Abstract
Objectives
To determine the incidence of invasive Group B streptococcal (iGBS) diseases and the factors significantly associated with iGBS mortality in adult patients.
Material and methods
This retrospective study included adults with a positive culture for GBS isolated from a sterile site at Siriraj Hospital – Thailand’s largest tertiary care hospital – during January 2013 to December 2017.
Results
Of the 224 included patients, 75.9% had bacteraemia. The median age of patients was 63 years (interquartile range [IQR]: 53–73) and 52.7% were female. Among the 80% of all patients with comorbid diseases, diabetes mellitus (38.8%), cancer (18.8%), and heart disease (12.5%) were the most common. Skin and soft tissue infection (30.8%), septic arthritis (21.4%), primary bacteraemia (21.0%), and meningitis (7.1%) were the most common manifestations of iGBS diseases. The overall 30-day mortality was 11%. Patients that died were older and had more chronic kidney disease, bacteraemia, urinary tract infection, pneumonia, and iGBS-related morbidities than survivors. Pneumonia was the only factor independently associated with 30-day mortality with an adjusted odds ratio of 24.96 (95% confidence interval [CI]: 5.95–104.75).
Conclusions
Invasive GBS is not uncommon in non-pregnant adults, particularly among older adults and those with diabetes. Concomitant bacteraemia was frequently observed in iGBS patients. The overall mortality was low, but significant morbidities were observed.
KEY MESSAGES
In our study, iGBS was not uncommon among older adults and those with diabetes.
Two-thirds of patients with iGBS had bacteraemia, and the overall 30-day mortality was 11%.
Keywords: Streptococcus agalactiae, Group B Streptococcus, epidemiology, mortality
Introduction
Streptococcus agalactiae, which is commonly referred to as Group B Streptococcus (GBS), remains the leading cause of neonatal sepsis and postpartum infection in both high and low-income countries [1–3]. Over the last 2 decades, GBS has also emerged as an important cause of invasive disease in nonpregnant adults [4–7]. The increase in the incidence of invasive GBS (iGBS) disease was largest among older adult patients and those with underlying medical conditions, particularly diabetes mellitus [7]. The clinical spectrum of iGBS disease varies substantially, ranging from mild to severe life-threatening illness depending on host factors and the site and extent of the infection [3,4,7]. Sequence type (ST) 283 was found to be more commonly associated with meningoencephalitis, septic arthritis, and spinal infection in a large outbreak in Singapore [8]. The mortality rate also varies widely with a reported range of 8.9–19% [3,9,10]. Previous studies of iGBS in adults mainly focussed on pregnant women or were reported from high-income countries [11–13]. Studies of iGBS disease in non-pregnant adults from low-to-middle-income countries (LMICs) are scarce [3,10]. Improved understanding of the current epidemiology of iGBS disease and the factors associated with iGBS mortality will improve prevention, treatment, and outcomes.
Materials and methods
Study design and population
This retrospective cohort study was conducted at Siriraj Hospital (Bangkok, Thailand), which is Thailand’s largest university-based national tertiary referral centre. Patients aged 18 years or older who had positive culture for GBS from sterile sites from 1 January 2013 to 31 December 2017 were identified and included. This study aimed to determine the incidence of and mortality in iGBS diseases, and the factors significantly associated with iGBS mortality in adult patients. The protocol for this study was approved by the Siriraj Institutional Review Board (SIRB) of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (COA number 500/2560), and the requirement to obtain written informed consent was waived due to the anonymous retrospective design of this study.
Data collection
Demographic data, clinical manifestations, and outcomes were retrieved from medical records. Invasive GBS infection was defined as isolation of GBS from a normally sterile site, such as blood, cerebrospinal fluid, synovial fluid, vitreous fluid, or operative samples of pus or tissue obtained from a site of focal suppuration, accompanied by symptoms and signs of clinical disease. Patients with skin and soft tissue infection (SSTI), such as non-necrotizing bacterial cellulitis, necrotizing fasciitis, pyomyositis, and diabetic foot ulcers caused by GBS, were also included. Diagnosis of SSTI was based on the presence of local or systemic signs and symptoms of inflammation. Diagnosis of osteomyelitis was made when imaging showed characteristic features, including loss of bone cortex with bony erosion or demineralization, focal loss of trabecular pattern or marrow radiolucency, periosteal reaction or bone sclerosis, and/or presence of sinus tract formation and/or sequestrum [14]. Community-acquired infection was defined as GBS infections occurring within 48 h after hospital admission. Bacteraemia was defined as isolation of GBS from one or more blood cultures. Primary bacteraemia was defined as bacteraemia of unidentified origin with no associated clinical disease at other organs. Secondary bacteraemia was defined as bacteraemia accompanied by positive culture from other sites or associated clinical disease at other organs. Blood samples were processed using the BACTEC or BacT/Alert system (bioMérieux SA, Marcy l’Etoile, France). Positive blood samples were then further subcultured on blood agar. The presence of beta-hemolysis of bacterial colonies on blood agar heightened the suspicion of GBS. GBS was then identified by Gram staining showing Gram-positive cocci in the chain, with negative PYR, positive CAMP test, and negative bile-esculin test. Lancefield grouping (serogroup B) was used to confirm the diagnosis. Islam’s Medium, which is a selective GBS medium, was also used as described in the literature [15]. In patients from whom multiple or repeated specimens were obtained for culture, we included only one in our analysis.
Sample size calculation and statistical analysis
Using a previously reported prevalence of invasive GBS of 44% [3], we calculated the sample size required to estimate proportion with a precision of 15% and a type 1 error of 5%. Using nQuery Advisor sample size software (Statistical Solutions, Ltd., Cork Ireland), the sample size required for estimating the incidence of invasive GBS was 220 patients.
Descriptive statistics were used to characterize the study population. Categorical variables are presented as numbers and percentages, and continuous variables are presented as the median and interquartile range (IQR). The clinical characteristics between patients with and without bacteraemia, and between those who survived and those who died were compared using Chi-square test, Fisher’s exact test, Student’s t-test, or Mann–Whitney U test, as appropriate. The factors significantly associated with 30-day mortality were identified. Variables with a p-value less than .1 in univariate analysis was entered into a multivariate logistic regression model after assessment for multicollinearity of variance inflation. A p-value less than .05 was considered statistically significant for all other tests. All statistical analyses were performed using SPSS Statistics version 20 (SPSS, Inc., Chicago, IL, USA).
Results
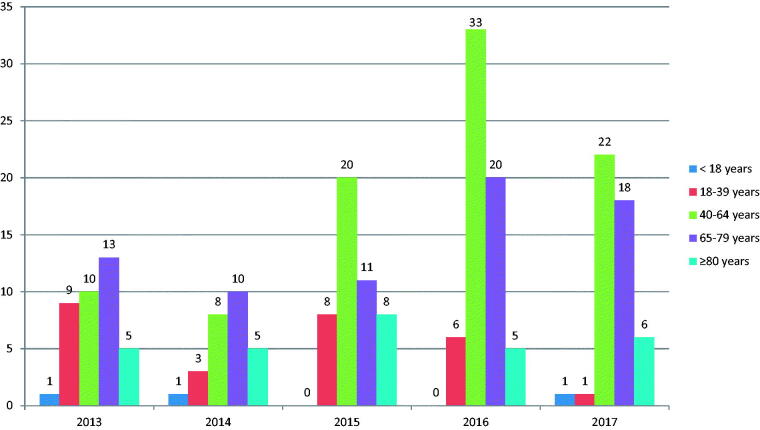
A total of 224 patients were identified during the 5-year study period. Three patients aged less than 18 years were also included in the study (a 16-year-old pregnant woman with chorioamnionitis, a 17-year-old with meningitis, and a 17-year-old with iliopsoas abscess). The median age of patients was 63 years (IQR: 53.3–73.7), and 52.7% were female. Patients aged 65 years or older accounted for 45% of all patients. The number of cases stratified by age category for each year from 2013 to 2017 seemed to increase (Figure 1), whereas the number of patients discharged per year from 2013-2017 was stable, as follows: 83,747 cases in 2013, 82,302 cases in 2014, 82,433 cases in 2015, 82,613 cases in 2016, and 83,578 cases in 2017. Almost 80% of patients had underlying medical conditions. Only 4 patients (1.8%) were pregnant. Among 224 patients, 182 patients (81.2%) were hospitalized, and most of those had community-acquired infection (176 cases: 96.7%). Polymicrobial infection was found in 45 patients (20%), of which 26 and 11 patients had SSTIs and osteomyelitis, respectively. The four most common comorbidities were diabetes mellitus (38.8%), solid malignancy (18.8%), heart disease (12.5%), and chronic kidney disease (CKD) (11.2%). SSTI was the most common (30.8%) presentation, followed by septic arthritis (21.4%), primary bacteraemia (21.0%), meningitis (7.1%), and pneumonia (6.2%).
Figure 1.
Number of cases by age category for each year from 2013 to 2017.
Demographic and clinical characteristics of patients compared between those with and without GBS bacteraemia are shown in Table 1. Among the 170 patients (75.9%) who had bacteraemia, 47 (21.0%) had bacteraemia without identified focus. Patients with GBS bacteraemia were more likely to have solid malignancy, heart disease, or neurological disorder than those without bacteraemia. All patients with meningitis and pneumonia had GBS isolated from blood. Patients with bacteraemia had higher mortality (14.4% vs. 1.9%, p = .011) and morbidities (48.2% vs. 11.1%, p < .001) than those without bacteraemia. Patients without bacteraemia commonly presented with SSTIs and osteomyelitis. Polymicrobial infections were found more frequently among those without bacteraemia (46.3% vs. 11.8%, p < .001). Comparing by gender, male patients had more osteomyelitis (9.4% vs. 2.5%, p = .028) and mycotic aneurysm (3.8% vs. 0%, p = .049) than female patients. The median duration of treatment was 14 days (IQR: 14–42).
Table 1.
Demographic and clinical characteristics of patients with invasive Group B Streptococcus (iGBS) diseases compared between those with and without bacteraemia.
| Characteristics | All patients | Bacteraemia | No bacteraemia | p-Value |
|---|---|---|---|---|
| (n = 224) | (n = 170) | (n = 54) | ||
| Age (years) | 63 (53.3–73.7) | 64.5 (52–75) | 59.5 (54–67) | .778 |
| Age ≥65 years | 101 (45.1%) | 85 (50.0%) | 16 (29.6%) | .009 |
| Female gender | 118 (52.7%) | 93 (54.7%) | 25 (46.3%) | .281 |
| Pregnant/postpartum within 30 days | 4 (1.8%) | 4 (4.3%) | 0 (0.0%) | .737 |
| Comorbid disease | 178 (79.5%) | 139 (81.8%) | 39 (72.2%) | .130 |
| Diabetes mellitus | 87 (38.8%) | 61 (35.9%) | 26 (48.1%) | .107 |
| Solid malignancy | 42 (18.8%) | 38 (22.4%) | 4 (7.4%) | .014 |
| Heart disease | 28 (12.5%) | 26 (15.3%) | 2 (3.7%) | .025 |
| Chronic kidney disease | 25 (11.2%) | 22 (12.9%) | 3 (5.6%) | .133 |
| Chronic liver disease | 19 (8.5%) | 17 (10.0%) | 2 (3.7%) | .259 |
| Neurological disorder | 15 (6.7%) | 15 (8.8%) | 0 (0.0%) | .025 |
| Chronic lung disease | 6 (2.7%) | 5 (2.9%) | 1 (1.9%) | 1.000 |
| Immunosuppressive agent | 5 (2.2%) | 5 (2.9%) | 0 (0.0%) | .34 |
| Autoimmune disease | 3 (1.3%) | 2 (1.2%) | 1 (1.9%) | .565 |
| Hematologic malignancy | 2 (0.9%) | 2 (1.2%) | 0 (0.0%) | 1.000 |
| Others | 75 (33.4%) | 17 (10.0) | 0 (0.0%) | |
| Clinical manifestationsa | ||||
| Skin and soft tissue infection | 69 (30.8%) | 35 (20.6%) | 34 (63.0%) | <.001 |
| Septic arthritis | 48 (21.4%) | 36 (21.2%) | 12 (22.2%) | .87 |
| Bacteraemia without focus | 47 (21.0%) | 47 (27.6%) | 0 (0.0%) | <.001 |
| Meningitis | 16 (7.1%) | 16 (9.4%) | 0 (0.0%) | .014 |
| Pneumonia | 14 (6.2%) | 14 (8.2%) | 0 (0.0%) | .025 |
| Osteomyelitis | 13 (5.8%) | 2 (1.2%) | 11 (20.4%) | <.001 |
| Infective endocarditis | 9 (4.0%) | 9 (5.3%) | 0 (0.0%) | .118 |
| Eye infection | 2 (0.9%) | 2 (1.2%) | 0 (0.0%) | 1.000 |
| Urinary tract infection | 2 (0.9%) | 2 (1.2%) | 0 (0.0%) | 1.000 |
| Endometritis | 1 (0.4%) | 1 (0.6%) | 0 (0.0%) | 1.000 |
| Chorioamnionitis | 1 (0.4%) | 1 (0.6%) | 0 (0.0%) | 1.000 |
| Others | 25 (11.1%) | 25 (14.7%) | 0 (0.0%) | .994 |
| Polymicrobial infections | 45 (20.1%) | 20 (11.8%) | 25 (46.3%) | <.001 |
| Duration of treatment (days)b | 14 (14–42) | 14 (10–28.7) | 55 (7–405) | <.001 |
| Length of hospital stay among hospital inpatients (days)c | 17 (1–344) | 14 (2–344) | 15.5 (1–118) | .846 |
| Morbidities | 88 (39.3%) | 82 (48.2%) | 6 (11.1%) | <.001 |
| Mortalityc | 23 (11.2%) | 22 (14.4%) | 1 (1.9%) | .011 |
Data reported as median and interquartile range (IQR) or number and percentage.
Bold p-values indicate statistical significance.
aSome patients had more than one clinical syndrome.
bExcluded 16 cases: 14 referral cases (12 cases in the bacteraemia group, and 2 cases in the no bacteraemia group) and 2 cases against advice.
cCalculated from 206 patients (excluded 18 cases: 15 referral cases and 3 against advice, of which 17 were in the bacteraemia group and 1 was in the no bacteraemia group).
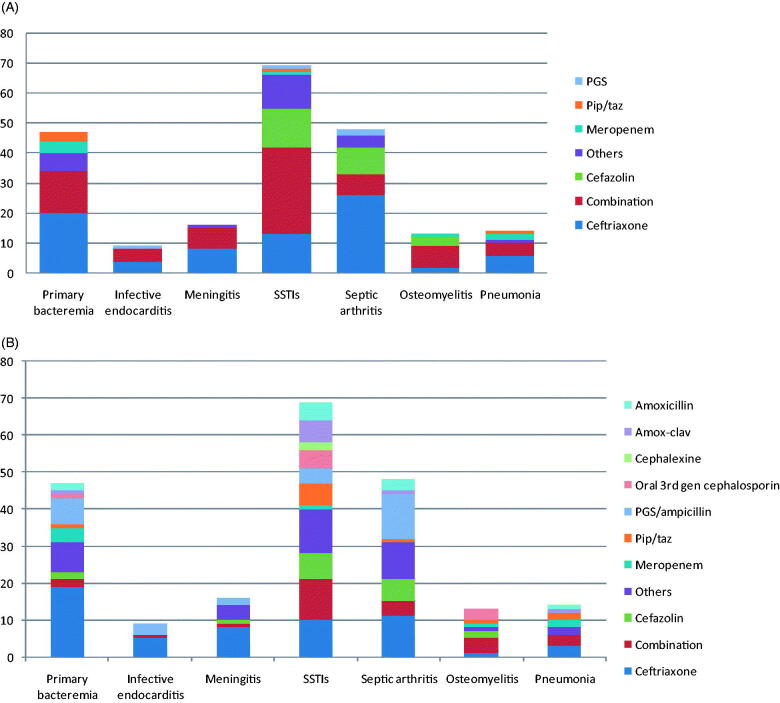
Among the 216 patients with 1 of 7 main clinical syndromes, including primary bacteraemia, infective endocarditis (IE), meningitis, SSTIs, septic arthritis, osteomyelitis, and pneumonia, ceftriaxone was the most commonly prescribed empirical antibiotic for most clinical presentations (excluding SSTIs and osteomyelitis) (79 patients, 36.5%), followed by combination antibiotic therapy (including multiple regimens) (72 patients, 33.3%) (e.g. ceftriaxone with clindamycin or metronidazole) and cefazolin (25 patients, 11.6%). The most common definite antibiotics included ceftriaxone (57 patients, 26.4%), Penicillin G Sodium (PGS)/ampicillin (28 patients, 12.9%), combination antibiotics with multiple regimens (26 patients, 12%), and cefazolin (18 patients, 8.3%). Empirical and definitive antibiotic therapies categorized by clinical presentation are shown in Figure 2(A,B), respectively.
Figure 2.
(A) Empirical antibiotic therapy and (B) definite antibiotic therapy of invasive Group B Streptococcus (iGBS) disease categorised by clinical manifestation. SSTIs: skin and soft tissue infections; Amox-clav: Amoxicillin/clavulanic acid; PGS: Penicillin G Sodium; Pip/taz: Piperacillin/tazobactam.
The overall 30-day mortality was 11%. Morbidities, including shock, renal failure, and respiratory failure, were found in 39.3% of all patients. Demographic and clinical characteristics compared between those who survived and those who died from iGBS are shown in Table 2. Patients who died were significantly older (76 vs. 63 years, p = 0.028); significantly more likely to have CKD (26.1% vs. 9.8%, p = 0.022), pneumonia (39.1% vs. 2.2%, p < 0.001), urinary tract infection (4.3% vs. 0.0%, p = 0.005), or bacteraemia (95.7%vs.71.6%, p = 0.013); and, had significantly higher morbidities (100%vs. 37.1%, p < 0.001) than those who survived.
Table 2.
Demographic and clinical characteristics compared between patients that survived and that died within 30 days (n = 206a).
| Characteristics | Survived | Deceased | p-Value |
|---|---|---|---|
| (n = 183) | (n = 23) | ||
| Age (years) | 63 (54–72) | 76 (61–82) | .028 |
| Age ≥65 years | 79 (43.2%) | 17 (73.9%) | .006 |
| Female gender | 101 (55.2%) | 9 (39.1%) | .146 |
| Comorbid disease | 145 (79.2%) | 20 (87.0%) | .008 |
| Diabetes mellitus | 73 (39.9%) | 10 (43.5%) | .741 |
| Solid malignancy | 34 (18.6%) | 7 (30.4%) | .180 |
| Heart disease | 26 (14.2%) | 0 (0.0%) | .053 |
| Chronic kidney disease | 18 (9.8%) | 6 (26.1%) | .022 |
| Chronic liver disease | 15 (8.2%) | 3 (13.0%) | .438 |
| Neurological disorder | 11 (6.0%) | 2 (8.7%) | .618 |
| Chronic lung disease | 6 (3.3%) | 0 (0.0%) | .378 |
| Immunosuppressive agent | 3 (1.6%) | 1 (4.3%) | .375 |
| Autoimmune disease | 3 (1.6%) | 0 (0.0%) | .536 |
| Hematologic malignancy | 1 (0.5%) | 1 (4.3%) | .080 |
| Bedridden state | 7 (3.8%) | 3 (13.0%) | .053 |
| Bacteraemia | 131 (71.6%) | 22 (95.7%) | .013 |
| Clinical manifestations | |||
| Skin and soft tissue infection | 61 (33.3%) | 3 (13.0%) | .047 |
| Primary bacteraemia | 36 (19.7%) | 7 (30.4%) | .231 |
| Septic arthritis | 43 (23.5%) | 2 (8.7%) | .105 |
| Meningitis | 10 (5.5%) | 3 (13.0%) | .159 |
| Pneumonia | 4 (2.2%) | 9 (39.1%) | <.001 |
| Osteomyelitis | 13 (7.1%) | 0 (0.0%) | .187 |
| Infective endocarditis | 9 (4.9%) | 0 (0.0%) | .277 |
| Eye infection | 2 (1.1%) | 0 (0.0%) | .614 |
| Urinary tract infection | 0 (0.0%) | 1 (4.3%) | .005 |
| Endometritis | 1 (0.5%) | 0 (0.0%) | .722 |
| Chorioamnionitis | 1 (0.5%) | 0 (0.0%) | .722 |
| Polymicrobial infections | 35 (19.1%) | 7 (30.4%) | .205 |
| Morbidities | 57 (31.1%) | 23 (100%) | <.001 |
| Shock | 17 (9.3%) | 13 (56.5%) | |
| Renal failure | 17 (9.3%) | 4 (17.4%) | |
| Respiratory failure | 5 (2.7%) | 4 (17.4%) | |
| Others | 18 (9.8%) | 2 (20.9%) | |
| Duration of treatment (days) | 21 (14–44) | 7 (1–14) | .015 |
Data reported as median and interquartile range (IQR) or number and percentage.
Bold p-values indicate statistical significance.
aA total of 206 patients were included in this analysis because 18 patients were excluded (15 were referred and 3 against advice).
Mulivariate analysis for factors independently associated with 30-day mortality is shown in Table 3. Pneumonia was identified as the only independent predictor of 30-day mortality with an adjusted odds ratio (aOR) of 24.96 (95% confidence interval [CI]: 5.95-104.75). Further analysis excluding polymicrobial infection revealed very similar results with a slightly lower mortality rate of 9.8%. When comparing between the 148 patients who survived and the 16 patients who died, the patients who died were more likely to be aged older than 65 years (75% vs. 45.3%, p = .024), more likely to have CKD (31.2% vs. 6.8%, p = .008) or pneumonia (25% vs. 2.7%, p = .003), and had higher morbidities (100% vs. 34.5%, p < .001) than those who survived.CKD and pneumonia were identified as the independent predictors of 30-day mortality with an adjusted odds ratio (aOR) of 4.60 (95% CI: 1.82–17.91) and 9.06 (95% CI: 1.78–46.21), respectively.
Table 3.
Analysis for factors independently associated with 30-day mortality.
| Factors | Crude OR (95% CI) | p-Value | Adjusted ORa (95% CI) | p-Value |
|---|---|---|---|---|
| Pneumonia | 28.768 (7.86–105.28) | <.001 | 24.96 (5.95–104.75) | <.001 |
| Bacteraemia | 8.733 (1.15–66.47) | .036 | 3.66 (0.43–30.92) | .234 |
| CKD | 3.235 (1.13–9.25) | .028 | 2.79 (0.79–9.85) | .111 |
| Age | 1.035 (1.00–1.07) | .030 | 1.030 (0.99–1.07) | .100 |
| SSTIs | 0.3 (0.09–1.05) | .059 | 0.706 (0.18–2.80) | .621 |
Bold p-values indicate statistical significance.
aAnalysis adjusted for all factors listed in the table.
OR: odds ratio; CI: confidence interval; CKD: chronic kidney disease; SSTIs: skin and soft tissue infections.
Discussion
Invasive GBS diseases among nonpregnant adults are being increasingly reported worldwide [3–7,10]. These diseases occur more frequently among older adults [3,7,10,16]. The increase in iGBS disease was more pronounced among patients aged 40 to 79 years in our study, which is similar to the finding from a previous study in the United States [6]. The higher incidence in this age group may be due to the higher prevalence of pre-existing comorbidities, which increase the susceptibility to or severity of GBS infections. Almost 80% of our patients had at least one underlying condition, of which diabetes and cancer were the most common, which is consistent with the findings of several prior studies [6,9,11,12,17]. The incidence of iGBS in pregnant women was very low in this study. This finding may be explained by the low rate of GBS colonization (11%) in pregnant Thai women [18]. Polymicrobial infection was found in approximately 20% of patients in our study, which is similar to reported findings from Latin America [9]. We also found that polymicrobial infection occurs mainly in those with osteomyelitis or SSTIs, such as diabetic foot or wet gangrene.
Bacteraemia with or without focus was common in patients with iGBS disease, accounting for 76% of patients in our study. We found a higher rate of bacteraemia than the rates reported from prior studies that varied from 36% to 60% [3,9,17], and most of those were secondary bacteraemia (72.4%). Among those with identified sources, bacteraemia was found in approximately 50% of patients with SSTIs, in 75% of those with septic arthritis, and in 100% of those with meningitis, pneumonia, or infective endocarditis. Primary bacteraemia was found in only 21% of our patients, which is considerably lower than the 32% to 35% rates reported from previous studies [3,6]. This difference among studies is likely due to how we defined secondary bacteraemia since we classified those who initially presented with abnormal symptoms and signs of organ involvement, such as septic arthritis, meningitis, or infective endocarditis accompanied with bacteraemia, as secondary to an identified source. Nevertheless, it is not easy in routine clinical practice to determine whether organ involvement is an original source or metatstatic foci. Patients without bacteraemia received a longer duration of antibiotic treatment than those with bacteraemia. This finding may be explained by the higher proportion of patients with SSTIs with or without osteomyelitis in the non-bacteraemia group that required a longer duration of antimicrobial therapy (2–6 weeks) for diabetic foot infection or acute osteomyelitis [19,20].
Ceftriaxone was the most often used antibiotic for both empirical and definitive treatment for all clinical syndromes, except SSTIs and osteomyelitis. Even though resistance to erythromycin and clindamycin has been increasingly reported over time [6,21], GBS remains uniformly susceptible to penicillin in vitro. Ceftriaxone was the most selected empiric and definitive treatment probably due to its convenience and its positive impact on patient compliance.
Overall 30-day mortality in our study was 11%, which is quite similar to the rates reported from previous studies over the past decade [3,9–12]. Although we found multiple factors to be associated with 30-day mortality in univariate analysis, only pneumonia was found to be independently associated with mortality, which is consistent with the finding from a study among military veterans in the United States [22]. To our knowledge, this is the largest study of invasive GBS diseases in Thailand. The strength of this study includes our recruitment of patients over a 5-year period, which facilitated observation of an increase in iGBS in various age groups. Furthermore, our study was performed in a university hospital where extensive diagnostic testing is performed, information on clinical manifestation is collected and recorded, and comprehensive management is routinely provided. This study also has some mentionable limitations. First, its retrospective design renders it vulnerable to missing and/or incomplete data. Second, the antibiotic susceptibility test of GBS was not performed. This is because routine testing for penicillin or ampicillin resistance is not currently recommended since GBS is rarely resistant to penicillins. Third and last, the fact that our data were derived from a single centre could limit the generalisability of our findings to other hospitals or healthcare settings.
Conclusions
Invasive GBS is not uncommon in non-pregnant adults, particularly among older adults and those with diabetes mellitus. Concomitant bacteraemia was frequently observed in iGBS patients. The overall mortality was low, but significant morbidities were observed.
Acknowledgements
The authors gratefully acknowledge Ms. Khemajira Karaketklang of the Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand for assistance with statistical analysis.
Funding Statement
NA and PP each received a Siriraj Chalermphrakiat Grant from the Faculty of Medicine Siriraj Hospital, Mahidol University. The funder had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding upon reasonable request.
References
- 1.Le Doare K, Heath PT.. An overview of global GBS epidemiology. Vaccine. 2013;31:D7–D12. [DOI] [PubMed] [Google Scholar]
- 2.Medugu N, Iregbu K, Iroh Tam PY, et al. Aetiology of neonatal sepsis in Nigeria, and relevance of group B Streptococcus: a systematic review. PLoS One. 2018;13(7):e0200350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaiwarith R, Jullaket W, Bunchoo M, et al. Streptococcus agalactiae in adults at Chiang Mai University Hospital: a retrospective study. BMC Infect Dis. 2011;11:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhhazmi A, Hurteau D, Tyrrell GJ.. Epidemiology of invasive group B Streptococcal disease in Alberta, Canada, from 2003 to 2013. J Clin Microbiol. 2016;54(7):1774–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farley MM. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis. 2001;33(4):556–561. [DOI] [PubMed] [Google Scholar]
- 6.Francois Watkins LK, McGee L, Schrag SJ, et al. Epidemiology of invasive group B Streptococcal infections among nonpregnant adults in the United States, 2008-2016. JAMA Intern Med. 2019;179(4):479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skoff TH, Farley MM, Petit S, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990-2007. Clin Infect Dis. 2009;49(1):85–92. [DOI] [PubMed] [Google Scholar]
- 8.Kalimuddin S, Chen SL, Lim CTK, et al. 2015 epidemic of severe Streptococcus agalactiae sequence type 283 infections in Singapore associated with the consumption of raw freshwater fish: a detailed analysis of clinical, epidemiological, and bacterial sequencing data. Clin Infect Dis. 2017;64(2):S145–S152. [DOI] [PubMed] [Google Scholar]
- 9.Crespo-Ortiz MdP, Castañeda-Ramirez CR, Recalde-Bolaños M, et al. Emerging trends in invasive and noninvasive isolates of Streptococcus agalactiae in a Latin American hospital: a 17-year study. BMC Infect Dis. 2014;14:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunyasontigul K, Chongtrakool P, Santanirand P, et al. High morbidity associated with invasive group B Streptococcal disease among nonpregnant adults in Thailand, 1999 to 2009. J Infect Dis Antimicrob Agents. 2011;28:169–177. [Google Scholar]
- 11.Collin SM, Shetty N, Lamagni T.. Invasive group B Streptococcus infections in adults, England, 2015-2016. Emerg Infect Dis. 2020;26(6):1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graux E, Hites M, Martiny D, et al. Invasive group B Streptococcus among non-pregnant adults in Brussels-Capital Region, 2005-2019. Eur J Clin Microbiol Infect Dis. 2021;40(3):515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vuillemin X, Hays C, Plainvert C, et al. Invasive group B Streptococcus infections in non-pregnant adults: a retrospective study, France, 2007-2019. Clin Microbiol Infect. 2021;27(1):129.e1–129.e4. [DOI] [PubMed] [Google Scholar]
- 14.Lipsky BA, Senneville E, Abbas ZG, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(1):e3280. [DOI] [PubMed] [Google Scholar]
- 15.Islam AKM. Rapid recognition of group-B Streptococci. The Lancet. 1977;309(8005):256–257. [DOI] [PubMed] [Google Scholar]
- 16.Camuset G, Picot S, Jaubert J, et al. Invasive group B Streptococcal disease in non-pregnant adults, Reunion Island, 2011. Int J Infect Dis. 2015;35:46–50. [DOI] [PubMed] [Google Scholar]
- 17.Matsubara K, Yamamoto G.. Invasive group B streptococcal infections in a tertiary care hospital between 1998 and 2007 in Japan. Int J Infect Dis. 2009;13(6):679–684. [DOI] [PubMed] [Google Scholar]
- 18.Akkaneesermsaeng W, Petpichetchian C, Yingkachorn M, et al. Prevalence and risk factors of group B Streptococcus colonisation in intrapartum women: a cross-sectional study. J Obstet Gynaecol. 2019;39(8):1093–1097. [DOI] [PubMed] [Google Scholar]
- 19.Lew DP, Waldvogel FA.. Osteomyelitis. Lancet. 2004;364(9431):369–379. [DOI] [PubMed] [Google Scholar]
- 20.Lipsky BA, Berendt AR, Cornia PB, et al. 2012 infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Li S, Li L, et al. Alarming regional differences in prevalence and antimicrobial susceptibility of group B streptococci in pregnant women: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2016;7:169–177. [DOI] [PubMed] [Google Scholar]
- 22.Jump RLP, Wilson BM, Baechle D, et al. Risk factors and mortality rates associated with invasive group B Streptococcus infections among patients in the US Veterans Health Administration. JAMA Netw Open. 2019;2(12):e1918324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding upon reasonable request.