Abstract
Background:
Egg-white ovomucoid, that is, Gal d 1, is associated with IgE-mediated allergic reactions in most egg-allergic children. Epitope-specific IgE levels have been correlated with the severity of egg allergy, while emerging evidence suggests that other antibody isotypes (IgG1, IgG4, IgA, and IgD) may have a protective function; yet, their epitope-specific repertoires and associations with atopic comorbidities have not been studied.
Methods:
Bead-based epitope assay (BBEA) was used to quantitate the levels of epitope-specific (es)IgA, esIgE, esIgD, esIgG1, and esIgG4 antibodies directed at 58 (15-mer) overlapping peptides, covering the entire sequence of ovomucoid, in plasma of 38 egg-allergic and 6 atopic children. Intraclass correlation (ICC) and coefficient of variation (CV) were used for the reliability assessment. The relationships across esIgs were evaluated using network analysis; linear and logistic regressions were used to compare groups based on egg allergy status and comorbidities.
Results:
BBEA had high reliability (ICC >0.75) and low variability (CV <20%) and could detect known IgE-binding epitopes. Egg-allergic children had lower esIgA1 (P = .010) and esIgG1 (P = .016) and higher esIgE (P < .001) and esIgD (P = .015) levels compared to the atopic controls. Interestingly, within the allergic group, children with higher esIgD had decreased odds of anaphylactic reactions (OR =0.48, P = .038). Network analysis identified most associations between esIgE with either esIgG4 or esIgD; indicating that IgE-secreting plasma cells could originate from either sequential isotype switch from antigen-experienced intermediate isotypes or directly from the IgD+ B cells.
Conclusions:
Collectively, these data point toward a contribution of epitope-specific antibody repertoires to the pathogenesis of egg allergy.
Keywords: anaphylaxis, antibody repertoire, egg allergy, epitopes, ovomucoid
Graphical Abstract
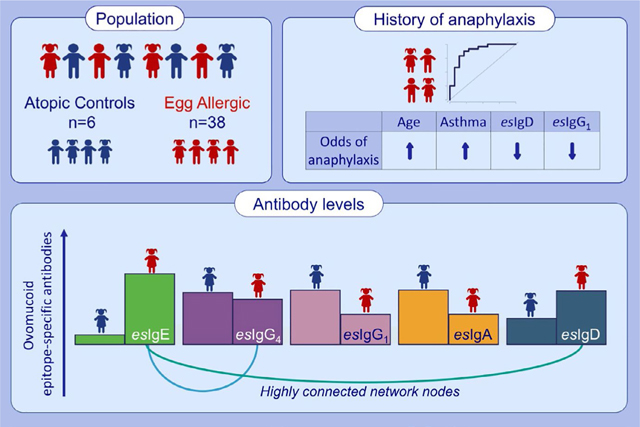
Egg-allergic children have lower levels of epitope-specific (es)IgA1 and esIgG1 but higher esIgE and esIgD compared to the atopic controls. Within the allergic group, children with higher esIgD antibodies have decreased odds of anaphylactic reactions. Network analysis identifies most associations between esIgE with either esIgG4 or esIgD, indicating potentially different origins of IgE.
1 |. INTRODUC TION
Hen’s egg allergy is one of the most common pediatric food allergies affecting 0.5%−10% of infants and children.1–9 Allergic reactions occur when antigen-specific immunoglobulin E (IgE) binds to its high-affinity receptors on granulocytes, activating their effector functions and release of inflammatory mediators.10,11 Several egg-white proteins contribute to IgE sensitization, of which ovomucoid (OVM) is considered a major allergen, designated as Gal d 1 (WHO-IUIS).12–16 Ovomucoid is resistant to heat and digestive enzymes, and IgE recognition of the OVM protein is indicative of a more persistent allergic phenotype.15,17–21 Additionally, IgE recognition of a greater number of sequential epitopes is shown to be associated with greater severity of allergic reactions.22–24
Over the past few years, different groups demonstrated that induction of other antibody isotypes, such as IgA, IgG1, IgG4, and IgD, can ameliorate IgE-mediated reactions.15,25–40 Specifically, several clinical trials of egg oral immunotherapy reported increases of serum antigen-specific IgA, IgG1, and IgG4 in patients that responded to treatment.32,33 Interestingly, the ability of the antibodies to block IgE-mediated responses could be largely dependent on their antigen specificity rather than isotype.31 However, recent research in food allergy has been mostly focused on understanding epitope-specific repertoire of IgE and IgG4, with only a limited number of studies addressing epitope specificity of IgG1, IgA, and IgD.41,42
We have previously presented a bead-based epitope assay that is highly reliable and sensitive for detecting milk43,44 and peanut45 epitope-specific IgE and IgG4. In this work, we developed an assay to measure levels of five antibody classes, that is, IgE, IgG4, IgG1, IgA1, and IgD, directed at the peptides covering the entire sequence of the OVM protein. We then investigated how epitope-specific antibody repertoires are associated with egg allergy and anaphylaxis, as well as atopic comorbidities, such as asthma, rhinitis, and eczema. To our knowledge, this is the first report investigating the relationships among epitope-specific adaptive humoral responses in pediatric food allergy.
2 |. MATERIALS AND METHODS
2.1 |. Study participants
Plasma from children with specific IgE (sIgE) to the egg-white extract >0.35 kUA/L (ImmunoCAP) was obtained from the Food Allergy Research Initiative (FARI) biorepository at Mount Sinai. Allergy status was determined by sIgE or skin prick testing and/or a convincing history, with moderate-to-severe, clear-cut allergic reactions following the ingestion of egg, as documented by a patient’s local physician. A negative control pool (a mix of sera from four adults with no history of any food or environmental allergies), a positive control pool (plasma from three children with egg sIgE >100 kUA/L), and atopic controls with food allergies other than egg were also included. The study was approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai. All the study subjects provided informed consent.
2.2 |. Measurement and quantification of epitope-specific (es) Ig levels
A peptide library consisting of 58 15-mer biotinylated peptides (12-mer overlap; CS Bio) was generated covering the entire sequence of hen’s egg white OVM (UniProt ID P01005). The bead-based epitope assay (BBEA) was carried out as described previously,45 with a few modifications. A master mix of biotinylated peptides, conjugated to LumAvidin beads (Luminex Corporation), was prepared in PBS-TBN buffer (1xPBS, 0.02% Tween-20, 0.1% BSA), and 100 μL/well of mix was added to 96-well filter plates. Plates were washed twice with PBS-TBN, and 100 μL/well of 1:10 plasma dilution was added in duplicates and incubated on a shaker for 2 hours at room temperature. To account for potential well position and plate effect, samples were randomized to wells using PlateDesigner.46 For the nonspecific signal detection, each plate included two wells with only PBS-TBN. Plates were washed and incubated for 30 minutes with 50 μL/well of mouse anti-human PE-labeled secondary antibody (2 μg/mL of IgE, clone BE5, Cat. MA1–10375, Thermo Fisher Scientific; or 0.25 μg/mL of IgG4, clone HP6025, Cat. 9200–09, Southern Biotech; or 62.5 ng/mL of IgG1, clone HP6001, Cat. 9054–28, Southern Biotech; or 0.2 μg/mL of IgA1, clone IS11–8E10, Miltenyi Biotec; or 0.17 μg/mL of IgD, clone IADB6, Cat. OB903009, Southern Biotech). Plates were washed three times, and beads were resuspended in 100 μL of PBS-TBN. The signal was quantified as a median fluorescence intensity (MFI) on the Luminex200 instrument (Luminex Corporation). For each peptide, MFIs were log2-normalized and converted to nMFI values by subtracting the average of the non-specific binding wells, as previously described.45 Since MFI is representative of antibody concentrations,47 these epitope-specific immunoglobulin (esIg) nMFI levels represent a relative quantity of an antibody in plasma directed at a particular peptide.
2.3 |. Statistical analyses
The plate effect for the IgG4 experiment was quantified using the principal variance component analysis (PVCA),48 with the principal component threshold set to 0.8. Plate effect was estimated for each peptide by fitting linear models with “plate” as a covariate; then, the coefficients for each plate were subtracted from the original nMFI values.45
To assess the reliability of the technical duplicates, a two-way intraclass correlation coefficient (ICC) for agreement was used, with the reliability thresholds defined as excellent (0.75–1.00), good (0.60–0.74), fair (0.40–0.59), or poor (<0.40).49 Unsupervised hierarchical clustering of the duplicates was performed using Pearson’s r correlation as a distance metric and an average-linkage algorithm. The coefficient of variation (CV) was calculated for each pair of replicates as (standard deviation [MFI]/average [MFI])*100. After the reliability assessment, the duplicates were averaged for downstream analyses.
An esIg network was constructed from the Pearson’s r using all epitope pairs with the WGCNA algorithm50: The epitope-epitope signed correlation matrix was converted to an adjacency matrix using a power function that optimizes a scale-free topology (power = 22). A normalized number of neighbors shared by the nodes on either side of each edge were calculated by transforming the adjacency into a topological overlap matrix (TOM). Then, esIgs were joined using the hierarchical clustering with an average linkage of the TOM and split into modules using a hybrid dynamic tree-cut algorithm (height = 0.99). A network layout was generated using the ForceAtlas2 method in Gephi51 and visualized using the Cytoscape software.52
The pairwise associations among epitopes of each antibody isotype were measured using Spearman’s ρ correlation and cosine similarity. Antibody-level summary statistics for the pairwise correlation were obtained after using the Fisher Z-transformation of the correlation coefficients and reverse-transformed those summary statistics to Spearman’s ρ.53 The association of esIgE and log10-normalized egg sIgE was calculated using Spearman’s ρ and clustered with mcquitty agglomeration algorithm.
Analysis of the egg-allergic (EA) vs atopic children (AC): Comparisons for each esIg were carried out using linear modeling in the limma framework, where an empirical Bayes approach allows estimation of the variance parameters acquiring information across all esIgs.54,55 Then, the levels of the significant esIgs (P < .05) were averaged for the downstream Spearman’s ρ estimations; since none of the esIgG4s were significant, the analyses were performed with the average of all 58 esIgG4s. Using either the averages of significant epitope-binding antibodies or the mean of all 58, logistic models were fitted to estimate the area under the curve (AUC) for each immunoglobulin. Similarly, analysis of anaphylaxis and the atopic comorbidities, that is, asthma, eczema, and rhinitis, for only EA children was done using limma methodology. Results are summarized as in a Manhattan plot using the -log10 (P-value) for each epitope but including the direction of the association (<0 if negative and >0 if positive). As the resultant plot resembles the skyline of a city by a lake, we have termed “Chicago” plot. Then, for each disease, logistic models were built using a stepwise selection of predictors that included age, atopic comorbidities, and top 5–10 esIgs (based on the smallest P-value). Due to a small sample size, the P-values were not adjusted for multiple comparisons. Statistical analyses were performed in R version 3.2.2.
3 |. RESULTS
3.1 |. Characteristics of the study cohort
Thirty-eight egg-allergic (EA) children, with an average egg-white sIgE of 54 kUA/L, and 6 atopic controls (AC) were included in the study (Table 1). EA children were on average 6.6 (standard deviation (SD) = 3.6) years old, 22 (57%) were male, and 27 (80%) were White/Caucasian. Twenty-four (63%) children in the EA group had a history of egg-related anaphylaxis, 29 (76%) had eczema, 28 (74%) had asthma, and 22 (58%) had rhinitis. The AC group was slightly older, 10.6 (SD = 3.8) years of age, but comparable in other demographics and medical history.
TABLE 1.
Patients’ characteristics
| AC (n = 6a) | EA (n = 38) | P-valueb | |
|---|---|---|---|
| Age | 10.6 (3.8) | 6.6 (3.6) | .016 |
| Male (%) | 3 (50.0) | 22 (57.9) | .999 |
| Race (%) | |||
| Asian | 0 (0.0) | 5 (14.7) | .166 |
| Back/African American | 1 (20.0) | 0 (0.0) | |
| Other | 0 (0.0) | 2 (5.9) | |
| White/Caucasian | 4 (80.0) | 27 (79.4) | |
| Not Hispanic or Latino (%) | 5 (100.0) | 30 (93.8) | .999 |
| Asthma (%) | 2 (40.0) | 28 (73.7) | .153 |
| Rhinitis (%) | 2 (40.0) | 22 (57.9) | .64 |
| Eczema (%) | 3 (60.0) | 29 (76.3) | .589 |
| Anaphylaxis (%) | . | 24 (63.2) | |
| Egg sIgE (kUA/L) | 0.1 [0.1–0.3] | 49.9 [22.8–90.5] | <.001 |
AC group had demographic and medical history data available for five out of six patients.
Continuous variables are presented as mean (SD) or median [1st and 3rd quartile]; categorical variables are reported as frequencies and percentages. Groups were compared using Wilcoxon-Mann-Whitney and Fisher’s exact test. AC, atopic controls; and EA, egg allergic.
3.2 |. BBEA to measure antibodies specific to ovomucoid epitopes
The bead-based epitope assay (BBEA) was developed to measure levels of OVM’s esIgs. We previously established in the peanut and milk BBEAs that plate effect (that is, technical experimental artifact common to high-throughput assays) can be detected.45 The IgG4 experiment was performed on multiple plates that included repeats of the same positive and negative pooled samples. The plate effect accounted for 57.2% of the overall variability and samples cluster by experimental date on a PCA plot (Figure S1A). This effect was estimated and eliminated using linear models, and in the adjusted data, the plate effect was contributing to 1.2% of total variability (Figure S1B).
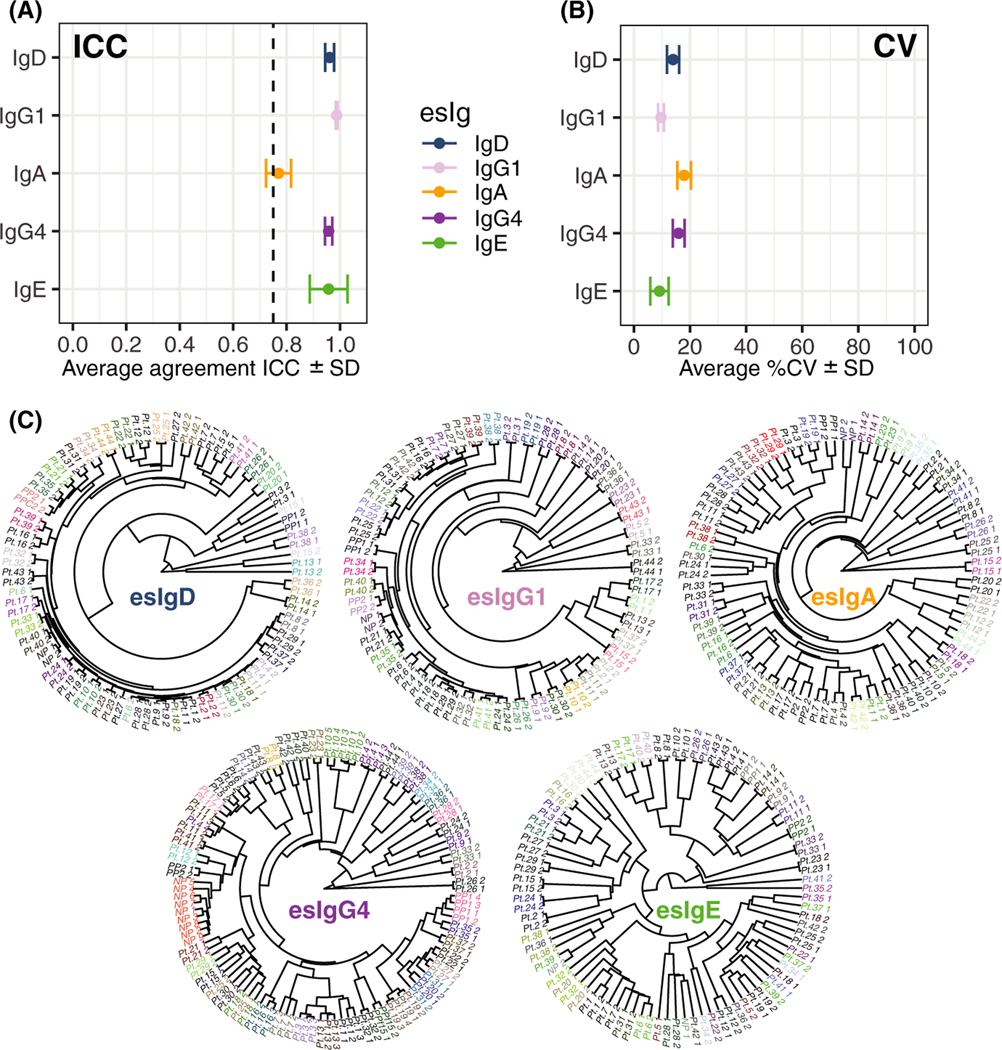
Experimental reliability of the BBEA was assessed using multiple metrics. First, technical duplicates showed excellent agreement based on the intraclass correlation coefficient (ICC) with the average (±SD) of 0.96 ± 0.07 for esIgE, 0.96 ± 0.01 for esIgG4, 0.99 ± 0.003 for esIgG1, 0.77 ± 0.05 for esIgA, and 0.97 ± 0.02 for esIgD (Figure 1A), which was consistent across individual peptides (Figure S1C). The average coefficient of variation (CV) was below 20% for all antibodies (Figure 1B, Figure S1D). Additionally, using unsupervised hierarchical clustering, duplicates of the same sample clustered together (Figure 1C).
FIGURE 1.
Characterization of egg-BBEA. A, ICC measured among duplicates and averaged across 58 peptides shows high agreement (ICC >0.75) for all antibody isotypes, presented as mean and SD. B, CV averaged across 58 peptides shows low variability among technical replicates, presented as mean and SD C, Unsupervised clustering presented as a phylogenetic tree of the technical duplicates constructed with the Pearson correlation as distance metric and “average” agglomeration algorithm shows that samples from the same patients cluster together
3.3 |. IgE shows most epitope specificity and is “connected” to esIgG4 and esIgD
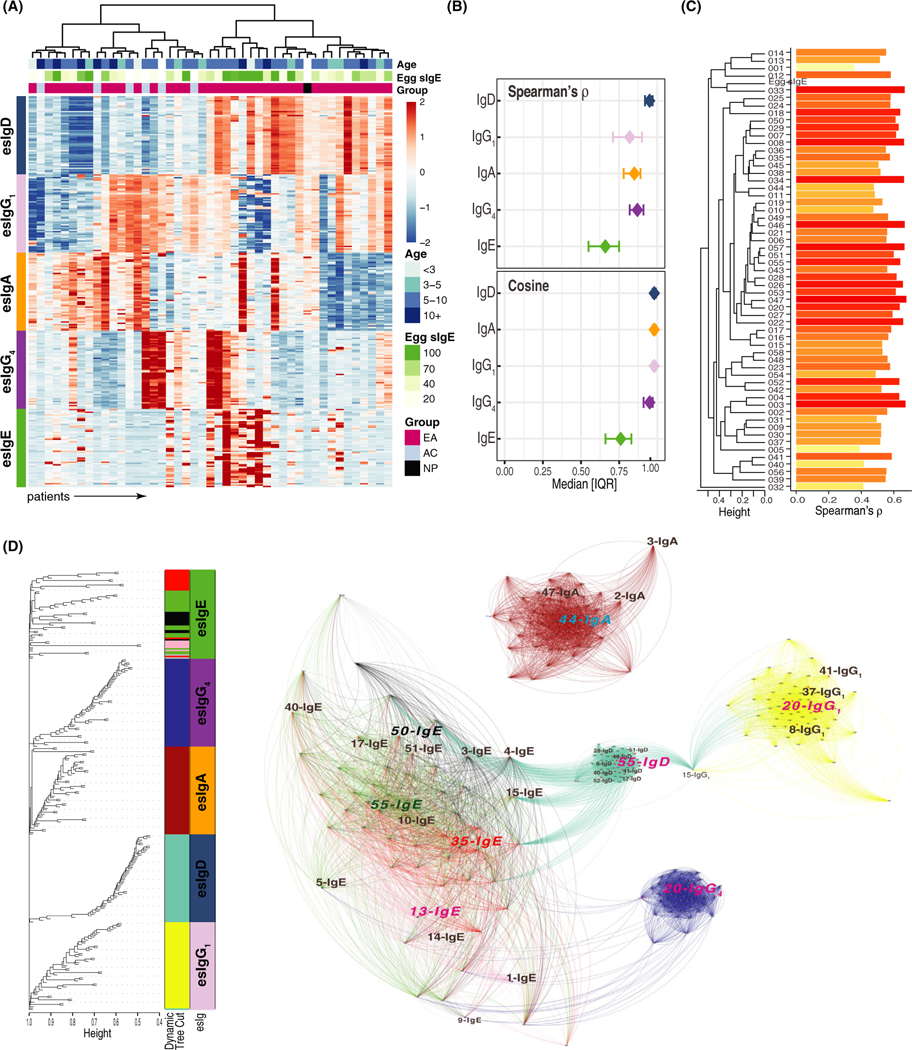
Epitope-specific antibody levels, presented as a heatmap (Figure 2A), show heterogeneity among patients. esIgE had greater intra-patient variability with the lowest average epitope-epitope pairwise correlation (ρ = .68) and cosine similarity (0.74, Figure 2B), preferentially recognizing only a few peptides, that is, IgE epitopes. All 58 IgE antibodies were positively associated with the egg white sIgE, with correlations ranging from 0.20 to 0.61 (Figure 2C). Other immunoglobulin isotypes were present at either low or high levels but with similar specificity to all 58 OVM peptides, as demonstrated by an average pairwise correlation of 0.95 for esIgD, 0.87 for esIgG4, 0.84 for esIgA, and 0.83 for esIgG1 (Figure 2B).
FIGURE 2.
Epitope-specific immunoglobulin repertoire. A, Heatmap representing the (standardized) levels of five antibody isotypes to 58 peptides (rows, ordered by sequential peptide number) for all 44 patients (columns). B, A median and interquartile range of epitope-epitope pairwise Spearman correlations or cosine similarity calculated separately for each antibody isotype. esIgEs have lowest correlations, potentially indicating epitope specificity C, Barplot showing that all esIgEs have positive Spearman correlation with egg-white sIgE (all P-values < 0.05), ordered by unsupervised hierarchical clustering. D, Left, a dendrogram of the hierarchical clustering of the TOM matrix (colored tips represent module assignment and corresponding classes of the esIgs) shows that aside from esIgE, other antibody isotypes were assigned to the self-containing modules. Right, a multiscale network of esIgs constructed with ForceAtlas2 algorithm and colored by module assignment; larger letters in color (module color for esIgE, blue for esIgA, and pink of the rest) are most connected nodes, that is, module hubs. esIgs in black larger letters highlight epitopes associated with allergy or history of anaphylactic reactions identified in subsequent analysis
The relationship across esIgs was further investigated through the network analysis, which separates distinct modules based on the overall connectivity. Consistent with the previous observations, esIgE antibodies showed the most variability and were split into four modules (green, black, pink, and red), while other esIgs belonged to self-containing groups: brown—esIgA, blue—esIgG4, turquoise—esIgD, and yellow—esIgG1 (Figure 2D). There was one exception: 015.esIgG1 was assigned to the turquoise (IgD) module, while the rest of the 57 esIgG1 antibodies belonged to the yellow cluster. Interestingly, this esIgG1 antibody had the most connections to both esIgD (turquoise) and esIgG1 (yellow) modules, serving as an intermediate link between the two isotypes. The interconnectivity was mostly observed between esIgE nodes and esIgG4 or esIgD, with no edges connecting esIgA to any other esIg.
3.4 |. Higher esIgE and esIgD and lower esIgA and esIgG1 are associated with egg allergy
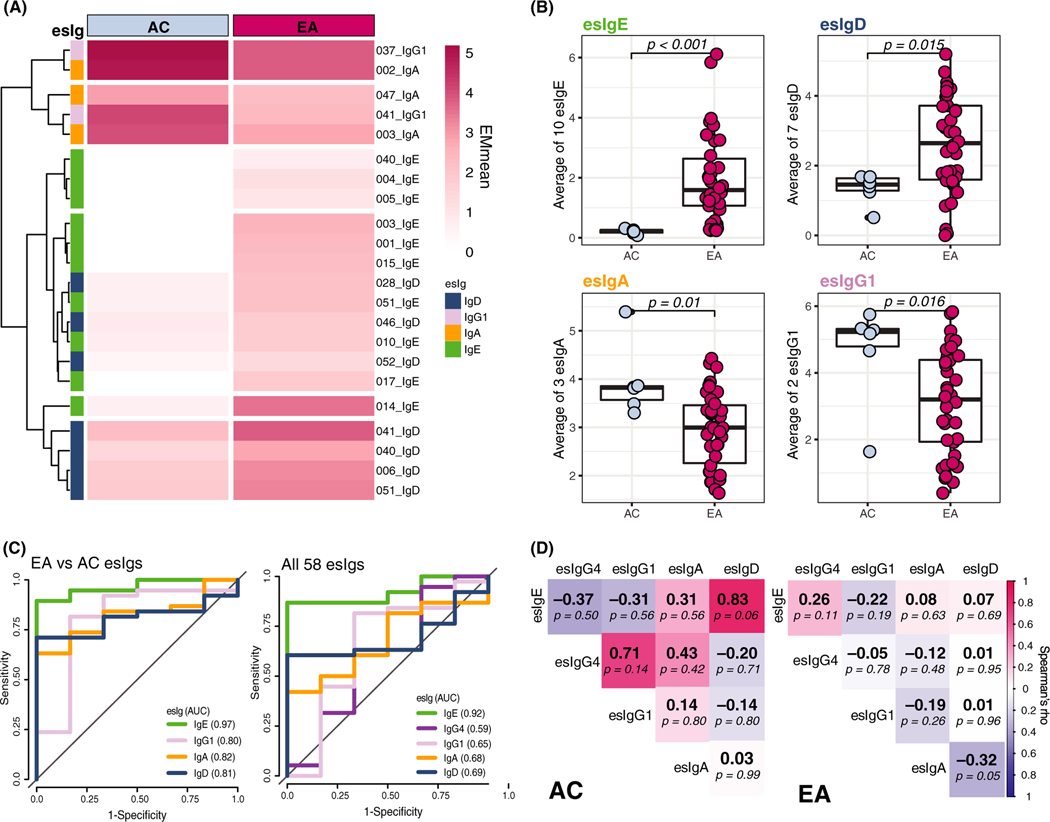
To determine which esIgs are associated with egg allergy, we compared repertoires of EA and AC groups. Ten esIgE and 12 esIgD were higher in the EA group (P < .05), while 2 esIgG1 and 3 esIgA were higher in the AC patients; none of the esIgG4 reached significance (Figure 3A). The averages of these esIgs were also significantly different among groups (Figure 3B) and provided better group distinction than averaging all 58 epitopes (Figure S2), with AUCs of 0.97 vs 0.92 for esIgE, 0.81 vs 0.69 for esIgD, 0.82 vs 0.62 for esIgA, and 0.80 vs 0.65 for esIgG1 (Figure 3C).
FIGURE 3.
esIg repertoire in EA vs AC groups. A, Heatmap representing the mean levels of esIgs that were significantly different among EA and AC groups (P < .05): 10 esIgE, 7 esIgD, 3 esIgA, 2 esIgG1, and none of the esIgG4. B, Boxplots of the average of the significant esIgs compared using Wilcoxon-Mann-Whitney U test (each point is an individual patient). C, The ROC curves of the logistic models for either the average of only significant esIgs (left) or all esIgs (right) and their respective AUCs in the legend. D, Spearman correlations of the averages of the significant esIgs (and all 58 esIgG4s) by group; color represents direction and strength of association, and correlation coefficient is a number in bold, followed by a p-value
The associations among esIgs were also different in the EA and AC (Figure 3D, Figure S3A). In the EA children, esIgD was negatively correlated with esIgA (ρ = −0.32, P = .05), while in the AC group, a strong positive trend was observed between esIgE and esIgD (ρ = 0.83, P = .06). Of note, while most patients had some IgD and IgA directed at OVM epitopes, their relative quantities were different: higher levels of one isotype generally meant lower level of the other one, as shown by patients clustering into the opposite groups in Figure S3B.
3.5 |. Lower esIgD and esIgG1 are associated with increased odds of anaphylaxis in egg-allergic patients
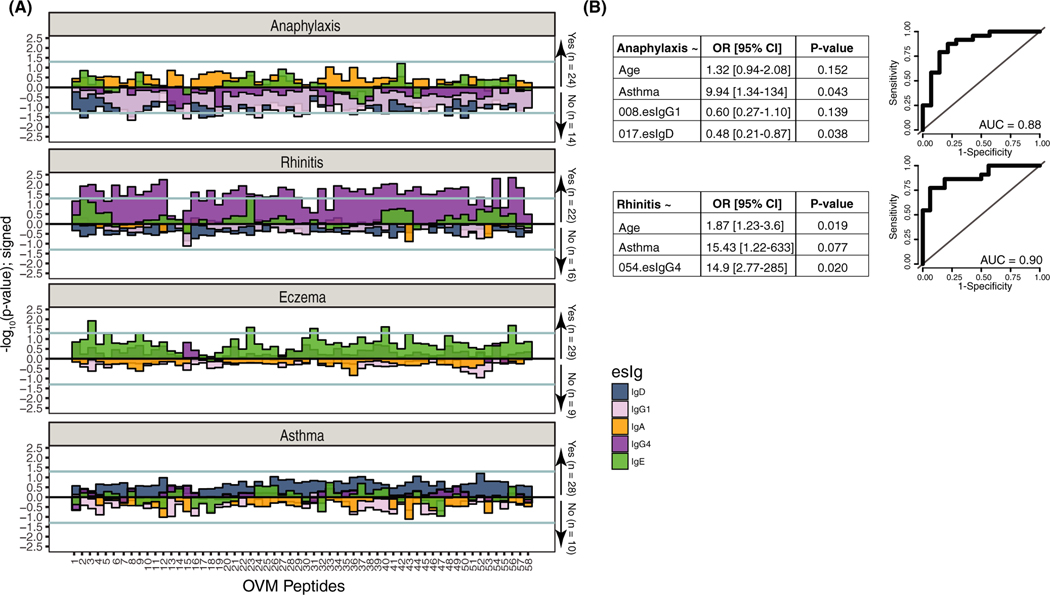
Univariate models of esIgs and anaphylaxis, as well as asthma, rhinitis, and eczema, showed different profiles of immunoglobulin isotypes. Eight esIgD and 14 esIgG1 were lower in patients with a history of anaphylaxis (Figure 4A); and the 017.esIgD antibody was significantly associated with reduced odds of anaphylactic reactions (OR = 0.48, P = .038) in a multivariable model adjusted for asthma, age, and esIgG1 (AUC = 0.88, Figure 4B). Additionally, nonanaphylactic patients had a positive correlation of esIgD with esIgE (ρ = 0.48, P = .037), which was similar to the AC group, while esIgD and esIgA (ρ = −0.76, P < .001) and esIgE and esIgG1 (ρ = −0.45, P = .057) were negatively correlated (Figure S4). These associations were in the same direction but with a much lower magnitude in the anaphylactic group.
FIGURE 4.
Associations of atopic comorbidities and anaphylaxis with esIgs in EA patients. A, “Chicago” plot representing –log10(P-value) for univariate associations; values above 0 represent positive association and negative association if below 0. B, Multivariable logistic models selected by a stepwise procedure for anaphylaxis (top) and rhinitis (bottom) and their respective ROCs
Of the atopic comorbidities, EA children with rhinitis had 44 esIgG4 antibodies that demonstrated significantly greater levels compared to patients without rhinitis; 5 esIgE were higher in children with eczema; and no differences in esIgs were observed in patients with or without asthma (Figure 4A,B). In a multivariable model, only a positive association of rhinitis and esIgG4 to epitope #054 remained significant, even when adjusted for age and asthma.
4 |. DISCUSSION
Understanding the molecular mechanism of allergic sensitization may help improve diagnostic, prognostic, and treatment approaches in food allergy. Previously, we demonstrated that milk-BBEA and peanut-BBEA have excellent reliability and sensitivity in detecting allergenic epitopes,45 providing further insights into the role of IgE and IgG4 repertoires in disease phenotypes44 and prognosis of milk oral immunotherapy outcome.43 We have now developed an assay, termed egg-BBEA to measure the levels of five antibody isotypes, that is, IgE, IgG4, IgG1, IgA, and IgD to peptides covering the entire sequence of a major hen’s egg white allergen—ovomucoid. We showed that egg-BBEA, similarly to other high-throughput technologies, has plate effects that can be detected and eliminated; and is a reliable assay with high agreement and low variation among technical replicates (Figure 1, Figure S1).
We have identified IgE directed at sequential peptides associated with egg allergy (Figure 3A), constituting immunogenic IgE epitopes previously identified by other groups15,23–57 and demonstrating sensitivity of egg-BBEA for epitope detection. We also found that EA children had variable but on average higher esIgD, while AC showed greater levels of esIgA and esIgG1 antibodies (Figure 3A,B, Figure S3). Furthermore, we found that esIgE was highly correlated with esIgD in AC but not EA patients (Figure 3D), which may indicate that while atopic patients have low levels of IgE, it is produced by plasma cells coming from a direct isotype switch from IgD+ B cells, resulting in lower-affinity IgE antibodies.58 Different antibody isotypes may inhibit IgE-mediated allergic reactions, given they have similar specificity to an antigen,31 and in this cohort, EA patients had high egg sensitization with median egg sIgE of 49.9 kUA/L, which might have rendered antibody repertoire profiles different from patients with lower sIgE levels. However, these results provide basis for further studies that should include broader allergic population and a larger number of control participants.
To identify association patterns across repertoires of antibody isotypes, we applied a network analysis and showed that esIgE had large variability with four distinct modules and low correlations, suggesting higher epitope specificity of this antibody (Figure 2B,D). On the other hand, the rest of the isotypes were assigned into self-containing modules. Interestingly, across antibodies connectivity was observed between esIgE and esIgG4 or esIgD, while esIgA was disconnected from other modules, and esIgG1 was only connected to esIgD via one epitope. This may indicate that IgE-secreting plasma cells come from either sequential isotype switch from the intermediate upstream isotypes (ie, IgG4+ B cells) or directly from the IgD+ B cells. This concept was shown by several groups that sequenced the heavy-chain loci of the human immunoglobulin gene (IgH) and compared the mutation rates of the variable segment, concluding that high-affinity IgE is a product of sequential isotype switch form “antigen-experienced” B cells of the IgG subclasses; while lower-affinity IgE derived from a direct switch from the IgM/IgD B cells.58–64
Early food allergy and eczema, especially an extrinsic subtype defined by high IgE levels, are often followed by progression to asthma and allergic rhinitis, commonly referred to as the “atopic march”.65,66 The development of atopic manifestations progresses with age and having one of the comorbidities is considered a risk factor for the others. Additionally, food-related anaphylaxis was shown to be associated with age as well as the presence of atopic dermatitis.67,68 We further investigated whether the repertoire of immunoglobulins was associated with atopic diseases and anaphylaxis. While the EA children had higher esIgD than AC (Figure 3B), within the EA group patients with lower esIgD and esIgG1 had greater odds of anaphylaxis (Figure 4). This finding is consistent with a recent study by Shan et al36 showing that antigen-bound IgD can attenuate IgE-mediated basophils degranulation, potentially increasing immunity against soluble antigens. High levels of esIgG1 could result in more antigen molecules being cleared through phagocytosis69,70 rendering them unavailable to IgE. Additionally, while no correlation was observed between esIgE and esIgD in the EA group (Figure 3D, Figure S3A), after stratifying by history of anaphylaxis, this correlation was positive (Figure S4) among children with no history of anaphylaxis to egg, similarly to that of the atopic controls. Zhang et al71 observed that antigen-specific IgE was correlated with antigen-specific IgD for several aero-allergens, for example, birch pollen, timothy pollen, and cat dander, with higher IgD levels to these antigens in atopic compared to nonatopic patients, which they hypothesized was indicative of similar regulation of the two antibodies.
Of the atopic comorbidities, high levels of almost all esIgG4 antibodies were associated with rhinitis, and one IgG4 epitope had significant association in a model adjusted for asthma and age (Figure 4). This association of IgG4 is not commonly known, but IgG4 specific to house-dust mite was shown to be on average higher in the rhinitis group than atopic or healthy controls.72,73 Several esIgE antibodies were associated with eczema; and none of the esIgs reached significance for asthma.
Despite a small sample size and limited clinical information for these biorepository samples, we showed that examining the relationships across several antibody isotypes, including less studied IgG1, IgA, and IgD antibodies, could distinguish additional endotypes among egg-allergic children at a molecular level. Further investigation of egg epitope-specific humoral immune profiles in patients with egg allergy confirmed by supervised oral food challenges and a control population with serological sensitization to egg in the absence of clinical allergy is likely to provide insight into the potential severity, natural history, and response to immunotherapy in egg-allergic individuals.
Supplementary Material
ACKNOWLEDG MENTS
The authors would like to thank Dr Andrea Cerutti, MD/PhD, for the valuable discussions and insights; and the Food Allergy Research Initiative (FARI) biorepository at the Icahn School of Medicine at Mount Sinai for patients’ plasma samples. The FARI repository was supported in part by a grant from Food Allergy Research and Education, a nonprofit organization supporting food allergy research. The study (HS and M.SF.) was funded in part by a grant from National Institute of Allergy and Infectious Diseases (NIAID, AI-66738), the David H. and Julia Koch Research Program in Food Allergy Therapeutics, and AllerGenis LLC. MS was funded by the Integrated Pharmacological Sciences Training Program grant from the National Institute of General Medical Sciences (NIGMS, T32GM062754). Other funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding information
National Institute of General Medical Sciences, Grant/Award Number: T32GM062754; National Institute of Allergy and Infectious Diseases, Grant/Award Number: AI-66738; David H. and Julia Koch Research Program in Food Allergy Therapeutics; AllerGenis LLC
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
CONFLICT OF INTEREST
MS has nothing to disclose. RG is an employee of Genisphere LLC and scientific consultant of AllerGenis LLC; in addition, RG has a patent PCT/US15/020715 (WO) pending. GG has nothing to disclose. AT has nothing to disclose. M.SF. received research funding to Mount Sinai by a grant from AllerGenis LLC. HS reports nonfinancial support from AllerGenis LLC during the conduct of the study; grants from Immune Tolerance Network; NIAID/NIH, personal fees from N-Fold Therapeutics, other from DBV Technologies, outside the submitted work; and Mount Sinai has licensed the technology for a bead-based epitope assay for food-allergen epitope analyses to AllerGenis LLC. HS serves as an unpaid Board of Directors member and advisor to AllerGenis LLC.
REFERENCES
- 1.Eggesbo M, Botten G, Halvorsen R, Magnus P. The prevalence of allergy to egg: a population-based study in young children. Allergy. 2001;56(5):403–411. [DOI] [PubMed] [Google Scholar]
- 2.Rona RJ, Keil T, Summers C, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–646. [DOI] [PubMed] [Google Scholar]
- 3.Janzi M, Kull I, Sjoberg R, et al. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clin Immunol. 2009;133(1):78–85. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–e17. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594–602. [DOI] [PubMed] [Google Scholar]
- 6.Osborne NJ, Koplin JJ, Martin PE, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668–676.e2. [DOI] [PubMed] [Google Scholar]
- 7.Peters RL, Koplin JJ, Gurrin LC, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: healthNuts age 4-year follow-up. J Allergy Clin Immunol. 2017;140(1):145–153.e8. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RS, Warren CM, Smith BM, et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2(1):e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osterlund J, Winberg A, West CE. A 10-year review found increasing incidence trends of emergency egg allergy reactions and food-induced anaphylaxis in children. Acta Paediatr. 2019;108(2):314–320. [DOI] [PubMed] [Google Scholar]
- 10.Tordesillas L, Berin MC, Sampson HA. Immunology of food allergy. Immunity. 2017;47(1):32–50. [DOI] [PubMed] [Google Scholar]
- 11.Sampson HA, O’Mahony L, Burks AW, Plaut M, Lack G, Akdis CA. Mechanisms of food allergy. J Allergy Clin Immunol. 2018;141(1):11–19. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda T, Watanabe K, Nakamura R. Immunochemical and physical properties of peptic-digested ovomucoid. J Agric Food Chem. 1983;31(5):942–946. [DOI] [PubMed] [Google Scholar]
- 13.Honma K, Aoyagi M, Saito K, et al. Antigenic determinants on ovalbumin and ovomucoid: comparison of the specificity of IgG and IgE antibodies. Arerugi. 1991;40(9):1167–1175. [PubMed] [Google Scholar]
- 14.Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol. 1994;93(6):1047–1059. [DOI] [PubMed] [Google Scholar]
- 15.Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997;159(4):2026–2032. [PubMed] [Google Scholar]
- 16.Walsh BJ, Hill DJ, Macoun P, Cairns D, Howden ME. Detection of four distinct groups of hen egg allergens binding IgE in the sera of children with egg allergy. Allergol Immunopathol (Madr). 2005;33(4):183–191. [DOI] [PubMed] [Google Scholar]
- 17.Urisu A, Yamada K, Tokuda R, et al. Clinical significance of IgE-binding activity to enzymatic digests of ovomucoid in the diagnosis and the prediction of the outgrowing of egg white hypersensitivity. Int Arch Allergy Immunol. 1999;120(3):192–198. [DOI] [PubMed] [Google Scholar]
- 18.Ando H, Moverare R, Kondo Y, et al. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008;122(3):583–588. [DOI] [PubMed] [Google Scholar]
- 19.Andorf S, Borres MP, Block W, et al. Association of clinical reactivity with sensitization to allergen components in multifood-allergic children. J Allergy Clin Immunol Pract. 2017;5(5): 1325–1334.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Yanagida N, Sato S, Ebisawa M. Predictive power of ovomucoid and egg white specific IgE in heated egg oral food challenges. J Allergy Clin Immunol Pract. 2018;6(6):2115–2117.e6. [DOI] [PubMed] [Google Scholar]
- 21.Berin MC, Grishin A, Masilamani M, et al. Egg-specific IgE and basophil activation but not egg-specific T cells correlate with phenotypes of clinical egg allergy. J Allergy Clin Immunol. 2018;142(1):149–158. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shreffler WG, Lencer DA, Bardina L, Sampson HA. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J Allergy Clin Immunol. 2005;116(4):893–899. [DOI] [PubMed] [Google Scholar]
- 23.Jarvinen KM, Beyer K, Vila L, Bardina L, Mishoe M, Sampson HA. Specificity of IgE antibodies to sequential epitopes of hen’s egg ovomucoid as a marker for persistence of egg allergy. Allergy. 2007;62(7):758–765. [DOI] [PubMed] [Google Scholar]
- 24.Flinterman AE, Knol EF, Lencer DA, et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121(3):737–743.e10. [DOI] [PubMed] [Google Scholar]
- 25.Urisu A, Ando H, Morita Y, et al. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997;100(2):171–176. [DOI] [PubMed] [Google Scholar]
- 26.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116(3):833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122(5):977–983 e971. [DOI] [PubMed] [Google Scholar]
- 28.Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Wegrzyn A. Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol. 2011;127(4):990–997. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard SA, Sampson HA, Sicherer SH, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012;130(2):473–480.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konstantinou GN, Nowak-Wegrzyn A, Bencharitiwong R, Bardina L, Sicherer SH, Sampson HA. Egg-white-specific IgA and IgA2 antibodies in egg-allergic children: is there a role in tolerance induction? Pediatr Allergy Immunol. 2014;25(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodev TS, Bowen H, Shamji MH, et al. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy. 2015;70(6):720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugimoto M, Kamemura N, Nagao M, et al. Differential response in allergen-specific IgE, IgGs, and IgA levels for predicting outcome of oral immunotherapy. Pediatr Allergy Immunol. 2016;27(3):276–282. [DOI] [PubMed] [Google Scholar]
- 33.Wright BL, Kulis M, Orgel KA, et al. Component-resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy. 2016;71(11):1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natsume O, Kabashima S, Nakazato J, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10066):276–286. [DOI] [PubMed] [Google Scholar]
- 35.Maeta A, Katahira R, Matsushima M, Onishi H, Nakamura Y, Takahashi K. Stepwise oral immunotherapy for 10 days in an egg-white allergy mouse model did not ameliorate the severity of allergy but induced the production of allergen-specific IgA. Biosci Biotechnol Biochem. 2018;82(12):2176–2179. [DOI] [PubMed] [Google Scholar]
- 36.Shan M, Carrillo J, Yeste A, et al. Secreted IgD amplifies humoral T helper 2 cell responses by binding basophils via galectin-9 and CD44. Immunity. 2018;49(4):709–724.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lupinek C, Hochwallner H, Johansson C, et al. Maternal allergen-specific IgG may protect the child against allergic sensitization. J Allergy Clin Immunol 2019;144(2):536–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez-Saiz R, Ellenbogen Y, Koenig JFE, et al. IgG1(+) B-cell immunity predates IgE responses in epicutaneous sensitization to foods. Allergy. 2019;74(1):165–175. [DOI] [PubMed] [Google Scholar]
- 39.Leung DYM, Calatroni A, Zaramela LS, et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med. 2019;11(480).eaav2685. 10.1126/scitranslmed.aav2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutzeit C, Chen K, Cerutti A. The enigmatic function of IgD: some answers at last. Eur J Immunol. 2018;48(7):1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klockenbring T, Boese A, Bauer R, Goerlich R. Comparative investigations of wheat and spelt cultivars: IgA, IgE, IgG1 and IgG4 binding characteristics. Food Agric Immunol. 2001;13(3):171–181. [Google Scholar]
- 42.Seppo AE, Savilahti EM, Berin MC, Sampson HA, Jarvinen KM. Breast milk IgA to foods has different epitope specificity than serum IgA-Evidence for entero-mammary link for food-specific IgA? Clin Exp Allergy. 2017;47(10):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suarez-Farinas M, Suprun M, Chang HL, et al. Predicting development of sustained unresponsiveness to milk oral immunotherapy using epitope-specific antibody binding profiles. J Allergy Clin Immunol. 2018;143(3):1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sackesen C, Suarez-Farinas M, Sillva R, et al. A new Luminex-based peptide assay to identify reactivity to baked, fermented, and whole milk. Allergy. 2018;74(2):327–336. [DOI] [PubMed] [Google Scholar]
- 45.Suprun M, Getts R, Raghunathan R, et al. Novel bead-based epitope assay is a sensitive and reliable tool for profiling epitope-specific antibody repertoire in food allergy. Sci Rep. 2019;9(1):18425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suprun M, Suarez-Farinas M. PlateDesigner: a web-based application for the design of microplate experiments. Bioinformatics. 2018;35(9):1605–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breen EJ, Tan W, Khan A. The statistical value of raw fluorescence signal in luminex xMAP based multiplex immunoassays. Sci Rep. 2016;6:26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Bushel PR, Chu TM, Wolfinger RD. Principal variance components analysis: estimating batch effects in microarray gene expression data. In: Scherer A, eds. Batch effects and noise in microarray experiments: sources and solutions. UK: John Wiley & Sons, Ltd; 2009:141–154. [Google Scholar]
- 49.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6(4):284. [Google Scholar]
- 50.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4(1): [DOI] [PubMed] [Google Scholar]
- 51.Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media. 2009. http://www.aaai.org/ocs/index.php/ICWSM/09/paper/view/154 [Google Scholar]
- 52.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27(3):431–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zar J. Spearman rank correlation: overview. In: Balakrishnan N, Colton T, Everitt B, Piegorsch W, Ruggeri F, Teugels JL, eds: Wiley StatsRef: Statistics Reference Online. Hoboken, NJ: Wiley; 2014. 10.1002/9781118445112.stat05964 [DOI] [Google Scholar]
- 54.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. [DOI] [PubMed] [Google Scholar]
- 55.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovacs-Nolan J, Zhang JW, Hayakawa S, Mine Y. Immunochemical and structural analysis of pepsin-digested egg white ovomucoid. J Agric Food Chem. 2000;48(12):6261–6266. [DOI] [PubMed] [Google Scholar]
- 57.Holen E, Bolann B, Elsayed S. Novel B and T cell epitopes of chicken ovomucoid (Gal d 1) induce T cell secretion of IL-6, IL-13, and IFN-gamma. Clin Exp Allergy. 2001;31(6):952–964. [DOI] [PubMed] [Google Scholar]
- 58.Mine Y, Wei ZJ. Identification and fine mapping of IgG and IgE epitopes in ovomucoid. Biochem Biophys Res Commun. 2002;292(4):1070–1074. [DOI] [PubMed] [Google Scholar]
- 59.Takagi K, Teshima R, Okunuki H, et al. Kinetic analysis of pepsin digestion of chicken egg white ovomucoid and allergenic potential of pepsin fragments. Int Arch Allergy Immunol. 2005;136(1):23–32. [DOI] [PubMed] [Google Scholar]
- 60.Martinez-Botas J, Cerecedo I, Zamora J, et al. Mapping of the IgE and IgG4 sequential epitopes of ovomucoid with a peptide microarray immunoassay. Int Arch Allergy Immunol. 2013;161(1):11–20. [DOI] [PubMed] [Google Scholar]
- 61.Looney TJ, Lee JY, Roskin KM, et al. B-cell isotype switching origins of IgE. J Allergy Clin Immunol. 2016;137(2):579–586.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong H, Dolpady J, Wabl M, Curotto de Lafaille MA, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. 2012;209(2):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horns F, Vollmers C, Croote D, et al. Lineage tracing of human B cells reveals the in vivo landscape of human antibody class switching. Elife. 2016;5. e16578. 10.7554/eLife.16578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118–S127. [DOI] [PubMed] [Google Scholar]
- 65.Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol. 2017;139(6):1723–1734. [DOI] [PubMed] [Google Scholar]
- 66.Worm M, Edenharter G, Rueff F, et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy. 2012;67(5):691–698. [DOI] [PubMed] [Google Scholar]
- 67.Worm M, Babina M, Hompes S. Causes and risk factors for anaphylaxis. J Dtsch Dermatol Ges. 2013;11(1):44–50. [DOI] [PubMed] [Google Scholar]
- 68.Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S41–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18(1):46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang M, Niehus J, Brunnee T, Kleine-Tebbe J, O’Connor A, Kunkel G. Measurement of allergen-specific IgD and correlation with allergen-specific IgE. Scand J Immunol. 1994;40(5):502–508. [DOI] [PubMed] [Google Scholar]
- 71.Aydogan M, Mete N, Yazi D, et al. Comparison of Der p1-specific antibody levels in children with allergic airway disease and healthy controls. Pediatr Allergy Immunol. 2007;18(4):320–325. [DOI] [PubMed] [Google Scholar]
- 72.Miranda DO, Silva DA, Fernandes JF, et al. Serum and salivary IgE, IgA, and IgG4 antibodies to Dermatophagoides pteronyssinus and its major allergens, Der p1 and Der p2, in allergic and nonallergic children. Clin Dev Immunol. 2011;2011:302739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.