of designs and methods, and was conducted with the rigor and transparency required [27]. It provides an overview of the available literature on the factors that favor or discourage the habit of physical activity in adults with DM2. However, possible limitations are the publication bias inherent in the literature, the lack of guidelines for carrying out specific methods, and the difficulty of summarizing the results of different studies. This review took a considerable amount of time to complete, due to the extensive search coverage required by the approach. There may be a risk of selection bias if not all the available data were identified.
7. Conclusions
This scoping review identified the main barriers and facilitators of the performance of physical activity in adults with DM2. Sociodemographic characteristics and six components—personal, motivation, social, mental, clinical, and self-efficacy—were identified as factors. Motivation and social support were the factors most frequently identified. Few studies published to date have explored the impact of these factors. It is important that future research should devise strategies for promoting adherence to PA that take into account the barriers and facilitators in populations with DM2 identified here in order to improve the effectiveness of intervention programs.
Implications for Practice
This review can help health professionals and educators to reorient the focus of interventions and programs mainly dedicated to this topic. Effort should be made to create intervention tools that provide patients with DM2 with motivational engagement and encouragement of self-care.
Preventative measures and promotion of health would empower patients with DM2 with information, instruments, and strategies to obtain the most positive perspective, achieve better adherence to PA, and motivate them to adopt healthy behaviors and achieve maximum personal satisfaction.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18105359/s1, Table S1: CASPE screening.
Author Contributions
Conceptualization, all authors (M.V.C., G.T.-N. and E.R.A.); methodology, M.V.C., G.T.-N.; formal analysis, M.V.C.; data curation, M.V.C., G.T.-N.; writing—original draft preparation, M.V.C.; writing—review and editing, G.T.-N., E.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Diabetes. [(accessed on 8 September 2019)];2018 Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 2.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.Aranceta-Bartrina J., Pérez-Rodrigo C., Alberdi-Aresti G., Ramos-Carrera N., Lázaro-Masedo S. Prevalencia de obesidad general y obesidad abdominal en la población adulta española (25–64 años) 2014–2015: Estudio ENPE. Rev. Española Cardiol. 2016;69:579–587. doi: 10.1016/j.recesp.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Islam S.M.S., Lechner A., Ferrari U., Laxy M., Seissler J., Brown J., Niessen L.W., Holle R. Healthcare use and expenditure for diabetes in Bangladesh. BMJ Glob. Health. 2017;2:e000033. doi: 10.1136/bmjgh-2016-000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz M., Ortiz E., Gatica A., Gómez D. Factores Psicosociales Asociados a la Adherencia al Tratamiento de la Diabetes Mellitus Tipo 2. Ter. Psicol. 2011;29:5–11. doi: 10.4067/S0718-48082011000100001. [DOI] [Google Scholar]
- 6.Broadbent E., Donkin L., Stroh J.C. Illness and Treatment Perceptions Are Associated With Adherence to Medications, Diet, and Exercise in Diabetic Patients. Diabetes Care. 2011;34:338–340. doi: 10.2337/dc10-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin E.H.B., Katon W., Von Korff M., Rutter C., Simon G.E., Oliver M., Ciechanowski P., Ludman E.J., Bush T., Young B. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 8.Cradock K.A., Ólaighin G., Finucane F.M., Gainforth H.L., Quinlan L.R., Ginis K.A.M. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017;14:1–17. doi: 10.1186/s12966-016-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Márquez J., Ramón G., Márquez J. Actualidad en ejercicio y diabetes tipo 2 (I) Arch. Med. Deporte. 2011;28:113–120. [Google Scholar]
- 10.Oliveira C., Simões M., Carvalho J., Ribeiro J. Combined exercise for people with type 2 diabetes mellitus: A systematic review. Diabetes Res. Clin. Pract. 2012;98:187–198. doi: 10.1016/j.diabres.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Yang P., Oh P. Predicting Aerobic Fitness Improvements after Participation in a Hybrid Supervised and Home-Based Exercise Program in People with Type 2 Diabetes. Can. J. Diabetes. 2013;37:388–393. doi: 10.1016/j.jcjd.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Chan A.W.K., Chair S.Y., Lee D.T.F., Leung D.Y.P., Sit J.W.H., Cheng H.Y., Taylor-Piliae R.E. Tai Chi exercise is more effective than brisk walking in reducing cardiovascular disease risk factors among adults with hypertension: A randomised controlled trial. Int. J. Nurs. Stud. 2018;88:44–52. doi: 10.1016/j.ijnurstu.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Centis E., Trento M., Cas A.D., Pontiroli A.E., De Feo P., Bruno A., Sasdelli A.S., Arturi F., Strollo F., Kreutzenberg S.V.D., et al. Stage of change and motivation to healthy diet and habitual physical activity in type 2 diabetes. Acta Diabetol. 2014;51:559–566. doi: 10.1007/s00592-013-0551-1. [DOI] [PubMed] [Google Scholar]
- 14.Vähäsarja K., Salmela S., Villberg J., Rintala P., Vanhala M., Saaristo T., Peltonen M., Keinänen-Kiukaanniemi S., Korpi-Hyövälti E., Kujala U.M., et al. Perceived need to increase physical activity levels among adults at high risk of type 2 diabetes. A cross-sectional analysis within a community-based diabetes prevention project FIN-D2D. BMC Public Health. 2012;12:514. doi: 10.1186/1471-2458-12-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caetano I.R.C.E.S., Santiago L.M., Marques M. Impact of written information on control and adherence in type 2 diabetes. Rev. Assoc. Médica Bras. 2018;64:140–147. doi: 10.1590/1806-9282.64.02.140. [DOI] [PubMed] [Google Scholar]
- 16.Alonso-Domínguez R., Gómez-Marcos M.A., Patino-Alonso M.C., Sánchez-Aguadero N., Agudo-Conde C., Castaño-Sánchez C., García-Ortiz L., Recio-Rodríguez J.I. Effectiveness of a multifactorial intervention based on an application for smartphones, heart-healthy walks and a nutritional workshop in patients with type 2 diabetes mellitus in primary care (EMID): Study protocol for a randomised controlled trial. BMJ Open. 2017;7:e016191. doi: 10.1136/bmjopen-2017-016191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso-Domínguez R., Patino-Alonso M.C., Sánchez-Aguadero N., García-Ortiz L., Recio-Rodríguez J.I., Gómez-Marcos M.A. Effect of a multifactorial intervention on the increase in physical activity in subjects with type 2 diabetes mellitus: A randomized clinical trial (EMID Study) Eur. J. Cardiovasc. Nurs. 2019;18:399–409. doi: 10.1177/1474515119835048. [DOI] [PubMed] [Google Scholar]
- 18.Koponen A.M., Simonsen N., Suominen S.B. Success in increasing physical activity (PA) among patients with type 2 diabetes: A self-determination theory perspective. Health Psychol. Behav. Med. 2018;6:104–119. doi: 10.1080/21642850.2018.1462707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller S.T., Marolen K. Physical Activity-Related Experiences, Counseling Expectations, Personal Responsibility, and Altruism among Urban African American Women with Type 2 Diabetes. Diabetes Educ. 2012;38:229–235. doi: 10.1177/0145721712437558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koponen A.M., Simonsen N., Laamanen R., Suominen S.B. Health-care climate, perceived self-care competence, and glycemic control among patients with type 2 diabetes in primary care. Health Psychol. Open. 2015;2 doi: 10.1177/2055102915579778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchino B.N. Social support and health: A review of physiological processes potentially underlying links to disease outcomes. J. Behav. Med. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 22.Van Dyck D., De Greef K., Deforche B., Ruige J., Tudor-Locke C.E., Kaufman J.-M., Owen N., De Bourdeaudhuij I. Mediators of physical activity change in a behavioral modification program for type 2 diabetes patients. Int. J. Behav. Nutr. Phys. Act. 2011;8:105. doi: 10.1186/1479-5868-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortier M.S., Duda J.L., Guerin E., Teixeira P.J. Promoting physical activity: Development and testing of self-determination theory-based interventions. Int. J. Behav. Nutr. Phys. Act. 2012;9:20. doi: 10.1186/1479-5868-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teixeira P.J., Carraça E.V., Markland D., Silva M.N., Ryan R.M. Exercise, physical activity, and self-determination theory: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2012;9:78. doi: 10.1186/1479-5868-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koponen A.M., Simonsen N., Suominen S.B. Determinants of physical activity among patients with type 2 diabetes: The role of perceived autonomy support, autonomous motivation and self-care competence. Psychol. Health Med. 2016;22:332–344. doi: 10.1080/13548506.2016.1154179. [DOI] [PubMed] [Google Scholar]
- 26.Gómez-Zúñiga B., Pousada M., Hernandez M.M., Colberg S., Gabarrón E., Armayones M. The Online Big Blue Test for Promoting Exercise: Health, Self-Efficacy, and Social Support. Telemed. J. e-Health. 2015;21:852–859. doi: 10.1089/tmj.2014.0158. [DOI] [PubMed] [Google Scholar]
- 27.Arksey H., O’Malley L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 28.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sánchez-Meca J. Cómo Realizar Una Revisión Sistemática y un Meta-análisis. Aula Abierta. [(accessed on 24 November 2019)];2010 Available online: https://dialnet.unirioja.es/descarga/articulo/3316651.pdf.
- 30.Alharbi M., Gallagher R., Neubeck L., Bauman A., Prebill G., Kirkness A., Randall S. Exercise barriers and the relationship to self-efficacy for exercise over 12 months of a lifestyle-change program for people with heart disease and/or diabetes. Eur. J. Cardiovasc. Nurs. 2017;16:309–317. doi: 10.1177/1474515116666475. [DOI] [PubMed] [Google Scholar]
- 31.Balducci S., Sacchetti M., Haxhi J., Orlando G., Zanuso S., Cardelli P., Cavallo S., D’Errico V., Ribaudo M.C., Biase N.D., et al. The Italian Diabetes and Exercise Study 2 (IDES-2): A long-term behavioral intervention for adoption and maintenance of a physically active lifestyle. Trials. 2015;16:569. doi: 10.1186/s13063-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bekele H., Asefa A., Getachew B., Belete A.M. Barriers and Strategies to Lifestyle and Dietary Pattern Interventions for Prevention and Management of TYPE-2 Diabetes in Africa, Systematic Review. J. Diabetes Res. 2020;2020:1–14. doi: 10.1155/2020/7948712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallé F., Di Onofrio V., Cirella A., Di Dio M., Miele A., Spinosa T., Liguori G. Improving Self-Management of Type 2 Diabetes in Overweight and Inactive Patients Through an Educational and Motivational Intervention Addressing Diet and Physical Activity: A Prospective Study in Naples, South Italy. Diabetes Ther. 2017;8:875–886. doi: 10.1007/s13300-017-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laranjo L., Neves A.L., Costa A., Ribeiro R.T., Couto L., Sá A.B. Facilitators, barriers and expectations in the self-management of type 2 diabetes—a qualitative study from Portugal. Eur. J. Gen. Pract. 2015;21:103–110. doi: 10.3109/13814788.2014.1000855. [DOI] [PubMed] [Google Scholar]
- 35.Liebreich T., Plotnikoff R.C., Courneya K.S., Boulé N. Diabetes NetPLAY: A physical activity website and linked email counselling randomized intervention for individuals with type 2 diabetes. Int. J. Behav. Nutr. Phys. Act. 2009;6:18. doi: 10.1186/1479-5868-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel N., Ferrer H.B., Tyrer F., Wray P., Farooqi A., Davies M.J., Khunti K. Barriers and Facilitators to Healthy Lifestyle Changes in Minority Ethnic Populations in the UK: A Narrative Review. J. Racial Ethn. Health Disparities. 2017;4:1107–1119. doi: 10.1007/s40615-016-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt S.K., Hemmestad L., Macdonald C.S., Langberg H., Valentiner L.S. Motivation and Barriers to Maintaining Lifestyle Changes in Patients with Type 2 Diabetes after an Intensive Lifestyle Intervention (The U-TURN Trial): A Longitudinal Qualitative Study. Int. J. Environ. Res. Public Health. 2020;17:7454. doi: 10.3390/ijerph17207454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson C.R., Buis L.R., Janney A.W., Goodrich D.E., Sen A., Hess M.L., Mehari K.S., Fortlage L.A., Resnick P.J., Zikmund-Fisher B.J., et al. An online community improves adherence in an internet-mediated walking program. Part 1: Results of a randomized controlled trial. J. Med. Internet Res. 2010;12:e71. doi: 10.2196/jmir.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider K.L., Panza E., Handschin B., Ma Y., Busch A.M., Waring M.E., Appelhans B.M., Whited M.C., Keeney J., Kern D., et al. Feasibility of Pairing Behavioral Activation with Exercise for Women With Type 2 Diabetes and Depression: The Get It Study Pilot Randomized Controlled Trial. Behav. Ther. 2016;47:198–212. doi: 10.1016/j.beth.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soderlund P.D. Effectiveness of motivational interviewing for improving physical activity self-management for adults with type 2 diabetes: A review. Chronic Illn. 2018;14:54–68. doi: 10.1177/1742395317699449. [DOI] [PubMed] [Google Scholar]
- 41.Wycherley T.P., Mohr P., Noakes M., Clifton P.M., Brinkworth G.D. Self-reported facilitators of, and impediments to maintenance of healthy lifestyle behaviours following a supervised research-based lifestyle intervention programme in patients with type 2 diabetes. Diabet. Med. 2012;29:632–639. doi: 10.1111/j.1464-5491.2011.03451.x. [DOI] [PubMed] [Google Scholar]
- 42.Collins T.C., Lunos S., Ahluwalia J.S. Self-efficacy is associated with walking ability in persons with diabetes mellitus and peripheral arterial disease. Vasc. Med. 2010;15:189–195. doi: 10.1177/1358863X10362604. [DOI] [PubMed] [Google Scholar]
- 43.CASPe|Programa de Habilidades en Lectura Crítica EspañolCritical Appraisal Skills Programme Español. [(accessed on 10 December 2019)]; Available online: http://www.redcaspe.org/
- 44.Terechenko N., Baute A., Zamonsky J. Adherencia al tratamiento en pacientes con Diagnóstico de Diabetes Mellitus Tipo II. Miomed. Fam. Comunitaria. 2014;10:20–33. [Google Scholar]
- 45.Mendes R., Dias E., Gama A., Castelo-Branco M., Themudo-Barata J.L. Prática de exercício físico e níveis de atividade física habitual em doentes com diabetes tipo 2–estudo piloto em Portugal. Rev. Port. Endocrinol. Diabetes Metab. 2013;8:9–15. doi: 10.1016/j.rpedm.2012.05.001. [DOI] [Google Scholar]
- 46.La Osa A.P.-D., Villaquirán-Hurtado A., Jácome-Velasco S., Galvis-Fernández B., Granados-Vidal Y.A. Actividad física en pacientes con diabetes mellitus tipo 2 y relación con características sociodemográficas, clínicas y antropométricas. Univ. Salud. 2017;20:72–81. doi: 10.22267/rus.182001.111. [DOI] [Google Scholar]
- 47.Figueroa A., Quingalombo G. Correlación Entre las Barreras para Realizar Actividad Física y el Nivel de Hemoglobina Glicosilada a1c en Pacientes Diabéticos que Acuden a la Clínica de Diabetes del Hospital Vozandes de abril a junio de 2017 Usando la Herramienta “Percepción de Barrera. Pontificia Universidad Católica del Ecuador. [(accessed on 12 June 2020)]; Available online: http://repositorio.puce.edu.ec/handle/22000/13730.
- 48.Blanchard C., Arthur H.M., Gunn E. Self-efficacy and outcome expectations in cardiac rehabilitation: Associations with women’s physical activity. Rehabil. Psychol. 2015;60:59–66. doi: 10.1037/rep0000024. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira P.J., Silva M.N., Mata J., Palmeira A.L., Markland D. Motivation, self-determination, and long-term weight control. Int. J. Behav. Nutr. Phys. Act. 2012;9:22. doi: 10.1186/1479-5868-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Granados J., Gutiérrez C., González L., Masso A. Ayudando al paciente diabético con actividad física. Rev. Digit. Act. Física Deporte. 2018;2:134–139. [Google Scholar]
- 51.Papín C., Martín R., González X. Descripción del tipo de motivación de pacientes con diabetes tipo 2 para realizar actividad física. RIdEC. 2018;11:8–45. [Google Scholar]
- 52.Dabaghi P., Dashti S., Tofangchiha S. The effectiveness of training program based on protective motivation theory on improving nutritional behaviors and physical activity in military patients with type 2 diabetes mellitus. J. Fam. Med. Prim. Care. 2020;9:3328–3332. doi: 10.4103/jfmpc.jfmpc_70_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alarcón-Mora C., Veracruzana M.I.D.S.P.D.L.U., Hernández-Barrera L., Argüelles-Nava V., Campos-Uscanga Y., Pública M.I.N.D.S. Social support and its association with diet self-care in patients with diabetes. Liberabit Rev. Peru. Psicol. 2017;23:111–121. doi: 10.24265/liberabit.2017.v23n1.08. [DOI] [Google Scholar]
- 54.Kadirvelu A., Sadasivan S., Ng S.H. Social support in type II diabetes care: A case of too little, too late. Diabetes Metab. Syndr. Obes. Targets Ther. 2012;5:407–417. doi: 10.2147/DMSO.S37183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daneta A., Prieto M., Gamboa E., de Retana Garcia L.O., March J. Peer training for patients with diabetes mellitus A quantitative and qualitative evaluation in the Basque Country and Andalusia. Aten. Primaria. 2016;58:507–517. doi: 10.1016/j.aprim.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salinero-Fort M.A., Gómez-Campelo P., Andrés-Rebollo F.J.S., Cárdenas-Valladolid J., Abánades-Herranz J.C., Pau E.C.D.S., Chico-Moraleja R.M., Beamud-Victoria D., De Miguel-Yanes J.M., Jimenez-Garcia R., et al. Prevalence of depression in patients with type 2 diabetes mellitus in Spain (the DIADEMA Study): Results from the MADIABETES cohort. BMJ Open. 2018;8:e020768. doi: 10.1136/bmjopen-2017-020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semenkovich K., Brown M.E., Svrakic D.M., Lustman P.J. Depression in Type 2 Diabetes Mellitus: Prevalence, Impact, and Treatment. Drugs. 2015;75:577–587. doi: 10.1007/s40265-015-0347-4. [DOI] [PubMed] [Google Scholar]
- 58.Bair M.J., Brizendine E.J., Ackermann R.T., Shen C., Kroenke K., Marrero D.G. Prevalence of pain and association with quality of life, depression and glycaemic control in patients with diabetes. Diabet. Med. 2010;27:578–584. doi: 10.1111/j.1464-5491.2010.02971.x. [DOI] [PubMed] [Google Scholar]
- 59.Bandura A. Social Foundations of thought and Action. SAGE Publications Ltd.; New Delhi, India: 1986. pp. 23–28. [Google Scholar]
- 60.Adam J., Folds L. Depression, Self-efficacy, and Adherence in Patients with Type 2 Diabetes. J. Nurse Pract. 2014;10:646–652. doi: 10.1016/j.nurpra.2014.07.033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.