Abstract
T cell-based immunotherapies have revolutionized treatment paradigms in various cancers, however, limited response rates secondary to lack of significant T-cell infiltration in the tumor site remain a major problem. To address this limitation, strategies for redirecting T cells to treat cancer are being intensively investigated, while the bispecific T cell engager (BiTE) therapy constitutes one of the most promising therapeutic approaches. BiTE is a bispecific antibody construct with a unique function, simultaneously binding an antigen on tumor cells and a surface molecule on T cells to induce tumor lysis. BiTE therapy represented by blinatumomab has achieved impressive efficacy in the treatment of B cell malignancies. However, major mechanisms of resistance to BiTE therapy are associated with antigen loss and immunosuppressive factors such as the upregulation of immune checkpoints. Thus, modification of antibody constructs and searching for combination strategies designed to further enhance treatment efficacy as well as reduce toxicity has become an urgent issue, especially for solid tumors in which response to BiTE therapy is always poor. In particular, immunotherapies focusing on innate immunity have attracted increasing interest and have shown promising anti-tumor activity by engaging innate cells or innate-like cells, which can be used alone or complement current therapies. In this review, we depict the landscape of BiTE therapy, including clinical advances with potential response predictors, challenges of treatment toxicity and resistance, and developments of novel immune cell-based engager therapy.
Keywords: Immunotherapy, Bispecific T cell engager, Cancer
Introduction
T cell-based cancer immunotherapies have transformed the clinical practice of cancer treatment by targeting and mobilizing T cells to eradicate malignant cells. Depending on the mechanisms of action, T cell-based cancer immunotherapies can be mainly divided into two classes: one against immunosuppressive factors represented by immune checkpoint inhibitors (ICIs), the other one focusing on immunostimulatory pathways represented by chimeric antigen receptor (CAR) T cells and T-cell engaging bispecific antibodies (bsAbs) [1–3]. ICIs have revolutionized cancer treatment in the clinic, especially in several advanced solid tumors, for instance, melanoma and non-small-cell lung cancer [4–7]. They hamper the tumor immune escape by blocking key immunosuppressive molecules such as programmed cell death 1 (PD-1) and its ligand (PD-L1) and releasing the “brake” of cytotoxic T cells to eliminate tumor cells, however, response rates of ICIs remain limited [8]. A major reason for this is the lack of a sufficient number of tumor-infiltrating immune cells (TILs), primarily T cells, in the tumor site, which is referred to as exhibiting cold phenotype [9]. CAR T-cell therapy is a newly developed adoptive cell therapy by genetically engineering T cells to express a CAR comprising intracellular T-cell signaling domains and an extracellular antigen-recognition structure targeting tumor-associated antigens (TAAs), redirecting and activating T cells to eradicate malignant cells specifically [10]. The preparation of CAR T Cells primarily includes isolation of T cells from patients, genetic modification of T cells, expansion of T cells in vitro, and infusion of edited T cells to patients, however, which is a complex and time-consuming process [11]. The other alternative approach to redirect T cells against target cells is T-cell engaging bsAbs with unique function engaging TAAs on cancer cells and cell surface molecules on T cells. Bispecific T-cell engager (BiTE) stands out as a novel subclass of T-cell engaging bsAbs with promising clinical results in the treatment of cancers. And the comparison of these three T-cell based immunotherapies is summarized in Table 1.
Table 1.
Comparison of three main T cell-based immunotherapies: ICI, CAR T cell, and BiTE
| ICI | CAR-T cell | BiTE | |
|---|---|---|---|
| Structure | Monoclonal antibody targeting immune checkpoint proteins | A T cell from patients that are genetically re-engineered to present a synthetic transmembrane receptor on the surface to target a tumor antigen on tumor cells | A recombinant antibody comprised of two tandem scFv, one binding CD3 on T cells, the other targeting a tumor antigen on tumor cells |
| Anti-tumor mechanisms | Blocking the inhibitory immune checkpoint proteins that result in cytotoxic T cell-mediated immune response | Inducing tumor cell lysis by the formation of immune synapse between T cells and tumor cells | Inducing tumor cell lysis by the formation of immune synapse between T cells and tumor cells |
| Recruitment of T cells | Passive, acting on tumor-infiltrating and endogenous T cells to kill tumor cells | Active, redirecting engineered T cells outside of body to kill tumor cells | Passive, dependent on endogenous T cells and redirecting them to kill tumor cells |
| Production | Hybridoma technology, pervasive for all patients | Genetically engineering patient’s T cells outside of body, individualized for each patient | Genetically engineered and purified from mammalian cell lines, pervasive for all patients |
| Indications | Mainly in solid tumors with approval in a small part of hematologic neoplasms | All in hematologic neoplasms | All in hematologic neoplasms but with promising results for solid tumors |
| Toxicity | Immune-mediated AEs such as hyperactivation and hypersensitivity | On-target off-tumor effects, CRS, neurotoxicity | On-target off-tumor effects, CRS, neurotoxicity |
| Advantages | Broad-spectrum anti-tumor activity, easy production | MHC-independent, TCR-independent, endogenous T cell-independent | MHC-independent, TCR-independent, relatively easy production, tumor-infiltrating T cell-independent |
| Disadvantages | Tumor-infiltrating T cell-dependent, immune checkpoint expression-dependent, MHC-dependent, TCR-dependent | Lack of efficacy for solid tumors, long-term and complex production, antigen-dependent | Antigen-dependent, continuous administration due to a short half-life |
ICI Immune checkpoint inhibitor, CAR Chimeric antigen receptor, BiTE Bispecific T cell engager, AEs Advert effects, CRS Cytokine release syndrome, MHC Major histocompatibility complex, TCR T cell receptor
BiTE design and mechanism of action
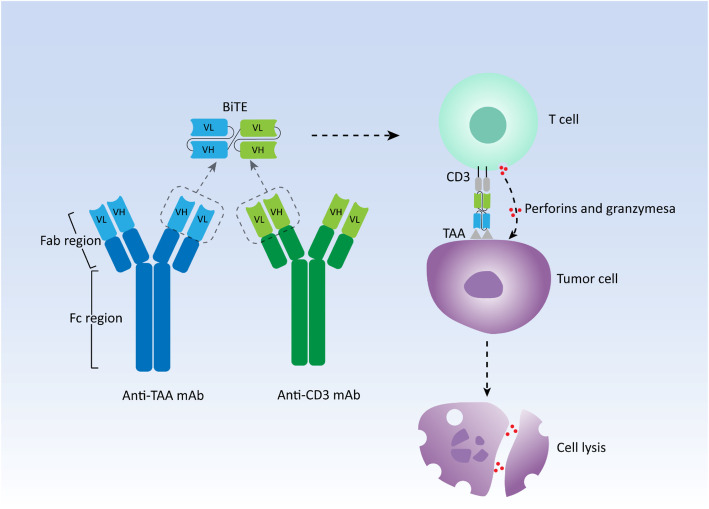
In general, human antibodies are monospecific that usually recognize only one targeted antigen. bsAbs simultaneously target two different antigens for cancer treatment via redirecting immune cells to tumor cells, delivering drugs to tumors, and blocking two biological pathways significant for tumors [12]. Among them, redirecting immune cells, primarily T cells, to target cells is the most successful and widely used function to induce specific and powerful anti-tumor activity. Due to the invariant property of CD3 chains in the T cell receptor (TCR), CD3 is always selected as a cell surface target [13]. Thus, numerous bsAbs targeting CD3 are developed in a variety of different constructs, they can be broadly classified into two categories: bsAbs with Fc domains and bsAbs without Fc domains. Fc domains contribute to the maintenance of stability, simplification of the purification process, and extending of half-life for bsAbs [14]. However, the interaction between Fc domains and their receptors on various types of immune effector cells such as natural killer (NK) cells, monocytes, and macrophages, is capable of inducing antibody-dependent cell-mediated cytotoxicity (ADCC), while Fc domains can also bind complement to elicit complement-dependent cytotoxicity (CDC), leading to the unnecessary non-specific immune response during bsAbs treatment [15]. BiTE falls into the latter category with a small molecular size. Two single-chain variable fragments (scFvs) from an anti-TAA and an anti-CD3 monoclonal antibody, respectively, form the canonical BiTE molecule with a short linker connecting them in tandem [16]. scFvs consisting of heavy chain variable regions (VH) and light chain variable regions (VL) of immunoglobulin (Ig) are commonly used as antigen-binding domains (Fig. 1). Therefore, BiTE is a small and flexible molecule, which is easily diffusible and can quickly move from the site of administration to the site of lesions, redirecting cytotoxic T cells to cancer cells with high affinity.
Fig. 1.
The schematic representation of structure and mechanism of action of canonical bispecific T-cell engager (BiTE). mAb: Monoclonal antibody; VH: Heavy chain variable region; VL: Light chain variable region; TAA: Tumor-associated antigen
Generally, TAAs are presented by major histocompatibility complex (MHC) molecules expressed on tumor cells, interacting with TCR on T cells and inducing T-cell activation to eliminate malignant cells, which are known as MHC restriction. However, the impairment or loss of the ability to present antigens for MHC molecules is one of the reasons for the intrinsic resistance to immunotherapies such as ICI therapy [17–19]. Moreover, effective T-cell activation and immune response also require costimulatory signals, primarily CD28 signals [20]. Nevertheless, BiTE can crosslink cytotoxic T cells and cancer cells independently from MHC restriction as well as costimulatory signals like a bridge, and then these T cells are activated and start to proliferate, inducing the formation of the immunologic synapse [21–23]. Perforins and granzymes are secreted by these activated T cells at the site of the immunologic synapse, which results in the cytotoxic lysis of target cells [24]. Compared with other bsAbs and monoclonal IgG antibodies, in cellular models, BiTE has a 100 ~ 10,000 fold higher efficacy in tumor cell lysis with a low ratio of T cells to target tumor cells [25]. In addition, BiTE can be produced by mammalian cell lines in large quantities, resulting in a relatively simple and fast production process compared with the lengthy and complicated culture of CAR T cells [16]. In the next section, we will present the latest clinical advances of BiTE therapy with published results in both hematologic neoplasms and solid tumors.
Clinical advances of BiTE in hematological malignancies
A primary requirement for successful BiTE therapy is the identification of appropriate TAAs expressed on target cells other than normal cells to avoid on-target/off-tumor toxicity. Abnormal expression of CD19 can be observed in many hematologic malignancies associated with B cells, contributing to tumorigenesis, besides, expression of CD19 is restricted to malignant and normal B-lineage cells [26–28]. Thus, CD19 is an attractive target for immunotherapies against B-cell malignancies. Blinatumomab is the first-in-class CD19 × CD3 canonical BiTE construct with a low molecular weight of 55 kDa. While adults with newly diagnosed B-cell precursor acute lymphoblastic leukemia (B-ALL) have achieved a complete remission (CR) rate of 80–90% with chemotherapy [29], patients with relapsed or refractory (R/R) disease, the cytogenetic abnormality with Philadelphia (Ph) chromosome and the minimal residual disease (MRD) after initial therapy remain major challenges and are associated with poor prognosis. Moreover, R/R non-Hodgkin lymphoma (NHL) that most frequently originate from B cells is another challenge in treating patients with B-cell malignancies. Nevertheless, blinatumomab has demonstrated remarkable clinical efficacy in addressing these thorny issues, which is summarized by other recent reviews [3, 30, 31] (Table 2). Blinatumomab has been approved by FDA for the treatment of both children and adults with R/R B-ALL and gained accelerated approval to treat patients with MRD-positive B-ALL, which is the first and only BiTE antibody reaching the market. A phase 1 clinical trial evaluating next-generation BiTE with extended half-life (HLE) AMG 562 is ongoing in patients with R/R NHL (NCT03571828). Other main T-cell engaging bsAbs targeting CD19 and CD3 include TNB-486 [48], dual affinity retargeting antibody (DART) duvortuxizumab [49], and tandem diabody (TandAb) AFM11 [50] developed for B-cell malignancies. However, clinical trials for DART duvortuxizumab and TandAb AFM11 were discontinued due to severe toxicity during treatment.
Table 2.
Clinical advances of blinatumomab in the treatment of B-cell malignancies
| Study/NCT identifier | Phase | Indications | Number of patients | Blinatumomab dosing | Efficacy | Toxicity | Reference |
|---|---|---|---|---|---|---|---|
| GMALL (original study NCT00198991, follow-up study NCT00198978) | 2 | Adults (≥18 years) with MRD-positive B- ALL | 21 (20 evaluable for response) | Continuous IV infusion at a dose of 15 μg/m2 /d | MRD-negative response rate: 80% (16/20) 5-year RFS rate: 50% (10/20) Median RFS: 19.1 months in 16 MRD responders; 3.2 months in 4 nonresponders CR in HSCT patients: 5/9 CR in non-HSCT patients: 5/11 | Grade ≥ 3 AEs: 81% Grade 3 CNS AEs: 19% | [32–34] |
| BLAST (NCT01207388). | 2 | Adults with MRD-positive B-ALL | 116 (113 evaluable for response) | Continuous IV infusion at a dose of 15 μg/m2 /d | MRD-negative response rate: 78% (88/113) 18 months RFS rate: 54% Median RFS: 18.9 months Median OS: 36.5 months 25% patients without HSCT after blinatumomab remained in continuous CR 49% with HSCT remained in continuous CR |
Grade ≥ 3 AEs: 60% Any grade CRS: 3% Grade ≥ 3 CRS: 2% Any grade CNS AEs: 53% Grade ≥ 3 CNS AEs: 13% |
[35, 36] |
| NCT02412306 | 1/2 | Japanese adults with R/R Ph-negative B-ALL | 26 | Continuous IV infusion at a dose of 9 μg/m2 /d for the first 7 days and 28 μg/m2 /d thereafter |
Phase 1b: CR/CRh rate: 80% (4/5) MRD-negative response rate in responders: 100% (4/4) Phase 2: CR/CRh rate: 38% (8/21) MRD-negative response rate in responders: 38% (3/8) Median RFS: 5 months |
Grade ≥ 3 AEs: 96% Any grade CRS: 46% Grade ≥ 3 CRS: 4% Any grade CNS AEs: 46% Grade ≥ 3 CNS AEs: 5% |
[37] |
| NCT01209286 | 2 | Adults with R/R Ph-negative B-ALL | 36 | Continuous IV infusion at a dose of 5–30 μg/m2 /d | CR/CRh rate: 69%(25/36) MRD-negative response rate in responders: 88% (22/25) Median OS: 9.8 months Median RFS: 7.6 months |
CNS AEs requiring intervention: 17% Grade ≥ 3 CRS: 6% |
[38] |
| NCT01466179 | 2 | Adults (≥18 years) with R/R Ph-negative B-ALL | 189 | Continuous IV infusion from 9 to 28 μg/m2/d | CR/CRh rate: 43%(81/189) MRD-negative response rate in responders: 82% (60/73) Median OS: 6.1 months Median RFS: 5.9 months |
Grade ≥ 3 AEs: 83% Grade ≥ 3 CRS: 2% Any grade CNS AEs: 52% Grade ≥ 3 CNS AEs: 13% |
[39] |
| TOWER (NCT02013167) | 3 | Adults (≥18 years) with R/R Ph-negative B-ALL | 405 (271 for blinatumomab, 134 for chemotherapy) | Continuous IV infusion from 9 to 28 μg/m2/d | Median OS: 7.7 vs. 4.0 months CR rate: 34% vs. 16%; CR/CRh/CRi rate: 44% vs. 25% 6-month EFS: 31% vs. 12% Median DOR: 7.3 vs. 4.6 months |
Grade ≥ 3 AEs: 87% vs. 92% Grade ≥ 3 CNS AEs: 9.4% vs.8.3% Grade ≥ 3 CRS: 4.9% vs. 0% (blinatumomab vs. chemotherapy) |
[40] |
| RIALTO (NCT02187354) | Expanded Access | Children (≤17 years) with R/R B-ALL | 110 (98 evaluable for response) |
≤ 25% blasts: 15 μg/m2/d > 25% blasts: 5 μg/m2/d for first 7 days, 15 μg/m2/d thereafter, |
CR rate: 59% (58/98) MRD-negative response rate in responders: 79% (46/58) Median OS:13.1 months Median RFS: 8.5 months 62% (36/58) proceeded to HSCT after achieving CR |
Grade ≥ 3 AEs: 65% Grade ≥ 3 CRS: 3% Any grade CNS AEs: 42% Grade ≥ 3 CNS AEs: 5.5% |
[41] |
| MT103–205 (NCT01471782) | 1/2 | Children (≤17 years) with R/R B-ALL | 94 (70 evaluable for response) | Continuous IV infusion from 5 to 15 μg/m2/d | CR rate: 39% (27/70) MRD-negative response rate in responders: 52% (14/27) 48% (13/27) proceeded to HSCT after achieving CR |
Grade ≥ 3 AEs: 87% Any grade CRS: 11% Grade ≥ 3 CRS: 6% Any grade CNS AEs: 24% Grade ≥ 3 CNS AEs: 4% |
[42] |
| NCT02000427 | 2 | Adults (≥18 years) with R/R Ph-positive B-ALL | 45 | Continuous IV infusion from 9 to 28 μg/m2/d | CR/CRh rate: 36%(16/45) MRD-negative response rate in responders: 88% (14/16) Median OS: 7.1 months Median RFS: 6.7 months 44% (7/16) proceeded to HSCT after achieving CR |
Grade ≥ 3 AEs: 82% Any grade CRS: 7% Grade ≥ 3 CNS AEs: 7% |
[43] |
| MT103–104 (NCT00274742) | 1 | Adults (≥18 years) with R/R B-NHL | 76 | Dose-escalation: 0.5–90 μg/m2/d Dose-expansion: 60 μg/m2/d |
Patients with dose of 60 μg/m2/d: ORR: 69% across NHL subtypes and 55% for DLBCL Median DOR: 404 days Median OS in overall population: 4.6 years Median OS in responders: 7.7 years Median OS in nonresponders: 1.1 years |
Grade 3, 4, and 5 AEs: 90, 66, and 4%, respectively Any grade CNS AEs: 71% Grade 3 CNS AEs: 22% |
[44, 45] |
| NCT02910063 | 2 | Adults with R/R B-NHL | 41 | Continuous IV infusion from 9 to 28 to 112 μg/d |
ORR: 37% (15/41) including CMR: 22% (9/41) and PMR: 15% (6/41) HSCT was performed in 53.3% (8/15) responders. |
Grade ≥ 3 AEs: 71% Grade ≥ 3 CRS: 2% Any grade CNS AEs: 56% Grade ≥ 3 CNS AEs.: 24% |
[46] |
| NCT01741792 | 2 | Adults with R/R B-DLBCL | 25 (21 evaluable for response) |
Cohort I + III: Stepwise dose 9 to 28 to 112 μg/d Cohort II: Flat dose 112 μg/d |
Patients with dose of 112 μg/d at least 1 week: ORR: 43% (9/21) CR rate: 19% (4/21) |
Cohort I: n = 23 Grade ≥ 3 AEs: 95.7% Any grade CRS: 0% Grade ≥ 3 CNS AEs: 22% Cohort II: n = 2 Grade ≥ 3 CNS AEs: 100% |
[47] |
MRD Minimal residual disease, B-ALL B-precursor acute lymphoblastic leukemia, IV Intravenous, HSCT Allogeneic hematopoietic stem cell transportation, AEs Adverse events, R/R Refractory/relapsed, CRS Cytokine release syndrome, OS Overall survival, RFS Relapse-free survival, CR Complete remission, CRh Complete remission with partial hematologic recovery, CRi Complete remission with incomplete hematological recovery, Ph Philadelphia chromosome, NHL Non- Hodgkin lymphoma, DLBCL Diffuse large B cell lymphoma, DLT Dose-limiting toxicity, DOR Duration of remission, EFS Event-free survival, ORR Objective response rate, CMR Complete metabolic response
CD33 mediates the proliferation and differentiation of myeloid cells and it can be detected on the malignant myeloid blasts in the majority of patients with acute myeloid leukemia (AML), suggesting it is a potential target for cancer immunotherapy [51]. In 2020 ASCO, updated data from an ongoing phase 1 study (NCT02520427) conducted for AMG 330, a CD33 × CD3 canonical BiTE antibody, has been reported. AMG 330 was given at doses ranging from 0.5–720 μg/d in the manner of continuous IV infusion among 55 patients with R/R AML. The most common treatment-related AEs was cytokine release syndrome (CRS) with a grade ≥ 3 CRS rate of 13%. CRS was reversible and was associated with the dose level and leukemic burden at baseline. Among 42 patients evaluable for response, there were 3 CR and 4 complete remission with incomplete hematological recovery (CRi) observed at the dose of ≥120 μg/d [52]. In addition, preliminary results from an ongoing phase 1clincal trial (NCT03224819) have shown anti-leukemic activity of HLE-BiTE AMG 673 in the treatment of R/R AML [53]. Currently, other main anti-CD33 T-cell engaging bsAbs have entered clinical development and are in phase 1 clinical study in patients with R/R AML, including JNJ-67571244 [54], affinity-tailored adaptors for T-cells (ATAC) GEM333 [55], and TandAb AMV 564 [56].
B-cell maturation antigen (BCMA) essential for the long-term survival of plasma cells is highly expressed on the malignant plasma cells in multiple myeloma (MM) with almost no expression on normal cells, which can be the promising target for T-cell based immunotherapy [57]. A first-in-human clinical trial recently demonstrated favorable efficacy and low toxicity of AMG 420, an anti-BCMA canonical BiTE molecule, treating patients with R/R MM. Forty-two patients who had previously been treated with more than 2 lines of therapies were enrolled and received 6-week cycles of AMG 420 at the dose of 0.2–800 μg/d. Among 10 patients in the maximum tolerated dose (MTD) cohort at the dose of 400 μg/d, the overall response rate (ORR) was 70% with 5 MRD-negative CR, 1 very good partial response (VGPR), and 1 PR. The serious AEs rate was 48%, of these, infections were the most common with a rate of 33%. The incidence of grade ≥ 3 CRS was 2% with no grade ≥ 3 central nervous system (CNS) toxicity [58]. AMG 420 has already been fast-tracked for development by the FDA. In addition, the second-generation HLE-BiTE AMG 701 has achieved an 83% ORR in patients with R/R MM, the majority of responders experienced triple therapy failure [59]. Teclistamab [60], REGN5458 [61], CC-93269 [62] PF-06863135 [63], and TNB-383B [64] are other investigational T-cell engaging bsAbs designed to bind to BCMA with structural differences, all of them have entered the early clinical stage for treating patients with R/R MM with promising efficacy. The clinical advances of these newly developed BiTE antibodies and other T-cell engaging bsAbs are summarized in Table 3.
Table 3.
Clinical advances with promising published results in other BiTE and T-cell engaging bsAbs
| NCT identifier | Phase | Indications | Number of patients | Drug | Format | Target | Results | Reference |
|---|---|---|---|---|---|---|---|---|
| NCT03625037 | 1/2 | R/R B-NHL | 67 | Epcoritamab | Fully human IgG1-based bsAb | CD20/CD3 |
In DLBCL ≥12 mg (n = 18), ORR: 66.7% (6CRs) In FL ≥ 0.76 mg (n = 8), ORR:100% (2CRs) In MCL (n = 4) ORR: 50% (1 CR, 1 PR) |
[65] |
| NCT02290951 | 1 | R/R B-NHL | 127 | Odronextamab | Fully human IgG4-based bsAb | CD20/CD3 |
In FL ≥ 5 mg (n = 28), ORR: 92.9% (CR rate: 75.0%) In DLBCL without prior CAR T therapy ≥80 mg (n = 10), ORR: 60% (CR rate: 60%) In DLBCL with prior CAR T therapy ≥80 mg (n = 21), ORR: 33.3% (CR rate: 23.8%) |
[66] |
| NCT02500407 | 1 | R/R FL | 62 | Mosunetuzumab | Fully humanized IgG1-based bsAb | CD20/CD3 | ORR: 68% (CR rate: 50%, PR rate: 18%) | [67] |
| NCT02924402 | 1 | R/R NHL and CLL | 44 | XmAb13676 | Fully humanized bsAb | CD20/CD3 |
In NHL 80–125 μg/kg (n = 18), ORR: 33.3%. In CLL 20 μg/kg (n = 5): ORR: 25% |
[68] |
| NCT03075696 | 1 | R/R NHL | 38 | Glofitamab | Fully humanized IgG1-based bsAb with a 2:1 molecular format | CD20/CD3 |
ORR:62.5%, CMR rate: 40.6% In aNHL (n = 24), ORR: 50.0%, CMR rate: 29.2%. In iNHL (n = 8), ORR: 100.0%, CMR rate: 75.0% |
[69] |
| NCT04082936 | 1 | R/R NHL | 14 | IGM-2323 | Fully humanized IgM-based bsAb | CD20/CD3 | Tumor size reduction rate: 64% (2 PRs) | [70] |
| NCT02520427 | 1 | R/R AML | 55 | AMG 330 | Two tandem scFvs | CD33/CD3 | ORR: 19% (CR rate: 7%, CRi rate: 10%) | [52] |
| NCT03224819 | 1 | R/R AML | 30 | AMG 673 | Two tandem scFvs with Fc region | CD33/CD3 | Decrease in blasts in bome marrow rate: 44% | [53] |
| NCT02514239 | 1 | R/R MM | 42 | AMG 420 | Two tandem scFvs | BCMA/CD3 |
ORR: 31% At dose of 400 mg/d (n = 10), ORR: 70% (5 MRD-negative CRs, 1 PR, and 1 VGPR) |
[58] |
| NCT03287908 | 1 | R/R MM | 75 | AMG 701 | Two tandem scFvs with Fc region | BCMA/CD3 |
At the dose of 3–12 mg (n = 45), ORR: 36%; At the dose of the 9 mg (n = 6), ORR: 83% (3PRs, 2VGPRs) |
[59] |
| NCT03145181 | 1 | R/R MM | 128 | Teclistamab | Fully humanized IgG1-based bsAb | BCMA/CD3 |
At the dose of 1500 μg/kg sc (n = 22), ORR: 73% (≥ CR rate: 23% and ≥ VGPR rate: 55%) |
[71] |
| NCT03761108 | 1 | R/R MM | 49 | REGN5458 | Fully humanized IgG1-based bsAb | BCMA/CD3 |
At the dose of 96 mg ORR: 63% (≥ VGPR rate: 95%) |
[61] |
| NCT03269136 | 1 | R/R MM | 30 | PF-06863135 | Fully humanized IgG1-based bsAb | BCMA/CD3 |
At the dose of 215 to 1000 μg/kg SC (n = 20), ORR: 80% (6CRs, 3 VGPRs,6 PRs) |
[72] |
| NCT03933735 | 1 | R/R MM | 38 | TNB-383B | Fully humanized IgG1-based bsAb | BCMA/CD3 |
At the dose of ≥40 mg, ORR: 80% (VGPR rate: 75%) |
[64] |
| NCT03275103 | 1 | R/R MM | 51 | Cevostamab | Fully humanized IgG1-based bsAb | FcRH5/CD3 |
At the dose of ≥3.6-20 mg (n = 29), ORR: 51.7% (6 CRs, 4 VGPRs, and 5 PRs) |
[73] |
| NCT03399799 | 1 | R/R MM | 137 | Talquetamab | Fully humanized IgG4-based bsAb | GPRC5D/CD3 |
At the dose of 20–180 μg/kg IV (n = 18), ORR: 78% (6/6 responded at the 60 μg/kg) At the dose of 135–405 μg/kg SC (n = 12) ORR: 67% (3/4 responded at the 405 μg/kg) |
[74] |
| NCT02152956 | 1/2 | PIF and ER AML | 38 | Flotetuzumab | Two inter-exchange of Fv domains | CD123/CD3 |
CRR: 42.1% (7 CR, 4 CRh, 4 CRi, and 1 MLFS) In PIF AML (n = 24), CRR: 45.8% (5 CR, 3 CRh, and 3 CRi) In ER AML (n = 14), CRR: 35.7% (2 CR, 1 CRh, 1CRi and 1 MLFS) |
[75] |
R/R Refractory/relapsed, IG Immunoglobin, bsAb Bispecific antibody, NHL Non- Hodgkin lymphoma, DLBCL Diffuse large B cell lymphoma, FL Follicular lymphoma, MCL Mantle cell lymphoma, ORR Objective response rate, CR Complete remission, CRh Complete remission with partial hematologic recovery, CRi Complete remission with incomplete hematological recovery, PR Partial response, VGPR Very good partial response, CMR Complete metabolic response, CAR Chimeric antigen receptor, CLL Chronic lymphocytic leukemia, aNHL aggressive NHL, iNHL indolent NHL, scFv Single-chain fragment variable, MRD Minimal residual disease, AML Acute Myeloid Leukemia, MM Multiple myeloma, Fc Fragment crystallizable, IV Intravenous, SC Subcutaneous, Fv Fragment variable, PIF Primary induction failure, LR Late relapse, MLFS Morphologic leukemia-free state
Clinical advances of BiTE in solid tumors
Given the favorable results of BiTE therapy in hematological malignancies, numerous BiTE antibodies targeting TAAs expressed on solid tumors are currently in clinical development (Table 4). Prostate-specific membrane antigen (PSMA) belongs to the type II transmembrane protein family highly confined to the prostate tissues. It is overexpressed in most prostate cancer (PC) and can be used as an ideal tumor antigen target [76]. Pasotuxizumab (AMG 212) is a canonical BiTE antibody targeting PSMA [77]. The anti-tumor activity which was defined by PSA level decrease was dose-dependent. Two patients achieved long-term PSA responses with more than 1 year of treatment at doses of 40 μg/d and 80 μg/d, respectively. The grade 3 AEs occurred in 81% of patients and the most common of them were decreased lymphocytes (44%) and infections (44%). Treatment-related SAE was observed in only one patient (fatigue). This is the first clinical study supporting BiTE immunotherapy’s role as an effective intervention in the treatment of patients with solid tumors. Moreover, in 2020 ESMO, HLE-BiTE AMG 160 has demonstrated a safety profile and showed evidence of preliminary efficacy in patients with mCRPC [78]. Another PSMA-targeting T-cell engager HPN424 has also entered a phase 1 clinical trial with early signs of clinical activity in patients with mCRPC [79].
Table 4.
Ongoing clinical trials of BiTE and other CD3-targeting T-cell engager bsAbs in solid tumors
| NCT | Phase | Indications | Number of patients | Drug | Target | Results | Reference |
|---|---|---|---|---|---|---|---|
| NCT01723475 | 1 | Metastatic castration-resistant prostate cancer | 47 | Pasotuxizumab | CD3/PSMA |
In SC group (n = 31), > 50% PSA decline rate:21% In cIV group (n = 16), > 50% PSA decline rate:19% |
[77] |
| NCT03792841 | 1 | Metastatic castration-resistant prostate cancer | 43 | AMG 160 | CD3/PSMA |
Any PSA decline rate: 68.6% > 50% PSA decline rate:34.3% In patients with evaluable disease (n = 15), there are 3 PRs and 8 SD |
[78] |
| NCT03296696 | 1 | R/R Glioblastoma | 15 | AMG 596 | CD3/EGFRvIII |
In patients with sufficient follow-up (n = 8), there are 1 PR and 2 SD |
[80] |
| NCT03319940 | 1 | R/R Small-cell lung cancer | 40 | AMG 757 | CD3/DLL3 |
In patients with evaluable disease (n = 38), there are 6 confirmed PRs, 11 SD, and 1 unconfirmed PR |
[81] |
| NCT04117958 | 1 | Gastric cancer | – | AMG 199 | CD3/MUC 17 | Not reported | [82] |
| NCT04260191 | 1` | Gastric cancer | – | AMG 910 | CD3/CLDN18.2 | Not reported | [83] |
| NCT04128423 | 1 | R/R solid tumors | 11 | AMV564 | CD3/CD33 |
In patients with evaluable disease (n = 9), there are 1 PR and 4 SD |
[84] |
| NCT02324257 | 1 | CEA+ advanced solid tumors | 80 | Cibisatamab | CD3/CEA |
In patients with mCRC at doses ≥60 mg (n = 36) Metabolic PR rate: 28% |
[85] |
| NCT04501770 | 1 | HER2+ advanced solid tumors | – | M802 | CD3/HER2 | Not reported | [86] |
| NCT04501744 | 1 | Malignant ascites | – | M701 | CD3/EpCAM | Not reported | [87] |
| NCT02748837 | 1 | Solid malignancies | – | ERY974 | CD3/GPC3 | Not reported | [88] |
| NCT02248805 | 1 | R/R Metastatic colorectal carcinoma | 95 | MGD007 | CD3/gpA33 | Not reported | [89] |
| NCT02262910 | 1 | Metastatic castration-resistant prostate cancer | 35 | ES414 | CD3/PSMA | Not reported | [90] |
| NCT03860207 | 1 | R/R Neuroblastoma, osteosarcoma, and other GD2+ solid tumors | – | Hu3F8-bsAb | CD3/GD2 | Not reported | [91] |
| NCT03983395 | 1/2 | HER2+ metastatic breast cancers | – | ISB 1302 | CD3/HER2 | Not reported | – |
| NCT03448042 | 1 | Locally advanced or metastatic HER2+ cancers | – | BTRC4017A | CD3/HER2 | Not reported | – |
| NCT04424641 | – | Locally advanced or metastatic solid tumors | – | GEN1044 | CD3/5 T4 | Not reported | – |
R/R Refractory/relapsed, PSMA Prostate-specific membrane antigen, cIV Continuous intravenous, SC Subcutaneous, PSA Prostate-specific antigen, CR Complete remission, PR Partial response, SD Stable disease, EGFRvIII The epidermal growth factor receptor variant III, DLL3 Delta-like 3, MUC 17 Mucin 17, CLDN18.2 Claudin-18 splice variant 2, CEA Carcinoembryonic antigen, Her2 Human epidermal growth factor receptor 2, EpCAM Epithelial cellular adhesion molecule, GPC3 Glypican 3, gpA33 Glycoprotein A33, GD2 Disialoganglioside
AMG 596 is a canonical BiTE molecule targeting the class III variant of the epidermal growth factor receptor (EGFRvIII). EGFR is a transmembrane cell surface receptor frequently overexpressed in patients with glioblastoma (GBM) that contributes to the formation of the tumor, meanwhile, EGFRvIII is the most common EGFR mutation in EGFR-positive patients with GBM [92]. Interim analysis of a phase 1 study (NCT03296696) evaluating the safety and efficacy of AMG 596 against recurrent GBM has shown 1 PR (12.5%) and 2 SD (25%) in 8 patients with sufficient follow-up. Among 14 evaluable patients, serious AEs occurred in 50% of patients, the most common treatment-related grade ≥ 3 AEs were headache and depressed consciousness (both 14.3%) [80].
Delta-like ligand 3 (DLL3) is particularly upregulated in small cell lung cancer (SCLC) but with minimal normal tissue expression. In 2020 the society for immunotherapy of cancer (SITC), preliminary results from an ongoing phase 1 study (NCT03319940) has demonstrated anti-tumor activity of AMG 757, a DLL3-targeting HLE-BiTE, in patients with R/R SCLC with tolerable toxicity [81]. Among 38 evaluable patients, there were 6 PR, 11 SD, and 1 unconfirmed PR. With a median follow-up of 8.8 months, five of the six responses were still in duration. The most common treatment-related AE was CRS with a rate of 43% and all CRS events were grade 1 or 2.
Additionally, AMG 199 [82] targeting mucin-17 (MUC17) and AMG 910 [83] targeting claudin18.2 (CLDN18.2) in patients with gastric cancer are in phase 1 clinical trials of NCT04117958 and NCT04260191, respectively.
Predictors of response to BiTE therapy
Blinatumomab represents BiTE therapy because it the only one approved for clinical use with the most outcome data. Some studies have reported that the high disease burden defined as ≥50% bone-marrow blasts was associated with the lower response rate of blinatumomab in the treatment of R/R B-ALL [39, 40, 42, 93]. For instance, in the phase 3 study, the remission rate including CR with full, partial, or incomplete hematologic recovery was 65.5% in R/R B-ALL patients with less than 50% of bone-marrow blasts, whereas it was only 34.4% for ≥50% [40]. A recent statistical study confirmed the negative correlation between disease burden and treatment response was statistically significant in R/R B-ALL patients treated with blinatumomab (P = 0.039) [94]. Moreover, the negative correlations between disease burden and response rate were also observed in CAR T cell therapy [95]. Therefore, before the initiation of T-cell engaging therapies such as blinatumomab treatment, administration of cytoreductive therapies such as dexamethasone alone and chemotherapy combined with dexamethasone can be a reasonable strategy for improving response rates for patients with high disease burden [96]. However, it is noted that no standard measurement has been established for disease burden.
Extramedullary disease (EMD) can be regarded as the surrogate for disease burden to an extent, which is one of the known manifestations of advanced hematological malignancies. A retrospective study suggested that a history of the EMD at baseline, as well as the occurrence of EMD during blinatumomab treatment, was associated with lower CR rates in patients with R/R B-ALL (P = 0.005 and P = 0.05, respectively) [93]. Bone marrow MRD constitutes another surrogate for disease burden. A post hoc analysis evaluated several early parameters for predicting response to blinatumomab in children with R/R B-ALL and identified that day 15 bone marrow MRD could predict complete MRD response to blinatumomab with high accuracy of up to 95% within the first two treatment cycles [97]. On day 15 of treatment, 59 patients were assessed for MRD, among 46 MRD positive patients, 44/46 patients had no complete MRD response with an accuracy of 96%, meanwhile, 12/13 patients achieved complete MRD response with an accuracy of 92% for 13 MRD negative patients [97].
Both BiTE therapy and CAR T cell therapy are dependent on targeting TAAs on the tumor cell surface, leading to T cell activation, proliferation, and cytokine production. Thus the density of TAAs should be one of the key factors for effective anti-tumor activity which is associated with treatment efficacy [98]. A previous study suggested that the CD20 density was no less than 200 molecules per target cell for inducing CAR T cell-mediated cytolysis while ∼10-fold higher antigen density was required for cytokine production for an anti-CD20 CAR T cell construct in vitro experiments [99]. A quantitative systems pharmacology (QSP) model was developed to assess some other factors affecting blinatumomab anti-tumor efficacy in NHL patients, besides tumor antigen density, factors significant for anti-tumor efficacy were blinatumomab density, rate of redirected T cell lysis, tumor cell growth rate, and E/T ratio [100, 101]. Potential biomarkers for predicting response included tumor-infiltrating lymphocytes (TILs), primarily T cells, and tumor growth rate [101]. However, it should be noted that the number of regulatory T cells (Tregs) was inversely correlated with the response rate to blinatumomab in patients with R/R B-ALL [102]. Blinatumomab responders (n = 22) had an average Tregs of 4.82% (CI: 1.79–8.34%) in the peripheral blood, whereas it was 10.25% (CI: 3.36–65.9%) for non-responders (n = 20). All of the blinatumomab responders were successfully identified by the Treg cutoff of 8.525%, while 70% of the non-responders were excluded by this threshold.
The analysis of the relationship between tumor genetic features and treatment response is a useful way to select potential biomarkers for predicting outcomes of patients and guiding treatment decisions in the clinic. A recent study characterized the genetic profile in 44 adults with R/R B-ALL treated with blinatumomab to find out potential biomarkers to blinatumomab therapy [94]. Sixteen Ph-like patients with CRLF2 rearrangement had a higher response rate of 75%, whereas the response rate was 55% in the overall cohort. More importantly, the partial deletion of exon 2 of CD19, CD19 ex2part, was identified as a potential biomarker that predicted response to blinatumomab. In contrast to the responders, CD19 ex2part levels were significantly higher in blinatumomab-unresponsive patients by analyzing samples from pre-treatment patients (P = 0.025). Additionally, samples from post-treatment patients with relapse presented higher CD19 ex2part levels compared with those from pre-treatment patients, which indicated that CD19 ex2part accumulated during treatment and likely led to treatment failure (P = 0.0002) [94]. The predictive role of CD19 ex2part as a biomarker should be confirmed by future research for CD19-targeted immunotherapies, as well as exploring other potential biomarkers relative to TAAs.
Toxicity
The safety profile of BiTE immunotherapy is of considerable concern to physicians and patients. As the only marketed member of BiTE family, the safety profile of blinatumomab is fully elucidated. In the phase 3 TOWER study, grade ≥ 3 AEs were ordered according to the frequency of occurrences, the top five were neutropenia, infection, elevated liver enzyme, neurotoxicity, and CRS [40]. Among them, the most concerning AEs are CRS and neurotoxicity which have proven dose-limiting toxicities (DLTs) and can even lead to death [103, 104]. CRS is an uncontrolled systemic inflammatory response with elevated levels of pro-inflammatory cytokines, primarily IL-6, which is triggered by T cell activation in T cell-engaging immunotherapies such as BiTE therapy and CAR T cell therapy [105]. CRS-related symptoms can range from mild flu-like symptomatology to severe and fatal multi-organ failure [105]. CRS often occurs in the first several days after BiTE infusion and the risk of grade ≥ 3 CRS in pivotal studies ranges from 0 to 6% for B-cell malignancies, in addition, a higher incidence of CRS is associated with higher tumor burden and drug doses [35, 38–40, 43, 46, 47]. The strategy of prophylactic use of dexamethasone combined with stepwise administration of blinatumomab is useful to decrease the risk of CRS [38]. After initiation of blinatumomab, interleukin (IL)-6 receptor inhibitor tocilizumab and corticosteroid are effective to treat severe CRS, while mild CRS can be addressed by symptomatic treatment [105].
Neurotoxicity is another unique treatment-related AEs for patients treated with T-cell engaging therapies, which is also termed as immune effector cell-associated neurotoxicity syndrome (ICANS). The incidence of grade ≥ 3 neurotoxicity ranges from 5.5 to 24% in these clinical studies related to blinatumomab [32, 35, 38–44, 47]. Neurotoxicity occurs mainly in treatment cycle 1 and the risk of neurotoxicity is increased when given higher doses of blinatumomab with the most common symptoms of dizziness, tremor, confusional state, and encephalopathy [104]. However, pathogenic mechanisms responsible for neurotoxicity induced by T cell-based anti-cancer therapies are complex and incompletely understood [106]. There was preclinical and clinical evidence that the adhesion of T cells to endothelial cells contributed to neurotoxicity induced by blinatumomab based on analyses of selected patients from 5 clinical trials and data from in vitro experiments [107]. Blinatumomab induced peripheral T-cell recruitment to the brain involving the following process: T cells adhered to cerebral microvascular endothelium, endothelial cells were activated with an increased level of Ang-2 (a marker of endothelial cell activation), T cells transmigrated across the blood-brain barrier into the brain. And then these T cells eliminated resident B cells with the release of cytokines, leading to an excessive immune response and ultimately resulting in neurotoxicity. The study suggested that agents inhibiting the adhesion between T cells and blood vessel endothelium could be an effective modality to reduce the incidence of neurotoxicity [107]. Interventions used routinely to treat neurotoxicity are interrupting treatment and dexamethasone [104].
To reduce minimize the risk of key systemic toxicity such as CRS, the safety, PK profile, and efficacy with subcutaneous (SC) administration of blinatumomab, a novel route of administration, is being evaluated in R/R B-ALL (NCT04521231) and R/R NHL (NCT02961881). In comparison to IV infusion, other potential benefits of SC injection include improved convenience and compliance and a reduction of cost for patients receiving treatment. Preliminary results regarding other bAbs that redirect T cells have been reported, suggesting SC injection did reduce the maximum concentration associated with CRS with high bioavailability, SC dosing is therefore a preferable route of administration for mitigating CRS and increasing the dose intensity [108, 109].
Resistance to BiTE therapy
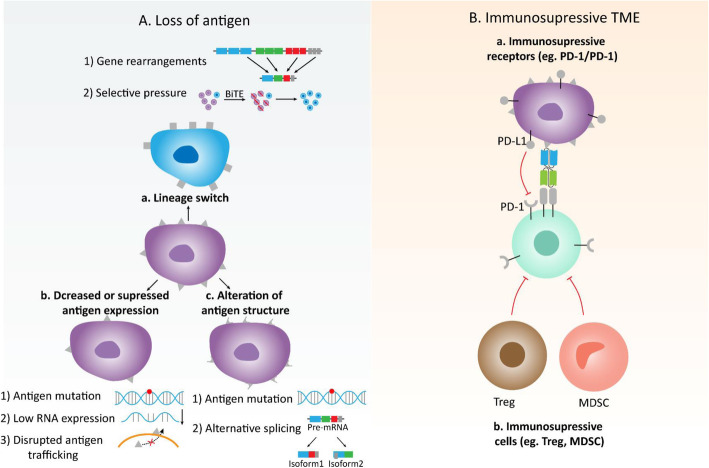
Despite the success of BiTE therapy with blinatumomab against B-cell malignancies expressing CD19, a significant portion of patients does not respond to treatment or they eventually relapse even with initial responses. The resistance to blinatumomab appears to involve multiple mechanisms, at present, studies have mainly focused on two aspects including immunosuppressive factors and loss of CD19 antigen (Fig. 2). Numerous studies have elucidated the key role of immune checkpoints in the suppression of anti-tumor immune responses, antibodies blocking the PD-1/PD-L1 axis have led to remarkable clinical efficacy for various malignancies [110]. Five years ago a case report described a patient with R/R B-ALL who experienced an increase in PD-1 and PD-L1 positivity after treatment with blinatumomab, in vitro experiments showed the ability of T cells inducing tumor cell lysis was weakened with a lower level of interferon γ (IFN-γ) in this patient [111]. Subsequent studies confirmed the finding that the expression of inhibitory immune checkpoints, mainly PD-L1, was increased after BiTE treatment in ex vivo cytotoxicity experiments and patients with different hematologic neoplasms, suggesting the addition of checkpoint inhibitors to BiTE therapy was a feasible strategy to improve BiTE-mediated cytotoxicity [100, 112]. Various solid tumor cells express immune checkpoint proteins on their surface that bind to inhibitory receptors on T cells, dampening the T-cell immune response and compromising the efficacy of T cell-based immunotherapies such as CAR T cell therapy and BiTE therapy [113]. Thus with the inhibition of immune checkpoints, the indications of BiTE therapy as well as CAR T cell therapy will expand including for both hematologic malignancies and solid tumors. The immunosuppressive cells in tumor microenvironment (TME) also present critical obstacles for effective immunotherapies, the most prominent representative is glioblastoma which is known to contain a dominance of immunosuppressive myeloid cells [114]. Tumors utilize Treg to suppress the tumor-specific immune response and promote their development, inhibition of blinatumomab efficacy by increasing levels of Treg measured by CD4/CD25/FOXP3 expression has been identified during blinatumomab treatment in patients with R/R B-ALL [102, 115]. Myeloid-derived suppressor cells (MDSCs), as another important component of immunosuppressive cells in TME, have received increasing attention in recent years that contribute to tumor development growth and progression [116]. A recent study identified neutrophils with the expression of CD11b/CD13/CD16 belonged to granulocytic MDSCs (G-MDSCs) in patients with MM, moreover, depleting these immunosuppressive cells could improve the cytotoxic activity of BCMA/CD3 bsABs [117]. Collectively, with the deep understanding of immunosuppressive factors in TME further related targeted therapies will combat resistance to current T-cell based immunotherapies.
Fig. 2.
Summary of currently identified mechanisms of tumor evasion to BiTE therapy. A. Loss of antigen---possible mechanisms: a. Lineage switch associated with 1) Gene rearrangement 2) Selective pressure due to bispecific T cell engager (BiTE) therapy b. Decreased or suppressed antigen expression associated with 1) Antigen mutation 2) Low RNA levels 3) Disrupted antigen trafficking process c. Alteration of antigen structure associated with 1) Antigen mutation 2) Alternative spicing B. Immunosuppressive tumor microenvironment (TME)---possible mechanisms: a. Immunosuppressive receptors such as PD-1/PD-L1 axis b. Immunosuppressive immune cells such as T regular (Treg) cells and MDSCs: Myeloid-derived suppressor cells; PD-1: Programmed cell death 1; PD- L1: Programmed cell death 1 ligand 1
Although B-ALL patients with initial CD19 positivity achieved CR after blinatumomab treatment, a considerable proportion of patients experienced relapse and CD19-negative relapse contributed to 8–50% of overall relapse [32, 33, 38, 42, 93, 118]. The targeted antigen loss was also observed in patients who were refractory to anti-CD19 CAR T cell treatment, suggesting the critical role of antigen loss in resistance to T-cell based cancer immunotherapies [119, 120]. Antigen loss can be interpreted as the loss of antigen expression and the loss of antigen-binding to targeted antibodies or cells, the presence of either situation or both can lead to the CD19-negative relapse. A study analyzed data from four B-ALL patients who had been treated with blinatumomab and experienced CD19-negative relapse and found that CD19 trafficking from the intracellular space to the membrane of B cells was prevented with the lack of CD81 that provided docking sites for CD19 signal transduction, resulting in absent CD19 expression [121]. CD19 mutations including frame-shift insertion or deletions, in-frame deletion, non-sense mutations, and splice-site single nucleotide variants (SNVs) were observed in five of seven R/R B-ALL patients who had CD19-negative relapse after blinatumomab treatment, suggesting CD19 mutations were common. Moreover, mechanisms of CD19 mutant allele-specific expression, low CD19 RNA expression, and mutations in CD81 which were in line with the previous study were also identified as partial causes of loss of CD19 expression [94]. Besides decreased or suppressed CD19 expression, notably, it was observed that usage of CD19 RNA isoform ex2part was increasing due to alternative splicing in both CD19-positive and CD19-negative relapses, which caused antigen escape by changing CD19 epitopes and ultimately disrupting the binding of blinatumomab to CD19 molecule rather than reducing CD19 expression [94]. One patient with CD19-negative relapse presented both antigen mutations and increased alternatively spliced RNA isoforms, suggesting they are likely to occur in parallel and responsible for antigen loss [94]. Alternative splicing and CD19 mutations were also demonstrated in other CD19-targeted immunotherapies such as CAR T cell therapy [122, 123]. Interestingly, the lineage switch of B lymphocytes from the lymphoid lineage to the myeloid lineage can be seen in patients with B-ALL who experienced CD19-directed immunotherapy in which CD19 expression was lost while myeloid marker levels such as CD33 were upregulated [124, 125]. The reasons for lineage switch may be associated with a strong selective advantage for subclones who did not express CD19 and gene rearrangements such as KMT2A/AFF1 and ZNF384 [126–128]. Therefore, multitargeted strategies may be effective to overcome antigen loss including developing a single drug that can simultaneously target dual or multiple TAAs or combining various immunotherapies that each of these targets a different TAA [129, 130].
Development of novel T-cell engager antibodies
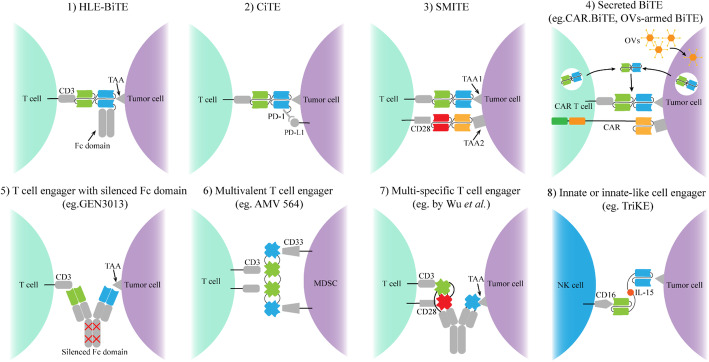
Despite the clinical success of blinatumomab against B-cell malignancies, many obstacles remain such as inconvenient routes of administration, treatment resistance, and limited efficacy in solid tumors. To overcome these limitations, considerable efforts have been dedicated to structural modifying canonical BiTE construct and developing multifunctional T-cell engaging antibodies, while some of them have entered the clinical stage (Fig. 3).
Fig. 3.
Schematic overview of the development of novel immune cell-based engager antibodies 1) Half-life extended-bispecific T cell engager (HLE-BiTE) 2) Checkpoint inhibitory T cell-engaging (CiTE) 3) Simultaneous multiple interaction T-cell engaging (SMiTE) 4) Secreted BiTE (eg. CAR.BiTE, OVs-armed BiTE) 5) T cell engager with silenced Fc domain 6) Multivalent T cell engager 7) Multi-specific T cell engager 8) Innate or innate-like cell engager CAR: chimeric antigen receptor OVs: Oncolytic viruses
HLE-BiTE
Canonical BiTE antibody is composed of only two scFvs without the Fc domain that possesses the ability to extend circulation time, thus this small molecule can be quickly cleared through the kidney with a short half-life, which requires continuous intravenous infusion to maintain therapeutic serum concentrations [131]. To overcome this limitation and also to further reduce treatment cost, HLE-BiTE has been designed by adding an Fc domain to a BiTE molecule and patients require only a single administration per week [132]. However, there is concern that prolonged serum drug concentration may be associated with increased toxicity compared with canonical BiTE that quickly gets cleared by stopping the infusion. Clinical trials for a variety of HLE-BiTE antibodies are currently underway in both hematological malignancies and solid tumors, they have been summarized by the previous review [30]. For example, there are published preliminary data from a phase 1 first-in-human study for HLE-BiTE AMG 673, which targets CD33 in patients with R/R AML (NCT03224819) [53]. Thirty patients were enrolled and 67% of them had been treated with more than 4 anti-AML treatments. The mode of administration was a short IV infusion once a week in a two-week cycle, and patients were equally distributed in 10 cohorts with doses ranging from 0.05–72 μg. The median treatment cycle was 1.5. The incidence of treatment-related grade ≥ 3 AEs was 50% (15/30) including 5 abnormal hepatic enzyme, 4 CRS, 4 leukopenia, 2 thrombocytopenia, and 2 febrile neutropenia. The incidence of grade 3 CRS was 13.3%. Of the 27 evaluable patients, 11 (41%) presented a decrease in bone marrow blasts with one patient achieving a CRi at the dose of 36 μg [53]. Updated results showed 2 DLTs were observed in the additional dose cohort (110 μg), thus 110 μg was identified as MTD for further dose escalation [133]. Subsequent results from ongoing clinical trials for AMG 673 as well as other HLE-BiTE antibodies are expected.
CiTE and SMiTE
It is established that the upregulation of immune checkpoints, such as PD-1/PD-L1, is one of the acquired resistance mechanisms to BiTE therapy [111]. The previous study found a significantly increased expression of PD-L1 when AMG 330 was added to AML cells in vitro, whereas immune blockade of the PD-1/PD-L1 interaction can greatly enhance the AMG 330-mediated cytotoxicity [100]. Thus, a novel bifunctional checkpoint inhibitory T cell-engaging (CiTE) antibody has been developed by fusing the PD-1 extracellular domain with low affinity to the canonical anti-CD33 BiTE construct [134]. Decreasing the affinity of the PD-1 extracellular domain to PD-L1 aims to restrict the development of immune-related AEs due to on-target off-leukemia toxicity, meanwhile, the affinity to CD33 tumor antigen is still high, which aims to maintain high specificity for CD33 + PD-L1+ double-positive cells other than all PD-L1+ cells. In vitro experiments, the CiTE antibody indeed showed a potent ability to activate T cells with an increased level of IFN -γ and cause cytotoxic lysis with high specificity for both CD33 + PD-L1 + cells and patient-derived AML cells [134]. CiTE can be a promising modified BiTE construct and is needed to be evaluated in more humanized models.
In general, activation of naive T cells requires at least two signals including TCR signal and costimulatory signal [135]. BiTE can generate TCR signal by binding to both CD3 and TAAs and form a powerful immunologic synapse between cytotoxic T cells and tumor cells, independent of the costimulatory signal [23]. However, a locally improved cytotoxic effect was observed in vitro and vivo studies when adding CD137, a potent costimulatory immunoreceptor, to BsAbs [136, 137]. Moreover, studies reported that activation of another costimulatory molecule CD28 enhanced the cytotoxicity of the BiTE antibody AMG 330 in primary human AML cells [138]. Thus, simultaneous multiple interaction T-cell engaging (SMITE) consisting of two separated canonical BiTE antibodies has been developed, both of the antibodies target TAAs on the one side while binding to either CD3 or CD28 on the other, providing additional costimulatory activation to enhance anti-tumor activity than either BiTE antibody alone [139]. In addition to targeting two different TAAs, one of BiTE antibodies can also target inhibitory immune checkpoints to overcome adaptive immune escape of tumor cells, mainly PD-L1, thus the SMITE pair was composed of CD3/TAA BiTE and CD28/PD-L1 BiTE [139].
Secreted BiTE
The current delivery route for BiTE and other T cell engaging antibodies is through intravenous administration passively, while standard human antibodies are actively secreted by plasma cells under normal physiological conditions. If these therapeutic antibodies with a potent anti-tumor function are also secreted by living cells in the human body, it may maintain a stable therapeutic concentration and exert durable anti-tumor effects without continuous infusion, furtherly, BiTE-secreting cells should specifically target the tumor regions or these secreted cells themselves are even tumor cells to provide local production of BiTE antibody without increased systemic toxicity. Currently, CAR T cells and oncolytic viruses (OVs) are selected to express BiTE antibody by gene modification, which integrates two complimentary cancer immunotherapies into one vehicle that is expected to synergistically improve anti-tumor activity, especially in solid tumors for which the efficacy of either therapy alone is insufficient [140, 141]. In patients with GBM, although CAR-T cells targeting EGFRvIII decreased the number of EGFRvIII-expressing tumor cells, subsequent analysis of surgical samples showed a high level of wild-type EGFR in the tumor site. Therefore, Choi et al. proposed the concept of CART.BiTE and developed a kind of CART.BiTE product that was the special CAR-T cells targeting EGFRvIII that secreted EGFR-targeted BiTE antibodies. These CART.BiTE cells had effective anti-tumor activity against GBM with heterogeneous and even negative EGFRvIII expression in mouse models, precluding immune escape due to antigen loss with no toxicity observed in mice transplanted with human skin [140].
In recent years, BiTE and OVs have been merged into a single platform by arming OVs with a therapeutic transgene encoding BiTE, which is termed as BiTE-armed OVs. These modified viruses preferentially infect and replicate in tumor cells, ultimately inducing oncolysis, meanwhile, tumor cells infected with BiTE-armed OVs can trigger T-cell activation and lead to bystander killing of non-infected tumor cells, which greatly enhances anti-tumor activity than unmodified OVs [141]. By the use of OVs as BiTE gene delivery vehicles, BiTE can be secreted locally at the tumor site due to the tumor specificity of OVs, which minimizes BiTE-mediated systemic toxicity. In addition to targeting tumor cells, BiTE-armed OVs can be also used to target cells that promote tumor progression in TME such as immunosuppressive stromal cells and M2-like tumor-associated macrophages (TAMs) [142, 143]. Moreover, in addition to a gene encoding BiTE antibodies, OVs can be also equipped with expression cassettes encoding cytokine, checkpoint inhibitors, and other molecules that have effective anti-tumor activity. Recently, OVs that were capable of producing BiTE antibodies targeting TAA of CD44 variant 6 (CD44v6), interleukin-12 (IL-12, an immunostimulatory cytokine), and anti-PD-L1 antibodies were developed, while these multifunctional OVs in combination with CAR T cells targeting TAA of HER2 enabled a substantial improvement of disease control in CD44v6 positive tumors with heterogeneity HER2 expression, overcoming multiple resistance mechanisms of tumor antigen loss and inhibitory effect of immune checkpoints which are major obstacles for single immunotherapy in the treatment of solid tumors [144]. Consequently, integrating BiTE therapy with other bioactive cancer immunotherapies possessing tumor-targeting ability including CAR T cell and OVs therapy into one platform that enables actively secret BiTE antibodies is a promising research direction, retaining their respective advantages and compensating for their limitations to maximize therapeutic benefits while reducing BiTE-induced systemic toxicity.
T cell engager with silenced Fc domain
The interaction between the Fc domain and its receptor on multiple immune cells can induce nonspecific immune activation such as ADCC, which leads to unwanted toxicity, however, the existence of the Fc domain enables antibodies to prolong the half-time. Thus, many antibodies with silent Fc domains have been developed by introducing point mutations that abrogate the binding of Fc receptors to Fc domains to reduce or avoid unnecessary effector functions while retaining the ability to extend the half-time [88, 145–151]. For example, GEN3013, a full-length human IgG1 bsAb recognizing CD3 and CD20, was generated by controlled Fab-arm exchange. Its Fc domain was silenced by the introduction of mutations L234F L235E D265A [151]. A phase 1/2 clinical trial is ongoing to evaluate the safety and efficacy of GEN3013 in patients with R/R B-cell lymphoma (NCT03625037). Moreover, recent studies have suggested that bsAbs with silenced Fc domains were able to improve the infiltration of T cells into solid tumors and enhance anti-tumor effects in the tumor xenograft mice, whereas bsAbs with intact Fc domains failed to induce T-cell trafficking to the tumor site because Fc-mediated effector functions enabled T cells to be sequestered in the reticuloendothelial system or depleted in circulation [152].
Multivalent and multi-specific T cell engager
BiTE is a bispecific and bivalent antibody, therefore, it should be possible to enhance the anti-tumor activity and mitigating antigen loss by using multivalent and/or multi-specific T cell engager antibodies that carry multiple antigen-binding sites to increase binding affinity and/or recognize more different TAAs and molecules involving T-cell activation. Numerous multivalent T cell engager molecules are under active development and some of them have yielded promising results in early clinical trials [56, 62]. For instance, AMV 564, a tetravalent bispecific molecule, has 2 binding sites for CD3 on T cells and 2 binding sites for CD33 on MDSCs with 2:2 antigen-binding valency. MDSCs play an important role in mediating immune suppression and promoting tumor growth, which can serve as a novel target for many malignant diseases, thus, AMV 564 can eliminate immunosuppressive MDSCs and drive T cell activation and proliferation to effectively induce anti-tumor immunity [153]. In phase 1 first-in-human trial (NCT03144245), AMV564 was well-tolerated and effective in patients with R/R AML [56]. Moreover, interim results of an ongoing phase 1 study (NCT04128423) have shown that AMV 564 had anti-tumor activity in multiple solid tumors with tolerable toxicity [84]. Besides increasing antibody valency, developing multi-specific T-cell engager antibodies that direct at multiple molecules associated with anti-tumor immunity is intended to provide greater efficacy and overcome treatment resistance with lower toxicity. A trispecific T-cell engager antibody has been developed that simultaneously binds to CD3, CD28, and CD38, both CD3 and CD28 engagement resulted in superior T cell activation, while the recognition of CD38 redirects T cells against MM and other hematologic malignancies with powerful killing efficiency [154]. Although clinical trials regarding this trispecific antibody have not been performed in humans, the integration of multiple specificities into one protein is a promising next-generation T cell engager immunotherapy and deserves further investigation.
Combination strategies
Chemotherapy
Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD) is a commonly used chemotherapy regimen for patients with newly diagnosed B-ALL, which can be served as a backbone for the development of new regimens [155]. A phase 2 study is evaluating the regimen of Hyper-CVAD in sequential combination with blinatumomab in adults with newly diagnosed B-ALL [156]. The regimen included 4 cycles of Hyper-CVAD followed by 4 cycles of blinatumomab. Twenty-seven patients were included, the median Hyper-CVAD treatment cycles were 3 while the median blinatumomab treatment cycles were 2, the rate of patients who underwent HSCT for high-risk features was 30%. The overall CR rate was 100% with an MRD-negativity response rate of 96%. With a median follow-up of 17 months, the 12-month RFS and OS rates were 76 and 89%, respectively. The incidence of blinatumomab-related grade ≥ 3 neurotoxicity was 17% while it was 4% for grade ≥ 3 CRS [156]. Two additional studies have also demonstrated the role of blinatumomab in combination with chemotherapy as front-line therapy in adults with newly diagnosed Ph- B-ALL with tolerable toxicity [157, 158]. In addition, blinatumomab monotherapy after current standard-of-care chemotherapy: a regimen of rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) have achieved an ORR of 89% in patients with newly diagnosed high-risk DLBCL [159].
To further improve treatment outcome in patients with R/R disease, triple therapy based on the sequential addition of blinatumomab to the combination of low-intensity chemotherapy (mini-hyper-CVD) and inotuzumab ozogamicin, an anti-CD22 antibody-drug conjugate (ADC), has presented superior clinical benefits as the first salvage therapy compared to conventional intensive chemotherapy and single-agent inotuzumab ozogamicin in patients with R/R ALL with reducing the dose of inotuzumab ozogamicin and minimizing the risk of liver veno-occlusive disease (VOD) [160]. With a median follow-up of 3 years, the ORR was 80% with an MRD response rate of 83%, three-year CR duration and OS rates were 32 and 33%, respectively [161]. Numerous clinical studies evaluating the safety and efficacy of blinatumomab in combination with chemotherapy in the treatment of patients with newly diagnosed or R/R B-ALL are ongoing (Table 5).
Table 5.
Ongoing clinical trials of blinatumomab in combination with other therapeutic approaches
| Clinical Trials.gov identifier | Phase | Patient/Disease | Combination regimen | Class of combination drugs | Estimated enrollment | Primary endpoint | Status |
|---|---|---|---|---|---|---|---|
| NCT04554485 | 2 | Adultswith newly diagnosed Ph-negative CD19+ B-ALL | Single cycle of blinatumomab followed by high-dose chemotherapy in the induction phase | Chemotherapy | 45 | CMR | Recruiting |
| NCT03518112 | 2 | Adults with R/R Ph-negative B-ALL | Concurrent blinatumomab and low-intensity chemotherapy | Chemotherapy | 44 | EFS | Recruiting |
| NCT03480438 | 2 | Older adults with newly diagnosed CD19+ Ph-negative B-ALL | Sequential dose reduced chemotherapy and blinatumomab | Chemotherapy | 50 | CHR and CMR | Recruiting |
| NCT02877303 | 2 | Children and adults with newly diagnosed B-ALL | Sequential chemotherapy and blinatumomab | Chemotherapy | 60 | RFS | Recruiting |
| NCT03523429 | 2 | Adults with high-risk CD19 + Ph-negative B-ALL | Blinatumomab added to consolidation phase with chemotherapy | Chemotherapy | 38 | MRD negative response | Recruiting |
| NCT03541083 | 2 | Adults with newly diagnosed CD19+ B-ALL | Blinatumomab added to prephase and consolidation phase with chemotherapy | Chemotherapy | 80 | MRD negative response | Recruiting |
| NCT03367299 | 2 | Adults with newly diagnosed or R/R CD19 + Ph-negative B-ALL | Sequential chemotherapy and blinatumomab | Chemotherapy | 149 | MRD negative response | Recruiting |
| NCT03914625 | 3 | Children and adultswith newly diagnosed B-ALL | Addition of 2 cycles of blinatumomab to standard chemotherapy | Chemotherapy | 6720 a | DFS | Recruiting |
| NCT02003222 | 3 | Adults with newly diagnosed Ph-negative B-ALL | Sequential chemotherapy and blinatumomab | Chemotherapy | 488 | OS | Active, not recruiting |
| NCT02744768 | 2 | Adults with newly diagnosed Ph + B-ALL | Sequential dasatinib and blinatumomab | TKI | 60 | MRD negative response | Recruiting |
| NCT02997761 | 2 | Adults with R/R Ph-negative B-ALL | Concurrent Ibrutinib and blinatumomab | TKI | 20 | CR | Recruiting |
| NCT04530565 | 3 | Adults with newly diagnosed Ph + B-ALL | Concurrent TKI and blinatumomab | TKI | 330 | OS | Recruiting |
| NCT04524455 | 1 | Adults with R/R B-ALL | Blinatumomab in combination with AMG 404 (anti-PD-1) | ICI | 21 | DLTs and toxicity | Recruiting |
| NCT03605589 | 1 | Children and adults with R/R CD19+ Ph-negative B-ALL | Blinatumomab in combination with pembrolizumab (anti-PD-1) | ICI | 24 | DLTs and toxicity | Suspended |
| NCT02879695 | 1 | Children and adults with poor-risk R/R CD19+ B-ALL | Blinatumomab in combination with nivolumab with or without ipilimumab (anti-CTLA-4) | ICI | 36 | Toxicity and MTD | Recruiting |
| NCT03340766 | 1 | Adults R/R DLBCL | Blinatumomab in combination with pembrolizumab | ICI | 70 | DLTs | Recruiting |
| NCT03160079 | 1/2 | Adultswith R/R CD19+ Ph-negative B-ALL with high marrow lymphoblasts (≥50%) | Blinatumomab in combination with pembrolizumab | ICI | 24 | ORR | Recruiting |
| NCT03512405 | 1/2 | Adultswith R/R CD19+ B-ALL | Blinatumomab in combination with pembrolizumab | ICI | 36 | Toxicity and ORR | Recruiting |
| NCT04546399 | 2 | Children and adults with first relapse CD19+ B-ALL | Blinatumomab in combination with nivolumab (anti-PD-1) | ICI | 550 | MRD negative second response and EFS | Recruiting |
| NCT02143414 | 2 | Older adults with newly diagnosed Ph-negative B-ALL (Cohort 1) and Ph + B-ALL (Cohort 2) | Blinatumomab followed by chemotherapy (Cohort 1) or dasatinib followed by blinatumomab (Cohort 2) | Chemotherapy or TKI | 58 | DLTs and OS | Recruiting |
| NCT03263572 | 2 | Adults with Ph + B-ALL | Concurrent blinatumomab, chemotherapy, and ponatinib | Chemotherapy and TKI | 60 | CMR, ORR, RFS, EFS, and OS | Recruiting |
| NCT03147612 | 2 | Adults with Ph + B-ALL | Sequential low-Intensity chemotherapy and ponatinib followed by blinatumomab and ponatinib | Chemotherapy and TKI | 60 | CMR and ORR | Recruiting |
| NCT02568553 | 1 | Adults with R/R CD19+ B-NHL | Concurrent lenalidomide and blinatumomab | Others | 44 | Toxicity | Recruiting |
| NCT03751709 | 1 | Adults (≥18 years) with R/R CD19+ B-ALL | Blinatumomab in combination with HLA-Mismatched Cellular Therapy | Others | 10 | DLTs | Recruiting |
| NCT03739814 | 2 | Adults with newly diagnosed or R/R CD22+ Ph-negative B-ALL | Inotuzumab ozogamicin followed by blinatumomab | Others | 64 | EFS | Recruiting |
| NCT03982992 | 2 | Adults with treatment-resistant MC or MRD of CD19+ B-ALL after HSCT | Blinatumomab in combination with donor lymphocyte infusion | Others | 12 | Toxicity | Recruiting |
Ph Philadelphia chromosome, CR Complete remission, CHR Complete hematologic remission, MRD Minimal residual disease, DLTs Dose-limiting toxicities, ORR Overall response rate, EFS Event-free survival, MTD Maximum tolerated dose, RFS Relapse-free survival, OS Overall survival, DFS Disease free survival, MC Mixed chimerism, DLBCL Diffuse large B-cell lymphoma, CMR Complete molecular response, HSCT Allogeneic hematopoietic stem cell transportation
Targeted therapy
Tyrosine kinase inhibitors (TKIs) targeting BCR-ABL1 are effective in the treatment of patients with Ph + leukemia, however, acquired resistance to TKI therapy remains a clinical challenge with limited therapeutic options. The retrospective study reported the encouraging efficacy of the combination strategy of blinatumomab and TKIs in patients with R/R Ph + B-ALL [162]. Among 16 patients with R/R disease, the CR rate was 88% with an MRD-negativity response rate of 86%. With a median follow-up of 15 months, the 12-month survival rate was 80% and the median OS following blinatumomab therapy was 45 months. Several other retrospective studies have also shown that blinatumomab combined with TKIs was safe and effective in patients with R/R Ph + disease [163, 164]. However, these studies are limited by retrospective design and small sample sizes, a prospective trial is investigating the efficacy of dasatinib, a second-generation TKI, in combination with blinatumomab as a front-line treatment in adults with Ph + ALL [165]. The regimen mainly consists of induction-phrase with dasatinib for 85 days and consolidation-phrase with blinatumomab. Among 63 patients treated with this regimen, the CR rate was 98%, the molecular response rate was 29.3% after completion of dasatinib-induction treatment and increased as the number of blinatumomab cycles increased (56.3% after 2 cycles of blinatumomab treatment, 65.7% after three cycles and 80% after 4 cycles). With a median follow-up of 10 months, the 12-month OS and disease-free survival (DFS) were 94.2 and 87.8%, respectively. Moreover, a recent case report has shown the combination of venetoclax, a selective inhibitor of the B-cell lymphoma 2 (Bcl-2), and blinatumomab is a highly effective and safe treatment against MRD relapse in patients with B-ALL [166]. Two patients achieved MRD-negativity responses soon after the complete blinatumomab treatment followed by venetoclax and underwent HSCT with no AEs. Several prospective trials to provide reliable evidence for this combination are ongoing (NCT02744768, NCT02997761, NCT04530565).
Immune checkpoint inhibitors
The upregulation of immune checkpoints is one of the major mechanisms involved in resistance to BiTE therapy. The combination therapy with ICIs can reactivate an exhausted immune response and improve anti-tumor activity, thus several related clinical studies have been conducted. A small phase 1 study is evaluating the safety and tolerability of blinatumomab combined with nivolumab targeting PD-1 and/or ipilimumab targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4) in patients with R/R B-ALL [167]. Eight patients were enrolled with the median BM blast percentage of 73%. Preliminary results have shown that the CR rate was 80% while all of them were MRD-negativity among 5 patients treated with blinatumomab and nivolumab. DLT was associated with a grade 4 infusion-related reaction. Another ongoing phase 1/2 study has also shown blinatumomab in combination with PD-1 inhibitor pembrolizumab is effective and safe in patients with R/R B-ALL and high disease burden at baseline [168]. The CR rate was 50% for four evaluable patients, while this study continues to evaluate efficacy by enrolling patients in the dose-expansion phase. Despite the impressive efficacy of ICI therapy against various solid tumors, the lack of tumor-reactive immune infiltration into tumors is one of the critical factors for the limited response rates. BiTE therapy can redirect large numbers of cytotoxic T cells to the tumor site to overcome this limitation and exert antigen-specific anti-tumor immunity. Furtherly, the therapeutic efficacy will be amplified by the addition of ICIs that facilitate a favorable immune profile and immune function of T cells by blocking immune checkpoint pathways.
Clinical trials concerning blinatumomab in combination with other therapeutic approaches involving immunomodulatory drug (lenalidomide), ADC (inotuzumab ozogamicin), and adoptive cellular therapy (donor lymphocyte infusion) are also in progress, which is summarized in Table 5.
Beyond CD3 T cells
According to the diversity of expressed TCR, T cells are mainly divided into αβ and γδ T cells. Current cancer immunotherapies such as ICI therapy focus mostly on the MHC-restricted αβ T cells, while γδ T cells also present potent anti-tumor activity independently from MHC restriction, and thus immunotherapy based on Vγ9Vδ2 T cells, the predominant subtypes of γδ T cells, enable favorable clinical outcomes in various cancers with low toxicity [169, 170]. Bispecific γδ T cell engager is being developed, the antibody targeting Vγ9Vδ2 on γδ T cells and EGFR on tumors cells was demonstrated to induce strong activation of Vγ9Vδ2 T cells and cytotoxic lysis of tumor cells in a mouse xenograft model [171]. Further studies are needed to evaluate whether the BiTE for the recruitment of γδ T cells is more effective than classical BiTE targeting CD3 that is expressed on both αβ and γδ T cells [172]. In addition to T cells, NK cells are also essential effector cells as a part of innate immunity that contribute to anti-tumor responses, therefore, considerable interest has recently focused on the potential of NK cells for cancer immunotherapies [173]. Bispecific killer cell engager (BiKE) that ligate CD16 on NK cells and a tumor antigen on tumor cells has been developed to activate NK cells and induce target cell lysis, to further improve activation, expansion, and survival of NK cells, a modified IL-15 crosslinker is infused into BiKE to create trispecific killer engager (TriKE) which has shown superior anti-tumor activity compared with BiKE [174, 175] (Fig. 3). A phase1/2 clinical trial of GTB-3550 (CD16/IL-15/CD33) TriKE for the treatment of CD33-expressing high-risk myelodysplastic syndromes, R/R ALL, or advanced systemic mastocytosis is ongoing (NCT03214666). A new generation of trifunctional NK-cell engager (NKCE) targeting two activating receptors NKp46 and CD16 on NK cells and a specific antigen on tumor cells has been reported to have more potent anti-tumor activity than clinical therapeutic antibodies in vitro and in vivo [176]. Moreover, natural killer T cells (NKT) cells, a kind of innate-like lymphocytes, are quickly gaining attention as carries for CAR therapy termed as CAR-NKT cells which are currently being investigated in phase 1 clinical trial for patients with R/R neuroblastoma, preliminary results have shown CAR-NKT cells were safe and effective and can be applied to treat more patients (NCT03294954) [177]. Macrophages, another key component of innate immunity, can also be utilized as targeted immune effector cells in innate cell-based immunotherapies, the CAR macrophages (CAR-Ms) construct has been developed and has displayed excellent efficacy, reducing tumor burden and extending survival time in two solid tumor xenograft mouse models, as only one injection is needed [178]. Consequently, cancer immunotherapies for reactivating innate immunity hold great promise through targeting innate cells or innate-like cells such as NK cells, macrophages, NKT cells, and γδ T cells, complement or even replace current therapies.
Conclusions
As the landscape of T cells and their role in anti-cancer immunity evolve, the development of T-cell-based immunotherapies has made a huge breakthrough in the last decade. In particular, the BiTE antibodies redirecting T cells to kill tumor cells have exhibited favorable clinical outcomes in R/R hematopoietic malignancies, as well as in solid tumors with preliminary evidence of clinical benefits. However, there remains a considerable number of patients that are resistant to the BiTE therapy and can not achieve durable responses. Current research has demonstrated that antigen loss and immunosuppressive factors, especially upregulation of inhibitory immune checkpoint molecules, are the two main mechanisms responsible for treatment failure. Therefore, strategies regarding improving BiTE constructs and developing novel T cell-engager antibodies with higher antigen avidities and multiple targets are currently being investigated in a series of preclinical and clinical studies, as well as combination therapies with BiTE antibodies and other therapeutic approaches. In addition to T cells, innate cell or innate-like cell engagers focusing on innate immunity are gaining increasing attention and have shown potent anti-tumor activity in various cancers, which reflects an orientation toward the future.
Acknowledgements
I would like to thank my supervisor, Dr. Meng, for her guidance through each stage of the process.
Authors’ contributions
Shujie Zhou: Contributed to Writing - Original Draft, Investigation, and Resources. Mingguo Liu: Contributed to Resources. Fei Ren: Contributed to Resources. Jinming Yu: Contributed to the Writing - Review & Editing and Funding acquisition. Xiangjiao Meng: Contributed to Study design, Supervision, and Writing - Review & Editing. All authors read and approved the final manuscript.
Funding
The study was funded by the National Natural Science Foundation of China (No.81972796 and 81972863), the National Key Research and Development Project (2018YFC1313200), the Innovation Project of Shandong Academy of Medical Sciences (2019–04), the Natural Science Foundation of Shandong (No.ZR2019MH010), and the Academic Promotion Program of Shandong First Medical University (2019ZL002).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 2.June CH, Connor RSO, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 3.Goebeler M, Bargou RC. T cell-engaging therapies — BiTEs and beyond. Nat Rev Clin Oncol. 2020;17:418–434. doi: 10.1038/s41571-020-0347-5. [DOI] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. New Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. New Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich M, Jasinski-Bergner S, Lazaridou M, Subbarayan K, Massa C, Tretbar S, et al. Tumor-induced escape mechanisms and their association with resistance to checkpoint inhibitor therapy. Cancer Immunol Immunother. 2019;68:1689–1700. doi: 10.1007/s00262-019-02373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018;49:1148–1161.e7. doi: 10.1016/j.immuni.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21:e168–e178. doi: 10.1016/S1470-2045(19)30823-X. [DOI] [PubMed] [Google Scholar]
- 11.Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39:433–444. doi: 10.1200/EDBK_238691. [DOI] [PubMed] [Google Scholar]
- 12.Suurs FV, Lub-de Hooge MN, de Vries EGE, de Groot DJA. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol Therapeut. 2019;201:103–119. doi: 10.1016/j.pharmthera.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 14.Thakur A, Huang M, Lum LG. Bispecific antibody based therapeutics: strengths and challenges. Blood Rev. 2018;32:339–347. doi: 10.1016/j.blre.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang TT, Ravetch JV. Functional diversification of IgGs through Fc glycosylation. J Clin Invest. 2019;129:3492–3498. doi: 10.1172/JCI130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. doi: 10.1182/blood.V95.6.2098. [DOI] [PubMed] [Google Scholar]
- 17.DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–105. doi: 10.1038/s41586-020-2229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinger M, Benjamin J, Kischel R, Stienen S, Zugmaier G. Harnessing T cells to fight cancer with BiTE® antibody constructs - past developments and future directions. Immunol Rev. 2016;270:193–208. doi: 10.1111/imr.12393. [DOI] [PubMed] [Google Scholar]
- 22.Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100:690–697. doi: 10.1002/ijc.10557. [DOI] [PubMed] [Google Scholar]
- 24.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 25.Wolf E, Hofmeister R, Kufer P, Schlereth B, Baeuerle PA. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today. 2005;10:1237–1244. doi: 10.1016/S1359-6446(05)03554-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1:36. doi: 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel P, Zhou L, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte delevopment, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 28.Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmacol Therapeut. 2012;136:334–342. doi: 10.1016/j.pharmthera.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v69–v82. doi: 10.1093/annonc/mdw025. [DOI] [PubMed] [Google Scholar]
- 30.Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, et al. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer-Am Cancer Soc. 2020;126:3192–3201. doi: 10.1002/cncr.32909. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Zhao J, Song Y, Luo X, Yang T. Clinical trial update on bispecific antibodies, antibody-drug conjugates, and antibody-containing regimens for acute lymphoblastic leukemia. J Hematol Oncol. 2019;12:15. doi: 10.1186/s13045-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 33.Topp MS, Gökbuget N, Zugmaier G, Degenhard E, Goebeler M, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120:5185–5187. doi: 10.1182/blood-2012-07-441030. [DOI] [PubMed] [Google Scholar]
- 34.Gökbuget N, Zugmaier G, Klinger M, Kufer P, Stelljes M, Viardot A, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica. 2017;102:e132–e135. doi: 10.3324/haematol.2016.153957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–1531. doi: 10.1182/blood-2017-08-798322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gökbuget N, Zugmaier G, Dombret H, Stein A, Bonifacio M, Graux C, et al. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma. 2020;61:2665–2673. doi: 10.1080/10428194.2020.1780583. [DOI] [PubMed] [Google Scholar]
- 37.Kiyoi H, Morris JD, Oh I, Maeda Y, Minami H, Miyamoto T, et al. Phase 1b/2 study of blinatumomab in Japanese adults with relapsed/refractory acute lymphoblastic leukemia. Cancer Sci. 2020;111:1314–1323. doi: 10.1111/cas.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topp MS, Gökbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell–engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 39.Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 40.Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. New Engl J Med. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Locatelli F, Zugmaier G, Mergen N, Bader P, Jeha S, Schlegel P, et al. Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: results of the RIALTO trial, an expanded access study. Blood Cancer J. 2020;10:1–5. doi: 10.1038/s41408-020-00342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34:4381–4389. doi: 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 43.Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Topp MS, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome–positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35:1795–1802. doi: 10.1200/JCO.2016.69.3531. [DOI] [PubMed] [Google Scholar]
- 44.Goebeler M, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34:1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 45.Dufner V, Sayehli CM, Chatterjee M, Hummel HD, Gelbrich G, Bargou RC, et al. Long-term outcome of patients with relapsed/refractory B-cell non-Hodgkin lymphoma treated with blinatumomab. Blood Adv. 2019;3:2491–2498. doi: 10.1182/bloodadvances.2019000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coyle L, Morley NJ, Rambaldi A, Mason KD, Verhoef G, Furness CL, et al. Open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2020;61:2103–2112. doi: 10.1080/10428194.2020.1759055. [DOI] [PubMed] [Google Scholar]
- 47.Viardot A, Goebeler M, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–1416. doi: 10.1182/blood-2015-06-651380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malik H, Buelow B, Rangaswamy U, Balasubramani A, Boudreau A, Dang K, et al. TNB-486, a novel fully human bispecific CD19 x CD3 antibody that kills CD19-positive tumor cells with minimal cytokine secretion. Blood. 2019;134:4070. doi: 10.1182/blood-2019-123226. [DOI] [Google Scholar]
- 49.Izhak L, Cullen DE, Elgawly M, Luistro L, Johnson S, Bald J, et al. Abstract 3636: potent antitumor activity of duvortuxizumab, a CD19 x CD3 DART® molecule, in lymphoma models. Cancer Res. 2017;77:3636. [Google Scholar]
- 50.Reusch U, Duell J, Ellwanger K, Herbrecht C, Knackmuss SH, Fucek I, et al. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells. Mabs-Austin. 2015;7:584–604. doi: 10.1080/19420862.2015.1029216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 52.Ravandi F, Walter RB, Subklewe M, Buecklein V, Jongen-Lavrencic M, Paschka P, et al. Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML) J Clin Oncol. 2020;38:7508. doi: 10.1200/JCO.2020.38.15_suppl.7508. [DOI] [Google Scholar]
- 53.Subklewe M, Stein A, Walter RB, Bhatia R, Wei AH, Ritchie D, et al. Preliminary results from a phase 1 first-in-human study of AMG 673, a novel half-life extended (HLE) anti-CD33/CD3 BiTE® (bispecific T-cell engager) in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML) Blood. 2019;134:833. doi: 10.1182/blood-2019-127977. [DOI] [Google Scholar]
- 54.Nair-Gupta P, Diem M, Reeves D, Wang W, Schulingkamp R, Sproesser K, et al. A novel C2 domain binding CD33xCD3 bispecific antibody with potent T-cell redirection activity against acute myeloid leukemia. Blood Adv. 2020;4:906–919. doi: 10.1182/bloodadvances.2019001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anti-CD33/CD3 bispecific antibody GEM 333. Available at: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/anti-cd33-cd3-bispecific-antibody-gem-333. Accessed 20 Dec 2020.
- 56.Westervelt P, Cortes JE, Altman JK, Long M, Oehler VG, Gojo I, et al. Phase 1 first-in-human trial of AMV564, a bivalent bispecific (2:2) CD33/CD3 T-cell engager, in patients with relapsed/refractory acute myeloid leukemia (AML) Blood. 2019;134:834. doi: 10.1182/blood-2019-129042. [DOI] [Google Scholar]
- 57.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. Anti-B-cell maturation antigen BiTE molecule AMG 420 induces responses in multiple myeloma. J Clin Oncol. 2020;38:775–783. doi: 10.1200/JCO.19.02657. [DOI] [PubMed] [Google Scholar]
- 59.Harrison SJ, Minnema MC, Lee HC, Spencer A, Kapoor P, Madduri D, et al. A phase 1 first in human (FIH) study of AMG 701, an anti-B-cell maturation antigen (BCMA) half-life extended (HLE) BiTE® (bispecific T-cell engager) molecule, in relapsed/refractory (RR) multiple myeloma (MM) Blood. 2020;136:28–29. doi: 10.1182/blood-2020-134063. [DOI] [Google Scholar]
- 60.Pillarisetti K, Powers G, Luistro L, Babich A, Baldwin E, Li Y, et al. Teclistamab is an active T cell–redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020;4:4538–4549. doi: 10.1182/bloodadvances.2020002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madduri D, Rosko A, Brayer J, Zonder J, Bensinger WI, Li J, et al. REGN5458, a BCMA x CD3 bispecific monoclonal antibody, induces deep and durable responses in patients with relapsed/refractory multiple myeloma (RRMM) Blood. 2020;136:41–42. doi: 10.1182/blood-2020-139192. [DOI] [Google Scholar]
- 62.Costa LJ, Wong SW, Bermúdez A, de la Rubia J, Mateos M, Ocio EM, et al. First clinical study of the B-cell maturation antigen (BCMA) 2+1 T cell engager (TCE) CC-93269 in patients (Pts) with relapsed/refractory multiple myeloma (RRMM): interim results of a phase 1 multicenter trial. Blood. 2019;134:143. doi: 10.1182/blood-2019-122895. [DOI] [Google Scholar]
- 63.Raje NS, Jakubowiak A, Gasparetto C, Cornell RF, Krupka HI, Navarro D, et al. Safety, clinical activity, pharmacokinetics, and pharmacodynamics from a phase I study of PF-06863135, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM) Blood. 2019;134:1869. doi: 10.1182/blood-2019-121805. [DOI] [Google Scholar]
- 64.Rodriguez C, D'Souza A, Shah N, Voorhees PM, Buelow B, Vij R, et al. Initial results of a phase I study of TNB-383B, a BCMA x CD3 bispecific T-cell redirecting antibody, in relapsed/refractory multiple myeloma. Blood. 2020;136:43–44. doi: 10.1182/blood-2020-139893. [DOI] [Google Scholar]
- 65.Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, et al. Subcutaneous epcoritamab induces complete responses with an encouraging safety profile across relapsed/refractory B-cell non-Hodgkin lymphoma subtypes, including patients with prior CAR-T therapy: updated dose escalation data. Blood. 2020;136:45–46. doi: 10.1182/blood-2020-133820. [DOI] [Google Scholar]
- 66.Bannerji R, Allan JN, Arnason JE, Brown JR, Advani R, Ansell SM, et al. Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy. Blood. 2020;136:42–43. doi: 10.1182/blood-2020-136659. [DOI] [Google Scholar]
- 67.Assouline SE, Kim WS, Sehn LH, Schuster SJ, Cheah CY, Nastoupil LJ, et al. Mosunetuzumab shows promising efficacy in patients with multiply relapsed follicular lymphoma: updated clinical experience from a phase I dose-escalation trial. Blood. 2020;136:42–44. doi: 10.1182/blood-2020-135839. [DOI] [Google Scholar]
- 68.Patel K, Michot J, Chanan-Khan AA, Salles GA, Cartron G, Peyrade F, et al. Preliminary safety and anti-tumor activity of XmAb13676, an anti-CD20 x anti-CD3 bispecific antibody, in patients with relapsed/refractory non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Blood. 2019;134:4079. doi: 10.1182/blood-2019-128564. [DOI] [Google Scholar]
- 69.Hutchings M, Carlo-Stella C, Bachy E, Offner FC, Morschhauser F, Crump M, et al. Glofitamab step-up dosing induces high response rates in patients with hard-to-treat refractory or relapsed non-Hodgkin lymphoma. Blood. 2020;136:46–48. doi: 10.1182/blood-2020-136044. [DOI] [Google Scholar]
- 70.IGM Biosciences Presents First Clinical Data from IGM-2323 in Non-Hodgkin’s Lymphoma at 2020 ASH Annual Meeting. Available at: https://igmbio.com/2020/12/05/igm-biosciences-presents-first-clinical-data-from-igm-2323-in-non-hodgkins-lymphoma-at-2020-ash-annual-meeting/. Accessed 15 Mar 2021.
- 71.Garfall AL, Usmani SZ, Mateos M, Nahi H, van de Donk NWCJ, San-Miguel JF, et al. Updated phase 1 results of teclistamab, a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in relapsed and/or refractory multiple myeloma (RRMM) Blood. 2020;136:27. doi: 10.1182/blood-2020-138831. [DOI] [Google Scholar]
- 72.Pfizer Reports Positive Clinical Data for BCMA-CD3 Bispecific Antibody (PF-06863135) in Multiple Myeloma. Available at: https://stockhouse.com/news/press-releases/2020/12/07/pfizer-reports-positive-clinical-data-for-bcma-cd3-bispecific-antibody-pf. Accessed 16 Mar 2021.
- 73.Cohen AD, Harrison SJ, Krishnan A, Fonseca R, Forsberg PA, Spencer A, et al. Initial clinical activity and safety of BFCR4350A, a FcRH5/CD3 T-cell-engaging bispecific antibody, in relapsed/refractory multiple myeloma. Blood. 2020;136:42–43. doi: 10.1182/blood-2020-136985. [DOI] [Google Scholar]
- 74.Chari A, Berdeja JG, Oriol A, van de Donk NWCJ, Rodriguez P, Askari E, et al. A phase 1, first-in-human study of talquetamab, a G protein-coupled receptor family C group 5 member D (GPRC5D) x CD3 bispecific antibody, in patients with relapsed and/or refractory multiple myeloma (RRMM) Blood. 2020;136:40–41. doi: 10.1182/blood-2020-133873. [DOI] [Google Scholar]
- 75.Aldoss I, Uy GL, Vey N, Emadi A, Sayre PH, Walter RB, et al. Flotetuzumab as salvage therapy for primary induction failure and early relapse acute myeloid leukemia. Blood. 2020;136:16–18. doi: 10.1182/blood-2020-134576. [DOI] [Google Scholar]
- 76.Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007;50:472–483. doi: 10.1111/j.1365-2559.2007.02635.x. [DOI] [PubMed] [Google Scholar]
- 77.Hummel H, Kufer P, Grüllich C, Deschler-Baier B, Chatterjee M, Goebeler M, et al. Phase I study of pasotuxizumab (AMG 212/BAY 2010112), a PSMA-targeting BiTE (bispecific T-cell engager) immune therapy for metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2020;38:124. doi: 10.1200/JCO.2020.38.6_suppl.124. [DOI] [Google Scholar]
- 78.Tran B, Horvath L, Dorff T, Rettig M, Lolkema MP, Machiels J, et al. Results from a phase I study of AMG 160, a half-life extended (HLE), PSMA-targeted, bispecific T-cell engager (BiTE®) immune therapy for metastatic castration-resistant prostate cancer (mCRPC) Ann Oncol. 2020;31(suppl_4):S507–S549. doi: 10.1016/j.annonc.2020.08.869. [DOI] [Google Scholar]
- 79.Bendell JC, Fong L, Stein MN, Beer TM, Ross A, Gao X, et al. First-in-human phase I study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager in patients with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2020;38:5552. doi: 10.1200/JCO.2020.38.15_suppl.5552. [DOI] [Google Scholar]
- 80.Rosenthal MA, Balana C, van Linde ME, Sayehli C, Fiedler WM, Wermke M, et al. ATIM-49 (LTBK-01). AMG 596, AMG 596, a novel anti-EGFRvIII bispecific T cell engager (BITE®) molecule for the treatment of glioblastoma (GBM): planned interim analysis in recurrent GBM (RGBM) Neuro-Oncology. 2019;21:vi283. doi: 10.1093/neuonc/noz219.1195. [DOI] [Google Scholar]
- 81.Amgen. Amgen Presents First Clinical Data for AMG 757 at SITC 2020. https://www.amgen.com/newsroom/press-releases/2020/11/amgen-presents-first-clinical-data-for-amg-757-at-sitc-2020. Accessed 12 Dec 2020.
- 82.Chao J, Buxó E, Cervantes A, Dayyani F, Lima CMSP, Greil R, et al. Trial in progress: a phase I study of AMG 199, a half-life extended bispecific T-cell engager (HLE BiTE) immune therapy, targeting MUC17 in patients with gastric and gastroesophageal junction (G/GEJ) cancer. J Clin Oncol. 2020;38:TPS4649. doi: 10.1200/JCO.2020.38.15_suppl.TPS4649. [DOI] [Google Scholar]
- 83.Bailis JM, Lutterbuese P, Thomas O, Locher K, Harrold J, Boyle M, et al. Abstract 3364: preclinical evaluation of BiTE®immune therapy targeting MUC17 or CLDN18.2 for gastric cancer. Cancer Res. 2020;80:3364. [Google Scholar]
- 84.Piha-Paul S, Starodub A, Karim R, Shafique M, Suarez GT, Ruegg C, et al. 372 single-agent anti-tumor activity in relapsed/refractory solid tumors: interim data from the phase 1 solid tumor trial of AMV564, a novel T-cell engager. J Immunother Cancer. 2020;8:A397. doi: 10.1136/jitc-2020-SITC2020.0372. [DOI] [Google Scholar]
- 85.Tabernero J, Melero I, Ros W, Argiles G, Marabelle A, Rodriguez-Ruiz ME, et al. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC) J Clin Oncol. 2017;35:3002. doi: 10.1200/JCO.2017.35.15_suppl.3002. [DOI] [Google Scholar]
- 86.Yu S, Zhang J, Yan Y, Yao X, Fang L, Xiong H, et al. A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells. J Exp Clin Cancer Res. 2019;38:355. doi: 10.1186/s13046-019-1354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song L, Xue J, Zhang J, Li S, Liu D, Zhou T. Mechanistic prediction of first-in-human dose for bispecific CD3/EpCAM T-cell engager antibody M701, using an integrated PK/PD modeling method. Eur J Pharm Sci. 2021;158:105584. doi: 10.1016/j.ejps.2020.105584. [DOI] [PubMed] [Google Scholar]
- 88.Ishiguro T, Sano Y, Komatsu S, Kamata-Sakurai M, Kaneko A, Kinoshita Y, et al. An anti–glypican 3/CD3 bispecific T cell–redirecting antibody for treatment of solid tumors. Sci Transl Med. 2017;9:eaal4291. doi: 10.1126/scitranslmed.aal4291. [DOI] [PubMed] [Google Scholar]
- 89.Moore PA, Shah K, Yang Y, Alderson R, Roberts P, Long V, et al. Development of MGD007, a gpA33 x CD3-bispecific DART protein for T-cell immunotherapy of metastatic colorectal cancer. Mol Cancer Ther. 2018;17:1761–1772. doi: 10.1158/1535-7163.MCT-17-1086. [DOI] [PubMed] [Google Scholar]
- 90.Hernandez-Hoyos G, Sewell T, Bader R, Bannink J, Chenault RA, Daugherty M, et al. MOR209/ES414, a novel bispecific antibody targeting PSMA for the treatment of metastatic castration-resistant prostate cancer. Mol Cancer Ther. 2016;15:2155–2165. doi: 10.1158/1535-7163.MCT-15-0242. [DOI] [PubMed] [Google Scholar]
- 91.Xu H, Cheng M, Guo H, Chen Y, Huse M, Cheung NV. Retargeting T cells to GD2 pentasaccharide on human tumors using bispecific humanized antibody. Cancer Immunol Res. 2015;3:266–277. doi: 10.1158/2326-6066.CIR-14-0230-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013;280:5350–5370. doi: 10.1111/febs.12393. [DOI] [PubMed] [Google Scholar]
- 93.Aldoss I, Song J, Stiller T, Nguyen T, Palmer J, O'Donnell M, et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017;92:858–865. doi: 10.1002/ajh.24783. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, Aldoss I, Qu C, Crawford JC, Gu Z, Allen EK, et al. Tumor-intrinsic and -extrinsic determinants of response to blinatumomab in adults with B-ALL. Blood. 2021;137:471–484. doi: 10.1182/blood.2020006287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. New Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.King AC, Bolanos R, Velasco K, Tu H, Zaman F, Geyer MB, et al. Real world chart review of blinatumomab to treat patients with high disease burden of relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Blood. 2019;134:5079. doi: 10.1182/blood-2019-122745. [DOI] [Google Scholar]
- 97.Brown P, Zugmaier G, Gore L, Tuglus CA, Stackelberg A. Day 15 bone marrow minimal residual disease predicts response to blinatumomab in relapsed/refractory paediatric B-ALL. Brit J Haematol. 2019;188:e36–e39. doi: 10.1111/bjh.16306. [DOI] [PubMed] [Google Scholar]
- 98.Walker AJ, Majzner RG, Zhang L, Wanhainen K, Long AH, Nguyen SM, et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol Ther. 2017;25:2189–2201. doi: 10.1016/j.ymthe.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watanabe K, Terakura S, Martens AC, van Meerten T, Uchiyama S, Imai M, et al. Target antigen density governs the efficacy of anti–CD20-CD28-CD3 ζ chimeric antigen receptor–modified effector CD8+ T cells. J Immunol. 2015;194:911–920. doi: 10.4049/jimmunol.1402346. [DOI] [PubMed] [Google Scholar]
- 100.Krupka C, Kufer P, Kischel R, Zugmaier G, Lichtenegger FS, Köhnke T, et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia. 2016;30:484–491. doi: 10.1038/leu.2015.214. [DOI] [PubMed] [Google Scholar]
- 101.Yuraszeck T, Bartlett D, Singh I, Reed M, Pagano S, Zhu M. A quantitative systems pharmacology (QSP) model to assess the action of blinatumomab in NHL patients (pts) J Clin Oncol. 2016;34:e14511. doi: 10.1200/JCO.2016.34.15_suppl.e14511. [DOI] [Google Scholar]
- 102.Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. 2017;31:2181–2190. doi: 10.1038/leu.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016:567–572. doi: 10.1182/asheducation-2016.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stein AS, Schiller G, Benjamin R, Jia C, Zhang A, Zhu M, et al. Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: management and mitigating factors. Ann Hematol. 2019;98:159–167. doi: 10.1007/s00277-018-3497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zheng P, Kros JM, Wang G. Elusive neurotoxicity in T cell-boosting anticancer therapies. Trends Immunol. 2019;40:274–278. doi: 10.1016/j.it.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 107.Klinger M, Zugmaier G, Nägele V, Goebeler M, Brandl C, Stelljes M, et al. Adhesion of T cells to endothelial cells facilitates blinatumomab-associated neurologic adverse events. Cancer Res. 2020;80:91–101. doi: 10.1158/0008-5472.CAN-19-1131. [DOI] [PubMed] [Google Scholar]
- 108.Matasar MJ, Cheah CY, Yoon DH, Assouline SE, Bartlett NL, Ku M, et al. Subcutaneous mosunetuzumab in relapsed or refractory B-cell lymphoma: promising safety and encouraging efficacy in dose escalation cohorts. Blood. 2020;136:45–46. doi: 10.1182/blood-2020-135818. [DOI] [Google Scholar]
- 109.Lesokhin AM, Levy MY, Dalovisio AP, Bahlis NJ, Solh M, Sebag M, et al. Preliminary safety, efficacy, pharmacokinetics, and pharmacodynamics of subcutaneously (SC) administered PF-06863135, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM) Blood. 2020;136:8–9. doi: 10.1182/blood-2020-133355. [DOI] [Google Scholar]
- 110.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Köhnke T, Krupka C, Tischer J, Knösel T, Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol. 2015;8:111. doi: 10.1186/s13045-015-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feucht J, Kayser S, Gorodezki D, Hamieh M, Döring M, Blaeschke F, et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7:76902–76919. doi: 10.18632/oncotarget.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20:1100–1109. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 115.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 116.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perez C, Botta C, Zabaleta A, Puig N, Cedena M, Goicoechea I, et al. Immunogenomic identification and characterization of granulocytic myeloid-derived suppressor cells in multiple myeloma. Blood. 2020;136:199–209. doi: 10.1182/blood.2019004537. [DOI] [PubMed] [Google Scholar]
- 118.Jabbour E, Düll J, Yilmaz M, Khoury JD, Ravandi F, Jain N, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018;93:371–374. doi: 10.1002/ajh.24987. [DOI] [PubMed] [Google Scholar]
- 119.Yu H, Sotillo E, Harrington C, Wertheim G, Paessler M, Maude SL, et al. Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am J Hematol. 2017;92:E11–E13. doi: 10.1002/ajh.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bukhari A, El Chaer F, Koka R, Singh Z, Hutnick E, Ruehle K, et al. Rapid relapse of large B-cell lymphoma after CD19 directed CAR-T-cell therapy due to CD-19 antigen loss. Am J Hematol. 2019;94:E273–E275. doi: 10.1002/ajh.25591. [DOI] [PubMed] [Google Scholar]
- 121.Braig F, Brandt A, Goebeler M, Tony H, Kurze A, Nollau P, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129:100–104. doi: 10.1182/blood-2016-05-718395. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Z, Chen X, Tian Y, Li F, Zhao X, Liu J, et al. Point mutation in CD19 facilitates immune escape of B cell lymphoma from CAR-T cell therapy. J Immunother Cancer. 2020;8:e001150. doi: 10.1136/jitc-2020-001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gardner R, Wu D, Cherian S, Fang M, Hanafi L, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rayes A, McMasters RL, O'Brien MM. Lineage switch in MLL-rearranged infant leukemia following CD19-directed therapy. Pediatr Blood Cancer. 2016;63:1113–1115. doi: 10.1002/pbc.25953. [DOI] [PubMed] [Google Scholar]
- 126.Zoghbi A, Winkler B, Zur Stadt U, Müller I, Escherich G. Lineage switch under blinatumomab of a relapsed common ALL co-expressing myeloid markers without MLL rearrangement. Blood. 2016;128:5196. doi: 10.1182/blood.V128.22.5196.5196. [DOI] [Google Scholar]
- 127.Oberley MJ, Gaynon PS, Bhojwani D, Pulsipher MA, Gardner RA, Hiemenz MC, et al. Myeloid lineage switch following chimeric antigen receptor T-cell therapy in a patient with TCF3-ZNF384 fusion-positive B-lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65:e27265. doi: 10.1002/pbc.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He RR, Nayer Z, Hogan M, Cuevo RS, Woodward K, Heyer D, et al. Immunotherapy- (blinatumomab-) related lineage switch of KMT2A/AFF1 rearranged B-lymphoblastic leukemia into acute myeloid leukemia/myeloid sarcoma and subsequently into B/myeloid mixed phenotype acute leukemia. Case Rep Hematol. 2019;2019:7394619. doi: 10.1155/2019/7394619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13:30. doi: 10.1186/s13045-020-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- 132.Lorenczewski G, Friedrich M, Kischel R, Dahlhoff C, Anlahr J, Balazs M, et al. Generation of a half-life extended anti-CD19 BiTE® antibody construct compatible with once-weekly dosing for treatment of CD19-positive malignancies. Blood. 2017;130:2815. [Google Scholar]
- 133.Marion S, Anthony S, Roland BW, Ravi B, Andrew HW, David R, et al. Updated results from a phase 1 first-in-human dose-escalation study of AMG 673, a novel anti-CD33/CD3 BiTE® (bispecific T-cell engager) molecule in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML) EHA Library. 2020;294466:EP548. [Google Scholar]
- 134.Herrmann M, Krupka C, Deiser K, Lindl B, Mocikat R, Metzeler KH, et al. Development of a bifunctional checkpoint inhibitory T cell engager (CiTE) to reverse adaptive immune escape in AML. Blood. 2018;132:4069. doi: 10.1182/blood-2018-99-119624. [DOI] [PubMed] [Google Scholar]
- 135.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 136.Liu R, Jiang W, Yang M, Guo H, Zhang Y, Wang J, et al. Efficient inhibition of human B-cell lymphoma in SCID mice by synergistic antitumor effect of human 4-1BB ligand/anti-CD20 fusion proteins and anti-CD3/anti-CD20 diabodies. J Immunother. 2010;33:500–509. doi: 10.1097/CJI.0b013e3181d75c20. [DOI] [PubMed] [Google Scholar]
- 137.Arndt C, Feldmann A, von Bonin M, Cartellieri M, Ewen E, Koristka S, et al. Costimulation improves the killing capability of T cells redirected to tumor cells expressing low levels of CD33: description of a novel modular targeting system. Leukemia. 2014;28:59–69. doi: 10.1038/leu.2013.243. [DOI] [PubMed] [Google Scholar]
- 138.Laszlo GS, Gudgeon CJ, Harrington KH, Walter RB. T-cell ligands modulate the cytolytic activity of the CD33/CD3 BiTE antibody construct, AMG 330. Blood Cancer J. 2015;5:e340. doi: 10.1038/bcj.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Correnti CE, Laszlo GS, de van der Schueren WJ, Godwin CD, Bandaranayake A, Busch MA, et al. Simultaneous multiple interaction T-cell engaging (SMITE) bispecific antibodies overcome bispecific T-cell engager (BiTE) resistance via CD28 co-stimulation. Leukemia. 2018;32:1239–1243. doi: 10.1038/s41375-018-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 141.Guo ZS, Lotze MT, Zhu Z, Storkus WJ, Song X. Bi- and tri-specific T cell engager-armed oncolytic viruses: next-generation cancer immunotherapy. Biomedicines. 2020;8:204. doi: 10.3390/biomedicines8070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scott EM, Jacobus EJ, Lyons B, Frost S, Freedman JD, Dyer A, et al. Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J Immunother Cancer. 2019;7:320. doi: 10.1186/s40425-019-0807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Freedman JD, Duffy MR, Lei-Rossmann J, Muntzer A, Scott EM, Hagel J, et al. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res. 2018;78:6852–6865. doi: 10.1158/0008-5472.CAN-18-1750. [DOI] [PubMed] [Google Scholar]
- 144.Porter CE, Rosewell Shaw A, Jung Y, Yip T, Castro PD, Sandulache VC, et al. Oncolytic adenovirus armed with BiTE, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol Ther. 2020;28:1251–1262. doi: 10.1016/j.ymthe.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Klupsch K, Baeriswyl V, Scholz R, Dannenberg J, Santimaria R, Senn D, et al. Abstract 1787: COVA4231, a potent CD3/CD33 bispecific FynomAb with IgG-like pharmacokinetics for the treatment of acute myeloid leukemia. Cancer Res. 2018;78:1787. doi: 10.1038/s41375-018-0249-z. [DOI] [PubMed] [Google Scholar]
- 146.Liu L, Lam CK, Long V, Widjaja L, Yang Y, Li H, et al. MGD011, A CD19 x CD3 dual-affinity retargeting bi-specific molecule incorporating extended circulating half-life for the treatment of B-cell malignancies. Clin Cancer Res. 2017;23:1506–1518. doi: 10.1158/1078-0432.CCR-16-0666. [DOI] [PubMed] [Google Scholar]
- 147.Mayes P, Tacken P, Wang S, Loo PV, Condamine T, Maaden HVD, et al. Abstract 539: a bispecific Fc-silenced IgG1 antibody (MCLA-145) requires PD-L1 binding to activate CD137. Cancer Res. 2019;79:539. [Google Scholar]
- 148.Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31:396–410. doi: 10.1016/j.ccell.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 149.Schlothauer T, Herter S, Koller CF, Grau-Richards S, Steinhart V, Spick C, et al. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng Des Sel. 2016;29:457–466. doi: 10.1093/protein/gzw040. [DOI] [PubMed] [Google Scholar]
- 150.Chornoguz O, Leettola CN, Leander K, Brosnan K, Emmell E, Chiu ML, et al. Characterization of a novel bispecific antibody that activates T cells in vitro and slows tumor growth in vivo. Monoclon Antib Immunodiagn Immunother. 2019;38:242–254. doi: 10.1089/mab.2019.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Engelberts PJ, Hiemstra IH, de Jong B, Schuurhuis DH, Meesters J, Beltran Hernandez I, et al. DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. Ebiomedicine. 2020;52:102625. doi: 10.1016/j.ebiom.2019.102625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang L, Hoseini SS, Xu H, Ponomarev V, Cheung N. Silencing fc domains in T cell–engaging bispecific antibodies improves T-cell trafficking and antitumor potency. Cancer Immunol Res. 2019;7:2013–2024. doi: 10.1158/2326-6066.CIR-19-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu L, Seung E, Xu L, Rao E, Lord DM, Wei RR, et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat Can. 2020;1:86–98. doi: 10.1038/s43018-019-0004-z. [DOI] [PubMed] [Google Scholar]
- 155.Seftel MD. Hyper-CVAD: a regimen for all seasons. Lancet Haematol. 2020;7:e501–e502. doi: 10.1016/S2352-3026(20)30179-4. [DOI] [PubMed] [Google Scholar]
- 156.Richard-Carpentier G, Kantarjian HM, Short NJ, Ravandi F, Ferrajoli A, Schroeder HM, et al. Updated results from the phase II study of hyper-CVAD in sequential combination with blinatumomab in newly diagnosed adults with B-cell acute lymphoblastic leukemia (B-ALL) Blood. 2019;134:3807. doi: 10.1182/blood-2019-129657. [DOI] [Google Scholar]
- 157.Fleming S, Venn N, Reynolds J, Nguyen U, Kwan J, Moore J, et al. Preliminary minimal residual disease analysis of the Australasian Leukaemia & Lymphoma Group (ALLG) ALL8 study of front-line blinatumomab with chemotherapy in adults with Ph negative B-cell acute lymphoblastic leukaemia. Blood. 2019;134:1300. doi: 10.1182/blood-2019-132048. [DOI] [Google Scholar]
- 158.Short NJ, Kantarjian HM, Ravandi F, Huang X, Ferrajoli A, Kadia TM, et al. Hyper-CVAD and sequential blinatumomab in adults with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia: results from a phase II study. Blood. 2020;136:9–11. doi: 10.1182/blood-2020-138565. [DOI] [Google Scholar]
- 159.Katz DA, Chu MP, David KA, Thieblemont C, Morley NJ, Khan SS, et al. Open-label, phase 2 study of blinatumomab after first-line rituximab-chemotherapy in adults with newly diagnosed, high-risk diffuse large B-cell lymphoma. Blood. 2019;134:4077. doi: 10.1182/blood-2019-121708. [DOI] [PubMed] [Google Scholar]
- 160.Sasaki K, Kantarjian HM, Ravandi F, Short NJ, Kebriaei P, Huang X, et al. Sequential combination of inotuzumab ozogamicin (InO) with low-intensity chemotherapy (mini-hyper-CVD) with or without blinatumomab is highly effective in patients (pts) with Philadelphia chromosome-negative acute lymphoblastic leukemia (ALL) in first relapse. Blood. 2019;134:3806. doi: 10.1182/blood-2019-129018. [DOI] [Google Scholar]
- 161.Sasaki K, Kantarjian HM, Ravandi F, Short NJ, Kebriaei P, Huang X, et al. Long-term follow-up of the combination of low-intensity chemotherapy plus inotuzumab ozogamicin with or without blinatumomab in patients with relapsed-refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: a phase 2 trial. Blood. 2020;136:40–42. [Google Scholar]
- 162.McCloskey JK, Gagnon J, McCabe T, Charlon J, Wang S, Fan R, et al. Blinatumomab in combination with tyrosine kinase inhibitors safely and effectively induces rapid, deep, and durable molecular responses in relapsed and refractory Philadelphia positive acute leukemias. Blood. 2019;134:3812. doi: 10.1182/blood-2019-131838. [DOI] [Google Scholar]
- 163.Assi R, Kantarjian H, Short NJ, Daver N, Takahashi K, Garcia-Manero G, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17:897–901. doi: 10.1016/j.clml.2017.08.101. [DOI] [PubMed] [Google Scholar]
- 164.Couturier M, Thomas X, Huguet F, Berthon C, Simand C, Hicheri Y, et al. Blinatumomab + ponatinib for relapsed Ph1-positive acute lymphoblastic leukemia: the French experience. Blood. 2018;132:4014. doi: 10.1182/blood-2018-99-111546. [DOI] [Google Scholar]
- 165.Chiaretti S, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo C, et al. Dasatinib-blinatumomab combination for the front-line treatment of adult Ph+ ALL patients. Updated results of the gimema LAL2116 D-Alba trial. Blood. 2019;134:740. doi: 10.1182/blood-2019-128759. [DOI] [Google Scholar]
- 166.Campos-Cabrera G, Vega-Tapia N, Campos-Cabrera V, Campos-Cabrera S, Campos-Villagomez J, Mendez-Garcia E. Blinatumomab and venetoclax for minimal residual disease relapse in acute lymphoblastic leukemia. Blood. 2019;134:5128. doi: 10.1182/blood-2019-127296. [DOI] [Google Scholar]
- 167.Webster J, Luskin MR, Prince GT, DeZern AE, DeAngelo DJ, Levis MJ, et al. Blinatumomab in combination with immune checkpoint inhibitors of PD-1 and CTLA-4 in adult patients with relapsed/refractory (R/R) CD19 positive B-cell acute lymphoblastic leukemia (ALL): preliminary results of a phase I study. Blood. 2018;132:557. doi: 10.1182/blood-2018-99-111845. [DOI] [Google Scholar]
- 168.Schwartz M, Damon LE, Jeyakumar D, Costello CL, Tzachanis D, Schiller GJ, et al. Blinatumomab in combination with pembrolizumab is safe for adults with relapsed or refractory B-lineage acute lymphoblastic leukemia: University of California Hematologic Malignancies Consortium Study 1504. Blood. 2019;134:3880. doi: 10.1182/blood-2019-131061. [DOI] [Google Scholar]
- 169.Buccheri S, Guggino G, Caccamo N, Li DP, Dieli F. Efficacy and safety of gammadeltaT cell-based tumor immunotherapy: a meta-analysis. J Biol Regul Homeost Agents. 2014;28:81–90. [PubMed] [Google Scholar]
- 170.Sebestyen Z, Prinz I, Dechanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discov. 2020;19:169–184. doi: 10.1038/s41573-019-0038-z. [DOI] [PubMed] [Google Scholar]
- 171.de Bruin RCG, Veluchamy JP, Lougheed SM, Schneiders FL, Lopez-Lastra S, Lameris R, et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology. 2017;7:e1375641. doi: 10.1080/2162402X.2017.1375641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kuhns MS, Badgandi HB. Piecing together the family portrait of TCR-CD3 complexes. Immunol Rev. 2012;250:120–143. doi: 10.1111/imr.12000. [DOI] [PubMed] [Google Scholar]
- 173.Bachiller M, Battram AM, Perez-Amill L, Martín-Antonio B. Natural killer cells in immunotherapy: are we nearly there? Cancers. 2020;12:3139. doi: 10.3390/cancers12113139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schmohl JU, Felices M, Taras E, Miller JS, Vallera DA. Enhanced ADCC and NK cell activation of an anticarcinoma bispecific antibody by genetic insertion of a modified IL-15 cross-linker. Mol Ther. 2016;24:1312–1322. doi: 10.1038/mt.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res. 2016;22:3440–3450. doi: 10.1158/1078-0432.CCR-15-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gauthier L, Morel A, Anceriz N, Rossi B, Blanchard-Alvarez A, Grondin G, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019;177:1701–1713.e16. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 177.Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. 2020;26:1686–1690. doi: 10.1038/s41591-020-1074-2. [DOI] [PubMed] [Google Scholar]
- 178.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.