Abstract
Background:
Individuals with immune-mediated inflammatory diseases, such as inflammatory bowel disease, multiple sclerosis and rheumatoid arthritis, are at increased risk for influenza and related complications. We examined and compared the uptake of influenza vaccination among people with and without these diseases, as well as the influence of psychiatric comorbidity on vaccine uptake.
Methods:
Using administrative data from Apr. 1, 1984, to Mar. 31, 2016, we conducted a retrospective matched cohort study in Manitoba, Canada. We matched persons 18 years of age or older who had a diagnosis of inflammatory bowel disease, multiple sclerosis or rheumatoid arthritis (the immune-mediated inflammatory disease cohorts) with persons who did not have these diagnoses (the control cohorts) on age, sex and region. We then identified cohort members with any mood or anxiety disorder (depression, anxiety disorders, bipolar disorder). We identified influenza vaccinations through billing codes. Using binomial regression, we modelled the difference in the proportion of the immune-mediated inflammatory disease and matched cohorts vaccinated annually, with adjustment for sociodemographic characteristics, comorbidity and immune therapy. We tested additive interaction effects between a person’s cohort and presence of a mood or anxiety disorder.
Results:
We identified 32 880 individuals with 1 or more immune-mediated inflammatory diseases (10 148 with inflammatory bowel disease, 6158 with multiple sclerosis and 16 975 with rheumatoid arthritis) and a total of 164 152 controls. In fiscal year 2015, 8668 (41.3%, 95% confidence interval [CI] 40.6% to 42.0%) of the 20 982 persons with an immune-mediated inflammatory disease received an influenza vaccination, a rate higher than among controls (35 238 of 104 634; 33.7%, 95% CI 33.4% to 34.0%). After adjustment, participants with an immune-mediated inflammatory disease but no mood or anxiety disorder had 6.44% (95% CI 5.79% to 7.10%) greater uptake of vaccination than participants without such a disease. Among participants without an immune-mediated inflammatory disease, having a mood or anxiety disorder was associated with 4.54% (95% CI 4.20% to 4.89%) greater uptake of vaccination. However, we observed a subadditive interaction between immune-mediated inflammatory disease and psychiatric status (−1.38%, 95% CI −2.26% to −0.50%).
Interpretation:
Uptake of influenza vaccination was consistently low in populations with immune-mediated inflammatory disease, and although psychiatric morbidity is associated with greater vaccine uptake by Manitobans, it negatively interacts with these diseases to reduce uptake. Changes in care delivery are needed to mitigate this gap in care.
Individuals affected by immune-mediated inflammatory diseases (referred to hereafter as immune-mediated diseases), such as inflammatory bowel disease, multiple sclerosis and rheumatoid arthritis, share an increased risk for influenza and related complications;1 therefore, prevention of influenza is important. Effective implementation of influenza vaccination strategies requires knowledge of vaccine uptake or use among target populations. However, our understanding of influenza vaccine uptake in populations with immune-mediated diseases has been limited by studies with small sample sizes, selection bias, cross-sectional designs, variable study durations and variable study periods. Prior findings regarding vaccine uptake among persons with immune-mediated diseases have been highly variable.2–5 A population-based assessment of influenza vaccine uptake by persons with immune-mediated diseases was needed to clarify this potential gap in preventive care.
Some psychiatric disorders, such as schizophrenia, are associated with lower uptake of preventive health care such as vaccinations.6,7 It is unknown whether mood and anxiety disorders have a similar effect on preventive health behaviours; however, it is known that they affect people with immune-mediated diseases more often than people without such diseases. 8 Small studies have suggested that the presence of any comorbidity may increase vaccination uptake,5 but others reported no association.9
We compared the uptake of influenza vaccination in population-based cohorts with inflammatory bowel disease, multiple sclerosis or rheumatoid arthritis and in matched cohorts without these diseases; we then evaluated the influence of mood and anxiety disorders on vaccine uptake. We hypothesized that uptake would be higher in the cohorts with immune diseases than among those without immune disease, and that psychiatric disorders would be associated with reduced uptake. We focused on immune-mediated diseases, with care provided by different specialists, that are highly prevalent in Canada,10–12 affect individuals across the age spectrum and affect different organ systems.
Methods
Design and setting
We conducted this retrospective matched cohort study in Manitoba, Canada, where publicly funded health care is provided for medically necessary services. Manitoba Health, the provincial health department, maintains health services databases, and we accessed data for the period Apr. 1, 1984, to Mar. 31, 2016.
Data sources
We accessed high-quality databases housed in the Manitoba Population Research Data Repository at the Manitoba Centre for Health Policy, including the population registry, the Discharge Abstract Database, the medical services database and the Drug Program Information Network database.13–15 We linked these databases at the individual level using an encrypted unique identifier.
For provincial residents who are eligible to receive health services, the population registry captures sex, dates of birth and death, health care coverage and region of residence (by postal code). The Discharge Abstract Database captures hospital admissions, related dates and up to 25 diagnoses. Until Mar. 31, 2004, diagnoses were recorded using the International Classification of Diseases, 9th Revision (clinical modification) (ICD-9-CM) codes;16 thereafter, diagnoses were recorded by International Statistical Classification of Diseases and Related Health Problems, 10th Revision, enhanced Canadian version codes.17 The medical services database captures service date, 1 diagnosis assigned using ICD-9-CM codes and tariff (i.e., billing) codes (including codes for vaccinations). Since 1995, the Drug Program Information Network database has captured outpatient prescription dispensations, including the drug identification number (which links to the World Health Organization’s Anatomic Therapeutic Chemical Classification System18) and the date of dispensation.
Study populations
Using validated case definitions, we identified all Manitobans aged 18 years or older who had inflammatory bowel disease (including Crohn disease and ulcerative colitis), multiple sclerosis or rheumatoid arthritis (Appendix 1, Table e1, available at www.cmajopen.ca/content/9/2/E510/suppl/DC1).19–21 For each case, we defined the first health claim (hospital, physician, prescription) as the index date. Next, we identified a general population cohort excluding anyone with diagnosis codes for inflammatory bowel disease, multiple sclerosis or rheumatoid arthritis or use of any disease-modifying therapies specific to multiple sclerosis, as these were part of the case definition for multiple sclerosis.8 See Appendix 1 for more details about selection of the study cohorts. Then, we selected controls using a uniform approach for each disease group, that is, matched on sex, year of birth (within 5 years before or after the birthdate of the corresponding case) and forward sortation area (based on postal code). We sought 5 controls for each case, but were unable to do so for every case. We assigned controls the index date of their matched cases.
Comorbidities
We applied validated case definitions developed in Manitoba to identify members of each cohort affected by any mood or anxiety disorder (≥ 1 of depression, anxiety disorders, bipolar disorders), as well as by depression and anxiety disorders separately (Appendix 1, Tables e2 and e3).22 We classified the date of the first claim for each condition as the diagnosis date. We identified physical comorbidity using the Aggregated Diagnosis Groups (major physical, not time-limited) of the Johns Hopkins Adjusted Clinical Group Case-Mix System.23–25
Covariables
We included the following matching factors and other covariables in the regression models:26 sex (male as reference group), age (updated annually), socioeconomic status at index date, region of residence at index date, physical comorbidity (updated annually), immune-mediated disease-specific procedures (ever) and use of disease-modifying therapy (updated annually).27–29
We categorized age as 18 to 24 years (reference group), 25 to 44 years, 45 to 64 years, and 65 years or older, reflecting the emphasis on immunizing individuals in the oldest age group.
To determine socioeconomic status, we linked postal code to census data at the level of dissemination area, and then calculated the Socioeconomic Factor Index version 2, which incorporates information about average household income, percent of single parent households, unemployment rate and high school education rate. For this index, scores less than 0 indicate higher socioeconomic status,30 and missing values were imputed at the mean of 0. We categorized socioeconomic status into quintiles (highest quintile as reference). We classified the region of residence as urban (Winnipeg, population > 600 000; Brandon, population > 47 000) or rural.
Physical comorbidity was based on Aggregated Diagnosis Group scores and categorized as 0 (reference group), 1, or 2 or above, with higher scores indicating greater comorbidity.
We included immune-mediated disease-specific procedures as a measure of disease severity. For inflammatory bowel disease, these included surgical procedures related to gastrointestinal resection or ostomy placement (Appendix 1, Table e4), and for rheumatoid arthritis, they included joint-related surgical procedures (Appendix 1, Table e5); there were no relevant procedures for multiple sclerosis.
For inflammatory bowel disease and rheumatoid arthritis, we categorized immune therapies annually as none (reference group), any biologic (alone or in combination), or any anti-inflammatory or traditional immunosuppressive therapy or corticosteroids. For multiple sclerosis, we categorized therapies as none, first-line or second-line (Appendix 1, Table e6).
Influenza vaccination
Annually, we identified influenza vaccinations using tariff codes 8791, 8792 or 8799, which are used by primary care providers, public health nurses and pharmacy providers.
Statistical analysis
We summarized the characteristics of the cohort using descriptive statistics, specifically means (with standard deviations), medians (with interquartile ranges) and frequencies (as percentages). We report the crude percentage of each cohort who had the influenza vaccination annually, along with a 95% confidence interval (CI) based on the binomial distribution. We also report percentages standardized by age and sex to the 2010 Canadian census population.
We accessed data for the period Apr. 1, 1984, to Mar. 31, 2016; however, for the regression analyses, we initially limited the study period to fiscal years 2006 to 2015 to reduce secular trends. We modelled the difference in the proportion of each cohort immunized annually so that we could estimate the absolute effects of cohort (immune-mediated disease v. matched controls) and psychiatric comorbidity on this outcome, as absolute effects are more useful than relative effects for policy-makers.
We used a binomial regression model with an identity link, as well as generalized estimating equations with an exchangeable correlation structure, accounting for differences in follow-up time by including the natural logarithm of person-years as the model offset. Covariables were those defined above.31
We tested for the presence of additive interaction effects between cohort and mood or anxiety disorders (entered as cohort*disorders), which we report as interaction contrasts (i.e., the difference of risk differences). A positive (synergistic or superadditive interaction) would indicate that the joint effects of cohort and mood and anxiety disorders exceeded the sum of their individual effects, whereas a negative (subadditive) interaction would indicate that the joint effects were less than the sum of the individual effects.
We report differences in terms of percentages and 95% CIs. We repeated these analyses for each immune-mediated disease cohort separately. In a complementary analysis, we further adjusted for the annual number of physician visits.
We conducted all statistical analyses using SAS software, version 9.4 (SAS Institute Inc.).
Ethics approval
The University of Manitoba Health Research Ethics Board approved the study. Manitoba’s Health Information Privacy Committee approved data access.
Results
For the entire study period (fiscal years 1984–2015), we identified 32 880 individuals with 1 or more immune-mediated inflammatory diseases (10 148 with inflammatory bowel disease, 6158 with multiple sclerosis and 16 975 with rheumatoid arthritis), along with a total of 164 152 matched controls (Table 1). The characteristics of these 3 disease cohorts were similar to those of the entire immune-mediated disease population (referred to hereafter as “cases”) for the period 2006 to 2015. Cases and controls were well matched with respect to age, sex and socioeconomic status at the index date.
Table 1:
Characteristics of prevalent disease cohorts at the time of diagnosis and matched controls at the matched index date
| Characteristic | Disease and cohort; no (%) of patients* | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Inflammatory bowel disease | Multiple sclerosis | Rheumatoid arthritis | IMID† | |||||
|
|
|
|
|
|||||
| Controls n = 50 704 |
Cases† n = 10 148 |
Controls n = 30 690 |
Cases† n = 6158 |
Controls n = 84 756 |
Cases† n = 16 975 |
Controls n = 164 152 |
Cases† n = 32 880 |
|
| Whole population, 1984–2015 | ||||||||
|
| ||||||||
| Sex, female | 27 663 (54.6) | 5536 (54.6) | 21 564 (70.3) | 4322 (70.2) | 61 231 (72.2) | 12 263 (72.2) | 109 065 (66.4) | 21 841 (66.4) |
|
| ||||||||
| Age at diagnosis, yr, mean ± SD (range) | 41.7 ± 17.0 (18 to 99) | 41.7 ± 17.0 (18 to 99) | 42.1 ± 13.5 (18 to 97) | 42.1 ± 13.5 (18 to 96) | 54.0 ± 16.1 (18 to 105) | 54.0 ± 16.1 (18 to 103) | 48.0 ± 17.1 (18 to 105) | 48.0 ± 17.1 (18 to 103) |
|
| ||||||||
| Duration of follow-up from index date, yr, median (IQR) | 11.9 (4.92 to 21.8) | 13.3 (5.93 to 22.8) | 13.9 (5.91 to 23.6) | 13.9 (6.28 to 22.5) | 11.1 (4.93 to 19.3) | 11.4 (5.51 to 19.0) | 11.9 (5.12 to 20.8) | 12.4 (5.79 to 20.7) |
|
| ||||||||
| Urban region of residence | 33 799 (66.7) | 6763 (66.6) | 20 685 (67.4) | 4154 (67.5) | 50 281 (59.3) | 10 070 (59.3) | 103 468 (63.0) | 20 727 (63.0) |
|
| ||||||||
| Socioeconomic status,‡§ mean ± SD | −0.23 ± 0.88 | −0.26 ± 0.91 | −0.22 ± 0.88 | −0.25 ± 0.91 | 0.06 ± 1.01 | 0.03 ± 1.03 | −0.08 ± 0.96 | −0.11 ± 0.99 |
|
| ||||||||
| Physician visits in prior year | ||||||||
|
| ||||||||
| 0–4 | 41 688 (82.2) | 6569 (64.7) | 24 637 (80.3) | 3860 (62.7) | 63 997 (75.5) | 8416 (45.6) | 128 760 (78.4) | 19 997 (60.8) |
|
| ||||||||
| 5–9 | 6517 (12.9) | 2172 (21.4) | 4196 (13.7) | 1313 (21.3) | 14 230 (16.8) | 3718 (21.9) | 24 623 (15.0) | 7297 (22.2) |
|
| ||||||||
| ≥ 10 | 2499 (4.9) | 1407 (13.9) | 1857 (6.0) | 985 (16.0) | 6529 (7.7) | 4841 (28.5) | 10 769 (6.6) | 5586 (17.0) |
|
| ||||||||
| Comorbidity status at study start | ||||||||
|
| ||||||||
| No. of ADGs | ||||||||
|
| ||||||||
| 0 | 42 318 (83.5) | 6749 (66.5) | 26 373 (85.9) | 4389 (71.3) | 65 883 (77.7) | 11 216 (66.1) | 133 038 (81.0) | 22 275 (67.7) |
|
| ||||||||
| 1 | 7246 (14.3) | 2789 (27.5) | 3763 (12.3) | 1392 (22.6) | 15 605 (18.4) | 4643 (27.4) | 26 227 (16.0) | 8561 (26.0) |
|
| ||||||||
| ≥ 2 | 1140 (2.2) | 610 (6.0) | 554 (1.8) | 377 (6.1) | 3268 (3.9) | 1116 (6.6) | 4887 (3.0) | 2044 (6.2) |
|
| ||||||||
| Any mood or anxiety disorder | 10 979 (21.7) | 3147 (31.0) | 7431 (24.2) | 2485 (40.4) | 22 078 (26.0) | 5735 (33.8) | 39 911 (24.3) | 11 171 (34.0) |
|
| ||||||||
| Depression | 9590 (18.9) | 2764 (27.2) | 6546 (21.3) | 2197 (35.7) | 18 853 (22.2) | 4864 (28.7) | 34 480 (21.0) | 9645 (29.3) |
|
| ||||||||
| Anxiety disorder | 13 438 (26.5) | 3453 (34.0) | 8931 (29.1) | 2378 (38.6) | 25 922 (30.6) | 6074 (35.8) | 47 639 (29.0) | 11 707 (35.6) |
|
| ||||||||
| Bipolar disorder | 1562 (3.1) | 548 (5.4) | 1151 (3.8) | 368 (6.0) | 2963 (3.5) | 716 (4.2) | 5583 (3.4) | 1602 (4.9) |
|
| ||||||||
| Population, 2006–2015 | n = 40 364 | n = 8458 | n = 24 154 | n = 4748 | n = 64 510 | n = 12 984 | n = 127 310 | n = 25 832 |
|
| ||||||||
| Sex, female | 22 207 (55.0) | 4623 (54.7) | 17 247 (71.4) | 3399 (71.6) | 47 102 (73.0) | 9458 (72.8) | 85 349 (67.0) | 8602 (33.3) |
|
| ||||||||
| Age at diagnosis, yr, mean ± SD (range) | 41.2 ± 16.2 (18 to 99) | 41.0 ± 16.2 (18 to 99) | 40.9 ± 12.2 (18 to 97) | 40.2 ± 11.9 (18 to 94) | 51.8 ± 15.2 (18 to 105) | 51.4 ± 15.4 (18 to 102) | 46.4 ± 16.0 (18 to 105) | 45.9 ± 16.0 (18 to 102) |
|
| ||||||||
| Urban region of residence | 26 558 (65.8) | 5574 (65.9) | 15 925 (65.9) | 3153 (66.4) | 37 559 (58.2) | 7609 (58.6) | 78 949 (62.0) | 16 104 (62.3) |
|
| ||||||||
| Socioeconomic status,‡ mean ± SD | −0.22 ± 0.85 | −0.26 ± 0.89 | −0.21 ± 0.85 | −0.25 ± 0.89 | 0.06 ± 1.0 | 0.04 ± 1.0 | −0.08 ± 0.95 | −0.11 ± 0.98 |
|
| ||||||||
| Physician visits in prior year | ||||||||
|
| ||||||||
| 0–4 | 26 789 (66.4) | 3810 (45.0) | 15 224 (63.0) | 2260 (47.6) | 37 960 (58.8) | 4745 (36.5) | 78 891 (62.0) | 10 716 (41.5) |
|
| ||||||||
| 5–9 | 8700 (21.6) | 2358 (27.9) | 5711 (23.6) | 1313 (27.7) | 16 064 (24.9) | 3593 (27.7) | 30 072 (23.6) | 7171 (27.8) |
|
| ||||||||
| ≥ 10 | 4875 (12.1) | 2290 (27.1) | 3219 (13.3) | 1175 (24.7) | 10 486 (16.3) | 4646 (35.8) | 18 347 (14.4) | 7945 (30.8) |
|
| ||||||||
| Comorbidity status at index date | ||||||||
|
| ||||||||
| No. of ADGs | ||||||||
|
| ||||||||
| 0 | 33 895 (84.0) | 5546 (65.6) | 20 903 (86.5) | 3371 (71.0) | 51 284 (79.5) | 8594 (66.2) | 104 753 (82.3) | 17 434 (67.5) |
|
| ||||||||
| 1 | 5638 (14.0) | 2390 (28.2) | 2892 (12.0) | 1089 (22.9) | 11 140 (17.3) | 3577 (27.5) | 19 340 (15.2) | 6827 (26.4) |
|
| ||||||||
| ≥ 2 | 831 (2.0) | 522 (6.2) | 359 (1.5) | 288 (6.1) | 2086 (3.2) | 813 (6.3) | 3217 (2.5) | 1571 (6.1) |
|
| ||||||||
| Any mood and anxiety disorder | 9108 (22.6) | 2669 (31.6) | 6119 (25.3) | 1985 (41.8) | 17 008 (26.4) | 4490 (34.6) | 31 739 (24.9) | 8960 (34.7) |
|
| ||||||||
| Depression | 7992 (19.8) | 2343 (27.7) | 5410 (22.4) | 1742 (36.7) | 14 548 (22.6) | 3808 (29.3) | 27 517 (21.6) | 7723 (29.9) |
|
| ||||||||
| Anxiety disorder | 11 444 (28.4) | 2980 (35.2) | 7543 (31.2) | 1983 (41.8) | 21 332 (33.1) | 5063 (39.0) | 39 735 (31.2) | 9839 (38.1) |
|
| ||||||||
| Bipolar disorder | 1326 (3.3) | 465 (5.5) | 968 (4.0) | 301 (6.3) | 2347 (3.6) | 575 (4.4) | 4559 (3.6) | 1312 (5.1) |
Note: IMID = immune-mediated inflammatory disease (IMID cohort combines patients with inflammatory bowel disease, multiple sclerosis and rheumatoid arthritis).
Except where indicated otherwise.
A small number of individuals met the case definitions for more than 1 of the IMIDs of interest. These were included in the analysis, to ensure generalizability of the findings, but they were counted only once in the combined IMID cohort. In this situation, they were classified on the basis of the IMID with the earliest index date in the coverage period. Some cases did not have 5 matched controls (IMID, n = 90; inflammatory bowel disease, n = 14; multiple sclerosis, n = 36; rheumatoid arthritis, n = 42).
Socioeconomic status is reported as the Socioeconomic Factor Index score, incorporating information about average household income, percent of single-parent households, unemployment rate and high school education rate, with missing values imputed at the mean of 0. With this measure, values less than 0 indicate higher socioeconomic status.
Missing data: for multiple sclerosis, 20; for multiple sclerosis controls, 110; for inflammatory bowel disease, 21; for inflammatory bowel disease controls, 76; for rheumatoid arthritis, 40; for rheumatoid arthritis controls, 160; imputed at population mean = 0.
Influenza vaccination
The crude percentage of combined cases and controls who received influenza vaccination rose over time (Figure 1 and Appendix 1, Table e7). Moreover, the percentage of cases who received an influenza vaccination was similar across immune-mediated diseases after age and sex-standardization (Figure 2 and Appendix 1, Table e8).
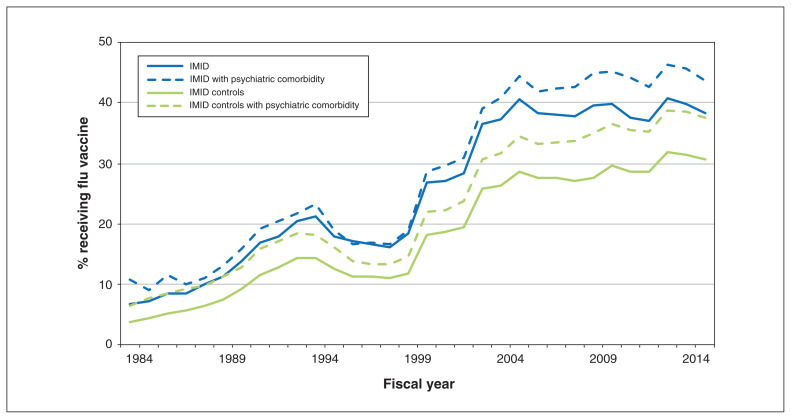
Figure 1:
Percentage of persons in the combined immune-mediated inflammatory disease (IMID) cohort and the combined cohort of matched controls who received an influenza vaccination, stratified by psychiatric comorbidity status, fiscal years 1984 to 2015 (i.e., Apr. 1, 1984, to Mar. 31, 2016).
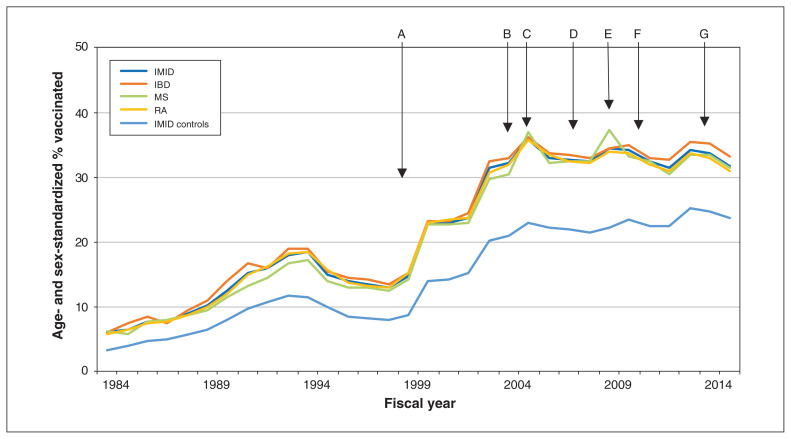
Figure 2:
Age- and sex-standardized percentage of persons in the combined immune-mediated inflammatory disease (IMID) cohort, the individual disease cohorts and the combined cohort of matched controls who received an influenza vaccination, fiscal years 1984 to 2015 (i.e., Apr. 1, 1984, to Mar. 31, 2016). Arrows indicate changes in groups eligible for provincial (public) funding of vaccination: in 1999, age ≥ 65 years, chronic conditions, health care workers (A); in 2004, children aged 6–23 months and their families (B); in 2005, patients with chronic respiratory diseases (C); in 2007, pregnant women (D); in 2009, H1N1 epidemic (E); in 2010, coverage for entire provincial population (F); and in 2014, pharmacists able to administer influenza vaccine (G). Note: IBD = inflammatory bowel disease, MS = multiple sclerosis, RA = rheumatoid arthritis.
In 2015, the crude percentage of cases who received an influenza vaccination was 41.3% (n = 8668 of 20 982; 95% CI 40.6% to 42.0%), 7.6 percentage points more than matched controls (n = 35 238 of 104 634; 33.7%, 95% CI 33.4% to 34.0%). Among cases, those with any mood or anxiety disorder had greater vaccine uptake than those without such a disorder (43.7%, 95% CI 42.8% to 44.6% v. 38.3%, 95% CI 37.3% to 39.3%). Similarly, among the controls, those with a mood or anxiety disorder had greater vaccine uptake than those without such a disorder (37.5%, 95% CI 37.1% to 37.9% v. 30.5%, 95% CI 30.1% to 30.9). The percentage of people immunized increased with age and was highest among those aged 65 years or older (Appendix 1, Figure e1).
Multivariable analysis
On multivariable analysis for the period 2006 to 2015, cases with no mood or anxiety disorder had 6.44% (95% CI 5.79 to 7.10) greater vaccination uptake than controls (Table 2). Among controls, having a mood or anxiety disorder was associated with 4.54% (95% CI 4.20% to 4.89%) more vaccination uptake. However, we observed a subadditive interaction between case and mood or anxiety disorder status (−1.38%, 95% CI −2.26% to −0.50%). Female as compared with male sex, older age, living in an urban not rural region, more physical comorbidities, prior disease-specific surgery and use of immune therapies were all associated with increased vaccine uptake. Our findings were similar after further adjustment for physician visits (Appendix 1, Table e9).
Table 2:
Multivariable adjusted risk differences for the association of immune-mediated disease, any mood or anxiety disorder and uptake of influenza vaccination
| Variable | Cohort; adjusted risk difference,* % (95% CI)† | |||
|---|---|---|---|---|
| Immune-mediated disease | Inflammatory bowel disease | Multiple sclerosis | Rheumatoid arthritis | |
| No. flu shots/PYs at risk | 402 542/1 235 712 | 111 983/401 123.6 | 72 424/248 553.6 | 223 725/601 042.5 |
| Controls without a mood/anxiety disorder | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Immune-mediated disease effect without mood/anxiety disorder effect | 6.44 (5.79 to 7.10) | 7.19 (6.12 to 8.26) | 6.70 (5.13 to 8.27) | 5.61 (4.64 to 6.59) |
| Mood/anxiety disorder effect without an immune-mediated disease effect | 4.54 (4.20 to 4.89) | 5.10 (4.52 to 5.68) | 5.31 (4.53 to 6.09) | 3.74 (3.23 to 4.26) |
| Interaction contrast between case effect and mood/anxiety disorder effect‡ | −1.38 (−2.26 to −0.50) | −1.43 (−2.90 to 0.05) | 0.08 (−1.97 to 2.13) | −1.89 (−3.16 to −0.63) |
| Age, yr | ||||
| 18–24 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| 25–44 | 6.12 (5.59 to 6.64) | 5.61 (4.98 to 6.24) | 6.94 (5.47 to 8.40) | 5.56 (4.41 to 6.71) |
| 45–64 | 15.10 (14.55 to 15.65) | 13.97 (13.28 to 14.67) | 15.15 (13.65 to 16.64) | 14.96 (13.79 to 16.14) |
| ≥ 65 | 33.33 (32.73 to 33.94) | 33.77 (32.87 to 34.67) | 32.61 (30.95 to 34.27) | 32.32 (31.11 to 33.53) |
| Sex | ||||
| Male | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Female | 4.97 (4.64 to 5.29) | 4.92 (4.41 to 5.43) | 3.66 (2.90 to 4.42) | 4.61 (4.09 to 5.13) |
| Region | ||||
| Rural | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Urban | 2.16 (1.83 to 2.49) | 1.88 (1.35 to 2.40) | 2.28 (1.52 to 3.04) | 2.56 (2.06 to 3.06) |
| Socioeconomic status | ||||
| Quintile 1 (lowest) | −4.69 (−5.19 to −4.19) | −4.66 (−5.48 to −3.84) | −3.38 (−4.52 to −2.23) | −6.03 (−6.82 to −5.25) |
| Quintile 2 | −3.74 (−4.25 to −3.23) | −4.00 (−4.79 to −3.22) | −3.45 (−4.57 to −2.34) | −4.01 (−4.83 to −3.18) |
| Quintile 3 | −3.09 (−3.61 to −2.58) | −3.40 (−4.18 to −2.61) | −2.74 (−3.85 to −1.63) | −3.39 (−4.23 to −2.55) |
| Quintile 4 | −2.25 (−2.75 to −1.75) | −2.16 (−2.93 to −1.39) | −2.21 (−3.30 to −1.12) | −2.66 (−3.49 to −1.84) |
| Quintile 5 (highest) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Comorbidity | ||||
| 0 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| 1 | 4.77 (4.32 to 5.22) | 3.43 (2.69 to 4.16) | 3.78 (2.68 to 4.87) | 5.66 (5.01 to 6.30) |
| ≥ 2 | 8.67 (7.60 to 9.74) | 8.64 (6.61 to 10.7) | 8.96 (6.10 to 11.81) | 8.34 (6.95 to 9.72) |
| Immune-mediated disease-specific procedure | 4.94 (4.21 to 5.67) | 6.90 (5.58 to 8.22) | – | 4.00 (3.12 to 4.88) |
| Immune therapy | ||||
| None | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Anti-inflammatory or immune-modulatory therapy | 3.09 (2.73 to 3.46) | 2.95 (2.39 to 3.52) | 4.43 (2.53 to 6.32) | 3.07 (2.58 to 3.56) |
| Any biologic | 9.08 (7.92 to 10.23) | 9.11 (7.09 to 11.13) | 5.60 (2.17 to 9.03) | 9.45 (7.93 to 10.97) |
Note: CI = confidence interval, PY = person-year, Ref. = reference category.
Adjusted for all other variables included in table.
Except where indicated otherwise.
A negative interaction contrast with a 95% CI that does not encompass 0 indicates a subadditive effect; that is, the joint effect of immune-mediated disease and a mood or anxiety disorder is less than the sum of their individual effects. A positive interaction with a 95% CI that does not encompass 0 indicates a superadditive effective that is greater than the sum of the individual immune-mediated disease and mood or anxiety disorder effects.
When we conducted separate analyses for depression and anxiety disorders, the findings were similar to those for mood and anxiety disorders combined (Table 3 and Table 4). When we conducted analyses for individual immune-mediated diseases, findings in the rheumatoid arthritis cohort were similar to those for the combined immune-mediated disease cohort. Findings in the inflammatory bowel disease cohort were similar in magnitude and direction to those in the combined immune-mediated disease cohort but were not statistically significant. In contrast, we did not observe a departure from additivity in the multiple sclerosis cohort for any mood or anxiety disorder, but we did observe a superadditive effect for depression and anxiety disorders (Tables 2, 3 and 4).
Table 3:
Multivariable adjusted risk differences for the association of immune-mediated inflammatory disease, depressive disorder and uptake of influenza vaccination
| Variable | Cohort; adjusted risk difference,* % (95% CI)† | |||
|---|---|---|---|---|
| Immune-mediated disease | Inflammatory bowel disease | Multiple sclerosis | Rheumatoid arthritis | |
| No. flu shots/PYs at risk | 402 542/1 235 712 | 111 983/401 123.6 | 72 424/248 553.6 | 223 725/601 042.5 |
| Controls without depression | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Immune-mediated disease effect without depression effect | 6.08 (5.49 to 6.66) | 6.81 (5.82 to 7.80) | 5.81 (4.45 to 7.18) | 5.52 (4.65 to 6.38) |
| Depression effect without immune-mediated disease effect | 4.15 (3.75 to 4.56) | 5.11 (4.40 to 5.82) | 4.28 (3.39 to 5.17) | 3.41 (2.82 to 4.00) |
| Interaction contrast between case effect and depressive disorder effect‡ | −0.98 (−1.93 to −0.04) | −1.03 (−2.70 to 0.64) | 2.43 (0.36 to 4.49) | −2.53 (−3.88 to −1.18) |
| Age, yr | ||||
| 18–24 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| 25–44 | 6.36 (5.83 to 6.89) | 5.87 (5.24 to 6.51) | 7.33 (5.90 to 8.77) | 5.74 (4.58 to 6.90) |
| 45–64 | 15.45 (14.90 to 16.00) | 14.36 (13.66 to 15.05) | 15.65 (14.19 to 17.11) | 15.22 (14.04 to 16.40) |
| ≥ 65 | 33.75 (33.14 to 34.35) | 34.26 (33.36 to 35.15) | 33.22 (31.59 to 34.86) | 32.60 (31.39 to 33.82) |
| Sex | ||||
| Male | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Female | 5.19 (4.87 to 5.52) | 5.12 (4.62 to 5.63) | 3.96 (3.21 to 4.72) | 4.80 (4.28 to 5.32) |
| Region | ||||
| Rural | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Urban | 2.28 (1.95 to 2.61) | 1.99 (1.47 to 2.52) | 2.47 (1.71 to 3.23) | 2.67 (2.17 to 3.16) |
| Socioeconomic status | ||||
| Quintile 1 (lowest) | −4.71 (−5.21 to −4.20) | −4.69 (−5.51 to −3.87) | −3.41 (−4.56 to −2.27) | −6.03 (−6.82 to −5.25) |
| Quintile 2 | −3.81 (−4.32 to −3.29) | −4.15 (−4.94 to −3.36) | −3.57 (−4.58 to −2.45) | −4.02 (−4.85 to −3.19) |
| Quintile 3 | −3.14 (−3.65 to −2.62) | −3.47 (−4.26 to −2.68) | −2.83 (−3.94 to −1.72) | −3.40 (−4.24 to −2.56) |
| Quintile 4 | −2.27 (−2.77 to −1.77) | −2.19 (−2.97 to −0.64) | −2.29 (−3.38 to −1.19) | −2.66 (−3.48 to −1.84) |
| Quintile 5 (highest) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Comorbidity | ||||
| 0 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| 1 | 4.86 (4.41 to 5.31) | 3.53 (2.80 to 4.27) | 3.89 (2.79 to 4.98) | 5.73 (5.09 to 6.37) |
| ≥ 2 | 8.73 (7.65 to 9.80) | 8.65 (6.62 to 10.68) | 9.04 (6.18 to 11.90) | 8.41 (7.02 to 9.79) |
| Immune-mediated disease-specific procedure | 4.96 (4.23 to 5.69) | 6.95 (5.63 to 8.27) | – | 4.01 (3.13 to 4.89) |
| Immune therapy | ||||
| None | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Anti-inflammatory or immune-modulatory therapy | 3.10 (2.74 to 3.47) | 2.97 (2.40 to 3.54) | 4.40 (2.51 to 6.29) | 3.06 (2.57 to 3.55) |
| Any biologic | 9.08 (7.92 to 10.23) | 9.08 (7.06 to 11.10) | 5.54 (2.11 to 8.96) | 9.46 (7.94 to 10.98) |
Note: CI = confidence interval, PY = person-year, Ref. = reference category.
Adjusted for all other variables included in table.
Except where indicated otherwise.
A negative interaction contrast with a 95% CI that does not encompass 0 indicates a subadditive effect; that is, the joint effect of immune-mediated disease and depressive disorder is less than the sum of their individual effects. A positive interaction with a 95% CI that does not encompass 0 indicates a superadditive effective that is greater than the sum of the individual immune-mediated disease and depressive disorder effects.
Table 4:
Multivariable adjusted risk differences for the association of immune-mediated inflammatory disease, anxiety disorder and uptake of influenza vaccination
| Variable | Cohort; adjusted risk difference,* % (95% CI)† | |||
|---|---|---|---|---|
| Immune-mediated disease | Inflammatory bowel disease | Multiple sclerosis | Rheumatoid arthritis | |
| No. flu shots/PYs at risk | 402 542/1 235 712 | 111 983/401 123.6 | 72 424/248 553.6 | 223 725/601 042.5 |
| Controls without anxiety disorder | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Immune-mediated disease effect without anxiety disorder effect | 6.58 (5.96 to 7.19) | 6.89 (5.86 to 7.91) | 7.78 (6.35 to 9.21) | 5.72 (4.80 to 6.64) |
| Anxiety effect without immune-mediated disease effect | 4.41 (4.05 to 4.77) | 4.93 (4.32 to 5.53) | 4.95 (4.65 to 5.75) | 3.75 (3.22 to 4.27) |
| Interaction contrast between case effect and anxiety disorder effect‡ | −1.74 (−2.62 to −0.86) | −0.78 (−2.27 to 0.71) | 2.43 (0.36 to 4.49) | −2.35 (−3.62 to −1.07) |
| Age, yr | ||||
| 18–24 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| 25–44 | 6.22 (5.70 to 6.74) | 5.72 (5.09 to 6.35) | 7.12 (5.67 to 8.58) | 5.62 (4.47 to 6.77) |
| 45–64 | 15.21 (14.67 to 15.76) | 14.08 (13.39 to 14.78) | 15.36 (13.88 to 16.85) | 15.03 (13.86 to 16.20) |
| ≥ 65 | 33.44 (32.83 to 34.04) | 33.88 (32.98 to 34.77) | 32.83 (31.17 to 34.48) | 32.38 (31.18 to 33.59) |
| Sex | ||||
| Male | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Female | 5.10 (4.78 to 5.42) | 5.06 (4.55 to 5.57) | 3.86 (3.10 to 4.62) | 4.69 (4.17 to 5.21) |
| Region | ||||
| Rural | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Urban | 2.20 (1.87 to 2.53) | 1.91 (1.38 to 2.43) | 2.32 (1.56 to 3.09) | 2.59 (2.09 to 3.09) |
| Socioeconomic status | ||||
| Quintile 1 (lowest) | −4.70 (−5.21 to −4.20) | −4.69 (−5.51 to −3.87) | −3.30 (−4.44 to −2.15) | −6.05 (−6.84 to −5.27) |
| Quintile 2 | −3.73 (−4.24 to −3.22) | −4.04 (−4.82 to −3.25) | −3.40 (−4.52 to −2.29) | −4.00 (−4.82 to −3.17) |
| Quintile 3 | −3.06 (−3.57 to −2.54) | −3.35 (−4.14 to −2.57) | −2.70 (−3.81 to −1.58) | −3.37 (−4.20 to −2.53) |
| Quintile 4 | −2.24 (−2.75 to −1.74) | −2.15 (−2.92 to −1.38) | −2.16 (−3.52 to −1.06) | −2.67 (−3.49 to −1.84) |
| Quintile 5 (highest) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Comorbidity | ||||
| 0 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| 1 | 4.81 (4.36 to 5.26) | 3.46 (2.72 to 4.20) | 3.86 (2.76 to 4.96) | 5.68 (5.04 to 6.33) |
| ≥ 2 | 8.75 (7.68 to 9.83) | 8.68 (6.65 to 10.71) | 9.17 (6.31 to 12.04) | 8.40 (7.02 to 9.79) |
| Immune-mediated disease-specific procedure | 4.98 (4.26 to 5.71) | 6.91 (5.59 to 8.23) | – | 4.04 (3.17 to 4.92) |
| Immune therapy | ||||
| None | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Anti-inflammatory/immune-modulatory therapy | 3.09 (2.72 to 3.45) | 2.97 (2.40 to 3.53) | 4.48 (2.58 to 6.37) | 3.06 (2.57 to 3.55) |
| Any biologic | 9.10 (7.95 to 10.30) | 9.17 (7.15 to 11.20) | 5.77 (2.31 to 9.24) | 9.46 (7.93 to 10.98) |
Note: CI = confidence interval, PY = person-years, Ref. = reference category.
Adjusted for all other variables included in table.
Except where indicated otherwise.
A negative interaction contrast with a 95% CI that does not encompass 0 indicates a subadditive effect; that is, the joint effect of immune-mediated disease and anxiety disorder is less than the sum of their individual effects. A positive interaction with a 95% CI that does not encompass 0 indicates a superadditive effective that is greater than the sum of the individual immune-mediated disease and anxiety disorder effects.
Interpretation
In this population-based study, vaccination uptake increased over the 30-year study period in 3 immune-mediated disease cohorts and in matched cohorts without immune-mediated disease, in concert with programmatic changes. In 1999, vaccinations became publicly funded for Manitobans aged 65 years and older, those with some chronic conditions and health care workers. In 2005, vaccination funding was extended to those with chronic respiratory disease. However, despite vaccination coverage for the entire provincial population as of 2010 and access through pharmacists as of 2014, vaccination rates in all populations remain lower than desired. In 2015, only 4 in 10 persons with immune-mediated disease were vaccinated annually, which was only slightly better than the 3 in 10 persons without immune-mediated disease who were vaccinated annually. In the matched population, having a mood or anxiety disorder was associated with increased vaccination uptake. However, the joint effect of an immune-mediated disease and mood or anxiety disorder on increasing uptake was subadditive.
In Canada, the target vaccination rate for high-risk populations and adults aged 65 years or older is 80%; vaccination is also recommended in immune-mediated disease guidelines.32–34 We found vaccination rates well below this target. In the 2013/14 Canadian Community Health Survey, only 37.8% of adults aged 18 to 64 years of age with a chronic medical condition were vaccinated.35
Findings from prior studies involving persons with inflammatory bowel disease, multiple sclerosis and rheumatoid arthritis have been inconsistent. Reported vaccination rates among those with inflammatory bowel disease have ranged from 6% to 80%.2,3 Investigation of temporal changes in vaccine uptake has been limited. In Israel, 16.1% of persons with Crohn disease were vaccinated in 2006; this increased to 38.3% by 2012.36 Findings were similar among persons with ulcerative colitis.37 Reported uptake of influenza vaccine has also ranged widely among persons with rheumatoid arthritis,5,38–40 from 26.6% in a German outpatient clinic40 to 85% in specialty rheumatology centres in the United Kingdom.5 Comparable findings for multiple sclerosis are more limited. In Israel, 37.6% of 101 participants with multiple sclerosis reported receiving the seasonal influenza vaccine during the winter of 2009/10.4 In Norway, 60.7% of persons with multiple sclerosis received the pandemic (H1N1) vaccine in 2009/10.41
We observed a subadditive interaction between immune-mediated disease and psychiatric comorbidity on vaccination uptake in the combined cohort and in the cohorts of persons with inflammatory bowel disease and rheumatoid arthritis. This finding may reflect competing demands during physician visits42 or reduced adherence to treatment recommendations by persons with psychiatric disorders, whether pharmacologic43–45 or health behaviours.46 However, we did not observe a subadditive interaction in the multiple sclerosis cohort, which may reflect differences in provincial programs of care for immune-mediated disease, which are more centralized for multiple sclerosis.
Older age, female sex, higher socioeconomic status, urban residence, greater disease severity (as evidenced by prior surgeries), physical comorbidities and use of immune therapies were associated with greater uptake of vaccination. These findings are consistent with those for the Canadian general population35 and earlier studies in immune-mediated disease populations.3,36,37
Factors contributing to low vaccination rates include a lack of perceived susceptibility to influenza, lack of perceived severity of infection and lack of belief in the vaccine’s effectiveness. 2,3,47–49 Interventions to improve vaccination rates in populations of persons with immune-mediated disease have been tested. Use of electronic medical record alerts improved vaccination rates in immunosuppressed people seen in rheumatology clinics (outpatient, hospital-based); having a nurse-led process improved vaccination rates further.50 At an inflammatory bowel disease clinic, distributing a vaccine questionnaire before attendance at the clinic and offering recommended vaccinations increased influenza vaccine uptake from 54% to 81%.51 Future studies aimed at improving implementation of such strategies are needed.
Limitations
Strengths of this study included the population-based design, application of validated case definitions for immune-mediated diseases and psychiatric comorbidity, and the extended study period. The female predominance and age distribution of our cohorts are consistent with the epidemiology of the diseases studied.52–54
Conduct of the study in a single province was a limitation. However, in the 2013/14 Canadian Community Health Survey, the proportion of individuals vaccinated was similar across provinces, apart from Nova Scotia, Quebec and Newfoundland and Labrador.55 As such, our findings are likely generalizable within Canada, although they may not be generalizable elsewhere.
We may not have identified all vaccinations administered, given that vaccinations in private workplaces would not be captured in our data; however, underascertainment was likely to be nondifferential among the cohorts and would not fully account for our findings. Reassuringly, the percentage of Manitobans reporting influenza vaccinations in the Canadian Community Health Survey (30%) was consistent with the percentage among our controls.55
We lacked clinical characteristics related to the immune-mediated diseases, but we included some measures of disease severity and treatment status.
Conclusion
Uptake of influenza vaccination was lower than desired in populations with immune-mediated disease. Given the increased susceptibility of these populations to influenza and related complications, it is essential that action be taken to ameliorate this gap in preventive care. Although having a mood or anxiety disorder was associated with increased influenza vaccine uptake among Manitobans without immune-mediated disease, comorbid mood or anxiety disorder interacted negatively with immune-mediated disease. This suggests that the association of psychiatric comorbidity with other preventive health behaviours should also be evaluated in people with immune-mediated diseases.
Supplementary Material
Acknowledgements
The Aggregated Diagnosis Groups (ADGs) codes for risk adjustment in regression models were created using The Johns Hopkins Adjusted Clinical Group (ACG) Case-Mix System version 9. The authors acknowledge the Manitoba Centre for Health Policy for use of the Population Health Research Data Repository under project #2014-030 (HIPC #2014/2015-19A). The results and conclusions presented are those of the authors; no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health or other data providers is intended or should be inferred.
Footnotes
Competing interests: For work outside the study reported here, Ruth Ann Marrie has received research funding from the Canadian Institutes of Health Research (CIHR), Research Manitoba, the Multiple Sclerosis Society of Canada, the Multiple Sclerosis Scientific Research Foundation, Crohn’s and Colitis Canada, the National Multiple Sclerosis Society and the Consortium of Multiple Sclerosis Centers; she has also participated in research funded by Roche and Biogen Idec (all funds to co-investigators). Jitender Sareen has received consulting fees from UpToDate and previously held stock in Johnson & Johnson. For work outside the study reported here, Scott Patten has received research funding from CIHR, the Hotchkiss Brain Institute and the Multiple Sclerosis Society of Canada (which includes contributions from Roche, Biogen and the Government of Alberta); he also holds the Cuthbertson and Fischer Chair in Pediatric Mental Health at the University of Calgary. Alexander Singer has received financial and in-kind support from an IBM/CIMVHR Advanced Analytics Grant and Calian Inc. For work outside the study reported here, Lisa Lix has received research funds from CIHR and the Arthritis Society. Carol Hitchon has received research funds for unrelated studies from UCB Canada and Pfizer. James Marriott has received grant funding from Roche (as site principal investigator for a clinical trial). Renée El-Gabalawy has received research funds for unrelated studies from University of Manitoba Start-Up Funds, the CIHR Chronic Pain Network, Health Sciences Centre foundation grant, Department of Anesthesia operating grant and the Tri-Agency New Frontiers in Research Fund. For activities unrelated to the current study, John Fisk has received research funds from CIHR, the Multiple Sclerosis Society of Canada, the Nova Scotia Health Authority Research Fund and the Dalhousie Medical Research Fund, as well as royalty fees from MAPI Research Trust. For activities unrelated to the current study, Charles Bernstein has received consultancy fees from Roche Canada, Mylan Pharmaceuticals and Takeda Canada; contract research funding from Pfizer, Janssen Canada and Roche; unrestricted educational or research grants from Abbvie Canada, Janssen Canada, Pfizer Canada, Takeda Canada, Sandoz and Medtronic Canada; speakers’ fees from Abbvie Canada, Janssen Canada, Pfizer Canada, Takeda Canada and Medtronic Canada; and has served on advisory boards for Abbvie Canada, Janssen Canada, Pfizer Canada, Takeda Canada, Sandoz, Amgen Canada, Bristol Myers Squibb Canada and Roche Canada. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Ruth Ann Marrie, James Bolton, Jitender Sareen, Scott Patten, Lisa Lix, Carol Hitchon, James Marriott, Alan Katz, John Fisk and Charles Bernstein obtained study funding and designed the study. Randy Walld analyzed the data, and all of the authors interpreted the data. Ruth Ann Marrie drafted the manuscript, and Randy Walld, James Bolton, Jitender Sareen, Scott Patten, Alexander Singer, Lisa Lix, Carol Hitchon, James Marriott, Renée El-Gabalawy, Alan Katz, John Fisk and Charles Bernstein revised the manuscript for important intellectual content. All of the authors approved the final version for publication and agreed to be accountable for the work.
Members of the CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease: Ruth Ann Marrie, James M. Bolton, Jitender Sareen, John R. Walker (deceased), Scott B. Patten, Alexander Singer, Lisa M. Lix, Carol A. Hitchon, Renée El-Gabalawy, Alan Katz, John D. Fisk, Charles N. Bernstein, Lesley Graff, Lindsay Berrigan, Ryan Zarychanski, Christine Peschken, James J. Marriott.
Funding: This study was funded by the Canadian Institutes of Health Research (CIHR; THC-135234), Crohn’s and Colitis Canada and the Waugh Family Chair in Multiple Sclerosis (to Ruth Ann Marrie). James Bolton has received research funding from the Brain & Behavior Research Foundation and the Multiple Sclerosis Society of Canada. Charles Bernstein is supported in part by the Bingham Chair in Gastroenterology. Jitender Sareen is supported by CIHR grant no. 333252. Lisa Lix is supported by a Canada Research Chair. Renée El-Gabalawy is supported by University of Manitoba Start-Up Funding. Alan Katz held a contract from the Government of Manitoba for maintenance of the data repository. The sponsors had no role in the design and conduct of the study; the collection, management, analysis or interpretation of the data; or the preparation, review or approval of the manuscript.
Data sharing: The authors of this study are not the data custodians and are therefore not authorized to make the study data available to others. With appropriate approvals, the data can be accessed through the Manitoba Centre for Health Policy.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/9/2/E510/suppl/DC1.
References
- 1.Blumentals WA, Arreglado A, Napalkov P, et al. Rheumatoid arthritis and the incidence of influenza and influenza-related complications: a retrospective cohort study. BMC Musculoskelet Disord. 2012;13:158. doi: 10.1186/1471-2474-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waszczuk K, Waszczuk E, Szenborn L. Can we better protect patients with inflammatory bowel disease against infections — patient attitude and personal immunization knowledge. Acta Gastroenterol Belg. 2018;81:257–61. [PubMed] [Google Scholar]
- 3.Wasan SK, Calderwood AH, Long MD, et al. Immunization rates and vaccine beliefs among patients with inflammatory bowel disease: an opportunity for improvement. Inflamm Bowel Dis. 2014;20:246–50. doi: 10.1097/01.MIB.0000437737.68841.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auriel E, Gadoth A, Regev K, et al. Seasonal and H1N1v influenza vaccines in MS: safety and compliance. J Neurol Sci. 2012;314:102–3. doi: 10.1016/j.jns.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Subesinghe S, Rutherford AI, Ibrahim F, et al. A large two-centre study in to rates of influenza and pneumococcal vaccination and infection burden in rheumatoid arthritis in the UK. BMC Musculoskelet Disord. 2016;17:322. doi: 10.1186/s12891-016-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martens PJ, Chochinov HM, Prior HJ, et al. Need To Know Team. Are cervical cancer screening rates different for women with schizophrenia? A Manitoba population-based study. Schizophr Res. 2009;113:101–6. doi: 10.1016/j.schres.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32:519–43. doi: 10.1016/j.genhosppsych.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Marrie RA, Walld R, Bolton JM, et al. CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J Psychosom Res. 2017;101:17–23. doi: 10.1016/j.jpsychores.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Pham HV, Hasan I, Udaltsova N, et al. Rates and predictors of vaccinations among inflammatory bowel disease patients receiving anti-tumor necrosis factor agents. Dig Dis Sci. 2018;63:209–17. doi: 10.1007/s10620-017-4716-6. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559–68. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 11.Arthritis in Canada: an ongoing challenge. Ottawa: Health Canada; 2003. Cat no H39-4/14-2003E. [Google Scholar]
- 12.Evans C, Beland SG, Kulaga S, et al. Incidence and prevalence of multiple sclerosis in the Americas: a systematic review. Neuroepidemiology. 2013;40:195–210. doi: 10.1159/000342779. [DOI] [PubMed] [Google Scholar]
- 13.Roos LL, Mustard CA, Nicol JP. Registries and administrative data: organization and accuracy. Med Care. 1993;31:201–12. doi: 10.1097/00005650-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Roos LL, Sharp SM, Cohen MM. Comparing clinical information with claims data: some similarities and differences. J Clin Epidemiol. 1991;44:881–8. doi: 10.1016/0895-4356(91)90050-j. [DOI] [PubMed] [Google Scholar]
- 15.Roos LL, Walld R, Wajda A, et al. Record linkage strategies, outpatient procedures, and administrative data. Med Care. 1996;34:570–82. doi: 10.1097/00005650-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 16.ICD-9-CM: international classification of diseases, 9th revision, clinical modification. 5th ed; Medicode; 1996. [Google Scholar]
- 17.ICD-10-CA: international statistical classification of diseases and related health problems, tenth revision, Canada. Ottawa: Canadian Institute for Health Information; 2015. [Google Scholar]
- 18.Anatomic therapeutic chemical classification system — structure and principles. WHO Collaborating Centre for Drug Statistics Methodology; [accessed 2012 Aug 9]. updated 2018 Feb 15Available: www.whocc.no/atc/structure_and_principles/ [Google Scholar]
- 19.Bernstein CN, Blanchard JF, Rawsthorne P, et al. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol. 1999;149:916–24. doi: 10.1093/oxfordjournals.aje.a009735. [DOI] [PubMed] [Google Scholar]
- 20.Al-Sakran LH, Marrie RA, Blackburn DF, et al. Establishing the incidence and prevalence of multiple sclerosis in Saskatchewan. Can J Neurol Sci. 2018;45:295–303. doi: 10.1017/cjn.2017.301. [DOI] [PubMed] [Google Scholar]
- 21.Hitchon CA, Khan S, Elias B, et al. Prevalence and incidence of rheumatoid arthritis in Canadian First Nations and non-First Nations people: a population-based study. J Clin Rheumatol. 2020;26:169–75. doi: 10.1097/RHU.0000000000001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrie RA, Fisk JD, Yu BN, et al. CIHR Team in the Epidemiology and Impact of Comorbidity on Multiple Sclerosis. Mental comorbidity and multiple sclerosis: validating administrative data to support population-based surveillance. BMC Neurol. 2013;13:16. doi: 10.1186/1471-2377-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starfield B, Weiner J, Mumford L, et al. Ambulatory care groups: a categorization of diagnoses for research and management. Health Serv Res. 1991;26:53–74. [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner JP, Starfield BH, Steinwachs DM, et al. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29:452–72. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Reid RJ, Roos NP, MacWilliam L, et al. Assessing population health care need using a claims-based ACG morbidity measure: a validation analysis in the province of Manitoba. Health Serv Res. 2002;37:1345–64. doi: 10.1111/1475-6773.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjölander A, Greenland S. Ignoring the matching variables in cohort studies — When is it valid and why? Stat Med. 2013;32:4696–708. doi: 10.1002/sim.5879. [DOI] [PubMed] [Google Scholar]
- 27.Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9:208–20. [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen RM. National health surveys and the behavioral model of health services use. Med Care. 2008;46:647–53. doi: 10.1097/MLR.0b013e31817a835d. [DOI] [PubMed] [Google Scholar]
- 29.Andersen R, Aday LA, Chen MS. Health status and health care utilization. Health Aff (Millwood) 1986;5:154–72. doi: 10.1377/hlthaff.5.1.154. [DOI] [PubMed] [Google Scholar]
- 30.Chateau D, Metge C, Prior H, et al. Learning from the census: the Socioeconomic Factor Index (SEFI) and health outcomes in Manitoba. Can J Public Health. 2012;103:S23–7. doi: 10.1007/BF03403825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman KJ, Greenland S. Matching. In: Rothman KJ, Greenland S, editors. Modern epidemiology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1998. pp. 147–61. [Google Scholar]
- 32.Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241–58. doi: 10.1038/ajg.2016.537. [DOI] [PubMed] [Google Scholar]
- 33.Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2019;93:584–94. doi: 10.1212/WNL.0000000000008157. [DOI] [PubMed] [Google Scholar]
- 34.Furer V, Rondaan C, Heijstek MW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79:39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 35.Roy M, Sherrard L, Dubé È, et al. Determinants of non-vaccination against seasonal influenza. Health Rep. 2018;29:12–22. [PubMed] [Google Scholar]
- 36.Boltin D, Gingold-Belfer R, Kimchi NA, et al. Utilization of influenza immunization in adults with Crohn’s disease-a longitudinal, population-based study. Inflamm Bowel Dis. 2014;20:240–5. doi: 10.1097/01.MIB.0000440816.76627.bf. [DOI] [PubMed] [Google Scholar]
- 37.Boltin D, Gingold-Belfer R, Kimchi NA, et al. Uptake of influenza vaccine in ulcerative colitis. Vaccine. 2014;32:5484–9. doi: 10.1016/j.vaccine.2014.07.080. [DOI] [PubMed] [Google Scholar]
- 38.Qendro T, de la Torre ML, Panopalis P, et al. Suboptimal immunization coverage among Canadian rheumatology patients in routine clinical care. J Rheumatol. 2020;47:770–8. doi: 10.3899/jrheum.181376. [DOI] [PubMed] [Google Scholar]
- 39.Costello R, Winthrop KL, Pye SR, et al. Influenza and pneumococcal vaccination uptake in patients with rheumatoid arthritis treated with immunosuppressive therapy in the UK: a retrospective cohort study using data from the clinical practice research datalink. PLoS One. 2016;11:e0153848. doi: 10.1371/journal.pone.0153848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krasselt M, Ivanov JP, Baerwald C, et al. Low vaccination rates among patients with rheumatoid arthritis in a German outpatient clinic. Rheumatol Int. 2017;37:229–37. doi: 10.1007/s00296-016-3608-y. [DOI] [PubMed] [Google Scholar]
- 41.Ghaderi S, Berg-Hansen P, Bakken IJ, et al. Hospitalization following influenza infection and pandemic vaccination in multiple sclerosis patients: a nationwide population-based registry study from Norway. Eur J Epidemiol. 2020;35:355–62. doi: 10.1007/s10654-019-00595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szilagyi PG, Shone LP, Barth R, et al. Physician practices and attitudes regarding adult immunizations. Prev Med. 2005;40:152–61. doi: 10.1016/j.ypmed.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Nigro G, Angelini G, Grosso SB, et al. Psychiatric predictors of noncompliance in inflammatory bowel disease: psychiatry and compliance. J Clin Gastroenterol. 2001;32:66–8. doi: 10.1097/00004836-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Mohr DC, Goodkin DE, Likosky W, et al. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch Neurol. 1997;54:531–3. doi: 10.1001/archneur.1997.00550170015009. [DOI] [PubMed] [Google Scholar]
- 45.Tarrants M, Oleen-Burkey M, Castelli-Haley J, et al. The impact of comorbid depression on adherence to therapy for multiple sclerosis. Mult Scler Int. 2011;2011:271321. doi: 10.1155/2011/271321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 47.Farmanara N, Sherrard L, Dubé È, et al. Determinants of non-vaccination against seasonal influenza in Canadian adults: findings from the 2015–2016 Influenza Immunization Coverage Survey. Can J Public Health. 2018;109:369–78. doi: 10.17269/s41997-018-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malhi G, Rumman A, Thanabalan R, et al. Vaccination in inflammatory bowel disease patients: attitudes, knowledge, and uptake. J Crohns Colitis. 2015;9:439–44. doi: 10.1093/ecco-jcc/jjv064. [DOI] [PubMed] [Google Scholar]
- 49.Hammami MB, Pandit P, Salamo RT, et al. Health maintenance and vaccination of patients with inflammatory bowel disease: practice and perception of responsibility of gastroenterologists vs primary care providers. Ochsner J. 2019;19:210–9. doi: 10.31486/toj.18.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ledwich LJ, Harrington TM, Ayoub WT, et al. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61:1505–10. doi: 10.1002/art.24873. [DOI] [PubMed] [Google Scholar]
- 51.Parker S, Chambers White L, Spangler C, et al. A quality improvement project significantly increased the vaccination rate for immunosuppressed patients with IBD. Inflamm Bowel Dis. 2013;19:1809–14. doi: 10.1097/MIB.0b013e31828c8512. [DOI] [PubMed] [Google Scholar]
- 52.Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-based differences in incidence of inflammatory bowel diseases — pooled analysis of population-based studies from western countries. Gastroenterology. 2018;155:1079–89.e3. doi: 10.1053/j.gastro.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 53.Dobson R, Giovannoni G. Multiple sclerosis — a review. Eur J Neurol. 2019;26:27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 54.Minichiello E, Semerano L, Boissier MC. Time trends in the incidence, prevalence, and severity of rheumatoid arthritis: a systematic literature review. Joint Bone Spine. 2016;83:625–30. doi: 10.1016/j.jbspin.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Gionet L. Flu vaccination rates in Canada. Ottawa: Statistics Canada; 2015. Cat no 82-624-X. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.