Abstract

Cellular life depends on transport and communication across membranes, which is emphasized by the fact that membrane proteins are prime drug targets. The cell-like environment of membrane proteins has gained increasing attention based on its important role in function and regulation. As a versatile scaffold for bottom-up synthetic biology and nanoscience, giant liposomes represent minimalistic models of living cells. Nevertheless, the incorporation of fragile multiprotein membrane complexes still remains a major challenge. Here, we report on an approach for the functional reconstitution of membrane assemblies exemplified by human and bacterial ATP-binding cassette (ABC) transporters. We reveal that these nanomachineries transport substrates unidirectionally against a steep concentration gradient. Active substrate transport can be spatiotemporally resolved in single cell-like compartments by light, enabling real-time tracking of substrate export and import in individual liposomes. This approach will help to construct delicate artificial cell-like systems.
Keywords: ABC transporter, giant liposomes, membrane transport, single-liposome analysis, synthetic biology
Introduction
ABC transporters represent one of the largest and probably most ancient protein superfamilies, shuttling a broad range of chemically distinct compounds across lipid bilayers.1−3 These machines share a common blueprint of two nucleotide-binding domains (NBDs) operating in concert with two transmembrane domains (TMDs).4,5 ATP binding and NBD dimerization switch the transporter from an inward-facing to an outward-facing state.6,7 Substrate translocation across membranes is assisted by large conformational changes before ATP hydrolysis, and phosphate release changes the transporter back to the inward-facing state.8,9 Owing to their versatile cellular function, ABC transporters are linked to many diseases. For example, the transporter associated with antigen processing (TAP1/2) plays a vital role in adaptive immunity.6,10 The heterodimeric ABC transporter TmrAB from Thermus thermophilus exhibits structural and functional homology with TAP and can restore antigen processing in TAP-deficient human cells.11 The related ABC transporter TAP-like (TAPL) consists of a core transporter (coreTAPL) and an extra membrane-embedded TMD0 domain, responsible for lysosomal targeting.12−14 The homodimeric complex transports polypeptides into lysosomes and has been linked to lysosome biogenesis.15,16
Giant liposomes have gained increasing attention as minimalistic model systems, which mimic the size and shape of cells, making them ideal targets for light microscopy and studies of membrane morphology.17−19 The reduced membrane tension and curvature can affect the properties of membrane proteins.20 Nevertheless, the functional reconstitution of fragile membrane proteins in giant unilamellar vesicles (GUVs) remains a major bottleneck because preparation methods such as electroformation21 or hydrogel-assisted swelling22,23 harm protein integrity by drying of proteoliposomes upon reconstitution.24 Inkjetting,25,26 vesicle fusion,27−30 or detergent-mediated reconstitution31,32 comprise the drawback of requiring specialized equipment, delicate handling, and specific fusogenic proteins or lipids, which limits their broader application. To date, only a few examples of ATP-dependent transporters reconstituted in giant liposomes are known.31,33−36 Large concentrative substrate translocation, which is a hallmark of primary active transporters, has not yet been demonstrated.33−36
Here, we established a versatile approach for the functional reconstitution of membrane protein complexes in giant liposomes via hydrogel-assisted swelling using poly(vinyl alcohol) (PVA) by carefully optimizing conditions for protein activity. As a proof of principle, human and bacterial ABC transporters were functionally reconstituted. Our proteoGUV preparation amalgamates three steps: (i) reconstitution in large unilamellar vesicles (LUVs), (ii) meticulous drying of proteoliposomes with additional sucrose on a PVA hydrogel in a fine-tuned humid environment, and (iii) giant liposome formation by rehydration in physiological buffer supplemented with sucrose. The detergent-mediated reconstitution was used to incorporate both peptide transporters in large liposomes.37,38
Results and Discussion
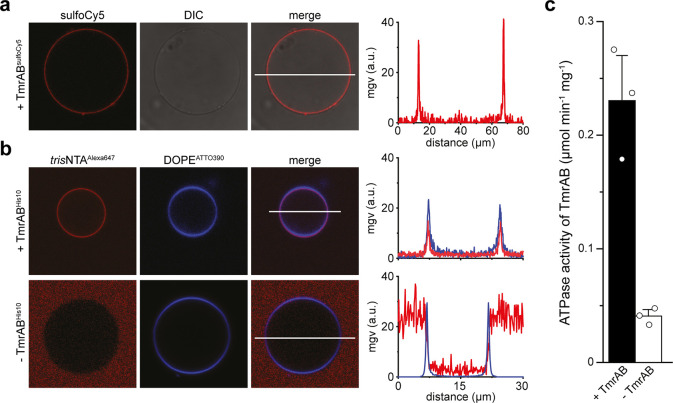
We first examined the reconstitution of TmrAB by direct and indirect fluorescence labeling. SulfoCy5-labeled TmrAB colocalizes with the lipid membrane after proteoGUV formation, indicating membrane incorporation (Figure 1a). Alternatively, TmrAB was site-specifically labeled by trisNTAAlexa647via a C-terminal His-tag,9,39−41 confirming that a fraction of the transporters is facing the NBDs to the external medium (Figure 1b, upper panel). In contrast, liposomes lacking TmrAB did not show trisNTAAlexa647 labeling (Figure 1b, lower panel). To address the function of the transport complex, we determined the ATP activity of TmrAB after reconstitution into GUVs. ProteoGUVs were generated, detached, and separated from the PVA hydrogel, followed by solubilization with a mild nonionic detergent. A high ATPase activity above background demonstrated TmrAB function (Figure 1c).
Figure 1.
Reconstitution of ABC transporters in giant liposomes. (a) SulfoCy5-labeled TmrAB was reconstituted in GUVs at a protein-to-lipid ratio of 1:20w/w. A line profile displays the mean gray values (mgv). (b) GUVs with and without reconstituted TmrAB were site-specifically labeled via the C-terminal His-tag of TmrA by trisNTAAlexa647. For dual-color visualization, the fluorescence gain was enhanced in the lower left and right image. The lipid bilayer was stained by DOPEATTO390. Line profiles demonstrate the colocalization of TmrAB and the lipid bilayer. (c) ATPase activity of TmrAB in isolated GUVs after detergent solubilization. The mean ± SD (n = 3) is shown.
ABC transporters are often assessed by their ATPase activity; however, the most important property is substrate translocation against a concentration gradient. We reconstituted fluorescently tagged human core TAPL, termed coreTAPLmVenus, in giant liposomes. Colocalization of mVenus fluorescence and the lipid membrane indicated incorporation of the complex (Figure 2a). TAPL function was assessed by translocation of the fluorescein-labeled peptide C4F in the presence of an ATP-regeneration system (ARS). Using confocal microscopy, we followed peptide accumulation above background, reaching a 4-fold increase at 37 °C (n = 11, number of imaged TAPL-containing GUVs). The membrane integrity was confirmed and no passive diffusion of C4F was observed in the absence of ATP (Figure S1).
Figure 2.

Functional reconstitution and active uphill transport of human TAPL in giant liposomes. (a) CoreTAPLmVenus incorporation and ATTO655 encapsulation in giant liposomes. (b) Peptide transport of coreTAPLmVenus for 2 h at 37 °C, after addition of ATP, ARS, and C4F peptides. Scale bars, 10 μm. All images are processed by ImageJ for better visualization.
Owing to the short functional lifetime of TAPL, which hinders long-term transport assays,12 we focused on the bacterial ABC transporter TmrAB. To track single-liposome transport over extended periods of time, we tethered biotinylated proteoGUVs via streptavidin to biotin-functionalized PEG glass slides (Figure 3a). Based on the thermophilic nature of TmrAB, all the following experiments were performed at 40 °C with an ARS and a protein-to-lipid reconstitution ratio of 1:15w/w. Under these conditions, the membrane integrity and leakage were monitored by Oyster647, a small, membrane-impermeable fluorophore (Figure 3b). After addition of ATP and C4F peptide, substrate accumulation in giant liposomes was monitored in real time. It is worth mentioning that the background fluorescence of C4F is lower at the beginning of the time-lapse recordings due to incomplete diffusion in the reservoir chamber. Notably, individual giant liposomes showed a >120-fold accumulation compared to background fluorescence, demonstrating active transport against a steep concentration gradient (Figure 3c). Before initiating the transport reaction by ATP addition, proteoGUVs show lower fluorescence in the interior compared to background (Figure S2). Building up high concentration gradients is a hallmark of primary-active transporters and has not been achieved in giant liposomes so far. Limitations in even higher accumulation capacities are most likely caused by ATP depletion and product (ADP) inhibition because protein stability for more than 2 h has been confirmed by transport studies with preincubated TmrAB at 40 °C (Figure S3). A 4000-fold accumulation in large unilamellar liposomes (∼100 nm) was previously observed, which requires high concentrations of phosphocreatine and ATP (24 and 3 mM, respectively).11 These conditions are incompatible with giant vesicle stabilities. The critical membrane tension resulting in membrane rupture is about 10 mN/m for 1,2-dioleyl-sn-glycero-3-phosphocholine (DOPC) membranes,42 which corresponds to a concentration gradient of a few millimolar.43
Figure 3.
Transport against a concentration gradient. (a) Transport assay of TmrAB in giant liposomes. The membrane-impermeable Oyster647 dye was encapsulated in proteoGUVs to control membrane integrity. GUVs were tethered to functionalized glass slides. ATP was added to drive the transport of fluorescent C4F peptides. (b) Time-lapse recordings demonstrate the transport activity of TmrAB. GUVs were filled with the Oyster647 fluorophore, and transport was induced by addition of ATP, ARS, and C4F peptides. Scale bar, 10 μm. (c) Representative analysis of two time-lapse traces. The accumulation of C4F peptides in giant liposomes was normalized to background fluorescence. (d) Rate constants (C4F per TmrAB per min) were determined for different C4F concentrations. Each dot represents the transport rate for an individual giant liposome. The median is given for each box plot (red line) and is illustrated in the inset for various peptide concentrations; 25–75% of the mean are shown as boxes and error bars as SD. The data were obtained from more than nine independent experiments (biological replicates).
It is worth noting that the volume of liposomes scales with the radius to the power of 3, while the surface area (proportional to the number of reconstituted transporters) scales to the power of 2. This scaling difference between surface and volume restricts the accumulation in giant liposomes for a given period of time compared to ∼100 nm vesicles. For example, for liposomes of 0.1 and 10.9 μm in diameter, 0.02 × 10–3 and 1.84 × 10–3 fL per transporter have to be filled, respectively (compare Table 1). In order to achieve a similar accumulation, a substantially larger volume must be filled with substrates per transporter. Passive diffusion, which could account for reduced accumulation, is negligible due to the high membrane integrity monitored by the control dye Oyster647. Table 1 resumes a calculation to compare the properties between large and giant liposomes.
Table 1. Comparison of Transport Properties in Large and Giant Proteoliposomes.
| diameter (μm) | surface (μm2) | volume (μm3) | TmrAB/vesicle | transport rate (1/min) | |
|---|---|---|---|---|---|
| LUVs | ∼0.1a | 0.06a | 0.5 × 10–3a | 25a | |
| GUVs | 10.9b | 820b | 920b | ∼0.5 × 106 | 0.31b |
| GUVs | 19.0c | 2600c | 5200c | ∼1.5 × 106c | 0.50c |
Derived from ref. (11) for proteoliposomes incubated in 10 μM C4F at 68 °C.
Parameters are means obtained from 19 individual single GUVs for the addition of 1 μM C4F.
Parameters are means obtained from 17 individual single GUVs for 3 μM C4F. Rate constants of GUVs are given as medians.
We examined the transport rates (C4F peptides per TmrAB per min) for different peptide concentrations (Figure 3d). Each dot represents the rate constant for an individual giant liposome. The median for the rate constant at different peptide concentrations is given as a red line, as it is less dependent on outliers. The transport rates increase with rising peptide concentration (Figure 3d, inset). We derived a Km value of 1.8 ± 1.0 μM and a turnover of TmrAB of approximately one peptide per minute at 40 °C. For TmrAB reconstituted in large liposomes, a Km,C4F of 8.3 μM and turnover of four peptides per TmrAB and minute were determined at 68 °C,11 which indicates a similar transporter activity considering the temperature dependence of the transport.44
We next examined whether the transport activity and directionality in individual liposomes can be controlled by light. Photocaged-ATP, ARS, Oyster647, and C4F peptides were encapsulated in the lumen of proteoGUVs (Figure 4a). Caged-ATP is a nonhydrolyzable nucleotide triphosphate analogue with a P3-(1-(4,5-dimethoxy-2-nitrophenyl)ethyl (DMNPE)-caged γ-phosphate, which can be cleaved by illumination at 405 nm using confocal scanning-laser microscopy. Before photoactivation, Oyster647 fluorophores and C4F peptides are stably encapsulated in the giant liposomes without membrane leakage (Figure S4).
Figure 4.
Light-controlled unidirectional export and sequential import. (a) Light-controlled export of TmrAB in giant liposomes. Caged-ATP was activated in situ by illumination at 405 nm using a confocal laser-scanning microscope. (b) Time-lapse images of the light-controlled export assay. Scale bar, 10 μm. (c) Time-dependent change in fluorescence intensity of C4F peptide. The inset illustrates the fluorescence signal of an exemplary GUV loaded with caged-ATP before photoactivation. (d) Export efflux constants for nine individual GUVs were summarized in a histogram. (e) Export and import of C4F peptides for the same giant liposome. The experiment was performed under identical conditions to those in (a), followed by addition of ATP and ARS driving peptide import. The focus was readjusted after addition of ATP/ARS, and the brightness of the red channel was enhanced by 6% for better visualization. Normalized and background-corrected fluorescence intensities (%) for the dyes inside the GUVs’ lumen are given below the images. Scale bar, 10 μm.
After illumination and confined ATP generation in the lumen of the GUV, we observed peptide export, reflected by a monoexponential decay of the C4F signal (Figure 4b). In contrast, the fluorescence of the membrane-impermeable Oyster647 dye, which is not transported by TmrAB, remains stable. A representative single-liposome export trace is shown in Figure 4c. Active transport exceeded the concentration equilibrium by exporting below the background concentration, additionally confirming active transport processes (Figure 4c). It is worth mentioning that only minimal fluorescence bleaching of C4F was observed without activation of caged-ATP (Figure 4c, inset). Liposomes of different diameters were photoactivated, and the export traces were monoexponentially fitted. The resulting rate constants are summarized in a histogram (Figure 4d). The majority of the proteoGUVs show export traces in the range of 0.05–0.1/min. This indicates that approximately 3 to 4 peptides are transported per TmrAB per minute (mean value), assuming a random (50:50) TmrAB orientation. Further information on the calculation is given in the Experimental Section. In very rare cases, an efflux of both C4F and Oyster647 was observed after in situ photoactivation, indicating membrane leakiness (Figure S5).
To compare export and import processes in individual giant liposomes, caged-ATP, ARS, Oyster647, and C4F peptides were encapsulated in their lumen. In situ photoactivation of an individual GUV results in export of C4F peptides, which can be reversed after addition of ATP and ARS to the external medium (Figure 4e). Photoinduced export followed by ATP-triggered import of an individual liposome in the vicinity of a cluster of vesicles additionally confirms the spatiotemporal specificity (Figure S6). Thereby, active export can be compared to active import in a single giant liposome, demonstrating the transport directionality and random orientation of TmrAB in giant liposomes.
Conclusion
In summary, we present a versatile approach for the functional reconstitution of membrane transport machineries in giant liposomes, exemplified by the bacterial ABC transport complex TmrAB and the human lysosomal exporter TAPL. Unidirectional transport against a steep gradient reveals activities for TmrAB peaking in a >120-fold accumulation in the lumen of the GUVs, with transport rates comparable to those of large unilamellar vesicles.11 These high accumulations are a hallmark of active transporters such as OpuA45,46 and have not been achieved in giant liposomes so far. Furthermore, active transport was controlled in time and in space by light, which enabled export and import processes on individual giant vesicles. Giant liposomes are activated in situ to follow active unidirectional export and import by nanomachineries in real time.
Experimental Section
Materials
All chemicals were purchased from Sigma-Aldrich, Carl Roth GmbH + Co. KG, or VWR International GmbH unless stated otherwise.
Expression and Purification TmrAB
Expression and purification of TmrAB were performed as previously described.11,47 In short, TmrAB was produced in Escherichia coli BL21(DE3) grown in LB high-salt media at 37 °C, with 180 rpm shaking and induced by 0.5 mM isopropyl β-d-1-thiagalactopyranoside (IPTG). All following steps were performed at 4 °C. Cells were harvested and resuspended in lysis buffer (20 mM HEPES/NaOH pH 7.5, 300 mM NaCl, 50 μg/mL lysozyme, 0.2 mM phenylmethylsulfonyl fluoride (PMSF)), followed by sonification and low-speed centrifugation at 10000g for 15 min to pellet cell debris. TmrAB was extracted from crude membranes by solubilization with 1%w/v β-n-dodecyl β-d-maltoside (β-DDM) in purification buffer (20 mM HEPES/NaOH pH 7.5, 300 mM NaCl, 0.2 mM PMSF) followed by centrifugation at 115000g for 30 min. Ni-NTA agarose beads (Qiagen) supplemented with 30 mM imidazole were used to bind the C-terminal His10-tagged TmrAB for 60 min and applied to a gravity flow column. Beads were washed with 20 column volumes (20 mM HEPES/NaOH pH 7.5, 300 mM NaCl, 0.05%w/v β-DDM, 50 mM imidazole), followed by elution (20 mM HEPES/NaOH pH 7.5, 300 mM NaCl, 0.05%w/v β-DDM, 300 mM imidazole). Fractions containing TmrAB were pooled and stored at 4 °C after buffer exchange (20 mM HEPES/NaOH pH 7.5, 150 mM NaCl, 0.05%w/v β-DDM). The concentration was determined by absorbance of 280 nm (ε280nm = 159 630 cm–1 M–1, Mw = 134 900 Da). Purity of TmrAB was analyzed by SDS-PAGE (10%), followed by Coomassie staining (InstantBlue; Expedeon) (SI Figure S7).
Expression and Purification of CoreTAPLmVenus
Expression and purification of coreTAPLmVenus were performed as described.48 In brief, coreTAPLmVenus was expressed in Pichia pastoris in a Labfors4 benchtop bioreactor (Infors HT). All following steps were performed at 4 °C. Cells (1/3 volume) were harvested and mixed with 1/3 volume of breaking buffer (50 mM KH2PO4 pH 7.5, 1 mM EDTA, 5 mM aminocaproic acid, 5%v/v glycerol) and 1/3 volume of washed glass beads. Cells were disrupted by shaking in a FastPrep-24 system (MP Biomedicals) for 45 s at 5 m/s for five cycles with 2 min cooling steps in between. Membranes were collected by centrifugation at 3500g for 15 min. Subsequently, crude membranes were extracted at 100000g for 45 min and resuspended in HEPES buffer (20 mM HEPES/NaOH pH 7.5, 500 mM NaCl, 2 mM benzamidine, 1 mM PMSF, 15%v/v glycerol) followed by Dounce homogenization. Crude membranes were solubilized (5 mg/mL total proteins) for 2 h in HEPES buffer containing 1%w/v β-DDM and 20 mM imidazole. Excess membranes were centrifuged by 100000g for 45 min, and the supernatant was incubated with Ni-NTA agarose beads for 3 h in an overhead rotor. Ni-NTA beads were loaded on a gravity flow column and washed with 20 column volumes (20 mM HEPES/NaOH pH 7.5, 500 mM NaCl, 2 mM benzamidine, 1 mM PMSF, 100 mM imidazole, 15%v/v glycerol, and 0.05%w/v β-DDM). CoreTAPLmVenus was eluted with HEPES buffer supplemented with 300 mM imidazole and 0.05%w/v β-DDM. Fractions containing coreTAPLmVenus were pooled and stored after buffer exchange (20 mM HEPES/NaOH pH 7.5, 150 mM NaCl, 0.05%w/v β-DDM, 5%v/v glycerol) at 4 °C. CoreTAPLmVenus concentration was determined by specific absorbance of mVenus (ε515 nm = 45 950 cm–1 M–1). Purity was analyzed by SDS-PAGE (10%) and Coomassie staining (InstantBlue; Expedeon), as well as by in-gel fluorescence of mVenus (λex/em = 480/520 nm) (Figure S8).
Peptide Labeling and Purification
The 9-mer peptide RRYCKSTEL was synthesized by solid-phase synthesis followed by fluorescein labeling. The peptide was dissolved in PBS (10 mM Na2HPO4, 2 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 6.5) supplemented with 3.5 mM dimethylformamide (DMF) and incubated for 1 h at RT with 1.2 molar excess of 5-iodoacetamidofluorescein (Merck) dissolved in DMF. Labeled peptides were purified by reverse-phase C18-HPLC (PerfectSil 300 ODS C18, 5 μm, 250 × 10 mm; MZ Analysentechnik) using a linear gradient from 5% to 100%v/v acetonitrile supplemented with 0.1%v/v trifluoroacetic acid. The identity of the peptides was verified by mass spectroscopy. Concentration was determined by absorption (ε492 nm = 75 000 cm–1 M–1, pH 9.0).
Fluorescently Labeled TmrAB
The single natural cysteine in wild-type TmrAB at position 416 in TmrA was labeled by maleimide chemistry. Therefore, TmrAB was incubated with sulfoCy5 maleimide (Lumiprobe) in PBS pH 6.8 for 1 h at room temperature. A protein concentration of 1 mg/mL and 20-fold molar excess of sulfoCy5 maleimide were used. Subsequently, the reaction was quenched with 1 mM β-mercaptoethanol. Dye excess was removed via size-exclusion spin columns (Bio-Rad) followed by size-exclusion chromatography (SEC).
Liposome Preparation
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(Cap biotinyl) (biotinylated-DOPE), E. coli polar lipids, and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) were purchased from Avanti Polar Lipids Inc., and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine coupled to ATTO390 (DOPEATTO390) was purchased from ATTO-TEC GmbH. POPC/POPG/POPE/biotinylated-DOPE (40:30:29:1 mol%), POPC/POPG/POPE/DOPEATTO390 (40:30:29.5:0.5 mol%), and E. coli polar lipids/DOPC (70:30 mol%) were mixed in chloroform to a concentration of 5 mg/mL, dried by a rotary evaporator, and subsequently resolved in 20 mM HEPES/NaOH pH 7.5, 150 mM NaCl. Large unilamellar vesicle formation was conducted as described including sonication and freeze–thaw cycles.49,50
Functional Reconstitution in Large Unilamellar Vesicles
TmrAB or coreTAPLmVenus were reconstituted in liposomes of POPC/POPG/POPE/biotinylated-DOPE (40:30:29:1 mol%) or E. coli polar lipids/DOPC (70:30 mol%) with protein-to-lipid ratios of 1:15w/w and 1:40w/w, respectively.9,51 Liposomes were extruded 11 times through 100 nm polycarbonate membranes, and all flowing steps were performed at 4 °C. Liposomes were destabilized by the addition of Triton X-100 4%v/v for 30 min, followed by incubation with the membrane protein complexes for 30 min on an overhead shaker. Detergent was gradually removed by four times addition of polystyrene beads (Bio-Beads SM-2, Bio-Rad). The first two additions of 40 mg/mL were incubated for 1 h and overnight, followed by two additions of 80 mg/mL each for 2 h. Proteoliposomes were harvested by centrifugation for 45 min at 300000g and resuspended in the appropriate buffer.
Formation of ProteoGUVs
Giant proteoliposomes were produced by hydrogel-assisted swelling of dried proteoliposomes.23 Therefore, 5%w/w solution of PVA (Merck KGaA) was dissolved in water and heated to 95 °C, followed by cooling to 38 °C. Afterward, 200 μL of the PVA solution was spread over a cover slide and dried at 50 °C for 30 min. Preformed proteoliposomes (5 mg/mL) were supplemented with sucrose (0.2 g sucrose/g lipid) and distributed in very small droplets on the dried PVA slide, to ensure fast drying. The PVA slide was placed under vacuum for at least 10 min in a humidity environment generated by NaCl. After drying, the PVA slide was rehydrated by the appropriate buffer containing 200 mM sucrose and incubated for 30 min before harvesting of proteoGUVs. Desired compounds for encapsulation must be included in the swelling buffer. The drying time drastically influences the protein activity and liposome yield. Here, we optimized the protocol for protein activity at the expense of GUV yield. Oyster647 and ATTO655 were purchased from Bio-Synthesis Inc. and ATTO-TEC GmbH, respectively. The sizes of the proteoGUVs were analyzed individually (n = 56, Figure S9).
ATPase Assay
The ATPase activity of DDM-solubilized proteoGUVs containing TmrAB was determined by using a Malachite Green-based colorimetric assay.52 Detergent-solubilized TmrAB (0.5 μM) was incubated in ATPase buffer (20 mM HEPES/NaOH pH 7.5, 150 mM NaCl, 2 mM MgCl2, 0.2%w/v β-DDM) in the presence of 2 mM Mg-ATP at 40 °C for 8 min and H2O as control. Transport was stopped by the addition of 20 mM H2SO4 supplemented with Malachite Green solution (3 mM Malachit Green, 0.2%v/v Tween 20, 1.5%w/v ammonium molybdate), and the absorbance of 620 nm was detected after 10 min of incubation at RT using the ELISA reader (CLARIOstar, BMG LABTECH).
Functionalized Glass Slides
Glass slides (170 ± 10 μm) were cleaned by oxygen plasma at 0.3 mbar and 80% power for 15 min (Diener Electronics), followed by silanization with (3-aminopropyl)triethoxysilane (APTES) as described.53 The amino group is coupled via an N-hydroxysuccinimide ester to biotinylated-PEG/PEG (1:10w/w, Rapp Polymere GmbH) followed by extensive washing with Milli Q water (Merck KGaA). Functionalized surfaces were covered by argon and stored at −20 °C. Sticky-slides (ibidi) were used for all transport experiments, and a lid was used to prevent evaporation.
Peptide Translocation into Giant Liposomes
Giant proteoliposomes were added to a precoated glass slide of β-casein (30 min incubation of 0.5 mg/mL) for coreTAPLmVenus containing proteoliposomes or to a biotinylated-PEG/PEG-functionalized glass slide (preincubated with streptavidin 0.2 mg/mL for 30 min) for TmrAB proteoliposomes. After streptavidin removal, biotinylated proteoGUVs were sedimented for 10 min before washing and addition of transport buffer (20 mM HEPES/NaOH pH 7.5, 150 mM NaCl, 1 mM MgCl2, 200 mM glucose, 2 mM phosphocreatine, 0.3 g/L phosphocreatine kinase, 0.2–3 μM C4F) at 40 °C. The transport of TmrAB was started by addition of 2 mM ATP, and substrate translocation was observed for around 2 h via confocal laser scanning microscopy (CLSM, Zeiss LSM 880, λex/C4F = 488 nm, λex/Oyster647/ATTO655/Alexa647 = 633 nm, λex/ATTO390 = 405 nm). Due to the short lifetime of TAPL at 37 °C, proteoliposomes were directly added in transport buffer containing 2 mM ATP, 3 μM C4F, 1 mM MgCl2, 2 mM phosphocreatine, and 0.3 g/L phosphocreatine kinase and subsequently imaged for 1–3 h. The fluorescein-labeled peptide C4F was used because it is considered a high-affinity substrate with well-described transport characteristics.9,11,54
Light-Triggered Peptide Export in Giant Liposomes
The export buffer (20 mM HEPES/NaOH pH 7.5, 150 mM NaCl, 1 mM MgCl2, 200 mM sucrose, 2 mM phosphocreatine, 0.1 g/L phosphocreatine kinase, 10–20 μM C4F, 2 mM DMNPE-caged ATP, Thermo Fischer Scientific) was encapsulated in giant proteoliposomes (TmrAB), and subsequently applied to a biotinylated-PEG/PEG-functionalized glass slide (preincubated with streptavidin 0.2 mg/mL for 30 min). The photocleavable ATP for individual liposomes was in situ activated by illumination of 405 nm for ∼30 s with 99% laser intensity (∼4.5 mW μm–2), followed by imaging via CLSM for 1–3 h.
Light-Triggered Peptide Export in Giant Liposomes Followed by ATP-Triggered Peptide Import
The export buffer (20 mM HEPES/NaOH pH 7.5, 150 mM NaCl, 1 mM MgCl2, 200 mM sucrose, 2 mM phosphocreatine, 0.1 g/L phosphocreatine kinase, 10–20 μM C4F, 2 mM DMNPE-caged ATP) was encapsulated in giant proteoliposomes (TmrAB), and subsequently applied to a biotinylated-PEG/PEG-functionalized glass slide (preincubated with streptavidin 0.2 mg/mL for 30 min). The photocleavable ATP for individual liposomes was in situ activated by illumination of 405 nm for ∼30 s with 99% laser intensity (∼4.5 mW μm–2), followed by imaging for 1–3 h via CLSM. Import processes were triggered by the addition of 2 mM ATP, and an ARS (0.3 g/L phosphocreatine kinase and 2 mM phosphocreatine), import was followed for 1–3 h by CLSM. More images of the export/import assay shown in Figure 4e are given in Figure S10.
Analysis of Import and Export Processes
Giant liposomes with minimal Oyster647 leakage were analyzed. The mean gray values of the fluorescent traces (import experiments) were extracted and processed by the open-source imaging analysis software ImageJ.55 Accumulations were extracted for each time point in each time trace (exemplary; an accumulation of two reflects a fluorescence intensity twice the background of the GUV exterior). The C4F concentration in the liposome was determined by calibrating the accumulation with the C4F concentration in the exterior. Subsequently, the number of C4F molecules was quantified by using the vesicle volume. The number of TmrAB (Mw = 134 900 Da) molecules per liposome was calculated for the total surface area (inner and outer leaflet surfaces were calculated independently with a membrane thickness of 5 nm), with the protein-to-lipid ratio of 1:15w/w, and with the lipid areas of 63.5, 61.0, and 62.8 Å2 for POPC, POPE, and POPG, respectively, in relation to the molar lipid ratios.56 Thereby, the number of C4F peptides transported per TmrAB per minute for individual liposome was calculated. The resulting saturation curves were fitted linearly to extract initial rate constants, which were plotted versus peptide concentrations. The mean and median values were calculated, with the latter being applied because of its lower dependence on outliers.
The mean gray values for export processes were analyzed by ImageJ (see above), including background correction. The resulting traces were fitted monoexponentially with the following equation:
Namely the export constant (k), the initial concentration (A), and an additional correction constant (y0). Subsequently, the resulting export rate constants were analyzed by descriptive statistics and summarized in a histogram (OriginPro 2020).
Liposome Geometry Calculations
The volume and surface area of giant and large liposomes were determined by assuming an ideal sphere. The membrane thickness was assumed to be 5 nm, and the inner and outer leaflet surface areas were calculated independently. The number of lipids per vesicle was quantified by dividing the total surface area by the average surface area of the used lipids including molar ratios. For POPC, POPE, and POPG, respectively, an lipid area of 63.5, 61.0, and 62.8 Å2 was taken into account.56 With the ratio of TmrAB (Mw = 134 900 Da) reconstituted per liposome of 1:15w/w, the number of TmrAB in a single liposome was derived. For large unilamellar vesicles, a protein-to-lipid ratio of 1:20w/w was used.11 A 50:50 orientation for ABC transporters was assumed for the calculations. All values in the table reflect mean values for the given number of GUVs (unless otherwise stated).
Calculation for the Export Experiments
As indicated in the analysis section, export traces were fitted monoexponentially, and the extracted efflux constants in 1/min were used to compute the number of substrate molecules per TmrAB per minute with the encapsulated substrate concentration and the liposome geometries. As described before, a membrane thickness of 5 nm was assumed for the surface area calculation of the inner and outer leaflet.
Data Presentation and Statistics
All measurements were performed at least in triplicates (n ≥ 3). In all column and XY diagrams, mean values ± SD were presented. Diagrams were prepared in OriginPro 2020 and further processed with Adobe Illustrator.
Acknowledgments
This research was supported by the German Research Foundation (SFB 807, TA 157/12-1, and GRK 1986 to R.T.), the LOEWE program (DynaMem A3 to R.T.), and the European Research Council (Advanced Grant789121 to R.T.). We thank Tina Zollmann for the expression of coreTAPLmVenus and Rupert Abele, Ralph Wieneke, Andrea Pott, Inga Nold, and all members of the Institute of Biochemistry, Goethe University Frankfurt, for discussions and comments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.0c10139.
Membrane integrity of giant liposomes containing coreTAPLmVenus; membrane integrity of a giant proteoliposome before transport initiation by ATP; long-term activity of TmrAB; direct comparison of a giant proteoliposome before and after photoactivation of caged-ATP; example of rarely observed membrane leakiness events after in situ photoactivation; export specificity and import of an individual in situ activated GUV, TmrAB purification; coreTAPLmVenus purification; size distribution of giant liposomes; export and import experiment; summary of substances for the export, import, and export/import assays (PDF)
Author Contributions
T.D. performed all experiments. T.D. and R.T. designed the experiments, analyzed the data, and wrote the manuscript. R.T. conceived the study and supervised the project.
The authors declare no competing financial interest.
Supplementary Material
References
- Dean M.; Rzhetsky A.; Allikmets R. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Res. 2001, 11, 1156–66. 10.1101/gr.GR-1649R. [DOI] [PubMed] [Google Scholar]
- Rees D. C.; Johnson E.; Lewinson O. ABC Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–27. 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.; Tampé R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2020, 89, 605–636. 10.1146/annurev-biochem-011520-105201. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC Transporters: From Microorganisms to Man. Annu. Rev. Cell Biol. 1992, 8, 67–113. 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Schmitt L.; Tampé R. Structure and Mechanism of ABC Transporters. Curr. Opin. Struct. Biol. 2002, 12, 754–60. 10.1016/S0959-440X(02)00399-8. [DOI] [PubMed] [Google Scholar]
- Abele R.; Tampé R. The ABCs of Immunology: Structure and Function of TAP, the Transporter Associated with Antigen Processing. Physiology 2004, 19, 216–24. 10.1152/physiol.00002.2004. [DOI] [PubMed] [Google Scholar]
- Higgins C. F.; Linton K. J. The ATP Switch Model for ABC Transporters. Nat. Struct. Mol. Biol. 2004, 11, 918–26. 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- Hofmann S.; Januliene D.; Mehdipour A. R.; Thomas C.; Stefan E.; Bruchert S.; Kuhn B. T.; Geertsma E. R.; Hummer G.; Tampé R.; Moeller A. Conformation Space of a Heterodimeric ABC Exporter under Turnover Conditions. Nature 2019, 571, 580–583. 10.1038/s41586-019-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan E.; Hofmann S.; Tampé R. A Single Power Stroke by ATP Binding Drives Substrate Translocation in a Heterodimeric ABC Transporter. eLife 2020, 9, e55943 10.7554/eLife.55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowitzsch S.; Tampé R. Multifunctional Chaperone and Quality Control Complexes in Adaptive Immunity. Annu. Rev. Biophys. 2020, 49, 135–161. 10.1146/annurev-biophys-121219-081643. [DOI] [PubMed] [Google Scholar]
- Nöll A.; Thomas C.; Herbring V.; Zollmann T.; Barth K.; Mehdipour A. R.; Tomasiak T. M.; Bruchert S.; Joseph B.; Abele R.; Olieric V.; Wang M.; Diederichs K.; Hummer G.; Stroud R. M.; Pos K. M.; Tampé R. Crystal Structure and Mechanistic Basis of a Functional Homolog of the Antigen Transporter TAP. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, E438–E447. 10.1073/pnas.1620009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirel O.; Jan I.; Wolters D.; Blanz J.; Saftig P.; Tampé R.; Abele R. The Lysosomal Polypeptide Transporter TAPL is Stabilized by Interaction with LAMP-1 and LAMP-2. J. Cell Sci. 2012, 125, 4230–40. 10.1242/jcs.087346. [DOI] [PubMed] [Google Scholar]
- Lawand M.; Evnouchidou I.; Baranek T.; Montealegre S.; Tao S.; Drexler I.; Saveanu L.; Si-Tahar M.; van Endert P. Impact of the TAP-Like Transporter in Antigen Presentation and Phagosome Maturation. Mol. Immunol. 2019, 113, 75–86. 10.1016/j.molimm.2018.06.268. [DOI] [PubMed] [Google Scholar]
- Tanji T.; Nishikori K.; Shiraishi H.; Maeda M.; Ohashi-Kobayashi A. Co-Operative Function and Mutual Stabilization of the Half ATP-Binding Cassette Transporters HAF-4 and HAF-9 in Caenorhabditis elegans. Biochem. J. 2013, 452, 467–75. 10.1042/BJ20130115. [DOI] [PubMed] [Google Scholar]
- Wolters J. C.; Abele R.; Tampé R. Selective and ATP-Dependent Translocation of Peptides by the Homodimeric ATP-Binding Cassette Transporter TAP-Like (ABCB9). J. Biol. Chem. 2005, 280, 23631–6. 10.1074/jbc.M503231200. [DOI] [PubMed] [Google Scholar]
- Tanji T.; Nishikori K.; Haga S.; Kanno Y.; Kobayashi Y.; Takaya M.; Gengyo-Ando K.; Mitani S.; Shiraishi H.; Ohashi-Kobayashi A. Characterization of HAF-4- and HAF-9-Localizing Organelles as Distinct Organelles in Caenorhabditis elegans Intestinal Cells. BMC Cell Biol. 2016, 17, 4. 10.1186/s12860-015-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain J. C.; Szostak J. W. Progress toward Synthetic Cells. Annu. Rev. Biochem. 2014, 83, 615–40. 10.1146/annurev-biochem-080411-124036. [DOI] [PubMed] [Google Scholar]
- Dimova R. Giant Vesicles and Their Use in Assays for Assessing Membrane Phase State, Curvature, Mechanics, and Electrical Properties. Annu. Rev. Biophys. 2019, 48, 93–119. 10.1146/annurev-biophys-052118-115342. [DOI] [PubMed] [Google Scholar]
- Schwille P.Giant Unilamellar Vesicles: From Minimal Membrane Systems to Minimal Cells? In The Minimal Cell; Springer: Dordrecht, 2010; pp 231–253. [Google Scholar]
- Tonnesen A.; Christensen S. M.; Tkach V.; Stamou D. Geometrical Membrane Curvature as an Allosteric Regulator of Membrane Protein Structure and Function. Biophys. J. 2014, 106, 201–9. 10.1016/j.bpj.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova M. I.; Dimitrov D. S. Liposome Electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–11. 10.1039/dc9868100303. [DOI] [Google Scholar]
- Horger K. S.; Estes D. J.; Capone R.; Mayer M. Films of Agarose Enable Rapid Formation of Giant Liposomes in Solutions of Physiologic Ionic Strength. J. Am. Chem. Soc. 2009, 131, 1810–9. 10.1021/ja805625u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger A.; Tsai F. C.; Koenderink G. H.; Schmidt T. F.; Itri R.; Meier W.; Schmatko T.; Schroder A.; Marques C. Gel-Assisted Formation of Giant Unilamellar Vesicles. Biophys. J. 2013, 105, 154–64. 10.1016/j.bpj.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P.; Pecreaux J.; Lenoir G.; Falson P.; Rigaud J. L.; Bassereau P. A New Method for the Reconstitution of Membrane Proteins into Giant Unilamellar Vesicles. Biophys. J. 2004, 87, 419–29. 10.1529/biophysj.104.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne C. W.; Patel K.; Heureaux J.; Stachowiak J.; Fletcher D. A.; Liu A. P. Lipid Bilayer Vesicle Generation Using Microfluidic Jetting. J. Vis. Exp. 2014, 84, e51510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond D. L.; Schmid E. M.; Martens S.; Stachowiak J. C.; Liska N.; Fletcher D. A. Forming Giant Vesicles with Controlled Membrane Composition, Asymmetry, and Contents. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 9431–6. 10.1073/pnas.1016410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. H.; van Lengerich B.; Boxer S. G. Effects of Linker Sequences on Vesicle Fusion Mediated by Lipid-Anchored DNA Oligonucleotides. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 979–84. 10.1073/pnas.0812356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishmukhametov R. R.; Russell A. N.; Berry R. M. A Modular Platform for One-Step Assembly of Multi-Component Membrane Systems by Fusion of Charged Proteoliposomes. Nat. Commun. 2016, 7, 13025. 10.1038/ncomms13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahya N.; Pecheur E. I.; de Boeij W. P.; Wiersma D. A.; Hoekstra D. Reconstitution of Membrane Proteins into Giant Unilamellar Vesicles via Peptide-Induced Fusion. Biophys. J. 2001, 81, 1464–74. 10.1016/S0006-3495(01)75801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnier A.; Kermarrec F.; Blesneac I.; Moreau C.; Liguori L.; Lenormand J. L.; Picollet-D’hahan N. A Simple Method for the Reconstitution of Membrane Proteins into Giant Unilamellar Vesicles. J. Membr. Biol. 2010, 233, 85–92. 10.1007/s00232-010-9227-8. [DOI] [PubMed] [Google Scholar]
- Dezi M.; Di Cicco A.; Bassereau P.; Levy D. Detergent-Mediated Incorporation of Transmembrane Proteins in Giant Unilamellar Vesicles with Controlled Physiological Contents. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 7276–81. 10.1073/pnas.1303857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijekoon C. J.; Udagedara S. R.; Knorr R. L.; Dimova R.; Wedd A. G.; Xiao Z. Copper ATPase CopA from Escherichia coli: Quantitative Correlation between ATPase Activity and Vectorial Copper Transport. J. Am. Chem. Soc. 2017, 139, 4266–4269. 10.1021/jacs.6b12921. [DOI] [PubMed] [Google Scholar]
- Doeven M. K.; Folgering J. H.; Krasnikov V.; Geertsma E. R.; van den Bogaart G.; Poolman B. Distribution, Lateral Mobility and Function of Membrane Proteins Incorporated into Giant Unilamellar Vesicles. Biophys. J. 2005, 88, 1134–42. 10.1529/biophysj.104.053413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeven M. K.; van den Bogaart G.; Krasnikov V.; Poolman B. Probing Receptor-Translocator Interactions in the Oligopeptide ABC Transporter by Fluorescence Correlation Spectroscopy. Biophys. J. 2008, 94, 3956–65. 10.1529/biophysj.107.120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger K. S.; Liu H.; Rao D. K.; Shukla S.; Sept D.; Ambudkar S. V.; Mayer M. Hydrogel-Assisted Functional Reconstitution of Human P-Glycoprotein (ABCB1) in Giant Liposomes. Biochim. Biophys. Acta, Biomembr. 2015, 1848, 643–53. 10.1016/j.bbamem.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.; Majd S. Reconstitution and Functional Studies of Hamster P-Glycoprotein in Giant Liposomes. PLoS One 2018, 13, e0199279 10.1371/journal.pone.0199279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geertsma E. R.; Poolman B. High-Throughput Cloning and Expression in Recalcitrant Bacteria. Nat. Methods 2007, 4, 705–7. 10.1038/nmeth1073. [DOI] [PubMed] [Google Scholar]
- Rigaud J. L.; Levy D. Reconstitution of Membrane Proteins into Liposomes. Methods Enzymol. 2003, 372, 65–86. 10.1016/S0076-6879(03)72004-7. [DOI] [PubMed] [Google Scholar]
- Brüchert S.; Joest E. F.; Gatterdam K.; Tampé R. Ultrafast In-Gel Detection by Fluorescent Super-Chelator Probes with HisQuick-PAGE. Commun. Biol. 2020, 3, 138. 10.1038/s42003-020-0852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata S.; Reichel A.; Brock R.; Tampe R.; Piehler J. High-Affinity Adaptors for Switchable Recognition of Histidine-Tagged Proteins. J. Am. Chem. Soc. 2005, 127, 10205–15. 10.1021/ja050690c. [DOI] [PubMed] [Google Scholar]
- Wieneke R.; Tampé R. Multivalent Chelators for in Vivo Protein Labeling. Angew. Chem., Int. Ed. 2019, 58, 8278–8290. 10.1002/anie.201811293. [DOI] [PubMed] [Google Scholar]
- Olbrich K.; Rawicz W.; Needham D.; Evans E. Water Permeability and Mechanical Strength of Polyunsaturated Lipid Bilayers. Biophys. J. 2000, 79, 321–7. 10.1016/S0006-3495(00)76294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande S.; Wunnava S.; Hueting D.; Dekker C. Membrane Tension-Mediated Growth of Liposomes. Small 2019, 15, e1902898 10.1002/smll.201902898. [DOI] [PubMed] [Google Scholar]
- Barth K.; Rudolph M.; Diederichs T.; Prisner T. F.; Tampé R.; Joseph B. Thermodynamic Basis for Conformational Coupling in an ATP-Binding Cassette Exporter. J. Phys. Chem. Lett. 2020, 11, 7946–7953. 10.1021/acs.jpclett.0c01876. [DOI] [PubMed] [Google Scholar]
- van der Heide T.; Poolman B. Osmoregulated ABC-Transport System of Lactococcus lactis Senses Water Stress via Changes in the Physical State of the Membrane. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 7102–6. 10.1073/pnas.97.13.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Der Heide T.; Poolman B. Glycine Betaine Transport in Lactococcus lactis Is Osmotically Regulated at the Level of Expression and Translocation Activity. J. Bacteriol. 2000, 182, 203–6. 10.1128/JB.182.1.203-206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutz A.; Hoffmann J.; Hellmich U. A.; Glaubitz C.; Ludwig B.; Brutschy B.; Tampé R. Asymmetric ATP Hydrolysis Cycle of the Heterodimeric Multidrug ABC Transport Complex TmrAB from Thermus thermophilus. J. Biol. Chem. 2011, 286, 7104–15. 10.1074/jbc.M110.201178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollmann T.; Moiset G.; Tumulka F.; Tampé R.; Poolman B.; Abele R. Single Liposome Analysis of Peptide Translocation by the ABC Transporter TAPL. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 2046–51. 10.1073/pnas.1418100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefen A.; Pedone D.; Grunwald C.; Wei R.; Firnkes M.; Abstreiter G.; Rant U.; Tampé R. Multiplexed Parallel Single Transport Recordings on Nanopore Arrays. Nano Lett. 2010, 10, 5080–7. 10.1021/nl1033528. [DOI] [PubMed] [Google Scholar]
- Diederichs T.; Nguyen Q. H.; Urban M.; Tampé R.; Tornow M. Transparent Nanopore Cavity Arrays Enable Highly Parallelized Optical Studies of Single Membrane Proteins on Chip. Nano Lett. 2018, 18, 3901–3910. 10.1021/acs.nanolett.8b01252. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Haase W.; Tampé R.; Abele R. Peptide Specificity and Lipid Activation of the Lysosomal Transport Complex ABCB9 (TAPL). J. Biol. Chem. 2008, 283, 17083–91. 10.1074/jbc.M801794200. [DOI] [PubMed] [Google Scholar]
- Baykov A. A.; Evtushenko O. A.; Avaeva S. M. A Malachite Green Procedure for Orthophosphate Determination and Its Use in Alkaline Phosphatase-Based Enzyme Immunoassay. Anal. Biochem. 1988, 171, 266–70. 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- Chandradoss S. D.; Haagsma A. C.; Lee Y. K.; Hwang J. H.; Nam J. M.; Joo C. Surface Passivation for Single-Molecule Protein Studies. J. Visualized Exp. 2014, 86. 10.3791/50549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C.; Zollmann T.; Lindt K. A.; Tampe R.; Abele R. Peptide Translocation by the Lysosomal ABC Transporter TAPL Is Regulated by Coupling Efficiency and Activation Energy. Sci. Rep. 2019, 9, 11884. 10.1038/s41598-019-48343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J.; Arganda-Carreras I.; Frise E.; Kaynig V.; Longair M.; Pietzsch T.; Preibisch S.; Rueden C.; Saalfeld S.; Schmid B.; Tinevez J. Y.; White D. J.; Hartenstein V.; Eliceiri K.; Tomancak P.; Cardona A. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–82. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzyn K.; Rog T.; Pasenkiewicz-Gierula M. Phosphatidylethanolamine-Phosphatidylglycerol Bilayer as a Model of the Inner Bacterial Membrane. Biophys. J. 2005, 88, 1091–103. 10.1529/biophysj.104.048835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.