ABSTRACT
Perioperative chemotherapy enhances the survival rates for patients with esophageal and gastric (EG) adenocarcinoma, but not all patients benefit from this additional treatment. Chemotherapeutic agents have been demonstrated to alter the immune cell (IC) composition in the tumor microenvironment. Hence, there is a rationale to investigate the influence of neoadjuvant chemotherapy (NAC) on different IC subsets, to better understand and compare their utility as complementary prognostic or predictive biomarkers in a clinically relevant context. The density of T cells (CD8+ and FoxP3+), B cells (CD20+) and the expression of PD-L1 on ICs and tumor cells (TC) was assessed by immunohistochemistry on paired biopsies from primary tumors (PT) pre-NAC, and resected PT and lymph node metastases post-NAC. The cohort encompasses 148 patients with resectable EG adenocarcinoma, all of whom received NAC. The density of CD8+ cells was decreased and the density of FoxP3+ cells and CD20+ cells was increased in PT post-NAC. PD-L1 expression was not altered following NAC. In pre-NAC specimens, high FoxP3+ density and high PD-L1 expression on ICs were favorable prognostic factors, whereas high CD8+ density was an unfavorable prognostic factor. In post-NAC specimens, however, high FoxP3+ density was an unfavorable prognostic factor, and high PD-L1 expression on TC was associated with a shorter survival. There were no significant associations between IC density or PD-L1 expression in PT pre-NAC and histopathological regression. These findings propose that NAC might alter the density and prognostic impact of some IC subsets in EG adenocarcinoma.
KEYWORDS: Esophageal cancer, gastric cancer, neoadjuvant chemotherapy, T-cells, B-cells; PD-L1 expression
Introduction
For patients with locally advanced esophageal and gastric (EG) adenocarcinoma, randomized studies have demonstrated perioperative (i.e. neoadjuvant and adjuvant) chemotherapy to enhance the 5-year overall survival (OS) rates from 23%-24% to 36–38% compared to surgery alone.1,2 Moreover, the current standard perioperative treatment regimen, FLOT (fluorouracil, leucovorin, oxaliplatin and docetaxel), has been shown to further improve the 5 year OS rate to 45%.3 However, the outcome for these patients remains poor and solely 15–20% of the patients benefit from the addition of chemotherapy to surgery.1–3 Therefore, there is a great need to identify suitable predictive and prognostic biomarkers for guidance in the clinical setting, with the aim to reduce overtreatment.
Accumulating evidence clearly demonstrates that the tumor microenvironment (TME) is of paramount importance for the development, progression, and prognosis of cancer.4,5 Tumor infiltrating lymphocytes (TILs) in general, and cytotoxic CD8+ T cells in particular, have mainly been shown to correlate with an improved outcome for patients with EG adenocarcinoma.6–9 The prognostic role of Forkhead box P3 (FoxP3+) T regulatory cells (Tregs) is more ambiguous, although the majority of studies points toward an association with an adverse outcome.8,10,11 Furthermore, while numerous studies have addressed the relevance of T cells, the clinical impact of B cells has only recently begun to attract interest. B cells are known to both positively and negatively regulate the cellular immune response against tumors. Several studies have shown associations between B cell infiltration and improved survival in patients with EG adenocarcinoma,9,12,13 although some studies are negative in this regard.6,14 Furthermore, the impact of the spatial distribution of B cell and T cell subsets and their ability to form aggregates or clusters has also been investigated, and the presence of tertiary lymphoid structures (TLS)15,16 has been associated with a favorable prognosis in several types of tumors, including gastric cancer.12,17–19
In addition to the well-established treatment with chemotherapy and chemoradiotherapy, the efficacy of immune checkpoint inhibitors (ICI) targeting the programmed-death 1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway, is being investigated in several ongoing trials in EG cancer. To date, ICI has no role in the neoadjuvant or adjuvant setting in clinical practice, however promising data are upcoming, e.g., from the CheckMate 577 trial, which demonstrated an increase in disease-free survival from 11.0 to 22.4 months with adjuvant nivolumab, a monoclonal antibody inhibitor of PD-1, compared to placebo in patients with esophageal or gastroesophageal junction cancer treated with neoadjuvant chemoradiotherapy followed by surgery.20 For patients with unresectable locally advanced disease or metastatic disease, a subset of patients can be considered for treatment with pembrolizumab, a monoclonal antibody inhibitor of PD-1, in the second line or later.21 Regarding treatment in the first line setting, the phase III trials CheckMate 649 and Keynote 590, comparing chemotherapy ± nivolumab or pembrolizumab, respectively, have both demonstrated superior results for combination therapy, hence this may potentially be established as new standard of care in the near future.22,23 The expression of PD-L1 on tumor cells (PD-L1TC) and tumor infiltrating immune cells (PD-L1IC) are putative biomarkers for response to ICI,21,24 and have also been investigated as prognostic biomarkers, with diverging results.25–29
Adding to the complexity, it has been revealed that chemotherapeutic agents have the capability to alter the immune composition in the TME, also in EG cancer.30–37 Whether neoadjuvant chemotherapy (NAC) has an influence on the immune landscape in EG adenocarcinoma is to date unknown. This is a fundamental lack of knowledge that needs to be covered to be able to increase the potential of future treatment options and to understand resistance mechanisms. The aim of this study was therefore to assess potential effects of NAC on the immune cell (IC) composition in resected EG adenocarcinoma; in biopsies from primary tumors (PT) pre-NAC, and resected PT and paired lymph node (LN) metastases post-NAC.
Materials and methods
Study design and participants
The study cohort has been described previously38,39 and encompasses a consecutive series of 148 patients diagnosed with resectable esophageal (n = 39) or gastric (n = 109) adenocarcinoma, all of whom received NAC ± adjuvant chemotherapy. More than 95% of the patients were treated with fluoropyromidine and oxaliplatin-based chemotherapy (EOX, FLOX or FOLFOX) at Skåne University Hospital, Sweden, between January 1, 2008 and December 31, 2014. After NAC, 118 (79.7%) patients underwent surgical resection. Last update on recurrence and vital status was performed on December 31, 2017. Clinical and histopathological classification of tumor stage was based on the 7th edition of the UICC/AJCC TNM classification, wherein esophago-gastric junction tumors Siewert type I–III are classified as esophageal tumors.40 However, as esophago-gastric junction Siewert type III tumors are managed as gastric cancer in the clinic, and according to the new 8th edition of the UICC/AJCC TNM classification,41 this definition is used in the present study. Prior to the neoadjuvant therapy, all patients were discussed at a multidisciplinary tumor board and clinical stage was determined based on endoscopy findings and computerized tomography. Patients who received neoadjuvant chemoradiotherapy or palliative chemotherapy followed by salvage surgery were excluded. Residual tumor status was classified as R0 = no residual tumor (free resection margins according to the pathology report), R1 = possible microscopic residual tumor (narrow or compromised resection margins according to the pathology report) or R2 = macroscopic residual tumor (according to the operative report).
Histopathological response
To determine the degree of residual carcinoma in the post-neoadjuvant resected PT the tumor regression grading system described by Chirieac was applied.42 This grading system divides the histopathological response into the following groups; 0%, 1–10%, 11–50% or >50% residual carcinoma.
Tissue microarray construction and immunohistochemistry
All cases were histopathologically reevaluated on hematoxylin and eosin-stained sections by a board-certified pathologist (KJ). Tissue microarrays (TMAs) were constructed using cores from separate donor blocks from the resected PT, whenever possible from both central and peripheral non-necrotic tumor areas, in 118 cases and from paired LN metastases in 56 cases, using a semi-automated arraying device (TMArrayer, Pathology Devices, Westminister, MD, USA), as described previously.43 For immunohistochemical (IHC) analysis of CD8, FoxP3, CD20, and PD-L1, 3 μm sections from the biopsies and 4 μm sections from the TMAs were automatically pre-treated using the PT Link system and then stained in an Autostainer Plus (Dako; Glostrup, Denmark) with the anti-CD8-antibody clone C8/144B, mouse; dilution, 1:50; product M7103; Dako, the anti-FoxP3-antibody clone 236A/E7, mouse, dilution 1:200, Abcam, Cambridge, UK, the anti-CD20-antibody HPA14341, rabbit, dilution 1:200, Atlas Antibodies AB, Stockholm, Sweden, and the anti PD-L1-antibody clone E1L3N, rabbit, dilution, 1:100, Cell Signaling Technology, Inc, Danvers MA, 01923, USA.
Evaluation of immunohistochemical staining
The estimated total (intratumoral, tumor-adjacent and stromal) percentage (0–100%) of stained CD8+ (cytotoxic T cell), FoxP3+ (Treg) and CD20+ (B cell) ICs was manually annotated in each biopsy and TMA core by two independent observers (MCS, KJ), one of whom is a pathologist (KJ), both blinded to clinical and outcome data. In addition, the infiltration of the CD8+ and FoxP3+ T cell populations was further classified regarding the infiltration into tumor nests (TN), 0 (none), 1 (sparse) or 2 (high) in each biopsy and TMA core. The absolute number of lymphoid aggregates of CD20+ B cells was annotated in each biopsy and TMA core. The estimated total percentage (0–100%) of PD-L1+ ICs (intratumoral, tumor-adjacent and stromal) and TC in each biopsy and TMA core was manually annotated independently by three different observers (AL, JN and KJ), all blinded to clinical and outcome data. A mean value of the assessable TMA cores and biopsies for each patient was calculated and used in the statistical analyses. For all immune markers, discrepant cases were reevaluated and discussed to reach consensus. Staining intensity was not taken into account, and only biopsies, TMA cores and LNs with the presence of clearly visible TC were accounted for.
Statistical analyses
Wilcoxon signed-rank test was applied to determine differences in the density of different IC subsets and PD-L1 expression in paired pre-NAC and post-NAC samples. Spearman’s rank correlation test was used to investigate the intercorrelation between the different immune markers in paired pre-NAC and post-NAC samples. Mann-Whitney U test was used to evaluate the association of different immune markers in pre-NAC PT with histopathological regression. To evaluate the associations of the different immune markers with clinicopathological factors in PT pre-NAC and post-NAC, Kruskal-Wallis or Mann-Whitney U test was used for continuous variables and Chi-Square test for categorical variables. To determine prognostic cutoff points for the survival analyses, dichotomous variables of low and high density of stained ICs and TC were constructed from median values as well as from classification and regression tree (CRT) analysis when applicable. Kaplan-Meier analysis and the log rank test were applied to detect differences in time to recurrence (TTR) and OS. Cox proportional hazards models were used to calculate univariable and multivariable hazard ratios (HR) for TTR and OS. The variables adjusted for in the multivariable analyses included age, sex, location, T-stage (T1-2 vs T3-4), N-stage (N0 vs N+), M-stage (M0 vs M1), differentiation grade (high vs low), resection margin (R0, R1, R2), and Laurén classification (intestinal vs diffused/mixed type). TTR was defined as time from resection to the date of biopsy or radiology proven recurrent disease. OS was defined as time from resection to the date of death. Patients who died within 90 days after surgery were excluded, as this was considered a postoperative complication. No survival analyses were performed on LN metastases for any of the immune markers due to the small number of cases. All tests were 2-sided and a p value of <0.05 was regarded as statistically significant. All calculations were performed using IBM SPSS Statistics for Mac version 24.0 or 26.0 (IBM Armonk, NY, USA).
Results
Immune marker density in pre-NAC and post-NAC specimens
Immunohistochemical sample images are shown in Supplementary Figure 1. Box plots visualizing the density of CD8+ T cells, FoxP3+ T cells, and CD20+ B cells in pre-NAC and post-NAC specimens in the entire cohort and by tumor location are shown in Figure 1. The density of CD8+ T cells was significantly higher in both PT and LN metastases post-NAC compared to PT pre-NAC, with similar findings when stratifying for tumor location. The density of FoxP3+ T cells was significantly higher in PT pre-NAC compared to PT post-NAC, with similar findings when stratifying for tumor location. The density of CD20+ B cells was significantly higher in PT pre-NAC than PT post-NAC, but significantly higher in LN metastases post-NAC than in PT both pre-NAC and post-NAC. Similar trends were seen when stratifying for tumor location.
Figure 1.
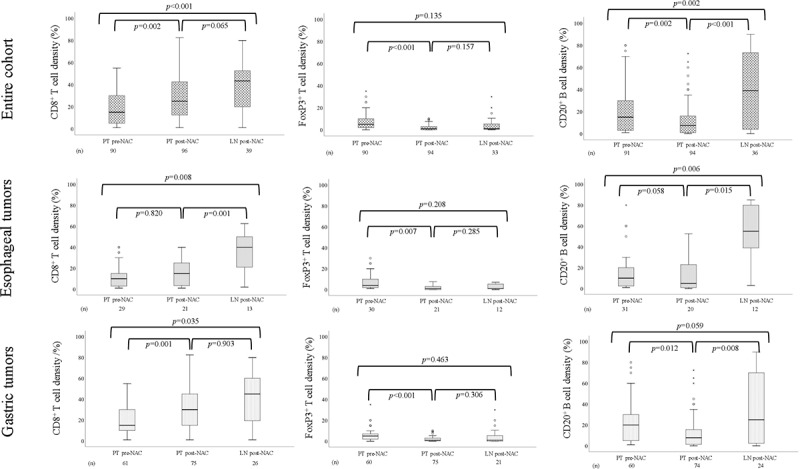
Boxplots visualizing the density of CD8+ T cells, FoxP3 + T cells and CD20+ B cells in PT pre-NAC, PT post-NAC and LN post-NAC, in the entire cohort, esophageal and gastric tumors, respectively
Box plots visualizing PD-L1IC and PD-L1TC expression in pre-NAC and post-NAC specimens are shown in Figure 2. No significant differences were found between PT pre-NAC, PT post-NAC or LN metastases post-NAC, neither in the entire cohort nor when stratifying for tumor location.
Figure 2.
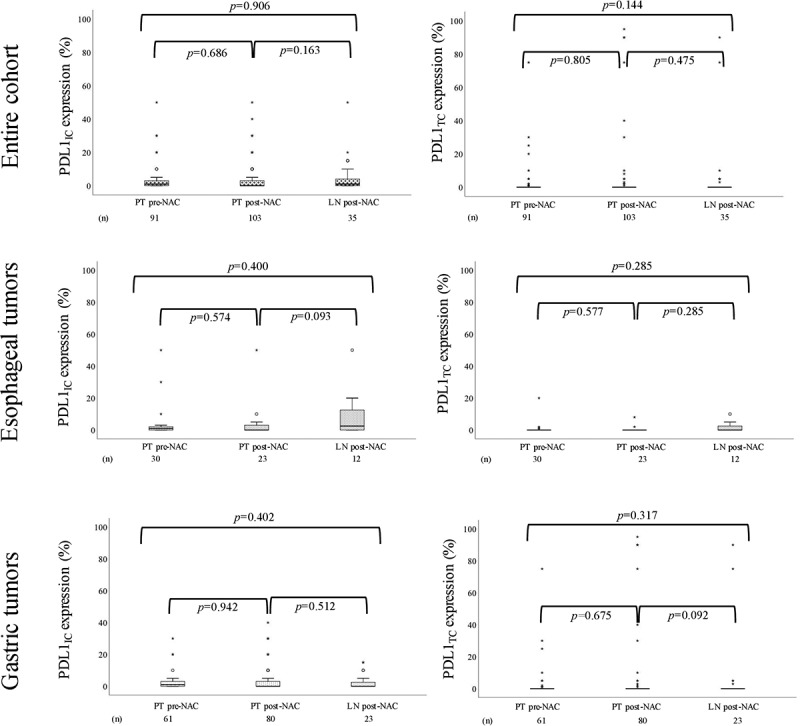
Boxplots visualizing the expression of PD-L1IC and PDL1TC in PT pre-NAC, PT post-NAC and LN post-NAC, in the entire cohort, esophageal tumors and gastric tumors, respectively
In summary, these results demonstrate that T and B cell density alters following NAC while PD-L1 expression is not affected.
Intercorrelation between the investigated immune markers in pre-NAC and post-NAC specimens
Intercorrelations between the different immune markers in pre-NAC and post-NAC specimens in the entire cohort are shown in Table 1. Significant correlations were found between CD8+ cells, FoxP3+ cells, and CD20+ cells in PT pre-NAC, PT post-NAC and LN metastases post-NAC. PD-L1IC expression was associated with FoxP3+ cells in PT pre-NAC, with CD8+ cells, FoxP3+ cells, and CD20+ cells in PT post-NAC, but not with any other immune markers in LN metastases post-NAC. PD-L1TC expression was associated with FoxP3+ cells and CD20+ cells in PT pre-NAC, with CD8+ cells, in PT post-NAC, but not with any other immune markers in LN metastases post-NAC. A significant correlation between PD-L1IC and PD-L1TC expression was only seen in PT pre-NAC.
Table 1.
Intercorrelation between the investigated immune markers in the entire cohort
| |
PT pre-NAC |
PT post-NAC |
LN metastasis post-NAC |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD8+ | FoxP3+ | CD20+ | PD-L1IC | PD-L1TC | CD8+ | FoxP3+ | CD20+ | PD-L1IC | PD-L1TC | CD8+ | FoxP3+ | CD20+ | PD-L1IC | PD-L1TC | |
| CD8+ | |||||||||||||||
| R | 0.247 | 0.509 | 0.100 | 0.194 | 0.292 | 0.273 | 0.248 | 0.292 | 0.491 | 0.520 | 0.143 | 0.163 | |||
| p | 0.019 | <0.001 | 0.349 | 0.068 | 0.005 | 0.009 | 0.015 | 0.004 | 0.004 | 0.002 | 0.469 | 0.391 | |||
| n | 89 | 89 | 89 | 89 | 90 | 91 | 96 | 96 | 33 | 32 | 28 | 30 | |||
| FoxP3+ | |||||||||||||||
| R | 0.247 | 0.135 | 0.395 | 0.237 | 0.292 | 0.351 | 0.310 | 0.029 | 0.491 | 0.508 | 0.314 | −0.188 | |||
| p | 0.019 | 0.206 | <0.001 | 0.025 | 0.005 | 0.001 | 0.002 | 0.783 | 0.004 | 0.005 | 0.092 | 0.338 | |||
| n | 89 | 90 | 89 | 89 | 90 | 86 | 93 | 93 | 33 | 29 | 30 | 28 | |||
| CD20+ | |||||||||||||||
| R | 0.509 | 0.135 | 0.143 | 0.236 | 0.273 | 0.351 | 0.310 | −0.048 | 0.520 | 0.508 | 0.334 | −0.085 | |||
| p | <0.001 | 0.206 | 0.178 | 0.025 | 0.009 | 0.001 | 0.002 | 0.643 | 0.002 | 0.005 | 0.061 | 0.644 | |||
| n | 89 | 90 | 90 | 90 | 91 | 86 | 94 | 94 | 32 | 29 | 32 | 32 | |||
| PD-L1IC | |||||||||||||||
| R | 0.100 | 0.395 | 0.143 | 0.290 | 0.248 | 0.310 | 0.310 | 0.092 | 0.314 | 0.143 | 0.334 | 0.125 | |||
| p | 0.349 | <0.001 | 0.178 | 0.005 | 0.015 | 0.002 | 0.002 | 0.355 | 0.092 | 0.469 | 0.061 | 0.475 | |||
| n | 89 | 89 | 90 | 91 | 96 | 93 | 94 | 103 | 30 | 28 | 32 | 35 | |||
| PD-L1TC | |||||||||||||||
| R | 0.194 | 0.237 | 0.236 | 0.290 | 0.292 | 0.029 | −0.048 | 0.092 | 0.163 | −0.188 | −0.085 | 0.125 | |||
| p | 0.068 | 0.025 | 0.025 | 0.005 | 0.004 | 0.783 | 0.643 | 0.355 | 0.391 | 0.338 | 0.644 | 0.475 | |||
| n | 89 | 89 | 90 | 91 | 96 | 93 | 94 | 103 | 30 | 28 | 32 | 35 | |||
R = Spearman’s correlation coefficient, p = p-value, n = number of cases available for analysis, *significance at the 5% level
Intercorrelations between the different immune markers in esophageal and gastric cancer are shown in Supplementary Table 1 and Supplementary Table 2, respectively. Significant associations between different immune markers were mainly seen in gastric cancer.
Together these findings show that lymphocyte infiltration of cytotoxic T lymphocytes (CTLs), Tregs and B cells are present in all tumors and time points investigated. The identified intercorrelations in pre- and post-NAC specimens were all weak or moderate.
Associations of different immune markers with histopathological regression
Box plots visualizing IC density in diagnostic biopsies pre-NAC in relation to histopathological response in the resected PT in the entire cohort are shown in Figure 3. Information regarding histopathological response was available for 117 (99.2%) of the 118 patients who underwent surgical resection after neoadjuvant chemotherapy. Complete response was noted in 13 (11.1%) patients, and the distribution of the other categories were 13 (11.1%) with 1–10%, 46 (39.3%) with 11–50% and 45 (38.5%) with >50% residual TC. The density of CD8+, FoxP3+ T cells and CD20+ B cells, PD-L1IC and PD-L1TC expression, in PT pre-NAC was not significantly altered in relation to histopathological response. No significant associations were found in separate analyses of esophageal and gastric tumors, as shown in Supplementary Figure 2 and Supplementary Figure 3, respectively.
Figure 3.
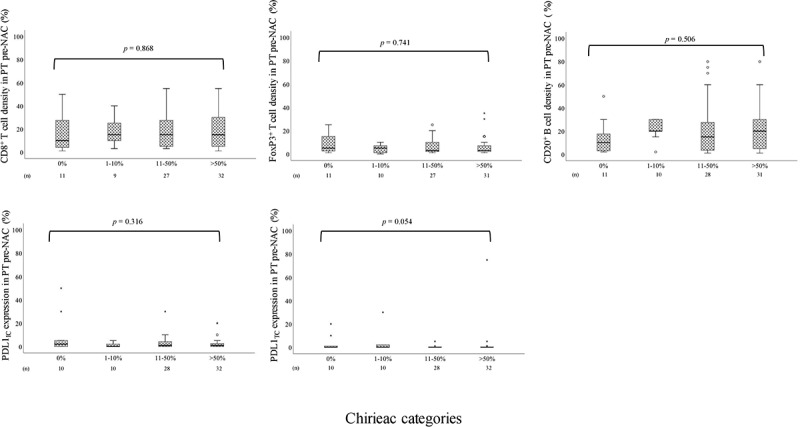
Boxplots visualizing the association between the density of CD8+ T cells, FoxP3 + T cells and CD20+ B cells in PT pre-NAC and PDL1IC and PDL1TC expression in PT pre-NAC and histopathological regression according to the different Chirieac categories (0%, 1–10%, 11–50% or >50% residual carcinoma) in the entire cohort
Altogether, this means that the changes in immune landscape pre-NAC to post-NAC is not associated with alterations in histopathological parameters.
Associations of different immune markers with clinicopathological factors in primary tumors pre-NAC and post-NAC
Associations of the different immune markers with clinicopathological factors in PT pre-NAC and PT post-NAC are shown in Supplementary Table 3 and Supplementary Table 4, respectively. In PT pre-NAC, high density of FoxP3+ T cells was significantly associated with male sex and Laurén mixed type. The density of CD8+ T cells was significantly higher in the stomach than in the esophagus, and high PD-L1IC expression was significantly associated with R0 resection (i.e no residual tumor in the resection margin). In PT post-NAC, high density of CD20+ B cells was significantly associated with female sex, and the density of CD20+ B cells was significantly higher in the stomach than in the esophagus. Also, high PD-L1TC expression was significantly associated with N2 nodal status.
In summary, PD-L1 expression was the only immune marker with significant associations to a favorable clinicopathological factor pre-NAC (PD-L1IC and R0 resection), and an unfavorable clinicopathological factor post-NAC (PD-L1TC and N2 nodal status).
Prognostic significance of T cells and B cells in primary tumors pre-NAC and post-NAC
The accumulation of T cells in pre- and post-NAC specimens in favor of B cells, was investigated in more detail. Kaplan-Meier analyses of TTR and OS according to low and high density of CD8+, FoxP3+ and CD20+ cells in PT pre-NAC based on CRT derived cutoffs are shown in Figure 4. All cutoff values are indicated in the figure legend. High CD8+ density was significantly associated with a shorter TTR and a shorter OS. High FoxP3+ density was significantly associated with a prolonged TTR and a similar trend, however non-significant, was seen for OS. Interestingly, CD20+ density in PT pre-NAC was not prognostic. As further shown in Table 2, high FoxP3+ density in PT pre-NAC was an independent favorable prognostic factor for both TTR and OS in multivariable analysis, whereas the opposite was seen for CD8+ density and TTR. Multivariable analysis for the latter could not be performed for OS due to the lack of events in the low category.
Figure 4.
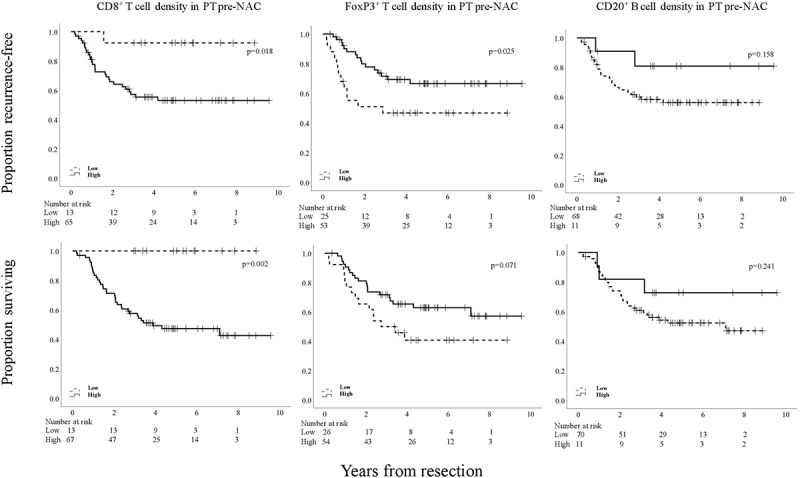
Kaplan-Meier estimates of TTR and OS in strata according to low and high total density of CD8+, FoxP3+, and CD20+ cells in PT pre-NAC in the entire cohort, defined by CRT analysis. Prognostic cutoff points based on CRT analyses for the different immune markers in PT pre-NAC, respectively, were as follows; high CD8+ >4.0%, high FoxP3+ >2.5%, high CD20+ >45.0%
Table 2.
Cox proportional hazards analysis of the impact of investigative lymphocyte subsets in PT pre-NAC, on time to recurrence and overall survival in the entire cohort
| TTR | OS | |||||
| n (events) | HR (95 % CI) | p | n (events) | HR (95 % CI) | p | |
| CD8+ | ||||||
| Univariable | ||||||
| Low | 13 (1) | 1.00 | 13 (0) | 1.00 | ||
| High | 65 (28) | 7.69 (1.05- 56.56) | 0.045 | 67 (35) | 29.22 (0.88-965.38) | 0.059 |
| Multivariable | ||||||
| Low | 12 (1) | 1.00 | 12 (0) | 1.0 | ||
| High | 61 (28) | 4.50 (0.57-35.93) | 0.155 | 63 (35) | 444052.13 (5.698E+203) | 0.955 |
| FoxP3+ | ||||||
| Univariable | ||||||
| Low | 25 (13) | 1.00 | 26 (15) | 1.00 | ||
| High | 53 (16) | 0.44 (0.21-0.92) | 0.029 | 54 (20) | 0.54 (0.28-1.06) | 0.075 |
| Multivariable | ||||||
| Low | 23 (13) | 1.00 | 24 (15) | 1.00 | ||
| High | 50 (26) | 0.23 (0.09-0.58) | 0.002 | 51 (20) | 0.309 (0.125-0.765) | 0.011 |
| CD20+ | ||||||
| Univariable | ||||||
| Low | 68 (28) | 1.00 | 70 (33) | 1.00 | ||
| High | 11 (2) | 0.37 (0.09-1.56) | 0.175 | 11 (3) | 0.50 (0.15-1.63) | 0.251 |
| Multivariable | ||||||
| Low | 63 (28) | 1.00 | 65 (33) | 1.00 | ||
| High | 11 (2) | 0.27 (0.06-1.18) | 0.083 | 11 (3) | 0.46 (0.14-1.53) | 0.203 |
Classification and regression tree-derived prognostic cutoffs are used to define ”low” and ”high” in all analyses. TTR = time to recurrence, OS = overall survival, HR = hazard ratio. Multivariable analysis includes age, sex, location, T-stage (T1-2 vs T3-4), N-stage (N0 vs N+), M-stage (M0 vs M1), differentiation grade (high vs low), resection margin (R0, R1, R2), Laurén classification (intestinal vs diffused/mixed type).
Median-derived cutoff values were not prognostic for either TTR or OS (Supplementary Figure 4), and no significant associations were found when stratifying for tumor location, as shown in Supplementary Figure 5 for esophageal tumors and Supplementary Figure 6 for gastric tumors, respectively. All cutoff values are indicated in the figure legend.
Kaplan-Meier analyses of TTR and OS according to low and high density of CD8+, FoxP3+ and CD20+ cells in PT post-NAC based on CRT-derived cutoff values are shown in Figure 5. All cutoff values are indicated in the figure legend. In sharp contrast to the findings for PT pre-NAC, for PT post-NAC high FoxP3+ density was significantly associated with a shorter TTR and a shorter OS. CD8+ and CD20+ densities in PT post-NAC were not prognostic. Median-based cutoff values were not prognostic for either TTR or OS (Supplementary Figure 7), and no significant associations were found when stratifying for tumor location, as shown in Supplementary Figure 8 for esophageal tumors and Supplementary Figure 9 for gastric tumors, respectively. All cutoff values are indicated in the figure legend.
Figure 5.
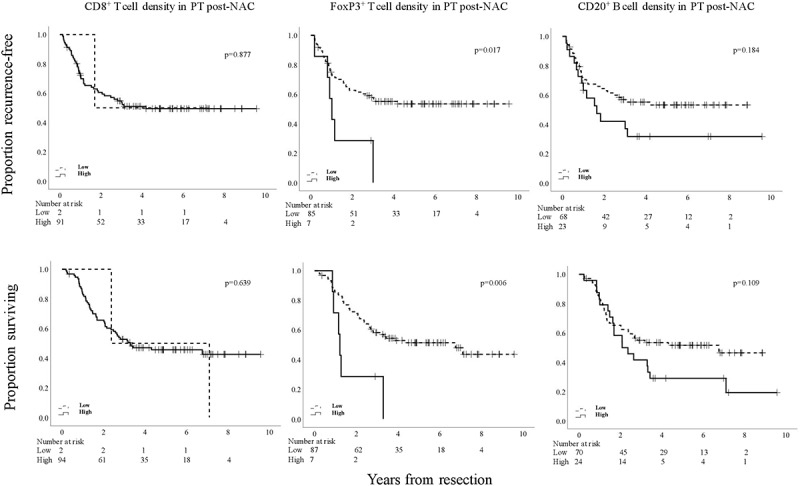
Kaplan-Meier estimates of TTR and OS in strata according to low and high total density of CD8+, FoxP3+, and CD20+ cells in PT post-NAC in the entire cohort, defined by CRT analysis. Prognostic cutoff points based on CRT analyses for the different immune markers in PT post-NAC, respectively, were as follows; high CD8+ >1.3%, high FoxP3+ >5.3%, high CD20+ >15.8%
As further shown in Table 3, high FoxP3+ density remained an independent unfavorable prognostic factor for OS but not for TTR in multivariable analysis.
Table 3.
Cox proportional hazards analysis of the impact of investigative lymphocyte subsets in PT post-NAC, on time to recurrence and overall survival in the entire cohort
| TTR | OS | |||||
| n (events) | HR (95 % CI) | P | n (events) | HR (95 % CI) | P | |
| CD8+ | ||||||
| Univariable | ||||||
| Low | 2 (1) | 1.00 | 2 (2) | 1.00 | ||
| High | 91 (44) | 1.17 (0.16- 8.49) | 0.878 | 94 (51) | 0.71 (0.17-2.94) | 0.641 |
| Multivariable | ||||||
| Low | 1 (1) | 1.00 | 1 (2) | 1.00 | ||
| High | 89 (44) | 161627.27 (0.00-2.24E+290) | 0.971 | 92 (51) | 2.29 (0.26-20.23) | 0.457 |
| FoxP3+ | ||||||
| Univariable | ||||||
| Low | 85 (38) | 1.00 | 87 (43) | 1.00 | ||
| High | 7 (6) | 2.77 (1.16-6.63) | 0.022 | 7 (6) | 3.18 (1.33-7.61) | 0.009 |
| Multivariable | ||||||
| Low | 82 (38) | 1.00 | 84 (43) | 1.00 | ||
| High | 7 (6) | 2.40 (0.87-6.58) | 0.090 | 7 (6) | 2.86 (1.03-7.90) | 0.043 |
| CD20+ | ||||||
| Univariable | ||||||
| Low | 68 (31) | 1.00 | 70 (34) | 1.00 | ||
| High | 23 (14) | 1.53 (0.81-2.88) | 0.188 | 24 (18) | 1.59 (0.90-2.82) | 0.112 |
| Multivariable | ||||||
| Low | 65 (31) | 1.00 | 67 (34) | 1.00 | ||
| High | 23 (14) | 1.45 (0.71-2.95) | 0.303 | 24 (18) | 1.49 (0.80-2.78) | 0.213 |
Classification and regression tree-derived prognostic cutoffs are used to define ”low” and ”high” in all analyses. TTR = time to recurrence, OS = overall survival, HR = hazard ratio. Multivariable analysis includes age, sex, location, T-stage (T1-2 vs T3-4), N-stage (N0 vs N+), M-stage (M0 vs M1), differentiation grade (high vs low), resection margin (R0, R1, R2), Laurén (intestinal vs diffused/mixed type).
Separate subgroup analyses of esophageal and gastric tumors, based on CRT-derived cutoffs were in general concordant with the analyses of the entire cohort, as shown in Supplementary Figure 10 and Supplementary Figure 11, respectively, for pre-NAC specimens and in Supplementary Figure 12 and Supplementary Figure 13, respectively, for post-NAC specimens. Multivariable analyses were not meaningful due to the small number of cases in some of the categories.
In light of our previous findings of an additive favorable prognostic impact of T and B cells in chemoradiotherapy naïve EG cancer,9 we also investigated the prognostic impact of low and high density of CD8+ and FoxP3+ T cells in relation to low and high density of CD20+ B cells on TTR and OS in PT pre-NAC and PT post-NAC in the entire cohort using the median as cutoff. As shown in Supplementary Figure 14, no additive prognostic information could be obtained from any of the combined variables.
Neither the infiltration of CD8+ or FoxP3+ T cells into TN, nor the presence of B cell aggregates, were prognostic for TTR or OS as shown in Supplementary Figure 15 for pre-NAC specimens and Supplementary Figure 16 for post-NAC specimens, respectively.
Altogether this indicates that the only prognostic factor for survival is T cell density, in particular FoxP3+ cells, which demonstrated to be an independent prognostic factor both pre- and post-NAC, with high-density pre-NAC being beneficial and high-density post-NAC being unbeneficial for survival.
Prognostic significance of PD-L1 expression in primary tumors pre-NAC and post-NAC
We next investigated the prognostic effect of PD-L1 expression. Kaplan-Meier analyses of TTR and OS according to low and high PD-L1IC and PD-L1TC expression in PT pre-NAC based on median derived cutoffs are shown in Figure 6. All cutoff values are indicated in the figure legend. High PD-L1IC expression was significantly associated with a prolonged TTR and a prolonged OS.
Figure 6.
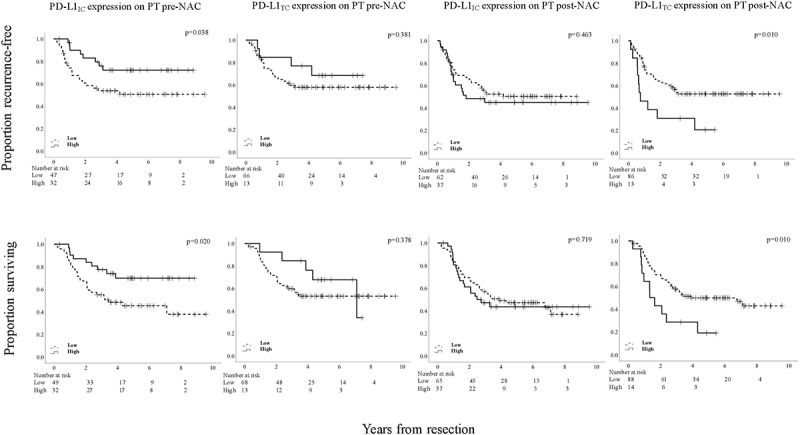
Kaplan-Meier estimates of TTR and OS in strata according to low and high PD-L1IC and PD-L1TC expression, respectively, in PT pre-NAC and PT post-NAC, in the entire cohort, using the median value as cutoff. Prognostic cutoff points based on the median values were as follows; PT pre-NAC: high PD-L1IC >1%, high PD-L1TC > 0%. PT post-NAC: high PD-L1IC >0%, high PD-L1TC > 0%
PD-L1TC expression in PT pre-NAC was not prognostic. As further shown in Table 4, high PD-L1IC expression remained an independent prognostic factor for TTR but not for OS in multivariable analysis. When stratifying for tumor location, similar associations were found for gastric tumors, whereas no significant associations were found for esophageal tumors, as shown in Supplementary Figure 17 for esophageal tumors and Supplementary Figure 18 for gastric tumors, respectively.
Table 4.
Cox proportional hazards analysis of the impact of PD-L1IC and PD-L1TC expression in PT pre-NAC and PT post-NAC, on time to recurrence and overall survival in the entire cohort
| TTR |
OS |
|||||
|---|---|---|---|---|---|---|
| n (events) | HR (95% CI) | p | n (events) | HR (95% CI) | p | |
| PD-L1IC PT pre-NAC | ||||||
| Univariable | ||||||
| Low | 47 (22) | 1.00 | 49 (27) | 1.00 | ||
| High | 32 (8) | 0.43 (0.19–0.98) | 0.043 | 32 (9) | 0.42 (0.20–0.89) | 0.024 |
| Multivariable | ||||||
| Low | 45 (22) | 1.00 | 47 (27) | 1.00 | ||
| High | 29 (8) | 0.32 (0.12–0.87) | 0.025 | 29 (9) | 0.572 (0.24–1.38) | 0.215 |
| PD-L1TC PT pre-NAC | ||||||
| Univariable | ||||||
| Low | 66 (26) | 1.00 | 68 (31) | 1.00 | ||
| High | 13 (4) | 0.63 (0.22–1.80) | 0.386 | 13 (5) | 0.66 (0.25–1.69) | 0.381 |
| Multivariable | ||||||
| Low | 61 (26) | 1.00 | 63 (31) | 1.00 | ||
| High | 13 (4) | 0.391 (0.11–1.42) | 0.153 | 13 (5) | 0.34 (0.10–1.09) | 0.069 |
| PD-L1IC PT post-NAC | ||||||
| Univariable | ||||||
| Low | 62 (30) | 1.00 | 65 (36) | 1.00 | ||
| High | 37 (19) | 1.24 (0.70–2.21) | 0.464 | 37 (20) | 1.11 (0.64–1.91) | 0.719 |
| Multivariable | ||||||
| Low | 59 (30) | 1.00 | 62 (36) | 1.00 | ||
| High | 37(19) | 1.39 (0.74–2.60) | 0.301 | 37 (20) | 1.39 (0.76–2.54) | 0.281 |
| PD-L1TC PT post-NAC | ||||||
| Univariable | ||||||
| Low | 86 (39) | 1.00 | 88 (45) | 1.00 | ||
| High | 13 (10) | 2.43 (1.21–4.88) | 0.013 | 14 (11) | 2.34 (1.20–4.55) | 0.012 |
| Multivariable | ||||||
| Low | 83 (30) | 1.00 | 85 (45) | 1.00 | ||
| High | 13 (10) | 2.15 (0.95–4.89) | 0.068 | 14 (11) | 2.09 (0.96–4.53) | 0.063 |
Prognostic cutoffs derived from the median are used to define ”low” and ”high” in all analyses. TTR = time to recurrence, OS = overall survival, HR = hazard ratio. Multivariable analysis includes age, sex, location, T-stage (T1-2 vs T3-4), N-stage (N0 vs N+), M-stage (M0 vs M1), differentiation grade (high vs low), resection margin (R0, R1, R2), Laurén classification (intestinal vs diffused/mixed type).
Kaplan-Meier analyses of TTR and OS according to low and high PD-L1IC and PD-L1TC expression in PT post-NAC based on median-derived cutoffs are also shown in Figure 6. All cutoff values are indicated in the figure legend. High PD-L1TC expression was significantly associated with a shorter TTR and a shorter OS. PD-L1IC expression in PT post-NAC was not prognostic. As further shown in Table 4, high PD-L1TC expression was not an independent prognostic factor for TTR or OS. When stratifying for tumor location, similar associations were found for both esophageal and gastric tumors, also shown in Supplementary Figure 17 and Supplementary Figure 18, respectively.
CRT derived cutoff values were not applicable to define low and high PD-L1IC and PD-L1TC expression, due to skewness of the data.
In summary, high PD-L1IC expression is a beneficial prognostic factor for survival, in pre-NAC specimens but not in post-NAC specimens, herein high PD-L1TC expression is associated with shorter survival, however not when adjusted for other prognostic factors.
Prognostic significance of immune marker heterogeneity pre-NAC and post-NAC
Next, we examined the potential prognostic impact of the temporal heterogeneity of the different immune markers in pre- and post-treatment specimens by constructing conversion variables based on combinations of low or high immune marker expression before and after NAC. This resulted in the following variables; low pre-NAC/low post-NAC, low pre-NAC/high post-NAC, high pre-NAC/low post-NAC, high pre-NAC/high post-NAC. In so doing, we determined whether different immune marker alteration patterns could have impact on clinical outcome. Kaplan-Meier analyses of OS according to the four different categories, based on CRT derived cutoffs for CD8+, FoxP3+ and CD20+ cells and median derived cutoffs for PD-L1 expression on ICs and TC, respectively, are shown in Figure 7.
Figure 7.
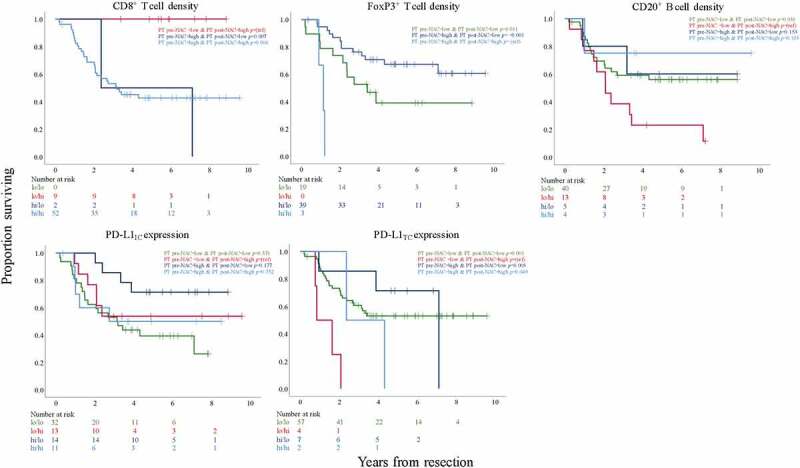
Kaplan-Meier estimates of OS in strata, in the entire cohort, with conversion-variables according to combinations of low or high CD8+, FoxP3+ and CD20+ cell density, respectively, in PT pre-NAC and PT post-NAC and low or high PD-L1IC and PD-L1TC expression, respectively, in PT pre-NAC and PT post-NAC. Prognostic cutoff points are defined by CRT analyses for CD8+, FoxP3+ and CD20+ cells and the median values for PD-L1IC and PD-L1TC expression
Patients with tumors displaying low CD8+ density pre-NAC and high CD8+ density post-NAC had a significantly better OS than those with high CD8+ density both pre-NAC and post-NAC as well as the small group of patients with high CD8+ density pre-NAC and low density post-NAC. No tumors had low CD8+ density both pre-NAC and post-NAC.
Patients with tumors displaying high FoxP3+ density pre-NAC and low FoxP3+ density post-NAC and patients with tumors displaying low FoxP3+ density both pre-NAC and post-NAC, had a significantly better OS compared to the small group of patients with high FoxP3+ density both pre-NAC and post-NAC. The group of patients with tumors converting from high to low FoxP3+ density had a borderline significantly better OS compared to the group with a consistent low density (p =.052). No tumors had low FoxP3+ density pre-NAC and high FoxP3+ density post-NAC.
Patients with tumors displaying low CD20+ density pre-NAC and high CD20+ density post-NAC had a significantly worse OS compared to patients with tumors displaying low CD20+ density both pre-NAC and post-NAC. Survival in the small groups of patients with tumors displaying high CD20+ density both pre- and post-NAC and with tumors converting from high density pre-NAC to low density post-NAC did not differ significantly from any of the other strata.
Regarding PD-L1IC expression, patients with tumors displaying a low expression both pre- and post-NAC had a significantly worse OS compared to patients with tumors converting from high pre-NAC to low post-NAC expression (p =.021). No other conversion variables differed significantly from any of the other strata.
Patients with tumors displaying low PD-L1TC expression pre-NAC with a conversion to high expression post-NAC had the worst OS with a significant difference to all other strata.
Altogether, these findings show that temporal heterogeneity is a highly interesting factor for prognosis, and significant alteration patterns were identified for all investigated immune markers, indicating either better or worse outcome.
Discussion
This study examined the density of CD8+ T cells, Tregs, B cells and expression of PD-L1 on ICs and TC, before and after neoadjuvant chemotherapy in a retrospective, consecutive cohort of patients with EG adenocarcinoma. The results revealed a significantly higher density of CD8+ T cells and significantly lower density of FoxP3+ T cells and CD20+ B cells in the PT after treatment. The IC densities in paired LN metastases were in general similar to the PT post-NAC, except for B cells, which were more abundant. This could however be due to the fact that B cells per se are expected in LN. PD-L1 expression was similar before and after NAC, both on ICs and TC. In a previous study by Yu et al., including 60 paired pre- and post-NAC samples of gastric cancer, similar results were seen regarding CD8+ T cell density, that was increased following NAC. PD-L1 expression was also increased, whereas FoxP3+ density did not change after chemotherapy.32
In the present study, neither IC density nor PD-L1 expression in pre-treatment samples was significantly associated with histopathological response, which is also in line with Yu et al..32 However, a study on gastric cancer exploring the effect of NAC on immune checkpoint proteins found that PD-L1 expression on ICs and TC increased with decreased histopathological response in post-treatment specimens, but of note no pre-treatment specimens were investigated in that study.44
This is, to our best knowledge, the only study comparing immune landscape, and one of few studies to compare IC density and PD-L1 expression, in pre- and post-treatment samples, or the relationship between these immune markers and histopathological response in EG adenocarcinoma.
The prognostic value of some IC subsets was not only shown to differ between pre-treatment and post-treatment samples, but also in relation to their temporal heterogeneity. CD8+ infiltration was not prognostic post-NAC, but high infiltration in pre-NAC specimens was associated with a shorter TTR and OS, however not independently of other factors, and the CRT analysis yielded a rather small subgroup with low expression. On the other hand, it is noteworthy that conversion from low CD8+ infiltration pre-NAC to high infiltration post-NAC was associated with the best prognosis. Since CD8+ cell density was found to be increased after NAC these findings could together suggest that patients with low CD8+ infiltration in the pre-treatment biopsy may have greater benefit from NAC. In the study by Yu et al., CD8+ infiltration pre-NAC was not found to be prognostic and the prognostic impact of CD8+ infiltration post-NAC was not evaluated.32 However, underpinning the importance of temporal immune marker heterogeneity, the authors suggest that it is the level of changes rather than the actual baseline or post-treatment levels of immune markers that brings the prognostic significance as a higher increase of CD8+ T cells, followed by NAC, was demonstrated as an independent beneficial prognostic factor for both progression-free survival and OS.32 The majority of prevailing studies on the clinical impact of CD8+ infiltration in EG adenocarcinoma support an association with a good outcome, particularly in a strictly chemoradiotherapy-naïve setting.6–9 However, a study on gastric cancer by Hennequin et al. encompassing a mixed cohort wherein approximately 50% of the patients received NAC, no association between CD8+ cell density and prognosis could be identified in the resected PT, regardless of the administration of chemotherapy, which is in line with the present results.12
Our study demonstrates that both the density and prognostic impact of CD8+ cells is altered following the administration of NAC. Fluoropyromidine has been suggested, besides from having a direct cytotoxic effect on TC, to possess the ability to eliminate myeloid-derived suppressor cells,45 which in turn triggers an increase in interferon-gamma (IFN-γ) production by CD8+ T cells thus promoting a T-cell dependent antitumoral effect.45 However, the secretion of IFN-γ may also upregulate PD-L1 expression on TC, hence enabling them to escape immune surveillance through disabling the CD8+ T cells to attack.46,47 Speculatively, different levels of IFN-γ may result in either a protumorigenic or antitumorigenic effect, thus contributing to divergent results regarding the prognostic impact of CD8+ T cells.
In the present study, the strongest prognostic significance was seen for FoxP3+ T cells, but quite contrastingly so in that high FoxP3+ infiltration in pre-NAC specimens was an independent prognostic factor for prolonged TTR and OS whereas high FoxP3+ infiltration in post-NAC specimens was an independent prognostic factor for shorter OS. Moreover, a better outcome was seen for patients with tumors converting from high FoxP3+ density pre-NAC to low FoxP3+ density post-NAC. Since NAC was demonstrated to decrease FoxP3+ density in the present study, these results could propose that patients with high FoxP3+ infiltration in the pre-treatment biopsy may have a greater benefit from NAC. In the study by Yu et al., FoxP3+ infiltration was not found to be prognostic in pre-NAC samples, and the association of post-NAC expression with survival was not explored.32 However, in line with the results in the present study, Hennequin et al. found high FoxP3+ density in post-treatment specimens to be associated with a worse prognosis, regardless of the administration of NAC, however not when adjusting for other prognostic factors.12
Most studies on the prognostic value of FoxP3+ T cells have been performed on gastric cancer, and the majority demonstrate an association with a worse outcome,8,10–12 with some contrasting reports.9,48,49 The role of FoxP3+ cells in esophageal adenocarcinoma has been less explored, with two studies showing an association with a prolonged survival,7,9 and another no prognostic impact.50 The association of high FoxP3+ density and a prolonged survival in the strictly chemoradiotherapy-naïve setting in EG adenocarcinoma might be due to the ability of Tregs to downregulate the inflammatory process that promotes carcinogenesis, as suggested by Haas et al. based on their study on chemoradiotherapy-naïve gastric cancer of the cardia.48 These authors also proposed that Tregs are particularly prevalent in earlier stages of the carcinogenesis and therefore more likely to have a prognostic impact at that time point.
The present study could not find any prognostic impact for B cells either in pre-NAC or in post-NAC samples. These results are in contrast to previous studies on chemoradiotherapy-naïve gastric cancer and EG cancer wherein B cells have been shown to be associated with a favorable prognosis.9,14,18 The picture is further complicated by the observation that conversion from low to high B cell infiltration following NAC was associated with a shorter OS, suggesting that chemotherapy may alter the functional competence of B cells. Moreover, no former studies have investigated the plausible impact by NAC on B cells by comparing pre and post-surgical specimens in EG adenocarcinoma. Solely the aforementioned study by Hennequin et al. has examined the influence of NAC on B cells and T cells in post-surgical specimens from 82 patients with gastric adenocarcinoma, of whom approximately fifty percent received NAC and their study showed no difference in density or prognostic value of any immune infiltrates between the untreated and pre-treated subgroups.12
No additive prognostic significance of T cell and B cell density was identified in the present study, either in pre-NAC or post-NAC samples, which is in contrast to findings in the chemoradiotherapy-naïve setting.9,13,18 In addition, the presence of CD20+ B lymphocyte aggregates in PT was not prognostic, either pre-NAC or post-NAC. We are not aware of any previous study investigating the additive prognostic impact of T cell and B cell infiltrates, nor the presence of B lymphocyte aggregates in pre-NAC and post-NAC samples. However, in post-surgical specimens, Hennequin et al. found a high number of peritumoral CD20+ B cell aggregates and a high degree of Tbet+ lymphocyte infiltration in the tumor stroma to be associated with better recurrence-free survival, the former independently of other prognostic factors and both independently of NAC. In their study additional double staining was performed to better characterize the peritumoral B cell aggregates and their interactions with T cells, and in so doing they demonstrated that the B cell aggregates were surrounded by T cells, hence the structures could be considered as TLS.12 Furthermore, a significant correlation between the density of TLS, B cell aggregates and Tbet+ cells was identified. Moreover, the combination of high CD20+ B cell aggregate density and high infiltration of Tbet+ cells was associated with the lowest risk of relapse, independently of NAC.12 In light of these findings, the lack of prognostic value of B cells in the present study might be due to a negative impact by NAC on the remaining B cells, or a shift of B cell type post-NAC.
We also investigated the prognostic impact of the infiltration of CD8+ and FoxP3+ T cells into TN, but no significant associations were found either in pre- or post-NAC specimens. To the best of our knowledge, only one former study has investigated these associations in EG adenocarcinoma. In line with the present results, the prognostic value was not improved for any of the investigated IC subsets, including CD8+ and FoxP3+ T cells, when analyzing the infiltration in intratumoral, tumor-adjacent and stromal compartments separately, of note the study was performed in a chemoradiotherapy-naïve setting.9 The prevailing studies investigating the prognostic impact of IC infiltration in different tumoral compartments in EG adenocarcinoma solely compares the infiltration in the center of the tumor versus the invasive margin or tumor periphery, hence not the infiltration into TN per se. These studies, performed on gastric cancer, utilizes whole tissue sections, or TMA cores specifically chosen from different tumor compartments, and taken together a prognostic impact is only present when investigating the infiltration in the center of the tumor.7,10,12
In the present study, high PD-L1 expression on ICs in pre-treatment specimens was associated with a prolonged TTR and OS, the former independently of other prognostic factors, while the expression on TC was not prognostic. In contrast, in post-treatment specimens, PD-L1 expression on ICs was not prognostic, while high expression on TC was associated with a shorter TTR and OS, and of note, also with a more advanced nodal status (N2) further strengthening the association with a worse prognosis. Furthermore, a consistent low expression of PD-L1 on ICs both pre- and post-NAC was associated with a worse prognosis as well as conversion from low PD-L1 expression on TC pre-NAC to high expression post-NAC, the latter possibly suggesting an evolutionary selection of more malignant TC following NAC. The result is however in contrast to the study by Yu et al, showing that the median expression of PD-L1 increased significantly after NAC and a higher increase was demonstrated to be an independent beneficial prognostic factor of OS, of note ICs and TC were analyzed in conjunction and not separately. Moreover in their study, a higher PD-L1 expression in pre-treatment specimens was associated with a shorter OS.32 The findings in the present study are however partly in line with a study on gastric cancer comparing the effects of NAC on immune checkpoint proteins between a pretreated cohort and a previously described chemoradiotherapy-naïve cohort.44 In the untreated cohort, high PD-L1 expression on both ICs and TC was significantly associated with a better OS while no significant associations between survival and PD-L1 expression on ICs and TC were found in post-treatment specimens. This led the authors to conclude that neoadjuvant treatment suppresses and modulates the beneficial effects of PD-L1 seen in untreated gastric cancer,44 which is further supported by findings in the present study. The prognostic impact of PD-L1 expression in the chemoradiotherapy-naïve setting is ambiguous. However, in line with the present results a previous study on EG adenocarcinoma demonstrate high PD-L1 expression on ICs as a beneficial prognostic factor while no prognostic impact was found regarding expression on TC.25
There is to date an unmet need for studies investigating the influence of NAC on immune marker expression by comparing pre- and post-treatment samples, especially when considering the increased use of neoadjuvant and adjuvant chemotherapy along with the implication of ICI in different settings. The prevailing literature on different tumor entities mapping the dynamic landscape of the TME following NAC is diverging. Several studies do however, in line with the present results, demonstrate a recruitment of CD8+ T cells following NAC.30–33 Regarding FoxP3+ T cells many studies demonstrate a decrease or unchanged density post-NAC.30,32–34 B-cells are much less investigated with one study demonstrating an increase in CD20+ B cell density35 and another a decrease following NAC.33 PD-L1 expression has been shown to increase in other tumor entities post-treatment,30–32,36 but a decrease in expression has also been demonstrated,37 while it was unchanged in the present study. The diverging results of how immune marker expression alters following NAC highlights the fact that chemotherapy may have different impact on ICs and the TME depending on which chemotherapeutic agents that is utilized, as well as what tumor entity we are treating.
There are some limitations to this study that must be acknowledged. The baseline specimens are derived from diagnostic biopsies, while the residual cancer tissues are derived from resected PT, which may contribute to a sampling bias. The use of the TMA-technique may also inaccurately reflect the heterogeneity of the immune landscape, as has been described in gastric cancer.51 In the herein used TMA, tumor areas, central as well as peripheral, had been sampled. Ideally, future studies should include TMA cores also from stromal and IC rich areas. However, to reduce this risk of sampling bias in the present study, multiple tissue cores were obtained from two different donor blocks from the PT, and from separate LN metastases when feasible.
Conclusion
In conclusion, the findings in this study suggest that NAC has the ability to alter the density, prognostic significance, and possibly even the functional competence, of different IC subsets in EG adenocarcinoma. Additional studies are needed to confirm these findings, and to sort out the potential implications for clinical decision making.
Supplementary Material
Funding Statement
This work was supported by peer reviewed grants from the Swedish Cancer Society under Grant 2016/483; the Mrs Berta Kamprad Foundation under Grant FBKS-2016-21; Regional grants, Region Halland under Grant 886941; Skåne University Hospital Funds and Donations, Lund University Faculty of Medicine, and Governmental Funding of Clinical Research within the National Health Service (ALF). The funders of this study had no role in the study design, data collection, analysis, interpretation or writing of the report.
Availability of data and materials
Part of the data generated in this study is included in the article. The raw data of immune cell expression can be made available upon request. Patient and clinicopathological data cannot be made publicly available due to their content of identifiable human data. Requests to access the datasets should be directed to the corresponding author.
Ethics approval and consent to participate
Approval was acquired from the Ethics committee of Lund University (reference nr 445/07) whereby the committee waived no need for consent other than the option to opt out. All EU and national regulations and requirements for handling human samples have been fully complied with during the conduct of this project; i.e.; decision no. 1110/94/EC of the Euopean Parliament and of the Council (OJL126 18,5,94), the Helsinki Declaration on ethical principles for medical research involving human rights and Biomedicine.
Consent for publication
All authors have given their consent for publication.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Author’s contributions
MCS: Carried out the immunohistochemical and statistical analyses, wrote the paper. AL and JN: Carried out immunohistochemical and statistical analyses. CH and DB: Collected histopathological and clinical data. BN: Constructed the tissue microarrays and carried out the immunohistochemical analyses. KL: Provided immunological expertise and critical reading of the manuscript. KJ: Conceived and supervised the study, helped writing the paper. All authors read and approved the final manuscript.
Abbreviations
- EG
– Esophageal and gastric;
- OS
– overall survival;
- TME
– tumor microenvironment;
- TILs
– tumor infiltrating lymphocytes;
- FoxP3
– Forkhead box P3;
- Tregs
– T regulatory cells;
- TLS
– Tertiary lymphoid structures;
- ICI
– Immune checkpoint inhibitors;
- PD-1
– programmed-death 1;
- PD-L1
- programmed death-ligand 1;
- PD-L1TC
– PD-L1 expression on tumor cells;
- PD-L1IC
– PD-L1 expression on immune cells;
- NAC
- Neoadjuvant chemotherapy;
- IC
– immune cell;
- PT
– primary tumors;
- LN
– lymph node;
- TMA
– tissue microarrays;
- TN;
tumor nests;
- TC
– tumor cells;
- CRT
– classification and regression tree;
- TTR
– time to recurrence;
- HR
– Hazard ratios;
- CTLs
– cytotoxic T lymphocytes;
- IFN-γ
– interferon-gamma
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van De Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–14. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 2.Ychou M, Boige V, Pignon J-P, Conroy T, Bouche O, Lebreton G, Ducourtieux M, Bedenne L, Fabre J-M, Saint-Aubert B, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 3.Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp H-G, Mayer F, Haag GM, Luley K, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Taube JM, Galon J, Sholl LM, Rodig SJ, Cottrell TR, Giraldo NA, Baras AS, Patel SS, Anders RA, Rimm DL, et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod Pathol. 2018;31(2):214–234. doi: 10.1038/modpathol.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99(10):1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein AV, Dislich B, Blank A, Guldener L, Kroll D, Seiler CA, Langer R. High intratumoural but not peritumoural inflammatory host response is associated with better prognosis in primary resected oesophageal adenocarcinomas. Pathology. 2017;49(1):30–37. doi: 10.1016/j.pathol.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Won HS, Sun S, Hong JH, Ko YH. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a systematic review and meta-analysis. Medicine. 2018;97(32):e11769. doi: 10.1097/MD.0000000000011769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svensson MC, Warfvinge CF, Fristedt R, Hedner C, Borg D, Eberhard J, Micke P, Nodin B, Leandersson K, Jirström K, et al. The integrative clinical impact of tumor-infiltrating T lymphocytes and NK cells in relation to B lymphocyte and plasma cell density in esophageal and gastric adenocarcinoma. Oncotarget. 2017;8(42):72108–72126. doi: 10.18632/oncotarget.19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Z, Zhou S, Wang Y, Li R-L, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136(10):1585–1595. doi: 10.1007/s00432-010-0816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou J, Yu Z, Xiang R, Li C, Wang L, Chen S, Li Q, Chen M, Wang L. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol. 2014;96(3):284–291. doi: 10.1016/j.yexmp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Hennequin A, Derangere V, Boidot R, Apetoh L, Vincent J, Orry D, Fraisse J, Causeret S, Martin F, Arnould L, et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2016;5(2):e1054598. doi: 10.1080/2162402X.2015.1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni Z, Xing D, Zhang T, Ding N, Xiang D, Zhao Z, Qu J, Hu C, Shen X, Xue X, et al. Tumor-infiltrating B cell is associated with the control of progression of gastric cancer. Immunol Res. 2021;69(1):43–52. doi: 10.1007/s12026-020-09167-z. [DOI] [PubMed] [Google Scholar]
- 14.Fristedt R, Borg D, Hedner C, Berntsson J, Nodin B, Eberhard J, Micke P, Jirström K. Prognostic impact of tumour-associated B cells and plasma cells in oesophageal and gastric adenocarcinoma. J Gastrointest Oncol. 2016;7(6):848–859. doi: 10.21037/jgo.2016.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 16.Schlosser HA, Thelen M, Lechner A, Wennhold K, Garcia-Marquez MA, Rothschild SI, Staib E, Zander T, Beutner D, Gathof B, et al. B cells in esophago-gastric adenocarcinoma are highly differentiated, organize in tertiary lymphoid structures and produce tumor-specific antibodies. Oncoimmunology. 2019;8(1):e1512458. doi: 10.1080/2162402X.2018.1512458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112(11):1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakimura C, Tanaka H, Okuno T, Hiramatsu S, Muguruma K, Hirakawa K, Wanibuchi H, Ohira M. B cells in tertiary lymphoid structures are associated with favorable prognosis in gastric cancer. J Surg Res. 2017;215:74–82. doi: 10.1016/j.jss.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Zhang D, He W, Chen T, Yan Z, Gao X, Chen L, Zheng X, Xu B, Lu B, et al. CD8+ T cells located in tertiary lymphoid structures are associated with improved prognosis in patients with gastric cancer. Oncol Lett. 2020;20(3):2655–2664. doi: 10.3892/ol.2020.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly RJ LBA9_PR - Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer (EC/GEJC) following neoadjuvant chemoradiation therapy (CRT): first results of the CheckMate 577 study; 2020. September 21. https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020.
- 21.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges J-P, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moehler M LBA6_PR - Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): first results of the CheckMate 649 study; 2020. September 21. https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020
- 23.Kato K LBA8_PR - Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: the phase 3 KEYNOTE-590 study; 2020. September 21. https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020
- 24.Walk EE, Yohe SL, Beckman A, Schade A, Zutter MM, Pfeifer J, Berry AB. The Cancer Immunotherapy Biomarker Testing Landscape. Arch Pathol Lab Med. 2020;144(6):706–724. doi: 10.5858/arpa.2018-0584-CP. [DOI] [PubMed] [Google Scholar]
- 25.Svensson MC, Borg D, Zhang C, Hedner C, Nodin B, Uhlen M, Mardinoglu A, Leandersson K, Jirström K. Expression of PD-L1 and PD-1 in chemoradiotherapy-naive esophageal and gastric adenocarcinoma: relationship with mismatch repair status and survival. Front Oncol. 2019;9:136. doi: 10.3389/fonc.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boger C, Behrens HM, Mathiak M, Kruger S, Kalthoff H, Rocken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7(17):24269–24283. doi: 10.18632/oncotarget.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0182692. doi: 10.1371/journal.pone.0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dislich B, Stein A, Seiler CA, Kroll D, Berezowska S, Zlobec I, Galvan J, Slotta-Huspenina J, Walch A, Langer R, et al. Expression patterns of programmed death-ligand 1 in esophageal adenocarcinomas: comparison between primary tumors and metastases. Cancer Immunol Immunother. 2017;66(6):777–786. doi: 10.1007/s00262-017-1982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kollmann D, Ignatova D, Jedamzik J, Chang YT, Jomrich G, Baierl A, Kazakov D, Michal M, French LE, Hoetzenecker W, et al. PD-L1 expression is an independent predictor of favorable outcome in patients with localized esophageal adenocarcinoma. Oncoimmunology. 2018;7(6):e1435226. doi: 10.1080/2162402X.2018.1435226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leduc C, Adam J, Louvet E, Sourisseau T, Dorvault N, Bernard M, Maingot E, Faivre L, Cassin-Kuo MS, Boissier E, et al. TPF induction chemotherapy increases PD-L1 expression in tumour cells and immune cells in head and neck squamous cell carcinoma. ESMO Open. 2018;3(1):e000257. doi: 10.1136/esmoopen-2017-000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuoka E, Yamashita K, Tanaka T, Sawada R, Sugita Y, Arimoto A, Fujita M, Takiguchi G, Matsuda T, Oshikiri T, et al. Neoadjuvant chemotherapy increases PD-L1 expression and CD8 + tumor-infiltrating lymphocytes in esophageal squamous cell carcinoma. Anticancer Res. 2019;39(8):4539–4548. doi: 10.21873/anticanres.13631. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y, Ma X, Zhang Y, Zhang Y, Ying J, Zhang W, Zhong Q, Zhou A, Zeng Y. Changes in expression of multiple checkpoint molecules and infiltration of tumor immune cells after neoadjuvant chemotherapy in gastric cancer. J Cancer. 2019;10(12):2754–2763. doi: 10.7150/jca.31755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Martinez E, Gil GL, Benito AC, Gonzalez-Billalabeitia E, Conesa MA, Garcia Garcia T, García-Garre E, Vicente V, De La Peña FA. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16(6):488. doi: 10.1186/s13058-014-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating Foxp3+ regulatory T cells. Clin Cancer Res. 2008;14(8):2413–2420. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 35.Lo CS, Sanii S, Kroeger DR, Milne K, Talhouk A, Chiu DS, Rahimi K, Shaw PA, Clarke BA, Nelson BH, et al. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin Cancer Res. 2017;23(4):925–934. doi: 10.1158/1078-0432.CCR-16-1433. [DOI] [PubMed] [Google Scholar]
- 36.Mesnage SJL, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, Gouy S, Morice P, Bentivegna E, Lhomme C, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann Oncol. 2017;28(3):651–657. doi: 10.1093/annonc/mdw625. [DOI] [PubMed] [Google Scholar]
- 37.Pelekanou V, Carvajal-Hausdorf DE, Altan M, Wasserman B, Carvajal-Hausdorf C, Wimberly H, Brown J, Lannin D, Pusztai L, Rimm DL, et al. Effect of neoadjuvant chemotherapy on tumor-infiltrating lymphocytes and PD-L1 expression in breast cancer and its clinical significance. Breast Cancer Res. 2017;19(1):91. doi: 10.1186/s13058-017-0884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borg D, Larsson AH, Hedner C, Nodin B, Johnsson A, Jirstrom K. Podocalyxin-like protein as a predictive biomarker for benefit of neoadjuvant chemotherapy in resectable gastric and esophageal adenocarcinoma. J Transl Med. 2018;16(1):290. doi: 10.1186/s12967-018-1668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedner C, Borg D, Nodin B, Karnevi E, Jirstrom K, Eberhard J. Expression and prognostic significance of human epidermal growth factor receptors 1, 2 and 3 in oesophageal and gastric adenocarcinomas preneoadjuvant and postneoadjuvant treatment. J Clin Pathol. 2018;71(5):451–462. doi: 10.1136/jclinpath-2017-204774. [DOI] [PubMed] [Google Scholar]
- 40.Sobin L, Wittekind C. TNM classification of malignant tumours, 7th Edition. Wiley-Blackwell; 2009. http://eu.wiley.com/WileyCDA/WileyTitle/productCd-1444332414.html. [Google Scholar]
- 41.Brierley J, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours, 8th Edition. 8th ed. Wiley-Blackwell; Bridgewater, NJ: 2017. January. p. 272. [Google Scholar]
- 42.Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, Roth JA, Rashid A, Hamilton SR, Wu -T-T, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 43.Hedner C, Borg D, Nodin B, Karnevi E, Jirstrom K, Eberhard J, St-Pierre Y. Expression and prognostic significance of human epidermal growth factor receptors 1 and 3 in gastric and esophageal adenocarcinoma. PLoS One. 2016;11(2):e0148101. doi: 10.1371/journal.pone.0148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoop H, Bregenzer A, Halske C, Behrens HM, Kruger S, Egberts JH, Röcken C. Therapy resistance in neoadjuvantly treated gastric cancer and cancer of the gastroesophageal junction is associated with an increased expression of immune checkpoint inhibitors-comparison against a therapy Naive Cohort. Transl Oncol. 2020;13(2):165–176. doi: 10.1016/j.tranon.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 46.Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109(1):43–53. doi: 10.1111/cas.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual faces of IFNgamma in cancer progression: a role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin Cancer Res. 2016;22(10):2329–2334. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- 48.Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009;9:65. doi: 10.1186/1471-230X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang B, Xu D, Yu X, Ding T, Rao H, Zhan Y, Zheng L, Li L. Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol. 2011;18(9):2585–2593. doi: 10.1245/s10434-011-1609-3. [DOI] [PubMed] [Google Scholar]
- 50.Zingg U, Montani M, Frey DM, Dirnhofer S, Esterman AJ, Went P, Oertli D. Tumour-infiltrating lymphocytes and survival in patients with adenocarcinoma of the oesophagus. Eur J Surg Oncol. 2010;36(7):670–677. doi: 10.1016/j.ejso.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Gullo I, Carneiro F, Oliveira C, Almeida GM. Heterogeneity in gastric cancer: from pure morphology to molecular classifications. Pathobiology. 2018;85(1–2):50–63. doi: 10.1159/000473881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Part of the data generated in this study is included in the article. The raw data of immune cell expression can be made available upon request. Patient and clinicopathological data cannot be made publicly available due to their content of identifiable human data. Requests to access the datasets should be directed to the corresponding author.


