ABSTRACT:
Atrial fibrillation (AF) is one of the main cardiac arrhythmias associated with higher risk of cardiovascular morbidity and mortality. AF can cause adverse symptoms and reduced quality of life. One of the strategies for the management of AF is rate control, which can modulate ventricle rate, alleviate adverse associated symptoms and improve the quality of life. As primary management of AF through rate control or rhythm is a topic under debate, the purpose of this review is to explore the rationale for the rate control approach in managing AF by considering the guidelines, recommendations and determinants for the choice of rate control drugs, including beta blockers, digoxin and non- dihydropyridine calcium channel blockers for patients with AF and other comorbidities and atrioventricular nodal ablation and pacing. Despite the limitations of rate control treatment, which may not be effective in preventing disease progression or in reducing symptoms in highly symptomatic patients, it is widely used for almost all patients with atrial fibrillation. Although rate control is one of the first line management of all patient with atrial fibrillation, several issues remain debateable.
Keywords: Atrial fibrillation, Rate control strategy, Electrophysiology, arrhythmias
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in adults [1]. It can increase the risk of stroke, heart failure (HF) and mortality [2]. The risk of developing AF increases progressively with age [3], from a prevalence of 0.1% in individuals under 55 years of age to 9.0% in individuals 80 years and older [4], and in adults, the current prevalence of AF is 2–4% [1], with estimated rise of 2.3 folds [5] due to extended longevity in general population [6]. The exact reasons for why age and certain medical disorders increase the risk of AF are not yet fully understood [7]. Although age is an independent risk factor for adverse AF outcomes [3], the increased burden of comorbidities (i.e. coronary heart disease, heart failure, diabetes mellitus, hypertension and obstructive sleep apnoea) are significant contributors to the development and progression of AF, as well as significant modifiable risk factors [8–10].It is likely that structural (e.g. dilatation and fibrosis) and electrophysiological changes of atrial myocytes, likely disturb the electrical substrate which causes AF [6],although the mechanisms vary among patients with AF. The main aim of AF treatment is to simply follow the ABC pathway, avoid strokes, better control symptoms, and reduce cardiovascular and comorbidity risks [2]. Another important aim is to recognize and manage the associated factors, whether acute (e.g. as a result of cardiac surgery and inflammatory diseases) or chronic (e.g. as a result of heart valve stenosis, coronary artery disease, hypertension or obesity) [11]. Rate control and rhythm control treatments are the two main strategies currently in use. The rate control is defined as use of any combination of β‐blocker, non- dihydropyridine calcium channel blocker, and digoxin or AV nodal ablation without aiming to restore normal sinus rhythm. Rate control is an essential part of AF management. The goal of rate control treatment is to modulate the ventricular rate, with the aim of better symptom control, the reductions in thromboembolic, cardiovascular and comorbidity risks.
The rhythm control strategy, which could involve pharmacological intervention, is relatively effective for the maintenance of sinus rhythm, but it can have adverse drug reactions. It reduces AF recurrences, but may not always eliminate them [12], the risk of serious adverse events in patients appears to be greater, compared to rate control strategies [13].
The AFFIRM trial [14] was a comparative study of rhythm versus rate control strategies in AF patients over five years. This demonstrated no significant difference in neither the ischemic stroke rate (7.1% vs. 5.5%; respectively; p = .79) or mortality (23.8% vs. 21.3%; respectively; p = .08). Similarly, meta-analyses of a few RCTs demonstrated no significant difference with regards to both stroke-related and general mortality, in spite of bias towards rate control [15]. Thus far, comparison of rhythm vs rate control as the superior strategy for AF management remains a controversial area in the literature; however, there is still little of evidence in the management of older patients. The superiority of rate control over rhythm control was evident in terms of cost-effectiveness [16], whereas rhythm control has demonstrated better outcomes in factors such as rates of stroke/TIA and health related QoL [17,18]. In spite of this, studies from the real world demonstrate that rate control strategies are used preferentially, and especially in the management of older patients [19], while rhythm control strategies are used primarily to reduce AF-related symptoms [20].
This can be achieved through the rate control approach, which is easier to establish and manage, and has a lower rate of hospitalization as well as major adverse events. The purpose of this review is to explore the rationale for employing the rate control approach, by considering the guidelines, recommendations and determinants for the choice of rate control drugs and atrioventricular nodal ablations. In spite of the limitations of rate control treatment, which may not be effective in preventing disease progression or in reducing symptoms insusceptible patients, it is still widely used for almost all AF patients (Figure 1).
Figure 1.
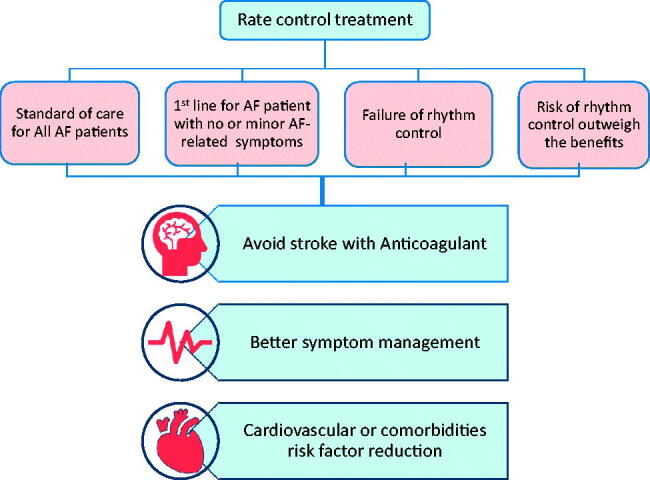
Indications of rate control treatment with the ultimate goal. AF: Atrial fibrillation [2].
The value of rate control drugs for management of atrial fibrillation
During AF, stroke volume and ventricle filling time are reduced as a result of fast and irregular ventricular rates [21]. Consequently, a reduction in the cardiac output by 20–30% [22] and irregular rhythm cause symptomatic consequences and contribute to the development or worsening of heart conditions, such as HF [23]. The dramatic increase in the heart rate also leads to substantial negative inotropic effects [21]. Patients with HF in whom the left ventricle ejection fraction (LVEF) is preserved or reduced are prone to more significant deterioration if they have AF [24]. In a nationwide large-scale AF cohort study [25], it was demonstrated that patients who received rate control treatment agents such as beta blockers (BB) or non-dihydropyridine calcium channel blockers (NDCC) had a lower mortality rate than patients who did not receive these treatments. In addition, the use of BB was associated with the lowest risk of mortality (adjusted HR: 0.76; 95% CI: 0.74–0.78), while digoxin usage was associated with the highest mortality risk (adjusted HR: 1.12; 95% CI: 1.10–1.14) [25].
Rate control therapy is preferred in three situations. First, although patients with new-onset AF (less than 1 year after diagnosis) and cardiovascular conditions are more preferred to use the rhythm control management (catheter ablation and/or pharmacological) compared to the usual care (rate control) [26]; rate control remains the mainstay treatment for almost all patients with AF. This is because maintaining well-controlled ventricular rates during AF deterioration is of the utmost importance. Second, it is the drug of choice for those who do not require rhythm control, that is, those older than 80 years of age who are asymptomatic or have mild symptoms [2,27]. Currently, the main aim of using rhythm control treatment is to improve AF-related symptoms. Third, it is the only alternative treatment used if rhythm strategy (e.g. pharmacological and catheter ablation) is unsuccessful or if the risks outweigh the benefits (e.g. bradycardia-tachycardia syndrome and high pacing risk). Therefore, it would be reasonable to consider rate control strategies in these situations, but the choice of treatment should be comprehensive and tailored to the severity of the symptoms, ventricular rates, associated co-morbidities and shared decision-making strategy [28,29].
Rate control definition and clinical guideline recommendations
Improvement of symptoms, preservation or prevention of left ventricle function impairment and QoL improvement are the aims of rate control drugs. This treatment should prevent tachycardiomyopathy and the development of HF. In order to maintain an adequate cardiac output, lower physiological demands, and prevent consequences, an appropriate ventricular rate should be achieved. If the ventricular rate is very rapid or slow, it could cause undesired adverse effects (e.g. pacemaker implantation, impaired QoL, and higher cost) [30].
However, in response to AF, ventricular rates have to increase in order to maintain homeostasis in the cardiovascular system due to the atrial conduction system being impaired and not contracting properly. Moreover, appropriate ventricular rates may differ from patient to patient. For instance, HF preserve ejection fraction (HFpEF) often requires slower ventricular rates to allow for more diastolic filling time [22]. However, American guidelines (American Heart Association/American College of Cardiology/Heart Rhythm Society) for the management of AF are more inclined to maintain a strict rate strategy and heart rate (defined as a ventricle rate <80 bpm at rest) in symptomatic patients [27].
The lenient strategy (defined as a ventricle rate <110 bpm at rest) could be reasonable in asymptomatic patients who have preserved left ventricle systolic function. Also, the optimal ventricle rate control in individuals with AF and HF with either preserved or reduced ejection fraction is unclear. The European Society of Cardiology for heart failure guidelines (2020) recommends a relatively lenient rate control strategy (60–100 beat per minutes at rest) in AF patients with HF [2]. In contrast, a ventricular rate < 80–90 bpm at rest and < 110–130 bpm during moderate-intensity exercise is supported by the ACC/AHA HF guideline [31]. Nevertheless, regardless of the recommendations in these guidelines, the main priority is based on the clinical decision.
Using the rate control strategy requires a comprehensive evaluation of AF patterns/types (e.g. paroxysmal, persistence, long standing persistence or permanent), symptoms, physical activity level, underlying diseases, age, assessment of cardiac functions and reconsideration of AF ablation. In some circumstances, a high heart rate is often necessary to maintain the physiological demands of physical activity and to prevent development of HF [32]. HFrEF and slow heart rates have been associated with higher mortality [32,33]. Often, symptoms such as fatigue and exercise intolerance may be due to chronotropic incompetence or relative bradycardia, such as bradycardia-tachycardia syndrome. Thereby, titrating rate control drugs should be initiated with or without the implementation of a pacemaker. To avoid the development of tachycardiomyopathy or persistent symptoms, increasing the rate control dosage may be necessary. Generally, no single regimen can exhibit a suitable approach to obtain an effective treatment in all AF patients, but it is worth noting that the lenient pharmacologic rate approach is convenient, safe and effective in a wide range of individuals and must be deemed as a first-line treatment.
Pharmacological rate control approaches
The purpose of the rate control approach is to closely monitor and assess the patient condition before establishing any treatments. It has been reported that a rapid ventricular rate could exacerbate the underlying disease which led to different pharmacological or non-pharmacological managements than the standard management of AF. The ventricular rate in AF is mediated by sympathetic and parasympathetic activity and dromotropic effect produced by the atrioventricular (AV) node. In order to slow the ventricular rate in patients with AF, there are three common classes of drugs that can be used for the rate control approach: BB, non- dihydropyridine calcium channel blocker (NDCC) and, to a certain extent, digoxin or amiodarone.
Several rhythm and rate control agents have been linked to a higher risk of mortality in patient with HF (e.g. dronedarone) [34] or structural heart disease (e.g. flecainide, propafenone and d-sotalol) [35]. So, adverse events, contraindications and non-invasive multimodality imaging are important considerations that provide all needed information in the selection of rhythm and rate control agents [36]. Also, based on the currently available evidence from RCTs, the primary indication for rhythm control is to reduce AF-related symptoms and improve QoL [2]. Thus, if symptoms are not AF related then rate control agents should be used instead of rhythm control agents. Similar to the selection between rate and rhythm control, the decision of single or concomitant use among rate control drugs is based on symptoms, potential adverse events, and the presence of comorbidities (e.g. HFpEF, HFrEF, severe chronic obstructive pulmonary disease (COPD) or asthma). In the recent European Society of Cardiology (ESC) guidelines on the management of AF, BB are the first-line treatment for those with HF regardless of their LV function/status (i.e. HFrEF or HFpEF) [2], and calcium channel blockers can be used in those with preserved ejection fraction and severe COPD and asthma [2]. However, in cases of suboptimal rate control (resting heart rate >110 bpm), worsening of symptoms or QoL, consider second-line treatments such as digoxin, and, if necessary, third-line treatments option of a combination of three drugs, or evaluation for CRT-P, CRT-D or AVN ablation and pacemaker implantation should be considered.
Beta blockers (BB)are sympatholytic agents that inhibit the activity of the beta-1 receptor in the AV node and thereby reduce accelerated ventricular rate. A randomized, double-blind study [37] in patients with persistent or permanent AF who were using the beta blocker carvedilol showed a significant graded reduction in heart rate with each dose up-titrating from 5 to 20 mg, which suggests a trend for dose-dependent heart rate reduction. Carvedilol is recommended for patients with HF and reduced LVEF because large randomized controlled trials have demonstrated a substantial reduction in morbidity and mortality rates in patient assigned to BB [38–40]. However, this result was not found in patients with AF [41]. This may be due to the large reduction in ventricle rate in AF patients. It is possible that a lower dose of BB applied to a faster heart rate could be beneficial [32–33]. It has been shown that BB have the advantage of improving the LVEF in patients with chronic HF. According to a retrospective analysis [42], in patients with established AF and chronic HF, carvedilol therapy showed a statistically significant improvement in LVEF and a potential decrease in the combined endpoint of death or hospitalization. However, the study had a sample size with more than 90% of the subjects on digoxin in both arms, which does not allow for determining which benefits resulted from carvedilol or digoxin. Nevertheless, this retrospective study shed light on subsequent studies of AF with HF, despite the prognostic benefit of BB seen in patients with HFrEF and sinus rhythm being questioned in patients with AF and HF [41].
Non-dihydropyridine calcium channel blockers (NDCC), such as verapamil or diltiazem, slow the conduction of the AV node and have negative inotropic and chronotropic effects. In AF patients, NDCC produce acceptable rate control levels [43], but it is better to avoid the use of NDCC in patients with HFrEF due to their negative inotropic effects [44]. NDCC can improve arrhythmia-related symptoms and exercise capacity as well as reduce the level of N-terminal prohormone brain natriuretic peptide (NT-proBNP; a marker for poor cardiac function) when compared with the use of BB, as demonstrated in a small trial of low-risk patients with preserved LVEF [45].
Digoxin reduces ventricular rate during AF by slowing AV nodal conduction but has no direct effect on the ventricles. It is not recommended for patients with high sympathetic activity (e.g. hyperthyroidism) [27]. In a recent study, it was demonstrated that verapamil significantly increased the concentration of digoxin levels in patients who simultaneously received digoxin, but this trend appears to be dependent on the digoxin dosage [46]. Therefore, low doses of digoxin in elderly patients combined with medication that elevates digoxin serum concentrations should be used with caution [47].
Several studies have called for digoxin safety in AF patients, yet whether these safety issues result from patient’s comorbidities or the drug itself is unclear. According to a retrospective analysis of a digoxin study [48], higher digoxin concentrations were shown to be associated with higher mortality rates in HF patients. The AFFIRM trial [49], which was conducted on patients with AF, showed that digoxin was independently associated with higher mortality. However, two post-hoc analyses of the AFFIRM database showed conflicting results on cardiovascular outcomes for AF patients using digoxin. For example, digoxin was independently associated with a 41% increased risk of death, regardless of HF status [50].
Another study showed that digoxin did not have a significant effect on mortality [51]. Even though the same database was used, different statistical analyses have resulted in different conclusions. These different conclusions were attributed to the differences in chosen study populations or exposure classification (i.e. fixed vs. time-varying) as differences in analytical techniques [52].
A retrospective analysis confirmed an increased risk of death with digoxin in AF patients [53], but a meta-analysis in patients with AF with or without HF showed a normal effect on mortality and all-cause hospital admission [54,55]
It is difficult to attribute poor outcomes to digoxin itself without taking into consideration the patient’s comorbidities and other treatment failures. Due to the lack of studies that support digoxin’s effectiveness during exercise as a rate control therapy, the use of digoxin has declined [56]. A systematic review with meta-analysis and trial sequential analysis showed that, based on current available evidence, the clinical effects of digoxin on serious adverse events, QoL, HF, stroke, and all-cause mortality are uncertain [57]. In reducing heart rate, digoxin appears to be superior compared with a placebo, but inferior compared with BB [57]. Despite this, in patients with AF and HF, the concomitant use of digoxin and carvedilol appears to be superior compared to using either drug alone [56]. The RATE-AF trial [58] compared two rate control strategies (digoxin vs. the beta blocker bisoprolol). This trial showed that digoxin therapy has a similar effect as BB on reducing heart rate and QoL in older patient with permanent AF and symptoms of HF. However, digoxin was associated with a higher reduction in natriuretic peptides and adverse events, and an improvement in some measures of QoL compared with BB. However, on the basis of this finding from a small, open-label in design, the clinical practice guidelines for rate control may not substantially changed; thus, digoxin may be favoured as a second-line therapy for those with permanent AF and intolerant or inadequate response to BB or NDCC [58].
Sotalol is a combination of BB with potassium channel blockers (Class III antiarrhythmic effect) [59] that slows the conduction velocity (i.e. dromotropic effect) [60] and can prolong the QT interval by blockading the rapid components of delayed rectifier potassium current. These effects are essential for cardiac action potential (i.e. the repolarization of phase 3) [61,62], but may cause a torsades de pointes as demonstrated in the PAFAC trials [63,64]. In post-myocardial infarction patients with left ventricle dysfunction, sotalol was associated with a higher mortality compared to the placebo group, which is probably because of ventricular arrhythmias [65,66]. However, in two controlled trials, there were no potential risks found as a result of the use of sotalol [67,68]. Overall, as a rate control strategy, sotalol is not recommended [69,70]. Some class III antiarrhythmic drugs have shown reverse use dependence (i.e. inverse correlation between heart rate and QT interval) on action potential duration, as seen with sotalol [61]. This means as the heart rate slows, the QT interval can be prolonged, which may elucidate the association between bradycardia and torsades de pointes, resulting in the ineffectiveness of sotalol for significant tachycardia.
Sotalol has a BB-like effect extending sinoatrial cycle length (i.e. reduced heart rate), decreasing AV node conduction, and increasing AV node refractoriness (i.e. PR interval prolongation) [71]. Since sotalol has rhythm control and rate control properties, it is indicated as sinus rhythm maintenance for post-cardioversion or post-cardiac surgery in AF patients with underlying coronary heart disease. A meta-analysis showed that compared to a placebo or even BB, sotalol was substantially more effect for the prevention of AF, and it had a similar effect to amiodarone [72]. In the DAPHNE trial, 135 patients with bradycardia-tachycardia syndrome were randomly assigned to sotalol or either of the beta receptor blockades metoprolol or atenolol one month after treatment using a rate-adaptive dual-chamber pacemaker [73]. As a result, almost 30% of patients were free from atrial tachyarrhythmia recurrences in both the beta blocker and sotalol group, and the rates of cardioversion and hospitalization in the sotalol or BB groups were not significantly different for either group. Survival analysis demonstrated a trend towards a lower incidence of cardioversion or hospitalization among the beta blocker group. In comparison with other antiarrhythmic medications, sotalol seems to be less effective, either when administrated orally [74] or intravenously [75,76]. Similarly, dronedarone slows the conduction velocity as a rate control drug [77]; however, in patients with permanent AF, it increases the risk of cardiovascular morbidity (i.e. stroke and HF) and mortality. For this reason, it is contraindicated for this group [34]. Lastly, the class III antiarrhythmic drugs (class III) have rate-control properties (e.g. sotalol, dronedarone, and amiodarone), but they should only be used for rhythm control, whereas class IA and IC antiarrhythmic drugs have no role in rate control and may paradoxically increase heart rate by reducing atrial rate.
Non-pharmacological rate control approaches
When pharmacological rate control medication fails, ablate and pace strategy can control ventricular rate with atrioventricular nodal (AVN) ablation and implantation of a pacemaker. It is a relatively simple procedure with low rate of complication [78], such as worsening left ventricle ejection fraction [79], and may even improve left ventricle ejection fraction in selected patients [80]. The timing of pacemaker implantation plays a major role in lowering the risk of long-term mortality, when the pacemaker implanted few weeks prior to AVN ablation and set at a pace rate of 70–90 bpm [81]. The selection of pacemaker type or pacing mode is still unclear; however, patient characteristics determine the choice of pacing therapy whether right or bi-ventricle pacing [82], although there are limited data on the advantage of RV vs. bi-ventricle pacing in HF patients. Interestingly, in a small RCT, in patients with permanent AF and who have severe symptoms, it was shown that ablate and cardiac resynchronized therapy was superior to pharmacological rate control agents in symptom relief and reducing HF hospitalization and mortality [83].Thus, atrioventricular nodal ablation in AF helps in limiting the rapid ventricular rate from aggravation symptoms and HF. Growing evidence suggests that His-bundle pacing, an attractive alternative pacing mode, could be useful in severely symptomatic patients with permanent AF [84], and it is being tested in a current ongoing clinical trial (NCT02805465).
Rate Control medications in different patient groups
Several studies have examined different medications with the aim of managing acute or chronic AF, but unfortunately the limitations of these trials, such as short-term follow-up and a small number of patients, have resulted in low-quality evidence. In acute care settings for AF, pharmacological rate control is recommended for patients who experience moderate symptoms or hemodynamic distress. However, it is recommended that the heart rate should not exceeds 100 bpm, but the exact optimal heart rate has still not properly been defined, and therapy is often symptom-guided. In acute settings of HF with reduced LVEF, amiodarone can be considered as a good alternative compared to BB and NDCC [85].
For chronic AF, no robust recommendations for a specific rate control drug have been made. The determinant of therapy relies on the pre-existence of comorbidities and HF. In a small study of 12 patients with permanent AF, the subjects underwent five different drug regimens over a two-week period. These drugs included atenolol (50 mg), diltiazem-CD (240 mg), digoxin (0.25 mg), digoxin plus diltiazem and lastly digoxin plus atenolol. Digoxin plus atenolol was the most effective rate control drug as it yielded the lowest ventricular rate during exercise; digoxin alone and diltiazem alone were the least efficient regimens [86]. The randomized study, RATAF [43], compared the effects of four rate-reducing drugs (metoprolol 100 mg/day, carvedilol 25 mg/day, verapamil 240 mg/day and diltiazem 360 mg/day) on ventricular rate and symptoms in permanent AF patients without reduced LVEF or HF. The results showed that diltiazem was the most effective at reducing heart rates, and both NDCC agents (verapamil and diltiazem) reduced the symptoms related to arrhythmia and improved exercise tolerance. Conversely, both BB were less effective on exercise tolerance, ventricular rate, and arrhythmia-related symptoms. RATAF II is an ongoing Phase 4 trial designed to compare the effects of two rate control drugs in patients with permanent AF, hypothesizing that a 6-month treatment with the NDCC diltiazem will lower the level of NT-pro BNP and increase exercise capacity (peak VO2; volume oxygen) compared to treatment with the beta blocker metoprolol, as low levels of NT-pro BNP are associated with sinus rhythm maintenance. The RATAF II trial may encourage more NDCC prescriptions if it yields positive outcomes (NCT02695992).
Moreover, patients with recent-onset AF could benefit from a wait-and-watch approach with the use of rate control agents only (i.e. beta blocking agents, non-dihydropyridine CCBs, or digoxin) and delayed cardioversion if needed within 2 days of symptom onset in paroxysmal AF. This wait-and-watch strategy was safe and non-inferior to the immediate cardioversion at4 weeks [87]. It also shows that using rate control agents instead reduces the need for cardioversion (whether pharmacological or electrical) and allows for spontaneous conversion, as frequently occurred in the delayed cardioversion group [87]. However, this specific rate control strategy has not been extensively studied in terms of the selection between the rate control agents. In observation study in acute AF patients, the clinical determinants of early spontaneous conversion were most likely recent-onset, short-duration AF episodes, lower BMI, and normal size of left atrium [88].
Rate control monitoring
Even though no specific recommendations suggest the necessity to monitor patients on rate control drugs, assessments of ventricular rates while resting and during exercise are highly recommended. It is necessary to assess ventricular rates during moderate exercise, since rapid increases in ventricular rate are accompanied by symptoms at rest that require strict rate control along with a 24-hour Holter monitor to assess safety concerns [89]. Adequate heart rate can be determined by an exercise test or remote monitoring devices.
For rate control monitoring, 12-lead ECG can only provide a time-point snapshot of cardiac rhythm in the clinical setting, while remote monitoring devices, such as (ZioPatch, AliveCor) have emerged as some of non-invasive AF detection and monitoring.
ZioPatch, an adhesive patch, is a single-lead ECG monitor provides a continuous monitoring for up to 14 days, and FDA approved [90], and allow the patients to perform their usual daily activities [91]. Several clinical trials have shown that the ZioPatch accurately identifies more episodes of AF compared to two-days ambulatory ECG device and high patient adherence with the device [91,92]. Thus, referred patients with AF symptoms such as, palpitations or syncope that often occurs more than 14-days period would more benefit from this way [92].
Similarly, AliveCor, mobile ECG recorder, that is FDA-approved (uses smartphone app) to capture arrythmias, including episodes of AF [93], with high sensitivity and specificity (94% and 98%; respectively). Studies have shown that the AliveCor is beneficial for detecting AF and it is easy to use [93,94], but this was based on case reports which successfully detect the recurrent episodes, however ongoing iHEART trial will evaluate the utility in recent-onset AF patient for larger scale in real world settings [95]. It will shed the light in the ability of this device for detection and treatment of recurrent AF, eventually improve the overall monitoring and management of atrial fibrillation. Thus, frequent and long episodes could be better captured by AliveCor, in contrast, shorter-time episode may be less suited for AliveCor.
Nevertheless, multimodalities imaging can offer a valuable information for the structural, functional and anatomical changes of both atrial and ventricles. A transthoracic echocardiogram (TTE) provides an evaluation of function and size of atrial and ventricles, such as valvular heart disease, LV hypertrophy, and most importantly LA appendages thrombus prior sinus rhythm restoration. CT coronary angiography assess the coronary heart disease, and the brain CT and MRI provides more insight in suspected stroke [96]. Thus, the decision between or among rate and rhythm control will depend on the functional and structural state of the heart as these imaging modalities provides an insightful information.
Conclusions
In summary, the rate control approach (i.e. maintaining an adequate ventricular rate for hemodynamic stability, haemostasis and prevention of serious adverse effects) is the first-line treatment for AF management and frequently adequate for improving AF-related symptoms. There is insufficient high-quality evidence to inform the intensity and type of rate control therapy. A lenient rate control strategy is convenient, safe, and extends across a wide spectrum of AF patients. It should be used as an initial therapy for patients with a good response to lenient rate control and who have mild symptoms. If symptoms persist or the left cardiac function begins to deteriorate, a strict rate control treatment should be considered. Special consideration is required for those with bradycardia-tachycardia syndrome, new-onset AF, and implantable cardioverter defibrillator. The optimal ventricular rates at rest and during exercise remain undetermined. Therefore, although rate control drugs are the initial management recommendation for all AF cases, a number of issues remain to be solved.
Funding Statement
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURP-000.
Disclosure statement
The authors report no conflict of interest
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-596. [DOI] [PubMed] [Google Scholar]
- 2.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. [DOI] [PubMed] [Google Scholar]
- 3.Schnabel RB, Yin X, Gona P, et al. 50-year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. J Am Med Assoc. 2001;285(18):2370–2375. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010. Study. Circulation. 2014;129(8):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staerk L, Sherer JA, Ko D, et al. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120(9):1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schotten U, Verheule S, Kirchhof P, et al. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91(1):265–325. [DOI] [PubMed] [Google Scholar]
- 8.Aune D, Feng T, Schlesinger S, et al. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: a systematic review and meta-analysis of cohort studies. J Diabet Complicat. 2018;32(5):501–511. [DOI] [PubMed] [Google Scholar]
- 9.Cadby G, McArdle N, Briffa T, et al. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest. 2015;148(4):945–952. [DOI] [PubMed] [Google Scholar]
- 10.Lip GY, Coca A, Kahan T, et al. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Ep Europace. 2017;19(6):891–911. [DOI] [PubMed] [Google Scholar]
- 11.Goudis CA, Korantzopoulos P, Ntalas IV, et al. Obesity and atrial fibrillation: a comprehensive review of the pathophysiological mechanisms and links. J Cardiol. 2015;66(5):361–369. [DOI] [PubMed] [Google Scholar]
- 12.Camm J. Antiarrhythmic drugs for the maintenance of sinus rhythm: risks and benefits. Int J Cardiol. 2012;155(3):362–371. [DOI] [PubMed] [Google Scholar]
- 13.Sethi NJ, Feinberg J, Nielsen EE, et al. The effects of rhythm control strategies versus rate control strategies for atrial fibrillation and atrial flutter: a systematic review with meta-analysis and Trial Sequential Analysis. PloS One. 2017;12(10):e0186856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. New Eng J Med. 2002;347(23):1825–1833. [DOI] [PubMed] [Google Scholar]
- 15.de Denus S, Sanoski CA, Carlsson J, et al. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med. 2005;165(3):258–262. [DOI] [PubMed] [Google Scholar]
- 16.Perez A, Touchette DR, DiDomenico RJ, et al. Comparison of rate control versus rhythm control for management of atrial fibrillation in patients with coexisting heart failure: a cost‐effectiveness analysis. Pharmacother. 2011;31(6):552–565. [DOI] [PubMed] [Google Scholar]
- 17.Tsadok MA, Jackevicius CA, Essebag V, et al. Rhythm versus rate control therapy and subsequent stroke or transient ischemic attack in patients with atrial fibrillation. Circulation. 2012;126(23):2680–2687. [DOI] [PubMed] [Google Scholar]
- 18.Ha AC, Breithardt G, Camm AJ, et al. Health-related quality of life in patients with atrial fibrillation treated with rhythm control versus rate control: insights from a prospective international registry (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation: RECORD-AF). Circulation. 2014;7(6):896–904. [DOI] [PubMed] [Google Scholar]
- 19.Fumagalli S, Said SA, Laroche C, et al. Age-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: the EORP-AF general pilot registry (EURObservational Research Programme-Atrial Fibrillation). J Am Coll Cardiol. 2015;1(4):326–334. [DOI] [PubMed] [Google Scholar]
- 20.Bunch TJ, Steinberg BA.. Revisiting rate versus rhythm control in atrial fibrillation-timing matters. N Engl J Med. 2020;383(14):1383–1384. [DOI] [PubMed] [Google Scholar]
- 21.Kerr AJ, Williams MJ, Stewart RA.. Ventricular rate and beat-to-beat variation of stroke volume in atrial fibrillation. Am J Cardiol. 2001;87(9):1116–1119. [DOI] [PubMed] [Google Scholar]
- 22.Wyse DG. Therapeutic considerations in applying rate control therapy for atrial fibrillation. J Cardiovasc Pharmacol. 2008;52(1):11–17. [DOI] [PubMed] [Google Scholar]
- 23.Daoud EG, Weiss R, Bahu M, et al. Effect of an irregular ventricular rhythm on cardiac output. Am J Cardiol. 1996;78(12):1433–1436. [DOI] [PubMed] [Google Scholar]
- 24.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao TF, Liu CJ, Tuan TC, et al. Rate-control treatment and mortality in atrial fibrillation. Circulation. 2015;132(17):1604–1612. [DOI] [PubMed] [Google Scholar]
- 26.Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–1316. [DOI] [PubMed] [Google Scholar]
- 27.January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. [DOI] [PubMed] [Google Scholar]
- 28.Peterson ED, Ho PM, Barton M, et al. ACC/AHA/AACVPR/AAFP/ANA Concepts for clinician–patient shared accountability in performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circulation. 2014;130(22):1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirchhof P, Breithardt G, Bax J, et al. A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace. 2016;18(1):37–50. [DOI] [PubMed] [Google Scholar]
- 30. Rienstra M, Van Gelder IC.. Who, when and how to rate control for atrial fibrillation. Curr Opin Cardiol. 2008;23(1):23–30. [DOI] [PubMed] [Google Scholar]
- 31.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): Developed in collaboration with the American College of Chest Physicians. Circulation. 2005;112:e154-235. [DOI] [PubMed] [Google Scholar]
- 32.Mareev Y, Cleland JG.. Should β-blockers be used in patients with heart failure and atrial fibrillation. Clin Ther. 2015;37(10):2215–2224. [DOI] [PubMed] [Google Scholar]
- 33.Laskey WK, Alomari I, Cox M, the AHA Get with The Guidelines‐Heart Failure Program, et al. Heart rate at hospital discharge in patients with heart failure is associated with mortality and rehospitalization. JAHA. 2015;4(4):e001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365(24):2268–2276. [DOI] [PubMed] [Google Scholar]
- 35.Lafuente-Lafuente C, Mouly S, Longás-Tejero MA, et al. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med. 2006;166(7):719–728. [DOI] [PubMed] [Google Scholar]
- 36.Donal E, Lip GY, Galderisi M, et al. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2016;17(4):355–383. [DOI] [PubMed] [Google Scholar]
- 37.Inoue H, Atarashi H, Okumura K, et al. Heart rate control by carvedilol in Japanese patients with chronic atrial fibrillation: the AF carvedilol study. J Cardiol. 2017;69(1):293–301. [DOI] [PubMed] [Google Scholar]
- 38.Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239. [DOI] [PubMed] [Google Scholar]
- 39.Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194–2199. [DOI] [PubMed] [Google Scholar]
- 40.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7–13. [DOI] [PubMed] [Google Scholar]
- 41.Kotecha D, Holmes J, Krum H, et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384(9961):2235–2243. [DOI] [PubMed] [Google Scholar]
- 42.Packer M, Bristow MR, Cohn JN, Colucci WS, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334(21):1349–1355. [DOI] [PubMed] [Google Scholar]
- 43.Ulimoen SR, Enger S, Carlson J, et al. Comparison of four single-drug regimens on ventricular rate and arrhythmia-related symptoms in patients with permanent atrial fibrillation. The American J Cardiol. 2013;111(2):225–230. [DOI] [PubMed] [Google Scholar]
- 44.Ponikowski P, Voors AA, Anker SD, Bueno H, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. [DOI] [PubMed] [Google Scholar]
- 45.Ulimoen SR, Enger S, Pripp AH, et al. Calcium channel blockers improve exercise capacity and reduce N-terminal Pro-B-type natriuretic peptide levels compared with beta-blockers in patients with permanent atrial fibrillation. Eur Heart J. 2014;35(8):517–524. [DOI] [PubMed] [Google Scholar]
- 46.Klein HO, Lang RO, Weiss EL, et al. The influence of verapamil on serum digoxin concentration. Circulation. 1982;65(5):998–1003. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27(2):178–186. Jan 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathore SS, Curtis JP, Wang Y, et al. Association of serum digoxin concentration and outcomes in patients with heart failure. J Am Med Assoc. 2003;289(7):871–878. [DOI] [PubMed] [Google Scholar]
- 49.Corley SD, Epstein AE, DiMarco JP.. Relationships between sinus rhythm, treatment and survival in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2003. 108:472–472. [DOI] [PubMed] [Google Scholar]
- 50.Whitbeck MG, Charnigo RJ, Khairy P, et al. Increased mortality among patients taking digoxin – analysis from the AFFIRM study. Eur Heart J. 2013;34(20):1481–1488. [DOI] [PubMed] [Google Scholar]
- 51.Gheorghiade M, Fonarow GC, Van Veldhuisen DJ, et al. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur Heart J. 2013;34(20):1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodman RJ. Opposing conclusions from post hoc analyses of the AFFIRM trial: was propensity score analysis to blame or just an innocent victim? Ther Adv Drug Saf. 2014;5(1):4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Washam JB, Stevens SR, Lokhnygina Y, et al. Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: a retrospective analysis of the rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Lancet. 2015;385(9985):2363–2370. [DOI] [PubMed] [Google Scholar]
- 54.Ziff OJ, Lane DA, Samra M, et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chamaria S, Desai AM, Reddy PC, et al. Digoxin use to control ventricular rate in patients with atrial fibrillation and heart failure is not associated with increased mortality. Cardiol Res Pract. 2015;2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khand AU, Rankin AC, Martin W, et al. Carvedilol alone or in combination with digoxin for the management of atrial fibrillation in patients with heart failure? J Am Coll Cardiol. 2003;42(11):1944–1951. [DOI] [PubMed] [Google Scholar]
- 57.Sethi NJ, Nielsen EE, Safi S, et al. Digoxin for atrial fibrillation and atrial flutter: a systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. PloS One. 2018;13(3):e0193924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotecha D, Bunting KV, Gill SK, et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life. JAMA. 2020;324(24):2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kochiadakis GE, Kanoupakis EM, Kalebubas MD, et al. Sotalol vs metoprolol for ventricular rate control in patients with chronic atrial fibrillation who have undergone digitalization: a single-blinded crossover study. Europace. 2001;3(1):73–79. [DOI] [PubMed] [Google Scholar]
- 60.Tse HF, Lam YM, Lau CP, et al. Comparison of digoxin versus low‐dose amiodarone for ventricular rate control in patients with chronic atrial fibrillation. Clin Exp Pharmacol Physiol. 2001;28(5-6):446–450. [DOI] [PubMed] [Google Scholar]
- 61.Numaguchi H, Mullins FM, Johnson JP, Jr, et al. Probing the interaction between inactivation gating and Dd-sotalol block of HERG. Circulat Res. 2000;87(11):1012–1018. [DOI] [PubMed] [Google Scholar]
- 62.Sanguinetti MC, Jurkiewicz NK.. Two components of cardiac delayed rectifier K current. J Gen Physiol. 1990;96(1):195–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fetsch T, Bauer P, Engberding R, et al. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. Eur Heart J. 2004;25(16):1385–1394. [DOI] [PubMed] [Google Scholar]
- 64.Haverkamp W, Hördt M, Breithardt G, et al. Torsade de Pointes Secondary to d, I‐sotalol after catheter ablation of incessant atrioventricular reentrant tachycardia—evidence for a significant contribution of the “cardiac memory”. Clin Cardiol. 1998;21(1):55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lafuente ‐Lafuente C, Valembois L, Bergmann JF, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochr Datab Syst Rev. 2015;(3):CD005049. [DOI] [PubMed] [Google Scholar]
- 66.Freemantle N, Lafuente-Lafuente C, Mitchell S, et al. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace. 2011;13(3):329–345. [DOI] [PubMed] [Google Scholar]
- 67.Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. N Engl J Med. 2000;342(13):913–920. [DOI] [PubMed] [Google Scholar]
- 68.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352(18):1861–1872. [DOI] [PubMed] [Google Scholar]
- 69.Kuck KH, Kunze KP, Roewer N, et al. Sotalol-induced torsade de pointes. Am Heart J. 1984;107(1):179–180. [DOI] [PubMed] [Google Scholar]
- 70.McKibbin JK, Pocock WA, Barlow JB, et al. Sotalol, hypokalaemia, syncope, and torsade de pointes. Heart. 1984;51(2):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson JL, Prystowsky EN.. Sotalol: an important new antiarrhythmic. Am Heart J. 1999;137(3):388–409. [DOI] [PubMed] [Google Scholar]
- 72.Kerin NZ, Jacob S.. The efficacy of sotalol in preventing postoperative atrial fibrillation: a meta-analysis. Am J Med. 2011;124(9):875-e1–875.e9. [DOI] [PubMed] [Google Scholar]
- 73.Capucci A, Botto G, Molon G, et al. DAPHNE Study Investigators. The Drug And Pace Health cliNical Evaluation (DAPHNE) study: a randomized trial comparing sotalol versus β-blockers to treat symptomatic atrial fibrillation in patients with brady-tachycardia syndrome implanted with an antitachycardia pacemaker. Am Heart J. 2008;156(2):373-e1–373.e8. [DOI] [PubMed] [Google Scholar]
- 74.Ferreira E, Sunderji R, Gin K.. Is oral sotalol effective in converting atrial fibrillation to sinus rhythm? Pharmacotherapy. 1997;17(6):1233–1237. [PubMed] [Google Scholar]
- 75.Vos MA, Golitsyn SR, Stangl K, et al. Superiority of ibutilide (a new class III agent) overdl-sotalol in converting atrial flutter and atrial fibrillation. Heart. 1998;79(6):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reisinger J, Gatterer E, Heinze G, et al. Prospective comparison of flecainide versus sotalol for immediate cardioversion of atrial fibrillation. Am J Cardiol. 1998;81(12):1450–1454. [DOI] [PubMed] [Google Scholar]
- 77.Davy JM, Herold M, Hoglund C, et al. ERATO Study Investigators. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J. 2008;156(3):527-e1–527.e9. [DOI] [PubMed] [Google Scholar]
- 78.Lim KT, Davis MJ, Powell A, et al. Ablate and pace strategy for atrial fibrillation: long-term outcome of AIRCRAFT trial. Europace. 2007;9(7):498–505. [DOI] [PubMed] [Google Scholar]
- 79.Chatterjee NA, Upadhyay GA, Ellenbogen KA, et al. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis and systematic review. Circ Arrhythm Electrophysiol. 2012;5(1):68–76. [DOI] [PubMed] [Google Scholar]
- 80.Bradley DJ, Shen WK.. Overview of management of atrial fibrillation in symptomatic elderly patients: pharmacologic therapy versus AV node ablation. Clin Pharmacol Ther. 2007;81(2):284–287. [DOI] [PubMed] [Google Scholar]
- 81.Wang RX, Lee HC, Hodge DO, et al. Effect of pacing method on risk of sudden death after atrioventricular node ablation and pacemaker implantation in patients with atrial fibrillation. Heart Rhythm. 2013;10(5):696–701. [DOI] [PubMed] [Google Scholar]
- 82.Chatterjee NA, Upadhyay GA, Ellenbogen KA, et al. Atrioventricular nodal ablation in atrial fibrillation: A meta‐analysis of biventricular vs. right ventricular pacing mode. Eur J Heart Fail. 2012;14(6):661–667. [DOI] [PubMed] [Google Scholar]
- 83.Brignole M, Pokushalov E, Pentimalli F, et al. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J. 2018;39(45):3999–4008. [DOI] [PubMed] [Google Scholar]
- 84.Abdelrahman M, Subzposh FA, Beer D, et al. Clinical outcomes of His bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. 2018;71(20):2319–2330. [DOI] [PubMed] [Google Scholar]
- 85.Hou ZY, Chang MS, Chen CY, et al. Acute treatment of recent-onset atrial fibrillation and flutter with a tailored dosing regimen of intravenous amiodarone: a randomized, digoxin-controlled study. Eur Heart J. 1995;16(4):521–528. [DOI] [PubMed] [Google Scholar]
- 86.Farshi R, Kistner D, Sarma JS, et al. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: a crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33(2):304–310. [DOI] [PubMed] [Google Scholar]
- 87.Pluymaekers NA, Dudink EA, Luermans JG, et al. Early or delayed cardioversion in recent-onset atrial fibrillation. N Engl J Med. 2019;380(16):1499–1508. [DOI] [PubMed] [Google Scholar]
- 88.Pluymaekers NA, Dudink EA, Weijs B, et al. Clinical determinants of early spontaneous conversion to sinus rhythm in patients with atrial fibrillation. Neth Heart J. 2021;29(5):255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Gelder IC, Van Veldhuisen DJ, Crijns HJ, et al. RAte Control Efficacy in permanent atrial fibrillation: a comparison between lenient versus strict rate control in patients with and without heart failure. Background, aims, and design of RACE II. Am Heart J. 2006;152(3):420–426. [DOI] [PubMed] [Google Scholar]
- 90.Turakhia MP, Hoang DD, Zimetbaum P, et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013;112(4):520–524. [DOI] [PubMed] [Google Scholar]
- 91.Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(1):95-e11–95.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheung CC, Kerr CR, Krahn AD.. Comparing 14-day adhesive patch with 24-h Holter monitoring. Future Cardiol. 2014;10(3):319–322. [DOI] [PubMed] [Google Scholar]
- 93.Haberman ZC, Jahn RT, Bose R, Tun H, et al. Wireless smartphone ECG enables large‐scale screening in diverse populations. J Cardiovasc Electrophysiol. 2015;26(5):520–526. [DOI] [PubMed] [Google Scholar]
- 94.Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone‐based photoplethysmographic application for atrial fibrillation screening in a primary care setting. JAHA. 2016;5(7):e003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hickey KT, Hauser NR, Valente LE, et al. A single-center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: the iHEART study protocol. BMC Cardiovasc Disord. 2016;16(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delgado V, Di Biase L, Leung M, et al. Structure and function of the left atrium and left atrial appendage: AF and stroke implications. J Am Coll Cardiol. 2017;70(25):3157–3172. [DOI] [PubMed] [Google Scholar]


