Dear Editor,
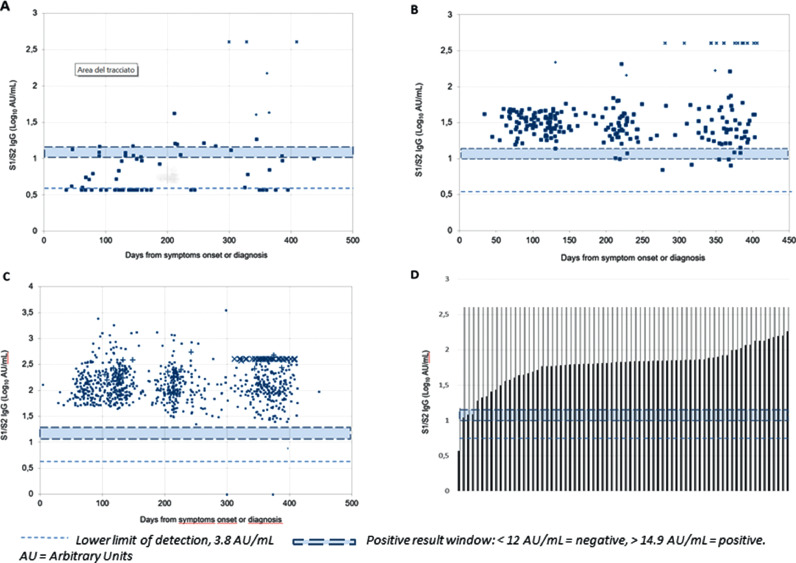
We read with interest the article by Ross J Harris and colleagues,1 showing assay-dependent durability of antibodies to SARS-CoV-2 after 6 months of symptomatic and asymptomatic COVID-19 as assessed by five different immunoassays with a prediction of durability at one year. We present our preliminary data on DiaSorin anti S1/S2 IgG production after one year from disease onset or detection of SARS-CoV-2 infection. The AntiCROWN study is being run in an hospital-based outpatient clinic open to all people who have serological or PCR evidence of SARS-Cov-2 infection. On May 13, 2020, the Infectious Diseases Department of Luigi Sacco Hospital, Milan, Italy, started an outpatient clinic for the follow-up of COVID-19 patients, diagnosed by a positive nasopharyngeal swab or a combination of clinical and epidemiological criteria and a positive serological test. Only patients whose onset was dated between February 20 and April 30, 2020 are included in this analysis (the so called “first wave”). The LIAISON® SARS-CoV-2 S1/S2 IgG solution (DiaSorin, Saluggia, Italy) used to quantify the antibody response shows a positive agreement of 94.4% (88.8%–97.2%) with in vitro neutralising antibody titre.2 The response was tested at the first outpatient visit (T1) set at week 12 ± 3 weeks from symptoms onset or diagnosis in asymptomatic subjects, at T2 (20 ± 3 weeks), T3 (32 ± 3 weeks) and T4 (52 ± 3 weeks). Since December, 2020, we were imposed a ceiling cut-off of 400 AU/mL. According to the WHO classification for COVID-19 severity patients were divided into mild, moderate, severe and critical.3 We calculated the relative risk of falling < 15 Arbitrary Units (AU)/mL with 95% confidence interval and statistical significance according to Altman and the significance of decay or increase over time through the Mann-Whitney and Wilcoxon log-rank test. The study was approved by the “Comitato Etico Interaziendale Area 1″. All patients signed a written informed consent. The full ARCOVID cohort counts 1048 outpatients, of whom 503 from the ‘first wave’. We present preliminary data of 368 patients who had a one-year control of serum anti-S1/S2 IgG levels (11 to 14 months, median 12.5 months). Our patients belonged to all severity classes according to the WHO definition. However, since response better correlated with baseline antibody production, we used this criterion to analyse data presented in Table 1 , stratifying by <15 (positive cut-off value), 15–50 (arbitrary cut-off for low antibody production) and >50 AU/mL. The mean age was 58.9 years (range 4–92 years) and 174 (41.8%) were females. Immune suppression or immune depression (HIV infection, cancer, immune diseases and autoimmunity, steroids, anti-cancer chemotherapy, monoclonal anti-B lymphocyte drugs) were present in patients. Our data suggest that loss of anti-S1/S2 IgG response at one year may be a rare event, occurring only in subjects who produce less than 50 AU/mL within the initial 4 months since disease onset. Of note, 12 patients had unexpected increase of antibody production in the absence of vaccination (+ 40 AU/mL and at least double compared to baseline), which suggests renewed exposure to the virus without developing symptoms. Stratifying for baseline antibody production did not show significant differences in such phenomenon. Moreover, only 6 patients had a new clinical COVID-19 event, four having IgG levels below 15 AU/mL. Events were mild and only one patient, who had recently been receiving monoclonal anti-B lymphocyte suppressive therapy for lymphoma, developed moderate pneumonia and was admitted to hospital, showing rapid clinical improvement during a 5-day stay. This patient subsequently responded minimally to recall vaccination with the BNT162b2 vaccine (15.1 AU/mL), whereas all the remaining 75 patients who were vaccinated showed an increase in antibody production over the ceiling cut-off, 67 (88.2%) having received a single vaccine shot. Fig. 1 allows overall visual understanding of the antibody production over time. In conclusion, our observation suggests that antiS1/S2 antibodies are fairly stable over one year. The few clinical events seem to occur almost only in those patients who had never responded and loss of protection in those who showed poor initial antibody response. This confirms similar observations reported in a shorter time frame by Lumley et al.4 Our aim now is to continue the observation until two years and widen the population with the “second wave”, which, by November, will bring our one-year observation to almost 1.100 patients, as well as to follow the response to single-dose vaccination over time in COVID-19 patients. If data are confirmed, we feel that COVID-19 provides long-lasting immunity to symptomatic subjects as well as to a proportion of asymptomatic subjects, although boosting immunity with a single dose, irrespective of the time lapsed from the clinical event, may improve the intensity5 and possibly the duration of such response. Similar observations may help inform health policy decisions,6 although their main limitation remains the fact they are necessarily performed in a setting influenced by the WHO advices7 and further restricted by local authorities’ measures. Indeed, nobody knows what this means in a world free of masks and social distancing.
Table 1.
Antibody response and new COVID-19 events, total 368/503 patients with results at one year.
| Baseline value (AU*/mL) | < 15 | 15–50 | >50 |
|---|---|---|---|
| N (%) | 29 (7.9) | 65 (17.7) | 274 (74.4) |
| one died of mesothelioma | |||
| Female sex, n (%) | 9 (31) | 41 (63.1) | 126 (46) |
| Median age (range) | 48.5 (18-92) | 46.5 (15–83) | 59 (4–87) |
| Immune depression/ immune suppression, n (%) | 1 (3.4) | 9 (14.3) | 43 (15.9) |
| WHO severity scale represented (m: mild; M: moderate; S: severe; C: critical, n, %) | 20 m (68.9); 7 M (24.2); 1 S (3.4); 1 C (3.4) | 49 m (77.8); 11 M (14.3); 2 S (3.2); 3 C (4.8) | 93 m (34.1); 73 M (26.3); 46 S (16.7); 62 C (23) |
| Lost response (<15 AU/mL) | 4 (6.1% of evaluable natural course) | 0 | |
| §RR vs >50 for losing reponse [95% †CI] | 37.5 [2.0–688.0]p = 0.0146 | ||
| Lost response (<3.8 AU/mL) | 0 | 0 | |
| Maintained natural response | 49 | 241 | |
| Lost AU/mL, median [‡IQR], variance; | -4.4 [13; +1-2.5], 1125.2 | -25 [-56; +7], 12184.2 | |
| P for lost AU/mL vs >50 | p = 0.0295 | ||
| Vaccinated, n (% achieved >400 AU/mL) | 4 (75), all baseline >10 AU/mL, 1 with recall dose | 15 (100), 3 with recall dose | 57 (100), 5 with recall dose |
| Acquired response later | 3 | ||
| Repeated clinical COVID-19, n (%=) | 4 (13.8) one admitted for pneumonia | 1 (1.5) | 1 (0.4) |
| RR vs >50 for repeating clinical COVID [95%CI] | 37.7 [4.4–326.9]p = 0.001 | 4.2 [0.3–66.5]p = 0.3067 |
*AU = Arbitrary Units
†CI = Confidence Interval
‡IQR = InterQuartile Range
§RR = Relative Risk
Fig. 1.
One Year follow-up of anti-S1/S2 antibody levels in subjects: A, with baselibe lavels <15 AU/mL; B, with baseline levels 15 to 50 AU/mL; C, with baseline levels >50 AU/mL. D: responses in vaccinated subjects (Results limited by ceiling cut-off effect at 400 AU/mL)).
Acknowledgements
We thank Simona Bocchio for her silent but substantial help in dealing with patients, visits and with the general organization of the clinic. No contribution was received for this study.
References
- 1.Harris RJ, Whitaker HJ, Andrews NJ. Serological surveillance of SARS-CoV-2: six-month trends and antibody response in a cohort of public health workers. J Infect. 2021;82(5):162–169. doi: 10.1016/j.jinf.2021.03.015. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiaSorin. LIAISON Ⓡ SARS-CoV-2 S1/S2 IgG the fully automated serology test for the detection of SARS CoV-2 IgG antibodies. https://www.diasorin.com/sites/default/files/allegati/liaisonr_sars-cov2_s1s2_igg_brochure.pdf (accessed on 12 May, 2021).
- 3.World Health Organization. Clinical management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of64covid-19 (accessed on 12 May, 2021).
- 4.Lumley SF, O'Donnell D, Stoesser NE, the Oxford University Hospitals Staff Testing Group Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capetti AF, Stangalini CA, Borgonovo F. Impressive boosting of anti-S1/S2 IgG production in COVID-19-experienced patients after the first shot of the BNT162b2 mRNA COVID-19 Vaccine. Clin Infect Dis. 2021 Mar 6 doi: 10.1093/cid/ciab214. ciab214Epub 2021 Feb. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Bringing together evidence to tackle COVID-19. https://www.who.int/alliance-hpsr/news/2020/bringing-evidence-together-for-covid-19/en/ (accessed on 12 May, 2021).
- 7.World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (accessed on 12 May, 2021).