ABSTRACT
Background: The Mediterranean diet (MedDiet) is a dietary pattern effective in terms of prevention of many diseases such as gestational diabetes mellitus (GDM). Recently, many studies have paid attention to nutritional factors during pregnancy as a modifiable contributor to GDM risk.
Objective: to investigate associations of nutrients intakes and MedDiet pattern of eating with risk of GDM.
Subjects/Methods: This study conducted on N = 120; Pregnant women with GDM (n = 60) and without controls (n = 60). The dietary habits were assessed by a dietary history method and a validated food frequency questionnaire. We calculated a MedDiet score which measures the degree of adherence to a Med Diet.
Result: A low Med Diet score was found in pregnant women with and without gestational diabetes in 46.7% and 38.8% of cases, respectively, with no significant difference. Our data showed that the higher the adherence score to the MedDiet, the lower the fasting blood glucose level and the plasma glucose 2 h post load. These findings concerned the two groups studied (P < 10−3). We also noted that controls had a significantly higher intake of legumes, vegetables and fish. Monounsaturated fatty acids and saturated fatty acids consumption was significantly higher in the control group (2.3 ± 0.8 vs 1.7 ± 0.7, P < 10−3). GDM subjects consumed significantly more dairy products and cereals (P < 10−3). After adjustment for confounders, no nutrient was associated with the risk of developing gestational diabetes except vitamin D intake (OR 0.29 [0.15−0.54], P < 10−3) which had a protective effect.
Conclusion: Our study underlines the importance of adequate vitamin D intake during pregnancy and suggests that the MedDiet may reduce the incidence of gestational diabetes.
KEYWORDS: Gestational diabetes, nutrients, Mediterranean diet
1. Introduction
Gestational diabetes is defined as a disorder of glucose tolerance first recognized during pregnancy [1]. It affects 1–28% of all pregnancies depending on the diagnostic threshold and the population studied [2,3]. Gestational diabetes mellitu (GDM) is associated with poor pregnancy outcomes as well as increased risk of longer-term morbidity for both mother and child [4].
Unmodifiable risk factors associated with GDM are known such as maternal age, prior history of GDM, family history of type 2 diabetes [5]. Identifying modifiable risk factors of GDM is needed for novel preventive strategies to avoid its associated adverse health outcomes [6].
Some studies have paid attention to nutritional factors during pregnancy as a modifiable contributor to GDM risk [7,8]. The findings of these researches are conflicting. High fiber intake is associated with lower risk of GDM in two studies, but such relation is not found in other studies [9,10]. A prospective study shows that high dietary glycemic load was associated with the development of GDM [10]. The role of fat subtypes seems interesting, as saturated fat increases risk of GDM polyunsaturated fat may have a protective effect [11,12].
Observational studies showed that achieving a healthier dietary pattern, such as Mediterranean dietary pattern seem to lower the risk of developing GDM [7,13]. In fact, MedDiet emphasizes consumption of fruits, vegetables, legumes, whole grains and foods rich in monounsaturated fatty acids (MUFAs), these beneficial components might contribute to the preventive effects on GDM [14].
However, until this day there is no Tunisian study who has investigated the relationship between adherence to MedDiet and the prevalence of GDM. The aim of this study was to investigate associations of nutrients intakes and Mediterranean diet (MedDiet) pattern of eating with risk of GDM.
2. Subjects and methods
2.1. Study population
This case-control study was conducted between March 2018 and June 2018. Pregnant women with (n = 60) and without (controls; n = 60) gestational diabetes were recruited from the National Institute of Nutrition. The two groups were matched for age and socioeconomic status. Women with a known history of diabetes (type 1 or 2), with metabolic or cardiovascular diseases and pregnant women using drugs in the long term were not included in the study. All other pregnant women who underwent a 75 g-OGTT at the 24th–32nd week of gestation were included. The definition of GDM was based on the recommendations of the International Association of the Diabetes and Pregnancy Study Groups [15]. GDM was diagnosed if one glucose value is equal to or above any cutoff point: fasting ≥0.92 g/l; 1 h ≥ 1.8 g/l; 2 h ≥ 1.53 g/l.
2.2. Data collection
We collected information about date of birth, family history of diabetes mellitus, personal medical history of GDM, pre-pregnancy weight (used to calculate pre-pregnancy BMI) and physical activity. Weight and height were measured. Weight gain was calculated by subtracting pre-pregnancy weight from the last measured weight and interpreted according to the recommendations of the Institute of medicine [16].
3. Dietary assessment
3.1. Dietary history method
The dietary habits were assessed by a dietary history method that has been used and validated in previous studies in the Mediterranean region [17]. The questionnaire was analyzed with ‘NUTRISOFT’ software to obtain a nutritional assessment including total caloric intake and the distribution of macronutrients (fat, protein and carbohydrate) and micronutrients (Magnesium,Vitamins C). The daily intakes of vitamin D, α-linolenic acid, linoleic acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were calculated manually based on the composition tables of Ciqual 2012.
3.2. Food frequency questionnaire (FFQ)
A semi-quantitative validated questionnaire compound of 134 items expressed as the number of daily, weekly and monthly portion and considered representative of the Tunisian food consumption was used. For quantification of the amount consumed, we used a validated manual including 380 food’s photography commonly consumed in Tunisia grouped into 5items: bread, vegetables, meats, grains, fruits and sweet products. Each food was presented with three portion sizes: small, medium and large considered representative of the consumption of the Tunisian population. The reference of the weight of the food (A, B, C) are shown on each photo. Food presentation in three sizes also provides intermediate and extreme sizes, corresponding to seven different sizes of food portions overall [18].
3.3. The score of adherence to the Mediterranean diet (MDS)
To calculate this score, we have identified foods derived from the FFQ data and nutrients from the dietary history (MUFAs and saturated fatty acids (SFAs)), which are the nine components of the MedDiet as defined in the Washington Heights-Inwood Columbia Aging Project (WHICAP) study [19]. Vegetables, fruits, legumes, cereals and bread, pasta, rice; fish and seafood; meat, poultry; dairy products; alcohol and ratio MUFAs/SFAs. The sub-scores were assigned as previously proposed by Trichopoulou et al. [20]: For the beneficia components of diet (vegetables, fruits, legumes, cereals, fish, MUFAs/SFAs ratio) individuals whose consumption was greater than or equal to the median received a score of 1 and zero for others. Conversely, for the supposed deleterious components (meat and dairy products), individuals whose consumption was greater than or equal to the median received a score of 0 and 1 for others. The total score quantifying adherence to the MedDiet was calculated by adding the binary scores awarded to nine components in a way that the higher the score the greater the adherence.
Adherence to the MedDiet was interpreted as, low adherence (score = [0–3]), moderate adherence (score = [4,5]), high adherence (score = [6–9]).
3.4. Statistical analysis
The statistical analysis was performed with STATA Version 11.1 software. Quantitative variables were presented as mean ± standard deviation. Quantitative values and means were compared with parametric Student’s t test. The univariate ANOVA was used to compare the mean blood glucose and post load glucose (2 h) in both strata based on the MedDiet score. The multiple comparison test ‘Post hoc student – Newman Keuls’ was used for pair wise comparison (for fasting glucose and post load glucose). Categorical variables were expressed as numbers and percentages. Comparisons of qualitative values and percentages were performed using the test Chi-square. We used logistic regression to assess the association between nutrient intakes and the development of GDM. The comparisons were done after adjustment for age, pre-pregnancy BMI, family history of type 2 diabetes, prior history of GDM, physical activity and weight gain during pregnancy. P-value ≤ 0.05 was considered significant.
3.5. Ethical approval
The study has been approved by the Ethic Committee of the National Institute of Nutrition. The study protocol was conducted in accordance with the guidelines of the Declaration of Helsinki. Informed consent was obtained from all participants prior to their inclusion in the study.
4. Results
The comparison between GDM subjects and controls concerning baseline characteristics is presented in Table 1. There was no significant difference in age between GDM and control subjects. The average pre-pregnancy BMI for GDM was significantly higher than the control group (27.5 ± 4.1 vs 25.4 ± 3.8; p = 0.01). Weight gain, family history of diabetes, prior history of GDM and physical activity were significantly higher in women with GDM than in controls.
Table 1.
Comparison of baseline characteristics between subjects with and without GDM GDM: gestational diabetes mellitus
| Characteristics | Control subjects, n = 60 |
GDM, n = 60 |
P (two-tailed) |
|---|---|---|---|
| Age (years) | 31.2 ± 5.7 | 31.2 ± 5.7 | NS |
| Pre- pregnancy BMI (kg/m2) Weight gain (kg) |
25.4 ± 3.811.5 ± 6 | 27.5 ± 4.19.4 ± 4.9 | 0.010.02 |
| Family history of diabetes (%) | 18 | 65 | <10−3 |
| Prior history of GDM (%) | 0 | 30 | 0.01 |
| Physical activity | |||
| Sedentary (%) | 33 | 67 | 0.01 |
| Moderate (%) | 68 | 37 | 0.03 |
Data are means ± SD, Student’s t test
BMI: Body mass Index
The comparison of foodstuff groups characterizing the MedDiet as described previously is shown in Table 2. Controls had a significantly higher intake of legumes, vegetables and fish. The MUFAs to SFAs ratio was significantly higher in the control group (2.3 ± 0.8 vs 1.7 ± 0.7, P < 10−3). Whereas GDM subjects consumed significantly more dairy products and cereals (P < 10−3).
Table 2.
Comparison of various foodstuffs intake of mediterranean diet between subjects with and without GDM
| Mean intake | Control subjects | GDM | P (two-tailed) |
|---|---|---|---|
| Vegetables (g/w) | 796.9 ± 365.7 | 584.2 ± 372.2 | 0.002 |
| Fruits (g/w) | 1129.5 ± 447.4 | 1321.7 ± 989.8 | NS |
| Legumes (g/w) | 63.2 ± 67.9 | 28.0 ± 38.8 | 0.001 |
| Cereals (g/w) | 2112.9 ± 625.1 | 2785.6 ± 373.9 | <10−3 |
| Fish (g/w) | 136.2 ± 128.0 | 49.9 ± 41.2 | <10−3 |
| MUFAs/SFAs | 2.3 ± 0.8 | 1.7 ± 0.7 | <10−3 |
| Meat (g/w) | 313.8 ± 125.9 | 342.4 ± 117.7 | NS |
| Dairy products (g/w) | 1540.7 ± 542.3 | 2793.5 ± 608.4 | <10−3 |
Data are means ± SD, Student’s t test
MUFAs: monounsaturated fatty acids
SFAs: saturated fatty acids
Figure 1 illustrates that a low score of adherence to the MedDiet was found in 38% of the control group and 47% of subjects with GDM. Adherence to the MedDiet was moderate in half of pregnant women of the control group and 37% of subjects with GDM. However, there was no significant difference concerning the adherence to the med Diet between the two groups. Means of fasting blood glucose and plasma glucose post load according to the MedDiet score are presented in Table 3. Our data shows that the higher the adherence score to the MedDiet, the lower the fasting blood glucose level and the plasma glucose 2 h post load. These findings concerned the two groups studied (P < 10−3). The pair wise comparison showed that mean fasting blood glucose was significantly higher in subjects who had low score to the Med diet comparing to those with a moderate (P < 0.05 for GDM and control groups) or high scores (P < 0.05 for GDM and control groups). Similarly, the average levels of plasma glucose 2 h post load were significantly higher in low adherence to the MedDiet compared to moderate and higher adherences for both groups. However, we found no differences in the averages of fasting blood glucose and glucose 2 h post load levels by comparing women with a moderate score with those having a high score in the two groups
Figure 1.
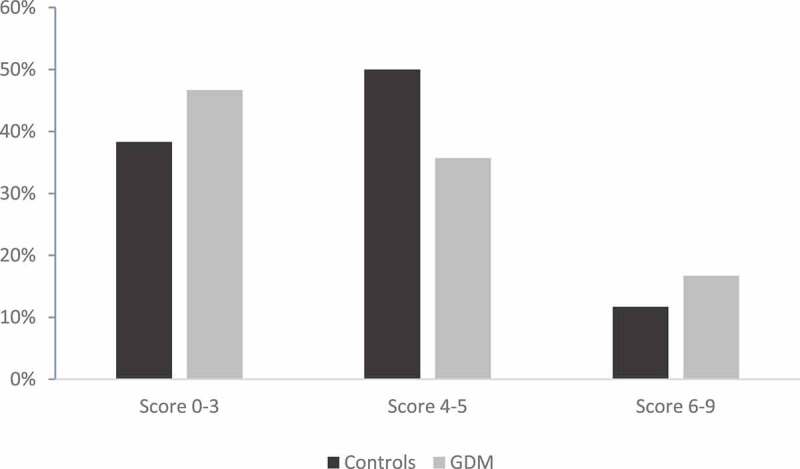
Distribution of the study population according to the mediterranean diet score
Table 3.
Means of fasting blood glucose and plasma glucose post load according to the Mediterranean diet score
| Gestational diabetes group | Parameters | Low score [0–3] |
Moderate score [4,5] |
High score [6–9] |
Pa |
|---|---|---|---|---|---|
| Fasting blood glucose (g/l) | 1.25 ± 0.30 b* | 1.08 ± 0.12d | 0.91 ± 0.12 c* | <10−3 | |
| Plasma glucose 2 h post load (g/l) |
1.76 ± 0.18 b* | 1.65 ± 0.10 d | 1.53 ± 0.12 c** | <10−3 | |
| Control group | Fasting blood glucose (g/l) |
0.88 ± 0.09 b* | 0.80 ± 0.06 d | 0.77 ± 0,09 c* | <10−3 |
| Plasma glucose 2 h post load (g/l) |
1.43 ± 0.15 b* | 1.11 ± 0.21 d | 1.04 ± 0.09 c** | <10−3 |
Data are means ± SD, a ANOVA Test
aPost hoc student – Newman Keuls Test for pair wise comparison. bComparison between low and moderate score. cComparison between low and high score. dComparison between moderate and high score.
*p < 0.05, **<10−3
Table 4 shows the reported dietary intake data of the two groups. The control subjects displayed a significantly higher fat intake than those with GDM (P = 0.007). However, women with GDM had a significantly higher intake of SFA, and lower consumptions of monosaturated fatty acid, n-6 fatty acid, eicosapentaenoic acid (EPA) and docosahexaenoic (DHA) compared to control subjects group. Furthermore, we found that vitamin D intake was lower in women with GDM than in controls (2.3 ± 2.1 µg/j vs 6.3 ± 3.3 µg/j, P < 10−3).
Table 4.
Comparison of nutritional data obtained by dietary history between subjects with and without GDM
| Control subjects | GDM | P (two-tailed) | |
|---|---|---|---|
| Total energy (kCal) | 2152 ± 791 | 2243.9 ± 803 | NS |
| Fat (% TEI) | 38.7 ± 6.5 | 35.5 ± 6.2 | 0.007 |
| Protein (%TEI) | 11.5 ± 3.1 | 12.6 ± 3.4 | NS |
| Carbohydrate (%TEIa) Sucrose (g/d) | 49.6 ± 7.443.5 ± 32.6 | 51.7 ± 6.966.57 ± 42.8 | NS0.001 |
| Fat (% total fat) Saturated Polyunsaturated Monounsaturated n-3 fatty acid (g/d)n-6 fatty acid (g/d)EPA(g/d)DHA(g/d) |
23.2 ± 6.427.1 ± 9.149.6 ± 8.31.5 ± 1.117.2 ± 8.070.2 ± 0.50.2 ± 0.6 | 30 ± 9.723.7 ± 10.846.1 ± 9.51 ± 0.514.1 ± 5.40.04 ± 0.10.06 ± 0.2 | <10−3NS0.03NS0.010.010.01 |
| Fiber (g/d)Magnesium(mg/d) Vitamin C(mg/d)Vitamin D (µg/d) | 22.1 ± 12288.1 ± 144.6126.6 ± 109.66.3 ± 3.3 | 23 ± 12.2286.1 ± 105.3192.8 ± 142.72.3 ± 2.1 | NSNS0.005<10−3 |
Data are means ± SD, Student’s t test
aTEI: total energy intake; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid
Multivariate logistic regression analysis of the relationship between nutrient intakes and the development of GDM are shown in Table 5. After adjustment for confounders, the only nutrient who remained significantly protective against GDM was vitamin D intake (OR 0.29 [0.15–0.54], P < 10−3).
Table 5.
Adjusteda associations of nutrient intake with the risk of gestational diabetes among the participants
| Daily intake | OR CI 95% | P |
|---|---|---|
| Energy (kCal) | 1.00 [0.99–1.00] | NS |
| Total fat (%TEI) | 0.98 [0.85–1.14] | NS |
| n-6 fatty acid (g/d) | 0.82 [0.66–1.01] | NS |
| n-3 fatty acid (g/d) | 1.77 [0.82–3.84] | NS |
| Cholesterol (mg/d) | 0.99 [0.99–1.00] | NS |
| Carbohydrates (%TEI) | 1.05 [0.97–1.10] | NS |
| Sucrose (g/d) | 1.00 [0.98–1.03] | NS |
| Protein (%TEI) | 1.25 [0.91–1.70] | NS |
| Fiber (g/d) | 1.07 [0.96–1.19] | NS |
| Magnesium (mg/d) | 0.99 [0.97–1.00] | NS |
| Vitamin C (mg/d) | 1.00 [0.99–1.01] | NS |
| Vitamin D (µg/d) | 0.29 [0.15–0.54] | <10−3 |
Logistic regression analysis
aCovariates included in the model were: maternal age, pre-pregnancy BMI, weight gain during pregnancy, previous gestational diabetes, family history of diabetes and physical activity.
TEI: total energy intake – CI: confidence intervals
5. Discussion
In the present study, there was no significant difference concerning the adherence to the MedDiet between the two groups. In contrary Zhang et al. in 2006 have shown that adherence to a diet rich in fruits, vegetables, poultry and fish was associated with reduction of GDM risk when compared to a diet high in red and processed meat [21]. Another study showed that adherence to the MedDiet was associated with a 24% lower risk of GDM [6]. An observational study reported that the incidence of gestational diabetes was lower among women with a high adherence to the MedDiet comparing them to those with low adherence [17]. A recent case control study reported that participants in the highest tertile of Med diet had 80% lower risk for GDM compared with those in the lowest tertile (P = 0.006) [22].
Our data shows that the higher the adherence score to the MedDiet, the lower the fasting blood glucose level and the plasma glucose 2 h post load in the two groups. These results are supported by several cross-sectional and interventional studies that emphasized reduction in fasting blood glucose and decrease in insulin resistance by the adoption of a MedDiet [23–26]. The study of Karamanos et al. showed that fasting plasma glucose and blood glucose an hour and two hours after a 75-g OGTT were negatively correlated with the score of the MedDiet (p < 0.001) [17]. Many components of The MedDiet can explain its benefits. This diet is rich in MUFAs due to the abundant use of olive oil for cooking or dressing salads, polyphenols, natural antioxidants, fiber and low in saturated fats and high glycemic index carbohydrates. Adherence to this diet may reduce GDM risk by improving systemic oxidative stress [27].
Our data showed that the daily intakes of fat, MUFA, n-6 fatty acids, EPA, DHA, P/S ratio and vitamin D had a protective effect against the development of gestational diabetes. In contrast, we found that daily intakes of SFA and sucrose significantly increased the risk of gestational diabetes. However, multivariate analysis showed that after adjustment for several covariates no nutrient was associated with risk of developing gestational diabetes, except vitamin D that kept its protective effect. Similar results were observed in a study which have shown that nutrients including lipids, carbohydrates, SFA, MUFA, PUFA, n-6 fatty acids, EPA, DHA, and fiber were not linked to GDM risk after adjustment for age, ethnicity, pre-pregnancy BMI, previous history of gestational diabetes, family history of type 2 diabetes and smoking during pregnancy [28]. In contrary, two case control studies showed that diet high in total fat and saturated fat and low in polyunsaturated fat was associated with the risk of GDM [11,12]. Saldana et al. in 2004 noted that higher total fat intake increases the risk of GDM, while carbohydrates effect was protective [29]. In a prospective cohort study, dietary glycemic load was positively related to GDM risk [10]. Controls consume significantly more vitamin D than cases. Insufficient intake of vitamin D can lead to hypovitaminosis D. Aljanahi et al. in 2020 showed that vitamin D dietary intake is higher among controls compared to GDM group [30]. Few studies have examined the association between spontaneous vitamin D intake and gestational diabetes risk. However, many trials have examined the link between hypovitaminosis D and gestational diabetes. Many studies reported that the rate of 25 (OH) D was significantly lower in women with gestational diabetes comparing them to normoglycemic subjects [31,32]. Recently a meta-analysis reported a significant association between vitamin D deficiency and an increased risk of GDM. The results of this study showed that women with vitamin D deficiency had a 26% greater risk of developing GDM than those with normal serum vitamin D concentrations (OR: 1.26; 95% CI: 1.13, 1.41) [33].
In conclusion, our study underlines the importance of adequate vitamin D intake during pregnancy and suggests that the MedDiet may reduce the incidence of gestational diabetes. However, further interventional studies will be needed to affirm this relationship.
Acknowledgments
The authors are indebted to all the subjects who volunteered in the study.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
References
- [1].Association AD. Classification and diagnosis of diabetes: standards of medical care in diabetes. Diabetes Care. 2020;43(1):S14–S31. [DOI] [PubMed] [Google Scholar]
- [2].Saeedi M, Cao Y, Fadl H, et al. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;172:108642. [DOI] [PubMed] [Google Scholar]
- [3].Behboudi-Gandevani S, Amiri M, Yarandi RB, et al. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetology & Metab Syndrome. 2019;11(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;51:47. PMID: 31296866. [DOI] [PubMed] [Google Scholar]
- [5].Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. [DOI] [PubMed] [Google Scholar]
- [6].Tobias DK, Zhang C, Chavarro J, et al. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr. 2012;96(2):289–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Donazar-Ezcurra M, López-Del Burgo C, Bes-Rastrollo M. Primary prevention of gestational diabetes mellitus through nutritional factors: a systematic review. BMC Pregnancy Childbirth. 2017;17(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mijatovic-Vukas J, Capling L, Cheng S, et al. Associations of diet and physical activity with risk for gestational diabetes mellitus: a systematic review and meta-analysis. Nutrients. 2018;10(6):698. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang Y, Storlien LH, Jenkins AB, et al. Dietary variables and glucose tolerance in pregnancy. Diabetes Care. 2000;23(4):460–464. [DOI] [PubMed] [Google Scholar]
- [10].Zhang C, Liu S, Solomon CG, et al. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care. 2006;29(10):2223–2230. [DOI] [PubMed] [Google Scholar]
- [11].Bo S, Menato G, Lezo A, et al. Dietary fat and gestational hyperglycaemia. Diabetologia. 2001;44(8):972–978. [DOI] [PubMed] [Google Scholar]
- [12].Moses RG, Shand JL, Tapsell LC. The recurrence of gestational diabetes: could dietary differences in fat intake be an explanation? Diabetes Care. 1997;20(11):1647–1650. [DOI] [PubMed] [Google Scholar]
- [13].Izadi V, Tehrani H, Haghighatdoost F, et al. Adherence to the DASH and mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition. 2016;32(10):1092–1096. [DOI] [PubMed] [Google Scholar]
- [14].Eckl MR, Brouwer-Brolsma EM, Küpers LK. Maternal adherence to the mediterranean diet during pregnancy: a review of commonly used a priori Indexes. Nutrients. 2021;13(2):582. 10. PMID: 33578689; PMCID: PMC7916386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Collège national des gynécologues et obstétriciens français et Société francophone du diabète . Recommandations pour la pratique clinique: le diabète gestationnel. J Gynecol Obstet Biol Reprod. 2010;39:S1–S342. [Google Scholar]
- [16].Truong YN, Yee LM, Caughey AB, et al. Weight gain in pregnancy: does the institute of medicine have it right? Am J Obstet Gynecol. 2015;212(3):362.e1–8. [DOI] [PubMed] [Google Scholar]
- [17].Karamanos B, Thanopoulou A, Anastasiou E, et al. Relation of the mediterranean diet with the incidence of gestational diabetes. Eur J Clin Nutr. 2014;68(1):8–13. [DOI] [PubMed] [Google Scholar]
- [18].Bouchoucha M et coll . Développement et validation d’un manuel tunisien de photos alimentaires comme un outil des enquêtes alimentaires. ESSTST/INNTA/SURVEN; 2015; pp. 45. http://www.surventunisie.rns.tn/images/2015/Annexe%203_2015/2.pdf
- [19].Scarmeas N, Stern Y, Tang M-X, et al. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, et al. Diet and overall survival in the elderly. BMJ. 1995;311(7018):1457–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang C, Schulze MB, Solomon CG, et al. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia. 2006;49(11):2604–2613. [DOI] [PubMed] [Google Scholar]
- [22].Hamer M, Chida Y. Intake of fruit, vegetables and antioxidants and risk of type 2 diabetes; systemic review and meta-analysis. J Hypertens. 2007;25(12):2361–2369. [DOI] [PubMed] [Google Scholar]
- [23].Estruch R, Martínez-González MA, Corella D, et al. PREDIMED Study Investigators. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1–11. [DOI] [PubMed] [Google Scholar]
- [24].Panagiotakos DB, Tzima N, Pitsavos C, et al. The association between adherence to the Mediterranean diet and fasting indices of glucose homoeostasis: the ATTICA Study. J Am Coll Nutr. 2007;26(1):32–38. [DOI] [PubMed] [Google Scholar]
- [25].Vincent-Baudry S, Defoort C, Gerber M, et al. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82(5):964–971. [DOI] [PubMed] [Google Scholar]
- [26].Tzima N, Pitsavos C, Panagiotakos DB, et al. Mediterranean diet and insulin sensitivity, lipid profile and blood pressure levels, in overweight and obese people; the Attica study. Lipids Health Dis. 2007;6(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yubero-Serrano EM, Fernandez-Gandara C, Garcia-Rios A, et al. Mediterranean diet and endothelial function in patients with coronary heart disease: an analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 2020;17(9):e1003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dempsey JC, Butler CL, Sorensen TK, et al. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2004;66(2):203–215. [DOI] [PubMed] [Google Scholar]
- [29].Saldana TM, Siega-Riz AM, Adair LS. Effect of macronutrient intake on the development of glucose intolerance during pregnancy. Am J Clin Nutr. 2004;79(3):479–486. [DOI] [PubMed] [Google Scholar]
- [30].Aljanahi A, Hadhiah H, Al-Nasr W, et al. The effect of dietary intake of vitamin d on gestational diabetes mellitus. Nutr Metab Insights. 2020;13:1178638820932164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Soheilykhah S, Mojibian M, Rashidi M, et al. Maternal vitamin D status in gestational diabetes mellitus. Nutr Clin Pract. 2010;25(5):524–527. [DOI] [PubMed] [Google Scholar]
- [32].Poel YH, Hummel P, Lips P, et al. D and gestational diabetes: a systemic review and meta-analysis. Eur J Intern Med. 2012;23(5):465–469. [DOI] [PubMed] [Google Scholar]
- [33].Milajerdi A, Abbasi F, Mousavi SM, et al. Maternal vitamin D status and risk of gestational diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Clin Nutr. 2021. 2April;40(5):2576–2586. In Press. [DOI] [PubMed] [Google Scholar]


