Abstract
Polycyclic aromatic hydrocarbons (PAHs) are attractive synthetic building blocks for more complex conjugated nanocarbons, but their use for this purpose requires appreciable quantities of a PAH with reactive functional groups. Despite tremendous recent advances, most synthetic methods cannot satisfy these demands. Here we present a general and scalable [2 + 2 + n] (n = 1 or 2) cycloaddition strategy to access PAHs that are decorated with synthetically versatile alkynyl groups and its application to seven structurally diverse PAH ring systems (thirteen new alkynylated PAHs in total). The critical discovery is the site-selectivity of an Ir-catalyzed [2 + 2 + 2] cycloaddition, which preferentially cyclizes tethered diyne units with preservation of other (peripheral) alkynyl groups. The potential for generalization of the site-selectivity to other [2 + 2 + n] reactions is demonstrated by identification of a Cp2Zr-mediated [2 + 2 + 1]/metallacycle transfer sequence for synthesis of an alkynylated, selenophene-annulated PAH. The new PAHs are excellent synthons for macrocyclic conjugated nanocarbons. As a proof of concept, four were subjected to alkyne metathesis catalysis to afford large, PAH-containing arylene ethylene macrocycles, which possess a range of cavity sizes reaching well into the nanometer regime. Notably, these high-yielding macrocyclizations establish that synthetically convenient pentynyl groups can be effective for metathesis since the 4-octyne byproduct is sequestered by 5 Å MS. Most importantly, this work is a demonstration of how site-selective reactions can be harnessed to rapidly build up structural complexity in a practical, scalable fashion.
An orthogonal [2 + 2 + n] cycloaddition/alkyne metathesis reaction sequence enables streamlined access to conjugated macrocyclic nanocarbons.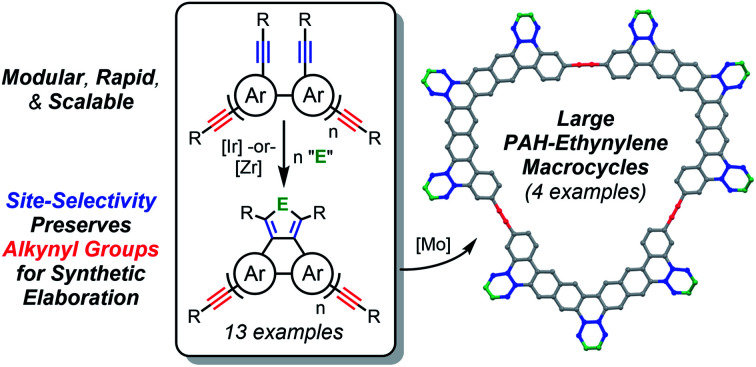
Introduction
The recent surge of interest in molecular carbon nanoscience is driven by the pursuit of atomic precision in larger nanostructures (e.g. graphene nanoribbons and carbon nanotubes)1 and by the extraordinary properties displayed by molecular nanocarbons themselves.2 Large polycyclic aromatic hydrocarbons (PAHs) represent a transition in the continuum between molecules and nanostructures. These structurally diverse compounds exhibit promise for a range of applications due to their exceptional photophysical and electronic properties, and their ability to organize into functional assemblies via π-stacking.3 Since most carbon nanostructures are comprised of fused rings, PAHs are their natural synthetic building blocks. For example, the polymerization of PAH monomers may offer the best approach for growing atomically precise graphene nanoribbons4 and the synthesis of carbon nanotubes and fullerenes from belt- and bowl-shaped PAH precursors should eventually be feasible.5 PAHs are also attractive as components of many other types of functional materials, including polymers,6 macrocycles,7 and metal and covalent organic frameworks.8
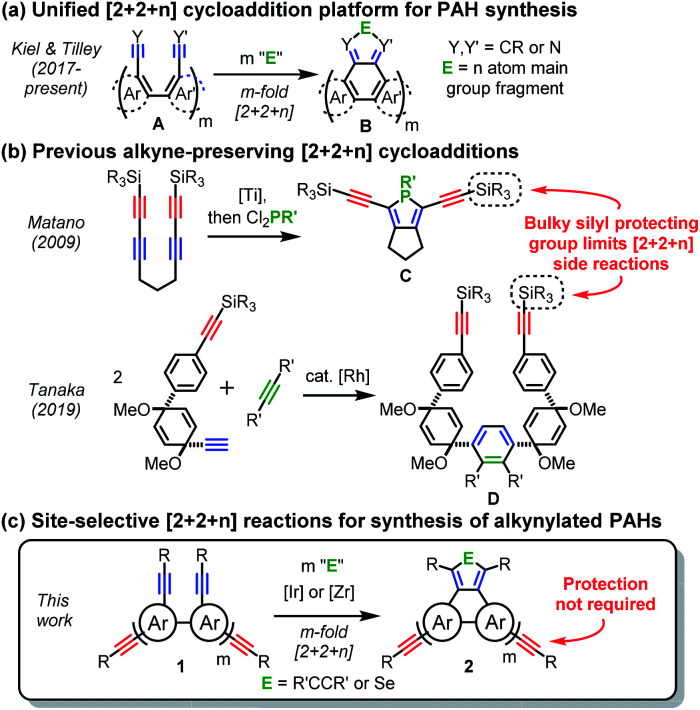
The synthesis of PAHs is challenging since it requires regioselective fusions of many rings. Recently, much progress has been made to address this challenge,1,2a,9 and metal-mediated cycloadditions have proven particularly valuable due to their efficacy in the formation of aromatic rings.10 This research group is focused on development of a unified approach to PAH synthesis (Scheme 1a), based on divergent [2 + 2 + n] cycloadditions of biaryl-tethered diynes or dinitriles (A) to give highly functionalized PAHs (B).11 Importantly, since only one conformational degree of freedom is frozen out with each cyclization, the minimal loss of entropy contributes to excellent yields11a,b and enables the use of more challenging dinitrile11c substrates.
Scheme 1.
For a PAH to be a useful synthon, it must be available in appreciable quantities and be suitably functionalized, but this set of requirements has proven difficult to achieve. Given their high carbon content and synthetic versatility, alkynyl substituents are attractive for the elaboration of PAHs into more complex functional materials.12 Such substituents are especially useful for macrocyclization reactions to give arylene ethynylene macrocycles (AEMs),13 cycloarylenes,14 and other exotic macrocycles.15 These and related macrocyclic nanocarbons5c,7,16 represent a broad structural class of tremendous interest for their internal cavities and unique spatial arrangements of carbon-rich building blocks.
Alkynyl groups are usually installed onto PAHs from the corresponding halide (via Sonogashira coupling) or carbonyl (via nucleophilic attack), but these precursors are limited in number given the established regiochemical preferences for halogenation or oxidation. Other widely employed methods to access carbonyl-containing precursors involve Friedel–Crafts-type cyclizations or Diels–Alder/oxidation sequences, but these also offer limited structural diversity. Perhaps most importantly, these routes add synthetic steps and usually involve harsh conditions. Thus, a more direct entry to alkynylated PAHs that bypasses traditional intermediates would be highly desirable. Relevant previous work by the Matano17 and Tanaka15b groups (Scheme 1b) demonstrated use of [2 + 2 + n] reactions to produce alkynylated synthetic intermediates C and Den route to oligomeric (non-PAH) products. Importantly, in both cases, one of the alkynes on the starting material was protected by a bulky silyl group, which minimizes the many possible deleterious cycloaddition side reactions.18 The efficiency described above for cycloadditions of biaryl-tethered diynes (A) suggested that these transformations might be site-selective19 and select for tethered diyne units (i.e. with preservation of peripheral, non-tethered alkynyl groups), as shown in Scheme 1c. This would alleviate the need to protect one of the alkynes, which would greatly simplify the syntheses of oligo(alkynyl)arylene precursors (1) and provide direct access to valuable alkynylated PAHs (2).
Here we describe development of the strategy of Scheme 1c, which was found to be possible with both Ir-catalyzed [2 + 2 + 2] and Cp2Zr-mediated [2 + 2 + 1] cycloadditions. This previously unrecognized site-selectivity gives rapid access to alkynylated PAHs, which is demonstrated with thirteen examples, representing seven different ring systems (Schemes 2–4). The [2 + 2 + 1] variant also offers the possibility of a divergent annulation with heterocyclic rings. Due to the streamlined precursor syntheses and generally high yields, multigram quantities of alkynylated PAHs are easily accessed, which is critical for their intended use as synthetic intermediates. As a proof of concept, four of the new PAHs were exploited as synthons for macrocyclic nanocarbons via alkyne metathesis reactions of their alkynyl groups (Scheme 5). Three of these structurally complex macrocycles are derived from previously unknown PAH ring systems. More importantly, they possess a range of internal cavity sizes reaching well into the nanometer regime.
Scheme 2. Model system for site-selective [2 + 2 + 2] reaction.
Scheme 3. Generalization of the site-selective [2 + 2 + 2] reaction to more complex PAHs.
Scheme 4. Extension of the strategy to include a Cp2Zr-mediated formal [2 + 2 + 1] reaction.
Scheme 5. New PAHs as synthons for large arylene ethynylene macrocycles.
Results and discussion
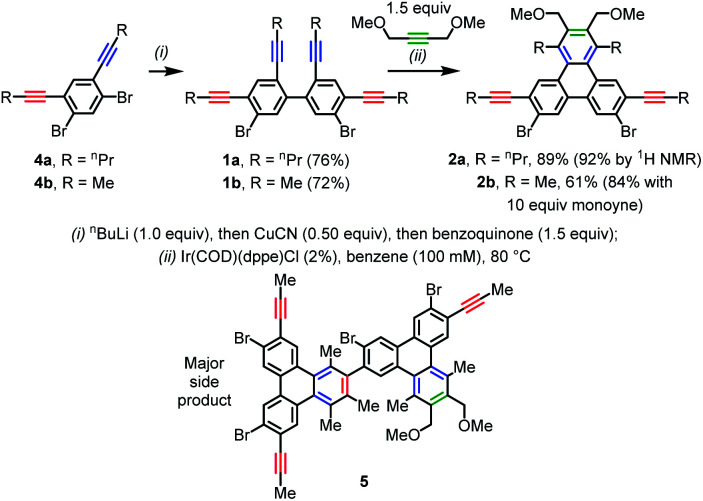
The new strategy was initially envisioned as a solution to difficulties encountered in the synthesis of alkynylated triphenylenes such as 2a and 2b (Scheme 2) using classical ring fusion techniques (e.g. the Scholl reaction20). Notably, there was no literature precedent for, or suitable synthetic precursors to, the deceptively simple 2,7-dialkynyl-3,6-dihalo triphenylene substitution pattern. Since the starting dialkynyl dihalides 4a and 4b are available in only two steps and can be easily homocoupled to yield tetra(alkynyl)biphenylene precursors 1a and 1b,11a this strategy would provide streamlined access to triphenylenes 2a and 2b if the subsequent site-selective [2 + 2 + 2] reaction was successful. The most important step in the synthesis of precursors 1a and 1b is the highly selective in situ desymmetrization via Li/Br exchange that precedes the Cu-mediated oxidative homocoupling. This desymmetrization also streamlines the synthesis of all other oligo(alkynyl)arylene substrates in this manuscript (e.g.1c–1l in Scheme 3; see ESI for more details†).
The [2 + 2 + 2] pre-catalyst Ir(COD)(dppe)Cl, which can be isolated21 or generated in situ22a,b from commercially available materials, proved to be highly effective for synthesis of model pentynylated triphenylene 2a. This compound was isolated in a remarkable 89% yield under optimized conditions. The preservation of the pentynyl groups in 2a was verified by 1H and 13C NMR spectroscopies (one propargylic methylene triplet and two quaternary alkynyl resonances, respectively) and high-resolution mass spectrometry. The choice of Ir(COD)(dppe)Cl as pre-catalyst was informed both by its exceptional efficiency in [2 + 2 + 2] reactions involving biphenyl-tethered diynes11b and by Takeuchi's demonstration of its ability to furnish alkynylated benzene products22a,b (in [2 + 2 + 2] reactions involving a 1,3-diyne reactant).
Since the peripheral pentynyl groups (shown in red) in the starting material (1a) and product (2a) are virtually identical, it was anticipated that competing intermolecular cycloadditions involving these groups would necessitate extensive screening and optimization efforts. In contrast, the yield of this transformation was fairly insensitive (82–93%) to wide ranges of values for several critical parameters, including concentration (10–300 mM), monoyne equivalents (1.0–5.7), catalyst loading (1–10%), and reaction time (1–10 h), as discussed further in the ESI (Tables S1–S3†). Under the optimized conditions, there was a notable decrease in yield (61%) for triphenylene 2b, which has less sterically demanding propynyl groups. The major side product was dimeric 5 (8–18% by 1H NMR, depending on concentration), resulting from a cycloaddition between the product (2b) and starting material (1b). Dilution from to 10 mM (from 100 mM) did not improve the yield. Notably, the yield of 2b increased to 84% with a larger excess of monoyne (10 equiv.), and side product 5 was not observed. Since bulkier pentynyl groups provided higher yields in the model system, they were used for the remaining examples below.
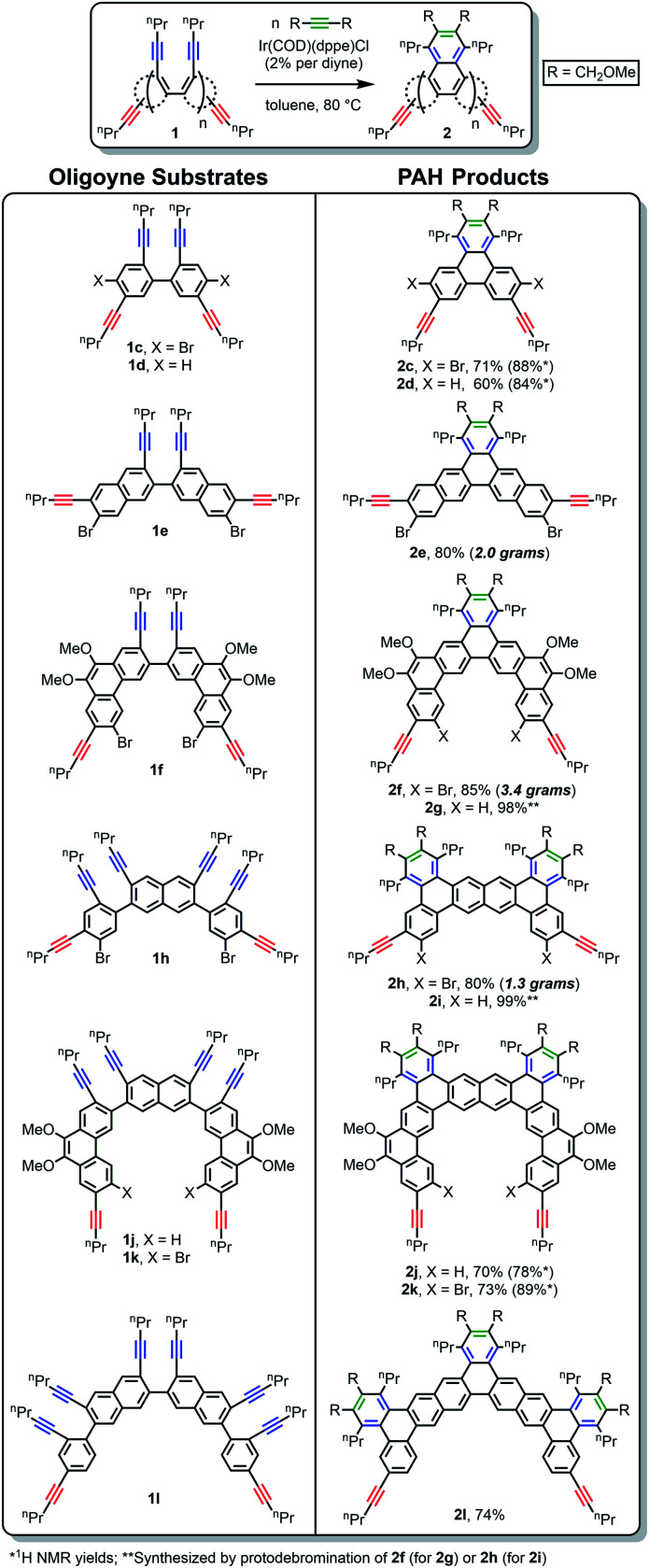
The site-selective [2 + 2 + 2] reaction proved to be a general means to access alkynylated PAHs, as demonstrated by the isolation of 2c–2l (Scheme 3). Precursors 1c–1l were synthesized in a modular fashion via standard C–C bond-forming reactions (see Schemes S1–S3 for details†), all of which were streamlined by Li-mediated desymmetrization reactions as mentioned above. From the same benzene, naphthalene, and phenanthrene building blocks, a range of different shapes, sizes, and alkynyl group substitution patterns were obtained via onefold, twofold, or threefold cycloadditions. Notably, the decrease in yields in the absence of the sterically demanding Br groups ortho to the alkynes (for 2d and 2j relative to 2c and 2k) was relatively small. This suggests that ortho substituents play a smaller role than the substituent on the alkynyl group in shutting down undesirable intermolecular side reactions. Also, the size of the PAH building blocks does not appear to play a large role. This can be seen by the comparable yields for 2a (89%), 2e (80%), and 2f (85%), which were derived from cycloadditions of biphenylene (1a), binaphthalene (1e), and biphenanthrene (1f) precursors, respectively. Interestingly, the generally good-to-excellent yields do not correlate with the number of required cycloadditions. For example, a twofold cycloaddition produced 2j in 70% yield, whereas a threefold cycloaddition produced 2l in 74% yield. The substitution patterns of the new PAHs would be difficult or impossible to access using previous synthetic methodology, even for the deceptively simple triphenylene systems 2a–d. Represented among these examples are the first known tribenzo[a,h,o]pentaphene (2f–g), tribenzo[a,h,o]phenanthro[2,3-q]hexaphene (2j–k), and pentabenzo[a,c,j,q,s]heptaphene (2l) ring systems. Perhaps most importantly, since high dilution is not necessary (diyne concentration = 100 mM), the new strategy is highly scalable. For example, 2e, 2f, and 2h were isolated on 2.0, 3.4, and 1.3 gram scales, respectively.
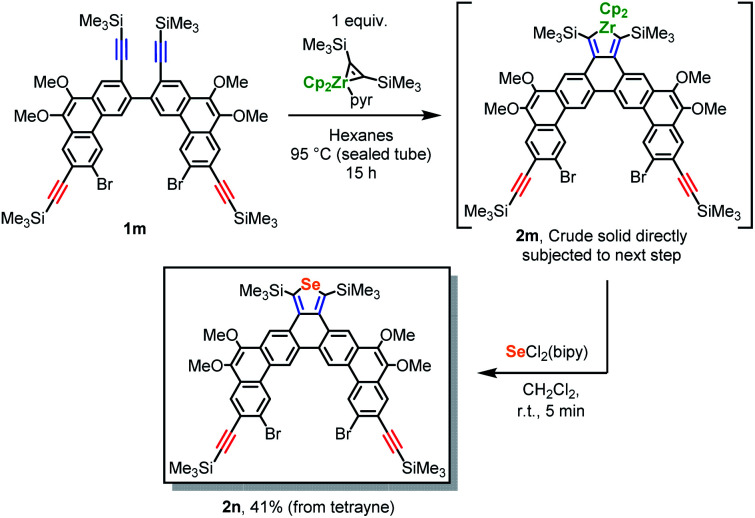
This strategy was subsequently generalized to include a formal [2 + 2 + 1] reaction, as shown in Scheme 4. Heating an equimolar mixture of 1m and Cp2Zr(pyr)(Me3SiC CSiMe3)23 (an isolable source of “Cp2Zr”) in hexanes, isolation of the crude zirconacyclopentadiene intermediate (2m) via filtration, and treatment of a CH2Cl2 solution of 2m with SeCl2(bipy)11b afforded selenophene-annulated dibenzo[a,o]pentaphene 2n in 41% isolated yield. The silyl groups on 1m were chosen since they often lead to reversible [2 + 2 + 1] cycloadditions involving zirconocene,14,24 and 2m was expected to be the thermodynamic product (entropy should favor monomeric 2m over the oligomeric products that would result from intermolecular cycloadditions14). Indeed, the site-selectivity of this reaction appears to occur under thermodynamic control. Thus, when 1m was treated with 1.0 equiv. of Cp2Zr(pyr)(Me3SiC CSiMe3) (in benzene-d6 due to solubility concerns), 2m was observed in only 20% yield after 10 min at 22 °C, along with starting 1m (30%), a complex mixture of unidentified products (likely oligomers), and no remaining Cp2Zr(pyr)(Me3SiC CSiMe3). Temperatures up to 90 °C did not lead to notable increases in 2m, but after 18 h at 105 °C the yield of 2m increased to 45%.25 Importantly, 2m is expected to be a versatile synthon for the installation of a range of other heterocycles using well-developed zirconacycle transfer chemistry.26
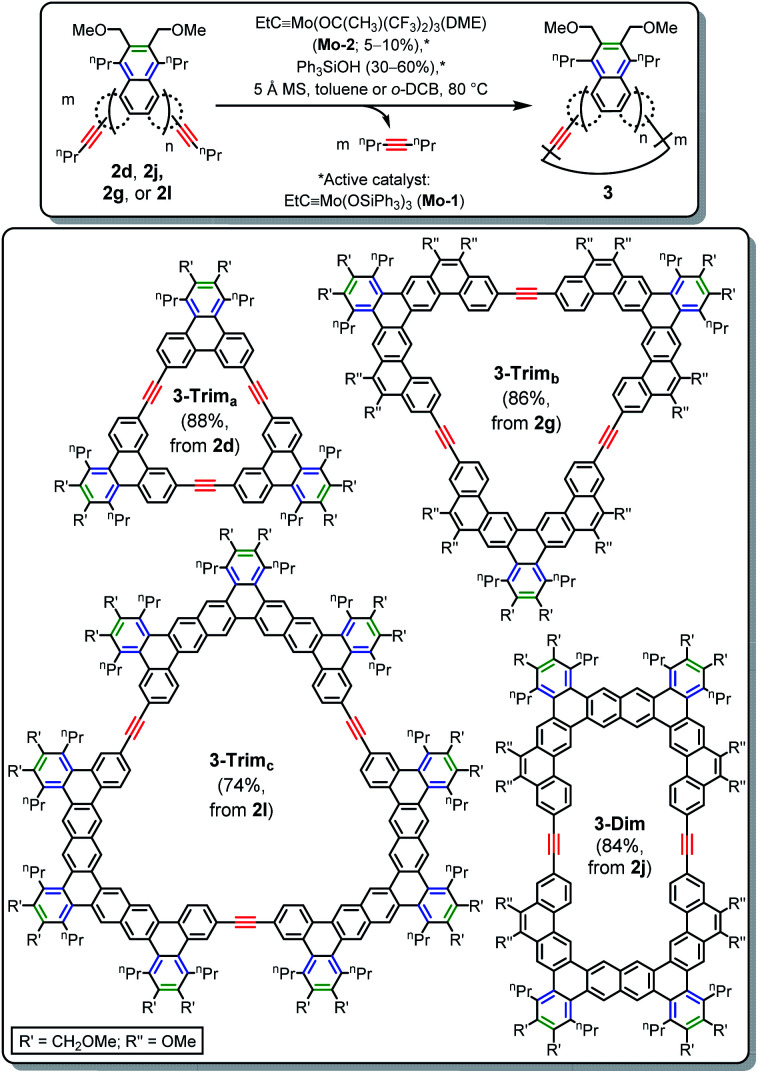
The angular disposition of the alkynyl groups in PAHs 2c–d and 2f–l makes them attractive for macrocyclization via alkyne metathesis, which often provides AEMs in a high yielding, scalable fashion.27 PAHs are intriguing building blocks for more complex AEMs, as has been demonstrated on a number of occasions.28 However, for all but the smallest PAHs (phenanthrene and carbazole), such AEMs have been produced by routes that are difficult to scale or generalize, probably due to difficulties associated with accessing appropriately functionalized PAH precursors. The site-selective [2 + 2 + 2] cycloaddition presented above directly addresses those difficulties.
For the macrocyclization reactions of Scheme 5, the Fürstner-type metathesis catalyst29 “EtC Mo(OSiPh3)3” (Mo-1) was employed. In analogy to the approach taken by Moore,30 we found that Mo-1 can be conveniently generated in situ by treatment of easily-prepared EtC Mo(OC(CH3)(CF3)2)3(DME) (Mo-2)31 with 6 equiv. of Ph3SiOH (see ESI for details on this ligand exchange†). Triphenylene ethynylene macrocycle 3-Trima was isolated in excellent yield (88%) after treatment of 2d with a 5% loading of Mo-1 in the presence of 5 Å molecular sieves29 (16 h at 80 °C in toluene). The identity of 3-Trima was unambiguously determined by 1H and 13C NMR spectroscopy (the former showed the absence of propargylic methylene resonances and the latter showed only one quaternary alkynyl resonance) and MALDI-TOF. As is often the case for alkyne metathesis macrocyclizations,27c,d minimal purification was required (elution of the reaction mixture through a plug of silica gel followed by precipitation with MeOH), and high dilution was not necessary. Attempts at the macrocyclization of the analogous, Br-substituted 2c yielded a complex mixture that contained only small amounts of macrocycle, even at higher (20%) catalyst loading.
Remarkably, the synthesis of 3-Trima demonstrates that 5 Å MS are effective for sequestration of the byproduct 4-octyne, which is imperative for the high yield. Fürstner's 2010 discovery29 that alkyne metathesis reactions can be driven by sequestration of (smaller) 2-butyne has played a critical role in the continued rise of this synthetic methodology.32 As a result, the use of propynyl groups has become ubiquitous since the easily-sequestered 2-butyne byproduct is produced, but the installation of propynyl groups requires either gaseous propyne (for Sonogashira coupling) or expensive propynyl magnesium bromide solution (for Kumada coupling). The high-yielding synthesis of 3-Trima (and the other macrocycles presented below) demonstrates that pentynyl groups may provide a cheaper, more convenient alternative to propynyl groups.
Since the presence of bulky Br substituents appears to inhibit macrocyclization with Mo-1 as catalyst, attention was focused on “Br-free” 2g, 2i, 2j, and 2l. Due to the larger sizes of these PAHs, the solvent was switched to o-dichlorobenzene (o-DCB) to limit the possibility of kinetic traps via precipitation of oligomeric or polymeric intermediates. Subjection of 2j, 2g, and 2l to the slightly modified metathesis conditions afforded the elliptical macrocycle 3-Dim and large, triangular macrocycles 3-Trimb and 3-Trimc in good to excellent isolated yields. While the yield of the largest of these macrocycles (3-Trimc) improved on dilution (from 43% at 25 mM to 74% at 9 mM), this is still quite concentrated for a macrocyclization reaction. Since no well-defined side products were observed in any case, purification was again streamlined. Unfortunately, subjection of 2i to the conditions of Scheme 5 produced an intractable mixture composed primarily (∼95% by mass) of insoluble material that could not be characterized. It is important to note that, except for the modest dilution in the case of 3-Trimc, we have not attempted to optimize any parameter in these reactions.
In general, the new PAHs (2a–n) and macrocycles (3) are highly soluble in chlorinated solvents (>100 mg mL−1) and insoluble in saturated hydrocarbons (e.g. hexanes) and more polar solvents (e.g. acetone and EtOH). The exception is 3-Dim, which is only moderately soluble in chloroform (15–20 mg mL−1). The solution behavior of the new AEMs (3) and their PAH precursors (2d, 2j, 2g, and 2l) were investigated by variable concentration 1H NMR spectroscopy. The chemical shifts in chloroform-d of all except 3-Dim and 3-Trimb are independent of concentration (see Fig. S74–S81†), which suggests that the high solubility results from limited aggregation via π-stacking. This is likely a result of the alkyl groups in the bay positions, which induce non-planarity. In contrast, despite this alkyl group substitution, the 1H NMR spectra of 3-Dim and 3-Trimb in chloroform-d solution exhibit significantly broadened aromatic resonances with chemical shifts that depend upon concentration, which suggests aggregation via π-stacking.
The UV/vis absorption and emission spectra of AEMs (3) and their monomeric precursors (2) display the typical features of large, highly benzenoid PAH systems,3a including high molar absorptivities (up to 5.0 × 105 M−1 cm−1, for 3-Trimc), relatively large optical HOMO–LUMO gaps (between 2.8 to 3.3 eV), and fluorescence (see Table S4 and Fig. S82–S86†). Despite the large increases in the sizes of the π-systems as a result of macrocyclization of 2 to form 3, the expected red shifts in absorption maxima range from small (21 nm for 3-Dim) to modest (54 nm for 3-Trima). More strikingly, very small decreases in optical HOMO–LUMO gap (≤0.15 eV) and emission maxima (≤12 nm) are observed. Thus, to a large extent, the new PAH-containing AEMs retain the properties of their PAH precursors.
Conclusions
The results presented above demonstrate how the newly discovered site-selectivity of an Ir-catalyzed [2 + 2 + 2] cycloaddition enables streamlined construction of complex nanocarbons. Here, the [2 + 2 + 2] reaction was used in concert with an orthogonal alkyne metathesis macrocyclization to provide scalable, two-step access to large, PAH-containing AEMs. Several opportunities are available for the introduction of structural and functional diversity, which might be systematically exploited to perturb self-assembly, photophysical, or electronic properties of a specific AEM, or to access entirely new AEMs. It is important to note, however, that the targeted AEMs represent only a proof of concept; the intermediate alkynylated PAHs 2a–n are valuable synthons for a range of other conjugated nanocarbons, including expanded helicenes, cycloarenes, and other macrocyclic nanocarbons. Such applications are the focus of continuing efforts in our laboratory.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was funded by the National Science Foundation under Grant No. CHE-1708210. We thank UC Berkeley's NMR facility for resources provided and the staff for their assistance. Instruments in the NMR facility are supported in part by NIH S10OD024998. We thank Stephen von Kugelgen and Felix R. Fischer for initial samples of Mo-2, and Stephen von Kugelgen for helpful discussions.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc06102a
Notes and references
- (a) Müllen K., Graphene as a Target for Polymer Synthesis, in Hierarchical Macromolecular Structures: 60 Years after the Staudinger Nobel Prize II, ed. V. Percec, Advances in Polymer Science; Springer International Publishing, 2013, pp. 61–92 [Google Scholar]; (b) Segawa Y. Ito H. Itami K. Structurally Uniform and Atomically Precise Carbon Nanostructures. Nat. Rev. Mater. 2016;1(1):15002. doi: 10.1038/natrevmats.2015.2. [DOI] [Google Scholar]
- (a) Polycyclic Arenes and Heteroarenes: Synthesis, Properties, and Applications, ed. Q. Miao, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2015 [Google Scholar]; (b) Carbon-Rich Compounds: From Molecules to Materials, ed. M. M. Haley and R. R. Tykwinski, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2006 [Google Scholar]; (c) Wu J. Pisula W. Müllen K. Graphenes as Potential Material for Electronics. Chem. Rev. 2007;107(3):718–747. doi: 10.1021/cr068010r. [DOI] [PubMed] [Google Scholar]
- (a) Rieger R. Müllen K. Forever Young: Polycyclic Aromatic Hydrocarbons as Model Cases for Structural and Optical Studies. J. Phys. Org. Chem. 2010;23(4):315–325. [Google Scholar]; (b) Sun Z. Ye Q. Chi C. Wu J. Low Band Gap Polycyclic Hydrocarbons: From Closed-Shell near Infrared Dyes and Semiconductors to Open-Shell Radicals. Chem. Soc. Rev. 2012;41:7857–7889. doi: 10.1039/C2CS35211G. [DOI] [PubMed] [Google Scholar]; (c) Chen Z. Lohr A. Saha-Möller C. R. Würthner F. Self-Assembled π-Stacks of Functional Dyes in Solution: Structural and Thermodynamic Features. Chem. Soc. Rev. 2009;38(2):564–584. doi: 10.1039/B809359H. [DOI] [PubMed] [Google Scholar]
- (a) Ruffieux P. Wang S. Yang B. Sánchez-Sánchez C. Liu J. Dienel T. Talirz L. Shinde P. Pignedoli C. A. Passerone D. Dumslaff T. Feng X. Müllen K. Fasel R. On-Surface Synthesis of Graphene Nanoribbons with Zigzag Edge Topology. Nature. 2016;531(7595):489–492. doi: 10.1038/nature17151. [DOI] [PubMed] [Google Scholar]; (b) Fogel Y. Zhi L. Rouhanipour A. Andrienko D. Räder H. J. Müllen K. Graphitic Nanoribbons with Dibenzo[e,l]Pyrene Repeat Units: Synthesis and Self-Assembly. Macromolecules. 2009;42(18):6878–6884. doi: 10.1021/ma901142g. [DOI] [Google Scholar]; (c) Rizzo D. J. Veber G. Cao T. Bronner C. Chen T. Zhao F. Rodriguez H. Louie S. G. Crommie M. F. Fischer F. R. Topological Band Engineering of Graphene Nanoribbons. Nature. 2018;560(7717):204–208. doi: 10.1038/s41586-018-0376-8. [DOI] [PubMed] [Google Scholar]
- (a) Fort E. H. Donovan P. M. Scott L. T. Diels–Alder Reactivity of Polycyclic Aromatic Hydrocarbon Bay Regions: Implications for Metal-Free Growth of Single-Chirality Carbon Nanotubes. J. Am. Chem. Soc. 2009;131(44):16006–16007. doi: 10.1021/ja907802g. [DOI] [PubMed] [Google Scholar]; (b) Jasti R. Bertozzi C. R. Progress and Challenges for the Bottom-up Synthesis of Carbon Nanotubes with Discrete Chirality. Chem. Phys. Lett. 2010;494(1):1–7. doi: 10.1016/j.cplett.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Omachi H. Nakayama T. Takahashi E. Segawa Y. Itami K. Initiation of Carbon Nanotube Growth by Well-Defined Carbon Nanorings. Nat. Chem. 2013;5(7):572–576. doi: 10.1038/nchem.1655. [DOI] [PubMed] [Google Scholar]
- Peurifoy S. R. Russell J. C. Sisto T. J. Yang Y. Roy X. Nuckolls C. Designing Three-Dimensional Architectures for High-Performance Electron Accepting Pseudocapacitors. J. Am. Chem. Soc. 2018;140(35):10960–10964. doi: 10.1021/jacs.8b07365. [DOI] [PubMed] [Google Scholar]
- (a) Iyoda M. Yamakawa J. Rahman M. J. Conjugated Macrocycles: Concepts and Applications. Angew. Chem., Int. Ed. 2011;50(45):10522–10553. doi: 10.1002/anie.201006198. [DOI] [PubMed] [Google Scholar]; (b) Ghasemabadi P. G. Yao T. Bodwell G. J. Cyclophanes Containing Large Polycyclic Aromatic Hydrocarbons. Chem. Soc. Rev. 2015;44:6494–6518. doi: 10.1039/C5CS00274E. [DOI] [PubMed] [Google Scholar]
- (a) Tran L. D. Ma J. Wong-Foy A. G. Matzger A. J. A Perylene-Based Microporous Coordination Polymer Interacts Selectively with Electron-Poor Aromatics. Chem.–Eur. J. 2016;22(16):5509–5513. doi: 10.1002/chem.201600526. [DOI] [PubMed] [Google Scholar]; (b) Fellows W. B. Rice A. M. Williams D. E. Dolgopolova E. A. Vannucci A. K. Pellechia P. J. Smith M. D. Krause J. A. Shustova N. B. Redox-Active Corannulene Buckybowls in a Crystalline Hybrid Scaffold. Angew. Chem., Int. Ed. 2016;55(6):2195–2199. doi: 10.1002/anie.201509557. [DOI] [PubMed] [Google Scholar]; (c) Dong R. Zhang Z. Tranca D. C. Zhou S. Wang M. Adler P. Liao Z. Liu F. Sun Y. Shi W. Zhang Z. Zschech E. Mannsfeld S. C. B. Felser C. Feng X. A Coronene-Based Semiconducting Two-Dimensional Metal-Organic Framework with Ferromagnetic Behavior. Nat. Commun. 2018;9(1):1–9. doi: 10.1038/s41467-017-02088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Qin J.-S. Yuan S. Zhang L. Li B. Du D.-Y. Huang N. Guan W. Drake H. F. Pang J. Lan Y.-Q. Alsalme A. Zhou H.-C. Creating Well-Defined Hexabenzocoronene in Zirconium Metal–Organic Framework by Postsynthetic Annulation. J. Am. Chem. Soc. 2019;141(5):2054–2060. doi: 10.1021/jacs.8b11042. [DOI] [PubMed] [Google Scholar]; (e) Suginome S. Sato H. Hori A. Mishima A. Harada Y. Kusaka S. Matsuda R. Pirillo J. Hijikata Y. Aida T. One-Step Synthesis of an Adaptive Nanographene MOF: Adsorbed Gas-Dependent Geometrical Diversity. J. Am. Chem. Soc. 2019;141(39):15649–15655. doi: 10.1021/jacs.9b07732. [DOI] [PubMed] [Google Scholar]
- (a) Narita A. Wang X.-Y. Feng X. Müllen K. New Advances in Nanographene Chemistry. Chem. Soc. Rev. 2015;44:6616–6643. doi: 10.1039/C5CS00183H. [DOI] [PubMed] [Google Scholar]; (b) Stępień M. Gońka E. Żyła M. Sprutta N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017;117(4):3479–3716. doi: 10.1021/acs.chemrev.6b00076. [DOI] [PubMed] [Google Scholar]; (c) Ito H. Segawa Y. Murakami K. Itami K. Polycyclic Arene Synthesis by Annulative π-Extension. J. Am. Chem. Soc. 2019;141(1):3–10. doi: 10.1021/jacs.8b09232. [DOI] [PubMed] [Google Scholar]
- General metal-mediated cycloaddition reviews: ; (a) Vollhardt K. P. C. Cobalt-Mediated [2 + 2 + 2]-Cycloadditions: A Maturing Synthetic Strategy [New Synthetic Methods (43)] Angew. Chem., Int. Ed. Engl. 1984;23(8):539–556. doi: 10.1002/anie.198405393. [DOI] [Google Scholar]; (b) Transition-Metal-Mediated Aromatic Ring Construction, ed. K. Tanaka, John Wiley & Sons, Inc., Hoboken, NJ, 2013, pp. 1−320 [Google Scholar]; ; Selected PAH applications:; (c) Jin T. Zhao J. Asao N. Yamamoto Y. Metal-Catalyzed Annulation Reactions for π-Conjugated Polycycles. Chem.–Eur. J. 2014;20(13):3554–3576. doi: 10.1002/chem.201304640. [DOI] [PubMed] [Google Scholar]; (d) Han S. Bond A. D. Disch R. L. Holmes D. Schulman J. M. Teat S. J. Vollhardt K. P. C. Whitener G. D. Total Syntheses and Structures of Angular [6]- and [7]Phenylene: The First Helical Phenylenes (Heliphenes) Angew. Chem., Int. Ed. 2002;41(17):3223–3227. doi: 10.1002/1521-3773(20020902)41:17<3223::AID-ANIE3223>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]; (e) Teplý F. Stará I. G. Starý I. Kollárovič A. Šaman D. Rulíšek L. Fiedler P. Synthesis of [5]-, [6]-, and [7]Helicene via Ni(0)- or Co(I)-Catalyzed Isomerization of Aromatic Cis, Cis-Dienetriynes. J. Am. Chem. Soc. 2002;124(31):9175–9180. doi: 10.1021/ja0259584. [DOI] [PubMed] [Google Scholar]; (f) Wu Y.-T. Hayama T. Baldridge K. K. Linden A. Siegel J. S. Synthesis of Fluoranthenes and Indenocorannulenes: Elucidation of Chiral Stereoisomers on the Basis of Static Molecular Bowls. J. Am. Chem. Soc. 2006;128(21):6870–6884. doi: 10.1021/ja058391a. [DOI] [PubMed] [Google Scholar]; (g) Pérez D. Peña D. Guitián E. Aryne Cycloaddition Reactions in the Synthesis of Large Polycyclic Aromatic Compounds. Eur. J. Org. Chem. 2013;2013(27):5981–6013. doi: 10.1002/ejoc.201300470. [DOI] [Google Scholar]; (h) Takahashi T. Kitamura M. Shen B. Nakajima K. Straightforward Method for Synthesis of Highly Alkyl-Substituted Naphthacene and Pentacene Derivatives by Homologation. J. Am. Chem. Soc. 2000;122(51):12876–12877. doi: 10.1021/ja003130g. [DOI] [Google Scholar]; (i) Sawada Y. Furumi S. Takai A. Takeuchi M. Noguchi K. Tanaka K. Rhodium-Catalyzed Enantioselective Synthesis, Crystal Structures, and Photophysical Properties of Helically Chiral 1,1′-Bitriphenylenes. J. Am. Chem. Soc. 2012;134(9):4080–4083. doi: 10.1021/ja300278e. [DOI] [PubMed] [Google Scholar]
- (a) Kiel G. R. Ziegler M. S. Tilley T. D. Zirconacyclopentadiene-Annulated Polycyclic Aromatic Hydrocarbons. Angew. Chem., Int. Ed. 2017;56(17):4839–4844. doi: 10.1002/anie.201700818. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kiel G. R. Patel S. C. Smith P. W. Levine D. S. Tilley T. D. Expanded Helicenes: A General Synthetic Strategy and Remarkable Supramolecular and Solid-State Behavior. J. Am. Chem. Soc. 2017;139(51):18456–18459. doi: 10.1021/jacs.7b10902. [DOI] [PubMed] [Google Scholar]; (c) Kiel G. R. Samkian A. E. Nicolay A. Witzke R. J. Tilley T. D. Titanocene-Mediated Dinitrile Coupling: A Divergent Route to Nitrogen-Containing Polycyclic Aromatic Hydrocarbons. J. Am. Chem. Soc. 2018;140(7):2450–2454. doi: 10.1021/jacs.7b13823. [DOI] [PubMed] [Google Scholar]
- Diederich F., Stang P. J. and Tykwinski R. R., Acetylene Chemistry: Chemistry, Biology and Material Science, John Wiley & Sons, 2006 [Google Scholar]
- (a) Zhao D. Moore J. S. Shape-Persistent Arylene Ethynylene Macrocycles: Syntheses and Supramolecular Chemistry. Chem. Commun. 2003:807–818. doi: 10.1039/B207442G. [DOI] [PubMed] [Google Scholar]; (b) Höger S. Shape-Persistent Macrocycles: From Molecules to Materials. Chem.–Eur. J. 2004;10(6):1320–1329. doi: 10.1002/chem.200305496. [DOI] [PubMed] [Google Scholar]; (c) Sadowy A. L. and Tykwinski R. R., Shape-Persistent Macrocycles Based on Acetylenic Scaffolding, in Modern Supramolecular Chemistry: Strategies for Macrocycle Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2008, pp. 185–231 [Google Scholar]; (d) Smith M. K. Miljanić O. Š. Arylene Ethynylene Macrocycles: From Molecular Hosts to Components of High-Performance Supramolecular Architectures. Org. Biomol. Chem. 2015;13(29):7841–7845. doi: 10.1039/C5OB01101A. [DOI] [PubMed] [Google Scholar]
- Gessner V. H. Tannaci J. F. Miller A. D. Tilley T. D. Assembly of Macrocycles by Zirconocene-Mediated, Reversible Carbon–Carbon Bond Formation. Acc. Chem. Res. 2011;44(6):435–446. doi: 10.1021/ar100148g. [DOI] [PubMed] [Google Scholar]
- (a) Sprafke J. K. Kondratuk D. V. Wykes M. Thompson A. L. Hoffmann M. Drevinskas R. Chen W.-H. Yong C. K. Kärnbratt J. Bullock J. E. Malfois M. Wasielewski M. R. Albinsson B. Herz L. M. Zigmantas D. Beljonne D. Anderson H. L. Belt-Shaped π-Systems: Relating Geometry to Electronic Structure in a Six-Porphyrin Nanoring. J. Am. Chem. Soc. 2011;133(43):17262–17273. doi: 10.1021/ja2045919. [DOI] [PubMed] [Google Scholar]; (b) Hayase N. Sugiyama H. Uekusa H. Shibata Y. Tanaka K. Rhodium-Catalyzed Synthesis, Crystal Structures, and Photophysical Properties of [6]Cycloparaphenylene Tetracarboxylates. Org. Lett. 2019;21(11):3895–3899. doi: 10.1021/acs.orglett.9b00820. [DOI] [PubMed] [Google Scholar]
- (a) Golder M. R. Jasti R. Syntheses of the Smallest Carbon Nanohoops and the Emergence of Unique Physical Phenomena. Acc. Chem. Res. 2015;48(3):557–566. doi: 10.1021/ar5004253. [DOI] [PubMed] [Google Scholar]; (b) Segawa Y. Yagi A. Matsui K. Itami K. Design and Synthesis of Carbon Nanotube Segments. Angew. Chem., Int. Ed. 2016;55(17):5136–5158. doi: 10.1002/anie.201508384. [DOI] [PubMed] [Google Scholar]; (c) Kayahara E. Kouyama T. Kato T. Takaya H. Yasuda N. Yamago S. Isolation and Characterization of the Cycloparaphenylene Radical Cation and Dication. Angew. Chem., Int. Ed. 2013;52(51):13722–13726. doi: 10.1002/anie.201306881. [DOI] [PubMed] [Google Scholar]; (d) Xu Y. von Delius M. The Supramolecular Chemistry of Strained Carbon Nanohoops. Angew. Chem., Int. Ed. 2020;59(2):559–573. doi: 10.1002/anie.201906069. [DOI] [PubMed] [Google Scholar]; (e) Miyoshi H. Nobusue S. Shimizu A. Tobe Y. Non-Alternant Non-Benzenoid Kekulenes: The Birth of a New Kekulene Family. Chem. Soc. Rev. 2015;44:6560–6577. doi: 10.1039/C5CS00185D. [DOI] [PubMed] [Google Scholar]; (f) Buttrick J. C. King B. T. Kekulenes, Cycloarenes, and Heterocycloarenes: Addressing Electronic Structure and Aromaticity through Experiments and Calculations. Chem. Soc. Rev. 2017;46:7–20. doi: 10.1039/C6CS00174B. [DOI] [PubMed] [Google Scholar]; (g) Gregolińska H. Majewski M. Chmielewski P. J. Gregoliński J. Chien A. Zhou J. Wu Y.-L. Bae Y. J. Wasielewski M. R. Zimmerman P. M. Stępień M. Fully Conjugated [4]Chrysaorene. Redox-Coupled Anion Binding in a Tetraradicaloid Macrocycle. J. Am. Chem. Soc. 2018;140(43):14474–14480. doi: 10.1021/jacs.8b09385. [DOI] [PubMed] [Google Scholar]; (h) Liu C. Sandoval-Salinas M. E. Hong Y. Gopalakrishna T. Y. Phan H. Aratani N. Herng T. S. Ding J. Yamada H. Kim D. Casanova D. Wu J. Macrocyclic Polyradicaloids with Unusual Super-Ring Structure and Global Aromaticity. Chem. 2018;4(7):1586–1595. doi: 10.1016/j.chempr.2018.03.020. [DOI] [Google Scholar]; (i) Iyoda M. Shimizu H. Multifunctional π-Expanded Oligothiophene Macrocycles. Chem. Soc. Rev. 2015;44(18):6411–6424. doi: 10.1039/C5CS00388A. [DOI] [PubMed] [Google Scholar]; (j) Lee S. Chen C.-H. Flood A. H. A Pentagonal Cyanostar Macrocycle with Cyanostilbene CH Donors Binds Anions and Forms Dialkylphosphate [3]Rotaxanes. Nat. Chem. 2013;5(8):704–710. doi: 10.1038/nchem.1668. [DOI] [PubMed] [Google Scholar]; (k) Schneebeli S. T. Frasconi M. Liu Z. Wu Y. Gardner D. M. Strutt N. L. Cheng C. Carmieli R. Wasielewski M. R. Stoddart J. F. Electron Sharing and Anion–π Recognition in Molecular Triangular Prisms. Angew. Chem., Int. Ed. 2013;52(49):13100–13104. doi: 10.1002/anie.201307984. [DOI] [PubMed] [Google Scholar]; (l) Liu Z. Liu G. Wu Y. Cao D. Sun J. Schneebeli S. T. Nassar M. S. Mirkin C. A. Stoddart J. F. Assembly of Supramolecular Nanotubes from Molecular Triangles and 1,2-Dihalohydrocarbons. J. Am. Chem. Soc. 2014;136(42):16651–16660. doi: 10.1021/ja509480u. [DOI] [PubMed] [Google Scholar]; (m) Ball M. Zhong Y. Fowler B. Zhang B. Li P. Etkin G. Paley D. W. Decatur J. Dalsania A. K. Li H. Xiao S. Ng F. Steigerwald M. L. Nuckolls C. Macrocyclization in the Design of Organic N-Type Electronic Materials. J. Am. Chem. Soc. 2016;138(39):12861–12867. doi: 10.1021/jacs.6b05474. [DOI] [PubMed] [Google Scholar]
- Matano Y. Nakashima M. Imahori H. A Convenient Method for the Synthesis of α-Ethynylphospholes and Modulation of Their π-Conjugated Systems. Angew. Chem., Int. Ed. 2009;48(22):4002–4005. doi: 10.1002/anie.200900542. [DOI] [PubMed] [Google Scholar]
- Note that Marder subsequently developed Rh- and Ir-mediated cyclizations on substrates similar to Matano's, but with aryl groups in place of the silyls. Here, the aryl groups may have played a similar role in shutting down competing intermolecular cycloaddition reactions. Rh: ; (a) Steffen A. Tay M. G. Batsanov A. S. Howard J. A. K. Beeby A. Vuong K. Q. Sun X.-Z. George M. W. Marder T. B. 2,5-Bis(p-R-Arylethynyl)Rhodacyclopentadienes Show Intense Fluorescence: Denying the Presence of a Heavy Atom. Angew. Chem., Int. Ed. 2010;49:2349–2353. doi: 10.1002/anie.200905697. [DOI] [PubMed] [Google Scholar]; ; Ir: ; (b) Steffen A. Costuas K. Boucekkine A. Thibault M.-H. Beeby A. Batsanov A. S. Charaf-Eddin A. Jacquemin D. Halet J.-F. Marder T. B. Fluorescence in Rhoda- and Iridacyclopentadienes Neglecting the Spin–Orbit Coupling of the Heavy Atom: The Ligand Dominates. Inorg. Chem. 2014;53:7055–7069. doi: 10.1021/ic501115k. [DOI] [PubMed] [Google Scholar]
- Huang Z. Dong G. Site-Selectivity Control in Organic Reactions: A Quest To Differentiate Reactivity among the Same Kind of Functional Groups. Acc. Chem. Res. 2017;50(3):465–471. doi: 10.1021/acs.accounts.6b00476. [DOI] [PubMed] [Google Scholar]
- Kivala M., Wu D., Feng X., Li C. and Müllen K., Cyclodehydrogenation in the Synthesis of Graphene-Type Molecules, in Materials Science and Technology, Wiley-VCH Verlag GmbH & Co. KGaA, 2006 [Google Scholar]
- Makino T. Yamamoto Y. Itoh K. Synthesis and Structure of Novel Iridium(I) Complexes Containing η4-1,6-Diene and Diphosphine Ligands: Remarkable Effect of Ligand Bite Angle upon Ligand Dissociation. Organometallics. 2004;23(8):1730–1737. doi: 10.1021/om034282y. [DOI] [Google Scholar]
- (a) Kezuka S. Tanaka S. Ohe T. Nakaya Y. Takeuchi R. Iridium Complex-Catalyzed [2 + 2 + 2] Cycloaddition of α,ω-Diynes with Monoynes and Monoenes. J. Org. Chem. 2006;71(2):543–552. doi: 10.1021/jo051874c. [DOI] [PubMed] [Google Scholar]; (b) Takeuchi R. Nakaya Y. Iridium Complex-Catalyzed Highly Selective Cross [2 + 2 + 2] Cycloaddition of Two Different Monoynes: 2:1 Coupling versus 1:2 Coupling. Org. Lett. 2003;5(20):3659–3662. doi: 10.1021/ol035330d. [DOI] [PubMed] [Google Scholar]
- (a) Rosenthal U. Ohff A. Baumann W. Tillack A. Görls H. Burlakov V. V. Shur V. B. Struktur, Eigenschaften und NMR-spektroskopische Charakterisierung von Cp2Zr(Pyridin)(Me3SiC CSiMe3) Z. Anorg. Allg. Chem. 1995;621(1):77–83. doi: 10.1002/zaac.19956210114. [DOI] [Google Scholar]; (b) Nitschke J. R. Zürcher S. Tilley T. D. New Zirconocene-Coupling Route to Large, Functionalized Macrocycles. J. Am. Chem. Soc. 2000;122(42):10345–10352. doi: 10.1021/ja0020310. [DOI] [Google Scholar]
- Erker G. Zwettler R. Reactions of in situ Generated (η2-Phenyltrimethylsilylacetylene)Zirconocene. J. Organomet. Chem. 1991;409(1):179–188. doi: 10.1016/0022-328X(91)86143-E. [DOI] [Google Scholar]
- The low yield may result from kinetic traps such as oxidative addition of the C–Br bond to “Cp2Zr”
- (a) Fagan P. J. Nugent W. A. Calabrese J. C. Metallacycle Transfer from Zirconium to Main Group Elements: A Versatile Synthesis of Heterocycles. J. Am. Chem. Soc. 1994;116(5):1880–1889. doi: 10.1021/ja00084a031. [DOI] [Google Scholar]; (b) Takahashi T. Hara R. Nishihara Y. Kotora M. Copper-Mediated Coupling of Zirconacyclopentadienes with Dihalo Aromatic Compounds. Formation of Fused Aromatic Rings. J. Am. Chem. Soc. 1996;118(21):5154–5155. doi: 10.1021/ja960407x. [DOI] [Google Scholar]; (c) Takahashi T. Li Y. Stepnicka P. Kitamura M. Liu Y. Nakajima K. Kotora M. Coupling Reaction of Zirconacyclopentadienes with Dihalonaphthalenes and Dihalopyridines: A New Procedure for the Preparation of Substituted Anthracenes, Quinolines, and Isoquinolines. J. Am. Chem. Soc. 2002;124(4):576–582. doi: 10.1021/ja016848k. [DOI] [PubMed] [Google Scholar]; (d) Yan X. Xi C. Conversion of Zirconacyclopentadienes into Metalloles: Fagan–Nugent Reaction and Beyond. Acc. Chem. Res. 2015;48(4):935–946. doi: 10.1021/ar500429f. [DOI] [PubMed] [Google Scholar]
- (a) Ge P.-H. Fu W. Herrmann W. A. Herdtweck E. Campana C. Adams R. D. Bunz U. H. F. Structural Characterization of a Cyclohexameric Meta-Phenyleneethynylene Made by Alkyne Metathesis with In Situ Catalysts. Angew. Chem., Int. Ed. 2000;39(20):3607–3610. doi: 10.1002/1521-3773(20001016)39:20<3607::AID-ANIE3607>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]; (b) Miljanić O. Š. Vollhardt K. P. C. Whitener G. D. An Alkyne Metathesis-Based Route to Ortho-Dehydrobenzannulenes. Synlett. 2003;1:29–34. [Google Scholar]; (c) Zhang W. Moore J. S. Arylene Ethynylene Macrocycles Prepared by Precipitation-Driven Alkyne Metathesis. J. Am. Chem. Soc. 2004;126(40):12796. doi: 10.1021/ja046531v. [DOI] [PubMed] [Google Scholar]; (d) Yu C., Jin Y. and Zhang W., Shape-Persistent Macrocycles through Dynamic Covalent Reactions, in Dynamic Covalent Chemistry, John Wiley & Sons, Ltd, 2017, pp. 121–163 [Google Scholar]
- (a) Höger S. Cheng X. H. Ramminger A.-D. Enkelmann V. Rapp A. Mondeshki M. Schnell I. Discotic Liquid Crystals with an Inverted Structure. Angew. Chem., Int. Ed. 2005;44(18):2801–2805. doi: 10.1002/anie.200462319. [DOI] [PubMed] [Google Scholar]; (b) Liu W.-J. Zhou Y. Zhou Q.-F. Ma Y. Pei J. Shape-Persistent Elliptic Macrocycles Composed of Polycyclic Aromatic Hydrocarbons: Synthesis and Photophysical Properties. Org. Lett. 2008;10(11):2123–2126. doi: 10.1021/ol800505y. [DOI] [PubMed] [Google Scholar]; (c) Chan J. M. W. Tischler J. R. Kooi S. E. Bulović V. Swager T. M. Synthesis of J-Aggregating Dibenz[a,j]Anthracene-Based Macrocycles. J. Am. Chem. Soc. 2009;131(15):5659–5666. doi: 10.1021/ja900382r. [DOI] [PubMed] [Google Scholar]; (d) Zhang L. Gopee H. Hughes D. L. Cammidge A. N. Antiaromatic Twinned Triphenylene Discotics Showing Nematic Phases and 2-Dimensional π-Overlap in the Solid State. Chem. Commun. 2010;46:4255–4257. doi: 10.1039/C0CC00311E. [DOI] [PubMed] [Google Scholar]; (e) Chen T. Pan G.-B. Wettach H. Fritzsche M. Höger S. Wan L.-J. Yang H.-B. Northrop B. H. Stang P. J. 2D Assembly of Metallacycles on HOPG by Shape-Persistent Macrocycle Templates. J. Am. Chem. Soc. 2010;132(4):1328–1333. doi: 10.1021/ja907220f. [DOI] [PubMed] [Google Scholar]; (f) Finke A. D. Gross D. E. Han A. Moore J. S. Engineering Solid-State Morphologies in Carbazole–Ethynylene Macrocycles. J. Am. Chem. Soc. 2011;133(35):14063–14070. doi: 10.1021/ja204795q. [DOI] [PubMed] [Google Scholar]; (g) Venkataramana G. Dongare P. Dawe L. N. Thompson D. W. Zhao Y. Bodwell G. J. 1,8-Pyrenylene–Ethynylene Macrocycles. Org. Lett. 2011;13(9):2240–2243. doi: 10.1021/ol200485x. [DOI] [PubMed] [Google Scholar]; (h) Toyota S. Construction of Novel Molecular Architectures from Anthracene Units and Acetylene Linkers. Pure Appl. Chem. 2012;84(4):917–929. [Google Scholar]; (i) He Z. Xu X. Zheng X. Ming T. Miao Q. Conjugated Macrocycles of Phenanthrene: A New Segment of [6,6]-Carbon Nanotube and Solution-Processed Organic Semiconductors. Chem. Sci. 2013;4(12):4525–4531. doi: 10.1039/C3SC52077C. [DOI] [Google Scholar]; (j) Yang H. Du Y. Wan S. Trahan G. D. Jin Y. Zhang W. Mesoporous 2D Covalent Organic Frameworks Based on Shape-Persistent Arylene-Ethynylene Macrocycles. Chem. Sci. 2015;6(7):4049–4053. doi: 10.1039/C5SC00894H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Takaki Y. Ozawa R. Kajitani T. Fukushima T. Mitsui M. Kobayashi K. Synthesis and Self-Assembly of Cyclic 2,7-Anthrylene Ethynylene 1,3-Phenylene Ethynylene Trimer with a Planar Conformation. Chem.–Eur. J. 2016;22(47):16760–16764. doi: 10.1002/chem.201603627. [DOI] [PubMed] [Google Scholar]
- Heppekausen J. Stade R. Goddard R. Fürstner A. Practical New Silyloxy-Based Alkyne Metathesis Catalysts with Optimized Activity and Selectivity Profiles. J. Am. Chem. Soc. 2010;132(32):11045–11057. doi: 10.1021/ja104800w. [DOI] [PubMed] [Google Scholar]
- Lee S. Yang A. Moneypenny T. P. Moore J. S. Kinetically Trapped Tetrahedral Cages via Alkyne Metathesis. J. Am. Chem. Soc. 2016;138(7):2182–2185. doi: 10.1021/jacs.6b00468. [DOI] [PubMed] [Google Scholar]
- Gdula R. L. Johnson M. J. A. Highly Active Molybdenum–Alkylidyne Catalysts for Alkyne Metathesis: Synthesis from the Nitrides by Metathesis with Alkynes. J. Am. Chem. Soc. 2006;128(30):9614–9615. doi: 10.1021/ja058036k. [DOI] [PubMed] [Google Scholar]
- (a) Fürstner A. Alkyne Metathesis on the Rise. Angew. Chem., Int. Ed. 2013;52:2794–2819. doi: 10.1002/anie.201204513. [DOI] [PubMed] [Google Scholar]; (b) Ortiz M. Yu C. Jin Y. Zhang W. Poly(Aryleneethynylene)s: Properties, Applications and Synthesis Through Alkyne Metathesis. Top. Curr. Chem. 2017;375(4):69. doi: 10.1007/s41061-017-0156-1. [DOI] [PubMed] [Google Scholar]; (c) Ehrhorn H. Tamm M. Well-Defined Alkyne Metathesis Catalysts: Developments and Recent Applications. Chem.–Eur. J. 2019;25(13):3190–3208. doi: 10.1002/chem.201804511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.