Abstract
Background and aims
Metabolic syndrome (MetS) is a chronic, low-grade inflammatory disease. This study aimed to investigate the impact of MetS on the risk and severity of COVID-19.
Methods and results
We investigated a nationwide cohort with COVID-19 including all patients who underwent the test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Korea. The COVID-19 group included 4070 patients with positive SARS-CoV-2 test results, and the age- and sex-matched control group included 27,618 subjects with negative SARS-CoV-2 test results. The endpoints were SARS-CoV-2 positivity and the severity of COVID-19. The prevalence of MetS was 24.7% and 24.5% in the COVID-19 and control groups, respectively. The presence of MetS was not associated with the risk of developing COVID-19. Among the components of MetS, central obesity was associated with a higher risk of COVID-19 infection (adjusted odds ratio [aOR], 1.17; 95% confidence interval [CI], 1.06–1.28, P = 0.001). The presence of MetS was significantly associated with severe COVID-19 (aOR, 1.25; 95% CI, 0.78–2.00, P = 0.352). Among the individual components of MetS, prediabetes/diabetes mellitus was associated with a higher risk of severe COVID-19 (aOR, 1.61; 95% CI, 1.21–2.13, P = 0.001). The risk of severe COVID-19 linearly increased according to the number of metabolic components (P for trend = 0.005).
Conclusion
In this nationwide cohort study, the individuals with MetS had a significant increase in the risk of severe COVID-19 infection. These patients, particularly those with central obesity and insulin resistance, deserve special attention amid the COVID-19 pandemic.
Keywords: Metabolic syndrome, COVID-19, SARS-CoV-2, Nationwide cohort study
Introduction
Coronavirus disease (COVID-19), first reported in Hubei, China in December 2019, is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. This disease has spread rapidly and has caused global health and economic crises. The World Health Organization declared a global pandemic of COVID-19 in March 2020 [2]. COVID-19 infection frequently causes severe pneumonia that requires hospitalization to the intensive care unit and invasive mechanical ventilation, and often results in acute respiratory distress syndrome [3,4]. In critically ill patients with COVID-19, the levels of inflammatory molecules and proinflammatory cytokines are highly increased, and the presence of the cytokine storm increases the severity and induces multiorgan dysfunctions and sepsis [5].
Cardiometabolic disorders are commonly observed in patients with COVID-19 infection, and dysmetabolic conditions are associated with severity and mortality [6,7]. Metabolic syndrome (MetS) is a constellation of multiple cardiometabolic risk factors, including insulin resistance, hypertension, and central obesity, which leads to endothelial and myocardial damage and cardiovascular events [8]. Visceral adipose tissue is metabolically bioactive and a source of proinflammatory cytokines [9]. The importance of MetS in increasing the risk and severity of COVID-19 infection has been investigated. In an urban population study conducted in the USA, MetS was associated with severe and fatal COVID-19 outcomes [10]. Patients with diabetes mellitus and MetS who were hospitalized for COVID-19 had an increased risk of adverse outcomes [11].
However, amid the ongoing pandemic, one key unanswered question is which individual components of MetS are associated with COVID-19 infection and severity. If individual components were strongly associated with COVID-19 infection, a preventive strategy should be established for patients with specific metabolic risk factors. Considering these, this study aimed to investigate the influence of MetS and its individual components on the positivity for SARS-CoV-2 and the severity of COVID-19 in a cohort covering the entire Korean population.
Methods
Study population
In this nationwide cohort study, the Korean cohort with COVID-19 included all and consecutive Koreans who underwent the test for SARS-CoV-2. South Korea experienced the Middle East respiratory syndrome outbreak in 2015, and the Korean government established a preparedness and response system for the next infectious disease [[12], [13], [14]]. In collaboration with the Korean Ministry of Health and Welfare, national and local governments, medical experts, and epidemic intelligence service officers applied the “trace, test, and treat” strategy for suspected patients with COVID-19 [12]. Amid the COVID-19 pandemic, the Korean Ministry of Health and Welfare provided a complimentary and obligatory medical service for all patients with COVID-19 infection. The Korean Ministry of Health and Welfare released the previous and current health insurance claims data of patients with COVID-19 infection for medical research with confidentiality. The Korea Centers for Disease Control and Prevention linked the medical records of patients with COVID-19 infection from the Korean National Health Insurance Service (NHIS) and the Health Insurance Review and Assessment Service of Korea database. Among consecutive patients who underwent the SARS-CoV-2 test between January 1 and July 4, 2020, those who underwent a recent national health check-up within 3 years were enrolled in this analysis. For the protection of personal information, personal identification was blinded and age was expressed as a categorical variable by 10 years. The detailed study protocol has been previously described [7,15,16].
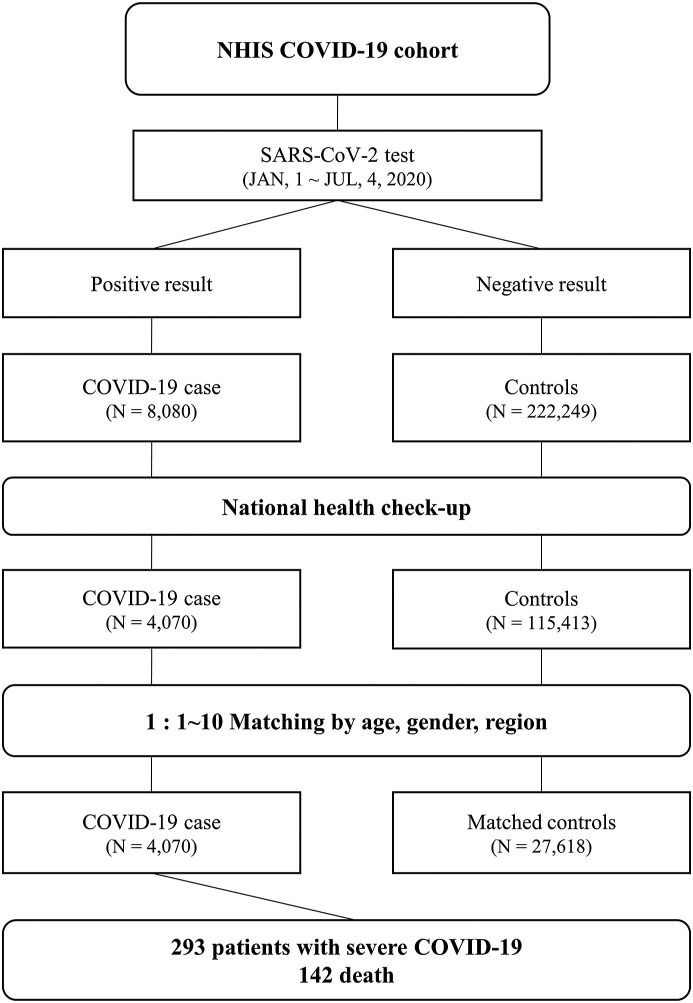
Attending physicians and medical experts performed the SARS-CoV-2 test, based on careful history taking for exposure to COVID-19, which was conducted by epidemic intelligence service officers. The institutional review board of the Korea University Medical Center approved the protocol of this study (2020AN0292). The flowchart of the study population is shown in Fig. 1 .
Figure 1.
Disposition of the patients with coronavirus disease and matched controls. The figure demonstrates the flowchart of the study population.
Case patients and matched controls
COVID-19 infection was confirmed by a positive result of the SARS-CoV-2 test with real-time reverse transcriptase PCR analysis of nasopharyngeal swab cultures [17]. Case patients were defined as those with positive SARS-CoV-2 test results, and for each case patient, up to 10 exact age- and sex-matched random controls were randomly selected and assigned from the patients who had been exposed to COVID-19, but with a negative result of the SARS-CoV-2 test.
Study variables and metabolic profiles
The Korean NHIS provides public health check-ups for manual workers annually and for all Koreans aged over 40 years biennially. The public health check-up investigated anthropometric parameters, medical history of cardiovascular risk factors, medications, metabolic laboratory tests, and lifestyle factors [18]. Attending physicians assessed basic physical examination parameters. Height, weight, waist circumference (WC), and systolic and diastolic blood pressures (SBP and DBP) were measured on the day of the health check-up. All participants fasted for 8 h before blood sampling. The total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride (TG), and fasting blood glucose (FBG) levels were measured. Underlying medical history was identified using health insurance claims data. The International Classification of Diseases, Tenth Revision was used for the operational definition of each disease, followed by a recently published article using the Korean National Health Information Database [19].
MetS was defined in accordance with the guidelines of the International Diabetes Federation and the American Heart Association [20]. Patients with at least three of the following metabolic risk factors were defined as having MetS: 1) central obesity (modified cutoff for Koreans) [21]: WC of ≥80 cm for women or ≥ 90 cm for men; 2) hypertension: SBP of ≥130 mmHg or DBP of ≥85 mmHg or use of antihypertensives; 3) low HDL cholesterol level: HDL cholesterol level of <50 mg/dL for women or < 40 mg/dL for men; 4) elevated TG level: TG level of ≥150 mg/dL or use of lipid-lowering medications; and 5) prediabetes/diabetes mellitus: FBG level of ≥100 mg/dL or use of diabetes medications.
Study outcomes
The primary outcome was positivity for SARS-CoV-2. The secondary outcome was severe COVID-19, which was defined as a composite of admission to intensive care units, use of mechanical ventilation, or COVID-19-related mortality. Admission to intensive care units was defined as a claim for the charge of intensive care units on medical bills; use of mechanical ventilation as medical insurance claim codes for mechanical ventilation (M5850 to M5860); and COVID-19-related mortality as termination of isolation owing to death.
Statistical analysis
The exact matching for age, sex, and region was performed between the COVID-19 and control groups. For each case patient, up to 10 controls were randomly selected. Continuous variables were presented as means (standard deviations) and categorical variables as frequencies and percentages. Using a conditional logistic regression model for matched data, we compared the differences in the demographic, anthropometric, clinical, and laboratory variables between the case and control groups. The difference in characteristics between the mild to moderate and severe COVID-19 groups was evaluated by a logistic regression model. We performed a multiple logistic regression analysis to investigate the association between MetS and its components and COVID-19 related outcomes after adjusting for age, sex, region, socioeconomic status, smoking history, alcohol consumption, physical activity, cardiovascular diseases (ischemic heart disease, peripheral artery disease, stroke, and heart failure), atrial fibrillation, chronic kidney disease, cancer, and nonalcoholic fatty liver disease. The results for association were represented as odds ratio and 95% confidence interval. Multivariable restricted cubic splines with 5 knots were used to detect the possible nonlinear relationship between continuous variables and COVID-19 infection. All analyses were performed using the SAS Enterprise Guide software version 7.1 (SAS Institute, Cary, NC, USA) and R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Table 1 denotes the demographic and clinical characteristics of 4070 patients with COVID-19 infection and 27,618 age- and sex-matched controls. Age and the proportion of sex did not differ between the groups. The parameters of obesity, body mass index, and WC were higher in the COVID-19 group than in the control group. The COVID-19 group had a higher prevalence of diabetes mellitus and dyslipidemia and a lower prevalence of hypertension than the control group. In the comparison of the metabolic laboratory parameters between the COVID-19 and control groups, the mean LDL cholesterol level was higher, and the TG level was lower in the COVID-19 group. The mean levels of HDL cholesterol and FBG were not significantly different between the groups.
Table 1.
Demographic and clinical characteristics of the patients with (case patients) and without COVID-19 (matched controls).
| Case patients |
Matched controls |
P | |||
|---|---|---|---|---|---|
| (N = 4070) |
(N = 27,618) |
||||
| n or mean | % or SD | n or mean | % or SD | ||
| Age (years) | 55.9 | (14.4) | 52.1 | (16.2) | – |
| Age group (y) | – | ||||
| 20–29 | 198 | (4.9) | 2472 | (9.0) | |
| 30–39 | 368 | (9.0) | 4582 | (16.6) | |
| 40–49 | 711 | (17.5) | 5549 | (20.1) | |
| 50–59 | 1213 | (29.8) | 6296 | (22.8) | |
| 60–69 | 945 | (23.2) | 4719 | (17.1) | |
| 70–79 | 436 | (10.7) | 2486 | (9.0) | |
| >80 | 199 | (4.9) | 1514 | (5.5) | |
| Men | 1530 | (37.6) | 12,726 | (46.1) | – |
| BMI (kg/m2) | 24.1 | (3.4) | 23.8 | (3.7) | <0.001 |
| WC (cm) | 80.7 | (9.5) | 80.4 | (10.3) | <0.001 |
| SBP (mmHg) | 121.5 | (15.4) | 121.7 | (15.1) | 0.015 |
| DBP (mmHg) | 74.9 | (10.0) | 75.1 | (10.0) | 0.136 |
| SES | <0.001 | ||||
| Low | 1347 | (33.1) | 5940 | (21.5) | |
| Middle | 1196 | (29.4) | 8582 | (31.1) | |
| High | 1527 | (37.5) | 13,096 | (47.4) | |
| Medical history | |||||
| HTN | 1323 | (32.5) | 9202 | (33.3) | <0.001 |
| DM | 590 | (14.5) | 3879 | (14.0) | 0.058 |
| Dyslipidemia | 1386 | (34.1) | 8945 | (32.4) | <0.001 |
| CVD | 1012 | (24.9) | 7140 | (25.9) | <0.001 |
| Asthma | 97 | (2.4) | 1421 | (5.1) | <0.001 |
| AF | 50 | (1.2) | 624 | (2.3) | <0.001 |
| CKD | 30 | (0.7) | 761 | (2.8) | <0.001 |
| Cancer | 459 | (11.3) | 6140 | (22.2) | <0.001 |
| NAFLD | 770 | (18.9) | 4961 | (18.0) | 0.007 |
| Laboratory parameters | |||||
| TC level (mg/dL) | 194.7 | (38.2) | 191.5 | (38.6) | 0.175 |
| HDL cholesterol level (mg/dL) | 57.7 | (29.6) | 57.2 | (20.6) | 0.928 |
| LDL cholesterol level (mg/dL) | 114.3 | (33.6) | 110.2 | (34.5) | 0.002 |
| TG level (mg/dL) | 121.2 | (84.1) | 126.8 | (100.4) | 0.002 |
| FBG level (mg/dL) | 101.5 | (27.2) | 100.1 | (26.3) | 0.127 |
Abbreviations: COVID-19, coronavirus disease; SD, standard deviation; BMI, body mass index; WC, waist circumference; SBP and DBP, systolic and diastolic blood pressures; SES, socioeconomic status; HTN, hypertension; DM, diabetes mellitus; CVD, cardiovascular disease; AF, atrial fibrillation; CKD, chronic kidney disease; NAFLD, non-alcoholic fatty liver disease; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; FBG, fasting blood glucose.
MetS and the risk of COVID-19
The impact of MetS and its components on the risk of COVID-19 infection was investigated. Table 2 depicts the risk of COVID-19 in relation to MetS and its components. The prevalence of MetS was not different between the COVID-19 and control groups (24.7% vs. 24.5%, P = 0.214). The presence of MetS was not associated with a higher risk of COVID-19 infection (adjusted odds ratio [aOR], 1.08; 95% confidence interval [CI], 0.99–1.18, P = 0.092). Regarding the association between the individual components of MetS and COVID-19 infection, only central obesity was significantly associated with a higher risk of COVID-19 infection (aOR, 1.17; 95% CI, 1.06–1.28, P = 0.001), whereas hypertension was associated with a lower risk of COVID-19 (aOR, 0.91; 95% CI, 0.84–1.00, P = 0.038).
Table 2.
Metabolic syndrome and the risk of COVID-19 infection.
| Case Patients |
Matched Controls |
OR for COVID-19 infection (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 4070) |
(N = 27618) |
|||||||||
| n or mean | % or SD | n or mean | % or SD | Unadjusted OR (CI) | P | Adjusted OR (CI) | Pa | |||
| Metabolic syndrome | 1006 | (24.7) | 6780 | (24.5) | 0.95 | (0.87, 1.03) | 0.214 | 1.08 | (0.99, 1.18) | 0.092 |
| Risk factors | ||||||||||
| Central obesity | 972 | (23.9) | 6405 | (23.2) | 1.06 | (0.98, 1.16) | 0.152 | 1.17 | (1.06, 1.28) | 0.001 |
| Hypertension | 1956 | (48.1) | 13407 | (48.5) | 0.84 | (0.78, 0.91) | <0.001 | 0.91 | (0.84, 1.00) | 0.038 |
| Elevated TG | 973 | (23.9) | 7199 | (26.1) | 0.91 | (0.83, 0.99) | 0.022 | 0.93 | (0.85, 1.02) | 0.109 |
| Low HDL-C | 879 | (21.6) | 5425 | (19.6) | 0.98 | (0.90, 1.07) | 0.703 | 1.04 | (0.95, 1.14) | 0.426 |
| Prediabetes/diabetes | 1612 | (39.6) | 10510 | (38.1) | 1.00 | (0.92, 1.08) | 0.950 | 1.07 | (0.99, 1.17) | 0.082 |
| No. of risk factors | ||||||||||
| 0 | 1071 | (26.3) | 7533 | (27.3) | 1.00 | 1.00 | ||||
| 1 | 1068 | (26.2) | 7106 | (25.7) | 0.92 | (0.83, 1.02) | 0.126 | 0.96 | (0.87, 1.07) | 0.456 |
| 2 | 925 | (22.7) | 6199 | (22.4) | 0.88 | (0.79, 0.99) | 0.026 | 0.95 | (0.85, 1.06) | 0.371 |
| 3 | 630 | (15.5) | 4221 | (15.3) | 0.86 | (0.76, 0.98) | 0.020 | 1.01 | (0.89, 1.15) | 0.895 |
| 4 | 296 | (7.3) | 2016 | (7.3) | 0.90 | (0.77, 1.05) | 0.189 | 1.11 | (0.95, 1.31) | 0.198 |
| 5 | 80 | (2.0) | 543 | (2.0) | 0.88 | (0.67, 1.15) | 0.337 | 1.08 | (0.82, 1.43) | 0.585 |
| Trend test | 0.97 | (0.94, 1.00) | 0.037 | 1.02 | (0.99, 1.05) | 0.267 | ||||
| Measurements | ||||||||||
| WC (per 10 cm) | 80.7 | (9.5) | 80.4 | (10.3) | 1.09 | (1.04, 1.13) | <0.001 | 1.16 | (1.11, 1.21) | <0.001 |
| HDL-C (per 10 mg/dL) | 57.7 | (29.6) | 57.2 | (20.6) | 1.00 | (0.99, 1.02) | 0.928 | 1.00 | (0.99, 1.02) | 0.930 |
| TG (per 10 mg/dL) | 121.2 | (84.1) | 57.2 | (20.6) | 0.99 | (0.99, 1.00) | 0.002 | 0.99 | (0.99, 1.00) | 0.004 |
| FBG (per 10 mg/dL) | 101.5 | (27.2) | 100.1 | (26.3) | 1.01 | (1.00, 1.02) | 0.127 | 1.02 | (1.01, 1.03) | 0.007 |
| SBP (per 10 mg/dL) | 121.5 | (15.4) | 121.7 | (15.1) | 0.97 | (0.95, 0.99) | 0.015 | 0.96 | (0.94, 0.99) | 0.006 |
Abbreviations: OR, odds ratio; CI, confidence interval; WC, waist circumference; SBP, systolic blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; FBG, fasting blood glucose.
Adjusted for social economic status, ever smoking, alcohol, physical activity, cardiovascular disease, asthma, atrial fibrillation, chronic kidney disease, cancer and non-alcoholic fatty liver disease.
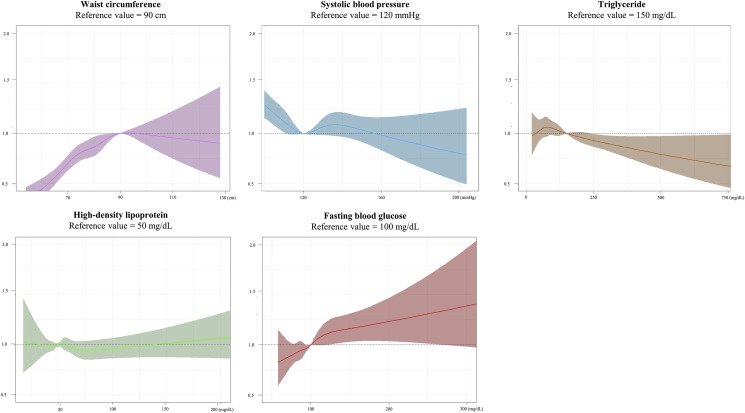
Among each continuous variable of MetS, the WC and FBG levels were significantly associated with a higher risk of COVID-19 infection (WC: aOR, 1.16; 95% CI, 1.11–1.21, P < 0.001; FBG level: aOR, 1.02; 95% CI, 1.01–1.03, P = 0.007). Fig. 2 with a cubic spine curve reveals the linear association of the continuous WC and FBG levels with a higher risk of COVID-19 infection. As shown in Fig. 3 , the odds for the risk of COVID-19 infection did not increase according to the number of metabolic components (P for trend = 0.267).
Figure 2.
Continuous association between the metabolic profile and COVID-19. The adjusted odds ratio with 95% confidence interval was demonstrated. The WC and FBG levels were associated with a higher risk of COVID-19 infection. COVID-19, coronavirus disease; WC, waist circumference; FBG, fasting blood glucose.
Figure 3.
Risk of COVID-19 according to the components of metabolic syndrome. The risk of COVID-19 infection increased in the patients according to the number of metabolic components. COVID-19, coronavirus disease
MetS and severe COVID-19 and mortality
Among the total of 4070 patients with COVID-19, 293 patients with severe COVID-19 and 142 mortalities were identified. Table 3 and supplementary table summarize the risk of severe COVID-19 in relation to MetS and its components. The prevalence of MetS was significantly higher in the patients with severe COVID-19 than in those with mild to moderate COVID-19 (44.7% vs. 23.2%, P < 0.001). The presence of MetS was significantly associated with severe COVID-19 infection and mortality (severe COVID-19: aOR, 1.46; 95% CI, 1.11–1.92, P = 0.007; mortality: aOR, 1.91; 95% CI, 1.27–2.86, P = 0.002). The percentage of subjects with individual metabolic components, including central obesity, hypertension, elevated TG level, and prediabetes/diabetes mellitus, but not low HDL cholesterol level, was higher in the severe COVID-19 group than in the mild to moderate COVID-19 group. However, only prediabetes/diabetes mellitus was significantly associated with a higher risk of severe COVID-19 (aOR, 1.61; 95% CI, 1.21–2.13, P = 0.001), and no individual components were associated with COVID-19 mortality.
Table 3.
Metabolic syndrome and the risk of severe COVID-19.
| Severe |
Mild-to-moderate |
OR for severe COVID-19 (95% CI) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 293) |
(N = 3777) |
||||||||||||
| n or mean | % or SD | n or mean | % or SD | Unadjusted OR (CI) | P | Adjusted OR (CI) | Pa | ||||||
| Metabolic syndrome | 131 | (44.7) | 875 | (23.2) | 2.68 | (2.11, 3.42) | <0.001 | 1.46 | (1.11, 1.92) | 0.007 | |||
| Risk factors | |||||||||||||
| Central obesity | 101 | (34.5) | 871 | (23.1) | 1.76 | (1.36, 2.26) | <0.001 | 1.09 | (0.81, 1.45) | 0.573 | |||
| Hypertension | 220 | (75.1) | 1736 | (46.0) | 3.54 | (2.70, 4.65) | <0.001 | 1.10 | (0.79, 1.52) | 0.574 | |||
| Elevated TG | 91 | (31.1) | 882 | (23.4) | 1.48 | (1.14, 1.92) | 0.003 | 1.11 | (0.82, 1.50) | 0.491 | |||
| Low HDL-C | 76 | (25.9) | 803 | (21.3) | 1.30 | (0.99, 1.70) | 0.061 | 0.94 | (0.68, 1.31) | 0.721 | |||
| Prediabetes/diabetes | 186 | (63.5) | 1426 | (37.8) | 2.87 | (2.24, 3.67) | <0.001 | 1.61 | (1.21, 2.13) | 0.001 | |||
| No. of risk factors | |||||||||||||
| 0 | 22 | (7.5) | 1049 | (27.8) | 1.00 | 1.00 | |||||||
| 1 | 61 | (20.8) | 1007 | (26.7) | 2.89 | (1.76, 4.74) | <0.001 | 1.55 | (0.92, 2.62) | 0.101 | |||
| 2 | 79 | (27.0) | 846 | (22.4) | 4.45 | (2.75, 7.20) | <0.001 | 1.58 | (0.94, 2.65) | 0.085 | |||
| 3 | 80 | (27.3) | 550 | (14.6) | 6.94 | (4.28, 11.24) | <0.001 | 2.18 | (1.29, 3.70) | 0.004 | |||
| 4 | 40 | (13.7) | 256 | (6.8) | 7.45 | (4.35, 12.76) | <0.001 | 2.10 | (1.16, 3.81) | 0.015 | |||
| 5 | 11 | (3.8) | 69 | (1.8) | 7.60 | (3.54, 16.31) | <0.001 | 2.01 | (0.86, 4.69) | 0.106 | |||
| Trend test | 1.52 | (1.39, 1.65) | <0.001 | 1.17 | (1.05, 1.30) | 0.005 | |||||||
| Measurements | |||||||||||||
| WC (per 10 cm) | 85.0 | (8.9) | 80.4 | (9.5) | 1.60 | (1.42, 1.80) | <0.001 | 1.08 | (0.91, 1.28) | 0.368 | |||
| HDL-C (per 10 mg/dL) | 51.8 | (13.4) | 58.1 | (30.5) | 0.78 | (0.71, 0.86) | <0.001 | 1.00 | (0.94, 1.06) | 0.913 | |||
| TG (per 10 mg/dL) | 139.3 | (76.3) | 119.8 | (84.5) | 1.02 | (1.01, 1.03) | 0.001 | 1.01 | (0.99, 1.02) | 0.365 | |||
| FBG (per 10 mg/dL) | 112.7 | (33.2) | 100.6 | (26.5) | 1.11 | (1.08, 1.15) | <0.001 | 1.05 | (1.01, 1.09) | 0.015 | |||
| SBP (per 10 mg/dL) | 127.9 | (15.7) | 121.0 | (15.2) | 1.34 | (1.24, 1.44) | <0.001 | 1.08 | (0.98, 1.18) | 0.116 | |||
Abbreviations: OR, odds ratio; CI, confidence interval; WC, waist circumference; SBP, systolic blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; FBG, fasting blood glucose.
Adjusted for age, gender, region, social economic status, ever smoking, alcohol, physical activity, cardiovascular disease, asthma, atrial fibrillation, chronic kidney disease, cancer and non-alcoholic fatty liver disease.
The linear association between the metabolic components and severe COVID-19 infection and mortality was also investigated. The FBG level was associated with a higher risk of severe COVID-19 infection (aOR, 1.05; 95% CI, 1.01–1.09, P = 0.015), and the WC was associated with a higher risk of mortality (aOR, 1.33; 95% CI, 1.03–1.72, P = 0.031). The association between the number of metabolic abnormalities and the risk of severe COVID-19 and mortality is illustrated in Fig. 3; the risk of severe COVID-19 and mortality linearly increased according to the number of metabolic components (severe COVID-19: P for trend = 0.005; mortality: P for trend = 0.003).
Discussion
Our study demonstrated that the presence of MetS was significantly associated with severe COVID-19 and mortality. Furthermore, prediabetes/diabetes mellitus and the FBG level were well correlated with a higher risk of severe COVID-19, and the risk of severe COVID-19 linearly increased according to the number of metabolic components. The presence of MetS was not associated with the risk of COVID-19, and among the individual components of MetS, central obesity was associated with a higher risk of COVID-19 infection. In general, our investigation revealed the association between MetS and the risk of COVID-19 infection and severity; especially, central obesity and impaired fasting glucose were significantly associated with the risk and severity of COVID-19 infection.
This is the first study to investigate the impact of MetS and its components on the risk and severity of COVID-19 infection. This study used a cohort with COVID-19 covering the entire Korean population who underwent the SARS-CoV-2 test. Amid the COVID-19 pandemic, it is a medical emergency to evaluate and stratify subjects with suspected COVID-19 infection. In the emergency medical situation, the epidemiologic investigation is mainly emphasized for the quarantine and isolation of patients, and detailed medical history recording and physical examination for underlying cardiometabolic disorders are sometimes neglected. Therefore, the previously evaluated metabolic status in participants with stable conditions is the novelty of our study.
Several studies have aimed to identify clinical and laboratory characteristics associated with severity [6,22,23]. However, studies focusing on metabolic profiles in patients with COVID-19 infection are rare. In a study of 287 patients with COVID-19, the prevalence of MetS was 66%, and had been associated with mortality and severity of COVID-19 [10]. In another multicenter retrospective study with 354 subjects, cardiometabolic disorders were associated with the severity of COVID-19 [11]. The findings of our study are consistent with those of previous studies. However, it is remarkable that these previous studies have included relatively limited patients with COVID-19 and did not evaluate the individual impact of metabolic components on the risks of COVID-19 infection and severity as an outcome. Our study also elaborates on the association of MetS with COVID-19 infection, demonstrating a dose-dependent association of central obesity and impaired fasting glucose with the risk of COVID-19 in a nationwide cohort. Furthermore, the increasing number of individual metabolic components linearly increased the severity of COVID-19 infection and COVID-19 mortality.
Though the exact pathogenic mechanism by which MetS is associated with a higher risk of COVID-19 infection remains unclear, several potential mechanisms have been suggested. Cardiometabolic disorders are characterized by enhanced low-grade systemic inflammation [24]. In middle-aged men with MetS, epicardial adipose tissue was associated with inflammation represented by high-sensitivity C-reactive protein level and subclinical myocardial dysfunction, suggesting that the inflammatory activity of epicardial adipose tissue induced myocardial dysfunction [9]. In a predominantly non-Hispanic black population with MetS, inflammatory biomarkers predicted COVID-19 mortality [10]. Metabolic inflammation may facilitate COVID-19 infection and intensify the inflammatory cytokine storm [25]. ACE2 physiologically counteracts the renin–angiotensin–aldosterone system and serves as the cellular entry for SARS-CoV-2 [26]. The high expression of angiotensin-converting enzyme 2 (ACE2) in the lungs and kidneys explains the most common manifestations of severe COVID-19: acute respiratory distress syndrome and acute kidney injury [27]. In a diabetic animal model, ACE levels were upregulated mainly in the serum, lung, liver, and heart, and ACE2 levels were elevated mainly in the serum, pancreas, and liver [28]. In another animal model, a high fat diet increased adipose mRNA expression of angiotensinogen and ACE2. This induced an increase in BP, suggesting that adipocytes express ACE2, which is dysregulated in individuals with metabolic disorders [29]. The elevated expression of ACE2 could be a possible link between the dysmetabolic status and the severity of COVID-19. The data mining from recent publications on COVID-19 and diabetes mellitus also revealed that dysregulation of ACE2 indicates a higher risk of COVID-19 infection [30]. Direct invasion of SARS-CoV-2 in the endothelial cells causes endotheliitis in several vital organs. This suggests that COVID-19 not only impairs the respiratory system, but also the vascular endothelial system [31]. Endothelial dysfunction is one of the key pathological mechanisms of MetS; thus, chronic endothelial dysfunction may predispose an individual to poor COVID-19 related outcomes [32]. However, further studies are required to clarify the pathophysiology between MetS and individual metabolic disorders, and the risk of a severe COVID-19 infection.
MetS is a constellation of cardiometabolic disorders. We investigated the impact of its individual components on COVID-19. The strong association of two metabolic components, central obesity and impaired fasting glucose with COVID-19, is a novel finding of this study. Impaired glucose homeostasis and insulin resistance induce alveolar microvascular angiopathy and interstitial fibrosis. Thus, diabetic patients frequently experience symptoms of respiratory involvement and are at a higher risk of developing respiratory infections, such as pneumonia [33]. Furthermore, the plasma level of IL-6, which is an inflammatory and metabolic biomarker, was significantly higher in diabetic patients than in nondiabetic patients [34]. Visceral adipose tissue is a bioactive organ that secretes several proinflammatory cytokines [9]. Therefore, central obesity may impair immune response to SARS-CoV-2. Furthermore, inflammatory cells, such as dendritic cells, macrophages, and cytotoxic T cells, accumulate in the adipose tissue and create an imbalance in the systemic immune cell population [35]. In obese individuals, their physique increases the chances of developing obstructive sleep apnea, and often induces the development of pulmonary hypertension and myocardial dysfunction [35].
Previous epidemiological studies report hypertension as a common cardiovascular disorder in patients with COVID-19 [22]. Additionally, poor BP control is associated with a higher risk of developing severe COVID-19 [36]. In hypertensive patients, the activation of the renin-angiotensin-aldosterone system results in a procoagulant and inflammatory response, which predisposes patients to COVID-19 induced multiorgan failure [37]. However, limited studies have reported the linear association between BP levels and the development of COVID-19 related morbidity. In the present study, higher blood pressure was associated with a lower risk of COVID-19 infection. The increased risk of COVID-19 in patients with lower BP may reflect unrecognized confounding factors such as cachexia or sarcopenia; however, the exact pathophysiology is unclear in the present study. Further studies are needed to investigate the linear association between BP levels and COVID-19 related morbidity.
This study acknowledges some limitations that warrant discussion. This study has a cross-sectional observational design, indicating that our findings only imply associations between MetS and COVID-19 infection, but not causality. We performed multiple logistic regression analyses; however, the effects of residual confounding factors may explain this association. The number of severe cases and mortality are relatively low and further studies with larger populations are required. Metabolic profiles were assessed from the data available from past national health check-ups. The status of these findings may differ from the metabolic characteristics at the time of COVID-19 testing. Further studies with metabolic profiles evaluated at the time of SARS-CoV-2 exposure are required. Finally, although the FBG level was well correlated with the severity of COVID-19, data on HbA1c, which reflects a longer-term trend of the glucose level, were not available in the study population.
Conclusion
In this nationwide cohort study, the patients with MetS had a significant increase in the risk of severe COVID-19 infection. These patients, particularly those with central obesity and diabetes mellitus, deserve special attention amid the COVID-19 pandemic.
Funding
None.
Ethics approval and consent to participate
The institutional review board of the Korea University Medical Center approved the protocol of this study (2020AN0292). Informed consent was waived because the present study is based on retrospective analysis.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
The authors thank the participants in this study.
Handling Editor: A. Siani
List of abbreviations
- COVID-19
coronavirus disease
- SARS-CoV-2,
evere acute respiratory syndrome coronavirus 2
- MetS
metabolic syndrome
- NHIS
National Health Insurance Service
- WC
waist circumference
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- TG
triglyceride
- FBG
fasting blood glucose
- aOR
adjusted odds ratio
- CI
confidence interval
- ACE2
angiotensin-converting enzyme 2
References
- 1.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in Wuhan, China: challenges for global health governance. J Am Med Assoc. 2020;323:709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed: Atenei Parmensis. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region—Case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho D.-H. The impact of COVID-19 on heart failure: what happened to the patients with heart failure who could not visit our clinic amid the COVID-19 pandemic? Int J Heart Failure. 2021;3:125–127. doi: 10.36628/ijhf.2021.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruscitti P., Berardicurti O., Iagnocco A., Giacomelli R. Cytokine storm syndrome in severe COVID-19. Autoimmun Rev. 2020;19:102562. doi: 10.1016/j.autrev.2020.102562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D.W., Byeon K.H., Kim J., Cho K.D., Lee N. The correlation of comorbidities on the mortality in patients with COVID-19: an observational study based on the Korean national health insurance big data. J Kor Med Sci. 2020;35:e243. doi: 10.3346/jkms.2020.35.e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho D.H., Kim M.N., Joo H.J., Shim W.J., Lim D.S., Park S.M. Visceral obesity, but not central obesity, is associated with cardiac remodeling in subjects with suspected metabolic syndrome. Nutr Metabol Cardiovasc Dis. 2019;29:360–366. doi: 10.1016/j.numecd.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Cho D.H., Joo H.J., Kim M.N., Lim D.S., Shim W.J., Park S.M. Association between epicardial adipose tissue, high-sensitivity C-reactive protein and myocardial dysfunction in middle-aged men with suspected metabolic syndrome. Cardiovasc Diabetol. 2018;17:95. doi: 10.1186/s12933-018-0735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J., Zu Y., Alkhatib A., Pham T.T., Gill F., Jang A. Metabolic syndrome and COVID-19 mortality among adult black patients in New Orleans. Diabetes Care. 2020;44:188–193. doi: 10.2337/dc20-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maddaloni E., D'Onofrio L., Alessandri F., Mignogna C., Leto G., Pascarella G. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II) Cardiovasc Diabetol. 2020;19:164. doi: 10.1186/s12933-020-01140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang T.U., Noh J.Y., Song J.-Y., Cheong H.J., Kim W.J. How lessons learned from the 2015 MERS outbreak affected the effective response to the COVID-19 epidemic in the Republic of Korea. Kor J Intern Med. 2020;36:271–285. doi: 10.3904/kjim.2020.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho D.H., Yoo B.S., Son J.W., Kim I.C., Park S.M., Choi D.J. COVID-19—implications for patients with heart failure: the Korean society of heart failure's clinical recommendations. Int J Heart Failure. 2020;2 doi: 10.36628/ijhf.2020.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J.-Y., Yun J.-G., Noh J.-Y., Cheong H.-J., Kim W.-J. Covid-19 in South Korea—challenges of subclinical manifestations. N Engl J Med. 2020;382:1858–1859. doi: 10.1056/NEJMc2001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.W., Ha E.K., Yeniova A.O., Moon S.Y., Kim S.Y., Koh H.Y. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2020;70:76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 16.Bae S., Kim J.H., Kim Y.-J., Lim J.S., Yun S.-C., Kim Y.-H. Effects of recent use of renin-angiotensin system inhibitors on mortality of patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7:ofaa519. doi: 10.1093/ofid/ofaa519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seong S.C., Kim Y.Y., Park S.K., Khang Y.H., Kim H.C., Park J.H. Cohort profile: the national health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi E.K. Cardiovascular research using the Korean national health information Database. Kor Circ J. 2020;50:754–772. doi: 10.4070/kcj.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood Institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.Y., Park H.S., Kim D.J., Han J.H., Kim S.M., Cho G.J. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauvais-Jarvis F. Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes. 2020;69:1857–1863. doi: 10.2337/dbi19-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaduganathan M., Vardeny O., Michel T., McMurray J.J., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupte M., Boustany-Kari C.M., Bharadwaj K., Police S., Thatcher S., Gong M.C. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marhl M., Grubelnik V., Magdic M., Markovic R. Diabetes and metabolic syndrome as risk factors for COVID-19. Diabetes Metab Syndrome. 2020;14:671–677. doi: 10.1016/j.dsx.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rask-Madsen C., King G.L. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metabol. 2013;17:20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khateeb J., Fuchs E., Khamaisi M. Diabetes and lung disease: a neglected relationship. Rev Diabet Stud. 2019;15:1–15. doi: 10.1900/RDS.2019.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randeria S.N., Thomson G.J., Nell T.A., Roberts T., Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc Diabetol. 2019;18:1–15. doi: 10.1186/s12933-019-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popkin B.M., Du S., Green W.D., Beck M.A., Algaith T., Herbst C.H. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ran J., Song Y., Zhuang Z., Han L., Zhao S., Cao P. Blood pressure control and adverse outcomes of COVID-19 infection in patients with concomitant hypertension in Wuhan, China. Hypertens Res. 2020;43:1267–1276. doi: 10.1038/s41440-020-00541-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bansal R., Gubbi S., Muniyappa R. Metabolic syndrome and COVID 19: endocrine-immune-vascular interactions shapes clinical course. Endocrinology. 2020:161. doi: 10.1210/endocr/bqaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]