Significance
GABAergic signaling is crucial for the physiological function and the pathological onset of neuropsychiatric disorder, but its roles in innate immunity remain unknown. Here, we reveal that pathogen Pseudomonas aeruginosa infection up-regulates the expression of synaptic components and enhances synaptic strength at GABAergic neuromuscular junctions, induces insulin-like peptide INS-31 in the muscles, and ultimately suppresses innate immunity in the intestine of C. elegans. This signaling axis of synapse–muscular insulin–intestinal innate immunity may play an important role in the maintenance of immunological homeostasis to promote host survival.
Keywords: innate immunity, GABAergic synapse, muscle, insulin signaling, intestine
Abstract
GABAergic neurotransmission constitutes a major inhibitory signaling mechanism that plays crucial roles in central nervous system physiology and immune cell immunomodulation. However, its roles in innate immunity remain unclear. Here, we report that deficiency in the GABAergic neuromuscular junctions (NMJs) of Caenorhabditis elegans results in enhanced resistance to pathogens, whereas pathogen infection enhances the strength of GABAergic transmission. GABAergic synapses control innate immunity in a manner dependent on the FOXO/DAF-16 but not the p38/PMK-1 pathway. Our data reveal that the insulin-like peptide INS-31 level was dramatically decreased in the GABAergic NMJ GABAAR-deficient unc-49 mutant compared with wild-type animals. C. elegans with ins-31 knockdown or loss of function exhibited enhanced resistance to Pseudomonas aeruginosa PA14 exposure. INS-31 may act downstream of GABAergic NMJs and in body wall muscle to control intestinal innate immunity in a cell-nonautonomous manner. Our results reveal a signaling axis of synapse–muscular insulin–intestinal innate immunity in vivo.
Innate immunity, an evolutionally conserved behavior, constitutes the first defense line of multiple organisms to prevent microbial infections (1). The nematode Caenorhabditis elegans has been used as a model host for human opportunistic pathogen Pseudomonas aeruginosa infection (2) to identify evolutionarily conserved mechanisms of innate immunity. Typically, p38/PMK-1 mitogen-activated protein kinases (MAPKs) (3) and insulin/insulin-like signaling (IIS)/DAF-2 signaling cascades are recognized as two key components of the C. elegans intestinal innate immune response upon P. aeruginosa strain PA14 infection (4), as they are in mammals (3, 4). Moreover, increasing evidence has revealed several neural mechanisms as also being involved in the regulation of innate immunity. For example, G protein–coupled receptor (GPCR) NPR-1– and soluble guanylate cyclase GCY-35–expressing sensory neurons actively suppress the immune response of nonneuronal tissues (5). Additionally, a putative octopamine GPCR, OCTR-1, which is expressed and functions in the C. elegans sensory neurons ASH and ASI (6), down-regulates the unfolded protein response genes pqn/abu to further suppress the immune response of nonneuronal tissues (5, 6).
Recent studies demonstrate that dopaminergic signaling inhibits innate immunity (7) whereas neuronal acetylcholine stimulates muscarinic signaling in the epithelium and activates the epithelial canonical Wnt pathway to promote the ability to defend against bacterial infection (8). Moreover, insulin-like peptide INS-7 secreted by the nervous system functions in a cell-nonautonomous manner to activate the IIS/DAF-2 pathway and modulate the intestinal innate immunity of C. elegans (9).
GABAergic signaling constitutes a major inhibitory neurotransmission system that plays crucial roles in the central nervous system, especially for maintaining the balance between excitation and inhibition of neuronal networks (10). Disruption of this balance is not only linked to several neuropsychiatric disorders including schizophrenia, autism, and epilepsy (11) but also implicated in autoimmune disease (12). Up to date, multiple lines of evidence have shown that GABAergic signaling cell-autonomously modulates the immune response in immune cells (13–15). However, the roles of GABAergic synapses in innate immunity remain unknown.
Here, we found that the nematode C. elegans harboring a deficiency in GABAergic neuromuscular junctions (NMJs) exhibits enhanced resistance to pathogens. P. aeruginosa PA14 infection increases synaptic expression of GABAergic synaptic components at the nerve cord of worms and enhances the strength of GABAergic transmission. Moreover, we identified an insulin-like peptide, INS-31, acting downstream of GABAergic NMJs and in body wall muscle (BWM) to control intestinal innate immunity in a cell-nonautonomous manner. This work reveals a signaling axis of synapse–muscular insulin–intestinal innate immunity in vivo.
Results
GABAergic NMJs Suppress Intestinal Innate Immunity.
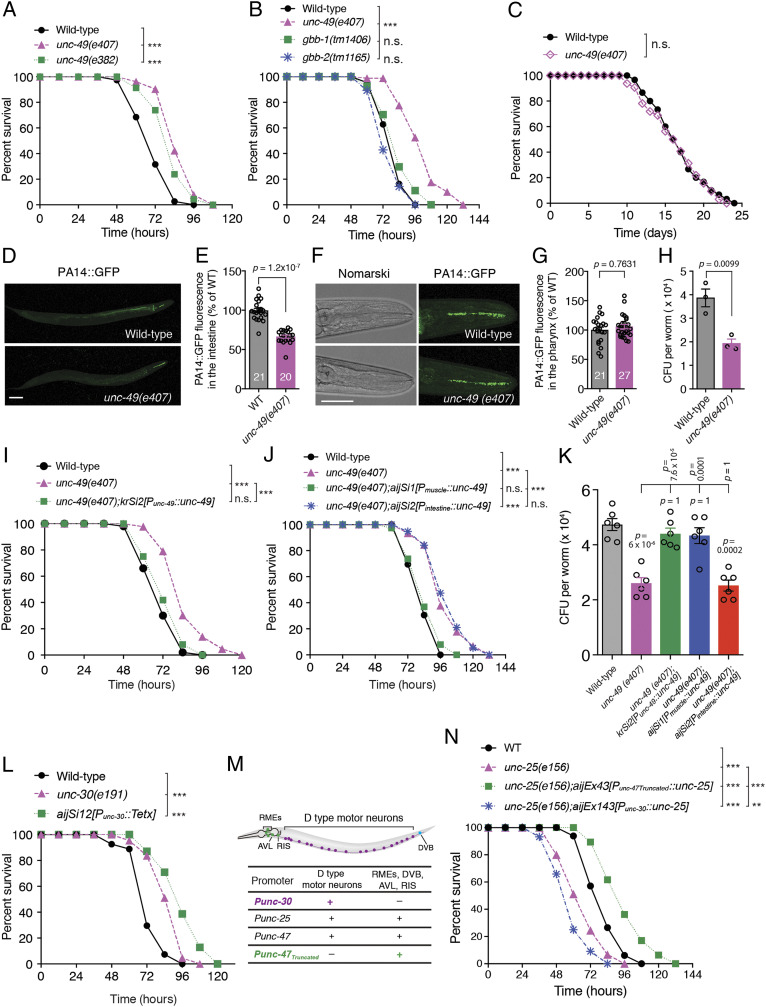
To investigate the role of GABAergic synapses with regard to innate immunity in response to pathogen infection, we first tested the survival of unc-49 mutant animals lacking the postsynaptic GABA neurotransmitter type A receptor (GABAAR) at GABAergic NMJs (16) in response to human opportunistic pathogen P. aeruginosa PA14 infection (2). We found that animals carrying either the unc-49(e407) or unc-49(e382) mutant allele exhibited enhanced resistance to killing by PA14 in a full lawn of this pathogen (Fig. 1A and SI Appendix, Fig. S1A), whereas the susceptibility to PA14 of mutant animals for either gbb-1 or gbb-2, which encodes the GABAB1 and GABAB2 subunits of the metabotropic GABA type B receptor (GABABR), respectively, was indistinguishable from that of wild-type animals (Fig. 1B). Nevertheless, although the unc-49(e407) mutant presented defective PA14 avoidance (SI Appendix, Fig. S1B), it exhibited normal pharyngeal pumping, intake of green fluorescent beads in the intestine of the animals fed on an Escherichia coli OP50 or PA14 lawn, and enteric muscle contraction (SI Appendix, Fig. S1 C–E). We also observed no difference in survival between wild-type and unc-49(e407) animals that were fed on heat-killed PA14 (Fig. 1C) or normal OP50 (17), suggesting that loss of GABAAR/UNC-49 function affects the immune response to living pathogenic bacteria without changing the basic life span of nematodes. Moreover, to determine whether the immune defense defect consequent to mutation in the unc-49 gene is specific for gram-negative bacterial P. aeruginosa infection, we exposed worms to gram-positive bacterial Staphylococcus aureus, another pathogen known to kill C. elegans (18–20). We found that unc-49(e407) animals also exhibited enhanced resistance to S. aureus (SI Appendix, Fig. S2A). These data indicate that the postsynaptic inotropic GABAAR, but not the metabotropic GABABR, could suppress the general innate immunity of the nematodes.
Fig. 1.
Deficiency in GABAergic NMJs extends survival of C. elegans upon P. aeruginosa PA14 exposure. (A–C) Survival of wild-type, unc-49(e407), and unc-49(e382) mutants (A) or of wild-type, unc-49(e407), gbb-1(tm1406), and gbb-2(tm1165) mutants (B) exposed to PA14 or of wild-type and unc-49(e407) mutants fed on heat-killed PA14 (C). The exact P values of statistics for all survival assays are listed in SI Appendix, Table S1. (D–G) Representative images (D and F) and fluorescence intensity (E and G) of wild-type and unc-49(e407) worms exposed to PA14-expressing green fluorescent protein (GFP) for 24 h in the intestine (D and E) and pharynx (F and G) of animals. Data are presented as mean ± SEM. The number of animals analyzed is indicated in E and G. (H) Quantitative analyses of the colony-forming units (CFU) of wild-type and unc-49(e407) worms exposed to PA14 for 24 h. (I–K) Survival (I and J) and CFU quantification (K) of wild-type, unc-49(e407), or indicated transgenic alleles for endogenous or tissue-specific rescue of unc-49(e407) worms exposed to PA14. (L) Survival of wild-type controls, unc-30(e191), and transgenic worms expressing the light chain of tetanus toxin (TeTx) under the control of the GABAergic D-type motor neuron-specific unc-30 promoter exposed to PA14. (M) Chart of GABAergic neuron expression patterns controlled by the corresponding specific promoters. (N) Survival of wild-type, unc-25(e156), and unc-25(e156) worms expressing UNC-25 in GABAergic non–D-type motor neurons under the control of an unc-47 truncated promoter (29) and in D-type motor neurons under unc-30 promoter, respectively, exposed to PA14. Statistical significance was determined by log-rank test for survival assays or nonparametric Mann–Whitney U test (E and G) or unpaired Student’s t test (H) or one-way ANOVA tests followed by Bonferroni’s multiple comparison tests (K). ***P < 0.001; n.s., not significant. (Scale bar, 100 μm in D and 50 μm in F.)
Certain mutant C. elegans resistant to the killing by a pathogen exhibits less pathogen accumulation in the intestine (6, 21). Therefore, we examined whether the unc-49 mutation affects PA14 accumulation in the intestine. Our results showed that the unc-49(e407) mutant indeed exhibited less accumulation of PA14 expressing green fluorescent protein (GFP) fluorescence in the intestine than that of the wild-type animals at 8 h, 12 h (SI Appendix, Fig. S1F), and 24 h after PA14 exposure (Fig. 1 D and E); although the PA14 accumulation in the intestine at 2 h and 4 h were indistinguishable (SI Appendix, Fig. S1F), the patterns in the pharynx (Fig. 1 F and G) and green fluorescent beads intake in the intestine (SI Appendix, Fig. S1D) between wild-type and the unc-49(e407) mutant were comparable. In addition, the number of bacterial cells in the animal body was dramatically decreased in the unc-49(e407) mutant as compared with wild-type animals (Fig. 1H). This indicated that the reduction of the bacterial accumulated in the intestine rather than the lower uptake of the pathogen PA14 into the animals contributed to the enhanced immunity of unc-49(e407) animals, suggesting that unc-49 mutant has an enhanced capacity to clear the pathogen.
P. aeruginosa infection induces the innate immunity of the nematode C. elegans in the intestine (2), whereas GABAAR/UNC-49 is mainly expressed in BWM cells of the animal (16). Therefore, to demonstrate the tissue in which GABAAR/UNC-49 acts to modulate the intestinal innate immunity of worms, rescue experiments were performed by using endogenous and tissue-specific promoters to drive GABAAR/UNC-49 expression. We first found that the enhanced resistance to and the reduced accumulation of P. aeruginosa in the unc-49(e407) mutant were well rescued by either single-copy insertions (Fig. 1 I and K) or extrachromosomal arrays (SI Appendix, Fig. S2B) expressing GABAAR/UNC-49 under its own endogenous promoter. Strikingly, only muscular but not intestinal expression of GABAAR/UNC-49 fully rescued the phenotype of enhanced innate immunity in the unc-49(e407) mutant (Fig. 1 J and K and SI Appendix, Fig. S2 C and D). These data indicate that postsynaptic GABAAR/UNC-49 functions to suppress innate immunity via a signaling axis from muscle to intestine.
GABAergic NMJs are primarily formed between GABAergic D-type motor neurons and the muscle arms of worm BWMs en passant (22). The unc-30 gene encodes a homeodomain transcription factor, an ortholog of the human paired–like homeodomain factor PITX2, which controls the expression of glutamic acid decarboxylase (GAD) UNC-25 that synthesizes the neurotransmitter GABA and the vesicular GABA transporter (VGAT) UNC-47, responsible for transporting GABA into vesicles (23). Both UNC-25 and UNC-47 are essential components of GABAergic NMJs (24, 25). To address whether GABAergic synaptic transmission is implicated in the enhanced resistance to PA14 of the unc-49(e407) mutant, we first tested the susceptibility to PA14 of unc-30(e191) mutants and found that unc-30(e191) also elicited enhanced resistance to PA14 exposure. Second, we generated transgenic lines expressing the light chain of tetanus toxin (TeTx), a specific protease of synaptobrevin (26), under the control of the D-type motor neuron-specific promoter unc-30, to inhibit synaptic transmission of GABAergic NMJs. We found that the transgenic animals expressing TeTx also exhibited enhanced resistance to PA14 exposure (Fig. 1L). Third, the nematode C. elegans has 26 GABAergic neurons, which are comprised of six dorsal D-type (DD) motor neurons, 13 ventral D-type (VD) motor neurons, four RMEs, one RIS, one AVL, and one DVB (22, 27, 28). These neurons fall into different classes based on their synaptic outputs: 19 D-type neurons (the six DD and 13 VD motor neurons) innervate the dorsal and ventral BWMs, respectively; the four RME motor neurons innervate the head muscles; the AVL and DVB motor neurons innervate the enteric muscles; and RIS is an interneuron (22, 27, 28). In the present study, both unc-25(e156) animals lacking GABA and the unc-47(n2409) mutant lacking VGAT exhibited enhanced sensitivity to PA14 (SI Appendix, Fig. S2E). This defective innate immunity of the unc-25(e156) mutant upon PA14 infection was fully rescued by the transgenic allele carrying the endogenous unc-25 locus (SI Appendix, Fig. S2E), or the D-type motor neurons promoter unc-30 driving unc-25 gene (Fig. 1 M and N), but not the endogenous unc-30 locus (SI Appendix, Fig. S2F). Strikingly, the enhanced sensitivity of unc-25(e156) animals was reverted to enhanced resistance to PA14 infection by the expression of UNC-25 under the control of the unc-47 truncated promoter, which drives gene expression only in RMEs, AVL, RIS, and DVB GABAergic neurons but not in D-type motor neurons (29) (Fig. 1 M and N and SI Appendix, Fig. S2E). Collectively, these data suggest that GABAergic synapses formed between D-type motor neurons and the BWM negatively control the intestinal innate immunity of nematodes.
Pathogen PA14 Infection Enhances the Strength of GABAergic Transmission.
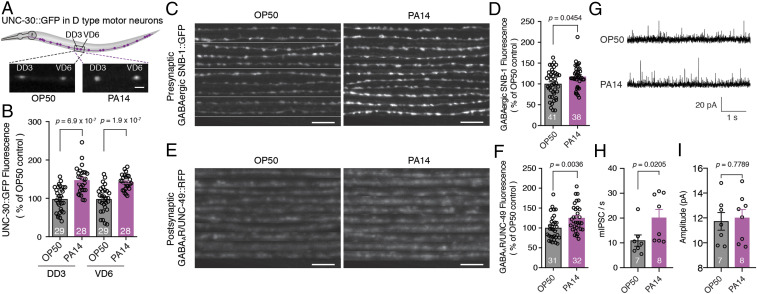
To address whether pathogen infection affects the plasticity of the synapse, we tested the effect of PA14 infection on the morphology and function of GABAergic NMJs. First, we found that the expressions of the homeodomain transcription factor UNC-30 were up-regulated in the D-type motor neurons of animals exposed to PA14 as compared with those fed on OP50 (Fig. 2 A and B). Second, the expression of both the presynaptic component SNB-1, an ortholog of mammalian vesicle-associated membrane protein 2 (VAMP2) (Fig. 2 C and D), and the postsynaptic GABAA receptor UNC-49 (Fig. 2 E and F) at GABAergic NMJs, but not that of the levamisole-sensitive acetylcholine receptor (L-AChR) UNC-29 at cholinergic NMJs (SI Appendix, Fig. S3 A and B), were significantly increased at the nerve cord of wild-type animals exposed to PA14 as compared with those fed on OP50 (Fig. 2 C–F). Intriguingly, only live but not heat-killed PA14 could stimulate synaptic expression of VAMP2/SNB-1 (SI Appendix, Fig. S4A) and GABAAR/UNC-49 (SI Appendix, Fig. S4B), suggesting that a yet unknown specific antigen or metabolite present in PA14 but absent in OP50 carries out this action. Third, as the short isoform of extracellular matrix protein muscle arm development defect 4 (MADD-4), MADD-4B, is required for proper postsynaptic localization of GABAAR/UNC-49, whereas the long isoform MADD-4L specifically localizes L-AChR/UNC-29 at the cholinergic synaptic site (30–32). We also examined the expression of both isoforms of MADD-4 at the nerve cords and observed that the level of MADD-4B (SI Appendix, Fig. S3 C and D) but not that of MADD-4L (SI Appendix, Fig. S3 E and F) was up-regulated at the nerve cords of animals exposed to PA14 as compared with those fed on OP50. Furthermore, electrophysiological recording analyses of GABAergic neurotransmission in the animals fed on OP50 or exposed to PA14 revealed that the frequency, but not the amplitude, of spontaneous miniature inhibitory postsynaptic currents in BWM cells of the animals exposed to PA14 was increased compared with that in animals fed on OP50 (Fig. 2 G–I). Taken together, these data strongly support the model that the pathogen infection not only up-regulates the expression of structural components of GABAergic synapses but also enhances synapse functional strength, thereby modulating the innate immunity of the nematode.
Fig. 2.
GABAergic neurotransmission between D-type motor neurons and BWM is enhanced upon PA14 exposure. (A–F) Diagram (A), representative images (A, C, and E), and fluorescence intensity (B, D, and F) of UNC-30::GFP in dorsal (DD3) or ventral (VD6) D-type motor neurons (A and B) or presynaptic GABAergic SNB-1::GFP (C and D) or postsynaptic GABAAR/UNC-49::red fluorescent protein (RFP) (E and F) at the dorsal cord of wild-type worms fed on OP50 or exposed to PA14. (G–I) Representative traces (G), average frequencies (H), and amplitudes (I) of spontaneous miniature inhibitory postsynaptic currents (mIPSCs) at GABAergic NMJs of wild-type worms fed on OP50 or exposed to PA14, respectively. Statistical significance was determined by unpaired Student’s t test (B and F) or nonparametric Mann–Whitney U test (D, H, and I). (Scale bar, 10 μM.) The number of animals analyzed is indicated.
GABAergic NMJ-Mediated Innate Immunity Requires the DAF-2/DAF-16 Pathway but Is Independent of p38/PMK-1 Signaling.
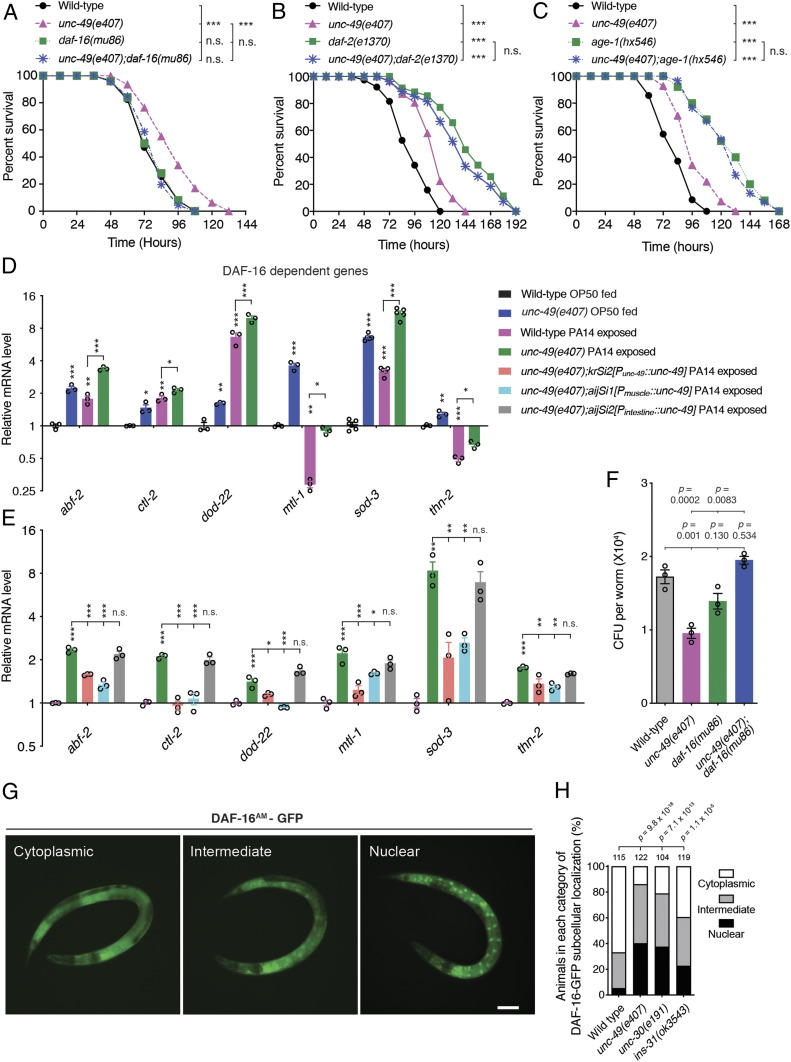
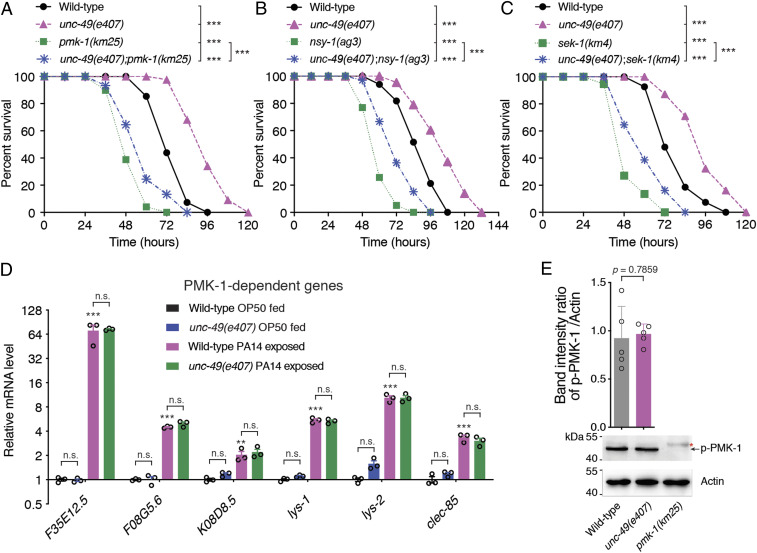
To understand the mechanisms underlying GABAergic NMJ-mediated innate immunity, we evaluated whether the pathways such as DAF-2/DAF-16 or p38/PMK-1 MAPK are involved in the GABAergic transmission-mediated intestinal innate immunity of C. elegans. We observed that the enhanced resistance to PA14-mediated killing of unc-49(e407) animals was robustly suppressed by a loss-of-function mutation or RNA interference of daf-16 (Fig. 3A) but not by loss-of-function mutations or RNA interference of pmk-1, nsy-1, or sek-1 genes in the p38/PMK-1 MAPK pathway (Fig. 4 A–C and SI Appendix, Fig. S5 A and B). Since mutations in daf-2 or age-1, which encodes a homolog of the mammalian insulin receptor and of the catalytic subunit of mammalian phosphatidylinositol 3-OH kinase, respectively, exhibited stronger resistance to PA14 infection than that of unc-49 or unc-30 mutant (33) (Fig. 3 B and C and SI Appendix, Fig. S5C), loss-of-function of daf-2 or age-1 in unc-49 or unc-30 mutant animals did not alter the enhanced resistance of daf-2 or age-1 mutant to PA14 infection (Fig. 3 B and C and SI Appendix, Fig. S5C), suggesting that unc-49 and unc-30 act in the same genetic pathway of daf-2 and age-1. Consistent with these findings, qRT-PCR analyses revealed that six DAF-16–dependent genes including abf-2 (34, 35), ctl-2 (36), dod-22 (37), mtl-1 (33), sod-3 (33), and thn-2 (35) (Fig. 3D) but not the p38/PMK-1–dependent genes F35E12.5 (7), F08G5.6 (9), K08D8.5 (38), lys-1 (39), lys-2 (7), and clec-85 (35) (Fig. 4D) were significantly up-regulated upon PA14 infection and dramatically increased by loss of GABAAR/UNC-49 in unc-49(e407) animals. The marked increase of the six DAF-16–dependent genes in unc-49(e407) mutants was fully restored by the expression of UNC-49 under the control of an endogenous or a muscle- but not an intestine-specific promoter (Fig. 3E). The colony-forming units (CFU) assay revealed that loss-of-function of DAF-16 blocked the enhanced pathogen-cleaning capacity of unc-49(e407) animals (Fig. 3F).
Fig. 3.
GABAergic synapse-mediated innate immunity is dependent on DAF-2/DAF-16 signaling. (A–C) Survival of wild-type, unc-49(e407), daf-16(mu86), and unc-49(e407);daf-16(mu86) (A) or wild-type, unc-49(e407), daf-2(e1370), and unc-49(e407);daf-2(e1370) (B) or wild-type, unc-49(e407), age-1(hx546), and unc-49(e407);age-1(hx546) (C) worms exposed to PA14. The exact P values of statistics for all survival assays are listed in SI Appendix, Table S1. (D and E) Quantitative RT-PCR analyses of the expression levels of six DAF-16–dependent genes in wild-type and unc-49(e407) worms fed on OP50 or exposed to PA14 (D) or in wild-type, unc-49(e407), and unc-49(e407) mutant animals with GABAAR/UNC-49 expressed under the control of endogenous (krSi2[Punc-49::unc-49]), muscle (aijSi1[Pmyo-3::unc-49])-, or intestine (aijSi2[Pges-1::unc-49])-specific promoters exposed to PA14 (E). The exact P values of statistics for comparison of qRT-PCR between groups are listed in SI Appendix, Table S2. (F) CFU quantification of wild-type, unc-49(e407), daf-16(mu86), and unc-49(e407);daf-16(mu86) animals. Data are presented as means ± SEM. (G and H) The representative images of three categories (G) and quantification analysis (H) of nuclear accumulation of DAF-16aAM−GFP in wild-type, unc-49(e407), unc-30(e191), and ins-31(ok3543) mutant animals, respectively. Statistical significance was determined by log-rank test for survival assays or one-way ANOVA tests followed by Bonferroni’s multiple comparison tests (D, E, and F) or χ2 test (H). *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant. (Scale bar, 100 μm in G.) The number of animals analyzed is indicated in H.
Fig. 4.
GABAergic synapse-mediated innate immunity is independent on PMK-1 pathway. (A–C) Survival of wild-type, unc-49(e407), pmk-1(km25), and unc-49(e407);pmk-1(km25) (A) or wild-type, unc-49(e407), nsy-1(ag3), and unc-49(e407);nsy-1(ag3) (B) or wild-type, unc-49(e407), sek-1(km4), and unc-49(e407);sek-1(km4) (C) animals exposed to PA14. The exact P values of statistics for all survival assays are listed in SI Appendix, Table S1. (D) qRT-PCR analyses of the expression levels of six PMK-1–dependent genes in wild-type and unc-49(e407) worms fed on OP50 or exposed to PA14. The exact P values of statistics for comparison of qRT-PCR between groups are listed in SI Appendix, Table S2. (E) Representative image and quantitative analysis of the phosphorylation level of PMK-1 in wild-type and unc-49(e407) mutant animals, respectively. Red star indicates nonspecific band. Data are presented as means ± SEM for D and E. Three independent experiments were performed. Statistical significance was determined by log-rank test for survival assays or one-way ANOVA tests followed by Bonferroni’s multiple comparison tests (D) or unpaired Student’s t test (E). *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant.
Furthermore, as activated DAF-16 is translocated to the nucleus in response to extracellular or cytoplasmic stimuli, we next tested the nuclear activity of DAF-16 using DAF-16aAM−GFP, a genetically engineered form of DAF-16 that is functional but cannot be sequestered in the cytosol as a reporter (40, 41). We found that more DAF-16aAM−GFP was accumulated in the nucleus of unc-49(e407) or unc-30(e191) mutants than in wild-type animals, indicating that the loss of function of GABAergic transmission in D-type motor neurons activates DAF-16 (Fig. 3 G and H). We also observed that the nuclear localization of wild-type DAF-16a–GFP (42) was dramatically stimulated by loss function of unc-49 or unc-30, respectively (SI Appendix, Fig. S5 E and F). The intensity of the intestinal SOD-3::GFP, a DAF-16 activity marker, (SI Appendix, Fig. S6 A and B) was dramatically increased. Furthermore, detoxification of reactive oxygen species (ROS) plays an important role in pathogen resistance (43). Here, we found the overall (SI Appendix, Fig. S6C) and intestinal (SI Appendix, Fig. S6D) ROS levels were significantly induced in unc-49(e407) as compared with wild-type. These data suggest that loss function of GABAergic NMJ stimulates intestinal DAF-16 and may increase antimicrobial activity.
Additionally, the phosphorylation level of p38/PMK-1 in unc-49(e407) mutants was indistinguishable from that in wild-type animals (Fig. 4E). Taken together, these data indicate that the suppression of innate immunity by GABAergic neurotransmission is dependent on insulin-like signaling DAF-2/DAF-16 but not p38/PMK-1 signaling.
Muscular Insulin-Like Peptide INS-31 Is Required for Intestinal Innate Immunity.
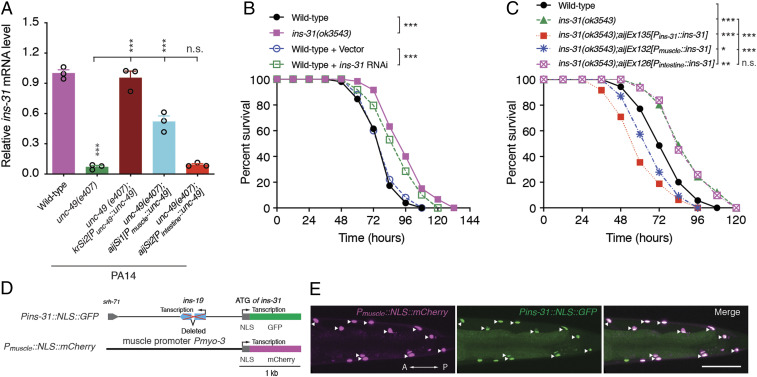
Previous study determined that any one mutant of two members of insulin-like peptide family, ins-27 and ins-31, exhibits enhanced resistance to PA14, while another insulin-like peptide ins-20 mutant is more susceptible to PA14 (44) than wild-type. To test whether the expression of these three insulin-like peptides is regulated by GABAergic NMJs, we first examined the messenger RNA levels of three peptide genes by qRT-PCR in wild-type and unc-49 mutants. We found that the ins-31 messenger RNA levels in unc-49(e407) mutants were dramatically down-regulated as compared with that in wild-type animals (Fig. 5A), while the messenger RNA levels of ins-20 and ins-27 in unc-49 mutants were indistinguishable from those in wild-type animals fed on OP50 or exposed to PA14 (SI Appendix, Fig. S7 A and B). The decrease of ins-31 messenger RNA levels in unc-49(e407) mutants was fully rescued by the expression of GABAAR/UNC-49 under the control of endogenous or muscular but not intestinal promoters (Fig. 5A). To determine whether ins-31 is involved in the GABAergic transmission of D-type motor neuron-mediated intestinal innate immunity, we examined the susceptibility of ins-31 mutant to PA14 infection and found that the animals harboring loss of function of ins-31 either through mutation or RNA interference, but not loss of the adjacent locus ins-19 (SI Appendix, Fig. S8 A–C), exhibited enhanced resistance to PA14 infection (Fig. 5B and SI Appendix, Fig. S8D), which is consistent with the previous observation (44). Notably, the enhanced resistance phenotype of ins-31(ok3543) animals was reverted by the overexpression of INS-31 in transgenic animals carrying ins-31 locus genomic DNA or in muscle cells but not in the intestine (Fig. 5C), further suggesting that the insulin-like peptide INS-31 is implicated in intestinal innate immunity and that it acts in the BWM of the nematodes.
Fig. 5.
Insulin-like peptide INS-31 is required for intestinal innate immunity activity in the BWM. (A) qRT-PCR analysis of ins-31 messenger RNA levels in indicated genotype worms exposed to PA14. Data are presented as means ± SEM. (B) Survival of wild-type and ins-31(ok3543) animals or of wild-type worms treated with control or ins-31 RNA interference vector exposed to PA14. (C) Survival of wild-type, ins-31(ok3543), and transgene-expressing INS-31 under the control of endogenous, muscle (Pmyo-3)-, or intestine (Pges-1)-specific promoters, respectively, in ins-31(3543) animals exposed to PA14. (D and E) Schematic diagram (D) and representative images (E) of NLS-tagged GFP or mCherry under the control of the ins-31 or muscle-specific promoters, respectively. Statistical significance was determined by one-way ANOVA tests followed by Bonferroni’s multiple comparison tests (A) or log-rank test for survival assays (B and C). *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant. Arrowhead indicates the nucleus of the BWM. (Scale bar, 10 μm.)
To identify the tissue in which ins-31 is endogenously expressed in C. elegans, we first generated a transgene expressing GFP trans-spliced by SL2 under the control of the ins-31 endogenous promoter (SI Appendix, Fig. S8E) and observed that GFP was mainly expressed in the seminal vesicles of male gonads and the intestines of both males and hermaphrodites (SI Appendix, Fig. S8F), which is consistent with a previous study (45). To further examine whether ins-31 is expressed in the muscle, we next created a transgene expressing nuclear localization signal (NLS)–tagged mCherry (NLS-mCherry) under the control of the muscle-specific myo-3 promoter and NLS-GFP under the control of the ins-31 promoter (Fig. 5D). Notably, we found that the muscular NLS-mCherry colocalized well with NLS-GFP (Fig. 5E), indicating that INS-31 is indeed expressed in muscle cells, albeit at a low level.
INS-31 Acts Downstream of GABAergic Transmission and Upstream of DAF-2 to Affect Intestinal Innate Immunity.
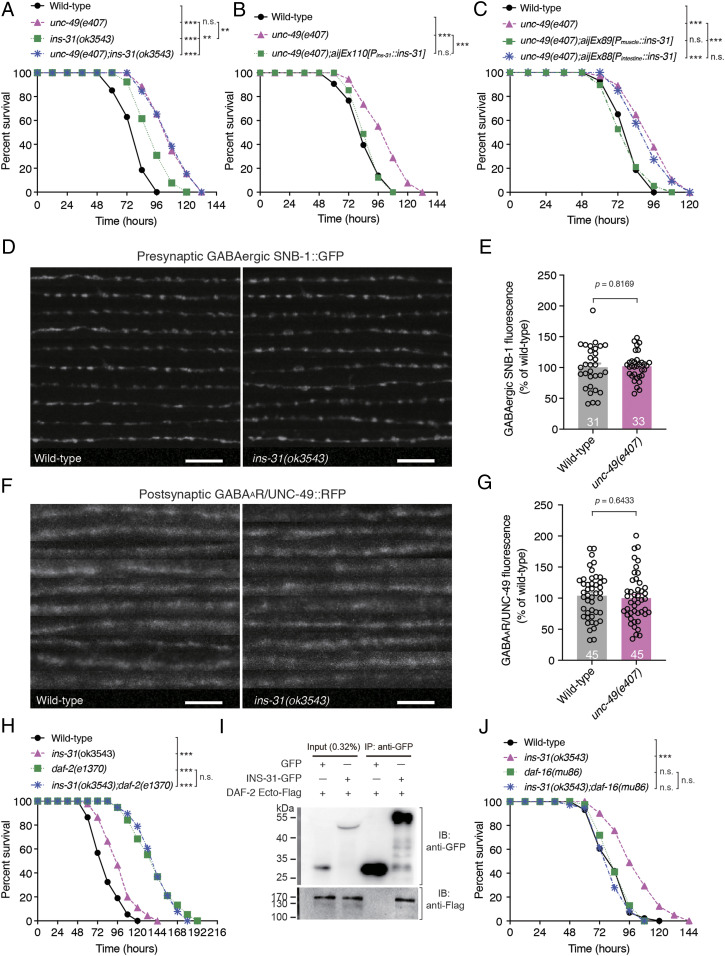
As INS-31 expression in the BWM of C. elegans is controlled by GABAergic neurotransmission of D-type motor neurons and loss function of INS-31 also exhibits enhanced susceptibility to PA14 infection, we tested whether INS-31 acts downstream of GABAergic NMJs to suppress intestinal innate immunity. We first examined the sensitivity of the unc-49;ins-31 double mutant to PA14 infection and observed that the enhanced resistance of the unc-49(e407);ins-31(ok3543) double mutant to PA14 infection did not differ from that of the unc-49(e407) single mutant (Fig. 6A). Notably, the enhanced resistance of ins-31(ok3543) animals was weaker than that of unc-49(e407) animals, indicating that ins-31 acts in the GABAergic transmission genetic pathway, and another signaling in parallel to ins-31 likely participates in the innate immunity. Second, the transgenes expressing INS-31 but not INS-19, endogenously or in the muscle, but not in the intestine, fully rescued the enhanced resistance of unc-49(e407) animals (Fig. 6 B and C and SI Appendix, Fig. S8G), suggesting that INS-31 cell-nonautonomously functions in the BWM to modulate the intestinal innate immunity. Third, INS-31 displays resistance to aldicarb, a specific acetylcholine esterase inhibitor, and may affect the function or development of C. elegans cholinergic NMJs (46). To address whether INS-31 affects the structure of GABAergic NMJs, we examined both the GFP-tagged presynaptic VAMP2 SNB-1 and red fluorescent protein-tagged postsynaptic GABAAR/UNC-49 contents of GABAergic NMJs at the nerve cord of ins-31(ok3543) mutant animals. Neither presynaptic nor postsynaptic structural compound contents were altered (Fig. 6 D–G), indicating that INS-31 does not affect the structure of GABAergic NMJs. Taken together, these data demonstrate that ins-31 acts downstream of GABAergic neurotransmission to modulate C. elegans innate immunity. Additionally, the phenotype of enhanced resistance to PA14 of the ins-31(ok3543);daf-2(e1370) double mutant was not distinguishable from that of the daf-2(e1370) single mutant (Fig. 6H), suggesting that ins-31 functions in the same pathway as daf-2 in response to PA14 exposure. Coimmunoprecipitation in vitro results showed that INS-31 binds to the extracellular domain of DAF-2 in the supernatant of human embryonic kidney 293T cells coexpressing INS-31 and the extracellular domain of DAF-2 (Fig. 6I). Obviously, more DAF-16aAM−GFP (Fig. 3 G and H) or wild-type DAF-16a–GFP (SI Appendix, Fig. S5 E and F) was accumulated in the nucleus of ins-31(ok3543) mutants as compared with wild-type animals, indicating that the loss of function of INS-31 activates DAF-16. Additionally, loss of function of daf-16 completely blocked the phenotype of enhanced resistance to PA14 in ins-31(ok3543) mutant (Fig. 6J). Collectively, these data demonstrate that GABAergic neurotransmission controls the expression of BWM-expressing insulin-like peptide INS-31, which in turn binds to and activates DAF-2 and might further modulate C. elegans intestinal innate immunity through the FOXO/DAF-16 pathway.
Fig. 6.
INS-31 acts downstream of GABAergic transmission and upstream of DAF-2 in intestinal innate immunity. (A) Survival of wild-type, unc-49(e407), ins-31(ok3543), and unc-49(e407);ins-31(ok3543) animals exposed to PA14. (B and C) Survival of wild-type, unc-49(e407), and transgene-expressing INS-31 under the control of endogenous (B), muscle (Pmyo-3)-, or intestine (Pges-1)-specific promoters (C), respectively, in ins-31(3543) animals exposed to PA14. (D–G) Ten representative images (D and F) and fluorescence intensity (E and G) of presynaptic GABAergic SNB-1::GFP (D and E) and postsynaptic GABAAR/UNC-49::red fluorescent protein (RFP) (F and G) in wild-type and ins-31(ok3543) animals, respectively. (H) Survival of wild-type, ins-31(ok3543), daf-2(e1370), and ins-31(ok3543);daf-2(e1370) animals exposed to PA14. (I) Immunoprecipitation of INS-31−GFP with anti-GFP antibodies followed by Western blot analysis with anti-Flag antibodies to detect the Flag-tagged extracellular domain of DAF-2 (DAF-2Ecto−Flag) from culture supernatant of human embryonic kidney 293 cells cotransfected with the plasmids encoding INS-31–GFP and DAF-2Ecto–Flag, respectively. (J) Survival of wild-type, ins-31(ok3543), daf-16(mu86), and ins-31(ok3543);daf-16(mu86) animals exposed to PA14. Statistical significance was determined by log-rank test for survival assays or unpaired Student’s t test (E and G). **P < 0.01, ***P < 0.001; n.s., not significant. The number of animals analyzed is indicated. (Scale bar, 10 μm.)
Discussion
In this study, we revealed a previously undescribed role of GABAergic NMJs formed between D-type motor neurons and BWM cells. Animals harboring a deficiency in GABAergic NMJs exhibited prolonged survival upon PA14 exposure, whereas the unc-25 mutant lacking GAD, which synthesizes the neurotransmitter GABA, and the unc-47 mutant lacking VGAT exhibited enhanced sensitivity to PA14 exposure as compared with that of wild-type animals. Conversely, the phenotype could be inverted by the expression of UNC-25 in GABAergic non–D-type motor neurons such as four RMEs, one AVL, one RIS, and one DVB neuron under the control of the unc-47 truncated promoter (29), suggesting that GABA signaling in these RMEs, AVL, RIS, and DVB GABAergic neuron antagonizes the effect of GABAergic NMJs in the innate immunity of C. elegans. Nevertheless, the molecular and cellular mechanisms remain to be fully elucidated.
C. elegans senses pathogenic bacteria and avoids aversive pathogens to obtain prolonged survival. Chemosensory detection of the secondary metabolites produced by PA14 leads to activation of a G protein–signaling pathway in the ASJ chemosensory neuron to provide environmental cues that contribute to pathogen recognition and host survival (47). OCTR-1, a neuronal GPCR analogous to human norepinephrine receptor, functions in ASI sensory neurons to promote pathogen avoidance behavior and acts in ASH neurons to regulate pathogen defense (6, 48). Notably, the intestine-secreted insulin-like peptide INS-11 affects the ASI and ADF neurons and negatively regulates aversive learning behavior (49). These studies revealed the neural regulation of pathogen recognition, avoidance behavior, and innate immunity defense. However, the effect of pathogen PA14 infection on the nervous system remains unknown. Our data support a mechanism in which the synaptic expression of GABAergic synaptic structural components at the nerve cord of worms is up-regulated and the spontaneous inhibitory postsynaptic currents of GABAergic NMJs are significantly stimulated upon pathogenic infection. Further investigation of the molecular and cellular mechanisms by which pathogens affect the morphology and neurotransmission of the nervous system is therefore warranted. In particular, the roles of GABAergic transmission in innate immunity remained unknown. In this study, we reveal a fundamental function of GABAergic NMJs in the pathogenic defense of intestinal innate immunity.
We identified that the insulin-like peptide INS-31 is involved in the GABAergic NMJ-mediated intestinal innate immunity. Our data suggest the model in which INS-31 may exclusively act in the BWM to control the intestinal innate immunity, although INS-31 is expressed in the vesicle of the gonads (45) and in the intestine. This specific action of INS-31 may be dependent on the tissue-specific posttranslational modification of INS-31 in BWM, for INS-31 is the unique insulin-like peptide with three repeats of B and A peptide chains in the insulin family of C. elegans (50). Additionally, the expression of INS-31 is tightly regulated by the iontrophic GABAAR/UNC-49 via an as-yet-uncharacterized transcriptional program.
Maintenance of the immune homeostasis is critical for host survival because either excessive immunosuppression or excessive cytokine and proinflammatory responses are harmful to organism (51). Since inhibitory GABA signaling also inhibits the activity of other neurons such as excitatory cholinergic motor neurons to modulate the balance of excitation and inhibition of neural circuit (22), the signaling axis of synapse–muscular insulin–intestinal innate immunity we revealed here may play a critical role in the maintenance of immunological homeostasis to promote host survival when the organism suffers environmental pathogen infection.
Our present studies indicate that pathogen infection up-regulates the expression of structural components at the GABAergic NMJ and enhances the strength of GABAergic neurotransmission, which in turn up-regulates insulin-like peptide INS-31 expression in muscle cells. Muscular INS-31 may genetically activate the insulin signaling receptor DAF-2, which subsequently inhibits the transcriptional activity of DAF-16 and down-regulates the expression of intestinal innate immune response genes, thereby leading to a decline in the antimicrobial ability of the nematode C. elegans (SI Appendix, Fig. S9). As many of the relevant genes have human orthologs, this signaling axis may be conserved across species.
Methods
Experimental C. elegans strains were maintained at 20 °C with standard procedures unless otherwise specified on nematode growth media plates seeded with E. coli OP50 as a food source (51). The Bristol strain C. elegans N2 was used as wild-type. Strains were maintained at 20 °C then shifted to 25 °C for P. aeruginosa PA14 lawn avoidance assays and pathogenesis survival assays. Transformation was conducted by microinjection of plasmid DNA into the gonad of C. elegans young adult animals as described previously (52). The alleles of single-copy insertion were generated by the Mos1-mediated homologous recombination (53). The plasmids were built by using isothermal assembly (54), otherwise stated specifically else. The killing assay by P. aeruginosa PA14 was performed as previously described (2) with minor modifications. Data were analyzed with Prism 8.0 (v8.4.3, GraphPad Software, Inc.) and SPSS Statistics Version 25 (IBM). For statistical analyses of all killing and life span assays, log-rank (Kaplan–Meier) method was used to calculate P values, which were listed in SI Appendix, Table S1. For comparison of category of DAF-16−GFP subcellular localization (cytoplasm, intermediate, nuclear), a χ2 test was used. For comparison of two groups, an unpaired Student’s t test or a nonparametric Mann–Whitney U test was performed. For comparison of more than two groups, one-way ANOVA tests followed by Bonferroni’s multiple comparison tests were performed. P values for all statistical analysis of quantitative RT-PCR are listed in SI Appendix, Table S2. The materials and methods in details were described in SI Appendix.
Supplementary Material
Acknowledgments
We thank W. Tan for critical reading of the manuscript; the Caenorhabditis Genetic Center, which is supported by the NIH–Office of Research Infrastructure Programs (P40 OD010440), National BioResource Project, J.-L. Bessereau, G. Shan, and J. Liu for providing strains; D. Wang for the PA14 strain; L. Ma, W. Li, and D. Chen for RNA interference bacterial clones; Z. Du and H. Wei for plasmids; P. Lou for experimental assistance; H. Zhang, B. Zhang, and Z. Li for technical advice; and Y. Peng for technical assistance regarding the bioinformatics analysis of RNA sequencing data. This work was supported by grants from the National Natural Science Foundation of China (31540020 and 31671048 to H.T. and 31800857 to C.C.), the Key Project of Research and Development Plan of Hunan Province (2020SK2092), the Hunan University–Jinxiang Pharmaceutical Cooperative R&D Project of New Drugs for Brain Diseases (201691490847), the Free Exploration Foundation of Shenzhen Science and Technology Innovation Committee (JCYJ20160530192506314 to H.T.), and the Provincial Natural Science Foundation of Hunan Province (2017JJ2041 to H.T. and 2018JJ3038 to C.C.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021063118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Irazoqui J. E., Urbach J. M., Ausubel F. M., Evolution of host innate defence: Insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 10, 47–58 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan M. W., Mahajan-Miklos S., Ausubel F. M., Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 96, 715–720 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D. H., et al., A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297, 623–626 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Garsin D. A., et al., Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300, 1921 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Styer K. L., et al., Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science 322, 460–464 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J., Singh V., Kajino-Sakamoto R., Aballay A., Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science 332, 729–732 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao X., Aballay A., Neural inhibition of dopaminergic signaling enhances immunity in a cell-non-autonomous manner. Curr. Biol. 26, 2398 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Labed S. A., et al., Intestinal epithelial Wnt signaling mediates acetylcholine-triggered host defense against infection. Immunity 48, 963–978.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawli T., Tan M. W., Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat. Immunol. 9, 1415–1424 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Isaacson J. S., Scanziani M., How inhibition shapes cortical activity. Neuron 72, 231–243 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu C. Q., Barberis A., Higley M. J., Preserving the balance: Diverse forms of long-term GABAergic synaptic plasticity. Nat. Rev. Neurosci. 20, 272–281 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Bhat R., et al., Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. U.S.A. 107, 2580–2585 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjurstöm H., et al., GABA, a natural immunomodulator of T lymphocytes. J. Neuroimmunol. 205, 44–50 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Jin Z., Mendu S. K., Birnir B., GABA is an effective immunomodulatory molecule. Amino Acids 45, 87–94 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J. K., et al., GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. Nat. Commun. 9, 4184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bamber B. A., Beg A. A., Twyman R. E., Jorgensen E. M., The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J. Neurosci. 19, 5348–5359 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun L., et al., Metabotropic GABA signalling modulates longevity in C. elegans. Nat. Commun. 6, 8828 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estes K. A., Dunbar T. L., Powell J. R., Ausubel F. M., Troemel E. R., bZIP transcription factor zip-2 mediates an early response to Pseudomonas aeruginosa infection in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 107, 2153–2158 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sifri C. D., Begun J., Ausubel F. M., Calderwood S. B., Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 71, 2208–2217 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visvikis O., et al., Innate host defense requires TFEB-mediated transcription of cytoprotective and antimicrobial genes. Immunity 40, 896–909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuhrman L. E., Goel A. K., Smith J., Shianna K. V., Aballay A., Nucleolar proteins suppress Caenorhabditis elegans innate immunity by inhibiting p53/CEP-1. PLoS Genet. 5, e1000657 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White J. G., Southgate E., Thomson J. N., Brenner S., The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340 (1986). [DOI] [PubMed] [Google Scholar]
- 23.McIntire S. L., Reimer R. J., Schuske K., Edwards R. H., Jorgensen E. M., Identification and characterization of the vesicular GABA transporter. Nature 389, 870–876 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Westmoreland J. J., McEwen J., Moore B. A., Jin Y., Condie B. G., Conserved function of Caenorhabditis elegans UNC-30 and mouse Pitx2 in controlling GABAergic neuron differentiation. J. Neurosci. 21, 6810–6819 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Y., Hoskins R., Horvitz H. R., Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature 372, 780–783 (1994). [DOI] [PubMed] [Google Scholar]
- 26.Macosko E. Z., et al., A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458, 1171–1175 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuske K., Beg A. A., Jorgensen E. M., The GABA nervous system in C. elegans. Trends Neurosci. 27, 407–414 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen E. M., Gaba. WormBook, 1–13 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eastman C., Horvitz H. R., Jin Y., Coordinated transcriptional regulation of the unc-25 glutamic acid decarboxylase and the unc-47 GABA vesicular transporter by the Caenorhabditis elegans UNC-30 homeodomain protein. J. Neurosci. 19, 6225–6234 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinan-Lucarré B., et al., C. elegans Punctin specifies cholinergic versus GABAergic identity of postsynaptic domains. Nature 511, 466–470 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Maro G. S., et al., MADD-4/Punctin and neurexin organize C. elegans GABAergic postsynapses through neuroligin. Neuron 86, 1420–1432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu H., Pinan-Lucarré B., Ji T., Jospin M., Bessereau J. L., C. elegans punctin clusters GABA(A) receptors via neuroligin binding and UNC-40/DCC recruitment. Neuron 86, 1407–1419 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Troemel E. R., et al., p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2, e183 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellegrino M. W., et al., Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 516, 414–417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans E. A., Kawli T., Tan M. W., Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog. 4, e1000175 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papp D., Csermely P., Sőti C., A role for SKN-1/Nrf in pathogen resistance and immunosenescence in Caenorhabditis elegans. PLoS Pathog. 8, e1002673 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alper S., McBride S. J., Lackford B., Freedman J. H., Schwartz D. A., Specificity and complexity of the Caenorhabditis elegans innate immune response. Mol. Cell. Biol. 27, 5544–5553 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shivers R. P., et al., Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet. 6, e1000892 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alper S., et al., Identification of innate immunity genes and pathways using a comparative genomics approach. Proc. Natl. Acad. Sci. U.S.A. 105, 7016–7021 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson S. T., Johnson T. E., daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 11, 1975–1980 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Lin K., Hsin H., Libina N., Kenyon C., Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 28, 139–145 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Mouchiroud L., et al., The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang H., Pang S., Proline catabolism modulates innate immunity in Caenorhabditis elegans. Cell Rep. 17, 2837–2844 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Fernandes de Abreu D. A., et al., An insulin-to-insulin regulatory network orchestrates phenotypic specificity in development and physiology. PLoS Genet. 10, e1004225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim B., Suo B., Emmons S. W., Gene function prediction based on developmental transcriptomes of the two sexes in C. elegans. Cell Rep. 17, 917–928 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sieburth D., et al., Systematic analysis of genes required for synapse structure and function. Nature 436, 510–517 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Meisel J. D., Panda O., Mahanti P., Schroeder F. C., Kim D. H., Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell 159, 267–280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao X., Kajino-Sakamoto R., Doss A., Aballay A., Distinct roles of sensory neurons in mediating pathogen avoidance and neuropeptide-dependent immune regulation. Cell Rep. 21, 1442–1451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee K., Mylonakis E., An intestine-derived neuropeptide controls avoidance behavior in Caenorhabditis elegans. Cell Rep. 20, 2501–2512 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Zheng S., et al., A functional study of all 40 Caenorhabditis elegans insulin-like peptides. J. Biol. Chem. 293, 16912–16922 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenner S., The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., Efficient gene transfer in C. elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frøkjaer-Jensen C., et al., Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40, 1375–1383 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibson D. G., Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 498, 349–361 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.