Significance
Our study demonstrates that CD34+ cells generated from human induced pluripotent stem cells (iPSCs) genetically engineered to confer resistance to HIV have engraftment capabilities if they are derived in vivo but not in vitro. We used iPSCs from HIV-infected patients under antiretroviral therapy to form teratomas in immunodeficient mice and isolated CD34+ cells from these teratomas. We showed not only that these CD34+ cells could engraft in recipient mice but also that their cell progeny found in the mouse peripheral blood was resistant to HIV infection. These findings confirm the potential for evaluating in an animal model the clinical development of cure therapies using genetically modified autologous iPSCs from patients with HIV.
Keywords: iPS cells, CCR5, HIV resistance, genetic editing, teratoma
Abstract
Genetic editing of induced pluripotent stem (iPS) cells represents a promising avenue for an HIV cure. However, certain challenges remain before bringing this approach to the clinic. Among them, in vivo engraftment of cells genetically edited in vitro needs to be achieved. In this study, CD34+ cells derived in vitro from iPS cells genetically modified to carry the CCR5Δ32 mutant alleles did not engraft in humanized immunodeficient mice. However, the CD34+ cells isolated from teratomas generated in vivo from these genetically edited iPS cells engrafted in all experiments. These CD34+ cells also gave rise to peripheral blood mononuclear cells in the mice that, when inoculated with HIV in cell culture, were resistant to HIV R5-tropic isolates. This study indicates that teratomas can provide an environment that can help evaluate the engraftment potential of CD34+ cells derived from the genetically modified iPS cells in vitro. The results further confirm the possibility of using genetically engineered iPS cells to derive engraftable hematopoietic stem cells resistant to HIV as an approach toward an HIV cure.
A major objective of recent HIV research is to develop a “cure” for this virus infection that avoids lifelong adherence to antiretroviral therapy (ART). One of the approaches toward reaching this objective has been to genetically delete or mutate genes encoding for proteins that promote HIV infection and spread. An attractive candidate for this strategy is the Ccr5 gene, for which a genetic mutation causing a 32-bp deletion has been shown to be associated with natural protection from HIV infection and disease (1, 2). The Ccr5 gene encodes CCR5, a human cell-surface chemokine receptor that is a coreceptor for HIV attachment and infection of cells (3, 4). The Ccr5 allele with its 32-bp deletion results in a truncated isoform of the CCR5 receptor, CCR5Δ32, which is not expressed at the cell surface. Thus, entry of the virus into the cell is blocked (5).
Induced pluripotent stem (iPS) cells (6), because of their capacity to differentiate into CD34+ hematopoietic stem cells (HSCs) (7), can reconstitute a full immune system (8, 9). These iPS cells are therefore a target of choice for genetic engineering. Our group and others have demonstrated that iPS cells generated from the peripheral blood mononuclear cells (PBMC) of both healthy individuals (10) and HIV-infected patients under ART (11) can have their wild-type allele of the Ccr5 gene genetically edited to carry the Ccr5 Δ32 mutation (12, 13). Notably, using CRISPR/Cas9 technology, the Ccr5 gene can be modified to have the naturally occurring Δ32 variant allele that has been associated with resistance to R5-tropic viruses. Moreover, while it is not present at the cell surface, the truncated CCR5Δ32 protein is still expressed and, as such, could have other important physiological roles (14–17).
We have confirmed that the genetically modified Ccr5 Δ32 iPS cells can be differentiated into CD34+ HSCs in vitro (10, 18). Under appropriate cell culture conditions, they can give rise to various myeloid and lymphoid cell lineages (10, 11, 18). This result can also be observed with the formation of teratomas following the injection of large quantities of iPS cells into mice. Teratomas are multicellular tumors composed of many different cell types including HSCs. Notably, immune cells with the CCR5Δ32 mutation differentiated in vitro from the genetically modified iPS cell-derived HSCs and inoculated with HIV are resistant to R5-tropic virus infection (10, 18).
These results have suggested that editing Ccr5 in iPS cells from HIV-infected subjects can be a promising strategy toward an HIV cure. The pluripotent stem cells can be induced from a small number of PBMC from the patients and genetically modified to become resistant to HIV infection (10, 11, 18). In this case, leukapheresis to obtain large amounts of these cells (19) is not required. The edited HSCs could then be transplanted back to the original patient without concern for immune cell rejection. Therefore, because these experiments were performed in cell culture, an important remaining question is whether in vitro-edited iPS cells can differentiate into HSCs that can be transplanted back into a recipient in vivo (20).
To address this question, transplantation of the in vitro-derived CD34+ cells was attempted under various conditions in animal models of humanized or immunodeficient mice (21). In approaches to obtain sufficient numbers of CD34+ cells for transplantation, our ability to grow them in vitro offered an opportunity. However, although we could expand CD34+ cells substantially in culture (18), we observed that engraftment of these cell culture-derived CD34+ cells in humanized NSG-BLT mice did not occur. Thus, alternatively, to study the genetically edited cells in vivo, we explored the use of differentiated CD34+ cells in vivo via the generation of teratomas from iPS cells. We found that not only did these teratomas successfully yield human CD34+ cells, but importantly, these CD34+ cells could engraft in recipient immunodeficient NSG mice. This observation has been made by Nakauchi and colleagues (22) with different mouse strains. Finally, we confirmed that the PBMC formed in mice from these teratoma-derived genetically edited CD34+ cells are resistant to ex vivo R5-tropic HIV infection when they carry the mutant Δ32 Ccr5 allele.
Results
iPS-Derived CD34+ Cells Differentiated In Vitro Do Not Engraft.
We have reported the generation of iPS cells derived from PBMC of control subjects and HIV-infected patients on ART (Table 1). Some of these iPS cells were genetically edited to carry mutant alleles of Ccr5 with the 32-bp deletion (10, 11). Subsequently, these Ccr5 mutant iPS cell lines were differentiated into CD34+ cells using the cell culture approaches described previously (10, 18). Our procedure could expand CD34+ cells 15 to 22 times to several hundred thousand cells in 7 d for the study (18).
Table 1.
HIV-infected subjects and iPS cell lines (18)
| Number of iPS cell lines used | ||||||
| Subject | ART* | HLA-A2 | Years infected | Gender | Wild type | Mutant |
| C | + | + | >20 | Female | 2 | 2 |
| D | + | + | >20 | Male | 2 | 3 |
| E | + | + | >20 | Male | 1 | 1 |
| F | + | + | >20 | Male | 1 | 2 |
Subjects were on treatment for 1 to 12 y.
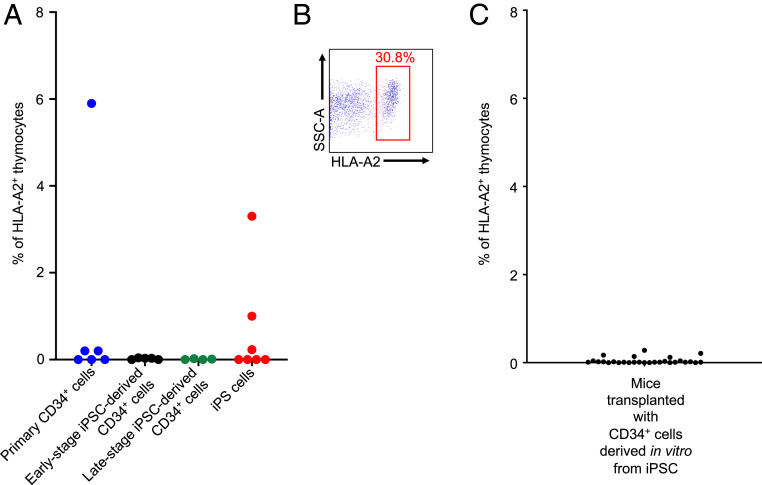
To demonstrate engraftment of the CD34+ cells derived in vitro from iPS cells, the protocol shown in Fig. 1 was used (Fig. 1A). We first inoculated primary or in vitro-derived human CD34+ cells into the implant of 15 humanized NSG-BLT mice (23). After 63 d, HLA-A2+ cells were detected in the implants of the NSG-BLT mice but only when primary, non–iPS-derived, CD34+ cells used as controls (n = 6) were transplanted (0.02 to 5.9% of thymocytes in three of six mice (Fig. 2A). However, no early- and late-stage iPS cell-derived transplanted CD34+ cells (n = 9) gave rise to any detectable HLA-A2+ cells in the BLT implants (Fig. 2A).
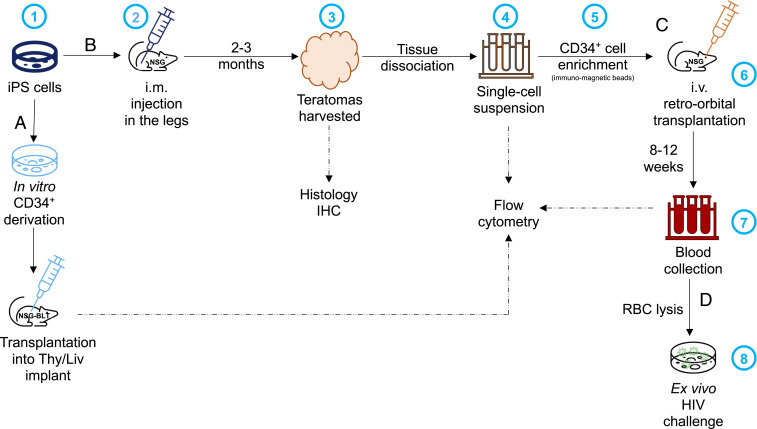
Fig. 1.
Schematic representation of the experimental approaches to assess engraftment of CD34+ cells derived (A) in vitro or (B) in vivo, from iPS cells. (B) 1) iPS cells are expanded in culture before 2) being injected intramuscularly (i.m.) into both hind legs of an NSG mouse; 3) when the teratomas reach 1 cm, the mice are killed and the teratomas harvested for histology and 4) following tissue dissociation, the preparation of a single-cell suspension used for flow cytometry or 5) enrichment of CD34+ cells by immunomagnetic separation. (C) Then, 6) the CD34+ cells are transplanted intravenously (i.v.) into the retro-orbital cavity of irradiated NSG mice; 7) after 6 to 8 wk, the mice are bled to check for engraftment (i.e., presence of HLA-A2+ cells) by flow cytometry. (D) If human cells are detected, 8) these PBMC are used for ex vivo inoculation with HIV isolates to assess HIV sensitivity. RBC, red blood cell.
Fig. 2.
Relative engraftment of human CD34+ cells in NSG-BLT mice. (A) 5 × 105 primary (non–iPS-derived) control CD34+ cells from a human subject (n = 6), early-stage (6 d after in vitro derivation) genetically modified iPS-derived CD34+ cells (n = 5), late-stage (16 d after in vitro derivation) genetically modified iPS-derived CD34+ (n = 4), or genetically modified Ccr5Δ32/Δ32 iPS cells (n = 7) were transplanted into the Thy/Liv implant of irradiated NSG-BLT mice. After 63 d, the mice were killed and the implant was harvested to determine the frequency of HLA-A2+ cells in the thymocytes by flow cytometry. (B) Density plot showing the presence of HLA-A2+ cells in the thymocytes from the Thy/Liv implant of an NSG-BLT mouse that developed a teratoma after being transplanted with 2 × 105 genetically modified CCR5Δ32/Δ32 iPS cells, 61 d postinjection. Only 2 of the 21 NSG-BLT mice that were injected with the same batch of iPS cells developed a teratoma. Data shown in A and B are from two independent experiments. (C) 0.5 to 1 × 106 CD34+ cells (n = 28) derived from iPS cells in vitro were transplanted into the Thy/Liv implant of irradiated BLT mice. After 3 to 13 wk, the mice were killed and the implants were harvested to determine the frequency of HLA-A2+ cells in the thymocytes by flow cytometry.
Notably, in the same experiment, seven other NSG-BLT mice were transplanted in the HLA-A2− human liver and thymus fragment (Thy/Liv) implant with genetically modified HLA-A2+ Ccr5Δ32/Δ32 iPS cells. In two of these mice, large teratomas formed, and HLA-A2+ cells represented 1.0% and 3.3% of the implant thymocytes present (Fig. 2A). In the implants of other NSG-BLT mice (n = 29) transplanted with 10,000 to 1 million iPS cells in three separate experiments independent from the experiment described above, nine teratomas formed, but only in mice receiving 100,000 iPS cells or more. In addition, HLA-A2+ cells were also detected in the implants of some of the mice transplanted with iPS cells (n = 10). Notably, one mouse that received 200,000 iPS cells had up to 30% of the implant thymocytes showing this human cell marker (Fig. 2B).
In contrast to these observations with primary CD34+ cells and iPS cells, many subsequent experiments involved injection of the in vitro-derived CD34+ cells into the Thy/Liv implant of NSG-BLT mice (n = 62). In all these experiments, no HLA-A2+ cells were detected in the implant thymocytes (representative experiment shown in Fig. 2C, n = 28). Thus, we found that the CD34+ cells derived in vitro from genetically modified iPS cells had no engraftment potential in the NSG mice.
Genetically Modified iPS Cells from ART-Treated, HIV-Infected Patients Form Teratomas in NSG Mice.
Because HLA-A2+ thymocytes could be detected in the NSG-BLT mice when large teratomas formed in the Thy/Liv implants (Fig. 2 A and B), we hypothesized that CD34+ cells isolated from the teratomas might be able to engraft in the immunodeficient mice with the same genetic background. This strategy was encouraged by the finding that CD34+ cells generated in teratomas can possess engraftment potential (22, 24).
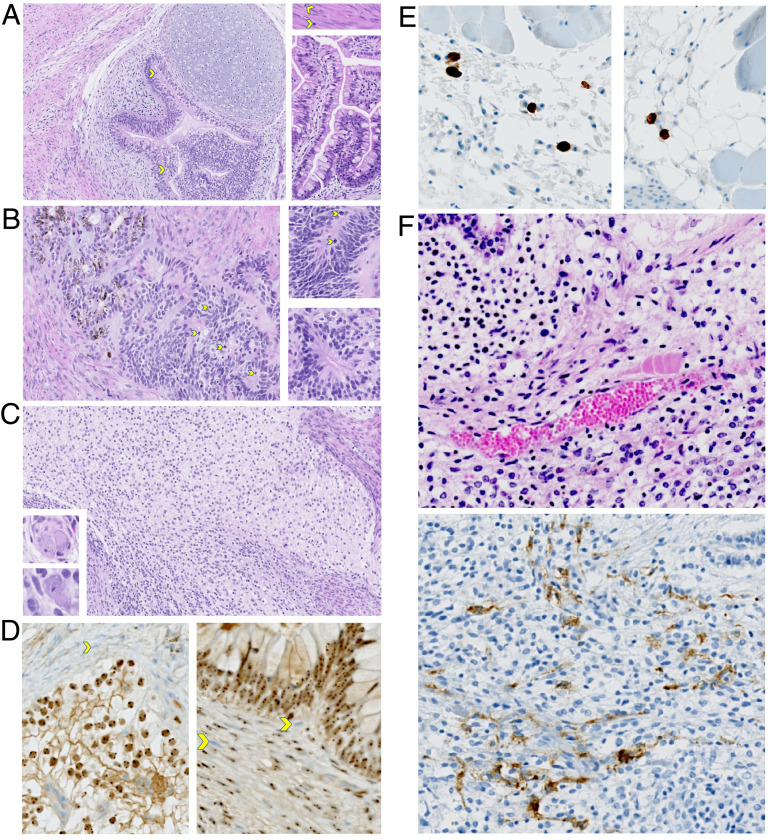
In our studies, wild-type or mutant iPS cells induced from the PBMC of four different HIV-infected subjects (18) (Table 1) were injected into the hind legs of NSG mice (Fig. 1B), where they generated teratomas. After the mice were killed, these teratomas were harvested and portions used for histology (n = 6). The histologic examination demonstrated typical features of immature teratomas containing tissue cells derived from all three classic germ layers. This finding confirmed our previous studies using the generation of teratomas in immunodeficient or humanized mice to show the pluripotency of genetically modified iPS cell lines (10, 11). These teratomas had abundant primitive neuroepithelial and neural cell types with frequent mitotic activity, mesodermal derivatives, and endodermal epithelium (Fig. 3 A–C).
Fig. 3.
Histologic examination of genetically modified iPS cell-derived teratomas. (A–C) These images demonstrate the typical features of an immature teratoma. (A) The main image shows the pseudostratified neuroepithelium, with mitotic figures (yellow arrows) and overlying cartilage. The Top Right Inset shows smooth muscle with mitotic figures. Bottom Right Inset shows abundant epithelium with goblet cells. Malignant cell populations resembling embryonal carcinoma, choriocarcinoma, and endodermal sinus tumors are completely absent. Magnification, 20×. (B) The main image shows the primitive neuroepithelium with abundant mitotic figures, which is pigmented and resembles early retinal pigment epithelium (RPE), in continuity with nonpigmented neuroepithelium. Both Insets show various types of pseudostratified neuroepithelial rosettes. Magnification, 20×. (C) The main image shows the neuroepithelial-derived neural cell populations that are abundant in this field. Both Lower Left Insets show abnormal ganglionic cells. Magnification, 20×. (D) IHC for human nucleoli was performed. The human cell nuclei are labeled by immuno-peroxidase staining in comparison to the negative mouse cell nuclei showing the blue counterstain in the two images. In the upper field of the Left image, the connective tissue surrounding the immature teratoma is composed of many mouse cells, some indicated by a yellow arrow. In the Right image, mouse cells infiltrating the teratoma are marked by yellow arrows. The microvasculature has both mouse cells and human cells, some of which are CD34+ (illustrated in F). Magnification, 20×. (E) IHC using an antibody specific for human CD3 was performed, labeling human T cells. The two representative images show that CD3+ cells are typically scattered, usually at the mouse–teratoma interface. Magnification, 20×. (F) IHC using an antibody specific for human CD34 was performed. Representative H&E staining (Top) and CD34 staining (Bottom) of fields from the same teratoma are shown. Magnification, 20×.
Using immunohistochemistry (IHC), human cells in the teratomas were labeled by peroxidase staining specific for the human nucleoli. In contrast, the nuclei of mouse cells that infiltrated the teratomas were negative for this staining and showed only a blue counterstain (Fig. 3D). The microvasculature stromal cells in the teratomas had both mouse cells and human cells, some of which were CD34+ (Fig. 3F). As expected, the primitive neuroepithelial and other ectodermal and mesenchymal cell populations were not immunoreactive with human CD34. The connective tissue surrounding the less mature parts of the teratomas was also composed of mostly mouse cells. Importantly, an antibody specific for human CD3 showed scattered human T cells, usually at the mouse–teratoma tissue interface (Fig. 3E). This finding indicated that iPS cells had differentiated into potentially HIV-sensitive immune cells in the teratomas.
Genetically Modified, iPS-Derived CD34+ Cells from Teratomas Can Engraft in NSG Mice.
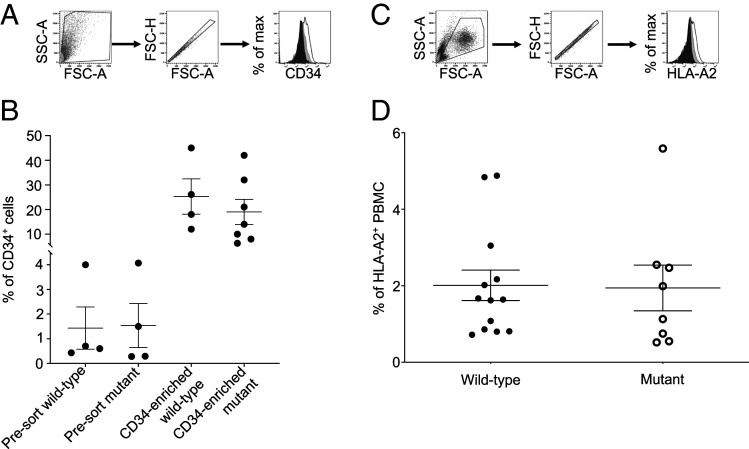
Portions of the two teratomas from each mouse (n = 42) that were not used for histologic analysis were processed into a single-cell suspension and analyzed by flow cytometry (Fig. 4A). Similar frequencies of human CD34+ cells were found in the single-cell suspension from both wild-type CCR5 and mutant CCR5Δ32 iPS-derived teratomas (0.43 to 4.0% and 0.28 to 4.1%, respectively) (Fig. 4B). The finding of CD34+ cells was confirmed by IHC (Fig. 3F). After immunomagnetic enrichment of human CD34+ cells from 11 samples (Fig. 4B), a comparable (P = 0.4889) frequency of CD34+ cells was present in the suspension of recovered wild-type cells (12 to 45%) and mutant cells (6.3 to 42%).
Fig. 4.
Engraftment of iPS-derived CD34+ cells from teratomas. (A and B) The teratomas generated from genetically modified wild-type and mutant iPS cells were harvested, pooled (one to five mice), and processed into a single-cell suspension. The frequency of human CD34+ cells was then determined by flow cytometry before (presort) and after enrichment of CD34+ cells by immunomagnetic cell sorting. The gating strategy used is represented in A: forward and size scatter, singlets, and CD34 staining (plain black: isotype control, plain grey: presort cells, black line: CD34-enriched cells). (C and D) The frequency of HLA-A2+ cells in the PBMC of NSG mice transplanted with genetically modified wild-type and mutant iPSC-derived CD34+ cells generated in teratomas was determined by flow cytometry, 6 to 8 wk posttransplantation. Each irradiated mouse received 56,000 to 500,000 CD34-enriched cells via intravenous retroorbital injection (n = 21). The gating strategy used is represented in C: forward and size scatter, singlets, and HLA-A2 staining (plain black: isotype control, plain grey: nontransplanted mouse, black line: transplanted mouse).
The enriched CD34+ cells from the teratomas (56,000 to 500,000) were then transplanted into new irradiated NSG recipient mice (n = 21) via intravenous injection in the retroorbital cavity (Fig. 1C). The mice were subsequently bled 6 to 8 wk following the transplantation and the presence of HLA-A2+ cells was checked by flow cytometry (Fig. 4C). In all the mice (n = 21) transplanted with teratoma-derived CD34+ cells from both wild-type and mutant iPS cells, a substantial frequency of human cells (0.72 to 4.88% in wild-type, 0.52 to 5.59% in mutant) was detected in the PBMC (Fig. 4D). Therefore, these results indicated that, in contrast to the lack of engraftment of CD34+ cells differentiated in vitro from iPS cells (Fig. 2 A and C), the genetically modified CD34+ cells generated in vivo in iPS-derived teratomas successfully engrafted in recipient hosts.
Ex Vivo Infection of Genetically Modified Ccr5-Mutant PBMC Confirms Their Resistance to HIV.
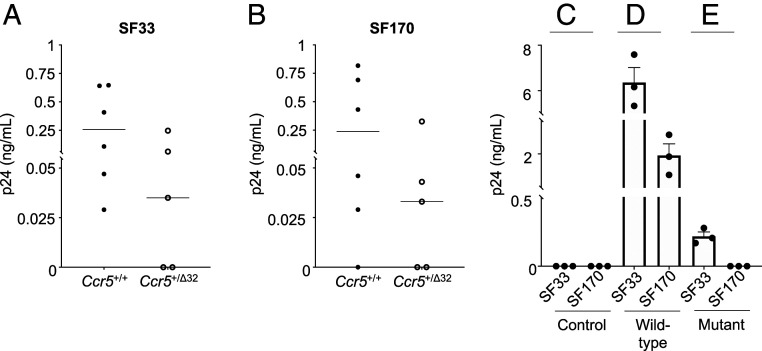
NSG mice transplanted with wild-type (n = 9) or mutant (n = 8) CD34+ cells were terminally bled (n = 17). The PBMC were obtained and inoculated with either the SF33 or SF170 HIV-1 isolates (Fig. 1D). Because of the low number of human cells harvested from the blood of the transplanted mice (Fig. 4D), PHA-activated human CD4+ cells from uninfected subjects were added twice at 7 and 14 d postinfection to the HIV-inoculated cultures to amplify virus detection (Materials and Methods). This strategy permitted detection of HIV infection in the PBMC obtained from mice transplanted with either wild-type Ccr5+/+ or heterozygous Ccr5+/Δ32 mutant CD34+ cells (Fig. 5 A and B). The range of virus replication in these cells was variable, most likely reflecting the relative number of HIV-sensitive human PBMC recovered from the mice (Fig. 4D).
Fig. 5.
In vitro HIV infection of PBMC harvested from NSG mice transplanted with Ccr5 wild-type and mutant CD34+ cells derived from genetically modified iPS cells. Virus replication in the HIV-inoculated PBMC harvested and pooled from up to three NSG mice was assessed by p24 antigen ELISA on the day 7 culture fluids following the second addition of stimulated CD4+ cells (Materials and Methods). When detected, virus replication in the HIV-inoculated cells ranged from low to high levels (0.029 to 7.58 ng/mL) of p24 antigen, depending on the relative number of HIV-sensitive human cells recovered from the mouse PBMC. The (A) X4-tropic (HIV-1SF33) and (B) R5-tropic (HIV-1SF170) isolates both replicated in human cells obtained from mice transplanted with the Ccr5+/+ (n = 6) or Ccr5+/Δ32 (n = 5) CD34+ cells derived from genetically modified iPS cells. (C) Virus replication was not detected in the HIV-inoculated control PBMCs of NSG mice that were not transplanted with human CD34+ cells (n = 6). X4-tropic (HIV-1SF33) and R5-tropic (HIV-1SF170) virus replication in the HIV-inoculated PBMC of NSG mice transplanted with (D) wild-type Ccr5+/+ (n = 6) or (E) mutant Ccr5Δ32/Δ32 (n = 6) CD34+ cells derived from genetically modified iPS cells. Resistance to R5-tropic virus replication was seen with the mutant cells.
In some cases, to increase human cell recovery from the mice, the harvested PBMC were pooled and differentiated into predominantly adherent cells using myeloid-inducing cytokines (18) (Fig. 5 C–E). Subsequently, the adherent PBMC obtained from the mice transplanted with wild-type CD34+ cells were inoculated with HIV-1 SF33 or SF170; viral replication occurred with both HIV isolates (Fig. 5D). In contrast, for PBMC obtained from the mice transplanted with mutant Ccr5Δ32/Δ32 CD34+ cells, viral replication was only observed with the X4-tropic SF33, but not the R5-tropic SF170 HIV-1 isolate (Fig. 5E). Notably, a reduction in replication of the SF33 in the mutant cells was noted (Fig. 5 D and E), as has been reported by others (15, 25).
Importantly, using cells from nontransplanted mice as controls (from which virus was not removed using trypsin) (Materials and Methods), no evidence of residual input virus after adding uninfected CD4+ cells was found (Fig. 5C). The studies confirmed that human hematopoietic cells can be reconstituted in mice from the iPS-derived CD34+ cells generated in teratomas. These cells remain sensitive to HIV infection but resistant to R5-tropic HIV infection in the case of the cells carrying the Ccr5 modified allele.
Discussion
Strategies for establishing a cure of HIV infection include attempts to identify infected cells and destroy them, enhance immune responses to recognize and control HIV-infected cells, and genetic editing (26, 27). With the latter approach, CRISPR/Cas 9 and TALENs technologies have permitted specific deletion or modification of genes within immune cells to affect their biologic activity (28, 29). Our laboratories have emphasized the use of genetic editing to remove CCR5, a major cell surface receptor for the AIDS virus, from the surface of immune cells so that their antiviral function can be preserved (10, 11, 18). We have reported methods to derive iPS cells from small amounts of PBMC; in this case, leukapheresis would not be needed (10). The iPS cells can then be edited with CRISPR/Cas9 not only to delete Ccr5 but also to replace the wild-type alleles with the naturally occurring Ccr5 allele with a mutant Δ32 deletion (10). This latter genetic modification prevents not just infection by R5-tropic HIV isolates via the loss of HIV binding to CCR5 for entry into the cells, but can reduce the replication of other HIV strains as well. Therefore, CCR5Δ32 most likely acts as an intracellular scavenger and prevents wild-type CCR5 (i.e., for Ccr5Δ32/+ individuals) as well as CXCR4 from reaching the cell surface. Thus, some resistance to X4-tropic viruses is observed, as has been reported (15, 25) (Fig. 5).
One of the challenges in achieving a clinical benefit from genetic engineering of immune cells is to confirm engraftment of modified HSCs in the recipient. In this report, we provide details on our approaches to determine if the genetically edited iPS cell-derived CD34+ cells obtained in vitro from the PBMC of HIV-infected patients can engraft and establish HIV-resistant cells in recipient subjects (Fig. 2A). In our studies, we first attempted to transplant these genetically modified CD34+ cells into humanized immunodeficient (NSG-BLT) mice. However, we were surprised to find that, in contrast to CD34+ cells obtained directly from human blood (Fig. 2A), the engraftment of these in vitro-derived cells did not take place (Fig. 2A). Subsequently, we took advantage of our observations that the injection of iPS cells into the Thy/Liv implants of BLT mice led to the formation of teratomas (Fig. 2 A and B), in which sometimes large numbers of CD34+ cells and even some CD3+ cells could be detected (Fig. 3 E and F). Based on this finding and reports by Nakauchi and colleagues (22, 24) using mouse and human iPS cells to form teratomas, we decided to isolate the CD34+ cells from the teratomas generated in NSG immunodeficient mice and transplant them into NSG recipient mice (Fig. 1B). The results indicated that engraftment by the genetically modified CD34+ cells from teratomas occurred in all the experiments (Fig. 4D), in contrast to the lack of engraftment by the in vitro-derived CD34+ cells (Fig. 2 A and C).
Subsequently, we determined that the cell progeny of the human CD34+ cells grown in vivo via teratomas and then transplanted into NSG mice maintained the biologic characteristics resulting from genetic editing of Ccr5. The Ccr5Δ32/Δ32 cells were resistant to the R5-tropic virus isolate, but not the X4-tropic virus isolate (Fig. 5 D and E). The PBMC obtained from mice transplanted with CD34+ cells carrying a single modified Ccr5 allele (Ccr5+/Δ32), as well as from the wild-type CD34+ cells, were susceptible to both viruses (Fig. 5 A and B). However, as mentioned above, the Ccr5Δ32/+ cells showed a reduction in both R5- and X4-tropic virus replication.
Our findings provide valuable information on experimental approaches to enhance engraftment of genetically modified iPS cell-derived HSCs that can differentiate into human hematopoietic cells (10, 18). While clinical applications would not allow the use of tumor-generated cells, future studies can help understand what in the teratomas elicits this engraftment potential. Perhaps cytokines produced by the teratoma stromal cells (Fig. 3) or cell-to-cell contact induce the in vitro-derived CD34+ cells to be capable of engraftment. Moreover, molecular analysis of the in vitro- versus in vivo-derived CD34+ cells could elucidate genes encoding proteins that are needed for engraftment. Future considerations should also emphasize the potential ability of teratoma-derived CD34+ cells to be expanded in vitro without losing their engraftment capacity. Thus, the findings in our studies should help future animal engraftment trials to evaluate in vitro genetic editing of iPS cells or HSCs. These animal teratoma studies would be precursors for eventual HIV cure approaches in human clinical trials.
Materials and Methods
Cell Lines, Mice, and Virus Isolates.
iPS cell lines were generated as described previously (11, 18) (Table 1). Briefly, these pluripotent stem cells were induced from the PBMC of HLA-A2+ HIV-infected subjects under ART. Subsequently, these iPS cells were genetically modified by CRISPR/Cas9 to carry the Δ32 mutation of the Ccr5 gene (10, 11) in one or both alleles. Control cell lines with a wild-type version of the gene were also generated (Table 1). The iPS cell-derived CD34+ cells were cultured as described previously (18).
For in vivo experiments, immunocompromised NSG mice were obtained from the Jackson Laboratory and housed at the Laboratory Animal Research Center at the University of California, San Francisco (UCSF) under Institutional Animal Care and Use Committee-approved protocols. They were used as recipients for CD34+ cell transplantation or generation of humanized BLT mice (23). Briefly, for the latter, the BLT mice were generated by implanting a mixture of HLA-A2− Thy/Liv under the renal capsule of NSG immunodeficient mice. Four months postimplantation, these Thy/Liv mice were irradiated, and the implant transplanted with primary CD34+ cells or early-stage (6 d postderivation) and late-stage (16 d postderivation) iPS cell-derived CD34+ cells from HLA-A2+ human subjects. The CD34+ cell derivation from iPS cells was conducted as described previously (10, 18). For experiments involving human cells, each subject provided informed consent and the studies received approval from the Institutional Review Board at UCSF.
The virus isolates used for the infection assays were HIV-1SF33 and HIV-1SF170 (30, 31). HIV-1SF33 is a molecular clone of an X4-tropic HIV isolate that can infect cells regardless of CCR5 expression at the cell surface; HIV-1SF170 is an R5-tropic virus that can only infect cells expressing the CCR5 coreceptor (18). The viruses were cultivated in human CD4+ cells cultured in RPMI-1640 growth medium containing 1% penicillin and streptomycin, 2 mM glutamine, 10% fetal calf serum (FCS), and 100 U/mL IL-2.
Teratoma Generation and Histology.
One million cultured iPS cells were injected either directly into the Thy/Liv implant of a BLT mouse, or intramuscularly into each hind leg of an NSG mouse (Fig. 1B). The mice were monitored weekly for teratoma generation by palpation and were killed once one of the teratomas reached 1 cm in size at their longest dimension. The teratomas were then harvested by surgical dissection and processed for flow cytometry, histology, or CD34+ cell enrichment. Histology examination was conducted as described previously (32).
Flow Cytometry.
Cells were stained for 20 to 30 min at 4 °C with one or several of the following fluorochrome-conjugated antibodies: APC-CD34 (clone 561, BioLegend), PE-TRA/1/81 (clone TRA-1-81, BioLegend), FITC-HLA-A2 (clone BB7.2, BioLegend). Cells were washed with PBS and fixed in buffer containing 1% paraformaldehyde. Sample acquisition was performed on a BD LSRii flow analyzer (UCSF Flow Cytometry Core) and the resulting FCS files were analyzed on FlowJo X (BD).
CD34+ Cell Recovery and Enrichment.
Pooled teratomas from one animal were cut into small pieces using a razor blade, and then minced into smaller pieces in DMEM culture medium (Gibco). These fragments were subsequently pushed twice through cell strainers with the help of syringe plungers. The resulting single-cell suspensions were assessed for CD34 expression via flow cytometry and used for CD34+ cell enrichment by positive selection with the Human CD34 MicroBead Kit (Miltenyi Biotech).
CD34+ Cell Transplantation.
NSG-BLT or NSG mice were irradiated at 175 cGy 1 h before the procedure, then anesthetized. The CD34+ cells derived in culture from iPS cells (18) or enriched from teratomas were then injected into the Thy/Liv implant of BLT mice or intravenously through the retro-orbital cavity of NSG mice (Fig. 1 A and C). The animals were then maintained under antibiotics provided in their water supply or food pellets. After 6 wk, the transplanted mice were bled to confirm engraftment of the human cells by flow cytometry using an HLA-A2 antibody. Four weeks postconfirmation of human cell engraftment, the mice were anesthetized and terminally bled. Cells from the heparinized blood were used for ex vivo HIV inoculation.
Ex Vivo HIV Infection.
The heparinized blood samples obtained from mice successfully engrafted with CD34+ cells from the same iPS cell line were pooled (one to three mice) and treated with ACK buffer to lyse red blood cells. The cells were washed and incubated for 1 h with polybrene (3 μg/mL), then washed and incubated for 1 h with a 1:1 dilution of the SF33 or SF170 HIV-1 isolate (at 10,000 TCID50 per 3 million cells) in RPMI-1640 growth medium at 10 million cells/mL. Subsequently, the infected cells were washed once, treated with 0.05% trypsin to remove input virus (18, 33), and washed again before being resuspended at 2 million cells/mL in the RPMI-1640 growth medium. After overnight incubation, the cells were washed once, treated with 0.05% trypsin, and washed again to remove any residual input virus. The cells were then resuspended and cultured at 1 million cells/mL in the RPMI-1640 growth medium for 14 d. Half of the culture medium was changed every 2 to 3 d. In some cases, the infected cells were kept in culture medium without IL-2 but with a mix of cytokines promoting differentiation into myeloid cells (18). Finally, after 7 to 10 d, PHA-stimulated human CD4+ cells were added to some of the infected cell cultures in order to amplify the detection of virus replication (18). Seven days later, cell culture supernatants were collected, and additional PHA-stimulated human CD4+ cells were added for another 7 d. For these cell cultures, half of the medium was changed every 2 d. All collected culture supernatants were kept at −80 °C until assayed by HIV p24 antigen ELISA for virus replication (18).
Acknowledgments
We thank the A. M. Dachs Foundation, the Hellman Foundation, and the Frank A. Campini Foundation for support. The research described in this article was supported by NIH Grants AI 102825, P01 DK 088760, and R21 AI108398 and Contract HHSN272201400002C.
Footnotes
The authors declare no competing interest.
Data Availability
All study data are included in the article.
References
- 1.Liu R., et al., Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86, 367–377 (1996). [DOI] [PubMed] [Google Scholar]
- 2.Lu Y., et al., Genotype and allele frequency of a 32-base pair deletion mutation in the CCR5 gene in various ethnic groups: Absence of mutation among Asians and pacific Islanders. Int. J. Infect. Dis. 3, 186–191 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Dragic T., et al., HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381, 667–673 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Brelot A., Chakrabarti L. A., CCR5 revisited: How mechanisms of HIV entry govern AIDS pathogenesis. J. Mol. Biol. 430, 2557–2589 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Dean M., et al., Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273, 1856–1862 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Karagiannis P., et al., Induced pluripotent stem cells and their use in human models of disease and development. Physiol. Rev. 99, 79–114 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Gori J. L., et al., Efficient generation, purification, and expansion of CD34(+) hematopoietic progenitor cells from nonhuman primate-induced pluripotent stem cells. Blood 120, e35–e44 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynes K., Menicanin D., Gronthos S., Bartold M. P., Differentiation of iPSC to mesenchymal stem-like cells and their characterization. Methods Mol. Biol. 1357, 353–374 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Bernareggi D., Pouyanfard S., Kaufman D. S., Development of innate immune cells from human pluripotent stem cells. Exp. Hematol. 71, 13–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye L., et al., Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Δ32 mutation confers resistance to HIV infection. Proc. Natl. Acad. Sci. U.S.A. 111, 9591–9596 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye L., et al., Generation of HIV-1-infected patients’ gene-edited induced pluripotent stem cells using feeder-free culture conditions. AIDS 34, 1127–1139 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Perez E. E., et al., Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 26, 808–816 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mussolino C., et al., A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 39, 9283–9293 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass W. G., et al., CCR5 deficiency increases risk of symptomatic West Nile virus infection. J. Exp. Med. 203, 35–40 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrawal L., et al., CCR5Δ32 protein expression and stability are critical for resistance to human immunodeficiency virus type 1 in vivo. J. Virol. 81, 8041–8049 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falcon A., et al., CCR5 deficiency predisposes to fatal outcome in influenza virus infection. J. Gen. Virol. 96, 2074–2078 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Martin-Blondel G., Brassat D., Bauer J., Lassmann H., Liblau R. S., CCR5 blockade for neuroinflammatory diseases—Beyond control of HIV. Nat. Rev. Neurol. 12, 95–105 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Teque F., et al., Genetically-edited induced pluripotent stem cells derived from HIV-1-infected patients on therapy can give rise to immune cells resistant to HIV-1 infection. AIDS 34, 1141–1149 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Uchida N., et al., High doses of purified stem cells cause early hematopoietic recovery in syngeneic and allogeneic hosts. J. Clin. Invest. 101, 961–966 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blümich S., Zdimerova H., Münz C., Kipar A., Pellegrini G., Human CD34+ hematopoietic stem cell–engrafted NSG mice: Morphological and immunophenotypic features. Vet. Pathol. 58, 161–180 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Pearson T., Greiner D. L., Shultz L. D., Creation of “humanized” mice to study human immunity. Curr. Protoc. Immunol. 81, 15.21.1–15.21.21 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukada M., et al., In vivo generation of engraftable murine hematopoietic stem cells by Gfi1b, c-Fos, and Gata2 overexpression within teratoma. Stem Cell Reports 9, 1024–1033 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoddart C. A., et al., Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ(-/-) (NSG) BLT mice. Virology 417, 154–160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki N., et al., Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol. Ther. 21, 1424–1431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal L., et al., Role for CCR5Δ32 protein in resistance to R5, R5X4, and X4 human immunodeficiency virus type 1 in primary CD4+ cells. J. Virol. 78, 2277–2287 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida M. J., Matos A., Designer nucleases: Gene-editing therapies using CCR5 as an emerging target in HIV. Curr. HIV Res. 17, 306–323 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Henderson L. J., Reoma L. B., Kovacs J. A., Nath A., Advances toward curing HIV-1 infection in tissue reservoirs. J. Virol. 94, e00375-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shifrut E., et al., Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell 175, 1958–1971.e15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azangou-Khyavy M., et al., CRISPR/Cas: From tumor gene editing to T cell-based immunotherapy of cancer. Front. Immunol. 11, 2062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tateno M., Levy J. A., MT-4 plaque formation can distinguish cytopathic subtypes of the human immunodeficiency virus (HIV). Virology 167, 299–301 (1988). [DOI] [PubMed] [Google Scholar]
- 31.Cheng-Mayer C., Homsy J., Evans L. A., Levy J. A., Identification of human immunodeficiency virus subtypes with distinct patterns of sensitivity to serum neutralization. Proc. Natl. Acad. Sci. U.S.A. 85, 2815–2819 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VandenBerg S. R., Herman M. M., Ludwin S. K., Bignami A., An experimental mouse testicular teratoma as a model for neuroepithelial neoplasia and differentiation. I. Light microscopic and tissue and organ culture observations. Am. J. Pathol. 79, 147–168 (1975). [PMC free article] [PubMed] [Google Scholar]
- 33.Tang S. B., Levy J. A., Inactivation of HIV-1 by trypsin and its use in demonstrating specific virus infection of cells. J. Virol. Methods 33, 39–46 (1991). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article.