Abstract
Background:
Lead exposure is associated with low birth-weight. The objective of this study is to determine whether lead exposure is associated with lower body weight in children, adolescents and adults.
Methods:
We analyzed data from NHANES 1999–2006 for participants aged ≥3 using multiple logistic and multivariate linear regression. Using age- and sex-standardized BMI Z-scores, overweight and obese children (ages 3–19) were classified by BMI ≥85th and ≥95th percentiles, respectively. The adult population (age ≥20) was classified as overweight and obese with BMI measures of 25–29.9 and ≥30, respectively. Blood lead level (BLL) was categorized by weighted quartiles.
Results:
Multivariate linear regressions revealed a lower BMI Z-score in children and adolescents when the highest lead quartile was compared to the lowest lead quartile (β (SE) = −0.33 (0.07), p < 0.001), and a decreased BMI in adults (β (SE) = −2.58 (0.25), p < 0.001). Multiple logistic analyses in children and adolescents found a negative association between BLL and the percentage of obese and overweight with BLL in the highest quartile compared to the lowest quartile (OR = 0.42, 95% CI: 0.30–0.59; and OR = 0.67, 95% CI: 0.52–0.88, respectively). Adults in the highest lead quartile were less likely to be obese (OR = 0.42, 95% CI: 0.35–0.50) compared to those in the lowest lead quartile. Further analyses with blood lead as restricted cubic splines, confirmed the dose-relationship between blood lead and body weight outcomes.
Conclusions:
BLLs are associated with lower body mass index and obesity in children, adolescents and adults.
Keywords: Obesity, Body mass index, Lead, NHANES, Children, Adults
Introduction
In the last few decades, the prevalence of obesity in United States has increased in all age groups. In adults, the prevalence of obesity rose from 14.5% in NHANES I (1972–1974) to 35.1% in NHANES 2005–2006 (http://www.cdc.gov/nchs/data/hestat/overweight/overweight_adult.pdf). The prevalence of obesity in children and adolescents also increased, from 6.1% in 1972–1974 (NHANES I) to 18.1% in NHANES 2007–2008 (http://www.cdc.gov/nchs/data/hestat/obesity_child_07_08/obesity_child_07_08.pdf).
The rise in obesity coincides with increased exposure to environmental toxins — particularly those that have the characteristic of being hormone-mimetic such as estrogen mimics and endocrine disrupting chemicals (EDCs). Recently, Grun and Blumberg (2006) coined the term ‘obesogens’ to identify “chemicals that promote obesity either by increasing the number of fat cells or the storage of fat into existing fat cells”. Prenatal exposure to these obesogens may be an important risk factor for childhood and adult obesity (Janesick and Blumberg, 2011).
Exposure to lead (Pb) is hypothesized to influence growth during childhood. Prenatal exposure to lead is associated with low birth weight (Jelliffe-Pawlowski et al., 2006). During pregnancy, lead passes across the placenta from the mother’s bloodstream to the fetal circulation (ATSDR, 2007). In the fetus, the lead competes with calcium for deposition in the fetal bone and may result in impaired growth (Zhu et al., 2010).
Epidemiological studies on the association of blood lead levels (BLL) with BMI or obesity are inconclusive. Kim et al. (1995) reported a weak but statistically significant positive association between childhood lead levels in teeth and BMI, whereas other studies did not or found an inverse association (Little et al., 2009). An inverse association between BMI and lead was also reported in adults (Padilla et al., 2010).
To investigate the correlation between BLL and weight outcomes, we analyzed data from NHANES 1999–2006 in children and adolescents (3–19 years old) and in adults (20 years and older). Body mass index was the measure of weight in the adult population (aged ≥20), and overweight and obese adults were defined based upon BMI measures of 25–29.9 and ≥30, respectively. We used age- and sex-standardized BMI Z-scores for children and adolescents as a measure of weight, and overweight and obese children were classified as having BMI Z-scores ≥85th and ≥95th percentiles, respectively.
Methods
Study population
The NHANES studies from 1999 to 2006, conducted by the U.S. National Center for Health Statistics (NCHS; Centers for Disease Control and Prevention, CDC, Atlanta, GA) with biomonitoring data evaluated by the National Center for Environmental Health (NCEH), are cross-sectional, nationally representative surveys of the non-institutionalized civilian population of the United States (NCHS, 2008a). The grouping we used consisted of 4 cycles (1999–2000, 2001–2002, 2003–2004 and 2005–2006) that were combined using NCHS recommendations (NCHS, 2008b). The survey employs a multistage stratified probability sample based on selected counties, blocks, households, and persons within households. Certain subgroups of the population, such as Mexican Americans, non-Hispanic blacks, and older adults were oversampled to improve the estimate precision for these groups.
Interviews were conducted in participants’ homes and extensive physical examinations, which included blood and urine collection, were conducted at mobile exam centers (MEC). For our analysis, the study population is limited to those who were examined.
Children and adolescents (3–19 years)
BMI Z-score and obesity.
Body mass index (BMI) was calculated by dividing measured weight in kilograms by measured height in meters squared, and was obtained from the physical examination. BMI varies by age and sex; therefore, we calculated age and sex specific BMI Z-scores using the methodology provided by the Centers for Disease Control and Prevention (http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm).
Overweight and obese were defined based on BMI as being greater than or equal to the 95th percentile (obese) or between the 85th and less than the 95th percentile (overweight).
Covariates.
Age, race/ethnicity, gender, hematocrit, poverty income ratio (PIR), serum cotinine, calorie intake, and television, videogame and computer usage were examined as independent predictors or confounders of the association between BLL and BMI Z-score. Calorie intake and television/videogame/computer usage have been associated with body weight. Hematocrit was included as a covariate because lead binds to ALAD, an enzyme in the heme pathway, and hematocrit is a measure of percentile of red blood cells within the blood. PIR is a measure of socioeconomic status and represents the calculated ratio of household income to the poverty threshold after accounting for inflation and family size. Environmental tobacco smoke is positively associated with childhood overweight/obesity (Hui et al., 2003; Weitzman et al., 2005). Serum cotinine as a biomarker of exposure to environmental tobacco smoke (ETS) was log natural transformed.
Adults
BMI and obesity.
The adult population (aged ≥20) was classified as overweight and obese with BMI measures of 25–29.9 and ≥30, respectively. Pregnant women (n = 1100), women who were breastfeeding (n = 208), and participants missing other variables of interest were excluded from the study, leaving 15,899 eligible participants who were included for the analyses of BLL and BMI.
Covariates.
We obtained information about age (years), sex, race/ethnicity, and education from the household interview. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, and other. Alcohol consumption (amount consumed per week) and smoking information were obtained from the physical examination and associated questionnaire. Serum cotinine was categorized as ETS low exposed non-smoker (<1 ng/mL), high exposed non-smokers (1–10 ng/mL), and exposed smokers (>10 ng/mL) (Hukkanen et al., 2005). Hematocrit was obtained through laboratory analyses.
BLL was determined by the CDC’s National Center for Environmental Health, Division of Laboratory Sciences using inductively coupled plasma mass spectrometry. The limit of detection (LOD) for blood lead was 0.3 μg/dL in NHANES (1999–2004) and there were 160 samples in the dataset below the LOD. The LOD for blood lead was 0.25 μg/dL in NHANES (2003–2006) and there were 21 samples in the dataset below the LOD. Lead concentration below the level of detection was assigned the limit of detection divided by the square root of 2, as recommended by NHANES.
Calorie intake was categorized as “normal” and “excessive” based on the U.S. Department of Agriculture calorie intake guidelines by age and sex (http://health.gov/dietaryguidelines/dga2010/DietaryGuidelines2010.pdf). The individual cut-off caloric need was the highest value for the range by age and gender assuming a moderate physical activity level. Information on daily hours of television, video or computer use was obtained through questionnaire and the covariate was categorized with a cut point of ≥2 h/d.
Statistical methods
Sample weights were used for analyses to account for the complex sampling design and non-response of NHANES. Weights for combined NHANES survey cycles were calculated according to NHANES guidelines (NCHS, 2008c). We estimated sampling errors using the Taylor series linearized method. We used the MULTILOG procedure in SUDAAN which implements the proportional odds model with a generalized multinomial logit model for nominal outcomes to produce separate parameter vectors for each of the generalized logit equations of interest to calculate adjusted odds ratios (ORs) for obesity or overweight status compared to normal/underweight in participants with BLL categorized by weighted quartile distribution. Linear regression analyses were used to investigate the correlation between BMI Z-scores (children and adolescent) or BMI (adults) with BLL categorized by weighted quartile distribution. Adults’ BMI outcome was used as continuous and log natural transformed variable, lead quartile distribution was calculated based on the combination of all four cycles (1999–2006); these quartile ranges were also used when assessing the potential association between lead and weight outcomes with only two cycles at a time (1999–2002 and 2003–2006).
To further characterize the shape of the relationship between blood lead and body weight outcomes we used blood lead as restricted cubic spline. The SAS macro-written Desquilbet and Mariotti (2010) modified to take in account NHANES weight was used to perform restricted cubic spline analyses. The knots used for restricted cubic spline were placed at the 10th, 50th, and 90th percentile as recommended by Harrell (2001).
We also assessed the collinearity between body weight parameters using Pearson correlation coefficients and multicollinearity diagnostic statistics (tolerance and variance inflation factors [VIF]). A value of VIF >10 often indicates multicollinearity. No covariates were multicollinear with each other.
SAS 9.2 (SAS Institute, Cary, NC) was used for all statistical analyses and SAS-Callable SUDAAN 10 (Research Triangle Institute, Research Triangle Park, NC) was used to account for the NHANES complex sample design. P-values from Satterthwaite statistics were presented at the significance level of 0.05.
Results
Children and adolescent (3–19 years old)
Table 1 illustrates the weighted characteristics of participants aged 3–19 years from NHANES 1999–2006 included in this study. The mean age of the participants was 11 years and approximately 51% were male. Non-Hispanic whites accounted for 60% of the total study group; 14.7% were non-Hispanic blacks, and 12.2% were Mexican-American. Approximately 23% of the participants were from families with income at the poverty level. The geometric mean (SE) BMI Z-score was 0.77 (0.01) and the geometric mean (SE) BLL was 1.12 (0.02) μg/dL. Table 1 also includes the weighted characteristics of children and adolescent participants broken down into 2-cycle analyses (1999−2002 and 2003−2006).
Table 1.
Weighted characteristics based on NHANES 1999–2006 participants aged 3–19.
| NHANES 1999–2006 | NHANES 1999–2002 | NHANES 2003–2006 | |
|---|---|---|---|
| Blood lead (μg/dL), GM (SE) | 1.12 (0.02) | 1.22(0.03) | 1.03 (0.02) |
| Hematocrit (%), GM (SE) | 40.36 (0.11) | 40.24 (0.14) | 40.48 (0.16) |
| Age (years), GM(SE) | 9.878 (0.07) | 9.74 (0.07) | 9.82 (0.12) |
| BMI Z-score, GM (SE) | 0.77 (0.01) | 0.76 (0.02) | 0.78 (0.02) |
| Serum cotinine, ng/mL, GM (SE) | 0.19 (0.02) | 0.22 (0.03) | 0.17 (0.02) |
| Weight | |||
| Obese, % (SE) | 16.04 (0.60) | 15.29 (0.72) | 16.77 (0.95) |
| Overweight, % (SE) | 15.05 (0.39) | 14.64 (0.50) | 15.45 (0.59) |
| Normal, % (SE) | 68.91(0.82) | 70.07(1.02) | 67.78 (1.28) |
| Sex | |||
| Male | 51.18 (0.51) | 50.94 (0.76) | 51.42 (0.69) |
| Female | 48.82 (0.51) | 49.06 (0.76) | 48.58 (0.69) |
| Income | |||
| Poverty income level: % (SE) | 23.26 (0.87) | 24.19 (0.88) | 22.39 (1.44) |
| Income > poverty level: % (SE) | 76.74 (0.87) | 75.81 (0.88) | 77.61 (1.44) |
| TV and video games | |||
| ≤2 h: % (SE) | 45.51 (0.92) | 43.29 (1.20) | 47.46 (1.30) |
| >2: % (SE) | 54.49 (0.92) | 56.71 (1.20) | 52.54 (1.30) |
| Calorie intake | |||
| Normal intake: % (SE) | 53.55 (0.65) | 53.58 (0.95) | 53.53 (0.89) |
| Excessive intake: % (SE) | 46.45 (0.65) | 46.42 (0.95) | 46.47 (0.89) |
| Race | |||
| White (non-Hispanic): % (SE) | 60.10 (1.74) | 59.40 (2.17) | 60.78 (2.69) |
| Non-Hispanic Black: % (SE) | 14.65 (1.20) | 14.41 (1.74) | 14.89 (1.65) |
| Mexican-American: % (SE) | 12.20 (1.03) | 11.84 (1.44) | 12.55 (1.48) |
| Other Hispanic: % (SE) | 6.20 (0.91) | 7.74 (1.69) | 4.68 (0.70) |
| Other: % (SE) | 6.86 (0.63) | 6.61 (0.85) | 7.09 (0.94) |
BMI Z-score outcome
The association between BMI Z-score and BLL was investigated using multivariate linear regressions. A statistically significant association was found between BMI Z-score and BLL: the third and fourth lead quartiles had a decreased BMI Z-score (β (SE) = −0.15 (0.06), p = 0.01 and β (SE) = −0.33 (0.07), p < 0.001, respectively) compared to the lowest lead quartile (Table 2).
Table 2.
Multilinear regression (β coefficients and SE) between BMI Z-score and BLL quartile in children and adolescent participants in NHANES 1999–2006 and by NHANES cycle 1999 = 2002 and 2003–2006.a
| NHANES 1999–2006 |
NHANES 1999–2002 |
NHANES 2003–2006 |
||||
|---|---|---|---|---|---|---|
| % (SE)b | β (SE) | % (SE)b | β (SE) | % (SE)b | β (SE) | |
| BLL quartile 1 (≤0.70 μg/dL) | 24.9(0.98) | 0 (reference) | 20.3 (1.02) | 0 (reference) | 29.4 (1.55) | 0 (reference) |
| BLL quartile 2 (0.71–1.09 μg/dL) | 24.6 (0.76) | −0.06 (0.04) p = 0.20 |
22.0 (0.96) | 0.01 (0.07) p = 0.95 |
27.1 (1.12) | −0.10 (0.06) p = 0.10 |
| BLL quartile 3 (1.10–1.60 μg/dL) | 25.6 (0.61) | −0.15 (0.06) p = 0.01 |
28.7 (0.77) | −0.16 (0.10) p = 0.13 |
22.6 (0.93) | −0.13 (0.07) p = 0.10 |
| BLL quartile 4 (≥1.61 μg/dL) | 24.9 (1.02) | −0.33 (0.07) p ≤ 0.01 |
29.0 (1.41) | −0.31 (0.12) p ≤ 0.01 |
20.9 (1.30) | −0.31 (0.08) p ≤ 0.01 |
| p trend | p ≤ 0.01 | p = 0.03 | p ≤ 0.01 | |||
Adjusted for age, gender, race/ethnicity, hematocrit, calorie intake, TV and video game use, serum cotinine, and poverty income ratio.
Percentages are sample-weighted for applicability to the U.S. population.
Analyses restricted to four-years of NHANES data, the relationship between BMI Z-score and BLL was maintained within the fourth lead quartile for both four-year analyses (1999–2002: β (SE) = −0.31 (0.12), p = 0.01 and 2003–2006: β (SE) = −0.31 (0.08), p < 0.001) (Table 2).
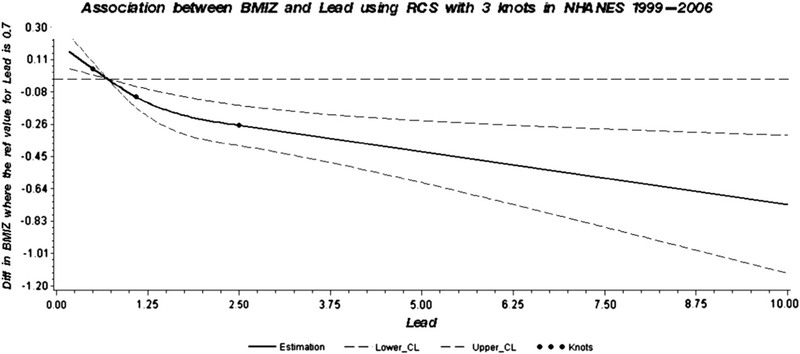
Analyses using restricted cubic spline confirmed the dose-relationship between lead and BMI Z-score in the NHANES 1999–2006 (Fig. 1), in the NHANES 1999–2002 (Supplement Fig. 1a) and in the NHANES (Supplement Fig. 1b) datasets.
Fig. 1.
Dose–response association between BMI Z score and blood lead in children and adolescents adjusted for age, gender, race/ethnicity, hematocrit, calorie intake, TV and video game use, serum cotinine, and poverty income ratio. A solid line shows dose–response curve between blood lead and BMI Z-score in NHANES 1999–2006. Y-axis represents the difference in BMI Z-score between individuals with any value of blood lead with individuals with 0.7 μg/dL of blood lead. The dashed lines represent the 95% confidence of interval. Knots are represented by dots.
Obesity and overweight outcomes
In multilog regression, we found a significant negative association between BLL and overweight and obesity in children and adolescents. Compared to persons in the lowest lead quartile, individuals in the second, third and highest lead quartiles had statistically significant lower adjusted odd ratios to be obese (aOR = 0.82, 95% CI: 0.67–1.00; aOR = 0.70, 95% CI: 0.54–0.90, and aOR = 0.42, 95% CI: 0.30–0.59, respectively). Also, compared to persons in the lowest BLL quartile individuals in the highest lead quartile had significantly lower adjusted odd ratios (aOR) risk to be overweight (aOR = 0.67, 95% CI: 0.52–0.88) (Table 3).
Table 3.
Multi-log regression (OR and 95% CI) in children and adolescent participants in NHANES 1999–2006, NHANES 1999–2002 and NHANES 2003–2006.a
| Participants, % (SE)b | Normal | Overweight | Obese | |
|---|---|---|---|---|
| NHANES 1999–2006; n = 10693 | 6923 | 1716 | 2054 | |
| BLL quartile 1 (≤0.70 μg/dL) | 1 (reference) | 1 (reference) | 1 (reference) | |
| BLL quartile 2 (0.71–1.09 μg/dL) | 1 (reference) | 0.93 (0.77–1.13) | 0.82 (0.67–1.00) | |
| BLL quartile 3 (1.10–1.60 μg/dL) | 1 (reference) | 0.87 (0.67–1.11) | 0.70 (0.54–0.90) | |
| BLL quartile 4 (≥1.61 μg/dL) | 1 (reference) | 0.67 (0.52–0.88) | 0.42 (0.30–0.59) | |
| NHANES 1999–2002; n=4920 | 3245 | 764 | 911 | |
| BLL quartile 1 (≤0.70 μg/dL) | 20.3 (1.02) | 1 (reference) | 1 (reference) | 1 (reference) |
| BLL quartile 2 (0.71–1.09 μg/dL) | 22.0 (0.96) | 1 (reference) | 1.11 (0.75–1.62) | 0.92 (0.67–1.28) |
| BLL quartile 3 (1.10–1.60 μg/dL) | 28.7 (0.77) | 1 (reference) | 0.96 (0.62–1.49) | 0.73 (0.49–1.10) |
| BLL quartile 4 (≥1.61 μg/dL) | 29.0 (1.41) | 1 (reference) | 0.63 (0.41–0.99) | 0.49 (0.27–0.89) |
| NHANES 2003–2006; n = 5773 | 3678 | 952 | 1143 | |
| BLL quartile 1 (≤0.70 μg/dL) | 29.4 (1.55) | 1 (reference) | 1 (reference) | 1 (reference) |
| BLL quartile 2 (0.71–1.09 μg/dL) | 27.1 (1.12) | 1 (reference) | 0.85 (0.69–1.05) | 0.76 (0.58–0.99) |
| BLL quartile 3 (1.10–1.60 μg/dL) | 22.6 (0.93) | 1 (reference) | 0.82 (0.60–1.14) | 0.70 (0.50–0.99) |
| BLL quartile 4 (≥1.61 μg/dL) | 20.9 (1.3) | 1 (reference) | 0.78 (0.55–1.10) | 0.38 (0.26–0.55) |
Adjusted for age, gender, race/ethnicity, hematocrit, calorie intake, TV and video game use, serum cotinine, and poverty income ratio.
Percentages are sample-weighted for applicability to the U.S. population.
Analyses restricted to four-years of NHANES data maintained the statistically significant association between weight outcomes and BLL. For the analyses done between 1999 and 2002, individuals in the highest lead quartile had a significantly lower adjusted odds ratio to be both obese and overweight compared to those in the lowest lead level (aOR= 0.49, 95% CI: 0.27–0.89, and aOR= 0.63, 95% CI: 0.41–0.99, respectively). For the years 2003–2006, individuals in the second, third and highest lead quartiles had a significantly lower adjusted odds ratio to be obese compared to persons in the lowest lead quartile (aOR = 0.76, 95% CI: 0.58–0.99; aOR = 0.70, 95% CI: 0.50–0.99, and aOR = 0.38, 95% CI: 0.26–0.55, respectively) (Table 3).
The dose-relationship between lead and obesity was confirmed by restricted cubic spline analyses using the NHANES 1999–2006 (Supplement Fig. 2a), NHANES 1999–2002 (Supplement Fig. 2b) and in the NHANES (Supplement Fig. 2c) datasets.
Adults (age 20 years and older)
Table 4 illustrates the weighted characteristics of participants aged 20 years and older from the NHANES 1999–2006 included in this study. The mean age of the participants was approximately 44 years and 50.8% were female. Non-Hispanic whites accounted for 71.8% of the total study group; 11% were non-Hispanic blacks, 7.2% were Mexican-American, 5.1% were other Hispanic, and 4.8% belong to other race/ethnicity or designated themselves as multiracial. Approximately 54%, 33%, and 50% of the people reported that they attended some college, never used alcohol, and never smoked, respectively. The geometric mean (SE) of BLL was 1.59 (0.02) μg/dL. The geometric mean (SE) BMI was 27.54 (0.09). Table 4 also includes the weighted characteristics of adult participants broken down into two-cycle analyses (1999–2002 and 2003–2006).
Table 4.
Weighted characteristics of the adults (20 years and older) participants in NHANES 1999–2006.
| NHANES 1999–2006 | NHANES 1999–2002 | NHANES 2003–2006 | |
|---|---|---|---|
| Blood lead (μg/dL), GM (SE) | 1.59 (0.02) | 1.70 (0.02) | 1.49 (0.02) |
| Hematocrit (%), GM (SE) | 42.60 (0.10) | 42.46 (0.14) | 42.73 (0.14) |
| Age (years), GM(SE) | 43.63 (0.26) | 43.49 (0.32) | 43.77 (0.41) |
| BMI, GM (SE) | 27.54 (0.09) | 27.37 (0.12) | 27.71 (0.12) |
| Serum cotinine, ng/mL, GM (SE) | 0.59 (0.05) | 0.62 (0.07) | 0.56 (0.06) |
| Weight | |||
| Obese, % (SE) | 31.90 (0.66) | 30.28 (0.93) | 33.41 (0.92) |
| Overweight, % (SE) | 33.56 (0.51) | 34.20 (0.82) | 32.96 (0.63) |
| Normal, % (SE) | 34.50(0.60) | 35.53(0.85) | 33.63 (0.84) |
| Sex | |||
| Male | 49.17 (0.34) | 49.09 (0.48) | 49.25 (0.48) |
| Female | 50.83 (0.34) | 50.91 (0.48) | 50.75 (0.48) |
| Smokers | |||
| Current smoker: % (SE) | 24.91 (0.59) | 24.70 (0.86) | 25.11 (0.81) |
| Former smoker: % (SE) | 25.10 (0.53) | 25.17 (0.84) | 25.03 (0.66) |
| Never smoked: % (SE) | 50.00 (0.74) | 50.14 (1.24) | 49.86 (0.85) |
| Alcohol consumption | |||
| No alcohol | 32.74 (0.98) | 32.63 (1.52) | 32.85 (1.26) |
| 1–4 drinks per week | 59.99 (1.05) | 59.92 (1.68) | 60.06 (1.29) |
| >4 drinks per week | 7.26 (0.36) | 7.45 (0.51) | 7.09 (0.51) |
| Education level | |||
| Less than high school | 20.04 (0.64) | 22.07 (0.84) | 18.10 (0.91) |
| Completed high school | 26.05 (0.59) | 25.81 (0.96) | 26.29 (0.68) |
| More than high school | 53.91 (0.90) | 52.12 (1.36) | 55.61 (1.17) |
| Calorie intake | |||
| Normal intake: % (SE) | 60.31 (0.59) | 60.69 (0.74) | 59.95 (0.92) |
| Excessive intake: % (SE) | 39.69 (0.59) | 39.31 (0.74) | 40.05 (0.92) |
| Race | |||
| White (non-Hispanic)% (SE) | 71.84 (1.44) | 71.37 (1.83) | 72.28 (2.19) |
| Non-Hispanic Black: % (SE) | 10.99 (0.89) | 10.66 (1.20) | 11.30 (1.31) |
| Mexican-American: % (SE) | 7.24 (0.67) | 6.87 (0.82) | 7.59 (1.06) |
| Other Hispanic: % (SE) | 5.12 (0.88) | 6.83 (1.72) | 3.49 (0.50) |
| Other: % (SE) | 4.81 (0.34) | 4.26 (0.52) | 5.34 (0.45) |
BMI outcome
Using multivariate linear regressions, a statistically significant association was found between BMI and BLL: the second, third, and fourth lead quartiles had lower BMI (β (SE) = −0.90 (0.20), p < 0.001, β (SE) = −1.41 (0.22), p < 0.001, and β (SE) = −2.58 (0.25), p<0.001 respectively) compared to the referent lead quartile (Table 5).
Table 5.
Multilinear regression (β coefficients and SE) between BMI score and BLL quartile in adult participants in NHANES 1999–2006.a
| NHANES 1999–2006 |
NHANES 1999–2002 |
NHANES 2003–2006 |
||||
|---|---|---|---|---|---|---|
| % (SE)b | β (SE) | % (SE)b | β (SE) | % (SE)b | β (SE) | |
| BLL quartile 1 (≤0.70 μg/dL) | 25.0 (0.72) | 0 (reference) | 21.8 (0.82) | 0 (reference) | 28.2 (1.12) | 0 (reference) |
| BLL quartile 2 (0.71–1.09 μg/dL) | 24.5 (0.47) | −0.90 (0.20) p ≤ 0.01 |
22.6 (0.70) | −0.70 (0.34) p = 0.05 |
26.1 (0.63) | −1.05 (0.20) p ≤ 0.01 |
| BLL quartile 3 (1.10–1.60 μg/dL) | 25.0 (0.55) | −1.41 (0.22) p ≤ 0.01 |
26.9 (0.66) | −1.29 (0.37) p ≤ 0.01 |
23.2 (0.82) | −1.42 (0.25) p ≤ 0.01 |
| BLL quartile 4 (≥1.61 μg/dL) | 25.5 (0.54) | −2.58 (0.25) p ≤ 0.01 |
28.7 (0.69) | −2.51 (0.41) p ≤ 0.01 |
22.5 (0.84) | −2.50 (0.29) p ≤ 0.01 |
| p trend | p ≤ 0.01 | p ≤ 0.01 | p ≤ 0.01 | |||
Adjusted for race/ethnicity, gender, age, Hematocrit, smoking status, serum cotinine, alcohol consumption, education, calorie intake and moderate and vigorous activity covariates.
Percentages are sample-weighted for applicability to the U.S. population.
Analyses restricted to four-years of NHANES data maintained the statistically significant association between BMI and BLL. For 1999–2002, the second, third and fourth lead quartiles had lower BMI compared to the referent lead quartile (β (SE) = −0.70 (0.34), p < 0.05, β (SE) = −1.29 (0.37), p < 0.001, and β (SE) = −2.51 (0.41), p < 0.001, respectively). Similarly, the analyses for the years 2003–2006 showed lower BMIs for the second, third, and fourth lead quartiles compared to the referent (β (SE) = −1.05 (0.20), p < 0.001, β (SE) = −1.42 (0.25), p < 0.001, and β (SE) = −2.50 (0.29), p < 0.001, respectively) (Table 5).
These significant associations were confirmed also when using as outcome log natural transformed BMI (Supplement Table 1).
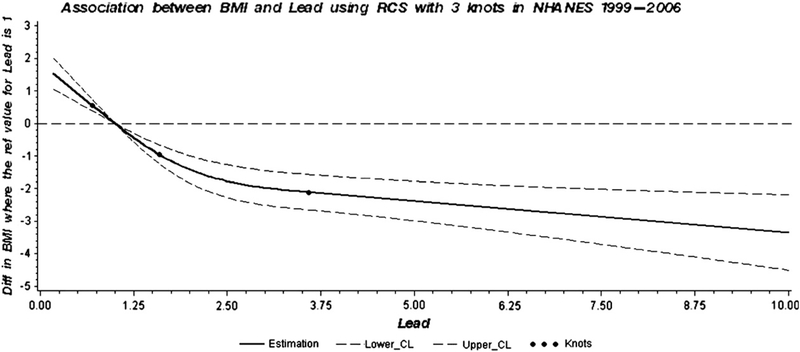
Further analyses using restricted cubic spline confirmed the dose-relationship between lead and BMI in the NHANES 1999–2006 (Fig. 2), in the NHANES 1999–2002 (Supplement Fig. 3a) and in the NHANES (Supplement Fig. 3b) datasets.
Fig. 2.
Dose–response association between BMI and blood lead in adults adjusted for race/ethnicity, gender, age, hematocrit, smoking status, serum cotinine, alcohol consumption, education, calorie intake and moderate and vigorous activity covariates. A solid line shows dose–response curve between blood lead and BMI in NHANES 1999–2006. Y-axis represents the difference in BMI between individuals with any value of blood lead with individuals with 1.0 μg/dL of blood lead. The dashed lines represent the 95% confidence of interval. Knots are represented by dots.
Obesity and overweight outcomes
In multiple logistic regression, BLL was negatively associated with obesity and with overweight status (Table 6). We found a statistically significant negative association between BLL and obesity in adults: compared to persons in the referent lead quartile, individuals in the second, third, and fourth lead quartiles had a statistically significant lower adjusted odd ratios (aOR) for obesity (aOR = 0.76, 95% CI: 0.66–0.87, aOR = 0.66, 95% CI: 0.56–0.77, and aOR = 0.42, 95% CI: 0.35–0.50, respectively). Also, compared to persons in the lowest BLL quartile individuals in the highest lead quartile had significantly lower adjusted odd ratios (aOR) risk to be overweight (aOR = 0.79, 95% CI: 0.65–0.95) (Table 6).
Table 6.
Multiple logistic regression (OR and 95% CI) in adult participants in NHANES.a
| Participants, (% + SE)b | Normal | Overweight | Obese | |
|---|---|---|---|---|
| NHANES 1999–2006; n = 15899 | 5060 | 5594 | 5245 | |
| BLL quartile 1 (≤1.00 μg/dL) | 25.0 (0.72) | 1 (reference) | 1 (reference) | 1 (reference) |
| BLL quartile 2 (1.01–1.59 μg/dL) | 24.5 (0.47) | 1 (reference) | 1.04 (0.87–1.25) | 0.76 (0.66–0.87) |
| BLL quartile 3 (1.60–2.39 μg/dL) | 25.0 (0.55) | 1 (reference) | 1.02 (0.87–1.19) | 0.66 (0.56–0.77) |
| BLL quartile 4 (≥2.40 μg/dL) | 25.5 (0.54) | 1 (reference) | 0.79 (0.65–0.95) | 0.42 (0.35–0.50) |
| NHANES 1999–2002; n = 7764 | 2530 | 2783 | 2451 | |
| BLL quartile 1 (≤0.70 μg/dL) | 21.8 (0.82) | 1 (reference) | 1 (reference) | 1 (reference) |
| BLL quartile 2 (0.71–1.09 μg/dL) | 22.6 (0.70) | 1 (reference) | 1.03 (0.78–1.36) | 0.76 (0.60–0.96) |
| BLL quartile 3 (1.10–1.60 μg/dL) | 26.9 (0.66) | 1 (reference) | 0.94 (0.77–1.14) | 0.62 (0.48–0.79) |
| BLL quartile 4 (≥1.61 μg/dL) | 28.7 (0.69) | 1 (reference) | 0.73 (0.58–0.93) | 0.39 (0.30–0.52) |
| NHANES 2003–2006; n = 8135 | 2530 | 2811 | 2794 | |
| BLL quartile 1 (≤0.70 μg/dL) | 28.2 (1.12) | 1 (reference) | 1 (reference) | 1 (reference) |
| BLL quartile 2 (0.71–1.09 μg/dL) | 26.1 (0.63) | 1 (reference) | 1.04 (0.81–1.33) | 0.77 (0.65–0.90) |
| BLL quartile 3 (1.10–1.60 μg/dL) | 23.2 (0.82) | 1 (reference) | 1.11 (0.88–1.39) | 0.74 (0.60–0.90) |
| BLL quartile 4 (≥1.61 μg/dL) | 22.5 (0.84) | 1 (reference) | 0.85 (0.64–1.12) | 0.47 (0.37–0.59) |
Adjusted for race/ethnicity, gender, age, hematocrit, smoking status, serum cotinine, alcohol consumption, education, calorie intake and moderate and vigorous activity covariates.
Percentages are sample-weighted for applicability to the U.S. population.
Analyses restricted to four-years of NHANES data maintained the statistically significant association between weight outcomes and BLL. For the analyses done between 1999 and 2002, individuals in the second, third, and fourth lead quartile had a significantly lower adjusted odds ratio to be obese compared to those in the lowest lead level (aOR = 0.76, 95% CI: 0.60–0.96, aOR = 0.62, 95% CI: 0.48–0.79, and aOR = 0.39, 95% CI: 0.30–0.52, respectively). Also, compared to persons in the lowest BLL quartile individuals in the highest lead quartile had significantly lower adjusted odd ratios (aOR) risk to be overweight (aOR = 0.73, 95% CI: 0.58–0.93). For the years 2003–2006, individuals in the second, third and highest lead quartiles had a significantly lower adjusted odds ratio to be obese compared to persons in the lowest lead quartile (aOR = 0.76, 95% CI: 0.65–0.90; aOR = 0.74, 95% CI: 0.60–0.90, and aOR = 0.47, 95% CI: 0.37–0.59, respectively) (Table 6).
The dose-relationship between lead and obesity was confirmed by restricted cubic spline analyses using the NHANES 1999–2006 (Supplement Fig. 4a), NHANES 1999–2002 (Supplement Fig. 4b) and in the NHANES (Supplement Fig. 4c) datasets.
Discussion
The results of this analyses support an inverse association of BLL and body weight outcomes in children and adolescents, and adults in the U.S. population. The inverse association is independent of confounding factors such as: age, gender, race/ethnicity, calorie intake, TV and video game use, poverty income rate, and serum cotinine in children and adolescents; and age, gender, race/ethnicity, education, alcohol consumption, cigarette smoking, environmental tobacco smoke exposure (through serum cotinine), calorie intake and physical activity in adults.
In children and adolescents, we found a lower BMI Z-score with higher BLL quartiles.
A lower BMI was also associated with higher BLL quartiles in adults. Additionally, after restriction of the analyses to only two-cycles of NHANES data (1999–2002 and 2003–2006), these relationships were maintained in children and adolescents as well as in adults. Since blood lead levels have been falling and overweight/obesity levels have continued to rise over the period of our study (1999–2006), we stratified our analyses to include only 2 cycles (4 years) at a time. We used 2 cycles combined instead of individual cycles to produce estimates with greater statistical reliability, Within these analyses, we maintained weighted quartiles based on all 4 cycles combined in order to more easily compare the association.
There was a statistically significant lower OR for obesity in children and adolescents, and adults in all blood lead quartiles when compared to the referent quartile. Additionally, a statistically significantly lower OR for overweight was found in children and adolescents as well as adults in the highest BLL quartile; this relationship was not found when analyses were restricted to the years 2003–2006.
Further analyses using blood lead as restricted cubic splines, confirmed the dose-relationship between blood lead and body weight outcomes in NHANES 1999–2006, as well in analyses done in NHANES 1999–2002 and NHANES 2003–2006 datasets.
Epidemiological studies on the association of BLL with BMI or obesity are scarce and inconclusive. Kim et al. (1995) reported a weak but statistically significant increased association between childhood lead levels in teeth and BMI. In the cross-sectional part of the study conducted in 1975–1978, with 236 children (mean age 7.4), a 10-fold increase of dentin lead level was associated with an increase of 1.02 kg/m2 in BMI. Follow-up analyses of this cohort in 1989–1990 (n = 58) found that a 10-fold increase of dentin lead level was associated with an increase of 2.65 kg/m2 in BMI change from age 7 to 20. However, bone lead measurement was not associated with BMI (Kim et al., 1995).
No association was found between BLL and BMI or obesity in several other cross-sectional studies done in children (Huzior-Balajewicz et al., 2001; Lamb et al., 2008) and in adults (Hauser et al., 2008; Ronco et al., 2010). Several studies reported an inverse dose–response association between BLL and BMI. A statistically significant negative correlation was reported between BLL and BMI in children (n = 250) aged 8–10 years attending a primary school in Alexandria, Egypt (Kamel et al., 2003). Dhooge et al. (2010) found that BMI was negatively associated with BLL in adult women aged 50–65, but not in adult men or in adolescents. Ignasiak et al. (2006) investigated the relationship between BLL and growth in children aged 7–15 years (463 males and 436 females). The BMI showed a statistically significant decrease with increased BLL among females, but not among males. Little et al. (2009) studied two cohorts of children from the city of Dallas (USA) at two points in time, one from the 1980s and one from 2002. They found that BLL had a significant (p<0.0001) inverse log-linear relationship with height, weight, and BMI Z-scores. A decreased association of BMI with increased BLL exposure in adults was also reported by Padilla et al. (2010). The authors found that decreased BMI and waste circumference were associated with increased urinary lead in NHANES 1999–2002 cycles. Recently, Park and Lee (2012), analyzing the Korean National Health and Nutrition Examination Survey data (2007–2009), found that BLL was negatively associated with fat intake with the effect being more prominent in men.
A reverse causality association may not be excluded. BMI is a good indicator for the measurements of bone mineral density (BMD), and low BMI is associated with lower BMD and osteoporosis in older people (Guthrie et al., 2000). Bone resorption results in lead mobilization from bone to blood (Tsaih et al., 2001). Therefore, one would expect that in elderly people, the decreased BMD will be associated with a decreased BMI and higher blood lead due to lead displacement from the bone compartment. We found that individuals 59 years and older with higher blood lead levels had lower BMI and lower OR to be obese; however, these relationships were not statistically significant (data not shown). Therefore, while we did see the relationship of lower BMI being associated with higher blood lead, this relationship lost its significance in the older age group. This suggests that the results we observed support a possible influence of lead on BMI.
Several animal studies found that animals exposed to lead experience body weight loss (Donald et al., 1988; Hamir et al., 1981). Recently, Leasure et al. (2008) demonstrated in their in vivo gestational lead exposure (GLE) model, that at 1 year of age, male rat pups exposed prenatally and during the first 10 days of life to the lowest GLE levels had a statistically significant increase of weight, and decreased levels of striatal dopamine and its main metabolite, DOPAC, compared to those exposed to the highest GLE level. While the exposed groups decreased in weight with higher lead exposure, the controls were statistically significantly smaller than all of the exposed animals making this study hard to interpret. Geiger et al. (2009) observed that obese female rats exhibited lower extracellular levels of dopamine as well as lower levels of the dopamine metabolite DOPAC suggesting that the linkage between body weight and lead exposure may be through perturbation of the hypothalamic dopaminergic system.
Increased cortisol levels in humans have been implicated in the pathophysiology of obesity (Rossi-George et al., 2009). Therefore, another potential explanation for the relationship between BLL and body weight observed in our study could be that lead acts to disrupt the hypothalamic–pituitary–adrenal (HPA) axis through an interaction with cortisol (Fortin et al., 2012; White et al., 2007). Dallman et al. (2003) proposed a mechanism through which chronic elevated stress increases levels of glucocorticoids leading to increased levels of abdominal energy stores, which inhibit the normal stress-response network. They hypothesize that this mechanism, in turn, leads to an increase in eating and obesity among patients chronically exposed to stress. White et al. (2007) showed that in the U.S., the largest populations at risk for lead exposure are low socioeconomic status (SES) populations. They commented that low SES environments are themselves a risk factor for many diseases as there is greater stress among these populations and thus elevated basal cortisol levels. Because of this environmental exposure risk, White et al. (2007) investigated how lead exposure and higher chronic stress levels interact. They found that although stress increases basal cortisol levels, lead actually acts to decrease basal cortisol levels. A previous study done by Virgolini et al. (2005) suggested similar results when looking at post-weaning lead and environmental stress exposure in rats. They also found significant reductions in basal corticosterone levels. A later study from Rossi-George et al. (2009), although finding that lead delays the reduction time of corticosterone levels after an injection stress challenge, found that lead exposed rats had lower basal corticosterone levels compared to controls, too. Taken together, these results suggest a possible mechanism through which lead acts to decrease basal levels of cortisol and potentially decrease the risk of obesity through a disruption in the HPA axis.
The major limitation of our study is the cross-sectional study design and the inferences that can be made based on the findings are limited. However, it can serve to generate hypotheses. Another limitation of this study is that although blood lead reflects both recent exogenous exposure and endogenous redistribution of lead stored in bone, blood-lead concentration may underestimate the internal dose of lead. Our results are based on BLL; because approximately 95% of the total body burden of lead is present in the skeleton, a preferred measure of chronic body burden would be bone lead (Hu et al., 1996). However, the measurement of bone lead in a large sample size, such as NHANES, is not feasible, and blood lead is known to be associated with bone lead (Gwiazda et al., 2005; Todd et al., 2001).
Conclusion
We found that BLLs are associated with lower body mass index and obesity in children and adolescents, as well as in adults both when analyzing eight years of NHANES data combined (1999–2006) and when restricted to only four years at a time (1999–2002 and 2003–2006). Considering the similar finding for the Korean NHANES study, this association deserves additional study.
Supplementary Material
Acknowledgment
This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC (MCB).
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.taap.2013.09.022.
References
- ATSDR. Agency for Toxic Substances and Disease Registry, 2007. Toxicological Profile for Lead. US Department of Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. , 2003. Chronic stress and obesity: a new view of “comfort food”. Proc. Natl. Acad. Sci. U. S. A. 100, 11696–11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L, Mariotti F, 2010. Dose–response analyses using restricted cubic spline functions in public health research. Stat. Med. 29, 1037–1057. [DOI] [PubMed] [Google Scholar]
- Dhooge W, Den Hond E, Koppen G, Bruckers L, Nelen V, Van De Mieroop E, et al. , 2010. Internal exposure to pollutants and body size in flemish adolescents and adults: associations and dose–response relationships. Environ. Int. 36, 330–337. [DOI] [PubMed] [Google Scholar]
- Donald JM, Bradley M, O’Grady JE, Cutler MG, Moore MR, 1988. Effects of low-level lead exposure on 24 h activity patterns in the mouse. Toxicol. Lett. 42, 137–147. [DOI] [PubMed] [Google Scholar]
- Fortin MC, Cory-Slechta DA, Ohman-Strickland P, Nwankwo C, Yanger TS, Todd AC, et al. , 2012. Increased lead biomarker levels are associated with changes in hormonal response to stress in occupationally exposed male participants. Environ. Health Perspect. 120, 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN, 2009. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 159, 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F, Blumberg B, 2006. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147 (6 Suppl.), S50–S55. [DOI] [PubMed] [Google Scholar]
- Guthrie JR, Dennerstein L, Wark JD, 2000. Risk factors for osteoporosis: a review. Medscape Womens Health 5 (4), E1 (Jul-Aug). [PubMed] [Google Scholar]
- Gwiazda R, Campbell C, Smith D, 2005. A noninvasive isotopic approach to estimate the bone lead contribution to blood in children: implications for assessing the efficacy of lead abatement. Environ. Health Perspect. 113, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamir AN, Sullivan ND, Handson PD, Wilkinson JS, Lavelle RB, 1981. Clinical signs, radiology and tissue lead distribution of dogs administered a mixture of lead chloride, lead bromide and lead sulphate. Aust. Vet. J. 57, 401–406. [DOI] [PubMed] [Google Scholar]
- Harrell FE, 2001. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. Springer. [Google Scholar]
- Hauser R, Sergeyev O, Korrick S, Lee MM, Revich B, Gitin E, et al. , 2008. Association of blood lead levels with onset of puberty in Russian boys. Environ. Health Perspect. 116, 976–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Aro A, Payton M, Korrick S, Sparrow D, Weiss ST, et al. , 1996. The relationship of bone and blood lead to hypertension. The normative aging study. JAMA 275, 1171–1176. [PubMed] [Google Scholar]
- Hui LL, Nelson EA, Yu LM, Li AM, Fok TF, 2003. Risk factors for childhood overweight in 6- to 7-y-old Hong Kong children. Int. J. Obes. Relat. Metab. Disord. 27, 1411–1418. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob III P, Benowitz NL, 2005. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 57, 79–115. [DOI] [PubMed] [Google Scholar]
- Huzior-Balajewicz A, Pietrzyk JJ, Schlegel-Zawadzka M, Piatkowska E, Zachwieja Z, 2001. The influence of lead and cadmium environmental pollution on anthropometric health factors in children. Przegl. Lek. 58, 315–324. [PubMed] [Google Scholar]
- Ignasiak Z, Slawinska T, Rozek K, Little BB, Malina RM, 2006. Lead and growth status of school children living in the copper basin of south-western Poland: differential effects on bone growth. Ann. Hum. Biol. 33, 401–414. [DOI] [PubMed] [Google Scholar]
- Janesick A, Blumberg B, 2011. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res. C Embryo Today 93, 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelliffe-Pawlowski LL, Miles SQ, Courtney JG, Materna B, Charlton V, 2006. Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. J. Perinatol. 26, 154–162. [DOI] [PubMed] [Google Scholar]
- Kamel NM, Ramadan AM, Kamel MI, Mostafa YA, Abo el-Naga RM, Ali AM, 2003. Impact of lead exposure on health status and scholastic achievement of school pupils in Alexandria J. Egypt. Public Health Assoc. 78, 1–28. [PubMed] [Google Scholar]
- Kim R, Hu H, Rotnitzky A, Bellinger D, Needleman H, 1995. A longitudinal study of chronic lead exposure and physical growth in Boston children. Environ. Health Perspect. 103, 952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb MR, Janevic T, Liu X, Cooper T, Kline J, Factor-Litvak P, 2008. Environmental lead exposure, maternal thyroid function, and childhood growth. Environ. Res. 106, 195–202. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Giddabasappa A, Chaney S, Johnson JE Jr., Pothakos K, Lau YS, et al. , 2008. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ. Health Perspect. 116, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little BB, Spalding S, Walsh B, Keyes DC, Wainer J, Pickens S, et al. , 2009. Blood lead levels and growth status among African-American and Hispanic children in Dallas, Texas—1980 and 2002: Dallas Lead Project II. Ann. Hum. Biol. 36, 331–341. [DOI] [PubMed] [Google Scholar]
- NCHS. National Center for Health Statistics, 2008a. National Health and Nutrition Examination Survey Home Page. Centers for Disease Control and Prevention, National Center for Health Statistics, Atlanta, GA: ((Accessed March 2012). http://www.cdc.gov/nchs/nhanes.htm). [Google Scholar]
- NCHS. National Center for Health Statistics, 2008b. National Health and Nutrition Examination. Survey Analytic Guidelines. Centers for Disease Control and Prevention, National Center for Health Statistics, Atlanta, GA: (Accessed October 2012). [Google Scholar]
- NCHS. National Center for Health Statistics, 2008c. Continuous National Health and Nutrition Examination Survey (NHANES) Web Tutorial. Constructing Weights for Combined NHANES Survey Cycles. Centers for Disease Control and Prevention, National Center for Health Statistics, Atlanta, GA: ((Accessed March 2012). http://www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/Weighting/intro.htm). [Google Scholar]
- Padilla MA, Elobeid M, Ruden DM, Allison DB, 2010. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99–02. Int. J. Environ. Res. Public Health 7, 3332–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee BK, 2012. Inverse relationship between fat intake and blood lead levels in the Korean adult population in the KNHANES 2007–2009. Sci. Total Environ. 430, 161–166. [DOI] [PubMed] [Google Scholar]
- Ronco AM, Gutierrez Y, Gras N, Munoz L, Salazar G, Llanos MN, 2010. Lead and arsenic levels in women with different body mass composition. l. Trace Elem. Res. 136, 269–278. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, Virgolini MB, Weston D, Cory-Slechta DA, 2009. Alterations in glucocorticoid negative feedback following maternal Pb, prenatal stress and the combination: a potential biological unifying mechanism for their corresponding disease profiles. Toxicol. Appl. Pharmacol. 234, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AC, Lee BK, Lee GS, Ahn KD, Moshier EL, Schwartz BS, 2001. Predictors of DMSA chelatable lead, tibial lead, and blood lead in 802 Korean lead workers. Occup. Environ. Med. 58, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaih SW, Korrick S, Schwartz J, Lee ML, Amarasiriwardena C, Aro A, Sparrow D, Hu H, 2001. Influence of bone resorption on the mobilization of lead from bone among middle-aged and elderly men: the Normative Aging Study. Environ. Health Perspect. 109 (10), 995–999 (October). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini MB, Chen K, Weston DD, Bauter MR, Cory-Slechta DA, 2005. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol. Sci. 87, 469–482. [DOI] [PubMed] [Google Scholar]
- Weitzman M, Cook S, Auinger P, Florin TA, Daniels S, Nguyen M, et al. , 2005. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation 112, 862–869. [DOI] [PubMed] [Google Scholar]
- White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, et al. , 2007. New and evolving concepts in the neurotoxicology of lead. Toxicol. Appl. Pharmacol. 225, 1–27. [DOI] [PubMed] [Google Scholar]
- Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM, 2010. Maternal low-level lead exposure and fetal growth. Environ. Health Perspect. 118, 1471–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.