Abstract
BACKGROUND AND AIMS:
Estrogen is an important risk factor for cholesterol gallstone disease because women are twice as likely as men to form gallstones. The classical estrogen receptor α (ERα), but not ERβ, in the liver plays a critical role in the formation of estrogen-induced gallstones in female mice. The molecular mechanisms underlying the lithogenic effect of estrogen on gallstone formation have become more complicated with the identification of G protein–coupled receptor 30 (GPR30), an estrogen receptor.
APPROACH AND RESULTS:
We investigated the biliary and gallstone phenotypes in ovariectomized female GPR30−/−, ERα−/−, and wild-type mice injected intramuscularly with the potent GPR30-selective agonist G-1 at 0 or 1 μg/day and fed a lithogenic diet for 8 weeks. The activation of GPR30 by G-1 enhanced cholelithogenesis by suppressing expression of cholesterol 7α-hydroxylase, the rate-limiting enzyme for the classical pathway of bile salt synthesis. These metabolic abnormalities led to an increase in biliary cholesterol concentrations in company with hepatic hyposecretion of biliary bile salts, thereby inducing cholesterol-supersaturated gallbladder bile and accelerating cholesterol crystallization. G-1 also impairs gallbladder emptying, leading to sluggish gallbladder motility and promoting the development of biliary sludge in the early stage of gallstone formation. The prevalence rates of gallstones were 80% in wild-type and ERα−/− mice treated with G-1 compared to 10% in wild-type mice receiving no G-1. However, no gallstones were formed in GPR30−/− mice treated with G-1.
CONCLUSIONS:
GPR30 produces additional lithogenic actions, working independently of ERα, to increase susceptible to gallstone formation in female mice; both GPR30 and ERα are potential therapeutic targets for cholesterol gallstone disease, particularly in women and patients exposed to high levels of estrogen.
Epidemiological investigations and clinical studies have clearly demonstrated that estrogen is a critical risk factor for cholesterol cholelithiasis because gallstones are more common in women than in men at all ages in every population studied.(1) The biological effects of estrogen are ascribed to transcriptional modulation of target genes through two classical estrogen receptors (ERs), subtypes α and β (ERα and ERβ). Using quantitative real-time PCR techniques, hepatic expression of the Erα gene is found to be ~50-fold higher compared with that of Erβ, suggesting that ERα is a major steroid hormone receptor for exerting the biological effects of estrogen in the liver.(2) To study the molecular mechanisms of how estrogen influences gallstone formation, we investigated gonadectomized (GOX) AKR/J mice that were implanted subcutaneously with pellets releasing various doses of 17β-estradiol (E2) and fed a lithogenic diet for 12 weeks.(2) Our results revealed that ERα, but not ERβ, plays a pivotal role in E2-mediated lithogenic actions on gallstone formation. We also explored whether hepatic cholesterol hypersecretion observed in E2-treated mice is attributable to increased hepatic cholesterogenesis by studying the contribution of newly synthesized hepatic cholesterol to biliary output.(3) We found that in GOX mice treated with high doses of E2, more newly synthesized cholesterol regulated by the estrogen–ERα–sterol regulatory element binding protein-2 (SREBP-2) pathway is secreted into bile, thereby leading to biliary cholesterol hypersecretion and cholesterol-supersatu-rated bile. Despite these observations, the metabolic abnormalities underlying the critical role of E2 in the pathogenesis of gallstone formation are still not fully understood because deletion of the Erα gene cannot completely prevent gallstone formation in GOX mice treated with E2.(4)
G protein–coupled receptor 30 (GPR30), an estrogen receptor, was identified and first reported in the late 1990s.(5) GPR30 is a member of the rhodopsin-like family of G protein–coupled receptors and is a seven-transmembrane protein.(6) Thus, the potential interplay between GPR30 and both ER subtypes has broad implications for the physiology of E2-mediated transcription and signaling pathways.(7) Notably, GPR30 is also expressed in the liver.(8,9) Using quantitative trait locus linkage analysis, lithogenic gene 18 (Lith18), a gallstone gene, has been identified and mapped to mouse chromosome 5 with its position at 78.7 cM near a genetic marker, DNA segment, chromosome 5, Massachusetts Institute of Technology 284.(10–13) GPR30 is colocalized with Lith18 on mouse chromosome 5 with its position at 78.6 cM. Further molecular and genetic analyses in mice supported the candidacy of Gpr30 as a compelling gene underlying Lith18.(10–13) However, it remains unclear whether or not activation of GPR30 produces an independent lithogenic action to promote the formation of estrogen-induced cholesterol gallstones in female mice.
To dissect the biological functions of each of the GPR30 and ER subtypes, a wide variety of compounds have been screened, and they are found to interact with GPR30, ERα, and ERβ. On the basis of affinity or efficacy as transcriptional activators, some ligands have been revealed to discriminate between GPR30 and ER subtypes.(14–17) Subsequently, a considerable effort has been focused on the development of receptor-selective agonists and antagonists for GPR30, ERα, or ERβ. A compound, G-1, has been found to be a cell-permeable, nonsteroidal, dihydroquinoline, high-affinity, selective agonist for GPR30, and it displays no activity on ERα or ERβ at concentrations up to 10 μM.(16) This synthetic estrogen-like compound with high-affinity to GPR30 is a very useful tool for elucidating the critical role of GPR30 in biliary lipid metabolism and cholesterol gallstone formation.
In the present study, to explore the potential pathophysiological roles between GPR30 and ERα in cholelithogenesis, we investigated whether the GPR30-selective agonist G-1 increases susceptibility to gallstone formation in ovariectomized (OVX) female wild-type mice with intact expression of the Gpr30, Erα, and Erβ genes compared to OVX GPR30−/− and ERα−/− mice fed the lithogenic diet for 8 weeks. Our results revealed that G-1 promotes the formation of cholesterol gallstones in OVX wild-type and ERα−/−, but not GPR30−/−, mice on the lithogenic diet. Taken together, GPR30 is involved in additional lithogenic actions, working independently of ERα, to enhance cholesterol cholelithogenesis in female mice.
Materials and Methods
CHEMICALS
G-1, the potent GPR30-selective agonist, was purchased from Tocris Bioscience (Ellisville, MO). The chemical name of G-1 is rel-1-[4-(6-bromo-1,3-benzodioxol-5-yl)-3αR,4S,5,9βS-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone.(16)
ANIMALS AND DIETS
Inbred AKR/J mice of both genders purchased from the Jackson Laboratory (Bar Harbor, ME) were bred to generate female mice for the studies. As we have found before, the AKR/J model is a gallstone-resistant strain; however, they are still susceptible to E2-induced cholesterol gallstone formation because E2 at 6 μg/day significantly increases cholelithogenesis in OVX AKR/J mice with intact expression of the Gpr30, Erα, and Erβ genes in the liver.(2) Notably, mRNA levels of Erβ in the liver are almost undetectable under normal physiological conditions, and its hepatic expression is 50-fold lower than that of Erα even under the stimulation of estrogen. Therefore, the inbred AKR/J strain, i.e., GPR30+/+/ERα+/+ wild-type mice, was used as the control for the studies. We have created GPR30−/− mice on the AKR/J genetic background and have established breeding colonies of the GPR30−/− mice in-house.(18) Of special note is that GPR30−/− mice are healthy and fertile. The ERα+/– heterozygotes of both genders on a C57BL/6J background were purchased from Taconic Biosciences (Germantown, NY). They were crossed with AKR/J mice for 10 generations to produce a strain of ERα+/– mice on an AKR/J genetic background. The ERα+/– mice are healthy and fertile and exhibit no obvious phenotypes that are associated with the disrupted ERα genotype. A cross between heterozygous ERα+/– mice resulted in the live birth of normal litter sizes of offspring containing the targeted ERα gene, i.e., homozygous ERα−/− mice. To exclude possible inter-individual differences in endogenous estrogen concentrations, all female mice, at the age of 4 weeks, were OVX. At the age of 8 weeks, the mice were intramuscularly injected with the GPR30 agonist G-1 at 0 or 1 μg/day for 8 weeks. Moreover, these mice were fed ad libitum a normal rodent chow diet containing trace cholesterol (< 0.02%) at day 0 or a lithogenic diet containing 1% cholesterol, 0.5% cholic acid, and 15% butter fat for 8 weeks. All animals were housed under controlled temperature (22 ± 1°C) and lighting (12-hour light/dark cycle) conditions with free access to water. All procedures were in accordance with current National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committees of Albert Einstein College of Medicine (Bronx, NY) and Saint Louis University (St. Louis, MO).
Additional methods are described in the Supporting Materials and Methods.
Results
THE GPR30-SELECTIVE AGONIST G-1 PROMOTES GALLSTONE FORMATION
Feeding the lithogenic diet for 8 weeks induced gallstone formation in 10% of OVX wild-type mice receiving no G-1 (Fig. 1A). Moreover, numerous solid plate-like cholesterol monohydrate crystals, as well as aggregated and fused liquid crystals were detected in 70% and 20% of these mice, respectively. By contrast, after 8 weeks of feeding the lithogenic diet, G-1 drastically increased gallstone prevalence to 80% in OVX wild-type and ERα−/− mice. Many solid cholesterol monohydrate crystals embedded within mucin gels were observed in the remaining (20%) mice. When gallbladder bile was examined by polarizing light microscopy, only some cholesterol monohydrate crystals and small nonbirefringent liquid crystals were detected in 60% and 40% of OVX GPR30−/− mice, respectively; and no gallstones were found in these mice treated with G-1 at 1 μg/day for 8 weeks (Fig. 1B).
FIG. 1.
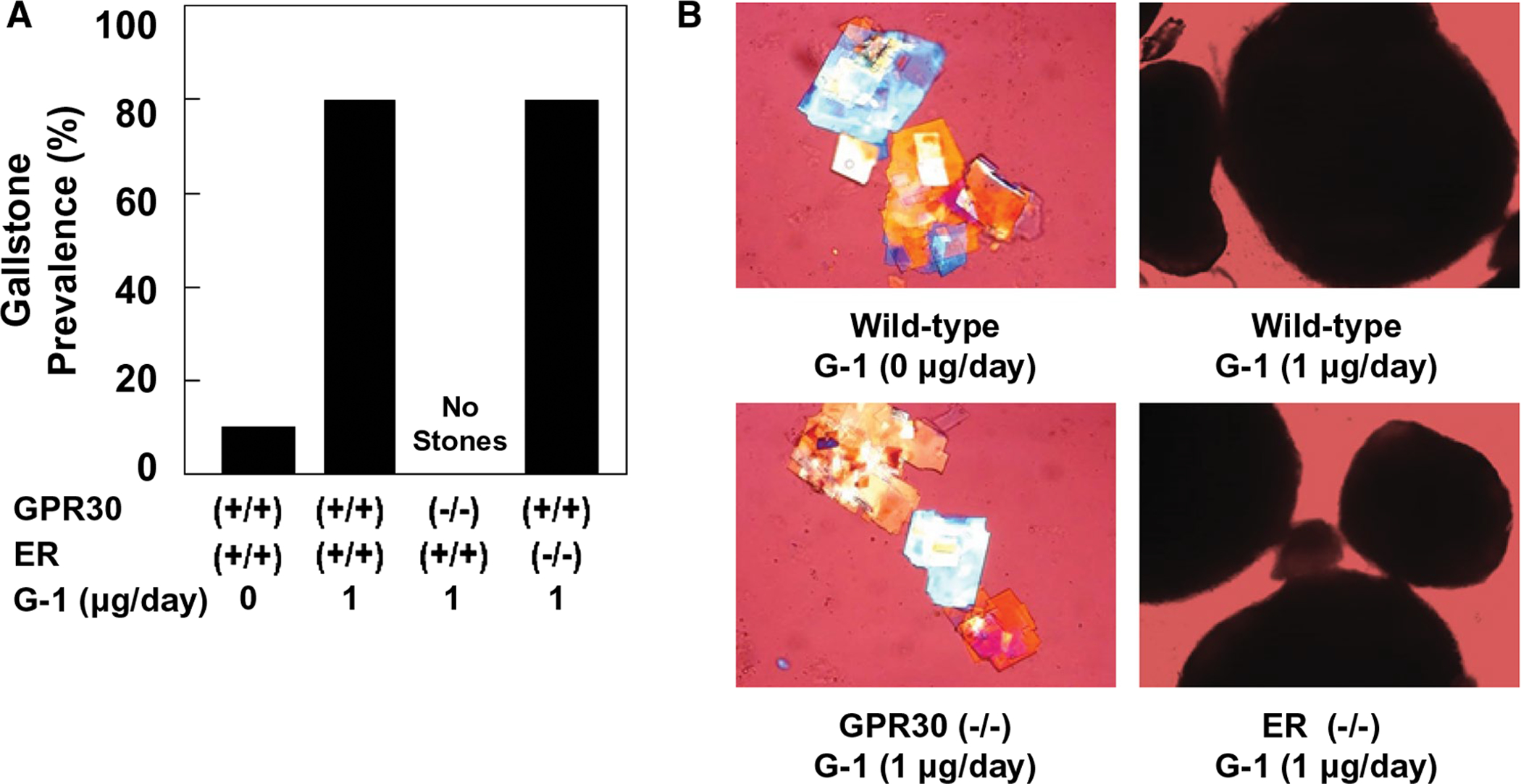
(A) At 8 weeks on the lithogenic diet, the prevalent rates of gallstones are 10% in OVX wild-type mice receiving no G-1, and most of these mice form solid cholesterol monohydrate crystals. The GPR30-selective agonist G-1 promotes gallstone formation in 80% of OVX wild-type and ERα−/− mice. Notably, only solid cholesterol monohydrate crystals, but not gallstones, are found in OVX GPR30−/− mice even treated with G-1 for 8 weeks. (B) Representative photomicrographs of solid plate-like cholesterol monohydrate crystals and gallstones as observed by polarizing light microscopy in gallbladder bile of OVX wild-type, GPR30−/−, and ERα−/− mice injected intramuscularly with G-1 at 0 or 1 μg/day and fed the lithogenic diet for 8 weeks. Magnifications are ×800 for left two panels and ×200 for right two panels.
G-1 TREATMENT ALTERS THE RELATIVE LIPID COMPOSITION OF GALLBLADDER BILE
As expected, feeding the lithogenic diet increased the mole percent cholesterol in pooled gallbladder bile, leading to the formation of cholesterol-super-saturated bile in OVX wild-type mice receiving no G-1 (Table 1). Moreover, in the lithogenic state, the cholesterol saturation index (CSI) values of pooled gallbladder bile were drastically increased in OVX wild-type and ERα−/− mice treated with G-1 for 8 weeks. However, the CSI value of pooled gallbladder bile was lower in OVX GPR30–/– mice treated with G-1 for 8 weeks compared to OVX wild-type mice receiving no G-1. The lithogenic effect of G-1 was further studied by phase analysis of gallbladder bile in OVX mice fed the lithogenic diet for 8 weeks. The relative lipid composition of pooled gallbladder bile with micellar phase boundary and cholesterol crystallization pathways for a total lipid concentration of 10 g/dL was plotted on a condensed phase diagram (Fig. 2).(19) The relative lipid composition of pooled gallbladder bile was located on the borderline between the one-phase micellar zone and the left two-phase area denoted region B in OVX wild-type mice receiving no G-1 and fed the lithogenic diet for 8 weeks. Moreover, the relative lipid composition of pooled gallbladder bile was plotted on the borderline between the micellar zone and region B in OVX GPR30−/− mice treated with G-1 for 8 weeks. By phase analysis, the bile in these mice consisted of solid cholesterol monohydrate crystals and saturated micelles, which is consistent with the results observed experimentally in model bile.(19) By contrast, under G-1 treatment, the relative lipid composition of pooled gallbladder bile in OVX wild-type and ERα−/− mice was plotted in the central three-phase zone of the phase diagram, denoted region C. As expected by phase analysis, the bile in region C was composed of solid cholesterol monohydrate crystals, liquid crystals, and saturated micelles.(19) These alterations were induced mainly by a striking increase in the cholesterol content of bile (Table 1). These results indicate that G-1 promotes gallstone formation in OVX mice with intact expression of Gpr30 in the liver when these mice are challenged to the lithogenic diet.
TABLE 1.
Biliary Lipid Composition of Gallbladder and Hepatic Bile
| Mice | G-1 (μg/day) | Ch (Mole%) | PL (Mole%) | BS (Mole%) | Ch/PL Ratio | Ch/BS Ratio | [TL] (g/dL) | CSI |
|---|---|---|---|---|---|---|---|---|
| Pooled gallbladder bile* | ||||||||
| Wild-type | 0 | 6.14 | 15.85 | 78.01 | 0.39 | 0.08 | 8.93 | 1.10 |
| Wild-type | 1 | 9.95 | 18.03 | 72.02 | 0.55 | 0.14 | 9.81 | 1.55 |
| GPR30−/− | 1 | 5.03 | 13.01 | 81.96 | 0.39 | 0.06 | 9.65 | 1.02 |
| ERα−/− | 1 | 8.71 | 16.77 | 74.52 | 0.52 | 0.12 | 10.15 | 1.43 |
| Individual hepatic bile* | ||||||||
| Wild-type | 0 | 6.38 ± 0.69 | 19.20 ± 1.04 | 74.42 ± 1.19 | 0.33 ± 0.04 | 0.09 ± 0.01 | 2.31 ± 0.38 | 1.31 ± 0.14 |
| Wild-type | 1 | 7.57 ± 1.53 | 22.07 ± 4.22 | 70.36 ± 5.21 | 0.35 ± 0.07 | 0.11 ± 0.03 | 1.57 ± 0.17 | 1.55 ± 0.28 |
| GPR30−/− | 1 | 4.36 ± 0.76 | 14.59 ± 3.13 | 81.06 ± 3.83† | 0.30 ± 0.03 | 0.05 ± 0.01‡ | 2.05 ± 0.45 | 1.17 ± 0.08† |
| ERα−/− | 1 | 7.29 ± 0.26 | 19.59 ± 1.66 | 73.12 ± 1.58 | 0.37 ± 0.04 | 0.10 ± 0.00 | 1.16 ± 0.19 | 1.75 ± 0.11 |
Values were determined from pooled gallbladder bile (n = 10 per group) and from individual gallbladder bile (n = 5 per group).
P < 0.001 compared to wild-type mice treated with G-1 at 1 μg/day and fed the lithogenic diet for 8 weeks.
P < 0.05 compared to wild-type mice treated with G-1 at 1 μg/day and fed the lithogenic diet for 8 weeks.
Abbreviations: [TL], total lipid concentration; BS, bile salts; Ch, cholesterol; PL, phospholipids.
FIG. 2.
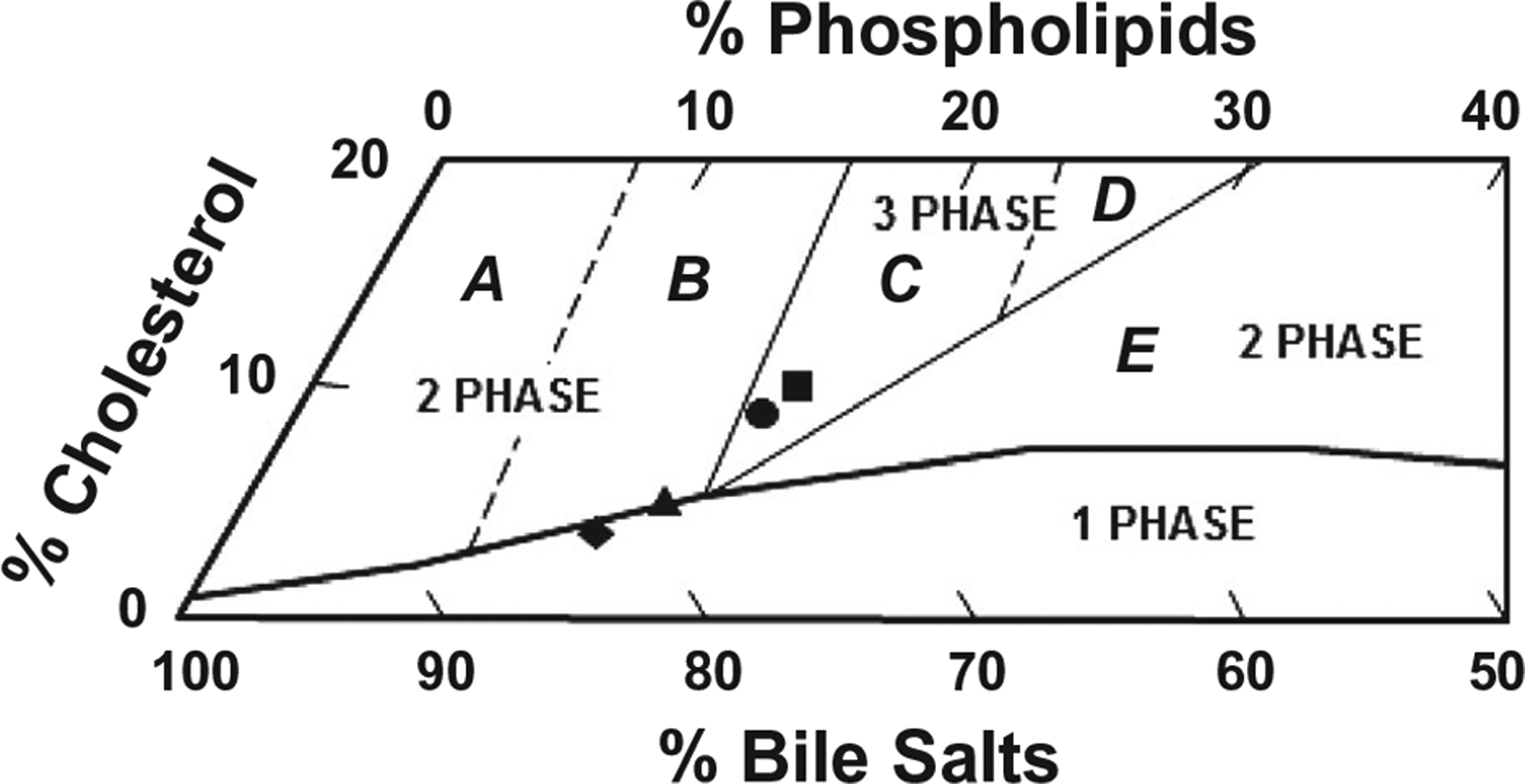
Effects of G-1 on the relative biliary lipid composition of pooled gallbladder bile at 8 weeks on the lithogenic diet, as plotted on a condensed phase diagram according to a total lipid concentration (~10 g/dL) of the bile. One-phase micellar zone at the bottom is enclosed by a solid curved line. Above it, two solid and two dashed lines divide the phase diagram into regions A-E with different crystallization pathways. After being fed the lithogenic diet for 8 weeks, the relative biliary lipid composition of pooled gallbladder bile in OVX wild-type mice receiving no G-1 and in OVX GPR30−/− mice treated with G-1 at 1 μg/day is located on the borderline between the one-phase micellar zone and left two-phase region B. By phase analysis, gallbladder bile is supersaturated with cholesterol. By contrast, G-1 administration to OVX wild-type and ERα−/− mice leads to the relative biliary lipid composition of pooled gallbladder bile plotted in the central three-phase zone denoted region C, where bile is composed of solid cholesterol monohydrate crystals, liquid crystals, and saturated micelles at equilibrium. The symbol ▲ represents the relative biliary lipid composition of pooled gallbladder bile at 8 weeks on the lithogenic diet in OVX wild-type mice receiving no G-1, as well as ■, ♦, and ● for OVX wild-type, GPR30−/−, and ERα−/− mice treated with G-1 at 1 μg/day, respectively.
G-1 ADMINISTRATION REDUCES HEPATIC OUTPUT OF BILIARY BILE SALTS
At 8 weeks on the lithogenic diet, bile flow rates were comparable in the four groups of mice, regardless of whether these OVX mice were treated with or without the GPR30 agonist G-1 (Fig. 3A). Although biliary cholesterol output during the first hour of biliary secretion was lower in OVX wild-type, GPR30−/−, and ERα−/− mice treated with G-1 for 8 weeks compared to OVX wild-type mice receiving no G-1, it was basically similar in the former three groups of mice (Fig. 3B). Moreover, hepatic output of biliary phospholipids varied slightly, and no significant statistical differences were found among the four groups of mice (Fig. 3C). More interestingly, compared to OVX wild-type mice receiving no G-1, biliary bile salt output was significantly reduced in OVX wild-type and ERα−/−, but not GPR30−/−, mice treated with G-1 for 8 weeks. Notably, biliary bile salt output was significantly higher in OVX GPR30−/− mice than in OVX wild-type and ERα−/− mice under G-1 treatment conditions (Fig. 3D). Further analysis of biliary lipid composition of individual hepatic bile reveals that the mole percent of bile acids was significantly higher and the ratios of cholesterol/bile salts and CSI values were significantly lower in OVX GPR30−/− mice than those in OVX wild-type mice treated with G-1 for 8 weeks (Table 1). Overall, it suggests that G-1 could produce the lithogenic actions through the GPR30 signaling pathway.
FIG. 3.
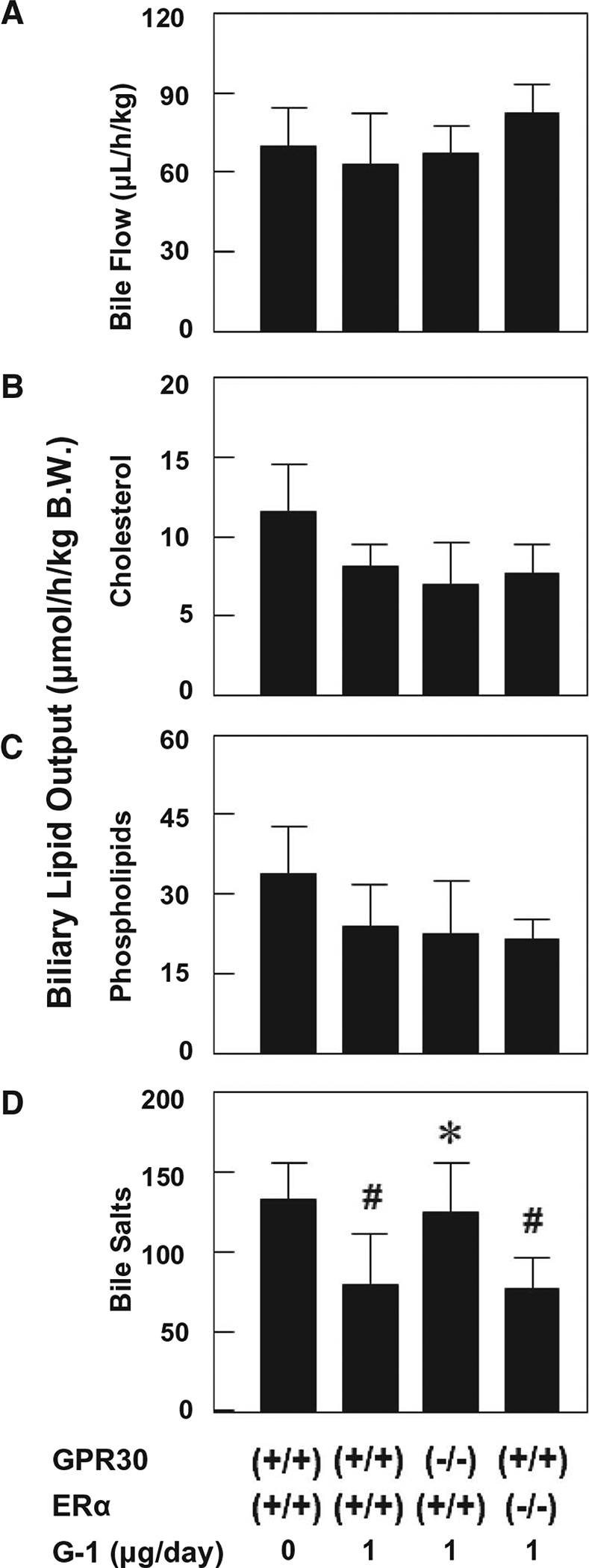
Effects of G-1 on bile flow rates (A) as well as hepatic output of biliary cholesterol (B), phospholipids (C), and bile salts (D) during the first hour of biliary secretion in OVX wild-type, GPR30−/−, and ERα−/− mice treated with G-1 at 0 or 1 μg/day and fed the lithogenic diet for 8 weeks. #P < 0.05 compared to OVX wild-type mice receiving no G-1 and fed the lithogenic diet for 8 weeks; *P < 0.05 compared to OVX wild-type mice treated with G-1 at 1 μg/day and fed the lithogenic diet for 8 weeks. Abbreviation: B.W., body weight.
EFFECTS OF G-1 ON GALLBLADDER EMPTYING FUNCTION
After feeding the lithogenic diet and treating G-1 at 0 or 1 μg/day for 2 weeks, a dynamic measurement of gallbladder emptying rate was performed (Fig. 4). As expected, phosphate-buffered saline (PBS) solution did not influence gallbladder motor function in all OVX mice. At 30 minutes after sulfated cholecystokinin octapeptide (CCK-8) injection compared to PBS, only a small portion of gallbladder bile was excreted and residual gallbladder volumes were large in OVX wild-type and ERα−/− mice treated with G-1 for 2 weeks. As a result, biliary sludge containing many solid plate-like cholesterol monohydrate crystals was found by polarizing light microscopy in sluggish gallbladders of these two groups of mice. By contrast, gallbladder emptying rates were significantly larger in response to exogenous CCK-8 stimulation compared to PBS in OVX wild-type mice receiving no G-1 and OVX GPR30−/− mice treated with G-1. Notably, only mucin gels and some small liquid crystals, but not solid cholesterol monohydrate crystals, were detected in gallbladder bile of these mice. Taken together, these results clearly imply that G-1 impairs gallbladder contractility in OVX wild-type and ERα−/−, but not GPR30−/−, mice, leading to the initiation of biliary sludge during the early stage of gallstone formation.
FIG. 4.

Gallbladder emptying rates in response to exogenous CCK-8 stimulation (as indicated by the arrows) during the early stage of gallstone formation in OVX mice treated with G-1 at 0 or 1 μg/day and fed the lithogenic diet for 2 weeks (left panels). PBS solution is used as control. Right panels show representative photomicrographs of mucin gels and biliary sludge as observed by polarizing light microscopy. Fresh gallbladder bile is examined in the same groups of OVX mice immediately after the gallbladder emptying study. G-1 impairs gallbladder contractile function in OVX wild-type and ERα−/− mice, leading to the formation of biliary sludge containing numerous plate-like cholesterol monohydrate crystals. By contrast, only mucin gels and small liquid crystals, but not solid cholesterol crystals, are found in OVX wild-type mice receiving no G-1 and GPR30−/− mice even treated with G-1 for 2 weeks. Magnifications are ×800 for all photos. *P < 0.01 and **P < 0.001 compared with PBS solution.
EFFECTS OF G-1 ON BILE SALT SPECIES OF HEPATIC BILE
We further examined the effect of G-1 treatment on individual bile salt species in hepatic bile by high-performance liquid chromatography (HPLC) at 8 weeks on the lithogenic diet. As shown by HPLC data, all bile salts were conjugated with taurine in hepatic bile, and the distribution of bile salt composition was similar in the four groups of OVX mice (Supporting Fig. S1). Because the lithogenic diet contained 0.5% cholic acid, taurocholate was increased in bile and became the major bile salt of the biliary pool, followed by taurochenodeoxycholate and taurodeoxycholate. This makes murine bile salt composition similar to humans in whom cholate is a predominant component.(20) Moreover, the concentrations of tauro-β-muricholate, tauro-ω-muricholate, and tauroursodeoxycholate were low in hepatic bile. Hydrophobicity indexes of bile salts in hepatic bile were comparable (−0.13 to −0.02) among the four groups of mice. These results indicate that G-1 does not influence bile salt species in bile.
EFFECTS OF G-1 ON EXPRESSION OF HEPATIC Gpr30, Erα, AND Erβ
It has been reported that G-1 binds with high affinity to GPR30 and functions as a full GPR30 agonist because it is devoid of activity on ERα and ERβ.(16) Compared to OVX wild-type mice receiving no G-1 and fed the lithogenic diet, G-1 treatment at 1 μg/day for 8 weeks significantly increased hepatic Gpr30 expression in OVX wild-type and ERα−/− mice (Fig. 5). As expected, mRNA levels of hepatic Gpr30 were not found in GPR30−/− mice even treated with G-1 for 8 weeks. In addition, G-1 did not influence expression of hepatic Erα or Erβ in all OVX mice. Notably, mRNA levels of Erα were approximately 50-fold higher than those of Erβ in the liver, regardless of whether these OVX mice were intramuscularly injected with G-1 at 0 or 1 μg/day.
FIG. 5.
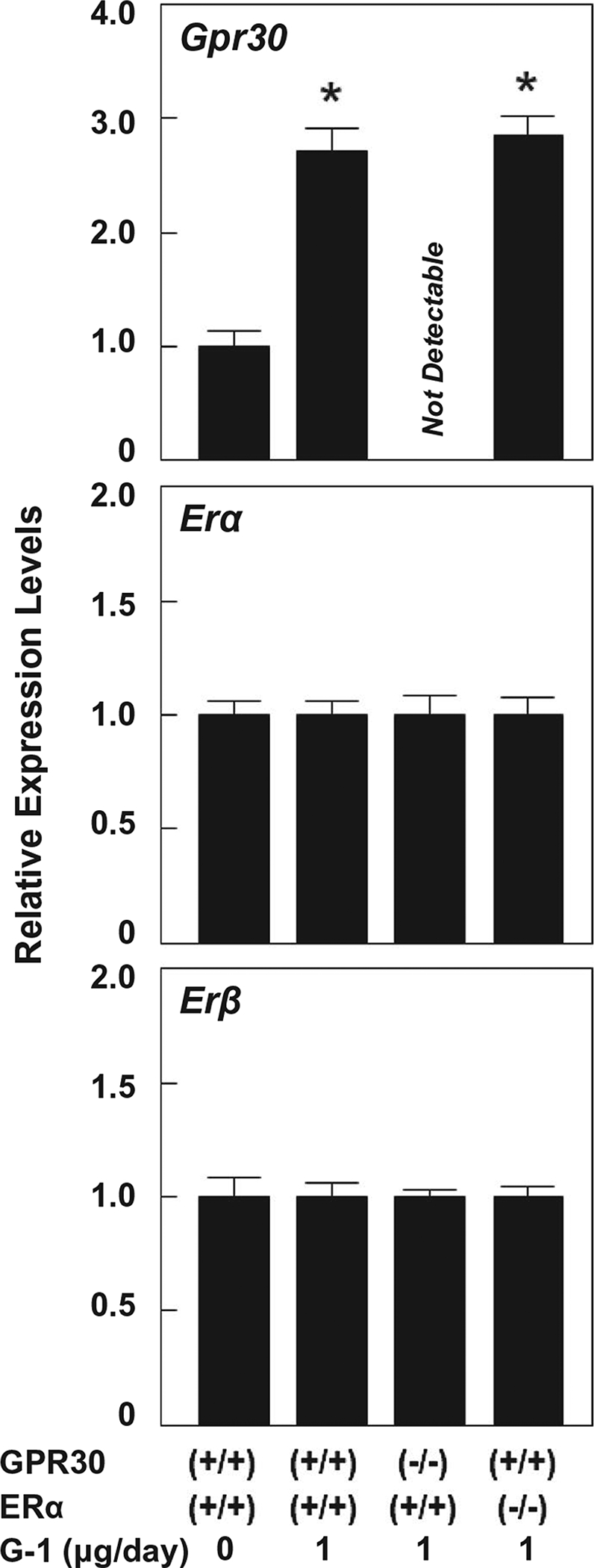
Effects of G-1 on mRNA levels of Gpr30, Erα, and Erβ in the liver. The relative mRNA levels of these genes in OVX wild-type mice receiving no G-1 and fed the lithogenic diet for 8 weeks are set at 1. Compared to OVX wild-type mice receiving no G-1, expression of Gpr30 in the liver is significantly increased in OVX wild-type and ERα (−/−), but not GPR30 (−/−), mice treated with G-1 at 1 μg/day and fed the lithogenic diet for 8 weeks. However, G-1 treatment does not influence expression of hepatic Erα or Erβ in these four groups of OVX mice. *P < 0.01 compared to OVX wild-type mice receiving no G-1 and fed the lithogenic diet for 8 weeks.
EFFECTS OF G-1 ADMINISTRATION ON mRNA LEVELS OF THE GENES INVOLVED IN THE REGULATION OF HEPATIC LIPID METABOLISM
It is well known that adenosine triphosphate–binding cassette G5/G8 (ABCG5/G8), ABCB4, and ABCB11 are the transporters located on the canalicular membrane of hepatocytes and are responsible for hepatic secretion of biliary cholesterol, phospholipids, and bile salts, respectively. Under lithogenic diet feeding conditions, G-1 treatment at 1 μg/day for 8 weeks significantly increased hepatic expression of Abcg5 and Abcg8 in OVX wild-type and ERα−/− mice compared to OVX wild-type mice receiving no G-1 (Fig. 6). No changes in Abcb4 expression were found between OVX wild-type and ERα−/− mice treated with G-1 for 8 weeks. Moreover, hepatic expression of Abcb11 was slightly reduced in OVX wild-type and ERα−/− mice treated with G-1, but it did not reach statistical significance. Notably, expression of the genes encoding these biliary lipid transporters was not influenced in OVX GPR30−/− mice treated with G-1 for 8 weeks. Moreover, mRNA levels of cholesterol 7-hydroxylase (Cyp7a1) and sterol 27-hydroxylase (Cyp27a1), which encode two rate-limiting enzymes for the classical and the alternative pathways of bile salt synthesis were carefully measured. Our results showed that under G-1 treatment, expression of Cyp7a1, but not Cyp27a1, in the liver was significantly reduced in OVX wild-type and ERα−/− mice fed the lithogenic diet for 8 weeks. By contrast, G-1 administration did not influence hepatic expression of Cyp7a1 or Cyp27a1 in OVX GPR30−/− mice compared to OVX wild-type mice receiving no G-1. Moreover, at 8 weeks on the lithogenic diet, G-1 did not appear to influence hepatic expression of acetyl-coenzyme A acetyltransferase 2 (Acat2), 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Hmgcr), Srebp2, low-density lipoprotein receptor (Ldlr), scavenger receptor class B type 1 (Sr-b1), and farnesyl diphosphate synthase (Fdps) genes in the four groups of OVX mice.
FIG. 6.

Effects of G-1 on mRNA levels of the genes involved in the regulation of hepatic lipid metabolism in the lithogenic state. The relative mRNA levels of the genes in OVX wild-type mice receiving no G-1 and fed the lithogenic diet for 8 weeks are set at 1. The data show the mRNA levels of the genes encoding hepatic lipid transporters, enzymes for the regulation of bile salt and cholesterol synthesis, and lipoprotein receptors in OVX wild-type, GPR30−/−, and ERα−/− mice treated with G-1 at 0 or 1 μg/day and fed the lithogenic diet for 8 weeks. *P < 0.01 compared with OVX wild-type mice receiving no G-1 and fed the lithogenic diet for 8 weeks. See text for further description and for abbreviations.
Because the high cholesterol and cholic acid in the lithogenic diet may complicate attempts to identify the pathways by which Gpr30 signals to the regulation of hepatic lipid metabolism, we further studied the effect of G-1 on these pathways in chow-fed OVX mice. G-1 significantly reduces hepatic expression of Cyp7a1 in OVX wild-type and ERα−/−, but not GPR30−/−, mice (Supporting Fig. S2). However, Cyp27a1 expression was comparable among the four groups of OVX mice. Again, mRNA levels of hepatic Abcg5 and Abcg8 were significantly increased in OVX wild-type and ERα−/−, but not GPR30−/−, mice. G-1 did not influence expression of hepatic Abcb4, Abcb11, Hmgcr, or Srebp2 in these OVX mice.
Discussion
Accumulated evidence from epidemiological and clinical studies has found that E2 enhances cholelithogenesis by promoting hepatic cholesterol hypersecretion, leading to a significant increase in cholesterol saturation of bile. Although ERα plays a critical role in the formation of E2-induced gallstones, our previous studies have found that 30% of OVX ERα−/− mice still form gallstones in response to high doses of E2 and the lithogenic diet for 8 weeks,(4) indicating that E2 also produces an ERα-independent action on the pathogenesis of gallstones. In the present study, the major findings are that (1) the potent GPR30-selective agonist G-1 promotes gallstone formation in OVX wild-type and ERα−/− mice by stimulating Gpr30 expression; (2) the activation of GPR30 by G-1 leads to an increase in biliary cholesterol concentrations, accompanied by reduced biliary bile salt output, thereby causing cholesterol-supersaturated bile; (3) G-1 treatment does not increase gallstone prevalence rate in OVX GPR30−/− mice; and (4) G-1 administration impairs gallbladder emptying through the GPR30 pathway. Our findings clearly demonstrate that GPR30 is an important Lith gene (i.e., Lith18) involved in E2-mediated lithogenic actions on gallstone formation, working independently of ERα. The results of this study may have highly relevant translational value as both GPR30 and ERα in the liver are responsible for gallstone formation through different lithogenic mechanisms in mice. Thus, inhibiting both GPR30 and ERα may be required for preventing the formation of E2-induced gallstones.
E2 has been found to play a pivotal role in the regulation of cellular functions through two signaling pathways: “nuclear-initiated steroid signaling” and “membrane-initiated steroid signaling.”(21–23) The nuclear-initiated signaling of E2 through ERα and ERβ, i.e., the “classical” signaling pathway, exerts diverse effects on a variety of tissues involved in the regulation of target genes. E2 is freely cell-permeable and can passively diffuse into cells to act as a transcription factor by binding to ERα and ERβ, causing a change in receptor tertiary and quaternary structure to form the active complex.(24) The active complex then binds a unique DNA sequence known as the “estrogen response elements” on the DNA upstream from estrogen-responsive genes, i.e., in estrogen-responsive gene promoters. Subsequently, transcription and translation of these genes result in the production of proteins that ultimately are responsible for mediating estrogen’s effects. Alternatively, the active complex may induce the production of a specific protein more indirectly through the activation of its transcription factor.
In addition, E2 regulates a plethora of biological processes through the membrane-initiated signaling cascade, i.e., the GPR30 pathway.(25) In the current study, we found that GPR30 activation by G-1 leads to a significant reduction in mRNA levels of Cyp7a1 in OVX wild-type and ERα−/−, but not GPR30−/−, mice. It is well known that ~50% of cholesterol is converted to bile salts in the liver each day in humans and mice.(26) CYP7A1 is the rate-limiting enzyme for the classical pathway of bile salt synthesis, converting cholesterol first to 7α-hydroxycholesterol and eventually to cholic acid. CYP27A1 is the rate-limiting enzyme for the alternative pathway, converting cholesterol first to 27-hydroxycholesterol and ultimately to chenodeoxycholic acid. In the normal physiological state, the classical pathway accounts for approximately 90% of the entire bile salt synthesis, and the alternative pathway produces the remaining 10% of bile salts.(27–32) However, the responses of the two enzymes to E2 may be different. We found that mRNA levels of Cyp7a1 are significantly reduced in OVX wild-type and ERα−/− mice treated with G-1 compared to OVX wild-type mice receiving no G-1, regardless of whether these OVX mice were fed chow or the lithogenic diet. However, G-1 treatment did not influence Cyp7a1 expression in OVX GPR30−/− mice. By contrast, mRNA levels of Cyp27a1 are comparable among the four groups of mice. These results show that the activation of GPR30 by G-1 inhibits the hepatic synthesis of bile salts by suppressing Cyp7a1 and the classical pathway, but not Cyp27a1 and the alternative pathway, in OVX mice. Furthermore, there is not a compensatory increase in Cyp27a1 expression in OVX wild-type and ERα−/− mice despite a marked decrease in Cyp7a1 expression, indicating that the alternative pathway does not provide a compensatory backup for bile salt synthesis when the classical pathway is repressed.
It is widely appreciated that hypersecretion of biliary cholesterol from the liver into bile is a prerequisite for cholesterol gallstone formation in humans and mice.(33) We have reported that the hepatic ERα activated by E2 interferes with the negative feedback regulation of cholesterol biosynthesis by stimulating expression of Srebp-2, which subsequently activates the SREBP-2-responsive genes for the cholesterol synthesis pathway.(3) We have found that during E2 treatment mice continue to synthesize cholesterol despite its excess availability from the high-cholesterol diet. This indicates that there is a loss in the negative feedback regulation of cholesterol synthesis in the liver, and more newly synthesized cholesterol regulated by the estrogen–ERα–SREBP-2 pathway is secreted into bile, thereby leading to biliary cholesterol hypersecretion and supersaturated bile.(3) These metabolic abnormalities promote the precipitation of solid cholesterol monohydrate crystals and the formation of gallstones. However, in the current study, we found that the activation of GPR30 by G-1 leads to an increase in biliary cholesterol concentrations in company with hepatic hyposecretion of biliary bile salts because of a marked reduction in bile salt synthesis and conversion of cholesterol to bile salts. Clinical studies have found that gallstone patients, who take oral contraceptive steroids and conjugated estrogens, demonstrate two types of biliary cholesterol hypersecretion, i.e., cholesterol hypersecretion into hepatic bile is accompanied by either (1) normal or (2) low secretion rates of biliary bile salts.(34–40) This implies that these clinical features may be attributable to the differences between GPR30 and ERα in varying responses to high levels of E2. Our findings indicate, therefore, that under E2 stimulation conditions GPR30 and ERα promote the formation of cholesterol-supersaturated bile by two distinct mechanisms through repressing Cyp7a1 expression and the classical pathway of bile salt synthesis, as well as enhancing hepatic cholesterogenesis, respectively.
We found that G-1 impairs gallbladder contractility in the early stage of gallstone formation, thereby inducing gallbladder hypomotility and subsequently promoting rapid formation of biliary sludge, a precursor of gallstones, in OVX wild-type and ERα−/−, but not GPR30−/−, mice. By immunohistochemical studies, we have revealed that GPR30 is expressed predominantly in the epithelial cells of gallbladder, whereas ERα is expressed mainly in the smooth muscle of gallbladder.(18) It is mostly likely that high levels of E2 could impair gallbladder contractile function by two different mechanisms through the GPR30 and the ERα pathways, respectively. A concept has been proposed to explain the potential lithogenic mechanisms of GPR30 on worsening gallbladder emptying.(18) GPR30 may have a vital effect on enhancing gallbladder cholesterol absorption by disrupting the function of gallbladder sterol transporters in the epithelial cells, whereas ERα may play a pivotal role in inhibiting expression of CCK-1 receptor in the muscle cells for inducing gallbladder stasis. The current study also constitutes the basic framework for further investigating the molecular and cellular mechanisms underlying the critical role of GPR30 in impairing gallbladder motility.
Notably, the molecular mechanism by which GPR30 regulates expression of Cyp7a1 and Abcg5/g8 is not defined yet. In preliminary experiments, not yet completed, we have found that epidermal growth factor receptor (Egfr) activated by Gpr30 may be involved in E2-mediated Cyp7a1 expression and the classical pathway of bile salt synthesis, as well as Abcg5/g8 expression possibly through a nontranscriptional regulatory mode because GPR30 is expressed exclusively in the endoplasmic reticulum, but not the nucleus, of hepatocytes. It is likely that upon E2 binding GPR30 activates heterotrimeric G proteins that stimulate multiple effectors, including cAMP and steroid receptor coactivator, a member of the nuclear receptor coactivator family. The latter pathway, in turn, activates matrix metalloproteinase, which cleaves pro-heparin-binding epidermal growth factor–like growth factor (HB-EGF) and promotes a release of free HB-EGF.(41) Then, HB-EGF trans-activates EGFR. Subsequently, this leads to multiple downstream events, including the activation of phospholipase C (PLC), mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K). PLC activation results in intracellular calcium mobilization by inositol triphosphate receptor through the actions of inositol triphosphate. The activation of MAPK and PI3K stimulates numerous cytosolic pathways and some transcription factors. These stimulations by E2 could induce the transcription of target genes whose promoters do not contain estrogen response elements. An in vitro study has found that the activation of GPR30 by estradiol-17β-D-glucuronide causes cholestasis by inhibiting the function of canalicular transporters, bile salt export pump (i.e., ABCB11), and multidrug resistance-associated protein 2 in the hepatocytes through the adenylyl cyclase and protein kinase A pathway, a membrane-initiated signaling cascade.(42) Obviously, the molecular mechanisms underlying the role of GPR30 in the regulation of expression of target genes is different from those of ERα and ERβ. In addition, GPR30 is activated by an external signal such as E2 and G-1, leading to a conformational change in the GPR30 structure and an increase in its expression levels. Subsequently, GPR30 is inactivated by guanosine triphosphatase activating proteins, known as the regulator of G protein signaling proteins.(43) However, under conditions of continued exposure to a ligand, it is likely that the feed-forward regulation may play a key role in determining Gpr30 expression.
In summary, the results reported herein are consistent with our recent findings showing that targeted disruption of the Erα gene alone does not completely protect against gallstone formation in OVX ERα−/− mice.(4) The current data support the concept that in the lithogenic state GPR30 is involved in additional lithogenic mechanisms of E2, working independently of ERα, to increase cholelithogenesis by suppressing Cyp7a1 expression and the classical pathway of bile salt synthesis in female mice (Fig. 7). Our results suggest that GPR30 may produce a synergistic lithogenic effect with ERα on the formation of E2-induced gallstones and that both GPR30 and ERα could be potential therapeutic targets for the prevention of cholesterol gallstones, particularly in women and patients exposed to high levels of E2.
FIG. 7.
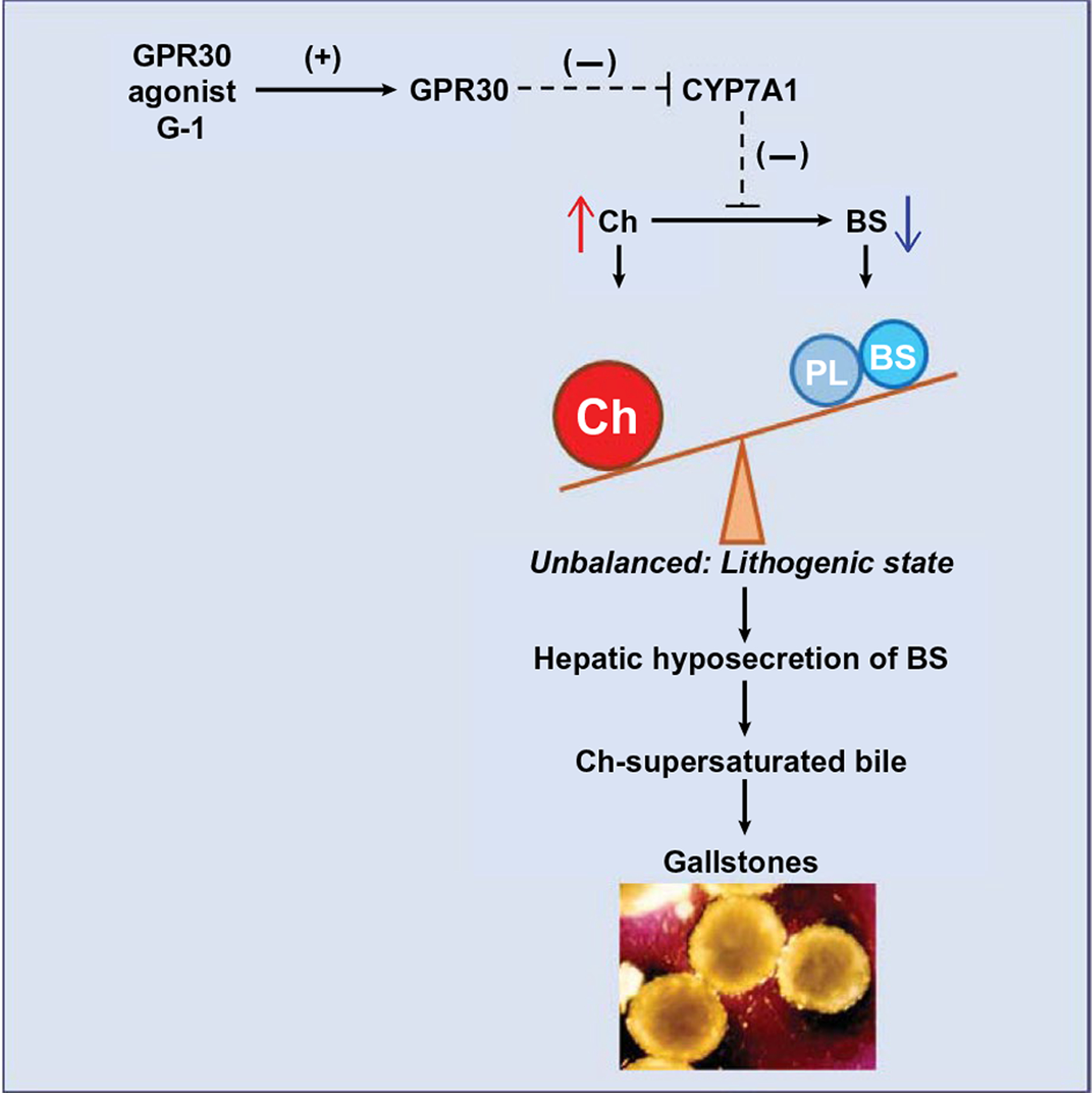
Working model for a possible G-1–GPR30–CYP7A1 pathway enhancing cholelithogenesis. We found that the GPR30-selective agonist G-1 activates hepatic Gpr30 expression, with the latter suppressing activity of Cyp7a1, the rate-limiting enzyme for the classical pathway of bile salt synthesis. As a result, these changes lead to increased biliary cholesterol concentration and hepatic bile salt hyposecretion, thereby reducing cholesterol solubility in bile and causing cholesterol-supersaturated bile that predisposes to solid cholesterol crystal precipitation and gallstone formation. Abbreviations: BS, bile salts; Ch, cholesterol; PL, phospholipids.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (US Public Health Service; DK101793, DK106249, DK114516, and AA025737, to D.Q.-H.W.; P30 DK041296, to the Marion Bessin Liver Research Center).
Abbreviations:
- ABC
adenosine triphosphate–binding cassette
- CCK-8
sulfated cholecystokinin octapeptide
- CSI
cholesterol saturation index
- CYP7A1
cholesterol 7α-hydroxylase
- CYP27A1
sterol 27-hydroxylase
- E2
17β-estradiol
- ER
estrogen receptor
- GOX
gonadectomized
- GPR30
the G protein-coupled receptor 30
- HB-EGF
heparin-binding epidermal growth factor-like growth factor
- Lith18
lithogenic gene 18
- OVX
ovariectomized
- PBS
phosphate buffered saline
- SREBP-2
sterol regulatory element binding proteins-2.
Footnotes
Potential conflict of interest: Nothing to report.
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.31212/suppinfo.
REFERENCES
- 1).Wang HH, Liu M, Clegg DJ, Portincasa P, Wang DQ. New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochim Biophys Acta 2009;1791:1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Wang HH, Afdhal NH, Wang DQ. Estrogen receptor alpha, but not beta, plays a major role in 17beta-estradiol-induced murine cholesterol gallstones. Gastroenterology 2004;127:239–249. [DOI] [PubMed] [Google Scholar]
- 3).Wang HH, Afdhal NH, Wang DQ. Overexpression of estrogen receptor alpha increases hepatic cholesterogenesis, leading to biliary hypersecretion in mice. J Lipid Res 2006;47:778–786. [DOI] [PubMed] [Google Scholar]
- 4).de Bari O, Wang HH, Portincasa P, Liu M, Wang DQ. The deletion of the estrogen receptor alpha gene reduces susceptibility to estrogen-induced cholesterol cholelithiasis in female mice. Biochim Biophys Acta 2015;1852:2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 1997;45:607–617. [DOI] [PubMed] [Google Scholar]
- 6).Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 2000;289:739–745. [DOI] [PubMed] [Google Scholar]
- 7).Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JA. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol 2012;212:3–12. [DOI] [PubMed] [Google Scholar]
- 8).O’Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, et al. Discovery of three novel G-protein-coupled receptor genes. Genomics 1998;47:310–313. [DOI] [PubMed] [Google Scholar]
- 9).Chagin AS, Savendahl L. GPR30 estrogen receptor expression in the growth plate declines as puberty progresses. J Clin Endocrinol Metab 2007;92:4873–4877. [DOI] [PubMed] [Google Scholar]
- 10).Lyons MA, Korstanje R, Li R, Sheehan SM, Walsh KA, Rollins JA, et al. Single and interacting QTLs for cholesterol gallstones revealed in an intercross between mouse strains NZB and SM. Mamm Genome 2005;16:152–163. [DOI] [PubMed] [Google Scholar]
- 11).Lyons MA, Wittenburg H. Cholesterol gallstone susceptibility loci: a mouse map, candidate gene evaluation, and guide to human LITH genes. Gastroenterology 2006;131:1943–1970. [DOI] [PubMed] [Google Scholar]
- 12).Wang HH, Portincasa P, Afdhal NH, Wang DQ. Lith genes and genetic analysis of cholesterol gallstone formation. Gastroenterol Clin North Am 2010;39:185–207. [DOI] [PubMed] [Google Scholar]
- 13).Wang TY, Portincasa P, Liu M, Tso P, Wang DQ. Mouse models of gallstone disease. Curr Opin Gastroenterol 2018;34:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, et al. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol 2000;74:279–285. [DOI] [PubMed] [Google Scholar]
- 15).Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology 2002;143:4172–4177. [DOI] [PubMed] [Google Scholar]
- 16).Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2006;2:207–212. [DOI] [PubMed] [Google Scholar]
- 17).O’Dea A, Sondergard C, Sweeney P, Arnatt CK. A series of indole-thiazole derivatives act as GPER agonists and inhibit breast cancer cell growth. ACS Med Chem Lett 2018;9:901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).de Bari O, Wang TY, Liu M, Portincasa P, Wang DQ. Estrogen induces two distinct cholesterol crystallization pathways by activating ERalpha and GPR30 in female mice. J Lipid Res 2015;56:1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res 1996;37:606–630. [PubMed] [Google Scholar]
- 20).Wang DQ, Portincasa P. Gallstones: Recent Advance in Epidemiology, Pathogenesis, Diagnosis and Management. New York: Nova Biomedical; 2017. [Google Scholar]
- 21).Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med 2002;346:340–352. [DOI] [PubMed] [Google Scholar]
- 22).Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005;307:1625–1630. [DOI] [PubMed] [Google Scholar]
- 23).Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 2008;70:165–190. [DOI] [PubMed] [Google Scholar]
- 24).Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 2006;38:1289–1297. [DOI] [PubMed] [Google Scholar]
- 25).Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci 2008;29:116–123. [DOI] [PubMed] [Google Scholar]
- 26).Wang DQ, Neuschwander-Tetri BA, Portincasa P. The Biliary System, 2nd ed. Princeton, NJ: Morgan & Claypool Life Sciences; 2017. [Google Scholar]
- 27).Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 2009;50:1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Hofmann AF. Bile acids: trying to understand their chemistry and biology with the hope of helping patients. Hepatology 2009;49:1403–1418. [DOI] [PubMed] [Google Scholar]
- 29).Vlahcevic ZR, Stravitz RT, Heuman DM, Hylemon PB, Pandak WM. Quantitative estimations of the contribution of different bile acid pathways to total bile acid synthesis in the rat. Gastroenterology 1997;113:1949–1957. [DOI] [PubMed] [Google Scholar]
- 30).Xu G, Salen G, Shefer S, Tint GS, Kren BT, Nguyen LB, et al. Increased bile acid pool inhibits cholesterol 7 alpha-hydroxylase in cholesterol-fed rabbits. Gastroenterology 1997;113:1958–1965. [DOI] [PubMed] [Google Scholar]
- 31).Pandak WM, Heuman DM, Redford K, Stravitz RT, Chiang JY, Hylemon PB, et al. Hormonal regulation of cholesterol 7alpha-hydroxylase specific activity, mRNA levels, and transcriptional activity in vivo in the rat. J Lipid Res 1997;38:2483–2491. [PubMed] [Google Scholar]
- 32).Xu G, Salen G, Shefer S, Tint GS, Nguyen LB, Parker TT, et al. Regulation of classic and alternative bile acid synthesis in hypercholesterolemic rabbits: effects of cholesterol feeding and bile acid depletion. J Lipid Res 1998;39:1608–1615. [PubMed] [Google Scholar]
- 33).Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res 2009;50(Suppl.):S406–S411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Bennion LJ, Mott DM, Howard BV. Oral contraceptives raise the cholesterol saturation of bile by increasing biliary cholesterol secretion. Metabolism 1980;29:18–22. [DOI] [PubMed] [Google Scholar]
- 35).Van Erpecum KJ, Van Berge Henegouwen GP, Verschoor L, Stoelwinder B, Willekens FL. Different hepatobiliary effects of oral and transdermal estradiol in postmenopausal women. Gastroenterology 1991;100:482–488. [DOI] [PubMed] [Google Scholar]
- 36).Nakagaki M, Nakayama F. Effect of female sex hormones on lithogenicity of bile. Jpn J Surg 1982;12:13–18. [DOI] [PubMed] [Google Scholar]
- 37).Lynn J, Williams L, O’Brien J, Wittenberg J, Egdahl RH. Effects of estrogen upon bile: implications with respect to gallstone formation. Ann Surg 1973;178:514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Bennion LJ, Ginsberg RL, Gernick MB, Bennett PH. Effects of oral contraceptives on the gallbladder bile of normal women. N Engl J Med 1976;294:189–192. [DOI] [PubMed] [Google Scholar]
- 39).Angelin B, Olivecrona H, Reihner E, Rudling M, Stahlberg D, Eriksson M, et al. Hepatic cholesterol metabolism in estrogen-treated men. Gastroenterology 1992;103:1657–1663. [DOI] [PubMed] [Google Scholar]
- 40).Everson GT, McKinley C, Kern F Jr. Mechanisms of gallstone formation in women. Effects of exogenous estrogen (Premarin) and dietary cholesterol on hepatic lipid metabolism. J Clin Invest 1991;87:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 2000;14:1649–1660. [DOI] [PubMed] [Google Scholar]
- 42).Zucchetti AE, Barosso IR, Boaglio AC, Basiglio CL, Miszczuk G, Larocca MC, et al. G-protein-coupled receptor 30/adenylyl cyclase/protein kinase A pathway is involved in estradiol 17beta-D-glucuronide-induced cholestasis. Hepatology 2014;59:1016–1029. [DOI] [PubMed] [Google Scholar]
- 43).Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature 1996;383:172–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


