ABSTRACT
During the last decade, inhibitors targeting immune checkpoint programmed death ligand 1/PD-1 and cytotoxic T-lymphocyte-associated protein 4 have been one of the most significant advances for cancer therapy in clinic. However, most of these therapies focused on stimulating the adaptive immune system-mediated elimination of tumor. Recent studies indicated that CD47/Signal-regulatory protein alpha (SIRPα), an innate anti-phagocytic axis between cancer cells and macrophages, could be a promising therapeutic target. Here, we review the current knowledge about developing CD47/SIRPα checkpoint inhibitors, avoiding potential side effect and designing optimal combination therapies, and highlight the key points for future clinical applications of CD47/SIRPα axis-targeted tumor immunotherapy.
Keywords: CD47/SIRPα axis, immune checkpoint inhibitors, adaptive immunity, immunotherapy, combination immunotherapy
Statement of Significance
Tumor immunotherapy targeting CD47/SIRPα axis has been one hotspot in cancer therapy. Here, we summarize the preclinical evidence and emerging data from clinical trials to support the development of CD47/SIRPα inhibitors, designing combination therapies and further application of CD47/SIRPα-based immunotherapy.
RISE OF IMMUNE CHECKPOINT INHIBITORS
In the last decade, cancer immunotherapy has rapidly translated into clinical strategy, yielding a new era of cancer therapy, alongside surgery, radiation and chemotherapy [1–3]. Inhibitors targeting immune checkpoint programmed death ligand 1 (PD-L1) and its receptor PD-1 and cytotoxic T-lymphocyte-associated protein 4, such as nivolumab, pembrolizumab and ipilimumab, are now moving from second-line treatment to first-line therapy of a broad range of malignancies [4–6]. These monoclonal antibodies work on the general premise that functions of immune cells are suppressed in tumor microenvironment and relief of these suppression stimulates anti-tumor immune response [7]. To date, most of the therapies are focused on stimulating the adaptive immunity, in particular T cells, to eliminate cancer cells. However, recent studies indicated that not only T cells associated with adaptive immunity but also innate immune responses mediated by myeloid cells, for example, macrophages are endued with specialized function to clear solid and hematopoietic cancers.
CD47/SIRPα, AN IMMUNE CHECKPOINT FORINNATE IMMUNE SYSTEM
Among cells of the myeloid lineage, macrophage has prominent potentials as the mediator of anti-cancer therapeutics based on its robust phagocytosis ability [8,9]. CD47, known as an integrin-associated protein, was first identified as a transmembrane protein from red blood cells (RBC) [10]. Signal-regulatory protein alpha (SIRPα), a transmembrane protein on macrophage, is the main receptor of CD47 [11]. CD47 binding to SIRPα triggers the coupling of SIRPα to these phosphatases, thereby delivering the ‘don’t eat me’ signals to macrophage then preventing their activation [8,12,13]. CD47 expression is regarded as a self-protective mechanism of normal cells, including transfused RBC, lymphocytes and platelets, to resist the elimination of macrophage phagocytosis [14]. It is well documented that kinetic hematopoietic stem cells (HSC) protect themselves from macrophage phagocytosis by upregulating the expression of CD47 as they pass across sinusoids then they decrease CD47 expression after relocating to the marrow [15]. In addition, the level of CD47 expression predicts the probability whether HSC could be phagocytized by macrophage while circulating [16]. While, the absence of CD47–SIRPα interaction could activate pro-phagocytic receptors to trigger macrophage phagocytosis of RBC [17].
In the process of carcinogenesis, overexpression of CD47 has been identified across most tumors (Fig. 1). In hematological cancer, CD47 expression on acute myeloid/lymphoblastic leukemia, non-Hodgkin’s lymphoma cells and bone marrow of multiple myeloma samples was detected as several folds increase compared with normal tissues and CD47 level was predictive of the overall survival to primary treatment [16,18]. Furthermore, studies also found that solid tumors, including breast, ovarian, bladder, colon, glioblastoma, prostate tumor and hepatocellular carcinoma, expressed about three- to five-fold more CD47 than the corresponding normal tissues [19]. When patients were assigned into ‘CD47 low’ and ‘CD47 high’ groups based on a univariate analysis, high CD47 mRNA expression level was shown to be associated with the decreased progression-free survival and overall survival [19]. Therefore, those evidences indicated that CD47/SIRPα axis was exploited by malignant cells to transmit ‘don’t eat me’ signal to evade macrophage surveillance and phagocytosis, which highlighted that blocking CD47/SIRPα axis could be used to promote the ability of macrophages to phagocytose and eliminate tumor cells.
Figure 1.
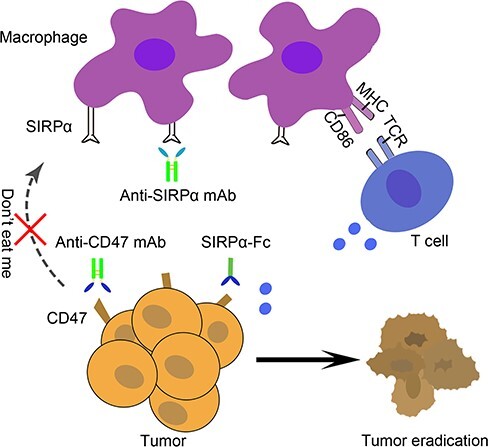
Targeting CD47/SIRPα axis for the therapy of cancer. Anti-CD47 mAb, Anti-SIRPα mAb and SIRPα-Fc fusion protein enhance tumor phagocytosis by macrophages, enabling the presentation of tumor antigens to T cells. TCR, T cell receptor. MHC, major histocompatibility complex.
CURRENT STATUS ABOUT CD47/SIRPα-TARGETED TUMOR IMMUNOTHERAPY
Similar to other immune checkpoint molecules, there are also two targets to choose from when disrupting the CD47/SIRPα axis. Different inhibitors targeting CD47/SIRPα axis have been generated to investigate their therapeutic effects on a variety of cancer types (Table 1). According to their design concepts, these agents fall into the following three main categories: anti-CD47 monoclonal antibody (mAb), anti-SIRPα mAb and SIRPα-Fc fusion protein (Fig. 1).
Table 1.
A summary of CD47/SIRPα-targeting immune checkpoint inhibitors under clinical development
| Agent | Description | Identifier | Strategy | Phase | Condition |
| Hu5F9-G4 | Humanized anti-CD47 antibody, human IgG4 subclass | NCT02216409 | Single agent | Phase I | Solid tumor [29,48] |
| NCT02678338 | Single agent | Phase I | Acute myeloid leukemia, Myelodysplastic syndrome [29,48] | ||
| NCT02953509 | Single agent; combination with rituximab | Phase I/II | Lymphosarcoma, Non-Hodgkin lymphoma, Diffuse large B-cell lymphoma | ||
| NCT02953782 | Single agent, combination with cetuximab | Phase I/II | Colorectal neoplasms, Solid tumors | ||
| NCT03248479 | Single agent; combination with azacitidine |
Phase I | Acute myeloid leukemia, Myelodysplastic syndromes | ||
| NCT03558139 | Single agent; combination with avelumab | Phase I | Ovarian cancer | ||
| CC-90002 | Humanized CD47-blocking antibody, human IgG4 subclass | NCT02367196 | Single agent; combination with rituximab | Phase I | Hematologic neoplasms [48] |
| NCT02641002 | Single agent | Phase I | Acute myeloid leukemia, High-risk myelodysplastic syndrome | ||
| TTI-621 | SIRPα-Fc fusion protein, human IgG1 subclass | NCT02663518 | Single agent; combination with rituximab; combination with nivolumab | Phase I | Hematologic malignancies, Solid tumor [34,35] |
| NCT02890368 | Single agent; combination with PD-1/PD-L1 inhibitor; combination with pegylated interferon-α2a | Phase I | Solid tumors [34] | ||
| ALX148 | High-affinity SIRPα variant, inactive Fc domain | NCT03013218 | Single agent; combination with atezolizumab; combination with trastuzumab; combination with rituximab |
Phase I | Advanced solid tumor, Non-Hodgkin lymphoma |
| SRF231 | High-affinity anti-CD47 antibody | NCT03512340 | Single agent | Phase I | Advanced solid cancers, Hematologic cancers |
| TTI-622 | SIRPα-Fc fusion protein, human IgG4 subclass | NCT03530683 | Single agent; combination with rituximab; combination with PD-1/PD-L1 inhibitor; combination with proteasome-inhibitor regimen | Phase I | Lymphoma, Myeloma |
Anti-CD47 antibodies
Preclinical studies employed the well-known anti-CD47 monoclonal antibodies, such as B6H12, Bric126 and Hu5F9-G4, to block CD47–SIRPα interactions, and these antibodies have shown to effectively facilitate the destruction of extensive solid and hematopoietic tumors [20–25]. There are three main mechanisms underlying the potent anti-tumor effect of these anti-CD47 antibodies. First, anti-CD47 antibodies targeting CD47-SIRPα could eliminate the ‘don’t eat me’ signaling and activate phagocytosis of malignant cells by macrophage, which was the primary task for the development of CD47/ SIRPα inhibitors. Second, using of a complete antibody of IgG1 type to target CD47 not only disrupt CD47/SIRPα axis, but also simultaneously induce antibody-dependent cellular cytotoxicity (ADCC) against cancer cells, thereby creating a dual signal to obliterate cancer cells [26–28]. Using no opsonizing F(ab′)2 fragment, the opsonization of B6H12, an intact antibody, was demonstrated to be essential for at least anti-tumor effect against both solid and hematological cancer cells in vitro [28]. Efforts have been devoted to minimize FcγR-mediated ADCC of anti-CD47 antibody, which might cause obvious side effects of the blood system. Hu5F9-G4, a novel CD47-blocking antibody with IgG4 Fc fragment, has been generated [29]. This specific design significantly reduced the anemia and improved the biosafety of anti-CD47 antibody. To determine the role of Fc function, EC Pietsch and colleagues generated anti-CD47 antibodies based on the Fc fragment of IgG1 and the effector function silent IgG2σ. The data showed that the therapeutic effect of anti-CD47 antibody was dependent on Fc effector function [30]. Third, adaptive T cell immunity is significantly activated in tumor-bearing mice after CD47 antibody-based immunotherapy. While xenograft models are widely employed for preclinical testing of antibodies targeting CD47/SIRPα, there are also some limitations. These mice lack T cells and therefore have oversimplified immune systems [9]. Using syngeneic immunocompetent models of B cell lymphoma and colon carcinoma, the therapeutic effects of MIAP301, an anti-mouse-CD47 antibody, depended on dendritic cell cross-priming of T cell responses and the effects could be abrogated in T cell-deficient mice. Underlying mechanism investigation showed that cytosolic sensing of DNA from the targeted cancer cells was increased by anti-CD47 antibody, bridging the innate and adaptive immunity [31]. In view of the excellent preclinical anti-tumor effects, therapeutics based on anti-CD47 antibodies, such as Hu5F9-G4, CC-90002 and SRF231, are now in clinical trials for solid and hematologic malignancies (Table 1). However, given the ubiquitous expression of CD47 on RBC and platelets, the general toxicity of anti-CD47 therapies, such as anemia and reduced platelets, should cause some concerns.
Anti-SIRPα antibodies
As the receptor of CD47, SIRPα has also been targeted to block the CD47–SIRPα interaction. KWAR23, an anti-SIRPα mAb, was generated and proved to increase macrophage-mediated phagocytosis of patient-derived tumor cells. While, administration of KWAR23 alone did not show obvious anti-tumor effects in lymphoma xenograft models. But when in combination with rituximab, a tumor-opsonizing antibody, KWAR23 augmented myeloid cell-mediated elimination of tumor cells in vitro and in vivo, indicating that anti-SIRPα antibody could be a promising agent for combination therapy [32]. High expression of SIRPα was observed in melanoma and renal cell carcinoma for the first time, and MY-1, an anti-SIRPα antibody of IgG2a type was generated and found to suppress the tumor formation. MY-1 showed potent effect via two mechanisms: induction of antibody-mediated macrophage phagocytosis and disruption of CD47-SIRPα axis [33]. Effi-DEM (OSE-172), an anti-SIRPα mAb, targeting tumor-associated macrophage and myeloid-derived suppressor cell, could modify the tumor microenvironment and facilitate cytotoxic immune cells infiltration. CD47 is ubiquitously expressed on cell surface, especially RBC and platelets. Some agents targeting CD47 have recently shown blood toxicity such as anemia or reduced platelets. Researches have showed that anti-SIRPα antibodies do not bind to RBC or platelets, which might be a hematological safety advantage compared with anti-CD47 antibody. These novel finding showed that anti-SIRPα antibodies could also be potential regents for malignant cancer immunotherapy.
SIRPα-Fc fusion proteins
Recently, several effective decoy receptors were engineered to target CD47: TT1-621, TT1-622, ALX148 and IMM01. TT1-621, a fusion protein consisting of IgG1 Fc fragment and the extracellular domain of SIRPα, enhanced macrophage phagocytosis against a broad spectrum of solid and hematologic tumor cells in vitro, and elicited potent effects in leukemia and lymphoma xenograft models. Importantly, different from anti-CD47 antibody, TT1-621 binds minimally to erythrocytes. Data from the clinical trial of TT1-621 (NCT02663518) showed that repeat dosing of TT1-621 overcame the ‘antigen sink’ (elimination of antibody that binding to membrane antigen is faster at low dose due to the fact that the unbound target will serve as a sink to ‘sop up’ antibody) and maintained acceptable levels of platelet in clinic [34,35]. TTI-622, Trillium’s second SIRPα-Fc fusion protein, has an IgG4 Fc region instead of the IgG1 Fc fragment and thus TTI-622 delivers a modest ‘eat me’ signal to macrophage than TTI-621. To minimize the potential hematologic toxicity, ALX148, a fusion protein containing high affinity CD47-binding domains of SIRPα and an inactive Fc domain, was generated. The preclinical data of ALX148 showed that it safely activated multiple immune cell types against non-Hodgkin lymphoma and advanced solid tumors. Another fusion protein for this came from our team that IMM01 consisting of IgG1 Fc fragment and the first domain extracellular domain of SIRPα was generated to target CD47 and triggered macrophage-mediated phagocytosis against non-small cell lung cancer and glioblastoma cells, eliciting significant efficacy in xenograft models. Using T-cell depleting antibody, further experiment demonstrated that adaptive immune response, especially CD8+ T cells, played a critical role in murine IMM01-triggered tumor rejection [36,37]. Thus, it appeared that blocking CD47 by fusion protein as a trap for CD47 in immunocompetent models has a beneficial effect and more work was still needed to confirm this observation in other types of tumors.
RATIONAL COMBINATION IMMUNOTHERAPY:TARGETING CD47/SIRPα AND AUTOPHAGY
Although increasing evidence showed that anti-CD47 therapies have potent anti-tumor effect in several hematologic and solid malignancies, additional investigation to increase the anti-tumor efficacy is ongoing [21]. Autophagy, a crucial player in microenvironment maintenance for tumor cells, could be activated by some condition, including nutrient deprivation and drug administration [38–41]. The first evidence is from our own studies in which we detected autophagosome formation and accumulation, autophagosome fusion with lysosome and autophagosome degradation in lysosome, the three main stages of complete autophagy, in the CD47-targeted cancer cells. Results showed that targeting CD47 by SIRPαD1-Fc, a CD47-blocking fusion protein, activated autophagy and complete autophagic flux in glioblastoma and non-small cell lung cancer cells [36,37]. Importantly, inhibition of autophagy by inhibitors or knockdown of autophagy-related genes significantly increased CD47 blockade-induced macrophage phagocytosis of non-small cell lung cancer and glioblastoma cells and potentiated the in vivo anti-tumor effects of CD47/SIRPα blockade. These data revealed that autophagy played a cyto-protective role in CD47-targeting tumor immunotherapy and highlighted the synergistic anti-tumor effects of blocking CD47 and autophagy, providing novel approach to further enhance the anti-tumor effect of CD47-SIRPα checkpoint inhibitors.
BISPECIFIC IMMUNOTHERAPY: TARGETINGCD47 AND CD20/CD19, TARGETING CD47 ANDMESOTHELIN AND TARGETING CD47 AND PD-L1
Based on the various evidence listed above, blocking CD47-SIRPα axis is certainly a very effective approach, but specificity in the response to the cancer cells was still a challenge. In order to find an effective solution, combining a CD47/SIRPα inhibitor with a tumor cell-specific opsonizing antibody was intensively studied. In non-Hodgkin lymphoma, anti-CD47 antibody (BRIC126 or B6H12) in combination with rituximab, the clinically used anti-CD20 antibody, resulted in synergetic elimination of human lymphoma cells in both disseminated and localized xenograft models. Subsequently, a bispecific antibody targeting CD20 and CD47 was engineered and the bispecific antibody has a relatively low affinity to CD47, decreasing its binding to normal cells that are expressing CD47, but maintaining the CD20 binding capacity at the same time. In comparison with the anti-CD47 antibody alone, the bispecific antibody reduced lymphoma burden and overcame the ‘antigen sink’ via selective binding to the lymphoma cells [42]. CD19, a transmembrane protein on B cells, has been proven to be a potential therapeutic target for the anti-CD20 resistant malignance. NI-1701, a novel bispecific antibody, was designed to co-engage CD47 and CD19, offering an alternative or adjunct therapeutic option to patients with B cell lymphoma and leukemia refractory/resistant to mAb therapy alone [43]. Meanwhile, NI-1801, another bispecific antibody based on human IgG molecular with bispecific antigen-binding regions, was generated by Novimmune (Geneva, Switzerland) to utilize the innate immunity to eliminate mesothelin-positive tumors. Due to the co-expression of CD47 and PD-L1 on some tumor cells, Yajun Guo and colleagues constructed IAB, a CD47 and PD-L1 bispecific fusion protein. The data of IAB indicated that dual-targeting CD47 and PD-L1 could induce synergistic therapeutic effect by activating innate and adaptive immunity simultaneously [44]. Combination therapy with multiple monoclonal antibodies has several advantages compared with monotherapy in non-Hodgkin lymphoma or other cancer. Primarily, treatment solely with antibody targeting cancer antigen will decrease off-target toxicity compared with the current approaches that are utilizing chemotherapy. Then, synergistic effect between two different antibody-mediated effector mechanisms could elicit potent therapeutic effect. Thirdly, antibodies that are targeting two cell-surface antigens would be more likely to clear tumor cells with epitope loss or pre-existing epitope variants, such as rituximab-resistant cancer patients [45–47]. Finally, a bispecific antibody with one arm binding to CD47 and the other arm binding to a validated target could retain the synergetic effect and reduce potential toxicity [18].
FUTURE CLINICAL APPLICATION AND CHALLENGE OF CD47-BASED TUMOR IMMUNOTHERAPY
Multiple researches published recently identified CD47/SIRPα axis as a promising immune checkpoint in tumors [48]. There are now a series of anti-CD47 antibodies and fusion proteins in clinical trials (NCT02216409, NCT02367196, NCT02678338, NCT02663518, NCT02641002, NCT02890368, NCT02953509, NCT02953782, NCT03013218, NCT03248479 and NCT03512340) and some other agents in preclinical investigation. These trials were aimed to detect the safety and effectiveness of these anti-CD47 antibodies or anti-CD47 fusion protein that consisted different IgG fragments such as IgG1 and IgG4. Hence, whether CD47-SIRPα disruption therapies are indeed safe and effective will be clearer in the coming years. However, there are some crucial issues that still need to be resolved. First, conceptual clarity is an important issue for the design of the most safe and effective CD47/SIRPα inhibitor and therapeutics. Second, given the ubiquitous expression of CD47 in normal tissues, there is some concern for off-target toxicity of anti-CD47 therapies. Third, what are the optimal combinations for cancer treatment by combinational use of checkpoint inhibitors and other therapeutic approaches? It could be expected that solving the above and related issues in the rapidly developing CD47-SIRPα blockade will have a major impact in cancer therapy.
ACKNOWLEDGEMENT
This work was supported by the National Key Basic Research Program of China [No. 2015CB931800], the National Natural Science Foundation of China [Nos 81573332 and 81773620] and Shanghai Science and Technology Funds [No. 18431902800].
Conflicts of Interest Statement. None declared.
REFERENCES
- 1. Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013; 342: 1432–3. [DOI] [PubMed] [Google Scholar]
- 2. Sharpe, AH. Introduction to checkpoint inhibitors and cancer immunotherapy. Immunol Rev 2017; 276: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma, P, Allison, JP. The future of immune checkpoint therapy. Science 2015; 348: 56–61. [DOI] [PubMed] [Google Scholar]
- 4. Hodi, FS, O’Day, SJ, McDermott, DFet al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garon, EB, Rizvi, NA, Hui, Ret al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 6. Borghaei, H, Paz-Ares, L, Horn, Let al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topalian, SL, Taube, JM, Anders, RAet al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16: 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiskopf, K. Cancer immunotherapy targeting the CD47/SIRPalpha axis. Eur J Cancer 2017; 76: 100–9. [DOI] [PubMed] [Google Scholar]
- 9. Matlung, HL, Szilagyi, K, Barclay, NAet al. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev 2017; 276: 145–64. [DOI] [PubMed] [Google Scholar]
- 10. Mawby, WJ, Holmes, CH, Anstee, DJet al. Isolation and characterization of CD47 glycoprotein: a multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem J 1994; 304: 525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barclay, AN, Van den Berg, TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 2014; 32: 25–50. [DOI] [PubMed] [Google Scholar]
- 12. Matozaki, T, Murata, Y, Okazawa, Het al. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol 2009; 19: 72–80. [DOI] [PubMed] [Google Scholar]
- 13. Alvey, C, Discher, DE. Engineering macrophages to eat cancer: from ‘marker of self’ CD47 and phagocytosis to differentiation. J Leukoc Biol 2017; 102: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsai, RK, Discher, DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol 2008; 180: 989–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang, C, Wang, H, Ide, Ket al. Human CD47 expression permits survival of porcine cells in immunodeficient mice that express SIRPα capable of binding to human CD47. Cell Transplant 2011; 20: 1915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaiswal, S, Jamieson, CH, Pang, WWet al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009; 138: 271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oldenborg, PA, Zheleznyak, A, Fang, YFet al. Role of CD47 as a marker of self on red blood cells. Science 2000; 288: 2051–4. [DOI] [PubMed] [Google Scholar]
- 18. Majeti, R, Chao, MP, Alizadeh, AAet al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138: 286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willingham, SB, Volkmer, JP, Gentles, AJet al. The CD47–signal regulatory protein alpha (SIRPα) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA 2012; 109: 6662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao, XW, vanBeek, EM, Schornagel, Ket al. CD47–signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci USA 2011; 108: 18342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chao, MP, Alizadeh, AA, Tang, Cet al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010; 142: 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim, D, Wang, J, Willingham, SBet al. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012; 26: 2538–45. [DOI] [PubMed] [Google Scholar]
- 23. Chao, MP, Tang, C, Pachynski, RKet al. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 2011; 118: 4890–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goto, H, Kojima, Y, Matsuda, Ket al. Efficacy of anti-CD47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur J Cancer 2014; 50: 1836–46. [DOI] [PubMed] [Google Scholar]
- 25. Edris, B, Weiskopf, K, Volkmer, AKet al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci USA 2012; 109: 6656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao, XW, Matlung, HL, Kuijpers, TWet al. On the mechanism of CD47 targeting in cancer. Proc Natl Acad Sci USA 2012; 109: E2843; author reply: E2844–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soto-Pantoja, DR, Miller, TW, Frazier, WAet al. Inhibitory signaling through signal regulatory protein-alpha is not sufficient to explain the antitumor activities of CD47 antibodies. Proc Natl Acad Sci USA 2012; 109: E2842; author reply: E2844–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao, XW, Kuijpers, TW, van denBerg, TK. Is targeting of CD47-SIRPα enough for treating hematopoietic malignancy? Blood 2012; 119: 4333–4; author reply 4334–5. [DOI] [PubMed] [Google Scholar]
- 29. Liu, J, Wang, L, Zhao, Fet al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One 2015; 10: e0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pietsch, EC, Dong, J, Cardoso, Ret al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J 2017; 7: e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu, X, Pu, Y, Cron, Ket al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 2015; 21: 1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ring, NG, Herndler-Brandstetter, D, Weiskopf, Ket al. Anti-SIRPalpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA 2017; 114: E10578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yanagita, T, Murata, Y, Tanaka, Det al. Anti-SIRPα antibodies as a potential new tool for cancer immunotherapy. JCI Insight 2017; 2: e89140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petrova, PS, Viller, NN, Wong, Met al. TTI-621 (SIRPαFc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res 2017; 23: 1068–79. [DOI] [PubMed] [Google Scholar]
- 35. Russ, A, Hua, AB, Montfort, WRet al. Blocking “don’t eat me” signal of CD47-SIRPα in hematological malignancies, an in-depth review. Blood Rev 2018; pii: S0268-960X(17)30093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang, X, Chen, W, Fan, Jet al. Disrupting CD47-SIRPα axis alone or combined with autophagy depletion for the therapy of glioblastoma. Carcinogenesis 2018; 39: 689–99. [DOI] [PubMed] [Google Scholar]
- 37. Zhang, X, Fan, J, Wang, Set al. targeting cd47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol Res 2017; 5: 363–75. [DOI] [PubMed] [Google Scholar]
- 38. Chen, Q, Ye, L, Fan, Jet al. Autophagy suppression potentiates the anti-glioblastoma effect of asparaginase in vitro and in vivo. Oncotarget 2017; 8: 91052–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shen, W, Zhang, X, Fu, Xet al. A novel and promising therapeutic approach for NSCLC: recombinant human arginase alone or combined with autophagy inhibitor. Cell Death Dis 2017; 8: e2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ji, Y, Li, L, Tao, Qet al. Deprivation of asparagine triggers cytoprotective autophagy in laryngeal squamous cell carcinoma. Appl Microbiol Biotechnol 2017; 101: 4951–61. [DOI] [PubMed] [Google Scholar]
- 41. Zhang, B, Fan, J, Zhang, Xet al. Targeting asparagine and autophagy for pulmonary adenocarcinoma therapy. Appl Microbiol Biotechnol 2016; 100: 9145–61. [DOI] [PubMed] [Google Scholar]
- 42. Piccione, EC, Juarez, S, Liu, Jet al. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs 2015; 7: 946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buatois, V, Johnson, Z, Salgado-Pires, Set al. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B cell lymphoma and leukemia. Mol Cancer Ther 2018; 17: 1739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu, B, Guo, H, Xu, Jet al. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses. MAbs 2018; 10: 315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Foran, JM, Norton, AJ, Micallef, INet al. Loss of CD20 expression following treatment with rituximab (chimaeric monoclonal anti-CD20): a retrospective cohort analysis. Br J Haematol 2001; 114: 881–3. [DOI] [PubMed] [Google Scholar]
- 46. Hiraga, J, Tomita, A, Sugimoto, Tet al. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood 2009; 113: 4885–93. [DOI] [PubMed] [Google Scholar]
- 47. Kennedy, AD, Beum, PV, Solga, MDet al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol 2004; 172: 3280–8. [DOI] [PubMed] [Google Scholar]
- 48. Vonderheide, RH. CD47 blockade as another immune checkpoint therapy for cancer. Nat Med 2015; 21: 1122–3. [DOI] [PubMed] [Google Scholar]


