Abstract
Background & objectives:
Gallbladder (GBC) is an aggressive form of cancer and most patients present with advanced unresectable disease due to lack of early signs and symptoms. This retrospective study was conducted to present the treatment outcomes with three lines of chemotherapies in a subset of patients with advanced, unresectable GBC with the primary objective to determine the response rates with nab-paclitaxel as the third-line chemotherapy after failure of the first-line gemcitabine and platinum and the second-line FOLFOX-4 (oxaliplatin, leucovorin and 5-FU) therapy. Another objective was to evaluate the toxicity, progression-free survival (PFS) and overall survival (OS).
Methods:
Treatment-naive patients with histologically proven inoperable GBC treated with gemcitabine/platinum, FOLFOX-4 and nab-paclitaxel as the first-, second- and third-line chemotherapy were included in this study. The dose of gemcitabine and cisplatin or carboplatin was 1 g/m2 on days 1 and 8 and 75 mg/m2 (or target AUC of 5) on day 1, in a 21-day cycle. FOLFOX-4 was administered every two weeks and nab-paclitaxel was administered as 125 mg/m2 on days 1, 8 and 15 in a 28-day cycle.
Results:
There were eight men and 13 women with a median age of 57 yr who received nab-paclitaxel therapy. The overall response rate of the first-, second- and third-line chemotherapy was 61.9, 57.1 and 52.4 per cent, respectively. The median PFS for the gemcitabine/platinum, FOLFOX-4 and nab-paclitaxel therapy was 5.5, 5.4 and 2.9 months, respectively. The median OS with three lines of therapies was 14.0 months. Common Terminology Criteria (CTC) grade 3 or 4 haematological toxicities were observed in 28.6, 38.1 and 23.8 per cent of patients on gemcitabine/platinum, FOLFOX-4 and nab-paclitaxel therapy, respectively.
Interpretation & conclusions:
Our study suggests the clinical benefit of nab-paclitaxel chemotherapy in prolonging OS in a selected subgroup of advanced, unresectable GBC patients after failure of the first-line gemcitabine and platinum and the second-line FOLFOX-4 therapy.
Keywords: FOLFOX-4, gallbladder cancer, nab-paclitaxel
Though gallbladder cancer (GBC) is one of the rare cancers with an annual incidence rate of 2.2 per 100,000 population, it is the sixth most common gastrointestinal cancer worldwide1. GBC is an aggressive disease, and majority of the patients have advanced unresectable disease at the time of presentation. This is due to the lack of early signs and symptoms, resulting in diagnosis either during the surgery or postoperatively2,3. Due to late presentation, patients with advanced GBC have a dismal prognosis with a five-year survival of less than five per cent4. Although surgical resection remains the treatment modality of choice, majority of the patients are left with no option other than the palliative chemotherapy5.
Gemcitabine in combination with different platinum compounds has shown an impressive response rate in GBC. A pooled analysis of 2810 patients across 104 clinical trials has suggested the superiority of gemcitabine and platinum combinations in improving the overall survival (OS) in GBC as compared to other regimens6. The ABC-02 (the advanced biliary cancer-02) trial7 from the UK and BT228 study from Japan have established gemcitabine and cisplatin as a standard of care in the management of advanced unresectable GBC. Two phase 2 trials on gemcitabine and carboplatin combination chemotherapy in GBC from India have reported a response rate ranging from 37 to 50.8 per cent and a median OS of 5.9-11 months9,10. Others have investigated the therapeutic role of FOLFOX-4 [oxaliplatin, leucovorin and 5-fluorouracil (5-FU)] as the second-line therapy after failure of gemcitabine and platinum combination in advanced GBC11,12. After progression on two lines of therapy including gemcitabine/platinum and FOLFOX-4, nab-paclitaxel has been investigated as a potential third-line therapy in metastatic GBC13.
This retrospective study was undertaken to evaluate three lines of therapy in patients with advanced unresectable GBC. The primary objective was to determine the response rates after nab-paclitaxel as the third-line chemotherapy in these patients after failure of the first-line gemcitabine and platinum combination chemotherapy and the second-line FOLFOX-4 therapy, and the secondary objective was to evaluate the toxicity, progression-free survival (PFS) and OS.
Material & Methods
In this retrospective study, patients with histologically proven inoperable GBCs treated with gemcitabine/platinum, FOLFOX-4 and nab-paclitaxel as the first-, second- and third-line chemotherapy at the department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India, between September 2012 and December 2015 were included. Patients were required to have a bi-dimensionally measurable disease with an age >18 years. Complete blood count and clinical assessment of non-haematological toxicities were carried out every week. Computed tomographic scan of the abdomen was done for response assessment at baseline, 3rd and 6th cycle and thereafter every six months or earlier as per the clinical judgement. The study protocol was approved by the Institutional Ethics Committee.
Treatment: As a first-line chemotherapy, patients received intravenous infusion of 1 g/m2 of gemcitabine on days 1 and 8 and 75 mg/m2 of cisplatin on day 1 of a 21-day treatment cycle. Cisplatin was administered after the gemcitabine dose and was preceded by pre-hydration and electrolyte supplementation. Patients who were not fit for therapy with cisplatin received carboplatin (target AUC of 5) on day one and were supported with granulocyte colony stimulating factor to reduce the myelosuppression and enhance the tolerability. After progression on the first-line chemotherapy, all the patients received FOLFOX-4 [oxaliplatin (85 mg/m2) as a 2 h infusion on day 1, leucovorin (200 mg/m2/day) as a 2 h infusion followed by bolus 5-FU (400 mg/m2/day) and 5-FU (600 mg/m2/day) as a 22 h infusion, repeated for two consecutive days every two weeks until disease progression] regimen as the second-line chemotherapy. After progression on the second-line chemotherapy, all the patients were considered for single-agent nab-paclitaxel as the third-line chemotherapy. Patients received 125 mg/m2 of nab-paclitaxel on days 1, 8 and 15 in a 28-day cycle via intravenous infusion until disease progression.
Efficacy and safety assessment: All patients who received at least one dose of nab-paclitaxel were included in the efficacy and safety assessment. Response assessment was performed using RECIST 1.1 (Response Evaluation Criteria In Solid Tumours 1.1) criteria14 and classified as: (i) complete response (CR) for disappearance of all target lesions; (ii) partial response (PR) for at least a 30 per cent decrease in the sum of the longest diameter (LD) of target lesions taking as reference the baseline sum LD; (iii) stable disease for neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease taking as reference the smallest sum LD since the treatment started; and (iv) progressive disease (PD) for at least a 20 per cent increase in the sum of the LD of target lesions along with an absolute increase of at least 5 mm taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions14. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v3.0), (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf). Survival was calculated from the start of chemotherapy until death or the last follow up.
Statistical analysis: The primary end point of this study was response rate. The width of the resultant confidence intervals (CIs) for parameters to be estimated was constructed with a significance level of 0.05, i.e. a 95 per cent CI. OS and PFS were analyzed with the use of Kaplan–Meier survival analysis, and estimates were provided with 95 per cent CIs. Statistical analysis was performed using SAS 8.02 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 217 patients received the gemcitabine/platinum-based first-line chemotherapy. Of these 70 patients received FOLFOX-4 as the second-line chemotherapy and 21 patients received the nab-paclitaxel as the third-line chemotherapy. This subgroup of 21 patients was included in the present study. There were eight men and 13 women with a median age of 57 yr (range 29-66 yr). Main baseline patient characteristics are given in Table I. All 21 patients had metastatic disease with a performance status of ≤2. The overall response rate of the first-line chemotherapy was 61.9 per cent. Of the 10 patients who received gemcitabine/cisplatin as the first-line chemotherapy, seven achieved a PR for a response rate of 70 per cent, whereas of the 11 patients who received gemcitabine/carboplatin, one patient achieved a CR and five achieved a PR for a response rate of 54.5 per cent. The median number of chemotherapy cycles administered was six (range 3-12), and the median PFS for the first-line gemcitabine/platinum therapy was 5.5 months (95% CI 3.7–7.4 months; Fig. 1). There was no significant difference (P=0.06) in the median PFS of gemcitabine/cisplatin group (7.4 months, 95% CI 1.6 months - upper limit not estimable) as compared to gemcitabine/carboplatin group (4.4 months, 95% CI 3.6-6.0 months). Common Terminology Criteria (CTC) grade 3 anaemia was seen in one (10%) and two (18.2%) patients in gemcitabine/cisplatin and gemcitabine/carboplatin groups, respectively. Grade 3 neutropaenia was observed in one (9.1%) patient of gemcitabine/carboplatin group, whereas grade 3 thrombocytopaenia was observed in one (10 and 9.1%) patient each of gemcitabine/cisplatin and gemcitabine/carboplatin group, respectively (Table II). None of the patients experienced grade 3 or 4 nausea and vomiting.
Table I.
Baseline characteristics of patient’s (n=21)
| Characteristic | n (%) |
|---|---|
| Gender | |
| Male | 8 (38.1) |
| Female | 13 (61.9) |
| Age (yr) | |
| Median | 57 |
| Range | 29-66 |
| Histopathology grade | |
| Well differentiated | 2 (9.5) |
| Moderately differentiated | 7 (33.3) |
| Poorly differentiated | 3 (14.3) |
| Unknown | 9 (42.9) |
Fig. 1.
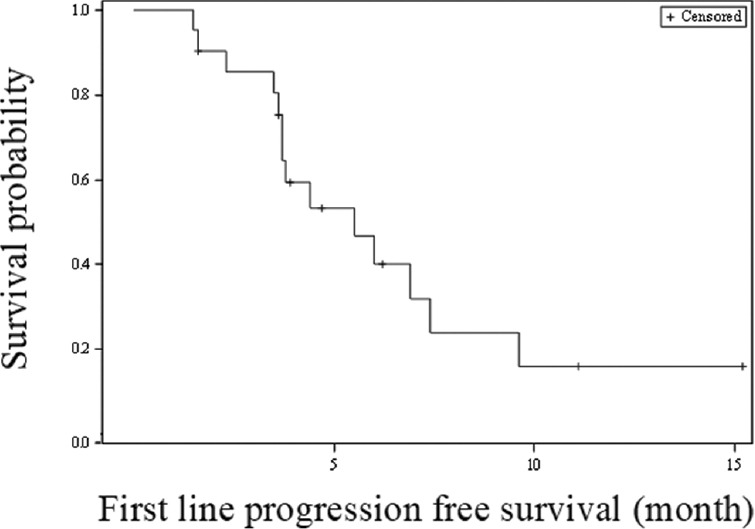
Kaplan-Meier survival analysis for progression-free survival (gemcitabine/platinum first-line therapy).
Table II.
Common Terminology Criteria Grade (CTC) toxicities
| Toxicity | Gemcitabine/platinum (n=21) | FOLFOX-4 (n=21) | Nab-paclitaxel (n=21) | |||
|---|---|---|---|---|---|---|
| Gem + Cis (n=10), n (%) | Gem + Carb (n=11), n (%) | Gem + Cis (n=10), n (%) | Gem + Carb (n=11), n (%) | Gem + Cis (n=10), n (%) | Gem + Carb (n=11), n (%) | |
| Anaemia | ||||||
| Grade 1 | 2 (20) | 1 (9.1) | 1 (10) | 3 (27.3) | 3 (30) | 2 (18.2) |
| Grade 2 | 4 (40) | 6 (54.5) | 8 (80) | 5 (45.5) | 5 (50) | 2 (18.2) |
| Grade 3 | 1 (10) | 2 (18.2) | 1 (10) | 1 (9.1) | 1 (10) | 4 (36.4) |
| Neutropaenia | ||||||
| Grade 1 | 4 (40) | 1 (9.1) | 2 (20) | 3 (27.3) | 1 (10) | 2 (18.2) |
| Grade 2 | - | - | 1 (10) | - | - | 2 (18.2) |
| Grade 3 | - | 1 (9.1) | 1 (10) | 1 (9.1) | - | - |
| Thrombocytopaenia | ||||||
| Grade 1 | - | 3 (27.3) | 3 (30) | 3 (27.3) | 1 (10) | 1 (9.1) |
| Grade 2 | - | 1 (9.1) | 3 (30) | - | - | 2 (18.2) |
| Grade 3 | 1 (10) | 1 (9.1) | - | 4 (36.4) | 1 (10) | - |
| Vomiting | ||||||
| Grade 1 | - | - | - | - | - | - |
| Grade 2 | 1 (10) | 2 (18.2) | 1 (10) | 4 (36.4) | 1 (10) | 2 (18.2) |
| Grade 3 | - | - | - | - | - | - |
| Abdominal pain | ||||||
| Grade 1 | - | 1 (9.1) | 2 (20) | 3 (27.3) | 3 (30) | 3 (27.3) |
| Fever | ||||||
| Grade 1 | - | 1 (9.1) | 4 (40) | 2 (18.2) | 3 (30) | 3 (27.3) |
| LFT deranged | ||||||
| Grade 1 | - | 1 (9.1) | 2 (20) | 3 (27.3) | 1 (10) | 2 (18.2) |
| Diarrhoea | ||||||
| Grade 1 | - | - | 2 (20) | 1 (9.1) | 1 (10) | - |
| Weakness | ||||||
| Grade 1 | - | - | - | 1 (9.1) | 3 (30) | 1 (9.1) |
| Peripheral neuropathy | ||||||
| Grade 1 | - | - | 1 (10) | 1 (9.1) | 2 (20) | 1 (9.1) |
| Grade 2 | - | - | 1 (10) | - | 2 (20) | - |
| Grade 3 | - | - | - | - | - | - |
-, represent no toxicity in a grade/group. LFT, liver function test; Cis, cisplatin; Carb, carboplatin
In the second-line chemotherapy with FOLFOX-4 regimen, seven (70%) patients in the gemcitabine/cisplatin group and five (45.5%) in the gemcitabine/carboplatin group achieved a PR for an overall response rate of 57.1 per cent. The median number of chemotherapy cycles administered was 12 (range 4-12) in the gemcitabine/cisplatin and six (range 3-12) in the gemcitabine/carboplatin group. The median PFS for the second-line FOLFOX-4 therapy was 5.4 months (95% CI 2.6-8.4 months; Fig. 2). CTC Grade 3 anaemia and neutropaenia were seen in one (10 and 9.1%) patient each of gemcitabine/cisplatin and gemcitabine/carboplatin group, respectively. Grade 3 thrombocytopaenia was observed in four (36.4%) patients of gemcitabine/carboplatin group (Table II). None of the patients experienced grade 3 or 4 nausea and vomiting.
Fig. 2.
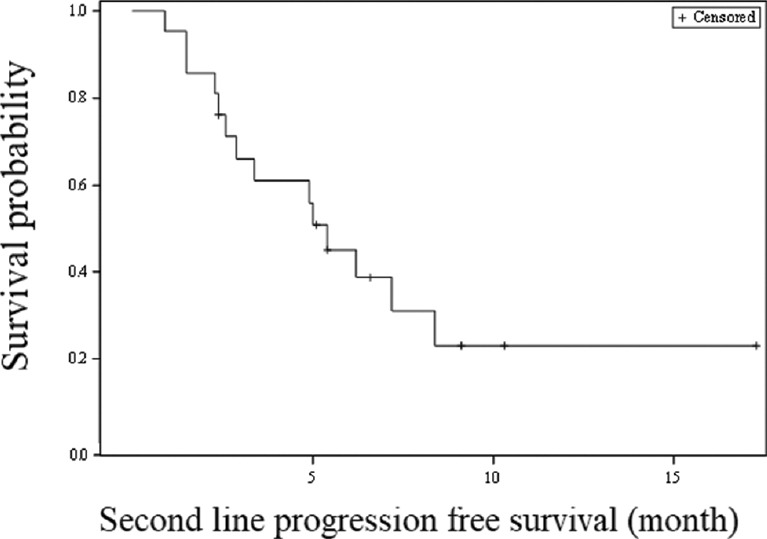
Kaplan-Meier survival analysis for progression-free survival (second-line FOLFOX-4 therapy).
In the third-line chemotherapy with nab-paclitaxel regimen, three (30%) patients in the gemcitabine/cisplatin group and four (36.4%) patients in the gemcitabine/carboplatin group achieved a PR for an overall response rate of 52.4 per cent. The median number of chemotherapy cycles administered was three (range 1-6) in the gemcitabine/cisplatin group and three (range 1-15) in the gemcitabine/carboplatin group. The overall median PFS for the third line nab-paclitaxel therapy was 2.9 months (95% CI 2.0-7.8 months; Fig. 3). CTC grade 3 anaemia was seen in one (10%) patient in the gemcitabine/cisplatin and 4 (36.4%) patients in the gemcitabine/carboplatin group, respectively. Grade 3 thrombocytopaenia was observed in one (10%) patient of the gemcitabine/cisplatin group Table II. None of the patients experienced Grade 3 or 4 nausea and vomiting.
Fig. 3.
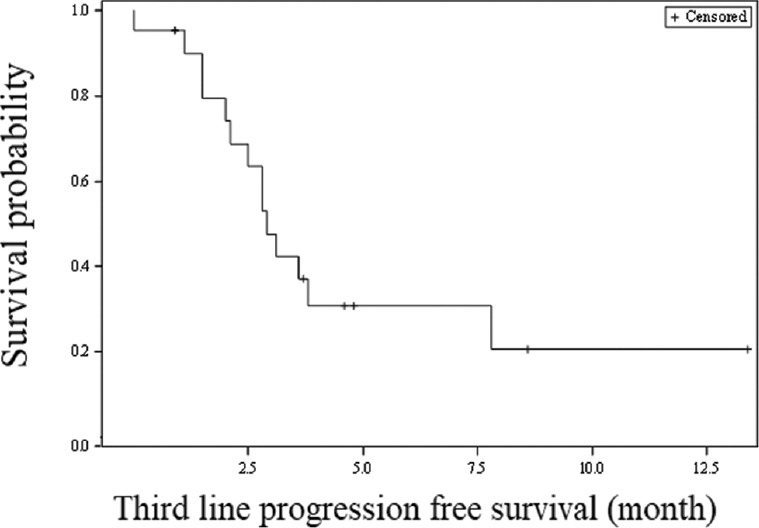
Kaplan-Meier survival analysis for progression-free survival (third-line nab-paclitaxel therapy).
The median OS with three line of therapies was 14.0 months (95% CI 9.8-17.1 months; Fig. 4). There was no significant difference (P=0.13) in the median OS of gemcitabine/cisplatin group as compared to gemcitabine/carboplatin group.
Fig. 4.
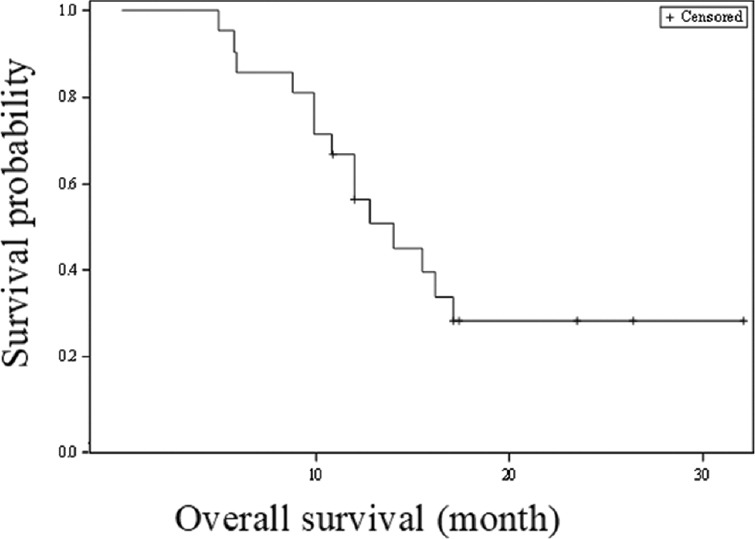
Kaplan-Meier survival analysis for overall survival (three lines of therapy).
Discussion
The ABC-02 trial from the UK and BT22 study from Japan established gemcitabine and cisplatin as a standard of care in the management of advanced unresectable GBC7,8. The ABC-02 trial reported a response rate of 37.7 per cent in the gemcitabine plus cisplatin arm and 21.4 per cent in the gemcitabine arm of the gallbladder subset. There was a significant improvement in the OS (11.7 vs. 8.1 months; P < 0.001) as well as median PFS (8 vs. 5 months; P < 0.001) in the gemcitabine plus cisplatin arm as compared to the gemcitabine arm of the gallbladder subset7. BT22 study reported a response rate of 19.5 per cent in the gemcitabine plus cisplatin arm as compared to 11.9 per cent in the gemcitabine arm. The median survival time (11.2 vs. 7.7 months) and median PFS (5.8 vs. 3.7 months) were better in the gemcitabine plus cisplatin arm although the same was not significant8. A study from India reported a high response rate of 55 per cent, median survival time of 8.5 months and median PFS of 5.4 months with gemcitabine plus cisplatin combination chemotherapy in 91 patients with inoperable, locoregionally advanced and metastatic GBC15. Two phase 2 trials on gemcitabine and carboplatin combination chemotherapy in GBC from India have reported a response rate ranging from 37 to 50.8 per cent and median OS of 5.9-11 months9,10.
The overall response rate of 61.9 per cent with the first-line gemcitabine/platinum chemotherapy in our study was much higher than the one reported in the previous studies with the limitation of having the less number of patients7,8,15. The median PFS of 7.4 months in the gemcitabine/cisplatin group and 4.4 months in the gemcitabine/carboplatin group in our study was comparable to previously published reports with the limitation of having the less number of patients7,8,9,10.
There are limited data on the role of second-line chemotherapy for advanced GBC after failure of gemcitabine and platinum combination. Lamarca et al16 conducted a systematic review of 761 patients across 25 studies to evaluate the role of second-line chemotherapy in advanced biliary tract cancers. With a response rate of 7.7 per cent and mean OS and PFS of 7.2 and 3.2 months, respectively, the evidence was not sufficient to recommend the second-line chemotherapy in advanced biliary tract cancers. Fornaro et al17 conducted a pooled analysis of 499 patients across five presented or published series to demonstrate a marginal activity of the second-line chemotherapy (response rate - 10.2%), with limited efficacy in unselected patient populations (median PFS - 3.1 months; median OS - 6.3 months). A study from India evaluated the role of FOLFOX-4 as the second-line therapy after failure of gemcitabine and platinum combination in 70 patients with advanced GBC11. With a response rate of 24.24 per cent (disease control rate - 59.1%) and median OS and PFS of 7.6 and 3.9 months, respectively, the study demonstrated clinical utility of FOLFOX-4 as an effective second-line treatment strategy in advanced GBC patients. The response rate of 57.1 per cent with the second-line FOLFOX-4 therapy in our study was higher compared to that of the previously published reports11,18. The median PFS of 5.4 months in the present study was also higher than the previous reports, confirming the clinical utility of FOLFOX-4 as the second-line therapy after failure of gemcitabine and platinum combination in patients with advanced GBC.
There is limited literature available on the role of the third-line nab-paclitaxel chemotherapy for advanced GBC after failure of gemcitabine/platinum and FOLFOX-4 therapies13,18. Manana et al13 evaluated the efficacy of nab-paclitaxel after failure of gemcitabine- and 5-FU-based combinations in 24 patients with advanced GBC. With a response rate of 37.5 per cent (disease control rate - 66.6%) and median PFS of 2.86 months, they demonstrated the initial efficacy of single-agent nab-paclitaxel as a third-line option for advanced GBC. The response rate of 52.4 per cent with nab-paclitaxel therapy in our study was higher compared to that of the previously published report13. The median PFS of 2.9 months in our study was comparable to the previous reports, confirming the clinical utility of nab-paclitaxel as a third-line option for advanced GBC after failure of gemcitabine/platinum and FOLFOX-4 therapies.
The overall median OS of 14.0 months with three lines of therapies in our study was higher compared to that of the previously published report13 and confirmed the clinical utility of FOLFOX-4 and nab-paclitaxel therapies in improving the OS of patients with advanced GBC.
The grade 3 or 4 toxicities of decreased neutrophil counts, abnormal liver function, fatigue and infection were reported in 70.7 per cent of patients in the gemcitabine plus cisplatin arm of ABC-02 study7. Gemcitabine plus cisplatin arm of BT22 study8 reported the most common grade 3 or 4 toxicities of neutropaenia, thrombocytopaenia and leucopenia in 56.1, 39 and 29.3 per cent patients, respectively. Talwar et al10,15 reported grade 3 or 4 toxicities of neutropaenia (10.9%), thrombocytopaenia (9.9%) and anaemia (4.4%) with gemcitabine/cisplatin and neutropaenia (12.1%), thrombocytopaenia (7.3%) and anaemia (4.8%) with gemcitabine/carboplatin in two separate studies. Our study showed 28.6 per cent grade 3 or 4 toxicities with anaemia (14.3%), neutropaenia (4.8%) and thrombocytopaenia in 9.5 per cent of patients, respectively. Dodagoudar et al11 reported 31.8 per cent haematological and 25.7 per cent gastrointestinal grade 3 or 4 toxicities with FOLFOX-4 therapy. Manana et al13 reported 33.3 per cent Grade 3 or 4 haematological toxicities with single-agent nab-paclitaxel therapy. Our study findings corroborated with these findings11,13. The other grade 1 or 2 toxicities in our study were vomiting, abdominal pain, fever, deranged liver function test, diarrhoea, weakness and peripheral neuropathy. The active follow up of a selected subgroup of advanced, unresectable GBC patients across three lines of chemotherapy along with the outcome data of third-line nab-paclitaxel chemotherapy was the key strength of the study. However, the retrospective data collection was the main limitation of the study.
In conclusion, our study showed encouraging results of third-line nab-paclitaxel therapy in the selected subgroup of advanced, unresectable GBC patients. The study demonstrated that addition of second-line and third-line chemotherapy helped in prolonging the OS in GBC patients after failure of the first-line therapy. However, prospective data collection is required to verify the advantage of these regimens.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Furlan A, Ferris JV, Hosseinzadeh K, Borhani AA. Gallbladder carcinoma update: multimodality imaging evaluation, staging, and treatment options. AJR Am J Roentgenol. 2008;191:1440–7. doi: 10.2214/AJR.07.3599. [DOI] [PubMed] [Google Scholar]
- 3.Jin K, Lan H, Zhu T, He K, Teng L. Gallbladder carcinoma incidentally encountered during laparoscopic cholecystectomy: How to deal with it. Clin Transl Oncol. 2011;13:25–33. doi: 10.1007/s12094-011-0613-1. [DOI] [PubMed] [Google Scholar]
- 4.Goetze TO. Gallbladder carcinoma: Prognostic factors and therapeutic options. World J Gastroenterol. 2015;21:12211–7. doi: 10.3748/wjg.v21.i43.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glazer ES, Liu P, Abdalla EK, Vauthey JN, Curley SA. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg. 2012;16:1666–71. doi: 10.1007/s11605-012-1935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 8.Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br J Cancer. 2010;103:469–74. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julka PK, Puri T, Rath GK. A Phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary Pancreat Dis Int. 2006;5:110–4. [PubMed] [Google Scholar]
- 10.Talwar V, Raina S, Goel V, Doval DC. Gemcitabine and carboplatin in inoperable, loco-regionally advanced and metastatic gallbladder cancer-A study from Northern Indian Cancer Institute. Br J Med Med Res. 2017;19:1–7. [Google Scholar]
- 11.Dodagoudar C, Doval DC, Mahanta A, Goel V, Upadhyay A, Goyal P, et al. FOLFOX-4 as second-line therapy after failure of gemcitabine and platinum combination in advanced gall bladder cancer patients. Jpn J Clin Oncol. 2016;46:57–62. doi: 10.1093/jjco/hyv148. [DOI] [PubMed] [Google Scholar]
- 12.Leal JL, Roa JC, Jarufe N, Madrid J, Ibanez C, Elisa M, et al. Second-line FOLFOX chemotherapy in patients with metastatic gallbladder cancer and cholangiocarcinoma. J Clin Oncol. 2014;32:322. [Google Scholar]
- 13.Manana SS, Goel V, Talwar V, Raina S. NAB-PACLITAXEL as third-line therapy after failure of gemcitabine and 5-FU based combinations in advanced gall bladder cancer patients. Ann Oncol. 2016;27:207–42. [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Talwar V, Raina S, Goel V, Doval DC. Gemcitabine and cisplatin in inoperable, loco-regionally advanced and metastatic gallbladder cancer: A study from Northern India cancer institute. Int J Hepatobiliary Pancreat Dis. 2016;6:108–13. [Google Scholar]
- 16.Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: A systematic review. Ann Oncol. 2014;25:2328–38. doi: 10.1093/annonc/mdu162. [DOI] [PubMed] [Google Scholar]
- 17.Fornaro L, Vivaldi C, Cereda S, Leone F, Aprile G, Lonardi S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: A multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res. 2015;34:156. doi: 10.1186/s13046-015-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tajima H, Ohta T, Shinbashi H, Hirose A, Tsukada T, Okamoto K, et al. Successful treatment of unresectable gallbladder cancer with low-dose paclitaxel as palliative chemotherapy after failure of gemcitabine and oral S-1: A case report. Oncol Lett. 2012;4:1281–4. doi: 10.3892/ol.2012.909. [DOI] [PMC free article] [PubMed] [Google Scholar]


