Abstract
Background: In dentistry, barrier membranes are used for guided tissue regeneration (GTR) and guided bone regeneration (GBR). Various membranes are commercially available and extensive research and development of novel membranes have been conducted. In general, membranes are required to provide barrier function, biosafety, biocompatibility and appropriate mechanical properties. In addition, membranes are expected to be bioactive to promote tissue regeneration.
Objectives: This review aims to organize the fundamental characteristics of the barrier membranes that are available and studied for dentistry, based on their components.
Results: The principal components of barrier membranes are divided into nonbiodegradable and biodegradable materials.
Nonbiodegradable membranes are manufactured from synthetic polymers, metals or composites of these materials. The first reported barrier membrane was made from expanded polytetrafluoroethylene (e-PTFE). Titanium has also been applied for dental regenerative therapy and shows favorable barrier function. Biodegradable membranes are mainly made from natural and synthetic polymers. Collagens are popular materials that are processed for clinical use by cross-linking. Aliphatic polyesters and their copolymers have been relatively recently introduced into GTR and GBR treatments. In addition, to improve the tissue regenerative function and mechanical strength of biodegradable membranes, inorganic materials such as calcium phosphate and bioactive glass have been incorporated at the research stage.
Conclusions: Currently, there are still insufficient guidelines for barrier membrane choice in GTR and GBR, therefore dentists are required to understand the characteristics of barrier membranes.
Keywords: Barrier membrane, GTR, GBR, dental materials, biodegradability
1. Introduction
In dentistry, barrier membranes are used to improve the prognosis in the regeneration of periodontal tissue, including in the bifurcation area and bone augmentation associated with implant treatment [1–3]. In 1982, Nyman et al. [4] succeeded in forming new attachment to tooth in human by directing periodontal tissue regeneration using a barrier membrane. Studies of dental regeneration have since advanced and operation protocols such as guided tissue regeneration (GTR) and guided bone regeneration (GBR) have been widely accepted for clinical application [5,6]. Barrier membranes implanted over the tissue defect area prohibit cell invasion from the gingival epithelium and connective tissue [7,8]. It has been reported that the shielding function is required to last 4–6 weeks for periodontal tissue regeneration and 16–24 weeks for bone augmentation [9,10], therefore barrier membranes need to persist between the gingiva and alveolar bone for longer than these time frames. This shielding function maintains the space for tissue regeneration and selectively guides the periodontal ligament derived cells or bone formation cells to the defect area [11,12].
To date, several clinical outcomes have been reported using various barrier membranes [13–15]. The required properties of barrier membranes are high biocompatibility, low permeability to cells, tight adhesion to host tissues, moderate mechanical strength, storage stability and handleability for clinical use [16,17]. In terms of the mechanical properties, the barrier membrane should be able to withstand the pressure of overhanging gingiva and keep its shape to maintain the regenerative space [18]. In addition, membranes should easily deform plastically without fracturing and maintain their morphology after implantation.
Barrier membranes are designed to promote tissue regeneration and can be divided according to the biodegradability of the base material. In recent years, the use of biodegradable membranes has been mainstreamed in GBR, however nonbiodegradable products are often applied to massive tissue loss and vertical bone defects owing to their advantages in space making [19–21]. The field of barrier membranes is expanding, and the evolution of biomaterials is inevitable. Consequently, selecting a membrane for clinical application will involve more than the current considerations of biodegradability, it will be necessary to understand other membrane components. Furthermore, even for currently commercially available membranes, guidelines for prescription protocols are not defined further than the biodegradability aspect, leaving the material selection highly dependent on the professional experience of clinician. This review aims to summarize the fundamental characteristics of the barrier membranes commercially available and currently studied in dentistry–based on their components–and provide an update from the material point of view. A better understanding of the available barrier membranes will lead to a better selection for each clinical situation.
2. Search methodology
A search at PubMed/MEDLINE and Scopus was performed for documents published in English using the following keywords: guided bone regeneration, guided tissue regeneration, bone augmentation, barrier membranes, functionally graded membranes, dental materials, biomaterials and biodegradability. Documents published within the past 20 years were selected and we further screened the bibliographies of the selected articles to identify relevant classical or groundbreaking studies. We discuss aspects of significant clinical impact, and opinions expressed here are also based on our own research and acquired knowledge.
3. Nonbiodegradable membranes
Barrier membranes composed of nonbiodegradable material is commonly used for relatively large-scale tissue regeneration owing to the ease of control of the shielding period (Table 1) [22]. Furthermore, they have the advantage that degradation by-products of the base materials do not need to be considered [23,24]. However, these membranes require surgical removal after tissue regeneration. It has been reported that nonbiodegradable membranes showed higher risk of complications related to membrane exposure during implantation than biodegradable membranes [25–27]. They can also be used in combination with metal pins and mini screws to avoid the collapse of their morphology [28,29].
Table 1.
Summary of nonbiodegradable barrier membranes.
| Materials | Advantages | Disadvantages | References |
|---|---|---|---|
| Polymers | |||
| Polytetrafluoroethylene (PTFE), expanded PTFE (e-PTFE), dense PTFE (d-PTFE), titanium-reinforced PTFE | - High chemical stability - High biocompatibility - High barrier function |
- Surgical removal required - Membrane exposure |
[25, 26, 30, 32, 34–38] |
| Metals | |||
| Titanium, titanium alloy | - High biocompatibility - High barrier function - Mechanical strength, durability |
- Surgical removal required - Expensive |
[51–55] |
| Cobalt, cobalt alloy | - Low cost - High mechanical strength - Solid space-making |
- Less biocompatibility | [56, 57] |
3.1. Nonbiodegradable synthetic polymers
Polytetrafluoroethylene (PTFE) is an example of a material used in nonbiodegradable membranes. The first reported barrier membrane was made from expanded PTFE (e-PTFE) [4,30–32]. PTFE is a stable polymer in vivo and it is categorized as a bioinert material [33,34]. This chemical stability, which counts in favor of biocompatibility, allows PTFE to endure biodegradation and prevents host immune responses. PTFE has high barrier function between tissues, therefore tends to reduce the blood supply resulting in dehiscence of the gingiva [35,36].
PTFE only membranes can be used for treatment, however titanium-reinforced membranes are common owing to their effective space-making. Recently, dense PTFE (d-PTFE), a compact form of PTFE, has been launched onto the market, and its efficacy for GBR has been evaluated [37]. Ronda et al. [38] reported that d- and e-PTFE membranes showed identical clinical results in the treatment of vertical bone defects. However, it was indicated that surgical removal was easier for the d-PTFE membrane than for the e-PTFE analogs, which could represent less disturbance to the regenerated tissue below.
3.2. Metals
Titanium—a popular material in dentistry as well as other medical fields—is used as both a pure metal and an alloy containing non-precious metals (e.g. aluminum, vanadium or nickel) [39–42]. Both the pure metal and alloys possess good biocompatibility, mechanical strength, durability, low density and corrosion resistance [43,44]. In addition, titanium is a bioinert material that can be used as a stable metal owing to the rapid formation of a passive layer [45,46]. Titanium is primarily used for bone fixation after maxillofacial surgery and has shown favorable outcomes [47]. As a result, titanium has been used for dental implants owing to its effective osseointegration showing direct joint to the bone through extracellular matrices [48–50]. Titanium membranes are more expensive than other membranes; however, they provide effective shape and can be applied for vertical and severe horizontal bone loss in combination with bone substitutes [51–55]. Recently, Hasegawa et al. [55] designed regular hexagonal honeycomb structure with 1 mm of inner circle in the titanium membrane. The authors also arranged microperforations with a diameter of 20 μm at 50-μm intervals within each honeycomb section. They implanted autologous bone to the bony defects created in the Beagle dogs and covered with the prototype membrane. In this study, it was demonstrated that mature osseous tissue was formed after 26 weeks of implantation. However, microperforations might be a difficulty when retrieving the membrane at the second surgery. Epithelial and connective tissue that invades the perforations will inevitably be removed with the membrane, potentially causing discomfort to the patient, and prolonging the healing period.
The use of cobalt and cobalt-chromium alloy in barrier membranes has been reported [56,57]. Decco et al. [56] applied a cobalt-chromium membrane to a noncritical bone defect in rabbit tibia and reported that the tested membrane showed solid space-making and favorable bone augmentation. Cobalt-chromium alloy is a bioinert metal like titanium, but wear processes can lead to the release of toxic chromium and cobalt ions into the body [58]. Cobalt-chromium alloy has the advantages of mechanical strength and low cost compared with titanium however it shows poorer biocompatibility than titanium, therefore there have been no clinical trials using cobalt-chromium membrane implants in humans.
The reliable space-making ability of nondegradable membranes is because of their high mechanical strength, however changes in the metal composition do not seem to provide a clear advantage of one composition over another. On the other hand, these materials require a retrieval surgery, which can damage the regenerated tissue, prolong the healing period and increase the risk of infection.
4. Biodegradable membranes
Biodegradable membranes, which are almost exclusively polymer-based (natural and synthetic polymers), have the advantages of few complications and low cost, as well as secondary surgeries not being necessary (Table 2) [59–61]. Therefore, biodegradable membranes are regarded a first-choice material when the treatment outcome is expected to be the same as that using non-biodegradable materials. However, biodegradable membranes are liable to show tissue regeneration failure due to the volume loss of the membrane and its degradation by-products [62,63]. For animal-derived collagen membranes, residual virus and cross-linker also present concerns [62,64,65]. In general, biodegradable membranes show lower mechanical strength and are therefore less efficient at space-making than nonbiodegradable PTFE and titanium mesh. Therefore, in GBR, bone substitutes are used in combination with biodegradable membranes to maintain the membrane shape and lead to space-making.
Table 2.
Summary of biodegradable barrier membranes.
| Materials | Advantages | Disadvantages | References |
|---|---|---|---|
| Natural polymers | |||
| Collagen | - High biocompatibility - Favorable barrier function - Promotion of wound healing - No surgical removal |
- Hard to control biodegradation - Low mechanical strength - Residual cross-linking agents - Possible disease transmission |
[62, 66, 71–73, 76–79] |
| Other natural polymers (alginate, chitosan) | - Favorable biocompatibility - No surgical removal |
- Questionable barrier function - Hard to control biodegradation - Few studies |
[80–87] |
| Synthetic polymers | |||
| Aliphatic polyesters (PLA, PGA and PCL), these copolymers | - Favorable biocompatibility - Favorable barrier function - High reproducibility - Controllable biodegradability and mechanical properties - No surgical removal |
- Relatively low mechanical strength - Cytotoxicity of degradation byproduct |
[95–100, 102] |
4.1. Natural polymers
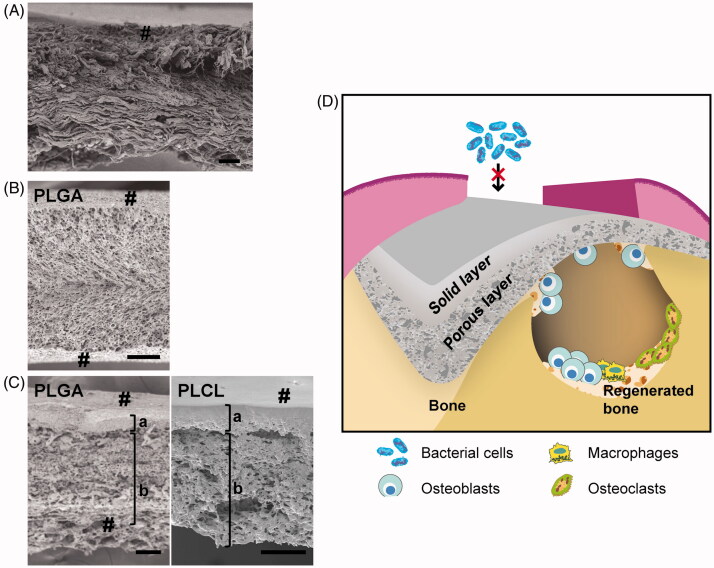
Type I and type III collagens derived from bovine and porcine are the commonly used materials for natural polymer membranes (Figure 1(A)) [66,67]. Collagen is an essential component of bone and connective tissue and supports these tissue structures [68–70]. Collagens are harvested from epidermis, tendon and intestine, then processed by decellularization, cross-linking treatment and sterilization to produce the barrier membranes [71]. There are various methods for cross-linking including ultraviolet irradiation and chemical treatment using glutaraldehyde, water-soluble carbodiimide and genipin [71,72]. These treatments increase hydrolysis resistance and membranes that can persist for six months within the human body have been developed for GBR treatment [9,73]. However, the residual chemical cross-linking agents were problematic for clinical usage due to their potential toxicity causing inflammation and interfering with cellular processes [74]. The cross-linking of structures improves the mechanical properties to an extent; however, the low stiffness of the membranes remains a drawback [71,75]. Therefore, collagen membranes were better suited to application in areas that are simple to set.
Figure 1.
Cross-sectional images and schematic illustration of GBR membrane. Cross-sectional electron micrographs of (A) collagen membrane (Bio-Gide®), (B) PLGA monolayer membrane and (C) bilayer membranes composed of PLGA (left) and PLCL (right). (D) Schematic illustration of a bilayer membrane for GBR. #: membrane surface; a: solid layer and b: porous layer (b) (scale bar: 100 μm). Reproduced with permission from Yoshimoto et al. [97] and Abe et al. [100]. Copyright Elsevier B.V.
There are some reports of the bone augmentation efficiency of collagen membranes in GBR [76,77]. The authors showed that the bone formation with collagen membranes was comparable to the amount observed for e-PTFE membrane. It was also reported that the collagen membranes contributed to bone regeneration as well as the passive barrier [78,79]. Stromal cells attached to the collagen membrane promoted the production of basic fibroblast growth factor (FGF)-2 and bone morphogenetic protein (BMP)-2 and these growth factors facilitated bone regeneration compared with other biodegradable membranes.
Chitosan and alginate have also been introduced for use as barrier membranes in the study phase [80–87]. Chitosan—a straight-chain polysaccharide, copolymer of glucosamine and N-acetyl-D-glucosamine—is industrially produced by the alkaline treatment of crustacean chitin [88,89]. Chitosan exhibits biodegradability, favorable biocompatibility and flexible workability, therefore chitosan processed in fibrous, film and spongy forms has been applied to surgical sutures and artificial skin [90,91]. It is thought that the biodegradability of chitosan depends on its molecular weight [92]. Although chitosan-based membranes showed favorable bioactive properties, such as bacteriostatic and homeostatic abilities, it still retains low mechanical properties as other natural polymers.
Alginate, a natural polymer extracted from seaweed, is a popular impression material in dentistry. Alginate hydrogel shows high biocompatibility and has a similar structure to extracellular matrix [89,93,94]. An in vivo study showed that chitosan and alginate membranes can function as barrier membranes, however there are currently no clinical trials assessing their performance in humans, so they are not applied in the clinic [80–82,87]. This may be because these materials have not been proven superior to their commercial counterparts.
4.2. Biodegradable synthetic polymers
Biodegradable aliphatic polyesters—such as polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL) and their copolymers (e.g. poly(lactic-co-glycolic acid) (PLGA) and poly(lactide-co-caprolactone) (PLCL))—are also used as barrier membranes [67,95–100]. Aliphatic polyester membranes can be made to be reproducible because these workable materials are processed industrially. The advantages of aliphatic polyester membranes include their adjustable biodegradability and mechanical properties, which can be controlled by regulating the polymer composition [101,102]. In addition, therapeutics and materials that promote tissue regeneration can be easily impregnated into the membranes [103]. The biodegradability of aliphatic copolymer varies significantly depending on the types and ratio of polyester. The addition of PCL, which shows greater hydrophobicity and lower degradability than PLA and PGA, allows the lifetime of the membrane to be extended [100]. However, improving the mechanical properties using only biodegradable polyester is challenging, therefore these membranes tend to be applied for small tissue defects or in combination with bone substitute.
Recently, our group developed bilayer membranes based on PLGA or PLCL [97,100]. These membranes comprised a solid layer and a porous layer, which respectively provided barrier function and cell support (Figure 1(B,C)). The thickness of each layer could be regulated by adjusting the temperature used for freeze drying. These membranes showed lower mechanical strength but better operability than the monolayer membranes. It was shown that the porous structure promoted cell proliferation and osteogenic differentiation of mesenchymal stem cells. It was also shown that the PLGA bilayer membrane was able to promote bone regeneration in vivo, where bone formation with the PLGA bilayer was significantly higher than that with a monolayer membrane [97]. As regards PLCL bilayer membrane, we have demonstrated that solid layer reduced bacterial adhesion and prevented bacterial invasion inside the membrane [104]. This characteristic could improve the prognosis and simplify the management of GTR/GBR complications. Furthermore, the PLCL membrane has a slower degradation rate than the other biodegradable polymers. Thus, PLCL bilayer membranes are considered promising biomaterials for GBR treatment (Figure 1(D)) [100].
No polymer, natural or synthetic, seem to be sufficient on its own. The association of different materials has the potential to combine their best features. Bioactivity and mechanical strength are the main target of the current attempts to improve polymeric membranes, and it is in this context that the incorporation of additives is proposed.
5. Membrane additives
There are some reports that incorporating inorganic components into barrier membranes promotes bone regeneration and improves the mechanical strength [86,105–108]. Hydroxyapatite, a calcium phosphate, is widely used in bone regenerative medicine and shows nonbiodegradability and osteoconductive properties [109,110]. Some research groups have attempted to add hydroxyapatite to barrier membranes for GTR and GBR applications [86,106,107,111]. Basile et al. [107] combined nanowhisker hydroxyapatites and modified PCL and reported that the composite membrane showed favorable proliferation and osteogenic differentiation of mesenchymal stromal cells in vitro. Veríssimo et al. [106] fabricated hydroxyapatite deposited collagen membranes and implanted them into critical-sized calvaria defects. The authors reported that the experimental membranes accelerated bone healing, but lost their biodegradability.
Beta-tricalcium phosphate (β-TCP)—categorized as a calcium phosphate material, similarly to hydroxyapatite—also possesses osteoconductivity and biodegradability [111–113]. Shim et al. [105] blended PLGA, PCL and β-TCP and fabricated a mesh membrane for GBR. The authors reported that this composite membrane promoted proliferation and osteogenic differentiation of mesenchymal stem cells in vitro, and increased bone formation without using bone substitute in vivo.
Bioactive glass composed mainly of silicon dioxide is an amorphous material that shows biodegradability [114–116]. Bioactive glass can release calcium and silicate ions, enhancing the activity of osteoblasts, and as a result forms a connection with bone [116–118]. It has been shown that bioactive glass contained in biodegradable membranes promoted mineral deposition on the surface and osteoblastic cell functions [84,87,98,119,120]. Hong et al. [119] combined a bioactive glass with collagen membrane, to which FGF-2 solution was infiltrated. The authors implanted the hybrid membranes in rat calvaria defects, which subsequently showed accelerated bone regeneration.
6. Conclusion
As a result of the progress in polymer science, barrier membranes have become widely used in dentistry. The membranes for GTR and GBR are expected to possess multiple properties that if effectively combined would develop an excellent material. More recently, bioactive functions of membrane have been experimented with, aiming at the enhanced promotion of tissue regeneration. To achieve these high standards, future research must explore the combination of growth factors and antibacterial agents in barrier membranes, as well as the dynamics of material degradation.
There are still no guidelines for choosing barrier membranes for GTR and GBR apart from the general considerations of selecting biodegradable versus nonbiodegradable membrane. Consequently, the protocols and materials used for these treatments depend greatly on the shortcomings of each membrane, and on the professional experience and skill of the dentists. Therefore, dentists must understand the characteristics of barrier membranes, as well as bone substitutes and growth factors, to select a suitable barrier membrane.
Funding Statement
This work was supported in part by Grants-in-Aid for Scientific Research [Nos. 17K11778, and 17H04383] from the Japan Society for the Promotion of Science. The funding sources were not involved in our study or our decision to publish the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Hämmerle CHF, Jung RE.. Bone augmentation by means of barrier membranes. Periodontol 2000. 2003;33:36–53. [DOI] [PubMed] [Google Scholar]
- 2.Khojasteh A, Kheiri L, Motamedian SR, et al. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann Maxillofac Surg. 2017;7(2):263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elgali I, Omar O, Dahlin C, et al. Guided bone regeneration: materials and biological mechanisms revisited. Eur J Oral Sci. 2017;125(5):315–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyman S, Lindhe J, Karring T, et al. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9(4):290–296. [DOI] [PubMed] [Google Scholar]
- 5.Murphy KG, Gunsolley JC.. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol. 2003;8(1):266–302. [DOI] [PubMed] [Google Scholar]
- 6.Faria-Almeida R, Astramskaite-Januseviciene I, Puisys A, et al. Extraction socket preservation with or without membranes, soft tissue influence on post extraction alveolar ridge preservation: A systematic review. J Oral Maxillofac Res. 2019;10:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masquelet AC, Begue T.. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41(1):27–37. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Kerns DG.. Mechanisms of guided bone regeneration: A review. Open Dent J. 2014;8:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoornaert A, d'Arros C, Heymann MF, et al. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed Mater. 2016;11(4):045012. [DOI] [PubMed] [Google Scholar]
- 10.Caballé-Serrano J, Sawada K, Miron RJ, et al. Collagen barrier membranes adsorb growth factors liberated from autogenous bone chips. Clin Oral Impl Res. 2017;28(2):236–241. [DOI] [PubMed] [Google Scholar]
- 11.Pelissier P, Masquelet AC, Bareille R, et al. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22(1):73–79. [DOI] [PubMed] [Google Scholar]
- 12.Viateau V, Guillemin G, Calando Y, et al. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: An ovine model. Vet Surgery. 2006;35(5):445–452. [DOI] [PubMed] [Google Scholar]
- 13.Gottlow J. Guided tissue regeneration using bioresorbable and non-resorbable devices: Initial healing and long-term results. J Periodontol. 1993;64(11s):1157–1165. [DOI] [PubMed] [Google Scholar]
- 14.Retzepi M, Donos N.. Guided bone regeneration: Biological principle and therapeutic applications. Clin Oral Implants Res. 2010;21(6):567–576. [DOI] [PubMed] [Google Scholar]
- 15.Moses O, Pitaru S, Artzi Z, et al. Healing of dehiscence-type defects in implants placed together with different barrier membranes: A comparative clinical study. Clin Oral Implants Res. 2005;16(2):210–219. [DOI] [PubMed] [Google Scholar]
- 16.Caballe-Serrano J, Munar-Frau A, Ortiz-Puigpelat O, et al. On the search of the ideal barrier membrane for guided bone regeneration. J Clin Exp Dent. 2018;10:e477–e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florjanski W, Orzeszek S, Olchowy A, et al. Modifications of polymeric membranes used in guided tissue and bone regeneration. Polymers. 2019;11(5):782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meinig RP. Clinical use of resorbable polymeric membranes in the treatment of bone defects. Orthop Clin North Am. 2010;41(1):39–47. [DOI] [PubMed] [Google Scholar]
- 19.Wiltfang J, Merten HA, Peters JH.. Comparative study of guided bone regeneration using absorbable and permanent barrier membranes: A histologic report. Int J Oral Maxillofac Implants. 1998;13(3):416–421. [PubMed] [Google Scholar]
- 20.Dimitriou R, Mataliotakis GI, Calori GM, et al. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaushal S, Kumar A, Khan MA, et al. Comparative study of nonabsorbable and absorbable barrier membranes in periodontal osseous defects by guided tissue regeneration. J Oral Biol Craniofac Res. 2016;6(2):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soldatos NK, Stylianou P, Koidou VP, et al. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017;48(2):131–147. [DOI] [PubMed] [Google Scholar]
- 23.Bergsma JE, Rozema FR, Bos RR, et al. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polyactide particles. Biomaterials. 1995;16(4):267–274. [DOI] [PubMed] [Google Scholar]
- 24.Evans GH, Yukna RA, Cambre KM, et al. Clinical regeneration with guided tissue barriers. Curr Opin Periodontol. 1997;4:75–81. [PubMed] [Google Scholar]
- 25.Murphy KG. Postoperative healing complications associated with Gore-Tex periodontal material. Part I. Incidence and characterization. Int J Periodontics Restorative Dent. 1995;15(4):363–375. [PubMed] [Google Scholar]
- 26.Murphy KG. Postoperative healing complications associated with Gore-Tex periodontal material. Part II. Effect of complications on regeneration. Int J Periodontics Restorative Dent. 1995;15(6):548–561. [PubMed] [Google Scholar]
- 27.Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: A meta-analysis. J Periodontol. 2001;72(4):512–516. [DOI] [PubMed] [Google Scholar]
- 28.Leinonen S, Suokas E, Veiranto M, et al. Holding power of bioabsorbable ciprofloxacin-containing self-reinforced poly-L/DL-lactide 70/30 bioactive glass 13 miniscrews in human cadaver bone. J Craniofac Surg. 2002;13:212–218. [DOI] [PubMed] [Google Scholar]
- 29.Chasioti E, Chiang TF, Drew HJ.. Maintaining space in localized ridge augmentation using guided bone regeneration with tenting screw technology. Quintessence Int. 2013;44(10):763–771. [DOI] [PubMed] [Google Scholar]
- 30.Simion M, Baldoni M, Rossi P, et al. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994;14(2):166–180. [PubMed] [Google Scholar]
- 31.Aaboe M, Pinholt EM, Hjørting-Hansen E.. Healing of experimentally created defects: A review. Br J Oral Maxillofac Surg. 1995;33(5):312–318. [DOI] [PubMed] [Google Scholar]
- 32.Trobos M, Juhlin A, Shah FA, et al. In vitro evaluation of barrier function against oral bacteria of dense and expanded polytetrafluoroethylene (PTFE) membranes for guided bone regeneration. Clin Implant Dent Relat Res. 2018;20(5):738–748. [DOI] [PubMed] [Google Scholar]
- 33.Kohal RJ, Trejo PM, Wirsching C, et al. Comparison of bioabsorbable and bioinert membranes for guided bone regeneration around non-submerged implants. An experimental study in the mongrel dog. Clin Oral Implants Res. 1999;10(3):226–237. [DOI] [PubMed] [Google Scholar]
- 34.Korzinskas T, Jung O, Smeets R, et al. In vivo analysis of the biocompatibility and macrophage response of a non-resorbable PTFE membrane for guided bone regeneration. Int J Mol Sci. 2018;19(10):2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiapasco M, Zaniboni M.. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin Oral Implants Res. 2009;20:113–123. [DOI] [PubMed] [Google Scholar]
- 36.Garcia J, Dodge A, Luepke P, et al. Effect of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(3):328–338. [DOI] [PubMed] [Google Scholar]
- 37.Gallo P, Díaz-Báez D.. Management of 80 complications in vertical and horizontal ridge augmentation with nonresorbable membrane (d-PTFE): A cross-sectional study. Int J Oral Maxillofac Implants. 2019;34(4):927–935. [DOI] [PubMed] [Google Scholar]
- 38.Ronda M, Rebaudi A, Torelli L, et al. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin Oral Impl Res. 2014;25(7):859–866. [DOI] [PubMed] [Google Scholar]
- 39.An SH, Matsumoto T, Sasaki JI, et al. In vitro bioactivity evaluation of nano- and micro-crystalline anodic TiO2: HA formation, cellular affinity and organ culture. Mater Sci Eng C Mater Biol Appl. 2012;32(8):2516–2522. [Google Scholar]
- 40.Gavini G, Santos MD, Caldeira CL, et al. Nickel-titanium instruments in endodontics: A concise review of the state of the art. Braz Oral Res. 2018;32(suppl 1):e67. [DOI] [PubMed] [Google Scholar]
- 41.García-Martínez E, Miguel V, Martínez-Martínez A, et al. Sustainable lubrication methods for the machining of titanium alloys: An overview. Materials. 2019;12(23):3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karre R, Kodli BK, Rajendran A, et al. Comparative study on Ti-Nb binary alloys fabricated through spark plasma sintering and conventional P/M routes for biomedical application. Mater Sci Eng C Mater Biol Appl. 2019;94:619–627. [DOI] [PubMed] [Google Scholar]
- 43.Wang RR, Fenton A.. Titanium for prosthodontic applications: A review of the literature. Quintessence Int. 1996;27:401–408. [PubMed] [Google Scholar]
- 44.Ottria L, Lauritano D, Andreasi BM, et al. Mechanical, chemical and biological aspects of titanium and titanium alloys in implant dentistry. J Biol Regul Homeost Agents. 2018;32(2 Suppl. 1):81–90. [PubMed] [Google Scholar]
- 45.An SH, Matsumoto T, Miyajima H, et al. Surface characterization of alkali- and heat-treated Ti with or without prior acid etching. Appl Surf Sci. 2012;258(10):4377–4382. [Google Scholar]
- 46.Hanawa T. Titanium-tissue interface reaction and its control with surface treatment. Front Bioeng Biotechnol. 2019;17(7):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyne PJ, Cole MD, Stringer D, et al. A technique for osseous restoration of deficient edentulous maxillary ridges. J Oral Maxillofac Surg. 1985;43(2):87–91. [DOI] [PubMed] [Google Scholar]
- 48.Roehling S, Schlegel KA, Woelfler H, et al. Zirconia compared to titanium dental implants in preclinical studies-A systematic review and meta-analysis. Clin Oral Implants Res. 2019;30(5):365–395. [DOI] [PubMed] [Google Scholar]
- 49.Jurczak P, Witkowska J, Rodziewicz-Motowidło S, et al. Proteins, peptides and peptidomimetics as active agents in implant surface functionalization. Adv Colloid Interface Sci. 2020;276:102083. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Jansen JA, Walboomers XF, et al. Mechanical aspects of dental implants and osseointegration: A narrative review. J Mech Behav Biomed Mater. 2020;103:103574. [DOI] [PubMed] [Google Scholar]
- 51.Zablotsky M, Meffert R, Caudill R, et al. Histological and clinical comparisons of guided tissue regeneration on dehisced hydroxylapatite-coated and titanium endosseous implant surfaces: A pilot study. Int J Oral Maxillofac Implants. 1991;6(3):294–303. [PubMed] [Google Scholar]
- 52.Sumi Y, Miyaishi O, Tohnai I, et al. Alveolar ridge augmentation with titanium mesh and autogenous bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(3):268–270. [DOI] [PubMed] [Google Scholar]
- 53.Degidi M, Scarano A, Piattelli A.. Regeneration of the alveolar crest using titanium micromesh with autologous bone and a resorbable membrane. J Oral Implantol. 2003;29(2):86–90. [DOI] [PubMed] [Google Scholar]
- 54.Rakhmatia YD, Ayukawa Y, Furuhashi A, et al. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013;57(1):3–14. [DOI] [PubMed] [Google Scholar]
- 55.Hasegawa H, Masui S, Ishihata H.. New microperforated pure titanium membrane created by laser processing for guided regeneration of bone. Br J Oral Maxillofac Surg. 2018;56(7):642–643. [DOI] [PubMed] [Google Scholar]
- 56.Decco O, Cura A, Beltrán V, et al. Bone augmentation in rabbit tibia using microfixed cobalt-chromium membranes with whole blood, tricalcium phosphate and bone marrow cells. Int J Clin Exp Med. 2015;8(1):135–144. [PMC free article] [PubMed] [Google Scholar]
- 57.Lin WC, Yao C, Huang TY, et al. Long-term in vitro degradation behavior and biocompatibility of polycaprolactone/cobalt-substituted hydroxyapatite composite for bone tissue engineering. Dent Mater. 2019;35(5):751–762. [DOI] [PubMed] [Google Scholar]
- 58.Eliaz N. Corrosion of metallic biomaterials: A review. Materials. 2019;12(3):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vert M. Aliphatic polyesters: Great degradable polymers that cannot do everything. Biomacromolecules. 2005;6(2):538–546. [DOI] [PubMed] [Google Scholar]
- 60.Sam G, Pillai BR.. Evolution of barrier membranes in periodontal regeneration-"Are the third generation membranes really here?". J Clin Diagn Res. 2014;8(12):ZE14–ZE17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bottino MC, Pankajakshan D, Nör JE.. Advanced scaffolds for dental pulp and periodontal regeneration. Dent Clin North Am. 2017;61(4):689–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Döri F, Huszár T, Nikolidakis D, et al. Effect of platelet-rich plasma on the healing of intra-bony defects treated with a natural bone mineral and a collagen membrane. J Clin Periodontol. 2007;34(3):254–261. [DOI] [PubMed] [Google Scholar]
- 63.Hoogeveen EJ, Gielkens PF, Schortinghuis J, et al. Vivosorb as a barrier membrane in rat mandibular defects. An evaluation with transversal microradiography. Int J Oral Maxillofac Surg. 2009;38(8):870–875. [DOI] [PubMed] [Google Scholar]
- 64.Gielkens PF, Schortinghuis J, de Jong JR, et al. Vivosorb, Bio-Gide, and Gore-Tex as barrier membranes in rat mandibular defects: An evaluation by microradiography and micro-CT. Clin Oral Implants Res. 2008;19(5):516–521. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Wang L, Zhou Z, et al. Biodegradable polymer membranes applied in guided bone/tissue regeneration: A review. Polymers. 2016;8(4):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wessing B, Lettner S, Zechner W.. Guided bone regeneration with collagen membranes and particulate graft materials: A systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2018;33(1):87–100. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z, Liang R, Jiang X, et al. Electrospun PLGA/PCL/OCP nanofiber membranes promote osteogenic differentiation of mesenchymal stem cells (MSCs). Mater Sci Eng C Mater Biol Appl. 2019;104:109796. [DOI] [PubMed] [Google Scholar]
- 68.Patino MG, Neiders ME, Andreana S, et al. Collagen: An overview. Implant Dent. 2002;11(3):280–285. [DOI] [PubMed] [Google Scholar]
- 69.Sasaki JI, Matsumoto T, Egusa H, et al. In vitro engineering of transitional tissue by patterning and functional control of cells in fibrin gel. Soft Matter. 2010;6(8):1662–1667. [Google Scholar]
- 70.Sasaki J, Matsumoto T, Egusa H, et al. In vitro reproduction of endochondral ossification using a 3D mesenchymal stem cell construct. Integr Biol (Camb). 2012;4(10):1207–1214. [DOI] [PubMed] [Google Scholar]
- 71.Sbricoli L, Guazzo R, Annunziata M, et al. Selection of collagen membranes for bone regeneration: A literature review. Materials. 2020;13(3):786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bottino MC, Thomas V, Schmidt G, et al. Recent advances in the development of GTR/GBR membranes for periodontal regeneration–A materials perspective. Dent Mater. 2012;28(7):703–721. [DOI] [PubMed] [Google Scholar]
- 73.Kozlovsky A, Aboodi G, Moses O, et al. Bio-degradation of a resorbable collagen membrane (Bio-Gide) applied in a double-layer technique in rats. Clin Oral Implants Res. 2009;20(10):1116–1123. [DOI] [PubMed] [Google Scholar]
- 74.Ferreira AM, Gentile P, Chiono V, et al. Collagen for bone tissue regeneration. Acta Biomater. 2012;8(9):3191–3200. [DOI] [PubMed] [Google Scholar]
- 75.Nair LS, Laurencin CT.. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8-9):762–798. [Google Scholar]
- 76.Zitzmann NU, Naef R, Schärer P.. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int J Oral Maxillofac Implants. 1997;12:844–852. [PubMed] [Google Scholar]
- 77.Bunyaratavej P, Wang HL.. Collagen membranes: A review. J Periodontol. 2001;72(2):215–229. [DOI] [PubMed] [Google Scholar]
- 78.Turri A, Elgali I, Vazirisani F, et al. Guided bone regeneration is promoted by the molecular events in the membrane compartment. Biomaterials. 2016;84:167–183. [DOI] [PubMed] [Google Scholar]
- 79.Gueldenpfennig T, Houshmand A, Najman S, et al. The condensation of collagen leads to an extended standing time and a decreased pro-inflammatory tissue response to a newly developed pericardium-based barrier membrane for guided bone regeneration. In Vivo. 2020;34(3):985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishikawa K, Ueyama Y, Mano T, et al. Self-setting barrier membrane for guided tissue regeneration method: Initial evaluation of alginate membrane made with sodium alginate and calcium chloride aqueous solutions. J Biomed Mater Res. 1999;47(2):111–115. [DOI] [PubMed] [Google Scholar]
- 81.Ueyama Y, Ishikawa K, Mano T, et al. Usefulness as guided bone regeneration membrane of the alginate membrane. Biomaterials. 2002;23(9):2027–2033. [DOI] [PubMed] [Google Scholar]
- 82.He H, Huang J, Ping F, et al. Calcium alginate film used for guided bone regeneration in mandible defects in a rabbit model. Cranio. 2008;26(1):65–70. [DOI] [PubMed] [Google Scholar]
- 83.Ma S, Adayi A, Liu Z, et al. Asymmetric collagen/chitosan membrane containing minocycline-loaded chitosan nanoparticles for guided bone regeneration. Sci Rep. 2016;6(1):31822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou T, Liu X, Sui B, et al. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed Mater. 2017;12(5):055004. [DOI] [PubMed] [Google Scholar]
- 85.Wu C, Su H, Karydis A, et al. Mechanically stable surface-hydrophobilized chitosan nanofibrous barrier membranes for guided bone regeneration. Biomed Mater. 2017;13(1):015004. [DOI] [PubMed] [Google Scholar]
- 86.Huang D, Niu L, Li J, et al. Reinforced chitosan membranes by microspheres for guided bone regeneration. J Mech Behav Biomed Mater. 2018;81:195–201. [DOI] [PubMed] [Google Scholar]
- 87.Shah AT, Zahid S, Ikram F, et al. Tri-layered functionally graded membrane for potential application in periodontal regeneration. Mater Sci Eng C Mater Biol Appl. 2019;103:109812. [DOI] [PubMed] [Google Scholar]
- 88.Tharanathan RN, Kittur FS.. Chitin–the undisputed biomolecule of great potential. Crit Rev Food Sci Nutr. 2003;43(1):61–87. [DOI] [PubMed] [Google Scholar]
- 89.Catoira MC, Fusaro L, Di Francesco D, et al. Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med. 2019;30(10):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sultankulov B, Berillo D, Sultankulova K, et al. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules. 2019;9(9):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Masi A, Tonazzini I, Masciullo C, et al. Chitosan films for regenerative medicine: Fabrication methods and mechanical characterization of nanostructured chitosan films. Biophys Rev. 2019;11(5):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lauritano D, Limongelli L, Moreo G, et al. Nanomaterials for periodontal tissue engineering: Chitosan-based scaffolds. A systematic review. Nanomaterials. 2020;10(4):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szabó L, Gerber-Lemaire S, Wandrey C.. Strategies to functionalize the anionic biopolymer Na-alginate without restricting its polyelectrolyte properties. Polymers. 2020;12(4):919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abasalizadeh F, Moghaddam SV, Alizadeh E, et al. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J Biol Eng. 2020;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geurs NC, Korostoff JM, Vassilopoulos PJ, et al. Clinical and histologic assessment of lateral alveolar ridge augmentation using a synthetic long-term bioabsorbable membrane and an allograft. J Periodontol. 2008;79(7):1133–1140. [DOI] [PubMed] [Google Scholar]
- 96.Annunziata M, Nastri L, Cecoro G, et al. The use of Poly-d,l-lactic acid (PDLLA) devices for bone augmentation techniques: A systematic review. Molecules. 2017;22(12):2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoshimoto I, Sasaki JI, Tsuboi R, et al. Development of layered PLGA membranes for periodontal tissue regeneration. Dent Mater. 2018;34(3):538–550. [DOI] [PubMed] [Google Scholar]
- 98.Haghighat A, Shakeri S, Mehdikhani M, et al. Histologic, histomorphometric, and osteogenesis comparative study of a novel fabricated nanocomposite membrane versus cytoplast membrane. J Oral Maxillofac Surg. 2019;77(10):2027–2039. [DOI] [PubMed] [Google Scholar]
- 99.Zhang HY, Jiang HB, Ryu JH, et al. Comparing properties of variable pore-sized 3D-printed PLA membrane with conventional PLA membrane for guided bone/tissue regeneration. Materials. 2019;12(10):1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abe GL, Sasaki JI, Katata C, et al. Fabrication of novel poly(lactic acid/caprolactone) bilayer membrane for GBR application. Dent Mater. 2020;36(5):626–634. [DOI] [PubMed] [Google Scholar]
- 101.Zamboulis A, Nakiou EA, Christodoulou E, et al. Polyglycerol hyperbranched polyesters: Synthesis, properties and pharmaceutical and biomedical applications. Int J Mol Sci. 2019;20(24):6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chi M, Qi M, A L, et al. Novel bioactive and therapeutic dental polymeric materials to inhibit periodontal pathogens and biofilms. Int J Mol Sci . 2019;20(2):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caballero Aguilar LM, Silva SM, Moulton SE.. Growth factor delivery: Defining the next generation platforms for tissue engineering. J Control Release. 2019;306:40–58. [DOI] [PubMed] [Google Scholar]
- 104.Tsuboi R, Abe GL, Kitagawa H, et al. Barrier effects of new bilayer GBR membrane against bacteria invasion. 2020 IADR/AADR/CADR General Session (Washington, D.C., USA). 2020. Abstract. No. 2506. [Google Scholar]
- 105.Shim JH, Huh JB, Park JY, et al. Fabrication of blended polycaprolactone/poly(lactic-co-glycolic acid)/beta-tricalcium phosphate thin membrane using solid freeform fabrication technology for guided bone regeneration. Tissue Eng Part A. 2013;19(3-4):317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verissimo DM, Leitao RF, Figueiro SD, et al. Guided bone regeneration produced by new mineralized and reticulated collagen membranes in critical-sized rat calvarial defects. Exp Biol Med. 2015;240:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Basile MA, d'Ayala GG, Malinconico M, et al. Functionalized PCL/HA nanocomposites as microporous membranes for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2015;48:457–468. [DOI] [PubMed] [Google Scholar]
- 108.Ezati M, Safavipour H, Houshmand B, et al. Development of a PCL/gelatin/chitosan/beta-TCP electrospun composite for guided bone regeneration. Prog Biomater. 2018;7(3):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matsumoto T, Okazaki M, Nakahira A, et al. Modification of apatite materials for bone tissue engineering and drug delivery carriers. Curr Med Chem. 2007;14(25):2726–2733. [DOI] [PubMed] [Google Scholar]
- 110.Elgali I, Turri A, Xia W, et al. Guided bone regeneration using resorbable membrane and different bone substitutes: Early histological and molecular events. Acta Biomater. 2016;29:409–423. [DOI] [PubMed] [Google Scholar]
- 111.Guillaume B. Filling bone defects with beta-TCP in maxillofacial surgery: A review. Morphologie. 2017;101(334):113–119. [DOI] [PubMed] [Google Scholar]
- 112.Rh Owen G, Dard M, Larjava H.. Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J Biomed Mater Res. 2018;106(6):2493–2512. [DOI] [PubMed] [Google Scholar]
- 113.Iviglia G, Kargozar S, Baino F.. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. JFB. 2019;10(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hench LL, Polak JM.. Third-generation biomedical materials. Science. 2002;295(5557):1014–1017. [DOI] [PubMed] [Google Scholar]
- 115.Miguez-Pacheco V, Hench LL, Boccaccini AR.. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015;13:1–15. [DOI] [PubMed] [Google Scholar]
- 116.Sasaki JI, Kiba W, Abe GL, et al. Fabrication of strontium-releasable inorganic cement by incorporation of bioactive glass. Dent Mater. 2019;35(5):780–788. [DOI] [PubMed] [Google Scholar]
- 117.Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering. Acta Biomater. 2011;7(6):2355–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoppe A, Güldal NS, Boccaccini AR.. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. [DOI] [PubMed] [Google Scholar]
- 119.Hong KS, Kim EC, Bang SH, et al. Bone regeneration by bioactive hybrid membrane containing FGF2 within rat calvarium. J Biomed Mater Res A. 2010;94(4):1187–1194. [DOI] [PubMed] [Google Scholar]
- 120.Leal AI, Caridade SG, Ma J, et al. Asymmetric PDLLA membranes containing Bioglass for guided tissue regeneration: Characterization and in vitro biological behavior. Dent Mater. 2013;29(4):427–436. [DOI] [PubMed] [Google Scholar]