Abstract
We report the synthesis and first characterisation of the novel chemical probe 3-bromotetrazine and establish its reactivity towards nucleophiles. This led to the synthesis of several novel classes of 3-monosubstituted s-tetrazines. A remarkable functional group selectivity is observed and is utilised to site-selectively functionalise different complex molecules. The stability of 3-bromotetrazine under the reaction conditions facilitated the development of a protocol for protein functionalisation, which enabled a “minimal”, bifunctional tetrazine unit as a bio-orthogonal handle for inverse electron demand Diels–Alder reactions. Additionally, a novel tetrazine-based chemical probe was developed and its application in the context of thiol-targeted natural product isolation and labelling of mammalian cells is demonstrated.
3-Bromotetrazine selectively labels small and macromolecules up to proteins and can then be used as a fluorophore or as a bio-orthogonal handle for downstream functionalisation.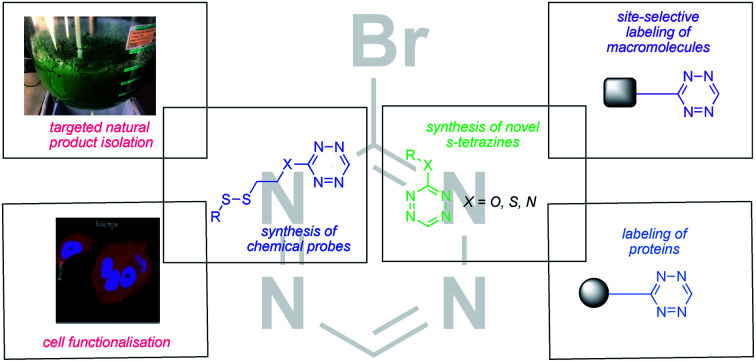
Introduction
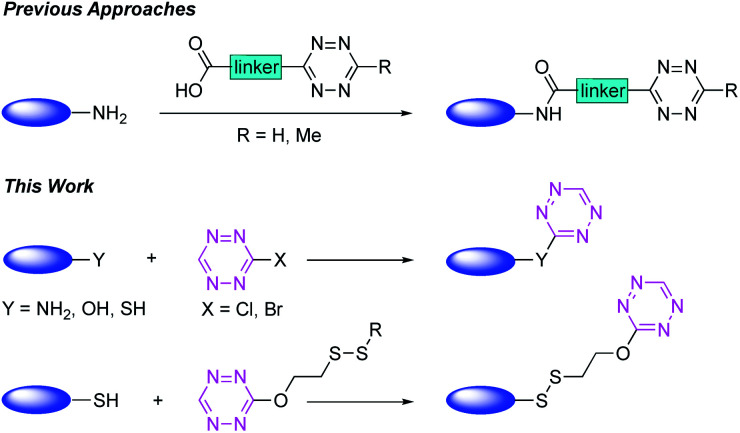
The nitrogen-rich heterocycle s-tetrazine was first described by Pinner at the end of the 19th century1 and since then has found applications in various fields, including organic electronics (e.g. OPVs and OFETs),2 energetic materials,3 total synthesis4 and coordination chemistry.5 More recently, s-tetrazines emerged as a powerful tool in bio-orthogonal chemistry, due to specific reactivity with alkynes and alkenes in the inverse-electron demand Diels–Alder reaction (iEDDA).6–8 However, classical methods for the synthesis of tetrazine-conjugates mainly rely on amide bond formation between a carboxylic acid derived tetrazine precursor and an amine.9 A major drawback of this method is the presence of a hydrophobic linker, which has a negative effect on the physiochemical properties of the moiety attached to the tetrazine (Scheme 1).10,11 In this context, the target engagement, metabolism and pharmacological profile of the payloads linked to the tetrazine can be altered drastically.10,12 Whereas there are several reports of the introduction of tetrazine moieties into proteins in response to an amber codon, there are few general methods available for the direct, chemical synthesis of small-/macromolecules functionalised with a minimal tetrazine.13,14
Scheme 1. Labelling of small-/macromolecules with tetrazines.
Recent advances towards simple tetrazine unit incorporation into proteins involve site-selective dual-labelling of cysteine residues with 3,6-disubstituted s-tetrazines.8 Nevertheless, the subsequent reaction with a strained alkyne proved to be demanding, as heating and prolonged reaction times were necessary, which demonstrated the generally slower kinetics when 3,6-disubstituted s-tetrazines are employed, compared with 3-s-tetrazines.6,8,15 A complementary method to the chemical introduction of the tetrazine moiety into proteins was recently demonstrated by the group of Lang and co-workers, who genetically encoded lysine-based Me-tetrazine amino acids.14 While this approach guarantees high degrees of labelling and high selectivity, in general, the heterologous expression of functionalised proteins and purification thereof can pose a challenge.11,14,16 Based on these observations, the development of novel, small tetrazine chemical probes for the fast and easy labelling of macromolecules, peptides and proteins in a selective fashion is of great interest. In this context, we envisioned the use of 3-halogenated s-tetrazines, since they display the unique profile of structurally simplified, bifunctional, fluorescent probes. Literature reports on these compounds are, however, limited. Whereas 3-chlorotetrazine (1) was synthesised previously by Spesivtseva and co-workers, the heavier analogues have so far not been characterised in the literature.17,18
In this article, we present a fast, robust, and operationally simple protocol for the synthesis of the previously uncharacterised 3-bromotetrazine (Br-Tet) (2), which avoids the use of elemental halogen.17 After investigation of the reactivity profile of Br-Tet (2) with different nucleophiles (NH2, OH, SH), we applied our findings to the site-selective labelling of natural products, peptides and proteins. Furthermore, Br-Tet (2) served as a building block for the synthesis of novel chemical probes, which were applied in the context of whole cell labelling and targeting of thiol-containing natural products.
Results and discussion
Synthesis of Br-Tet
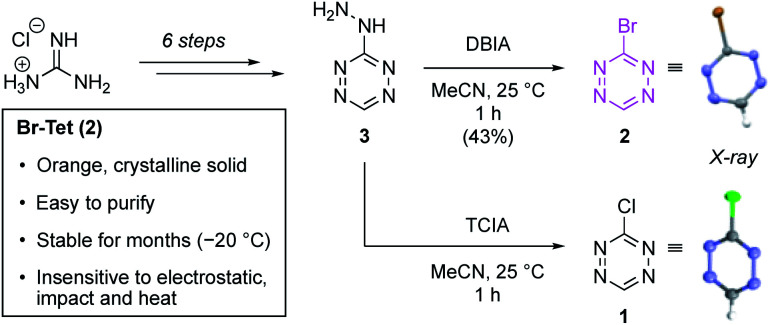
Our synthetic efforts towards Br-Tet (2) started with an operationally simple six step procedure, which delivers the key intermediate 3-hydrazinotetrazine (3) in an overall yield of 13% (Scheme 2).19 This route is easily scalable and all intermediates were found to be insensitive towards impact, friction and electrostatics and are thus safe to handle (see ESI for details†). With compound 3 in hand, we investigated the halogenation of the tetrazine core without using elemental halogen. Treatment of 3-hydrazinotetrazine (3) with dibromoisocyanuric acid (DBIA) delivered the desired Br-Tet (2) in a reproducible yield of 43%, and the product was easily purified via column chromatography to give an orange, crystalline solid. The structure was unambiguously assigned by an X-ray crystal structure analysis. Compared to previously known 3-chlorotetrazine (1), prepared from 3 using trichloroisocyanuric acid (TCIA), Br-Tet (2) displays significantly improved properties in terms of lower volatility and can be stored over months at −20 °C. Additionally, 2 was found to be insensitive towards impact, friction and electrostatics and thus is very safe to handle. Based on the superior properties, we chose Br-Tet (2) as a precursor for subsequent experiments.
Scheme 2. Synthesis of Br-Tet (2). Synthesis of 3-chlorotetrazine (1) and novel 3-bromotetrazine (2) from guanidine hydrochloride. DBIA = dibromoisocyanuric acid; TCIA = trichloroisocyanuric acid.
Nucleophilic aromatic substitution
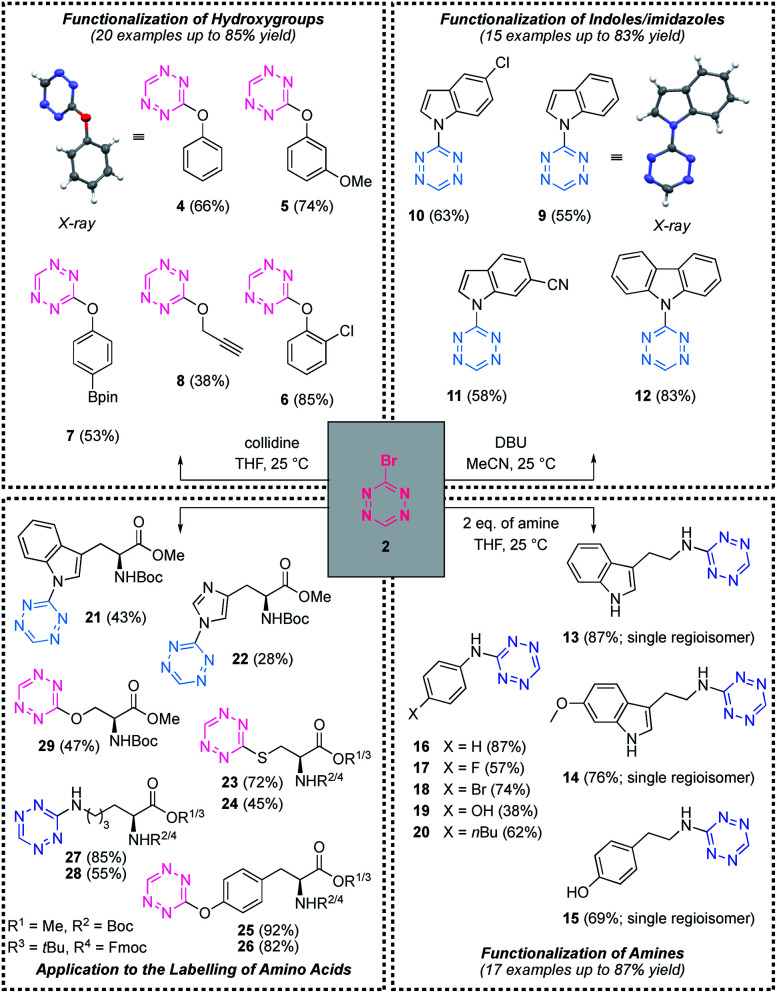
With the title compound in hand, we investigated its reactivity towards nucleophilic aromatic substitution with small molecules (Scheme 3).19 Initial experiments quickly revealed that different functional groups require slightly different conditions in order to react with Br-Tet (2). In this context, we synthesised several novel classes of 3-monosubstituted s-tetrazines (Scheme 3). To functionalise hydroxy groups, the mild base collidine was required and phenols as well as primary alcohols engaged in the reaction, which afforded compounds 4–8 in moderate to good yield (for further examples see ESI†). Note that the reaction with alkyl-hydroxy groups required prolonged reaction times and the yields tended to be lower than with phenols (Scheme 3). In order to functionalise indoles, the stronger base DBU was necessary; delivering N-substituted indoles with up to 83% yield. The site of functionalisation was verified by X-ray analysis of compound 9, which confirmed that the reaction took place at the ring-nitrogen of the indole. Chloride as well as nitrile containing substrates proved compatible with the presented method and delivered compounds 10 and 11, respectively (Scheme 3). To functionalise amines, 2 equivalents of the respective amine were sufficient as nucleophile and base in order to promote the reaction. Interestingly, when bifunctional nucleophiles like tyramine or tryptamine were examined, high regioselectivity was observed and compounds 13, 14 and 15 were obtained in excellent yield. Analogously, 4-aminophenol reacted to give compound 19 with high selectivity for amine-functionalisation and moderate yield.
Scheme 3. Investigation of the nucleophilic aromatic substitution with heteroatoms. Standard procedures and selected substrate scope for the functionalisation of hydroxy groups, indoles/imidazoles, amines and amino acids.
These standard conditions for each functional group were then applied to the functionalisation of amino acids. We could demonstrate that the previously established conditions transferred well to amino acids and several tetrazine-labelled amino acids were isolated in moderate to good yield (compounds 21–29).
Synthesis of amino acids for solid phase peptide synthesis
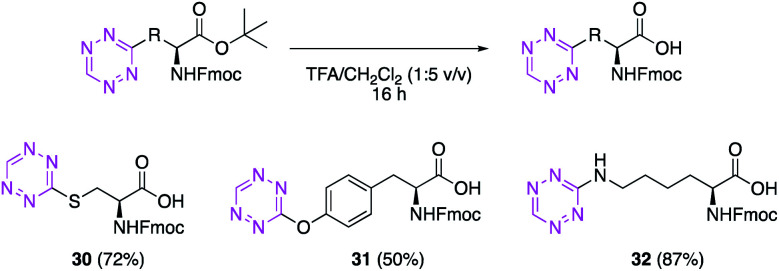
For the amino acids 24, 26 and 28 to have a practical application in the context of solid phase peptide synthesis (SPPS), we investigated the deprotection of the tert-butyl ester. Treatment of the labelled amino acids with TFA in dichloromethane delivered the required Fmoc-protected amino acids 30, 31 and 32 in moderate to good yield (Scheme 4).
Scheme 4. Amino acids for SPPS. Fmoc-Cys(Tet)-OH (30), Fmoc-Tyr(Tet)-OH (31) and Fmoc-Lys(Tet)-OH (32) for application to SPPS.
Application of Br-Tet as a chemical probe
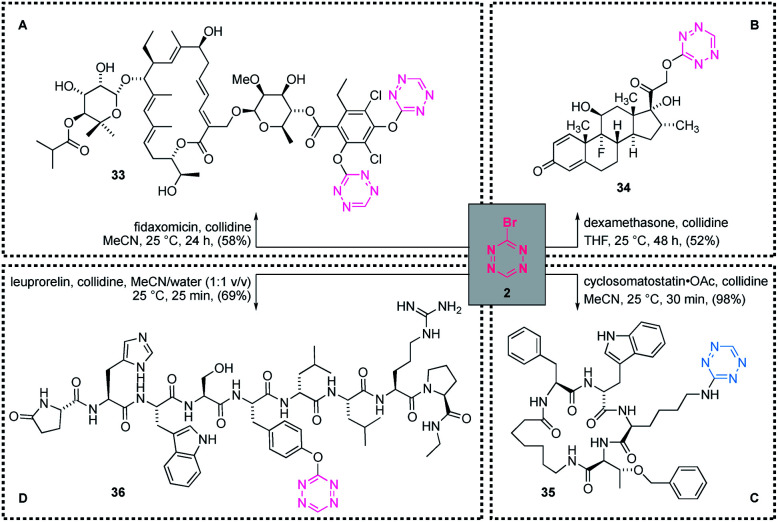
With the optimised conditions for functionalisation of different heteroatoms in hand and based on the intriguing functional group selectivity observed in the functionalisation of compounds 13–15 and 19, the envisioned application of Br-Tet (2) as a chemical probe in a more complex setting was explored. Due to the trends in reactivity of Br-Tet (2) with heteroatoms, (S > N > O (aromatic) ≫ O (alkyl)), high selectivity in combination with high predictability of the site of functionalisation was postulated and tested on a variety of different classes of compounds (Scheme 5).
Scheme 5. Site-selective labelling of macromolecules. (A) Synthesis of di-tet-fidaxomicin (33); (B) tet-dexamethasone (34); (C) lys(tet)-cyclosomatostatin (35); (D) tyr(tet)-leuprorelin (36).
The functional group selectivity predicted above could easily be validated on several complex molecules. Firstly, the labelling of the antibiotic fidaxomicin was tested. Although, the reaction proved to be sluggish under the standard conditions (collidine, THF), a switch of solvent to acetonitrile afforded the difunctionalised fidaxomicin 33 in 58% yield. The reaction proved to be highly selective and only functionalisation at the phenolic sites could be observed; NMR studies verified that all other hydroxy groups remained untouched.
However, given the high abundance of hydroxy groups in natural products, a demonstration of the successful labelling of hydroxy groups in complex molecules would add value to the presented method. Thus, we investigated the labelling of the steroid derived compound dexamethasone. After a prolonged reaction time (48 h), this compound could be functionalised selectively at the primary hydroxy group with our tetrazine to afford 34 in 52% yield, as verified by 1H-NMR spectroscopy. In consideration of the site of functionalisation in 33 and 34, it is most likely that the steric hindrance of secondary hydroxy groups diminishes their nucleophilicity and renders them unreactive towards Br-Tet (2). Thus, in general, phenolic sites are functionalised prior to primary hydroxy groups, whereas secondary hydroxy groups remain untouched under the reported conditions.
Next, we challenged this method in the context of site-selective functionalisation of amino acids in small peptides, with the ultimate goal of installing a tetrazine unit in proteins. Preliminary competition experiments using two different Boc-protected amino acid methyl esters (see ESI for details†) underlined the intriguing functional group selectivity and showed the following reactivity trend: Cys > Lys > Tyr ≫ Ser. To increase the potential of our method, we investigated its compatibility with an aqueous solution as solvent. Thus, the reaction of Boc-Cys-OMe and Boc-Lys-OMe with Br-Tet (2) in biologically relevant solvents (acetonitrile/water mixtures up to aqueous buffers) was demonstrated. Pleasingly, the yields were comparable with those obtained in pure organic solvents (see ESI for details†) and an unexpected compatibility of Br-Tet (2) with aqueous systems was observed, thereby indicating the potential of applying Br-Tet (2) in the labelling of proteins. Final competition experiments in aqueous buffered systems revealed that, in ammonium acetate buffer (0.11 M, pH = 4.15), Boc-Cys-OMe could be functionalised selectively, even in the presence of Boc-Lys-OMe.
Having established the reactivity profile of the relevant amino acids, we selected two simple peptides with which to probe this concept in a more complex environment. In this context, cyclosomatostatin was selectively functionalised in pure acetonitrile at the lysine residue (confirmed by MS/MS) rather than at the tryptophan residue, with quantitative recovery of the functionalised peptide 35 after purification (Scheme 5). Similar results were obtained with the more complex linear peptide leuprorelin in aqueous solution. The UHPLC analysis of the reaction mixture indicated that the single product 36 was formed during the reaction, additionally verifying our predicted reactivity scale. After isolation and purification of the obtained material by preparative HPLC, the structure was confirmed by MS/MS analysis, which revealed sole functionalisation at the phenolic moiety of leuprorelin, with 69% of functionalised peptide 36 recovered.
Overall, these results clearly demonstrate the potential of this method in the site-selective labelling of multifunctional small molecules and peptides and verify the high predictability of the functionalisation site, even in the presence of multiple nucleophilic functional groups.
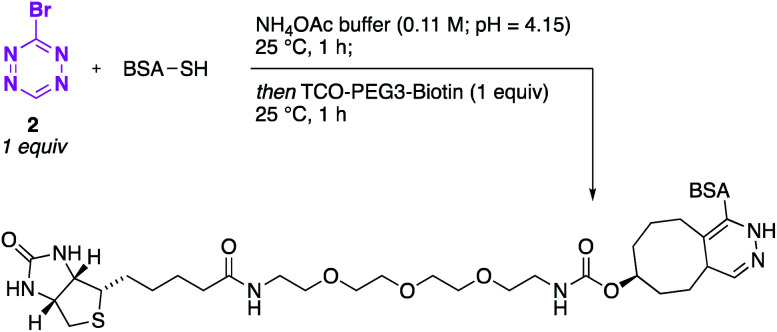
In order to demonstrate the feasibility of using Br-Tet (2) in a biochemical context, we set out to investigate the selective functionalisation of the protein bovine serum albumin (BSA). After verifying the presence of a reactive sulfhydryl group in BSA by using an Ellman assay with cysteine as a reference (see ESI for details†),20 the labelling of BSA with Br-Tet (2) under the previously established conditions (ammonium acetate buffer, 0.11 M, pH = 4.15) was performed. After 1 h, the reaction was analysed by ESI-MS and full conversion was observed, with the mass of the product corresponding to the mono-tetrazine-functionalised BSA. With the bio-orthogonal handle installed, we set out to demonstrate the feasibility of an iEDDA reaction by using TCO-PEG3-biotin (1 equiv.) as the dienophile. After 1 h at room temperature, full conversion to biotin-tagged-BSA was observed (Scheme 6), which demonstrated the very fast kinetics of the iEDDA reaction when utilising a 3-monosubstituted tetrazine as the diene. In order to estimate the rate constant of this transformation, Boc-Cys(Tet)-OMe was reacted with TCO-PEG3-biotin as a model system under pseudo-first order conditions; a rate constant of 22.7 M−1 s−1 was observed for the iEDDA reaction (see ESI†). In comparison, the kinetics of the iEDDA reaction of a diene functionalised with two thioethers required 14.5 h and heating to 37 °C.8 Ultimately, fast reaction rates are indispensable for bio-orthogonal reactions in order to ensure full conversion of the target to the labelled compound, especially at physiologically relevant μM-concentrations of the protein.11
Scheme 6. Labelling of BSA. One-pot procedure for the functionalisation of BSA with 3-Br-Tet (2) and subsequent iEDDA with TCO-PEG3-biotin.
Thus, this fast protocol clearly demonstrates the utility of Br-Tet (2) for bio-orthogonal labelling and shows that with this approach the most likely smallest fluorophore available can easily be attached onto a protein under biologically relevant conditions without the use of any additives such as base.21 More importantly, the very short reaction times required for the labelling procedure, as well as the subsequent iEDDA reaction, which can be carried out in a one-pot fashion, greatly enhance the utility of this approach.
Hypothesis on heteroatom selectivity
In order to rationalise the observed trends in reactivity of the investigated heteroatoms (O, N, S) in the nucleophilic substitution reaction with Br-Tet (2) and the relative inertness of 2 towards water as a solvent, we compared the nucleophilicity parameters of the different functional groups, as described of Mayr and co-workers.22 On careful evaluation of the data reported, S-nucleophiles have far superior nucleophilicity over nitrogen and oxygen based nucleophiles (N parameter for cysteine in water = 23.43, N parameter for primary amines in water = 12.99–14.02, N parameter for water with 20% MeCN = 5.04).22 Thus, the high selectivity most probably arises from a kinetic effect, in which the stronger nucleophile outcompetes other nucleophiles present in the mixture for Br-Tet (2). The experimentally observed selectivity towards heteroatoms aligns well with the nucleophilicity scale by Mayr and co-workers, which clearly explains the superiority of thiols over amines and hydroxy groups. Additionally, water and alcohols exhibit by far the lowest nucleophilicity parameters, as exemplified by the sluggish reactivity towards primary hydroxy groups and especially water.
Synthesis of disulphide targeting chemical probe
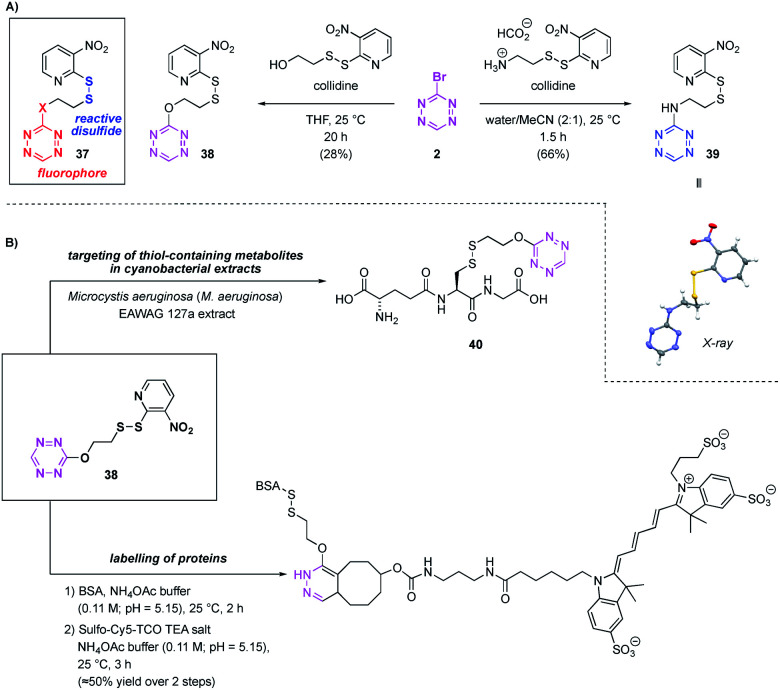
In order to not be restricted to low pH values to achieve high selectivity for thiols over amines, we set out to develop a disulphide based chemical probe derived from Br-Tet (2). Especially when targeting thiols, chemical probe assisted isolation can be of great value due to the instability of this functional group under standard isolation conditions.23 Whereas prior research has targeted thiols in a non-reversible fashion,24 there are only rare reports of thiols in extracts being targeted in a reversible fashion.23 Additionally, a difficult synthesis of the resin carrying the photolabile linker and the reactive disulphide render this approach impractical.23 Thus, we set out to synthesise disulphides of the general structure 37 as candidates for a small chemical probe for labelling thiols in a reversible fashion. Chemical probes of this type would bear the advantages of being very small compared to similar probes used, of being fluorescent and of exhibiting a characteristic UV-trace due to the tetrazine moiety. This would enable easy detection of functionalised metabolites via UHPLC-MS, even in complex mixtures. Hence, such a probe would greatly facilitate the separation of captured metabolites from the extract.
In this context, we synthesised two chemical probes, 38 and 39, in one step from Br-Tet (2). Compound 38 exhibited a much higher fluorescence intensity than disulphide 39, therefore 38 was selected for further studies (see ESI for emission spectra†). After establishing the best conditions for disulphide exchange under aqueous conditions by using l-glutathione as a model system to give disulphide 40 (Scheme 7), we set out to explore the disulphide exchange on a more complex system. In order to probe the disulphide exchange on proteins and to elaborate conditions for a subsequent iEDDA reaction with strained alkenes, we selected BSA as a model system. Gratifyingly, the conditions previously established for disulphide exchange with l-glutathione were directly applicable to BSA and full conversion was achieved in aqueous media within 2 h. A subsequent iEDDA reaction with Sulfo-Cy5-TCO in a one pot fashion afforded a BSA-Tet-Cy5 conjugate and the yield of this two-step procedure was established to be around 50% by using in-gel fluorescence measurements with Sulfo-Cy5-TCO triethylamine salt as the reference.
Scheme 7. Synthesis and application of disulphide targeting chemical probes. (A) Synthesis of tetrazine, disulphide based chemical probes; (B) application of the chemical probes to the isolation of natural products and labelling of proteins.
Having established that our chemical probe is stable in aqueous media and is compatible with complex systems such as proteins, we investigated the ability of this probe to target natural products. As our group is interested in the metabolites of cyanobacteria, we chose an extract of the cyanobacterium Microcystis aeruginosa (M. aeruginosa) EAWAG 127a as a model system.25 To demonstrate the applicability of our probe 38 in this context, we chose to target l-glutathione, one of the few metabolites of cyanobacteria known to contain a thiol.26 Treatment of the extract with our chemical probe 38 lead to successful functionalisation of l-glutathione (see ESI for details†).
As predicted, detection via the UV-trace of the UHPLC-MS chromatogram was possible, where the characteristic absorption of the tetrazine moiety was observed at 514 nm. Further studies on applications of the reported chemical probe in the isolation of novel, thiol containing metabolites are currently being conducted in our group.
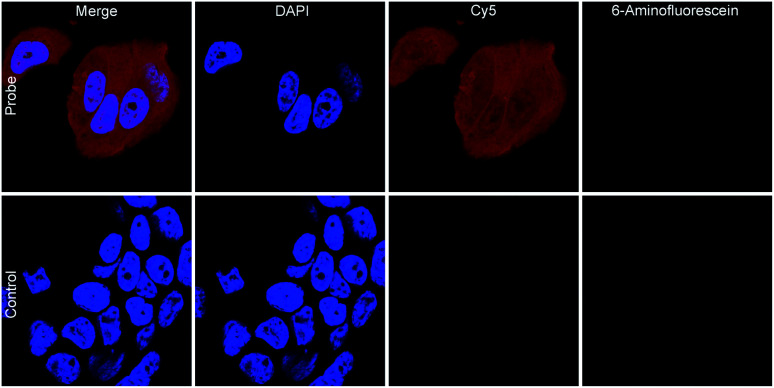
Having established the compatibility of our chemical probe with complex systems, we labelled mammalian cells with disulphide 38, which ultimately demonstrated the applicability of our tetrazine chemical probe to very complex environments. To this end, fixed MCF7 breast cancer cells were tagged with the chemical probe 38 by incubation at 25 °C for only 30 min. To verify the attachment of the tetrazine and to show the possibility of further functionalising the bio-orthogonal handle, the cells were incubated with Sulfo-Cy5-TCO trimethylamine salt (Fig. 1). Control experiments with 3-(2-(methylthio)ethoxy)-s-tetrazine (see ESI for details†) and Sulfo-Cy5-TCO triethylamine salt did not lead to successful labelling, since the control cannot attach covalently to sulphides within the cells. Dye accumulation due to unspecific non-covalent binding was excluded by additionally incubating tagged cells with 6-aminofluorescein. These results clearly demonstrate that this approach presents an easy and rapid method for covalently attaching an s-tetrazine to cells and the viability of this probe to function as a bio-orthogonal handle for further downstream functionalisation. Short incubation times additionally add value to the presented method for cell labelling.
Fig. 1. Labelling of fixed MCF7 cells tagged with tetrazine 38 and labelled with TCO-Cy5 dye (nuclei stained with DAPI).
Conclusion
In conclusion, we developed Br-Tet (2) as a versatile precursor for the synthesis of a broad range of novel classes of 3-monosubstituted s-tetrazines. Several families of 3-monosubstituted s-tetrazines, including 3-phenoxy-s-tetrazines, 3-indole substituted s-tetrazines, 3-thiol-s-tetrazines, 3-amino-s-tetrazines, and several functionalised amino acids, were synthesised in moderate to excellent yields. In addition, the antibiotic fidaxomicin, a steroid, as well as small peptides, were functionalised with excellent selectivity and the site of functionalisation can easily be predicted. Br-Tet (2) is compatible with aqueous media, which allowed the development of a protocol for protein labelling and subsequent modification through an iEDDA reaction to install a biotin linker. Furthermore, two chemical probes for targeting thiols were synthesised and were demonstrated to be highly applicable in the fields of targeted natural product isolation and the labelling of proteins and, ultimately cells.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank Andrea Turrin for laboratory assistance and Dr Olivier Blacque for acquiring the crystallographic data for compound 1. We express our gratitude to the Functional Genomics Center Zurich (FGCZ) for analysis of the functionalised proteins.
Electronic supplementary information (ESI) available: Experimental procedures, additional information and characterisation data. CCDC 1937215–1937219 and 1958903. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc06169j
Notes and references
- Pinner A. Ber. Dtsch. Chem. Ges. 1893;26:2126. doi: 10.1002/cber.189302602188. [DOI] [Google Scholar]
- (a) Li Z. Ding J. Song N. Du X. Zhou J. Lu J. Tao Y. Chem. Mater. 2011;23:1977. doi: 10.1021/cm200330c. [DOI] [Google Scholar]; (b) Hwang D. K. Dasari R. R. Fenoll M. Alain-Rizzo V. Dindar A. Shim J. W. Deb N. Fuentes-Hernandez C. Barlow S. Bucknall D. G. Audebert P. Marder S. R. Kippelen B. Adv. Mater. 2012;24:4445. doi: 10.1002/adma.201201689. [DOI] [PubMed] [Google Scholar]
- (a) Chavez D. E. Hiskey M. A. Gilardi R. D. Org. Lett. 2004;6:2889. doi: 10.1021/ol049076g. [DOI] [PubMed] [Google Scholar]; (b) Chavez D. E. Parrish D. A. Mitchell L. Imler G. H. Angew. Chem., Int. Ed. 2017;56:3575. doi: 10.1002/anie.201612496. [DOI] [PubMed] [Google Scholar]; (c) Wang G. Lu T. Fan G. Yin H. Chen F.-X. New J. Chem. 2019;43:1663. doi: 10.1039/C8NJ05220D. [DOI] [Google Scholar]
- (a) Boger D. L. Hong J. J. Am. Chem. Soc. 2001;123:8515. doi: 10.1021/ja011271s. [DOI] [PubMed] [Google Scholar]; (b) Hamasaki A. Zimpleman J. M. Hwang I. Boger D. L. J. Am. Chem. Soc. 2005;127:10767. doi: 10.1021/ja0526416. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Landry M. L. McKenna G. M. Burns N. Z. J. Am. Chem. Soc. 2019;141:2867. doi: 10.1021/jacs.8b12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Glöckle M. Kaim W. Angew. Chem., Int. Ed. 1999;38:3072. doi: 10.1002/(SICI)1521-3773(19991018)38:20<3072::AID-ANIE3072>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]; (b) Kaim W. Coord. Chem. Rev. 2002;230:127. doi: 10.1016/S0010-8545(02)00044-9. [DOI] [Google Scholar]; (c) Yuasa J. Mitsui A. Kawai T. Chem. Commun. 2011;47:5807. doi: 10.1039/C1CC11485A. [DOI] [PubMed] [Google Scholar]
- Oliveira B. L. Guo Z. Bernardes G. J. L. Chem. Soc. Rev. 2017;46:4895. doi: 10.1039/C7CS00184C. [DOI] [PubMed] [Google Scholar]
- Wu H. Devaraj N. K. Acc. Chem. Res. 2018;51:1249. doi: 10.1021/acs.accounts.8b00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canovas C. Moreau M. Bernhard C. Oudot A. Guillemin M. Denat F. Goncalves V. Angew. Chem., Int. Ed. 2018;57:10646. doi: 10.1002/anie.201806053. [DOI] [PubMed] [Google Scholar]
- Lambert W. D. Fang Y. Mahapatra S. Huang Z. am Ende C. W. Fox J. M. J. Am. Chem. Soc. 2019;141:17068. doi: 10.1021/jacs.9b08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañeque T. Müller S. Rodriguez R. Nat. Rev. Chem. 2018;2:202. doi: 10.1038/s41570-018-0030-x. [DOI] [Google Scholar]
- Baalmann M. Ziegler M. J. Werther P. Wilhelm J. Wombacher R. Bioconjugate Chem. 2019;30:1405. doi: 10.1021/acs.bioconjchem.9b00157. [DOI] [PubMed] [Google Scholar]
- Baker J. G. Middleton R. Adams L. May L. T. Briddon S. J. Kellam B. Hill S. J. Br. J. Pharmacol. 2010;159:772. doi: 10.1111/j.1476-5381.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Seitchik J. L. Peeler J. C. Taylor M. T. Blackman M. L. Rhoads T. W. Cooley R. C. Refakis C. Fox J. M. Mehl R. A. J. Am. Chem. Soc. 2012;134:2898. doi: 10.1021/ja2109745. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Blizzard R. J. Backus D. R. Brown W. Bazewicz C. G. Li Y. Mehl R. A. J. Am. Chem. Soc. 2015;137:10044. doi: 10.1021/jacs.5b03275. [DOI] [PubMed] [Google Scholar]; (c) Kamber D. N. Liang Y. Blizzard R. J. Liu F. Mehl R. A. Houk K. N. Prescher J. A. J. Am. Chem. Soc. 2015;137:8388. doi: 10.1021/jacs.5b05100. [DOI] [PubMed] [Google Scholar]; (d) Yang J. Liang Y. Šečkutė J. Houk K. N. Devaraj N. K. Chem.–Eur. J. 2014;20:3365. doi: 10.1002/chem.201304225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S. V. Murnauer A. von Wrisberg M.-K. Jokisch M.-L. Lang K. Angew. Chem., Int. Ed. 2019;131:16023. doi: 10.1002/ange.201908209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Karver M. R. Weissleder R. Hilderbrand S. A. Bioconjugate Chem. 2011;22:2263. doi: 10.1021/bc200295y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cserép G. B. Demeter O. Bätzner E. Kállay M. Wagenknecht H.-A. Kele P. Synthesis. 2015;47:2738. doi: 10.1055/s-0034-1380721. [DOI] [Google Scholar]; (c) Maggi A. Ruivo E. Fissers J. Vangestel C. Chatterjee S. Joossens J. Sobott F. Staelens S. Stroobants S. Van Der Veken P. Wyffels L. Augustyns K. Org. Biomol. Chem. 2016;14:7544. doi: 10.1039/C6OB01411A. [DOI] [PubMed] [Google Scholar]
- (a) Tokmakov A. A. Kurotani A. Takagi T. Toyama M. Shirouzu M. Fukami Y. Yokoyama S. J. Biol. Chem. 2012;287:27106. doi: 10.1074/jbc.M112.366351. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gomes A. R. Byregowda S. M. Balamurugan V. Adv. Anim. Vet. Sci. 2016;4:346. doi: 10.14737/journal.aavs/2016/4.7.346.356. [DOI] [Google Scholar]
- Counotte-Potman A. Van der Plas H. C. Van Veldhuizen B. Landheer C. A. J. Org. Chem. 1981;46:5102. doi: 10.1021/jo00338a009. [DOI] [Google Scholar]
- Rudakov G. F. Moiseenko Y. A. Spesivtseva N. A. Chem. Heterocycl. Compd. 2017;53:802. doi: 10.1007/s10593-017-2127-4. [DOI] [Google Scholar]
- (a) Chavez D. E. Hiskey M. A. J. Heterocycl. Chem. 1998;35:1329. doi: 10.1002/jhet.5570350616. [DOI] [Google Scholar]; (b) Zhu J. Hiltz J. Lennox R. B. Schirrmacher R. Chem. Commun. 2013;49:10275. doi: 10.1039/C3CC46325G. [DOI] [PubMed] [Google Scholar]; ; for selected examples of nucleophilic aromatic substitution of 3-bromo-5-substituted tetrazines see: ; Grakakauskas V. A. Tomasewski A. J. Horwitz J. P. J. Am. Chem. Soc. 1958;80:3155. doi: 10.1021/ja01545a060. [DOI] [Google Scholar]; Mangia A. Bortesi F. Amendola U. J. Heterocycl. Chem. 1977;15:587. doi: 10.1021/ja01545a060. [DOI] [Google Scholar]; Werbel L. M. Mcnamara D. J. Colbry N. K. Johnson J. L. Degnan M. J. Whitney B. J. Heterocycl. Chem. 1979;16:881. doi: 10.1021/ja01545a060. [DOI] [Google Scholar]; Seitz G. Görge L. Dietrich S. Tetrahedron Lett. 1985;26:4355. doi: 10.1021/ja01545a060. [DOI] [Google Scholar]; Seitz G. Richter J. Chem. Ber. 1989;122:2177. doi: 10.1021/ja01545a060. [DOI] [Google Scholar]
- (a) Eyer P. Worek F. Kiderlen D. Sinko G. Stuglin A. Simeon-Rudolf V. Reiner E. Anal. Biochem. 2003;312:224. doi: 10.1016/S0003-2697(02)00506-7. [DOI] [PubMed] [Google Scholar]; (b) Funk W. E. Li H. Iavarone A. T. Williams E. R. Riby J. Rappaport S. M. Anal. Biochem. 2010;400:61. doi: 10.1016/j.ab.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audebert P. Miomandre F. Clavier G. Vernières M.-C. Badré S. Méallet-Renault R. Chem.–Eur. J. 2005;11:5667. doi: 10.1002/chem.200401252. [DOI] [PubMed] [Google Scholar]
- (a) Minegishi S. Kobayashi S. Mayr H. J. Am. Chem. Soc. 2004;126:5174. doi: 10.1021/ja031828z. [DOI] [PubMed] [Google Scholar]; (b) Brotzel F. Chu Y. C. Mayr H. J. Org. Chem. 2007;72:3679. doi: 10.1021/jo062586z. [DOI] [PubMed] [Google Scholar]; (c) Brotzel F. Mayr H. Org. Biomol. Chem. 2007;5:3814. doi: 10.1039/B713778H. [DOI] [PubMed] [Google Scholar]
- Capehart S. L. Carlson E. E. Chem. Commun. 2016;52:13229. doi: 10.1039/C6CC07111B. [DOI] [PubMed] [Google Scholar]
- (a) Carlson E. E. Cravatt B. F. J. Am. Chem. Soc. 2007;129:15780. doi: 10.1021/ja0779506. [DOI] [PubMed] [Google Scholar]; (b) Carlson E. E. Cravatt B. F. Nat. Methods. 2007;4:429. doi: 10.1038/nmeth1038. [DOI] [PubMed] [Google Scholar]; (c) Castro-Falcón G. Hahn D. Reimer D. Hughes C. C. ACS Chem. Biol. 2016;11:2328. doi: 10.1021/acschembio.5b00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Portmann C. Blom J. F. Kaiser M. Brun R. Jüttner F. Gademann K. J. Nat. Prod. 2008;71:1891. doi: 10.1021/np800409z. [DOI] [PubMed] [Google Scholar]; (b) Gademann K. Portmann C. Blom J. F. Zeder M. Jüttner F. J. Nat. Prod. 2010;73:980. doi: 10.1021/np900818c. [DOI] [PubMed] [Google Scholar]; (c) Portmann C. Sieber S. Wirthensohn S. Blim J. F. Da Silva L. Baudat E. Kaiser M. Brun R. Gademann K. J. Nat. Prod. 2014;77:557. doi: 10.1021/np400814w. [DOI] [PubMed] [Google Scholar]
- Narainsamy K. Farci S. Braun E. Junot C. Cassier-Chauvat C. Chauvat F. Mol. Microbiol. 2016;100:15. doi: 10.1111/mmi.13296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.