Abstract
Objectives:
To assess the benefits of training in mindfulness-based stress reduction (MBSR) or moderate intensity exercise (EX) for improving sleep quality.
Design:
Randomized controlled trial.
Setting:
Outpatient, community-based.
Participants:
Healthy adults (n=413) aged 30–69 who did not regularly exercise or practice meditation, and who had no known prior sleep problems.
Interventions:
1) 8-weeks of MBSR training; 2) matched EX training; or 3) wait-list control.
Measurements:
The Pittsburgh Sleep Quality Index (PSQI) was administered at baseline and at 1, 3, 5, and 7-month follow-up visits.
Analysis:
Total PSQI scores and three PSQI factors (perceived sleep quality; daily disturbances, sleep efficiency) were assessed using linear mixed effects regression models for longitudinal data.
Results:
Compared to controls, PSQI global scores improved significantly for EX (mean change −0.98 points [95% CI −1.56, −0.41] p=0.001) and marginally for MBSR (−0.53 [−1.10, 0.04] p=0.07). The perceived sleep quality factor improved for both EX (−0.18 [−0.30, −0.07] p=0.002) and MBSR (−0.12 [−0.24, −0.01] p=0.035). The daily disturbances factor improved slightly more for MBSR (−0.13 [−0.22, −.033] p=0.008) than EX (−0.09 [−0.19, 0.004] p=0.06). The sleep efficiency factor did not improve after MBSR (0.08 [−.045, 0.21] p=0.2) or EX (−0.07 [−0.20, 0.06] p=0.3). Improvements in the sleep quality were sustained over 7 months for both groups.
Conclusions:
Training in MBSR and EX produced small but statistically significant and sustained improvements in sleep quality. For EX participants, this improvement was due primarily to improvements in perceived sleep quality. For MBSR, the decrease in daytime disturbance was more important.
Keywords: exercise, meditation, mindfulness, sleep, PSQI
INTRODUCTION
Sleep problems, ranging from mild sleep disturbance to debilitating insomnia, are among the most common health challenges occurring in adults, with prevalence estimated from 35% to 48%.1–4 Indeed, even moderate levels of sleep disturbance are associated with increases in daytime fatigue, and often with disturbed mood, depressive symptoms, and reduced quality of life. For people with mild to moderate sleep problems, medical treatments may not be appropriate. Instead, community-based behavioral interventions may be able to effectively address moderate sleep disturbance and related daytime dysfunction.
Despite the widespread health impact of mild to moderate sleep disturbance, such symptoms often go unrecognized or untreated, and few interventions have been properly assessed. This is in contrast to clinically diagnosed insomnia, for which medications are often used, and where cognitive behavioral therapy for insomnia (CBT-I) is considered the treatment of choice by the American College of Physicians, as well as the American Academy of Sleep Medicine.5,6 However, CBT-I requires highly trained therapists, and cannot be readily disseminated at the community level for cost-effective treatment of moderate sleep problems. Alternative behavioral treatments might be useful for mild to moderate sleep disturbances in community dwelling adults, with the potential to improve quality of life and prevent insomnia and related consequences.
Two behavioral treatments that might address this need are exercise and meditation. With regard to exercise, two recent reviews of 9 and 11 randomized controlled trials (RCTs) for sleep problems including insomnia have found that exercise training can improve sleep quality.7,8 A larger body of less rigorous evidence (66 studies, 2863 participants) also supports the thesis that exercise improves general sleep quality.9 Effects of exercise on sleep domains such as sleep onset latency, subjective sleep quality, sleep continuity, total sleep time, sleep efficiency, and daytime dysfunctions from sleep impairment are known with less confidence. We interpret the available data as providing moderately strong evidence that exercise improves sleep quality for both those with clinical insomnia as well as those with sleep disturbance who do not fulfil severity for insomnia diagnosis.
A limited but growing body of evidence suggests that mindfulness and other types of meditation may also improve sleep quality.10–13 Neuendorf et al. (2015) reviewed 112 research studies testing a variety of different mind-body interventions, including meditation, and found that even though the evidence was heterogeneous, limited, and potentially biased, mind-body training could be considered as a treatment option for patients with insomnia or sleep disturbance.14 However, the findings from trials testing meditation are limited and have yielded mixed results. For example, Black et al. (2015) identified significant improvements in sleep quality after 6 weeks of mindfulness training, as compared to sleep hygiene education (active control) in older adults with moderate sleep disturbance.12 Wong et al (2017) randomized 216 adults with insomnia to mindfulness-based cognitive therapy (MBCT) versus sleep psycho-education, and found significant benefits in the MBCT group initially, but these benefits diminished and were not statistically significant 6 months later.15 Both Innes et al. (2016) and Adler et al. (2017) reported non-significant trends towards sleep quality benefits in trials where meditation was compared to active control group.10,16 Importantly, no RCT has compared the effects of meditation vs. exercise on sleep quality among community dwelling adults.
To address this gap, this study examines data from the MEPARI-2 trial17 (Meditation or Exercise for Preventing Acute Respiratory Infection), looking at the effects of two relatively low-cost and community-accessible interventions, training in exercise or mindfulness meditation, on sleep quality. For the current analysis, we hypothesized that both exercise and mindfulness meditation would confer superior improvement in sleep quality as compared to an observational wait-list control, in this community sample of adults followed for 7 months. While participants in this trial were enrolled without regard to sleep disturbance, the sample does represent varying levels of mild-to-moderate sleep disturbance as assessed by the Pittsburgh Sleep Quality Index (PSQI). Hence, additional exploratory analyses also examined improvements in sleep quality in those who evidenced impairments in sleep with PSQI scores >5. It should be acknowledged, however, that while this was a high quality RCT with sleep quality as a pre-specified outcome, the trial was aimed at potential influences on respiratory infections rather than sleep quality, and thus this report represents a post hoc secondary analysis.
METHODS
Trial design
The MEPARI-2 trial randomized community-recruited adults to three groups: 1) 8 weeks of training in mindfulness based stress reduction (MBSR), 2) matched 8 weeks of moderate intensity aerobic exercise training (EX), or 3) observational wait-list control. Each of the 4 yearly cohorts was followed from September/October, when interventions were delivered, through May of the following year (37 weeks of observation). The targeted primary outcome for the MEPARI-2 trial was all-cause acute respiratory infection illness during one cold and flu season. Main outcomes of that trial,17–20 and the preceding MEPARI preliminary trial,21 are reported elsewhere. Sleep quality was self-reported using the Pittsburgh Sleep Quality Index (PSQI) at baseline, and at four additional time-points over 7 months of post-intervention monitoring. The MEPARI-2 trial was sponsored by the National Center for Complementary and Integrative Health at the U.S. National Institutes of Health (R01AT006970). The trial was registered at clinicaltrials.gov (NCT01654289), and data have been archived at the publicly accessible ICPSR data repository (www.openicpsr.org/openicpsr/project/103581/version/V2/view).
The protocol was approved and monitored by the University of Wisconsin-Madison Institutional Review Board. All subjects provided written consent.
Participants and setting
The MEPARI-2 trial was conducted in Madison, Wisconsin, USA, from 2012 to 2016. Participants were recruited through local advertisements, screened first by telephone, and then with an in-person visit, usually one or two weeks before consent and enrollment. Severity of sleep disturbance was not considered as a selection criterion. Inclusion criteria were: 1) 30–69 years of age; 2) self-report of an average of at least 1 cold per year, or at least 2 colds in the past year; 3) meeting American Heart Association guidelines22 for suitability for an exercise program; 4) willingness to participate in either meditation or exercise training (or neither, depending on randomized allocation); 5) willingness to be immunized against influenza virus and undergo periodic blood draws, nasal irrigation, questionnaires; 6) a score of 14 or lower on the 9-item depression module of the Patient Health Questionnaire (PHQ-9);23 7) fluency and literacy in English language sufficient for completing questionnaires; and 8) successful completion of run-in screening procedures, consisting of 2 visits and a few questionnaires. Exclusion criteria included: 1) current meditation practice or previous meditation experience; 2) inability to engage in moderate exercise more than twice per week or vigorous exercise more than once per week; 3) pregnancy or intention to become pregnant during the course of the study; 4) physical, medical, or mental conditions precluding adherence to study protocol (e.g., malignant disease, function-impairing psychopathology); 5) use or anticipated need for immunomodulatory drugs (e.g., steroids, immunosuppressants, chemotherapy); 6) immune deficiency or auto-immune disease.
Study interventions
The training in mindfulness meditation followed the standard MBSR format,24,25 and was led by experienced MBSR instructors. Classes of approximately 15 participants met weekly for 8 weeks. Each class lasted approximately 2.5 hours. Participants were expected to practice of 20 to 45 min daily. A 5-hour weekend retreat was held around the 6th week. Exercise (EX) training was matched to MBSR in terms of contact hours, class size, location, expected practice time, and the weekend retreat. EX practice focused on brisk walking or jogging on treadmill, with customized instruction for those with physical limitations, or access to specific equipment, such as stationary or road bicycle, or elliptical, stair-step, or rowing machine. Experienced exercise instructors led the EX classes. The goal for EX participants was to reach and sustain a Borg’s Rating of Perceived Exertion26 level of 12 to 16 points. Both MBSR and EX participants practiced under supervision during the classes, and on their own on other days. Practice was logged daily and reported weekly. Control participants who completed the study were offered free meditation training, or $300 remuneration and assistance with finding subsequent EX classes.
Randomization and blinding
Randomized allocation to intervention groups was accomplished using computer-generated randomization codes concealed in sealed envelopes, which were opened after baseline values were obtained and the participant signed the consent form. The statistician employed variable block size methods to keep group sizes approximately equal without jeopardizing blinded allocation. During telephone screening and in-person baseline assessment, participants had to declare that they were willing to be randomized to either of the interventions, or to wait-list control, and to carry out all related activities, regardless of assignment. Participants could not be blinded to the type of intervention once initiated, but investigators and data analysts were masked to group assignment until after the last participant exited the study and all data entry and cleaning was completed.
Assessment of sleep quality
Self-reported sleep quality, as assessed by the Pittsburgh Sleep Quality Index (PSQI),27,28 was specified a priori as an important secondary outcome of the MEPARI-2 trial. The PSQI was administered at baseline in August and at approximate 3-, 5-, 7-, and 9-month post-enrollment follow-ups. The PSQI assesses “usual sleep habits during the past month,” and includes 19 items yielding 7 components: sleep duration, sleep disturbances, sleep latency, daytime dysfunction, habitual sleep efficiency, subjective sleep quality, and sleep medication use. Each component is scored on a 4-point range (0 to 3), with higher scores indicating worse sleep. The PSQI global score is the sum of the 7 component scores. For the current analysis, the PSQI global score is considered as the primary outcome. Secondary outcomes include the 3 factors described and validated by Cole et al.27 Sleep efficiency (factor 1) represents the sum of the components sleep duration and habitual sleep efficiency. Perceived sleep quality (factor 2) is the sum of the components subjective sleep quality, sleep latency, and sleep medication use. Daily disturbances (factor 3) represents the sum of the components sleep disturbances and daytime dysfunction scores.27
Other measures
Sociodemographic factors including age, sex, education, income, race/ethnicity, and smoking status were collected at baseline, along with several validated self-report questionnaires, including: SF12 (general mental and physical health,12-item Short Form Medical Outcomes Study);29 PHQ9 (depression symptoms, 9-item Patient Health Questionnaire),23 PSS-10 (perceived stress, 10-item Perceived Stress Scale);30,31 Baseline body mass index (BMI) was derived from objectively measured height and weight. Blood pressure (BP) was measured by sphygmomanometer by experienced nurses. All baseline values were gathered prior to randomized allocation. See Table 1.
Table 1.
Participant Characteristics at Baseline
| Characteristic | Exercise | Meditation | Control |
|---|---|---|---|
| Sample size | 137 | 138 | 138 |
| Age (years), mean ± SD | 49.1 ± 11.4 | 49.2 ± 11.2 | 50.7 ± 12.1 |
| Female, n (%) | 107 (78.1) | 105 (76.1) | 101 (73.2) |
| Current smoker, n (%) | 9 (6.6) | 6 (4.3) | 11 (8.0) |
| Race, n (%) | |||
| White/Caucasian | 105 (76.6) | 121 (88.3) | 123 (89.1) |
| Black/African American | 14 (10.2) | 5 (3.6) | 6 (4.3) |
| Asian | 8 (5.8) | 5 (3.6) | 3 (2.2) |
| Other/More Than One Race | 10 (7.3) | 6 (4.4) | 6 (4.3) |
| Hispanic ethnicity, n (%) | 5 (3.8) | 11 (8.1) | 8 (6.0) |
| BMI (kg/m2), mean ± SD | 29.3 ± 7.0 | 29.8 ± 7.8 | 29.0 ± 6.6 |
| College graduate or more, n (%) | 108 (78.8) | 106 (76.8) | 102 (73.9) |
| Income > $50,000, n (%) | 79 (58.1) | 85 (63.4) | 85 (62.5) |
| Systolic BP (mmHg), mean ± SD | 122 ± 15 | 120 ± 16 | 124 ± 17 |
| Diastolic BP, mean ± SD | 75 ± 9 | 74 ± 8 | 76 ± 9 |
| Self-report scores, mean ± SD | |||
| SF12 Mental health | 47.8 ± 10.5 | 48.0 ± 10.2 | 47.7 ± 9.8 |
| SF12 Physical health | 51.6 ± 8.2 | 51.4 ± 7.8 | 51.5 ± 8.3 |
| PSS10 Perceived Stress | 13.3 ± 6.6 | 13.1 ± 6.4 | 12.4 ± 5.9 |
| PHQ9 Depressive Symptoms | 2.9 ± 2.9 | 2.4 ± 2.4 | 2.9 ± 3.1 |
| PSQI Global sleep quality | 6.2 ± 3.6 | 5.8 ± 3.3 | 5.7 ± 3.3 |
| PSQI PSQ | 0.94 ± 0.70 | 0.89 ± 0.67 | 0.90 ± 0.63 |
| PSQI SEf | 0.55 ± 0.71 | 0.42 ± 0.58 | 0.43 ± 0.73 |
| PSQI DD | 1.10 ± 0.50 | 1.13 ± 0.48 | 1.09 ± 0.49 |
Abbreviations: SD = standard deviation; BP = blood pressure; BMI = body mass index; SF12 = Medical Outcomes Study Short Form; PSS10 = Perceived Stress Scale; PHQ9 = depressive symptoms; PSQI = Pittsburgh Sleep Quality Index; PSQ = Perceived Sleep Quality PSQI factor; SEf = Sleep Efficiency PSQI factor; DD = Daily Disturbances PSQI factor
Data management
Data collection began in August, when participants were screened and enrolled, and continued through May of the following year. Baseline demographic and other data were directly entered by participants or study personnel, using a customized REDCap study database.32 Standardized questionnaire booklets including PSQI and other self-report instruments were filled out by participants at home, and were either brought to study visits or mailed in, and then scanned into the study database. Incoming data were monitored by study personnel blinded to group assignment; participants were occasionally contacted when data were missing or unclear. Adherence to protocol and data entry were exceptional, with less than 2% of intended data found to be missing. For these few cases where data was missing, we used Little’s method of testing for missing-completely-at-random (MCAR).33 Where MCAR criteria were accepted, data were imputed using Stata MICE multiple imputation methods, as described by Azur et al.34
Statistical analysis
Correlated mixed-effects linear models for cross-sectional time-series data (i.e., panel data) were used to compare PSQI scores for each of the intervention groups (MBSR, EX) to the control group across 4 post-intervention time periods. Similar models compared the 3 PSQI factors (perceived sleep quality, daily disturbances, sleep efficiency) of MBSR and EX to control across time. These methods are particularly appropriate here, as they take into account the longitudinal nature of the data (correlations between serial observations on the same person), and effectively summarize overall effects across multiple follow-up time points. These models control for time-invariant variables (i.e., baseline measures), which facilitates assessment of average longitudinal effects due to the randomized interventions.35 We fit a panel-data linear model by using generalized least squares estimator, with an AR1 variance-covariance matrix structure for y. Covariates used in these models were age, sex, education, and baseline PSQI score. All statistical models were constructed using Stata Version 15.1 software program.
RESULTS
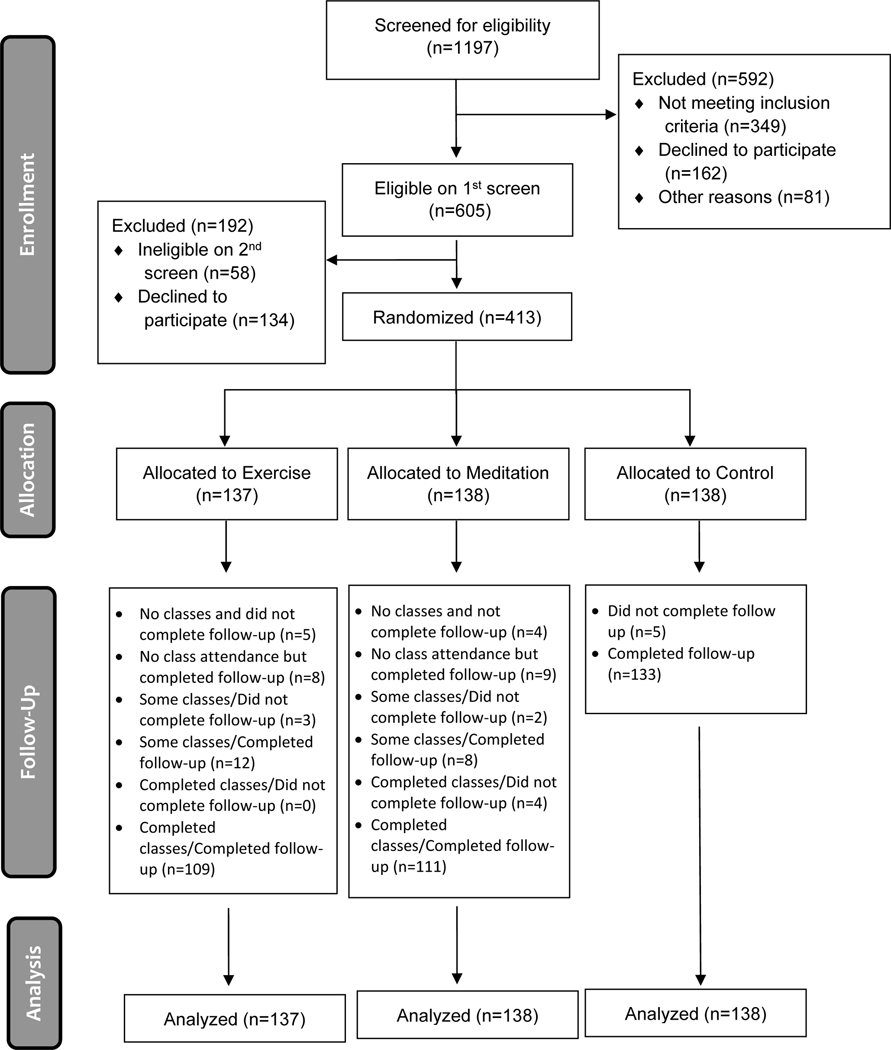
The complete trial was conducted in 4 annual cohorts from 2012–13 to 2015–16, with randomized assignments to all 3 arms in each year.17 There were 1197 telephone contacts with potential participants who responded to community advertising. Of these, 605 came in for in-person screening, and 413 completed baseline assessment and returned for written consent, enrollment, and randomized allocation to EX (137), MBSR (138), or control (138). Each yearly cohort included 2 separate classes for each intervention with class sizes of about 17 people each. Of the 413 randomized, 390 completed the trial (94.4% retention) (see Figure 1). Table 1 shows sample characteristics and baseline values for several relevant variables. Representative of the community demographics in Madison, WI, the participants were predominantly white, included more females, and with a moderately high income and educational attainment.
Figure 1. Participant Flow Diagram (CONSORT Figure).
Practice adherence
We prospectively defined “per protocol” intervention adherence as attending at least 5 of the 9 possible in-person training sessions (8 weekly classes, 1 weekend retreat). In the EX group, 109 participants (80%) met these criteria. For MBSR, 115 people (83%) attended at least 5 of 9 sessions. MBSR and EX practice was recorded on daily practice logs by participants, then self-reported once weekly on a study-specific REDCap-enabled online database. Averaged over the 37 weeks of observation, the median weekly amount of self-reported practice was 236 minutes/week for EX participants, and 220 minutes/week for MBSR participants.
Effects of MBSR and EX on PSQI Global Score
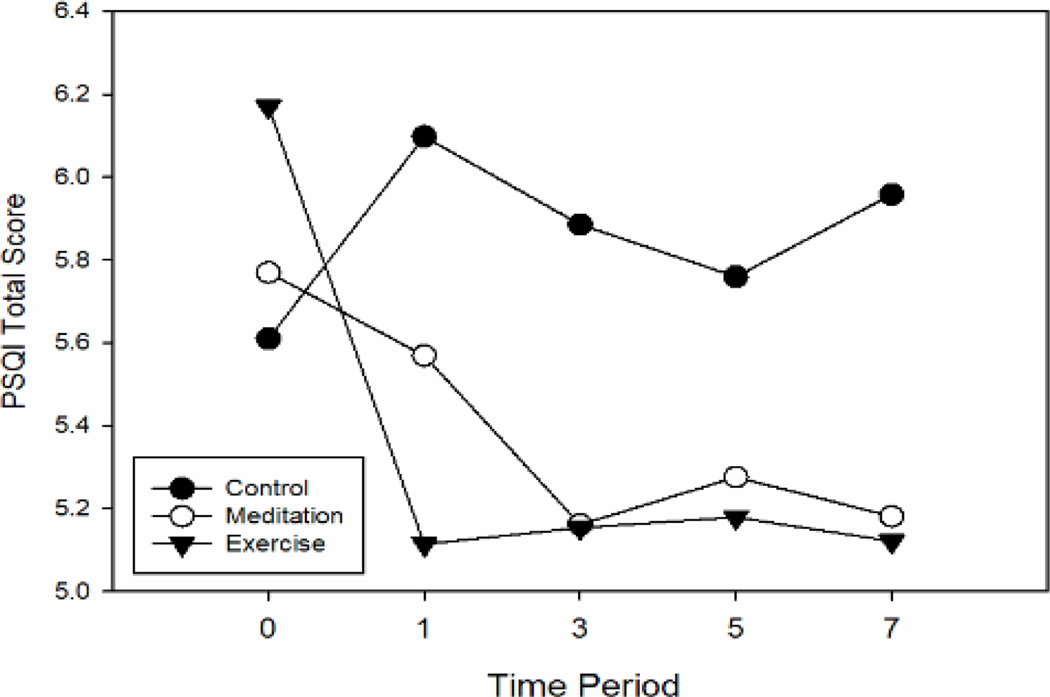
Compared to the control condition, PSQI global score improved modestly for both MBSR and EX groups, with benefits persisting throughout the 7-month post-intervention monitoring period (see Figure 2). Using mixed-effects general linear model methods to calculate intervention effects, mean improvements (reductions) in global sleep quality score were statistically significant for EX (−0.98 points [95% CI −1.56, −0.41] p=0.001) and marginal for MBSR (−0.53 [−1.10, 0.04] p=0.07). This primary model used all available data and took baseline values into account to delineate overall intervention effects on PSQI global sleep quality, using the control group as comparison. Table 2 shows model-generated means, mean differences, and Cohen’s d standardized effect sizes for the PSQI global score at each of the 4 follow-up time points. Coefficients and parameters for the primary statistical model are shown in Table 3.
Figure 2. PSQI global score across 7 months of post-intervention observation.
Mean values derived from random effects linear models.
Better sleep is indicated by lower scores.
Table 2.
PSQI Global Scores at 1-, 3-, 5- and 7- months follow-up
| Measure | November 1-mo. follow-up | January 3-mo. follow-up | March 5-mo. follow-up | May 7-mo. follow-up | |
|---|---|---|---|---|---|
| Exercise | MBSR and EX classes | ||||
| Mean ± SD | 5.11 ± 2.36 | 5.15 ± 2.34 | 5.18 ± 2.36 | 5.12 ± 2.37 | |
| Mean dif. c/w Ctl [95% CI] | 0.98 [−0.41, −1.56] | 0.73 [0.15, 1.32] | 0.58 [0.00, 1.16] | 0.84 [0.26, 1.41] | |
| P value | 0.0006 | 0.01 | 0.05 | 0.001 | |
| Cohen d ES [95% CI] | 0.42 [0.17, 0.66] | 0.31 [0.06, 0.56] | 0.25 [0.00, 0.49] | 0.35 [0.11, 0.60] | |
| Meditation | |||||
| Mean ± SD | 5.57 ± 2.36 | 5.16 ± 2.32 | 5.28 ± 2.35 | 5.18 ± 2.36 | |
| Mean dif. c/w Ctl [95% CI] | 0.53 [−0.04, 1.10] | 0.73 [0.14, 1.31] | 0.48 [−0.09, 1.06] | 0.78 [0.20, 1.35] | |
| P value | 0.07 | 0.02 | 0.10 | 0.01 | |
| Cohen d ES [95% CI] | 0.22 [−0.02, 0.47] | 0.31 [0.06, 0.56] | 0.21 [−0.04, 0.45] | 0.33 [0.08, 0.57] | |
| Control | |||||
| Mean ± SD | 6.10 ± 2.37 | 5.89 ± 2.35 | 5.76 ± 2.36 | 5.96 ± 2.36 |
SD = standard deviation; CI = confidence interval; ES = effect size
Mean dif c/w Ctl = Mean difference between intervention group and control group
P-values come from group comparison T-tests at each time point
Mean difference and effect size are shown in absolute values, so that a positive number indicates benefit (lower PSQI scores), compared to control
Table 3.
Model parameter estimates for PSQI global score
| 95% Confidence Interval | ||||||
|---|---|---|---|---|---|---|
| PSQI Global SCORE | Coefficient | Std. Err. | Z-value | P>|Z| | Lower | Upper |
| Intercept | 2.107 | 0.258 | 8.16 | 0.000 | 1.600 | 2.613 |
| PSQI Baseline (covariate) | 0.684 | 0.027 | 24.69 | 0.000 | 0.630 | 0.739 |
| TREATMENT | ||||||
| Control Group (reference) | ||||||
| Meditation Group | −0.528 | 0.291 | −1.82 | 0.070 | −1.099 | 0.042 |
| Exercise Group | −0.984 | 0.292 | −3.37 | 0.001 | −1.557 | −0.411 |
| TIME | ||||||
| Post-Intervention time 1 November (reference) | ||||||
| Post-Intervention time 2 January | −0.212 | 0.209 | −1.01 | 0.311 | −0.624 | 0.198 |
| Post-intervention time 3 March | −0.338 | 0.208 | −1.62 | 0.105 | −0.748 | 0.070 |
| Post-intervention time 4 May | −0.140 | 0.207 | −0.68 | 0.499 | −0.548 | 0.266 |
| INTERACTION | ||||||
| Meditation group X Time 2 | −0.196 | 0.303 | −0.65 | 0.518 | −0.790 | 0.398 |
| Meditation group X Time 3 | 0.045 | 0.299 | 0.15 | 0.879 | −0.540 | 0.631 |
| Meditation group X Time 4 | −0.247 | 0.297 | −0.83 | 0.405 | −0.831 | 0.335 |
| Exercise group X Time 2 | 0.252 | 0.301 | 0.84 | 0.401 | −0.337 | 0.843 |
| Exercise group X Time 3 | 0.404 | 0.298 | 1.36 | 0.175 | −0.180 | 0.988 |
| Exercise group X Time 4 | 0.147 | 0.297 | 0.50 | 0.619 | −0.434 | 0.730 |
Correlated random effects linear models for cross-sectional time-series data
Effects of MBSR and EX on PSQI Factors: Perceived Sleep Quality, Daily Disturbances, Sleep Efficiency
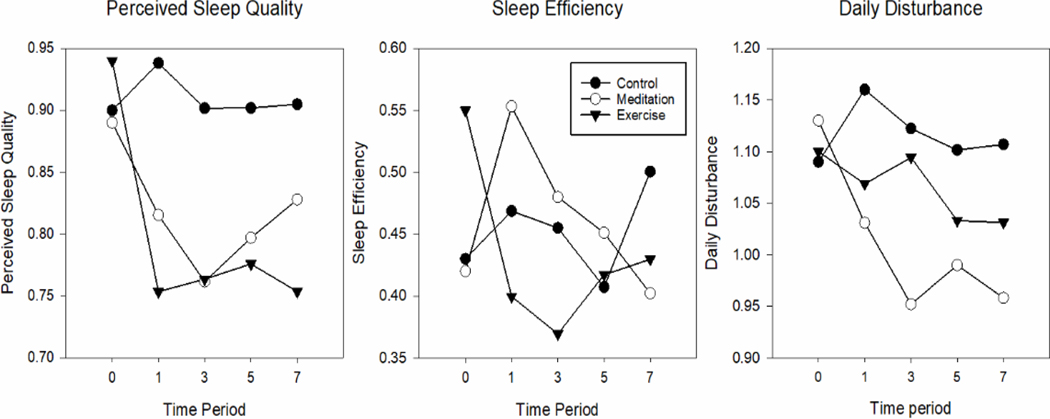
Mixed-effects general linear models similar to the primary model described above were used to test for longitudinal effects of EX and MBSR on each of the three PSQI factors identified by Cole et al.27 - perceived sleep quality, daily disturbances, and sleep efficiency. Table 4 and Figure 3 display model-generated means and 95% confidence intervals at each time point for the 3 comparison groups. The general effects across all follow-up time points suggest that the perceived sleep quality factor improved slightly more for those in the EX group (−0.18 [−0.30, −0.07] p=0.002) than for those receiving MBSR training (−0.12 [−0.24, −0.01] p=0.035). The daily disturbance factor, however, improved slightly more for the MBSR group (−0.13 [−0.22, −0.033] p=0.008) than for those assigned to EX training (−0.09 [−0.19, 0.004] p=0.06). The sleep efficiency factor did not improve significantly for either MBSR (0.08 [−0.045, 0.21] p=0.2) or EX (−0.07 [−0.20, 0.06] p=0.3).
Table 4.
PSQI Factor Perceived Sleep Quality (PSQ), Sleep Efficiency (SEf), and Daily Disturbances (DD) in intervention groups, compared with control
| Measure | November 1-mo. follow-up | January 3-mo. follow-up | March 5-mo. follow-up | May 7-mo. follow-up | |
|---|---|---|---|---|---|
| Exercise | MBSR and EX classes | ||||
| PSQ mean +/- SD | 0.75 +/- 0.47 | 0.76 +/- 0.47 | 0.78 +/- 0.47 | 0.75 +/- 0.47 | |
| PSQ mean dif. c/w Ctl [95% CI] | 0.18 [0.07, 0.30] | 0.14 [0.02, 0.25] | 0.13 [0.01, 0.24] | 0.15 [0.04, 0.27] | |
| PSQ P value | 0.002 | 0.02 | 0.03 | 0.01 | |
| SEf. mean +/- SD | 0.40 +/- 0.53 | 0.37 +/- 0.53 | 0.42 +/- 0.54 | 0.43 +/- 0.54 | |
| SEf mean dif. c/w Ctl [95% CI] | 0.07 [−0.35, 0.49] | 0.09 [−0.05, 0.22] | −0.01 [−0.14, 0.12] | 0.07 [−0.06, 0.20] | |
| SEf. P value | 0.75 | 0.21 | 0.87 | 0.29 | |
| DD mean +/- SD | 1.07 +/- 0.40 | 1.09 +/- 0.39 | 1.03 +/- 0.40 | 1.03 +/- 0.47 | |
| DD mean dif. c/w Ctl [95% CI] | 0.09 [−0.32, 0.51] | 0.03 [−0.07, 0.13] | 0.07 [−0.03, 0.17] | 0.08 [−0.03, 0.18] | |
| DD P value | 0.67 | 0.58 | 0.16 | 0.16 | |
| Meditation | |||||
| PSQ mean +/- SD | 0.82 +/- 0.47 | 0.76 +/- 0.46 | .80 +/- 0.47 | 0.83 +/- 0.47 | |
| PSQ mean dif. c/w Ctl [95% CI] | 0.12 [0.01, 0.24] | 0.14 [0.02, 0.26] | 0.10 [−0.01, 0.22] | 0.08 [−0.04, 0.19] | |
| PSQ P value | 0.04 | 0.02 | 0.07 | 0.19 | |
| SEf mean +/- SD | 0.55 +/- 0.54 | 0.48 +/- 0.53 | 0.45 +/- 0.53 | 0.40 +/- 0.54 | |
| SEf mean dif. c/w Ctl [95% CI] | −0.08 [−0.21, 0.05] | −0.02 [−0.16, 0.11] | −0.04 [−0.17, 0.09] | 0.10 [−0.03, 0.23] | |
| SEf. P value | 0.19 | 0.71 | 0.51 | 0.14 | |
| DD mean +/- SD | 1.03 +/- 0.40 | 0.95 +/- 0.39 | 0.99 +/- 0.39 | 0.96 +/- 0.40 | |
| DD mean dif. c/w Ctl [95% CI] | 0.13 [0.03, 0.23] | 0.17 [0.07, 0.27] | 0.11 [0.01, 0.21] | 0.15 [0.05, 0.25] | |
| DD P value | 0.01 | 0.0006 | 0.02 | 0.0005 | |
| Control | |||||
| PSQ mean +/- SD | 0.94 +/- 0.47 | 0.90 +/- 0.47 | 0.90 +/- 0.47 | 0.90 +/- 0.47 | |
| SEf mean +/- SD | 0.47 +/- 0.53 | 0.45 +/- 0.53 | 0.41 +/- 0.53 | 0.50 +/- 0.53 | |
| DD mean +/- SD | 1.16 +/- 0.40 | 1.12 +/- 0.39 | 1.10 +/- 0.40 | 1.11 +/- 0.40 |
SD = standard deviation; CI = confidence interval
PSQ = Perceived Sleep Quality factor
SEf = Sleep Efficiency factor
DD = Daily Disturbances factor
Mean dif c/w Ctl = Mean difference between intervention group and control group
P-values come from group comparison T-tests at each time point
Mean difference and effect size are shown in absolute values, so that a positive number indicates benefit (lower PSQI scores), compared to control
Figure 3. PSQI Factors: Perceived Sleep Quality, Sleep Efficiency, Daily Disturbances across 7 months of post-intervention observation.
Mean values derived from random effects linear models.
Better sleep is indicated by lower scores.
Maintenance of Sleep Benefits: PSQI Global Score and PSQI Factors
The results suggested that improvements in the PSQI global score of sleep quality were sustained over 7 months for both the EX and MBSR groups. As illustrated in Figure 2, EX training improved the PSQI Global score immediately after the 8-week training sessions; these benefits were maintained throughout the 4 follow-up periods. The degree of improvement in the MBSR group, however, appeared somewhat smaller at the first follow-up visit, but then incrementally larger and similar to EX at the next 3 follow-up assessments. Based on the three PSQI Factors, Figure 3 portrays that both EX and MBSR had modest but sustained benefits on the perceived sleep quality factor throughout the assessment period, with a trend for more benefit for EX. However, neither MBSR nor EX led to significant improvements in the sleep efficiency factor; for MBSR, the first follow-up period trended toward a worsening of this factor. The daily disturbances factor, however, improved more for those in the MBSR group than for EX, with beneficial trends continuing throughout 7 months of post-intervention monitoring.
Subset Analysis of Community Dwelling Adults with and without Poor Sleep (PSQI >5)
To assess whether EX and MBSR had benefit in poor sleepers similar to that in the whole sample, we conducted exploratory subset analyses on those with PSQI global scores > 5.0 points (n=186) and those with PSQI scores ≤ 5.0 points at baseline. In those with PSQI global scores > 5, general linear regression models estimated mean differences of −1.29 [−2.27, −0.32] for EX and −0.83 [−1.84, 0.17] for MBSR as compared to control condition. As expected, these improvements were slightly larger than the −0.98 points [−1.56, −0.41] for EX and −0.53 [−1.10, 0.04] points for MBSR seen with the full dataset. Tables 2a, 2b, 4a and 4b display mean differences compared to the wait-list controls for each of these subsets, along with confidence intervals, p-values, and Cohen d effect size, for each time point across the 7 month observation period.
Table 2a.
PSQI Global Scores at 1-, 3-, 5- and 7- months for subsample Global PSQI Score > 5
| Measure | November | January | March | May | |
|---|---|---|---|---|---|
| Exercise, n | MBSR and EX classes | ||||
| Mean ± SD | 8.08 ± 1.61 | 8.16 ± 0.80 | 8.49 ± 1.58 | 8.34 ± 1.25 | |
| Mean dif. c/w Ctl [95% CI] | −0.99 [−1.58, −0.41] | −0.86 [−1.32, −0.39] | −0.24 [−0.85, 0.38] | −1.05 [−1.73, −0.37] | |
| P value | 0.001 | 0.000 | 0.451 | 0.002 | |
| Cohen d ES [95% CI] | 0.59 [0.24, 0.95] | 0.71 [0.32, 1.10] | 0.15 [−0.23, 0.53] | 0.64 [0.27, 1.01] | |
| Meditation, n | |||||
| Mean ± SD | 8.79 ± 1.64 | 8.74 ± 0.90 | 9.08 ± 1.66 | 8.39 ± 1.70 | |
| Mean dif. c/w Ctl [95% CI] | −0.29 [−0.88, 0.31] | −0.28 [−0.74, 0.19] | 0.35 [−0.28, 0.98] | −1.00 [−1.73, −0.27] | |
| P value | 0.344 | 0.248 | 0.278 | 0.007 | |
| Cohen d ES [95% CI] | 0.17 [ −0.18, 0.52] | 0.22 [−0.17, 0.62] | −0.21 [−0.59, 0.17] | 0.55 [0.17, 0.93] | |
| Control, n | |||||
| Mean ± SD | 9.08 ± 1.72 | 9.02 ± 1.47 | 8.73 ± 1.65 | 9.40 ± 1.91 |
Table 2b.
PSQI Global Scores at 1-, 3-, 5- and 7- months for subsample Global PSQI Score ≤ 5
| Measure | November | January | March | May | |
|---|---|---|---|---|---|
| Exercise, n | MBSR and EX classes | ||||
| Mean ± SD | 3.04 ± 0.80 | 3.34 ± 0.38 | 3.25 ± 0.78 | 2.98 ± 0.95 | |
| Mean dif. c/w Ctl [95% CI] | −0.32 [−0.55, −0.09] | 0.02 [−0.14, 0.19] | 0.05 [−0.18, 0.28] | −0.15 [−0.46, 0.16] | |
| P value | 0.006 | 0.803 | 0.657 | 0.347 | |
| Cohen d ES [95% CI] | 0.16 [−0.18, 0.49] | −0.07 [−0.40, 0.26] | −0.09 [−0.41, 0.23] | 0.21 [−0.12, 0.53] | |
| Meditation, n | |||||
| Mean ± SD | 3.23 ± 0.85 | 2.90 ± 0.58 | 2.94 ± 0.80 | 3.18 ± 1.00 | |
| Mean dif. c/w Ctl [95% CI] | −0.13 [−0.36, 0.11] | −0.42 [−0.61, −0.22] | −0.26 [−0.49, −0.02] | 0.05 [−0.26, 0.36] | |
| P value | 0.288 | 0.000 | 0.032 | 0.759 | |
| Cohen d ES [95% CI] | 0.17 [ −0.16, 0.51] | 0.95 [0.61, 1.30] | 0.42 [0.10, 0.75] | −0.06 [−0.38, 0.26] | |
| Control, n | |||||
| Mean ± SD | 3.36 ± 0.54 | 3.31 ± 0.57 | 3.20 ± 0.66 | 3.13 ± 0.95 |
Table 4a.
PSQI Sleep Quality Factors, Subsample, Global PSQI Score > 5
| Measure | November | January | March | May | |
|---|---|---|---|---|---|
| Exercise | MBSR and EX classes | ||||
| PSQ mean ± SD | 1.27 ± 0.35 | 1.31 ±0.31 | 1.30 ± 0.34 | 1.27 ± 0.38 | |
| PSQ mean dif. c/w Ctl [95% CI] | −0.27 [−0.40, −0.14] | −0.26 [−0.39, −0.14] | −0.22 [−0.36, −0.09] | −0.35 [−0.49, −0.20] | |
| PSQ P value | 0.000 | 0.000 | 0.001 | 0.000 | |
| SEf mean ± SD | 0.81 ± 0.42 | 0.81 ± 0.36 | 0.88 ± 0.50 | 0.97 ± 0.56 | |
| SEf mean dif. c/w Ctl [95% CI] | −0.002 [−0.14, 0.14] | −0.05 [−0.19, 0.08] | 0.15 [−0.02, 0.33] | 0.05 [−0.16, 0.26] | |
| SEf P value | 0.983 | 0.456 | 0.083 | 0.630 | |
| DD mean ± SD | 1.30 ± 0.26 | 1.33 ± 0.25 | 1.31 ± 0.29 | 1.30 ± 0.33 | |
| DD mean dif. c/w Ctl [95% CI] | −0.09 [−0.18, −0.01] | −0.02 [−0.10, 0.07] | 0.002 [−0.098, 0.102] | −0.07 [−0.19, 0.047] | |
| DD P value | 0.03 | 0.68 | 0.97 | 0.24 | |
| Meditation | |||||
| PSQ mean ± SD | 1.39 ± 0.34 | 1.35 ± 0.26 | 1.47 ± 0.33 | 1.37 ± 0.37 | |
| PSQ mean dif. c/w Ctl [95% CI] | −0.15 [−0.28, −0.02] | −0.22 [−0.34, −0.10] | −0.05 [−0.18, 0.08] | −0.24 [−0.39, −0.09] | |
| PSQ P value | 0.021 | 0.000 | 0.412 | 0.001 | |
| SEf mean ± SD | 1.03 ± 0.50 | 1.04 ± 0.39 | 0.97 ± 0.47 | 0.84 ± 0.56 | |
| SEf mean dif. c/w Ctl [95% CI] | 0.21 [0.04, 0.38] | 0.18 [0.03, 0.32] | 0.25 [0.09, 0.42] | −0.08 [−0.29, 0.13] | |
| SEf P value | 0.014 | 0.015 | 0.003 | 0.472 | |
| DD mean ± SD | 1.36 ±0.30 | 1.34 ± 0.23 | 1.37 ± 0.27 | 1.32 ± 0.33 | |
| DD mean dif. c/w Ctl [95% CI] | −0.028 [−0.12, .07] | −0.006 [−0.09, .08] | 0.055 [−0.04, 0.15] | −0.05 [−0.17, 0.07] | |
| DD P value | 0.565 | 0.88 | 0.25 | 0.41 | |
| Control | |||||
| PSQ mean ± SD | 1.54 ± 0.39 | 1.57 ± 0.34 | 1.52 ±0.36 | 1.61 ± 0.41 | |
| SEf mean ± SD | 0.81 ± 0.37 | 0.86 ± 0.36 | 0.72 ± 0.38 | 0.91 ± 0.57 | |
| DD mean ± SD | 1.39 ± 2.21 | 1.35 ± 0.19 | 1.31 ± 0.22 | 1.37 ± 0.33 |
SD = standard deviation; CI= confidence interval
PSQ = Perceived Sleep Quality PSQI factor
SEf = Sleep Efficiency PSQI factor
DD = Daily Disturbances PSQI factor
Mean dif c/w Ctl = Mean difference between intervention group and control group
P-values come from group comparison T-tests at each time point
Mean difference and effect size are shown in absolute values, so that a positive number indicates benefit (lower PSQI scores), compared to control
Table 4b.
PSQI Sleep Quality Factors, Subsample, Global PSQI Score ≤ 5
| Measure | November | January | March | May | |
|---|---|---|---|---|---|
| Exercise | MBSR and EX classes | ||||
| PSQ mean ± SD | 0.36 ± 0.09 | 0.42 ± 0.15 | 0.43 ± 0.15 | 0.42 ± 0.21 | |
| PSQ mean dif. c/w Ctl [95% CI] | −0.09 [−0.14, −0.05] | 0.01 [−0.03, 0.06] | −0.04 [−0.10, 0.01] | 0.008 [−0.06, 0.08] | |
| PSQ P value | 0.000 | 0.572 | 0.112 | 0.278 | |
| SEf mean ± SD | 0.14 ± 0.18 | 0.18 ± 0.17 | 0.20 ± 0.18 | 0.19 ± 0.19 | |
| SEf mean dif. c/w Ctl [95% CI] | 0.004 [−0.05, 0.06] | 0.04 [−0.01, 0.12] | 0.06 [0.001, 0.11] | 0.02 [−0.04, 0.08] | |
| SEf P value | 0.883 | 0.153 | 0.046 | 0.505 | |
| DD mean ± SD | 0.89 ± 0.20 | 0.88 ± 0.15 | 0.80 ± 0.23 | 0.79 ± 0.28 | |
| DD mean dif. c/w Ctl [95% CI] | −0.04 [ −0.09, 0.01] | −0.06 [ −0.10, −0.01] | −0.08 [−0.15, −0.004] | −0.04 [−0.13, 0.05] | |
| DD P value | 0.122 | 0.009 | 0.038 | 0.387 | |
| Meditation | |||||
| PSQ mean ± SD | 0.40 ± 0.17 | 0.35 ± 0.10 | 0.38 ± 0.18 | 0.45 ± 0.22 | |
| PSQ mean dif. c/w Ctl [95% CI] | −0.05 [−0.11, 0.01] | −0.06 [−0.10, −0.02] | −0.09 [−0.15, −0.03] | 0.04 [−0.03, 0.11] | |
| PSQ P value | 0.107 | 0.005 | 0.002 | 0.822 | |
| SEf mean ± SD | 0.22 ± 0.18 | 0.21 ± 0.17 | 0.18 ± 0.19 | 0.17 ± 0.19 | |
| SEf mean dif. c/w Ctl [95% CI] | 0.08 [0.02, 0.14] | 0.07 [0.02, 0.12] | 0.03 [−0.02, 0.09] | 0.003 [−0.06, 0.07] | |
| SEf P value | 0.006 | 0.008 | 0.214 | 0.917 | |
| DD mean ± SD | 0.82 ± 0.24 | 0.75 ± 0.19 | 0.76 ± 0.22 | 0.76 ± 0.28 | |
| DD mean dif. c/w Ctl [95% CI] | −0.11 [ −0.17, −0.05] | −0.19 [−0.23, −0.14] | −0.12 [−0.19, −0.04] | −0.07 [−0.16, 0.02] | |
| DD P value | 0.000 | 0.000 | 0.001 | 0.110 | |
| Control | |||||
| PSQ mean ± SD | 0.45 ± 0.18 | 0.41 ± 0.14 | 0.47 ± 0.19 | 0.41 ± 0.22 | |
| SEf mean ± SD | 0.14 ± 0.16 | 0.14 ± 0.15 | 0.15 ± 0.16 | 0.17 ± 0.19 | |
| DD mean ± SD | 0.93 ± 0.11 | 0.94 ± 0.11 | 0.88 ± 0.23 | 0.83 ± 0.28 |
SD = standard deviation; CI= confidence interval
PSQ = Perceived Sleep Quality PSQI factor
SEf = Sleep Efficiency PSQI factor
DD = Daily Disturbances PSQI factor
Mean dif c/w Ctl = Mean difference between intervention group and control group
P-values come from group comparison T-tests at each time point
Mean difference and effect size are shown in absolute values, so that a positive number indicates benefit (lower PSQI scores), compared to control
Testing model assumptions
Assessment of model residuals indicated a fairly typical pattern of residuals for the PSQI global score, and for two factors (sleep quality and sleep disturbance). Assessment of residuals and raw distribution of the sleep efficiency scores failed to yield a normal pattern of residuals, with the distribution indicating that there was a large number of zero scores for certain questionnaire items. Both Tobit and Hurdle longitudinal models36 were run on the sleep efficiency measure and yielded results similar to the naïve mixed-effects model. Assumptions were also assessed for testing appropriateness of the mixed-effects approach versus a fixed approach using the Breusch-Pagan test for heteroskedasticity37 and the Hausman test of random effects consistency.38 In all cases, the results were consistent. No statistical approach indicated a significant effect of the interventions on sleep efficiency. The simpler and more interpretable raw data mixed-effects model results were thus used for this analysis; model parameters are shown in Table 3.
DISCUSSION
The findings from this randomized trial have significant implications for the field of sleep research, and for public health. Several large observational studies have found that short sleep duration is associated with the higher morbidity and mortality in the general population,39 as well as among people with diabetes, hypertension, and a cardiovascular disease.40 While the preponderance of evidence is focused on sleep duration, sleep disturbance is also important. For example, in a 19-year prospective cohort study among 16,989 participants, sleep disturbance predicted incidence of diabetes and hypertension, suicidality and all-cause mortality among men.41 While the mediating pathways are not understood with confidence, increased subclinical inflammatory activity has been implicated as one mechanism of action.42 Causal pathways are usually bidirectional, as poor sleep can worsen mental and physical health, and adverse health conditions negatively impact sleep quality.43,44
A growing body of evidence has documented that both exercise9 and meditation10–14 improve sleep quality among those with clinically significant insomnia as well as mild-to-moderate sleep problems. The results from our high quality RCT are consistent with the extant research and add at least two new findings. Perhaps most importantly, the data from the MEPARI-2 trial show that sleep quality can be improved in a community-recruited sample of healthy adults who did not have severe sleep problems when enrolled, and who reported only mild-to-moderate levels of sleep disturbance on the PSQI, similar to the general population.
Indeed, the statistical model demonstrating improvement in sleep quality was as robust in the total sample as when the analyses were limited to the 45.9% of our sample who had PSQI scores above 5 points at baseline (i.e., a level to be considered clinically significant).
A second conclusion is that EX may have slightly greater effectiveness than MBSR in terms of improving perceived sleep quality overall, and possibly some added benefit for better perceived sleep quality. However, MBSR appears to be more effective for reducing the disturbance in daily functioning that results from poor sleep.
We consider these findings and conclusions to be tentative because: A) our clinical trial was initially designed to determine if there was an effect on acute respiratory infections (sleep quality was not the primary outcome), and B) the magnitude of the effect size was relatively small for all sleep factors that were assessed. Nevertheless, the apparent differences in impact on the PSQI factors may point towards different ways that meditation versus exercise can improve sleep. This finding should guide future research, and has implications on the development and implementation of meditative and exercise behavioral health programs. We are now particularly interested in knowing whether the combination of exercise and meditation would have greater impact than either intervention alone.
Limitations
These data derived from a RCT that assessed the impact of MBSR and EX training on the incidence, duration, and severity of acute respiratory infections.17 We had not precisely defined hypotheses about sleep quality a priori, did not pre-specify tolerances for type 1 and 2 error, nor list criteria for hypothesis rejection or acceptance. While this randomized trial was reasonably large, and the mixed-effects linear models controlled for status at baseline, it is remotely possible that small differences at baseline contributed to results that emerged after the interventions. When designing the trial, improvements in sleep quality were considered to be secondary outcome, and a possible pathway that might contribute to immunomodulation and the prevention of bacterial and viral infections. Therefore, this paper presents a post hoc analysis, which should be considered exploratory. However, these data did provide a unique opportunity to discern whether there are specific aspects of sleep quality that are differentially improved by either increased EX or meditative practice. While the evidence presented here demonstrates statistically significant benefits for both EX and MBSR training, the extent of the clinical significance is not known. It is not clear that the degree of benefit is large enough to guide policy and lead to a change in clinical practice. To our knowledge, there has not been enough rigorous research on the PSQI or its factors to determine the “minimal important difference”45 or “sufficiently important difference” (i.e., “smallest worthwhile effect”).46 Thus, we cannot say with confidence whether the degree of improvement observed in this study should be interpreted as important or worthwhile to patients, clinicians, or policy-makers.
SUMMARY and CONCLUSIONS
Sleep quality is important for mental and physical health. Meditation and exercise can improve sleep quality for people with insomnia or other sleep problems, but the benefits for normal sleepers are not known. This RCT compared people receiving training in EX or MBSR to controls. Sleep quality was assessed at baseline and 4 more times during 7 months of follow-up. Both MBSR and EX improved sleep scores, with EX having larger effects on perceived sleep quality, and MBSR having more benefit for reducing adverse impact of poor sleep on daily life. This trial is the first to document improved sleep quality after meditation and exercise training in people without known major sleep problems.
The results from this moderately large, high quality randomized trial suggest that 8-week training programs in exercise or mindfulness meditation can lead to sustained and statistically significant improvements in sleep quality. Exercise may have greater effects on perceived sleep quality. Mindfulness may be more effective in ameliorating how poor sleep and the resulting fatigue compromise functioning and the response to daily events and disturbances.
ACKNOWLEDGMENTS
The MEPARI-2 trial was funded by the National Center for Complementary and Integrative Health (NCCIH) at the U.S. National Institutes of Health (NIH; R01AT006970). This research also received support from the University of Wisconsin (UW) Clinical and Translational Science Award from NIH (CTSA; UL1TR000427). Bruce Barrett was supported by a mid-career research and mentoring grant from NCCIH (K24AT006543). Christine Harden was supported by a NCCIH research training fellowship (5T32AT006956–04). We gratefully acknowledge Jodi Barnet, who assisted with early versions of data analysis, Mary Checovich who assisted with the formatting and submission of this manuscript, and the many contributions of the study participants and the MEPARI-2 research team.
Funded by: National Center for Complementary and Integrative Health (NCCIH) at the U.S. National Institutes of Health (R01AT006970)
LIST OF ABBREVIATIONS
- ANOVA
Analysis of Variance
- BP
Blood Pressure
- BMI
Body Mass Index
- EX
Exercise
- MBSR
Mindfulness Based Stress Reduction
- MEPARI-2
Mindfulness or Exercise for Prevention of Acute Respiratory Infection
- NCCIH
National Center for Complementary and Integrative Health
- PHQ9
Patient Health Questionnaire (depressive symptoms)
- PSQI
Pittsburgh Sleep Quality Index
- PSS10
Perceived Stress Scale
- SD
Standard Deviation
- SF12
Medical Outcomes Study Short Form (mental and physical general health)
Footnotes
Parent Trial: Meditation or Exercise to Prevent Acute Respiratory Infection-2 (MEPARI-2)
Data archived at: https://www.openicpsr.org/openicpsr/project/103581/version/V2/view
DISCLOSURE STATEMENT
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or significant non-financial interest in the subject matter or materials discussed in this manuscript.
References
- 1.Morin CM, Belanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447–453. [DOI] [PubMed] [Google Scholar]
- 2.Mai E, Buysse DJ. Insomnia: Prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3(2):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung KF, Yeung WF, Ho FY, Yung KP, Yu YM, Kwok CW. Cross-cultural and comparative epidemiology of insomnia: the Diagnostic and statistical manual (DSM), International classification of diseases (ICD) and International classification of sleep disorders (ICSD). Sleep Med. 2015;16(4):477–482. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 6.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415–1419. [PubMed] [Google Scholar]
- 7.Lowe H, Haddock G, Mulligan LD, et al. Does exercise improve sleep for adults with insomnia? A systematic review with quality appraisal. Clin Psychol Rev. 2019;68:1–12. [DOI] [PubMed] [Google Scholar]
- 8.Banno M, Harada Y, Taniguchi M, et al. Exercise can improve sleep quality: a systematic review and meta-analysis. PeerJ. 2018;6:e5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38(3):427–449. [DOI] [PubMed] [Google Scholar]
- 10.Adler E, Dhruva A, Moran PJ, et al. Impact of a mindfulness-based weight-loss intervention on sleep quality among adults with obesity: Data from the SHINE randomized controlled trial. J Altern Complement Med. 2017;23(3):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blake M, Schwartz O, Waloszek JM, et al. The SENSE study: Treatment mechanisms of a cognitive behavioral and mindfulness-based group sleep improvement intervention for at-risk adolescents. Sleep. 2017;40(6). [DOI] [PubMed] [Google Scholar]
- 12.Black DS, O’Reilly GA, Olmstead R, Breen EC, Irwin MR. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA Intern Med. 2015;175(4):494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong JC, Manber R, Segal Z, Xia Y, Shapiro S, Wyatt JK. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep. 2014;37(9):1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuendorf R, Wahbeh H, Chamine I, Yu J, Hutchison K, Oken BS. The effects of mind-body interventions on sleep quality: A systematic review. Evid Based Complement Alternat Med. 2015;2015:902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong SY, Zhang DX, Li CC, et al. Comparing the effects of mindfulness-based cognitive therapy and sleep psycho-education with exercise on chronic insomnia: A randomised controlled trial. Psychother Psychosom. 2017;86(4):241–253. [DOI] [PubMed] [Google Scholar]
- 16.Innes KE, Selfe TK, Khalsa DS, Kandati S. Effects of meditation versus music listening on perceived stress, mood, sleep, and quality of life in adults with early memory loss: A pilot randomized controlled trial. J Alzheimers Dis. 2016;52(4):1277–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett B, Hayney MS, Muller D, et al. Meditation or exercise for preventing acute respiratory infection (MEPARI-2): A randomized controlled trial. PLoS One. 2018;13(6):e0197778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein E, Topitzes J, Brown RL, Barrett B. Mediational pathways of meditation and exercise on mental health and perceived stress: A randomized controlled trial. J Health Psychol. 2018:1359105318772608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer JD, Torres ER, Grabow ML, et al. Benefits of 8-wk mindfulness-based stress reduction or aerobic training on seasonal declines in physical activity. Med Sci Sports Exerc. 2018;50(9):1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett B, Torres ER, Meyer J, Barnet JH, Brown RJM. Predictors of mindfulness meditation and exercise practice, from MEPARI-2, a randomized controlled trial. Mindfulness. 2019;March 2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett B, Hayney MS, Muller D, et al. Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Ann Fam Med. 2012;10(4):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabat-Zinn J. Mindfulness-based interventions in context: Past, present, future. Clin Psychol Sci Prac. 2003;10:144–156. [Google Scholar]
- 25.MBSR. Mindfulness Based Stress Reduction. https://www.umassmed.edu/cfm/mindfulness-based-programs/mbsr-courses/Web site. Published 2018. Updated 2018. Accessed.
- 26.Borg GV, Linderholm H. Perceived exertion and pulse rate during graded exercise in various age groups. Acta Med Scand. 1967;472(suppl):194–206. [Google Scholar]
- 27.Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29(1):112–116. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. User’s Manual for the SF-12v2 Health Survey. Boston: QualityMetric; 2008. [Google Scholar]
- 30.Cohen S, Janicki-Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006 and 2009. J Appl Psychol. 2012;42:1320–1334. [Google Scholar]
- 31.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. The Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83(404):1198–1202. [Google Scholar]
- 34.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsiao C. Analysis of Panel Data. Third ed. New York: Cambridge University Press; 2014. [Google Scholar]
- 36.Eggers J. On statistical methods for zero-inflated models. Project report 2015:9. Uppsala, Sweden: University of Uppsala Department of Mathematics; 2015. [Google Scholar]
- 37.Breusch TS, Pagan AR. A simple test for heteroscedasticity and random coefficient variation. Econometrica. 1979;47(5):1287–1294. [Google Scholar]
- 38.Hausman JA. Specification tests in econometrics. Econometrica. 1978;46(6):1251–1271. [Google Scholar]
- 39.da Silva AA, de Mello RG, Schaan CW, Fuchs FD, Redline S, Fuchs SC. Sleep duration and mortality in the elderly: a systematic review with meta-analysis. Bmj Open. 2016;6(2):e008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. [DOI] [PubMed] [Google Scholar]
- 41.Rod NH, Vahtera J, Westerlund H, et al. Sleep disturbances and cause-specific mortality: Results from the GAZEL cohort study. Am J Epidemiol. 2011;173(3):300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolezal BA, Neufeld EV, Boland DM, Martin JL, Cooper CB. Interrelationship between sleep and exercise: A systematic review. Adv Prev Med. 2017;2017:1364387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pettee GK, Sternfeld B, Shiroma EJ, Perez A, Cheung J, Lee IM. Bidirectional associations of accelerometer-determined sedentary behavior and physical activity with reported time in bed: Women’s Health Study. Sleep Health. 2017;3(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR, Group CSCM. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371–383. [DOI] [PubMed] [Google Scholar]
- 46.Barrett B. Sufficiently important difference: Concepts, caveats, and challenges. Med Decis Making. 2013;33(6):869–874. [DOI] [PubMed] [Google Scholar]