Abstract
Intensified use of disinfectants to control COVID-19 could unintentionally increase the disinfection byproducts (DBPs) in the environment. In indoor spaces, it is critical to determine the optimal disinfection practice to prevent the spread of the virus while keeping DBPs at relatively low levels in the air. The formation of DBPs exceed 0.1 μg/mg while hypochlorite dosed at >10 mg/m3. The total DBP concentrations in highly disinfected places (100–200 mg/m3 hypochlorite) were as high as 66.8 μg/m3, and the Hazard Index (HI) was up to 0.84, and both values were much higher than those in less disinfected places (<10 mg/m3 hypochlorite). Taking into account the HI, formation yields and the origin of the DBPs, we recommended 10 mg/m3 as the suggested hypochlorite dose to minimize DBPs generation during routine disinfection for controlling the coronavirus. DBPs in indoor air could be eliminated by ventilation, reducing the usage of personal care products, and wiping the solid surface with water before or after disinfection. These results highlighted the necessity to control air-borne DBPs and their associated health risks arising from intensified disinfection, and will guide the further development of evidence-based regulation on DBP exposure during disinfection and improve public health protection.
Keywords: COVID-19, Disinfection byproducts, Indoor air, Personal care products, Hazard quotient
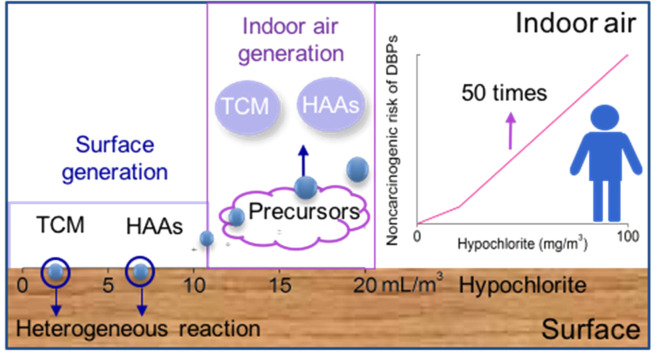
Graphical Abstract
1. Introduction
Since the breakout of Coronavirus Disease 2019 (COVID-19) in December 2019, more than 132,048,206 people had been infected until April 7, 2021 (WHO, 2021). The novel coronavirus (SARS-CoV-2) can survive in air and spread through airborne transmission, or human contact with virus-contaminated surface (Lu et al., 2020, Ong et al., 2020, Morawska and Cao, 2020). Hence, indoor space disinfection has been recommended by World Health Organization (WHO) and U.S. Environmental Protection Agency (EPA) to prevent the spread of the virus (WHO, 2020, U.S. Environmental Protection Agency, 2020a, United States Environmental Protection Agency, 2020b). The intensified disinfection, however, can significantly increase the concentration of residual chlorine and the formation of disinfection byproducts (DBPs) in diverse environments (Chu et al., 2020).
DBPs in drinking water are neurotoxic, cytotoxic, mutagenic, genotoxic and/or carcinogenic (Richardson et al., 2007). Acute and chronic exposures to DBPs have several adverse health effects, such as birth defect, increased risks of bladder, rectum and colon cancer (Bove et al., 2007a, Bove et al., 2007b, Rahman et al., 2010, Costet et al., 2011, Grazuleviciene et al., 2013, Horton et al., 2011, Nieuwenhuijsen et al., 2000, Nieuwenhuijsen et al., 2013, Villanueva et al., 2003, Villanueva et al., 2004, Villanueva et al., 2007, Waller et al., 1998, Wright et al., 2017). Haloacetic acids (HAAs) and trihalomethanes (THMs) are the two most prevalent DBPs detected in drinking water (Richardson et al., 2007), and thus regulated by many countries and organizations, e.g., WHO and the U.S. EPA (United States Environmental Protection Agency, 2006, WHO, 2006, Cortvriend, 2008). Thus far, most research on DBPs has concentrated on the aquatic environment (Richardson et al., 2007), whereas airborne DBPs have been rarely studied.
The application of chlorinated disinfectants on solid surface leads to the emission of reactive chlorine species into air. Wong et al. (2017) has stressed the importance of HOCl reaction on surface in indoor chemistry, especially in places receiving less ventilation. Indoor solid surface is an important site for DBPs formation, where the surface-to-volume ratio is high (2–4 m2/m3). Organic compounds react with HOCl continuously on solid surface until the disinfectant or compound is depleted (Weschler and Carslaw, 2018). Meanwhile, HOCl can evaporate and disintegrate to generate free chlorine (Cl2). Other volatile reactive species, such as chlorine radical (Cl.) and hydroxyl radical (.OH) are formed during HOCl and Cl2 photolysis (Mattila et al., 2020, Lou et al., 2021). These chemicals can trigger a series of chemical reactions to generate DBPs in indoor air. Therefore, the occurrence of DBPs on indoor surface and in the air at different hypochlorite doses need to be evaluated.
Inhalation has recently been recognized as another human exposure route for DBPs in addition to drinking, showering, and swimming (Gabriela et al., 2019). The composition of indoor air is complex due to the volatile compounds released from personal care products (PCPs), smoking, cooking, humans and building materials (Weschler and Carslaw, 2018). Numerous unsaturated volatile organic compounds (VOCs) are precursors of DBPs, such as 1,2,4-trimethylbenzene, dihydromyrcenol, limonene and phthalates (Wallace et al., 2017, Peder, 2020, Kosaka et al., 2017). Among them, limonene is a ubiquitous compound with various sources, including air fresheners, plants, food, cleaning products, wood products and PCPs (Ciriminna et al., 2014). The background concentrations ranged from a few ppb to 1000 ppb in the studied indoor environments or weekly sampling (Fellin and Otson, 1994, Yang et al., 2020). These precursors could also be emitted from PCPs, e.g., hand sanitizer. It is therefore essential to evaluate the impacts of other increasingly used PCPs on DBPs formation in the air, which can provide more available precursors.
Understanding the occurrence and potential risks of airborne DBPs resulting from intensified disinfection during the COVID-19 pandemic is urgent and crucial. In this work, we aimed to: (1) investigate the occurrence of HAAs and THMs in 40 indoor air samples during the COVID-19 pandemic and assess their associated health risks via inhalation exposure; (2) evaluate the influential factors (temperature, hypochlorite concentration and frequency, air ventilation and hand sanitizer/detergent) on the occurrence and concentration of DBPs in indoor air; (3) identify the formation sites and mechanisms of major DBP species depending on the hypochlorite dose.
2. Materials and methods
2.1. Chemicals and materials
Chloroacetic acid (MCAA), bromoacetic acid (MBAA), dichloroacetic acid (DCAA), dibromoacetic acid (DBAA), trichloroacetic acid (TCAA), bromochloroacetic acid (BCAA), bromoform (TBM), chloroform (TCM), dibromochloromethane (DBCM) and dichlorobromomethane (DCBM) were purchased from Supelco, Bellefonte, USA. Methyl tertbutyl ether (MtBE) was obtained from Fisher Scientific, USA. Chloroform-d (TCM-d) was obtained from Accelerating Scientific and Industrial Development Thereby Serving Humanity, Shanghai, China. Dichloromethane (Optima LC/MS grade) was purchased from Sigma-Aldrich, Shanghai, China. Sodium hypochlorite solution (reagent grade, available chlorine 5–10%), hydrochloric acid (HCl, ACS grade), methyl orange, potassium bromide (KBr, ACS grade), anhydrous sodium sulfate (Na2SO4, ACS grade), carbon disulfide (CS2) and concentrated sulfuric acid (H2SO4, ACS grade) were obtained from Aladdin, Shanghai, China. Hand sanitizer and detergent were purchased from local markets (Hangzhou, China). Based on the information on the labels of hand sanitizer, the dominant ingredients included salicylic acid, chlorhexidine acetate, plant extracts containing amino acids, vitamins and limonene. The major ingredients of the detergent were limonene, sodium alkylbenzene sulfonate, and cellulase.
2.2. Collection of indoor air samples
We collected air samples from 40 places in Hangzhou, China, from April to September 2020 (during the COVID-19 pandemic in the China). Both of the control samples before disinfection and experimental samples after disinfection for 30 min were collected in the 40 places. Notably, in the frequently sterilized places, the time interval between collecting control samples and the last disinfection should be longer than 24 h. When DBPs were detected in control samples, the repeated samples should be collected after 1 h and 2 h to confirm the results. If the three values were identical, we concluded that they were the background concentrations, otherwise they were the substantial residual DBPs from the last disinfection.
Supporting Information (SI) Table S1 shows the conditions of the 40 places. During each disinfection event, the volunteer prepared the hypochlorite solution at 1000 mg/L and applied the solution to indoor floors with a mop. We explored the effect of mop fibers in the degradation of hypochlorite by comparing three experimental groups: A) 50 mL of 10 mg/L hypochlorite with 1 g fibers; B) 50 mL of 10 mg/L hypochlorite and 2 g fibers; C) 50 mL of 10 mg/L hypochlorite without fiber. The concentrations of hypochlorite were found unchanged in the three groups after 2 h, and thus mop fibers would not affect the degradation of hypochlorite. We aimed to disinfect the indoor space at an initial hypochlorite concentration between 3 and 200 mg/m3 (weight of hypochlorite per unit volume of the indoor space), thus the dose of hypochlorite solution was calculated as: . We estimated the volume of hypochlorite solution applied to the floor by measuring the volume difference before and after each disinfection event. Ten of them were disinfected with 1000 mg/L hypochlorite at concentration of 100–200 mg/m3 hypochlorite, and half of the ten samples were collected at >26 °C places, the other half were sampled at <26 °C places. Thirty of them were conducted at 3–20 mg/m3 with concentration of 1000 mg/L hypochlorite. Among the thirty samples, twelve places were disinfected at frequencies of 1–3 times per week, other places were disinfected at frequencies of >3 times per week. Also, the thirty samples can be divided into two groups based on the recommended setting temperature, which was <26 °C (n = 12) and >26 °C (n = 18). Meanwhile, we investigated the disinfection frequency of the sampled indoor space before the COVID-19 outbreak, and the results showed that only 2 sites had disinfection frequencies of 2 times per week. The samples of HAAs and THMs were adsorbed by 20 mL 0.1 M NaOH solution and activated carbon tubes using the dual air path atmospheric sampler (QC-2AI, Qingdao Lubo Environment Technology, Shandong, China), respectively. The sampling period was set at 60 min with a flow rate of 2 L/min. The atmospheric samplers were installed at the height of 120 cm. The collected tubes were placed in a sealed bag. All samples were transported to the laboratory immediately and kept at 4 °C until analyzed.
2.3. Analytical methods
The samples for HAAs pretreated following previous methods with some modifications (Wang et al., 2019a, Wang et al., 2019b). Briefly, the pH of the 40 mL sample was adjusted to below 0.5 using 1 mL of H2SO4, and then 2 mL of MtBE and 4 g of NaCl were added into the sample. After a vigorous and consistent shaking for 5 min, the mixture was allowed to stand and separate for 5 min, and then 1.5 mL of the organic layer was acquired for followed derivatization using methanol containing 10% H2SO4 at 50 °C for 2 h. After derivatization, 7 mL of NaCl solution (250 g/L) was added into the sample and subjected to a short shaking. The solution was reduced to a volume of 1.5 mL by discarding the aqueous phase of the samples. One mL of saturated NaHCO3 solution was added and the mixed solution was vortexed until the two phases were separated. Finally, 1 mL of the organic layer was transferred into a polypropylene vial for gas chromatography-electron capture detection (GC-ECD) analysis.
The samples for THMs were pretreated following the reported method using activated carbon adsorption tubes (Chinese Ministry of Environmental Protection, CMEP, 2013). Briefly, 150 mg dry activated carbon was transferred into a brown bottle, mixed with 0.2 g Na2SO4 and submerged into 1.0 mL CS2 solution. The bottle was sealed and then shaken for 1 min. The role of CS2 was to desorb THMs from activated carbon. After desorption for 1 h at room temperature, the solution was transferred into a liquid vial for GC-ECD analysis.
The quantification of THMs and HAAs was performed using an Agilent 6890N GC-ECD coupled with a J&W HP-5MS UI column (0.25 µm film thickness, 30 m length, and 0.25 mm ID). One μL of each pretreated sample was injected in splitless mode. The injector and detector temperatures were 220 °C and 320 °C, respectively. The carrier gas and make-up gas were helium at a flow rate of 2.5 mL/min and nitrogen at a flow rate of 60 mL/min, respectively. The oven temperature program was set as follows: initial temperature 40 °C for 0 min; first ramp of 10 °C/min to 160 °C (2 min); second ramp of 20 °C/min to 280 °C (5 min). The method detection limits (MDLs) of HAAs and THMs were in the range of 0.8–2.8 ng/m3 and 18–30 ng/m3, the recoveries were in the range of 70–94% and 68–77%, respectively (Table S2).
2.4. Measurements of reactive chlorine species
The collection and preparation of reactive chlorine species referring to the methyl orange spectrophotometry (CMH, 2004). First, the absorption liquid consisted of 0.1 g methyl orange, 1.1 g KBr and 5 mL H2SO4 (2.57 mol/L), and the liquid preparation protocol has been used previously (CMH, 2004). The absorption liquid was used together with the atmospheric samplers to sample reactive chlorine species (Cl2) in indoor air at a flow rate of 2 L/min. The sampled duration was 20 min and the height was 120 cm. Then the samples were analyzed by UV-1800 spectrophotometer (Shimadzu) at 515 nm.
2.5. The experiments of influential factors
The effect of hand sanitizer on DBPs generation was examined in two typical home in Zhejiang University, Hangzhou, China (120°05′54.3″ E, 30°18′38.7″ N). The amount of 100 mL and 40 mL hand sanitizer product were consumed in the home 1# (around 48 m3) and home 2# (around 24 m3) during the experiments, respectively. The volunteer took out one pump (around 1 mL) of hand sanitizer product every time, and rubbed his/her hands for more than 30 s. The frequency was one time/min. The experiments were completed until 100 mL and 40 mL hand sanitizer were consumed in the experiment. We sterilized the homes immediately after the hand sanitizer experiment with hypochlorite dose at 20 mg/m3 by mopping the solid surface. The volume of hypochlorite solution was determined by: . The temperature was 12–14 °C during the experimental period.
To explore the time profile of DBPs under different ventilation rates, the experiments were conducted in the two typical home, respectively, the ventilation rate was controlled by the ventilation and air conditioning systems. The temperature was kept at 17–18 °C during our experiments. The maximum dose of hypochlorite used in the 40 investigated samples was 20 mg/m3 and the practical concentration in coronaviruses detected places was >100 mg/m3. A conservative concentration of 1000 mg/L hypochlorite will be adequate to inactivate the vast majority of pathogens, which was recommended by WHO (WHO, 2020). Thus, 100 mg/m3 and 20 mg/m3 hypochlorite were selected as experimental dose in our simulated experiments. Every sample was collected after disinfection for 30 min. The cleaning surface activities with water and detergent on the generation of DBPs was examined in the 48 m3 home 1# and 24 m3 home 2#. The experiment was conducted separately, and the room was ventilated for more than 48 h to ensure no residual DBPs detected. The volunteer prepared detergent solutions in a plastic bucket by mixing 20 g commercial detergent into 4 L of tap water. The starting time of disinfection was immediately after completing cleaning process. The sampling period was 60 min at a flow rate of 2 L/min in all experiments. The atmospheric samplers were installed at the height of 120 cm.
The formation potential experiments of DBPs were conducted in a 41.4 cm3 (47 cm × 40 cm × 22 cm) Teflon chamber filled with clean air. Seeds aerosols were injected into the chamber by atomizing 0.015 M AS solution to generate particles. Limonene was the common ingredient in detergent and hand sanitizer, thus limonene was brought into the chamber by clean air at a flow of 3 L/min over a Teflon tube, then 3 L/min clean air continued for 30 min to ensure these compounds at a stable level. Chlorine species were introduced into the chamber after limonene was well mixed after 30 min. Chlorine species were generated by bubbling clean air into 0.37 M NaOCl solution following reported method (Wang et al., 2019a, Wang et al., 2019b). The schematic diagram of the chamber was presented in Fig. S1. The chlorine was added into the chamber without further dilution, the concentrations of HOCl and Cl2 were 4.0 mg/m3 and 1.6 mg/m3 in the chamber at initial time. After mixing the reaction compounds at mixing ratio of 5:1 (Chlorine: limonene), the chamber was sealed for different periods to explore the time profile of THMs and HAAs.
2.6. Quality assurance and quality control
To avoid any contamination during sample collection, extraction and sequential GC-ECD analysis process, the 0.1 M NaOH solution, adsorption solution and active carbon tubes were analyzed during samples collection, pretreatment and analysis processes. Procedural blank experiments were carried out along with every five samples. All of experiments were performed in triplicate, and all of the mean values were reported with relative standard deviations of <5%. Pearson coefficients were used to examine the correlation of DBPs concentrations in indoor air, and a t-test was applied for comparative statistics. The significance level was set at p < 0.05.
2.7. Health risk assessment of DBPs
The persistence of DBPs varied with the dose of hypochlorite, and thus a 24 h time-inhalation model was used to assess the health risks (Allan and Richardson, 1998). The 24 h time-inhalation model was established according to the inhalation of contaminant, inhalation rates of individuals at different ages and body weight. The variation of DCAA, TCAA and TCM underwent two stages, DBPs first rapidly decreased to a relatively stable concentration, and then followed with a slight degradation period. The concentrations of DCAA, TCAA and TCM were calculated as the average concentrations between two sampling points in the 24 h time-inhalation model. Two scenarios of DBPs exposure were considered: one was mean exposure scenario and the other was high (95th) exposure scenario (U.S. Environmental Protection Agency: Washington, DC, 2011a, U.S. Environmental Protection Agency: Washington, DC, 2011b). Calculations for the two scenarios were conducted according to the different inhalation rates (U.S. Environmental Protection Agency: Washington, DC, 2011a, U.S. Environmental Protection Agency: Washington, DC, 2011b). The detailed calculation procedure of THMs for five ages in the 24 h time-inhalation model was demonstrated in Tables S3–S7.
The noncarcinogenic risk was evaluated based on the hazard quotient (HQ) (U.S. Environmental Protection Agency: Washington, DC, 2011a, U.S. Environmental Protection Agency: Washington, DC, 2011b, U.S. Environmental Protection Agency, 2020a, United States Environmental Protection Agency, 2020b). More than one DBP were generated after disinfection, and most of them shared the same health effect, i.e., hepatotoxicity. We therefore can add all HQ values to obtain the hazard index (HI) to estimate the risks of DBPs:
| HQ = (DDBPs,i × IR)/(BW× RfDi) |
| HI =ΣHQ |
where DDBPs,i is the concentration of DBPs in indoor air; Inhalation rate (IR, m3/day) is the daily inhalation volume of air, which is recommended by the “Exposure Factors Handbook: Chapter 6–Inhalation Rates” published by the U.S. Environmental Protection Agency: Washington, DC, 2011a, U.S. Environmental Protection Agency: Washington, DC, 2011b; Body weight (BW, kg) is recommended by the “Exposure Factors Handbook: Chapter 8–Body Weight Studies” published by the U.S. Environmental Protection Agency: Washington, DC, 2011a, U.S. Environmental Protection Agency: Washington, DC, 2011b; Chronic oral reference dose (RfDi, mg/kg-day) is suggested by the U.S. EPA (1989); and n is the number of DBPs. The parameters used in the model are shown in Table S8. The non-cancer risk of DBPs was positively correlated with the HI value, where HI > 1.0 represents likely adverse health effects, and HI ≤ 1.0 means the risk may be absent (Pan et al., 2018, Yu et al., 2018).
3. Results and discussions
3.1. Occurrence and risks of DBPs in indoor air at varied disinfection doses and frequencies
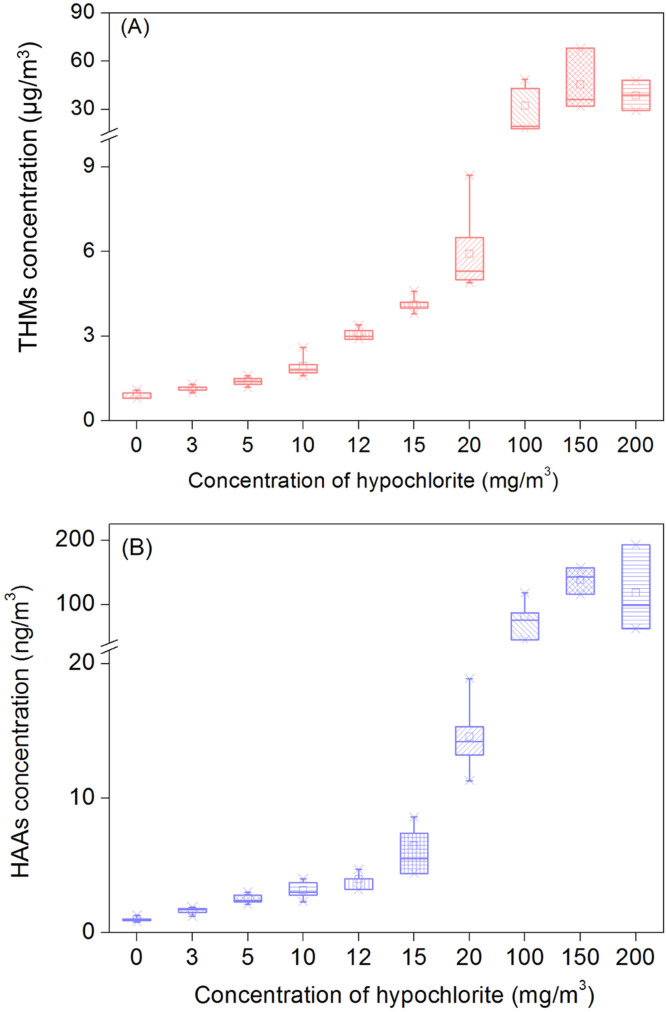
The occurrence and concentrations of THMs and HAAs significantly increased due to the intensified disinfection compared with control samples without disinfection ( Fig. 1). The occurrence of airborne DBPs was 100% in all disinfected spaces studies, which was 1.6 times higher than that in the control. The DBPs detected in control samples may be attributed to the volatility of chemicals in indoor environment. Previous study has pointed that TCM can release from nonionic surfactant, soap, tap water and fragrance and detect in indoor air (Odabasi, 2008). Meanwhile, the residual DBPs generated from last disinfection were also detected in daily disinfection places. The median concentrations of ∑DBPs occurring in indoor space disinfected with 100–200 mg/m3, 3–20 mg/m3 and 0 mg/m3 hypochlorite were 34.1 μg/m3, 2.8 μg/m3, and 1.0 μg/m3, respectively (Fig. 1). TCM was the most abundant DBP, contributing to up to 99% of the total DBPs in all air samples, followed by HAAs (0.1–0.3%). The median concentrations of TCM in the samples with 100–200 mg/m3 and 3–20 mg/m3 hypochlorite were approximately 34-fold and 2.8-fold greater than that of control samples (p < 0.05). As expected, when the dose of hypochlorite increased from 0 to 20 mg/m3, HAAs in these samples significantly increased from 0.9 to 9.6 ng/m3. The median concentration of HAAs was 93.8 ng/m3 in the places with more intensive disinfection (100–200 mg/m3) (Table S9). Overall, the concentrations of DBPs significantly increased due to intensified disinfection practice during the COVID-19 pandemic and the DBPs concentration was positively correlated with the dose of hypochlorite (p < 0.05, Fig. 1).
Fig. 1.
The concentrations of THMs (A) and HAAs (B) in indoor air after disinfection for 30 min with varied hypochlorite doses. The central lines, ends, squares and error bars of the box define the medians, 25th and 75th percentiles, mean and the 99% confidence intervals of the mean, respectively.
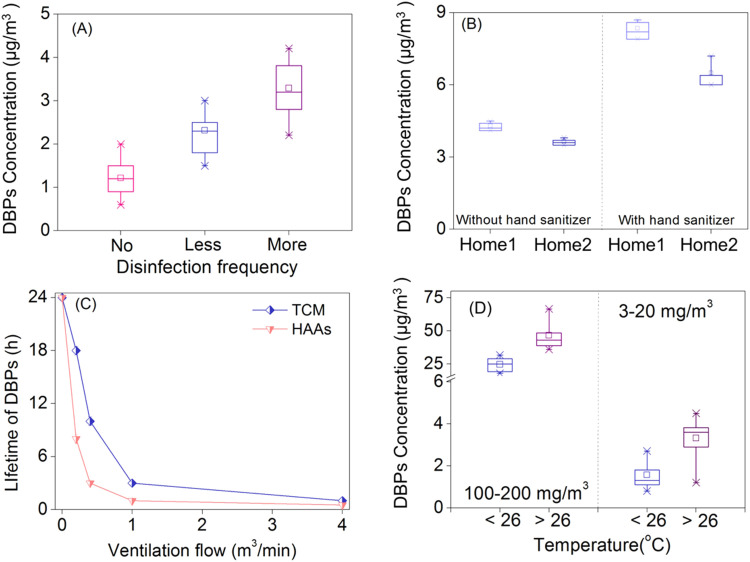
In the sampled indoor spaces, the disinfection frequency during the pandemic increased substantially. The median concentrations of ∑DBPs in the places with more frequent disinfection (frequencies: >3 times per week, ∑DBPs: 2.8 μg/m3, hypochlorite dose: 3–20 mg/m3) was higher than that of places with less or no disinfection (frequencies: 1–3 times per week, ∑DBPs:1.8 μg/m3, hypochlorite: 3–20 mg/m3 and ∑DBPs:1.0 μg/m3 in control) ( Fig. 2A and Table S10). The results supported that DBPs formation was positively correlated with hypochlorite dose and disinfection frequency in indoor environment.
Fig. 2.
Influential factors for DBPs generation in indoor air. (A) Disinfection frequency on the generation of DBPs in indoor. No, less and more represent no disinfecting, disinfection for 1–3 times per week, disinfection for >3 times per week with 3–20 mg/m3 hypochlorite; (B) DBPs generation with and without hand sanitizer; (C) The effect of ventilation on the lifetime of DBPs; (D) The contribution of temperature on the generation of DBPs. The central lines, ends, squares and error bars of the box define the medians, 25th and 75th percentiles, mean and the 99% confidence intervals of the mean, respectively. Error bars represent standard deviation (n = 3).
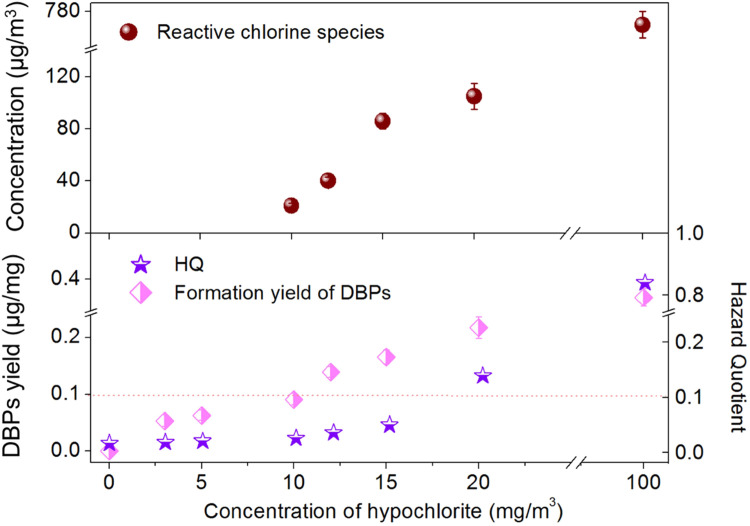
Notably, the correlation between the concentration of airborne DBPs and the dose of hypochlorite was not linear. At relatively low doses (0–10 mg/m3), the median concentration of DBPs increased by a maximum factor of 1.4 ( Fig. 3). However, from 10 to 100 mg/m3 hypochlorite, DBPs increased significantly by up to 16.4 folds. The yields of DBPs increased from 0.05 to 0.09 μg/mg as the hypochlorite increased from 3 to 10 mg/m3, which further increased rapidly to 0.14 μg/mg at 12 mg/m3 hypochlorite and 0.36 μg/mg at 100 mg/m3 hypochlorite (Fig. 3). This could result from additional reactive chlorine species and/or availability of precursors, when the hypochlorite dose exceeded 10 mg/m3. We therefore measured the concentrations of reactive chlorine species (e.g., Cl2, HOCl and Cl.) liberated into indoor air under varied hypochlorite doses. The amount of reactive chlorine species was well correlated with DBPs concentration when hypochlorite exceeded 10 mg/m3 (Fig. 3). On the contrary, no reactive chlorine species were detected in indoor air when hypochlorite was applied at <10 mg/m3. Therefore, 10 mg/m3 was a threshold concentration that initiated DBPs formation with reactive chlorine species in the air.
Fig. 3.
The profiles of reactive chlorine species, yield of DBPs, and HQ in indoor air at varied hypochlorite doses. The dotted line represents 10% of effect threshold. Error bars represent standard deviation (n = 3).
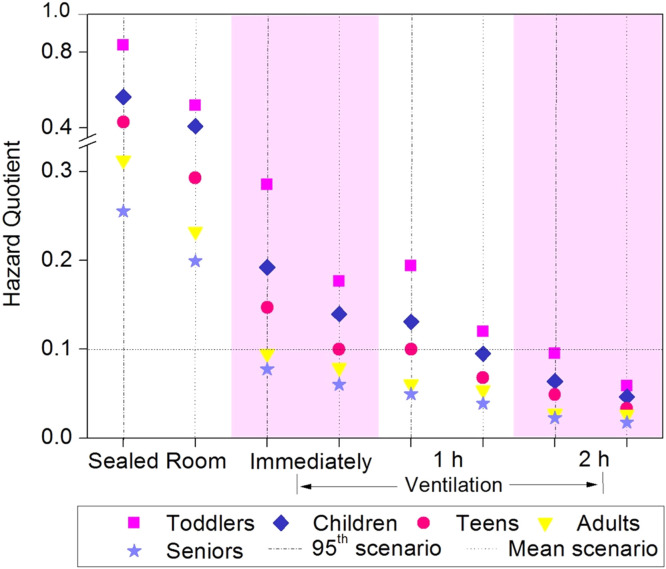
Due to the relatively high concentrations of DBPs detected in the air, it is critical to evaluate their health risks. The HI of DBPs in the 24 h time-inhalation model was calculated based on inhalation rate, BW, RfD, DBPs concentration and measured lifetime of DBPs. The HAAs and TCM have lifetime around 24 h, with the highest concentrations of 148.0 ± 6.0 ng/m3 (HAAs) and 43.9 ± 2.6 μg/m3 (TCM) after disinfection (100 mg/m3 hypochlorite), respectively (Fig. S2). Time-weighted average concentrations of DCAA, TCAA and TCM were used. The HI of 95th HQ values of DBPs for toddlers, children, teens, adults and seniors were obtained in the disinfected home 1# (0.84, 0.56, 0.43, 0.31 and 0.26, respectively, Fig. 4). They were approximately 50 times higher than that in samples without disinfection. The HI values for toddlers and children were greater than 0.5 (half of the HI threshold value, 1.0), and thus inhalation exposure to airborne DBPs may still pose risks to these groups. To expand the margin of safety, we determined to use HI = 0.1 (10% of effect threshold) as the targeted value.
Fig. 4.
Age health risks posed by DBPs via inhalation after disinfection in sealed rooms and ventilated rooms (0.2 m3/min, for 0, 1 h and 2 h). The dotted line represents 10% of effect threshold.
We further calculated the formation yield of airborne DBPs under different hypochlorite doses as the indicator in disinfected indoor spaces (Fig. 3). The noncarcinogenic risks associated with DBPs generated in the places with disinfection at 20 mg/m3 was above 0.1, while the risks were minimal as the dosage of hypochlorite was below 15 mg/m3 (all HI values < 0.1). Therefore, disinfection with 10 mg/m3 hypochlorite would not lead to significantly elevated noncarcinogenic risks of DBPs via inhalation exposure.
3.2. Another major influential factor for airborne DBPs formation: precursor availability
Notably, hypochlorite dose above 100 mg/m3 would not further increase DBPs formation, likely due to limited DBPs precursors in the sampled spaces (Fig. 1). There are two major sources of DBPs precursors in indoor environment, including components derived from human skin and those released from daily used PCPs. A typical adult sheds skin at 30–90 mg/h (Gowadia and Settles, 2001) and releases sebum at about 500 mg/h (Downing et al., 1982). Squalene (major component of human skin oil) and oleic acid (typical monounsaturated fatty acid derived from skin oil) have been commonly found in indoor environment at relative high concentrations, and could serve as DBPs precursors (Schwartz-Narbonne et al., 2019, Zhou et al., 2016). Limonene, another typical DBP precursor and universal component in PCPs, e.g., hand sanitizer, lotion, sunscreen, air fresheners, bleach fumes and detergent, has also been detected in indoor air at 10–1000 ppb (Wang et al., 2019a, Wang et al., 2019b). The usage of hand sanitizer increased dramatically during the pandemic, providing a significant amount of DBPs precursors and inducing DBP formation in the air. To confirm this, we compared the DBPs formed in a disinfected home 2# with and without the usage of hand sanitizer (Group A: 40 mL hand sanitizer + 20 mg/m3 hypochlorite, and Group B: 20 mg/m3 hypochlorite only). The median concentration of DBPs formed in the home 2# disinfected after hand sanitizer usage was 6.2 μg/m3 (Group A), significantly higher than that in group B (3.7 μg/m3) (Fig. 2B). Similarly, in the home 1#, airborne DBPs concentration increased by 1.5–2.1 folds during the lunch hour, when approximately 1000 mL hand sanitizer was used (Fig. 2B). To examine the formation potential of common solvents in hand sanitizer, we chlorinated limonene at a molar mixing ratio of 5:1 (Chlorine: limonene), and diverse DBPs were formed, including TCM (55.6 ± 2.6 μmol/mol), TCAA (66.6 ± 1.8 μmol/mol), and DCAA (69.9 ± 1.7 μmol/mol). The yields of DBPs were calculated as DBPs divided by limonene (Fig. S3).
Temperature affects DBPs formation by changing the reactivity and availability of precursors. Higher temperature leads to more DBPs formation in water (Wang et al., 2013), but its effects on airborne DBPs are unknown. We divided the samples into two groups (<26 °C and >26 °C) based on the temperature of sampled air. The total DBPs in places with higher temperature were consistently higher than those in colder places (median concentration of 43.2 μg/m3 v.s. 25.0 μg/m3, hypochlorite 100–200 mg/m3, p < 0.05) (Fig. 2D and Table S11). The similar results were observed at less disinfected places (3–20 mg/m3), where the median concentration was 3.6 μg/m3 (>26 °C) v.s. 1.4 μg/m3 (<26 °C, p < 0.05) (Fig. 2D and Table S10). Furthermore, in warmer places (>26 °C), TCM increased by 72.4% and 118.8% with 100–200 mg/m3 and 3–20 mg/m3 hypochlorite, which was significantly higher than those of HAAs (increased by 64.0% and 83.3%, respectively). This may be attributed to the heterogeneous reactions of DBPs occurring on the solid surface of the indoor space (Singer et al., 2004, Morrison, 2008). TCM is more volatile than HAAs, and thus more TCM can be transferred from surface to indoor air. The above results indicated that temperature can enhance the concentration of DBPs in indoor air.
3.3. Effective strategies for reducing airborne DBPs: ventilation and surface wiping
Ventilation has been proved effective in controlling DBPs in indoor air (Weschler and Carslaw, 2018). In this study, we further determined the optimal ventilation rate by measuring the concentration and lifetime of DBPs. In a 24 m3 sealed room disinfected with 100 mg /m3 hypochlorite for 30 min, the lifetime of DBPs was around 24 h (Lifetime refers to the time for the concentration of DBPs to decrease to below MDL). With an air exchange rate of 0.5 (flow = 0.2 m3/min), the lifetime of TCM and HAAs decreased to 18 h and 8 h, respectively (Fig. 2C). When the flow increased to 0.4 m3/min, the lifetime values further reduced to 10 h and 3 h, respectively. Ventilation at 0.4 m3/min for 3 h was equivalent to exchanging 300% of the whole air. TCM was no longer detected after 1 h of ventilation at 4 m3/min. Similar results were obtained with a lower amount of disinfectant (20 mg/m3 hypochlorite), where the lifetime of DBPs decreased from 10 h to 30 min as the flow increased from 0 to 4 m3/min (Fig. S4).
Meanwhile, we calculated the HI values to recommend appropriate ventilation duration. In the 100 mg/m3 hypochlorite disinfected place. With the ventilation at a flow of 0.2 m3/min for 2 h after disinfection, the HI values of DBPs for all five age groups decreased to <0.1 (10% of effect threshold) (Fig. 4), indicating that prolonged ventilation is needed.
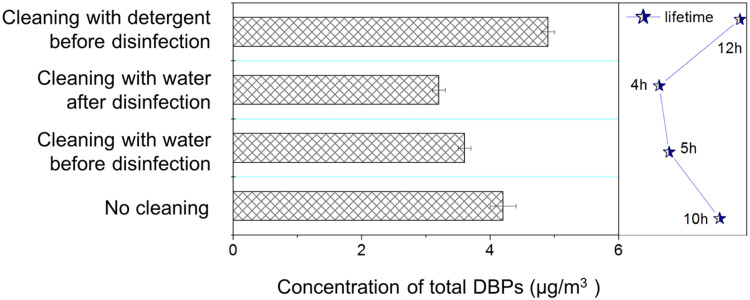
Despite the effectiveness of ventilation, DBPs were still detected even after exchanging 100% of the indoor air. We postulated that the continued DBPs formation on the indoor solid surface served as the major source of DBPs in the air. To confirm this, we firstly examined the volatility of TCM, a typical DBP, 500 μL TCM-d was applied on the floor at a concentration of 1000 mg/L, and TCM-d concentration in indoor air peaked at 3.6 μg/m3 after 5 h (Fig. S5). Secondly, we wiped the floor with a water-dipped cloth after disinfection, and the lifetime of DBPs in indoor air decreased by 22.2% compared to that without wiping. The above results collectively verified the hypothesis that continued DBPs formation contributed to their persistence in indoor air.
Wiping floor with water before disinfection can further reduce DBPs in the air. We found that the concentration of DBPs in indoor air after 30 min disinfection was 12–20% lower when the floor was wiped with water beforehand ( Fig. 5), where the lifetime of DBPs also decreased by 50%. Replacing water with detergent could otherwise increase DBPs formation. Surface cleaned with 0.5% detergent prior to disinfection resulted in 30–50% more DBPs (the highest concentration was 4.8 μg/m3 and the lifetime was prolonged to 12 h, Fig. 5). It is possibly because cleaning with detergents can introduce additional precursors into indoor air. Therefore, cleaning with water was more appropriate to remove DBPs precursors on solid surface before disinfection.
Fig. 5.
The effects of surface cleaning on DBPs in the air. Error bars represent standard deviation (n = 3).
4. Conclusions and implications
Intensive disinfection has been applied to combat the COVID-19 pandemic worldwide. Its associated environmental impacts have just started to gain attention. Recently, the increasing risks of antimicrobial resistance (AMR) spread caused by intensive disinfection was reported (Lu and Guo, 2021) and substantially enhanced disinfectant concentrations in indoor air have also been observed (Zheng et al., 2020). This study highlighted significantly increased airborne DBPs and their associated risks in disinfected indoor air. It is essential to determine the appropriate dose of disinfectants to efficiently inactive virus while maintaining the secondary pollution, e.g., DBPs and AMR at relatively low levels.
WHO has recommended 1000 mg/L hypochlorite to disinfect the indoor surface of non-healthcare settings by wiping, e.g. in the home, schools or restaurants (WHO, 2020). COVID-19 Prevention and Control Plan (Chinese Health Commission, CHC, 2020) has suggested to apply 1000 mg/L hypochlorite at 100 mL/m2–300 mL/m2 to inactivate coronavirus on floors and walls. In the routine indoor disinfection process, hypochlorite applied at 100 mL/m2–300 mL/m2 may be ascribed to over disinfection. In order to eliminate risks associated with airborne DBPs, we recommend 10 mL/m3 hypochlorite at 1000 mg/L (10 mg/m3) for routine indoor disinfection. Meanwhile, the effectiveness of virus inactivation at this dose needs further investigation. Additionally, less usage of PCPs and lower temperature are beneficial to the reduction of DBP precursor availability and reactivity. Ventilation should be performed immediately after the recommended disinfection time (varied for different places) until three-time air exchanges are achieved. Wiping the surface with water before and/or after disinfection is effective to remove precursors and prevent prolonged DBPs formation reactions occurring on solid surface, resulting in less airborne DBPs.
In addition to the factors mentioned above, there are other practices that we may adopt to decrease airborne DBPs in our living environment. Smoking releases nicotine, a critical DBP precursor, at as high as 8 mg per cigarette (Sleiman et al., 2010). More than 100 different DBP precursors can emit from air fresheners, including volatile organic compounds (VOCs, e.g. limonene, alpha-pinene and benzene) and semi-VOCs (e.g. phthalates) (Weschler and Carslaw, 2018). Nicotine and some of these VOC compounds stay on indoor surface and persist for weeks to months (Singer et al., 2003). Hence, reducing the consumption of air fresheners and cigarettes can help to control airborne DBPs, especially during the pandemic.
The results of our study call for the attention of the increased health risks associated with elevated DBPs formation during the control and prevention of COVID-19. In a foreseeable future, disinfectants will continuously be applied to control transmission and prevalence of diverse pathogens. Comprehensive evaluations on DBP contamination in the air and their associated health risks are urgently needed.
CRediT authorship contribution statement
Jinxiu Lou: Conceptualization, Methodology, Data curation, Writing - original draft. Wei Wang: Validation. Huijie Lu: Software, Visualization, Investigation, Supervision. Lin Wang: Software. Lizhong Zhu: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21836003, 21621005), and the consulting research project of Chinese Academy of Engineering (2020-ZD-15).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jhazmat.2021.126249.
Appendix A. Supplementary material
Supplementary material
.
References
- Allan M., Richardson G.M. Probability density functions describing 24-h inhalation rates for use in human health risk assessments. Hum. Ecol. Risk Assess. 1998;4(2):379–408. [Google Scholar]
- Bove G.E., Rogerson P.A., Vena J.E. Case control study of the geographic variability of exposure to disinfectant byproducts and risk for rectal cancer. Int. J. Health Geogr. 2007;6:18. doi: 10.1186/1476-072X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove G.E., Rogerson P.A., Vena J.E. Case-control study of the effects of trihalomethanes on urinary bladder cancer risk. Arch. Environ. Occup. Health. 2007;62:39–47. doi: 10.3200/AEOH.62.1.39-47. [DOI] [PubMed] [Google Scholar]
- Chinese Health Commission, 2020. COVID-19 Prevention and Control Plan. http://www.nhc.gov.cn/jkj/s3577/202002/573340613ab243b3a7f61df260551dd4/files/c791e5a7ea5149f680fdcb34dac0f54e.pdf.
- Chinese Ministry of Environmental Protection, 2013. Ambient air-Determination of volatile organic compounds-Sorbent adsorption and thermal desorption/gas chromatography mass spectrometry method. HJ 644-2013.
- Chinese Ministry of Health, 2004. Methods fordetermination of chlorides in the air of workplace. GBZ/T 160.37-2004.
- Chu W.H., Fang C., Deng Y., Xu Z.X. Intensified disinfection amid COVID-19 pandemic poses potential risk to water quality and safety. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c04394. [DOI] [PubMed] [Google Scholar]
- Ciriminna R., Lomeli-Rodriguez M., Demma C.P., Lopez-Sanchez J.A., Pagliaro M. Limonene: a versatile chemical of the bioeconomy. Chem. Commun. 2014;50:15288–15296. doi: 10.1039/c4cc06147k. [DOI] [PubMed] [Google Scholar]
- Cortvriend, J., 2008. ENV.D.2/ETU/2007/0077r. Establishment of a List of Chemical Parameters for the Revision of the Drinking Water Directive. http://circa.europa.eu/Public/irc/env/drinking_water_rev/library?l./chemical_parameters/parameters_26092008pdf/_EN_1.0_&a.d.
- Costet N., Villanueva C., Jaakkola J., Kogevinas M., Cantor K., King W., Lynch C., Nieuwenhuijsen M., Cordier S. Water disinfection by-products and bladder cancer: is there a European specificity? A pooled and meta-analysis of European case-control studies. Occup. Environ. Med. 2011;68:379–385. doi: 10.1136/oem.2010.062703. [DOI] [PubMed] [Google Scholar]
- Downing D.T., Stranieri A.M., Strauss J.S. The effect of accumulated lipids on measurements of sebum secretion in human skin. J. Invest. Dermatol. 1982;79:226–228. doi: 10.1111/1523-1747.ep12500066. [DOI] [PubMed] [Google Scholar]
- Fellin P., Otson R. Assessment of the influence of climatic factors on concentration levels of volatile organic compounds (VOCs) in Canadian homes. Atmos. Environ. 1994;28:3581–3586. [Google Scholar]
- Gabriela M.F., Felgueirasa F., Mourãoa Z., Fernandesb E.O. Assessment of the air quality in 20 public indoor swimming pools located in the Northern Region of Portugal. Environ. Int. 2019;133 doi: 10.1016/j.envint.2019.105274. [DOI] [PubMed] [Google Scholar]
- Gowadia H.A., Settles G.S. The natural sampling of airborne trace signals from explosives concealed upon the human body. J. Forensic Sci. 2001;46:1324–1331. [PubMed] [Google Scholar]
- Grazuleviciene R., Kapustinskiene V., Vencloviene J., Buinauskiene J., Nieuwenhuijsen M.J. Risk of congenital anomalies in relation to the uptake of trihalomethane from drinking water during pregnancy. Occup. Environ. Med. 2013;70:274–282. doi: 10.1136/oemed-2012-101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton B.J., Luben T.J., Herring A.H., Savitz D.A., Singer P.C., Weinberg H.S., Hartmann K.E. The effect of water disinfection byproducts on pregnancy outcomes in two southeastern US communities. Occup. Environ. Med. 2011;53:1172–1178. doi: 10.1097/JOM.0b013e31822b8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K., Nakai T., Hishida Y., Asami M., Ohkubo K., Akiba M. Formation of 2,6-dichloro-1,4-benzoquinone from aromatic compounds after chlorination. Water Res. 2017;110:48–55. doi: 10.1016/j.watres.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Lou J.X., Wang W., Zhu L.Z. Transformation of emerging disinfection byproducts Halobenzoquinones to haloacetic acids during chlorination of drinking water. Chem. Eng. J. 2021;418 [Google Scholar]
- Lu J., Guo J.H. Disinfection spreads antimicrobial resistance. Science. 2021;371:474. doi: 10.1126/science.abg4380. [DOI] [PubMed] [Google Scholar]
- Lu R.J., Zhao X., Li J., Niu P.H., Yang B., Wu H.L., Wang W.L., Song H., Huang B.Y., Zhu N., Bi Y.H., Ma X.J., Zhan F.X., Wang L., Hu T., Zhou H., Hu Z.H., Zhou W.M., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J.Y., Xie Z.H., Ma J.M., Liu W.J., Wang D.Y., Xu W.B., Holmes E.C., Gao G.F., Wu G.Z., Chen W.J., Shi W.F., Tan W.J. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila J.M., Lakey P.S.J., Shiraiwa M., Wang C., Abbatt J.P.D., Arata C., Goldstein A.H., Ampollini L., Katz E.F., DeCarlo P.F., Zhou S., Kahan T.F., Cardoso-Saldaña F.J., Ruiz L.H., Abeleira A., Boedicker E.K., Vance M.E., Farmer D.K. Multiphase chemistry controls inorganic chlorinated and nitrogenated compounds in indoor air during bleach cleaning. Environ. Sci. Technol. 2020;54:1730–1739. doi: 10.1021/acs.est.9b05767. [DOI] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730–105733. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison G. Interfacial chemistry in indoor environments. Environ. Sci. Technol. 2008;42:3494–3499. doi: 10.1021/es087114b. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen M.J., Toledano M.B., Eaton N.E., Fawell J., Elliott P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup. Environ. Med. 2000;57:73–85. doi: 10.1136/oem.57.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen M.J., Dadvand P., Grellier J., Martinez D., Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ. Health. 2013;12:6. doi: 10.1186/1476-069X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odabasi M. Halogenated volatile organic compounds from the use of chlorine-bleach-containing household products. Environ. Sci. Technol. 2008;42:1445–1451. doi: 10.1021/es702355u. [DOI] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. J. Am. Med. Assoc. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L.L., Sun J.T., Li Z.H., Zhan Y., Xu S., Zhu L.Z. Organophosphate pesticide in agricultural soils from the Yangtze River Delta of China: concentration, distribution, and risk assessment. Environ. Sci. Pollut. Res. 2018;25(1):4–11. doi: 10.1007/s11356-016-7664-3. [DOI] [PubMed] [Google Scholar]
- Peder W. Indoor air chemistry: terpene reaction products and airway effects. Int. J. Hyg. Environ. Health. 2020;225 doi: 10.1016/j.ijheh.2019.113439. [DOI] [PubMed] [Google Scholar]
- Rahman M.B., Driscoll T., Cowie C., Armstrong B.K. Disinfection byproducts in drinking water and colorectal cancer: a meta-analysis. Int. J. Epidemiol. 2010;39:733–745. doi: 10.1093/ije/dyp371. [DOI] [PubMed] [Google Scholar]
- Richardson S.D., Plewa M.J., Wagner E.D., Schoeny R., DeMarini D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection byproducts in drinking water: a review and roadmap for research. Mutat. Res. 2007;636:178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Schwartz-Narbonne H., Wang C., Zhou S.M., Abbatt J.P.D., Faust J. Heterogeneous chlorination of squalene and oleic acid. Environ. Sci. Technol. 2019;53(3):1217–1224. doi: 10.1021/acs.est.8b04248. [DOI] [PubMed] [Google Scholar]
- Singer B.C., Hodgson A.T., Nazaroff W.W. Gas-phase organics in environmental tobacco smoke: 2. Exposure-relevant emission factors and indirect exposure from habitual smoking. Atmos. Environ. 2003;37:5551–5561. [Google Scholar]
- Singer B.C., Revzan K.L., Hotchi T., Hodgson A.T., Brown N.J. Sorption of organic gases in a furnished room. Atmos. Environ. 2004;38:2483–2494. [Google Scholar]
- Sleiman M., Gundel L.A., Pankow J.F., Jacob P., Singer B.C., Destaillats H. Formation of carcinogens indoors by surface mediated reactions of nicotine with nitrous acid, leading to potential third hand smoke hazards. Proc. Natl. Acad. Sci. USA. 2010;107(15):6576–6581. doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency, update 2020a. Regional Screening Levels(RSLs)-Generic Tables. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables.
- U.S. Environmental Protection Agency . Office of Research and Development; Washington, DC: 1989. Risk Assessment Guidance for Superfund. Vol. I: Human Health Evaluation Manual (Part A). EPA/540/1-89/002; pp. 35–52. [Google Scholar]
- U.S. Environmental Protection Agency: Washington, DC, 2011a. Exposure Factors Handbook, Chapter 6: Inhalation Rates. https://www.epa.gov/expobox/efh-highlights-chapter-6.
- U.S. Environmental Protection Agency: Washington, DC, 2011b. Exposure Factors Handbook, Chapter 8: Body Weight Studies. https://www.epa.gov/expobox/efh-highlights-chapter-8.
- United States Environmental Protection Agency, 2020b. List N: Disinfectants for Use Against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. (Accessed June 23, 2020).
- United States Environmental Protection Agency, 2006. Stage 2 Disinfectants and disinfection byproducts rule. EPA-HQ-OW-2002-0043.
- Villanueva C.M., Fernandez F., Malats N., Grimalt J.O., Kogevinas M. Meta-analysis of studies on individual consumption of chlorinated drinking water and bladder cancer. J. Epidemiol. Community Health. 2003;57:166–173. doi: 10.1136/jech.57.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva C.M., Cantor K.P., Cordier S., Jaakkola J.J., King W.D., Lynch C.F., Porru S., Kogevinas M. Disinfection byproducts and bladder cancer: a pooled analysis. Epidemiology. 2004;15:357–367. doi: 10.1097/01.ede.0000121380.02594.fc. [DOI] [PubMed] [Google Scholar]
- Villanueva C.M., Cantor K.P., Grimalt J.O., Malats N., Silverman D., Tardon A., Garcia-Closas R., Serra C., Carrato A., Castano-Vinyals G. Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am. J. Epidemiol. 2007;165:148–156. doi: 10.1093/aje/kwj364. [DOI] [PubMed] [Google Scholar]
- Wallace L.A., Ott W.R., Weschler C.J., Lai A.C.K. Desorption of SVOCs from heated surfaces in the form of ultrafine particles. Environ. Sci. Technol. 2017;51:1140–1146. doi: 10.1021/acs.est.6b03248. [DOI] [PubMed] [Google Scholar]
- Waller K., Swan S.H., DeLorenze G., Hopkins B. Trihalomethanes in drinking water and spontaneous abortion. Epidemiology. 1998;2:134–140. [PubMed] [Google Scholar]
- Wang C., Collins G.B., Abbatt J.P.D. Indoor illumination of terpenes and bleach emissions leads to particle formation and growth. Environ. Sci. Technol. 2019;53:11792–11800. doi: 10.1021/acs.est.9b04261. [DOI] [PubMed] [Google Scholar]
- Wang W., Qian Y.C., Boyd J.M., Wu M.H., Hrudey S.E., Li X.-F. Halobenzoquinones in swimming pool waters and their formation from personal care products. Environ. Sci. Technol. 2013;47:3275–3282. doi: 10.1021/es304938x. [DOI] [PubMed] [Google Scholar]
- Wang X.J., Xiao Y., Han W., Wang C. GC determination of 9 haloacetic acids in air and exhaust gas with absorption liquid sampling. PTCA (Part B: Chem. Anal.) 2019;55:882–886. [Google Scholar]
- Weschler C.J., Carslaw N. Indoor chemistry. Environ. Sci. Technol. 2018;52:2419–2428. doi: 10.1021/acs.est.7b06387. [DOI] [PubMed] [Google Scholar]
- WHO, 2006. http://www.who.int/water_sanitation_health/dwq/gdwq0506.pdf.
- WHO, 2020. Cleaning and disinfection of environmental surfaces in the context of COVID-19.
- WHO, 2021. Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/table.
- Wong J.P.S., Carslaw N., Zhao R., Zhou S., Abbatt J.P.D. Observations and impacts of bleach washing on indoor chlorine chemistry. Indoor Air. 2017;27:1082–1090. doi: 10.1111/ina.12402. [DOI] [PubMed] [Google Scholar]
- Wright J.M., Evans A., Kaufman J.A., Rivera-Núñez Z., Narotsky M.G. Disinfection by-product exposures and the risk of specific cardiac birth defects. Environ. Health Perspect. 2017;125:269–277. doi: 10.1289/EHP103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.Q., Harris S.A., Jantunen L.M., Kvasnicka J., Nguyen L.V., Diamond M.L. Phthalates: relationships between air, dust, electronic devices, and hands with implications for exposure. Environ. Sci. Technol. 2020;54:8186–8197. doi: 10.1021/acs.est.0c00229. [DOI] [PubMed] [Google Scholar]
- Yu N.Y., Guo H.W., Yang J.P., Jin L., Wang X.B., Shi W., Zhang X.W., Yu H.X., Wei S. Non-target and suspect screening of per- and polyfluoroalkyl substances in airborne particulate matter in China. Environ. Sci. Technol. 2018;52:8205–8214. doi: 10.1021/acs.est.8b02492. [DOI] [PubMed] [Google Scholar]
- Zheng G.M., Filippelli G.M., Salamova A. Increased indoor exposure to commonly used disinfectants during the COVID-19 pandemic. Environ. Sci. Technol. Lett. 2020;7:760–765. doi: 10.1021/acs.estlett.0c00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Forbes M.W., Katrib Y., Abbatt J.P.D. Rapid oxidation of skin oil by ozone. Environ. Sci. Technol. Lett. 2016;3:170–174. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material