Abstract
By definition, autism spectrum disorder (ASD) emerges during early childhood. However, despite longstanding recommendations for earlier identification and intervention, there has been relatively slow progress in lowering the average age of diagnosis and enrollment in treatment for affected children. This has been due to several factors, including the inadequacy of behavioral risk markers and clinical practice entailing a “wait to see” or “wait to fail” approach to identification. Converging evidence now suggests that brain changes precede changes in behavior in children with ASD. This work has led to the discovery of potential biomarkers of presymptomatic or prodromal risk which may be used to accurately identify children at ultra-high risk during the first year of life. Such findings raise the possibility of intervention prior to the consolidation of core autistic features and during a period of substantial neural plasticity. While these avenues of research suggest strong potential for eventual clinical application, they also raise new questions regarding content, dosage, and timing of intervention as well as ethical, legal, and social considerations concerning presymptomatic identification and treatment.
Keywords: autism spectrum disorder, prediction, infant, presymptomatic, prodromal
Predicting Autism Risk in Infancy
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social-communication and interaction deficits and restricted, repetitive patterns of interests and behavior that are evident in early childhood. Its prevalence has grown substantially over the past several decades, with current estimates ranging from 1.7–2.5% in the United States.1,2 This represents over 1.5 million children with ASD, the vast majority of whom receive or will receive specialized services.2 Each year, approximately 100,000 (and growing) individuals with ASD reach adulthood, and many face a myriad of challenges related to employment, housing, mental health, and overburdened or insufficient support services.3–5 Although it varies widely by individual, there are a host of significant costs associated with ASD, from direct costs related to the provision of special education programs, housing, and medical care to indirect costs such as loss of productivity affecting both individuals with ASD and their families.6 Currently, overall lifetime cost of care per individual with ASD can exceed $3 million, totaling more than $265 billion annually in the United States and rising to an estimated $1 trillion by 2025.7,8
A central challenge to the field – and a significant barrier to improving public health and educational outcomes – has been in the identification and treatment of affected children as early as possible. In the United States, the current average age of first clinical diagnosis of ASD is 4.2 years.3 Upwards of 50% of children with the disorder are not identified until after age 6 years, suggesting that the educational system and its associated social and cognitive demands function as a de facto screener for many with the disorder.21 Crucially, these figures mean that the majority of children with ASD are never afforded access to early intervention, arguably the most effective and established form of treatment. And despite longstanding acknowledgement that early identification and intervention are essential to improving long term outcomes for children with ASD, there has been relatively modest change in average age of diagnosis over the past decade.1 Prevailing practice is, in many cases, a model of “wait-to-see” (if delays worsen or resolve on their own) or “wait-to-fail” (identification occurs only when a child is unable to meet the demands of their environment, such as elementary school).
Presymptomatic/prodromal period
As with previous diagnostic criteria, the DSM 5 specifies that symptoms of ASD must be present in early development, while acknowledging that such symptoms may not fully manifest until the demands of the child’s environment elicit them.10 Indeed, the defining behavioral features of ASD are not present at birth and instead emerge over the first years of life. For most children, overt symptoms of autism will not become apparent until toddlerhood or later. On average, the age of first developmental concerns reported by caregivers is 32 months, with a range spanning early infancy to school age.11 Often the initial concerns reported by parents are not specific to the diagnostic criteria for ASD, such as expressive language delay. Retrospective studies of the presymptomatic or prodromal period preceding a diagnosis have been limited in their capacity to accurately capture the unfolding of autism, particularly as they concern parental recall of timing and behavioral change.12
In just over the past decade, prospective studies of infant siblings of children with autism, who are themselves at higher risk for the disorder, have substantially clarified the presymptomatic or prodromal period of autism.13 In one such infant sibling study, Ozonoff and colleagues found that 6- and 12-month old infants later diagnosed with ASD appear no different from typically developing children on measures of core social features such as social smiling or gaze to faces.14 Many babies who will later be diagnosed with ASD will smile, make eye contact, and otherwise engage with caregivers no differently than babies who do not go on to receive a diagnosis. Rather, differences in social communicative features emerge gradually over the second year of life for many children who eventually receive a diagnosis. Restricted and repetitive behaviors, including atypical sensory responsivity, have been reported as elevated at age 12 months in high-risk infants later diagnosed with ASD.15,16 However, observed differences are relatively modest and there are limited data on these behaviors prior to toddlerhood. Studies of cognitive and adaptive behavior suggest that while early differences may be detected with sufficient statistical power, standard scores for most children later diagnosed with ASD are within the normal range at 6 and 12 months.17–19 In sum, while behavioral differences have been reported in the published literature at or prior to age 12 months, these differences have largely been observed only at the group level and are likely too subtle to rise to the level of detection by caregivers or clinicians.
Prediction* of ASD through biomarkers
Much of the impetus for identifying early and predictive biomarkers of ASD has been that assessment of risk on the basis of behavioral markers alone has been insufficient with regard to accuracy even at age 18 months.20,21 This lack of precision may stem in part from heterogeneity associated with the behavioral phenotype of ASD, particularly as it relates to early social-communication symptoms as these may not reliably differentiate children at this age.20 Critically, there are no tools available to accurately identify children during the first year of life. Experimental behavioral screeners for ASD risk in infants have shown poor sensitivity and are as of yet unsuitable for widespread adoption in clinical settings.22,23 Lacking options, providers continue to rely exclusively on behavioral screeners, such as the Modified Checklist for Autism in Toddlers (M-CHAT) at ages 18 months and later when core symptoms may be clearly observed.21 However, a recent universal screening study of the M-CHAT/F found evidence of poor accuracy, with an overall positive predictive value of about 15%.24 Perhaps more concerning was evidence that behavioral screening is significantly more accurate for children who are White and of higher socioeconomic status. The addition of biological markers could plausibly reduce such bias and enhance the accuracy of behavioral approaches to risk assessment or even replace them in some contexts. Evidence to this effect is seen in studies of other neurodevelopmental disorders, such as dyslexia, wherein neural markers have been shown as superior to behavioral measures in predicting long-term reading improvement in adolescents.25
What have we learned about autism that might inform prediction? There are numerous examples of biological findings related to a diagnosis of ASD in the published literature.26 However, the vast majority of these putative markers have been identified through cross-sectional studies of older children and adults, for whom the disorder is already clearly manifest, using approaches such as neuroimaging or eye tracking. Developmental variance largely precludes the downward extension of findings from older individuals to infants; children with autism are not tantamount to children who will later develop it. Data from prospective studies of infants later diagnosed with ASD support the position that the biological signature of the disorder is unique from that observed in older children and adults.27–30 As an example, we have found that infants later diagnosed with ASD have a larger corpus callosum relative to typically developing infants; this effect is diminished by around age 2 years.24 By preschool age or later, the corpus callosum in children with autism is relatively smaller.31 While this may be superficially considered inconsistency, it likely reflects qualitatively different developmental effects, such as early axonal pruning versus later experience-dependent myelination. Relatedly, several independent studies have identified early increases in fractional anisotropy, a measure of white matter structural connectivity, but slower growth rates among children with ASD, such that trajectories cross over with comparison children during early childhood.32 Such evidence of increased fractional anisotropy in ASD has only been reported in young children. These examples highlight a common theme in the published literature, that the neural signature of ASD is characterized by significant developmental variance.
The advent of infant sibling studies has dramatically enhanced our understanding of the early development of ASD and informed potential methods for prediction. Infant siblings of children with ASD are at elevated risk for the disorder, allowing researchers the opportunity to chart the early unfolding of the disorder in prospectively ascertained samples.13 A multitude of infant sibling studies have been published in the past decade, but only a handful have focused on characterizing the early biological signatures of ASD.32,33 The majority of these have examined temporal associations at the group level to address scientific questions related to issues of development or pathophysiology; relatively few have focused on prediction in individual infants. While group-level studies have informed potential developmental mechanisms underlying autism, clinically viable prediction of risk in infancy requires approaches that show evidence of strong, individual-level classification accuracy.
There are now seven published empirical papers reporting potential biomarkers for the presymptomatic prediction of ASD in infants. Based on resting state EEG data collected from infants and toddlers, for example, Bosl and colleagues examined whether features of signal complexity could accurately classify ASD risk in infants.34 The authors reported high classification accuracy for comparisons between infants later diagnosed and low-risk controls. However, classification accuracy was not reported for the high-risk infant comparison group (i.e., HR with vs. without ASD). Recently, Gabard-Durnam and colleagues35 reported that EEG power measures collected over 3 to 12 months differentiated HR infants later diagnosed with ASD from high-risk infants without ASD and low risk controls. Effects were found both for intercept at age 6 months as well as change (or slope) over the age interval, with frontal EEG power contributing the most to group differences. The authors reported 82% sensitivity, 86% sensitivity, and 72% positive predictive value for detection of ASD among high risk infants over the 3 to 12 month interval. Like EEG, eye tracking is another potentially scalable means of assessing risk for ASD in infants that has been utilized by several research groups. For example, Klin and Jones reported that change in duration of visual fixation from ages 2 to 6 months differentiated high-risk infants later diagnosed with ASD from typically developing infants.36 A key takeaway from this work is that autism risk may be best captured through change over time versus measurement at a single time point. Such developmental surveillance may be clinically feasible given the potential scalability of eye tracking. While eye tracking studies of infants are encouraging, the individual-level diagnostic accuracy, particularly comparing high risk children with and without autism, has generally not been examined or reported.
The Infant Brain Imaging Study has published four reports examining the accuracy of MRI screening at age 6 months in classifying a later diagnosis of ASD in high risk infants. Shen and colleagues37 reported on predictive values of extra-axial cerebral spinal fluid (CSF) measured at age 6 months in relation to diagnostic outcomes at age 2 years. Using a cross-validated machine learning approach, the authors found that an algorithm accounting for extra-axial CSF, cerebral volume, age and sex yielded a positive predictive value of 36% and overall accuracy of 69%. The approach accurately predicted 66% of those later diagnosed with ASD (sensitivity) and 68% of those without a diagnosis (specificity). In a diffusion-tensor imaging study, Wolff and colleagues27 reported that fractional anisotropy in the splenium of the corpus callosum and superior cerebellar peduncles at age 6 months significantly predicted diagnosis at age 2. Positive predictive values ranged from 65–68%, and negative predictive values ranged from 84–85%.
Using structural MRI, Hazlett and colleagues38 reported on brain structural growth in a study of 435 infants at low- and high-risk for ASD assessed at ages 6, 12, and 24 months. Of these, 148 contributed data at each time point. The authors identified increased rates of surface area expansion from age 6 to 12 months, followed by increased rate of total brain volume from 12 to 24 months. These findings directly support the hypotheses generated from previous work39 and may implicate the proliferation of neural progenitor cells prenatally.40 To assess predictive accuracy, a machine learning approach was applied to features of surface area growth from 6 to 12 months. Results indicated that this structural MRI data predicted diagnostic outcome at age 24 months with 88% sensitivity, 95% specificity and a PPV of 81%.
Finally, Emerson and colleagues41 utilized whole-brain resting state functional MRI (rsfMRI) data collected at age 6 months to predict diagnostic outcome at age two years in infants at high familial risk for ASD. Based on a cross-validated machine learning algorithm applied to the imaging data, the authors reported a positive predictive value of 100% and negative predictive value of 96%. Functional network pairs (n = 974) contributing to the predictive algorithm were derived on the basis of their association with clinical features at age two, such as social communication skills and restricted and repetitive behaviors. Results suggested that differences in functional connectivity contributing to the predictive algorithm were highly diffuse, with no single network or concentrated set of ROIs differentiating those who did versus did not develop ASD. Though based on a relatively modest sample (59 high risk infants, 11 of whom later received a diagnosis of ASD), this study along with the three other MRI studies of prediction suggest the possibility of developing clinically-viable MRI screening for ASD. Such a presymptomatic test for ASD could contribute to clinical decisions for infants who are already at elevated risk due to family history.
The use of MRI or some other biological measure to detect autism in infancy is in our view an important way forward given abundant evidence that brain changes precede behavioral change in children who develop autism. Put differently, intervention provided after the consolidation of defining symptoms may be too late to substantially impact neurodevelopment. This rationale is illustrated by studies of Parkinson’s disease (PD), where it has been well established that substantial loss of striatal dopaminergic function occurs prior to disease onset. Importantly, there are now several promising neuroimaging biomarkers for the prodromal identification of PD that are subjects of active investigation.42 Our network is now beginning a new project to replicate and refine neuroimaging methods for detecting autism, with the eventual goal of readying them for clinical use in high-risk infants. If used as a level 2 screener in conjunction with an approach involving a cost-effective level 1 (or universal) behavioral screener and/or polygenic risk score, MRI could be used to determine ultra-high risk status and allow for enrollment in very early intervention. If such an approach could be established in a high-risk sample, it could feasibly be adapted for the general population. While the field has only just begun to evaluate risk screeners for ASD in infants, there are several promising new measures, including a modification of the First Year Inventory that has recently been piloted in high-risk siblings.43
Presymptomatic intervention
It has long been acknowledged that early intervention is the preferred means of improving quality-of-life and long-term cognitive and behavioral outcomes for individuals with autism, as well as significantly decreasing lifetime costs associated with care.44–49 It is presumed that early intervention provides foundational skills that may prevent a developmental cascade culminating in the core features of ASD.50,51 Rather than responding to an entrenched behavioral excess or deficit or playing “catch-up” with regard to cognitive or adaptive skills, early intervention is tied to developmental processes in a manner individualized for each child and intended to support a healthy trajectory. This represents a qualitatively different intervention scenario than what is associated with the “wait to see” and “wait to fail” models of identification, for which providing treatment once a child is demonstrably behind their peers across multiple developmental domains is customary. Indeed, later intervention is likely to entail greater costs and significantly more effort to achieve the same effects as early intervention.52 Early intervention is likewise believed to capitalize on the highly malleable developing brain, leveraging a unique sensitive period of experience-dependent plasticity to foster the rapid accumulation of skills.53,54 It is during this time that the interplay between genes-brain-experience establish important foundations for ensuing development. As with behavior, early intervention should advantageously alter development before nervous system effects consolidate and become less responsive to treatment. However, early intervention provided after diagnosis, while desirable, may be insufficient to dramatically improve outcomes. Indeed, early intervention delivered outside of tightly controlled clinical intervention studies is associated with only modest gains in key outcome measures.55
If autism can be reliably predicted in infancy, a clear opportunity to deliver presymptomatic intervention presents itself. But is presymptomatic intervention a desirable goal, or one that represents an improvement over traditionally defined early intervention? Infant intervention would be uniquely proactive, allowing children who are at ultra-high risk to contact specialized services before the accumulation of developmental delays and significant changes to brain structure and function. For autism, and neurodevelopmental disorders more broadly, infant intervention is relatively new territory. In this section, we will briefly discuss pragmatic and conceptual considerations and provide an overview of nascent work in the area of infant intervention for ASD.
Aside from a handful of promising preliminary studies, there are no established practices for use in infants who will later develop ASD. Developing a presymptomatic intervention package for infants is not a simple matter of downward extending evidence-based interventions for preschool aged children. First, by definition, most children who are identified as eligible for presymptomatic intervention will not yet exhibit overt behavioral signs associated with ASD.18,56 Thus, the treatment targets themselves will qualitatively differ, with a focus more on developmentally appropriate targets than the symptoms themselves. The goal of a presymptomatic intervention might be to interrupt a developmental cascade that has downstream implications related to ASD, for example targeting attentional skills, such as visual orienting and sustained attention, in infancy to support later executive function or language acquisition.57 Presymptomatic intervention packages for infants identified as ultra-high risk will therefore entail the scaffolding of behavioral priors relevant to distal features of ASD, such as working on skills such as orienting to faces in anticipation of later joint attention. If a child enrolled in presymptomatic intervention begins showing signs of autism or receives a diagnosis (e.g., in the second year of life), intervention would likely transition to an established, ASD-specific approach, such as a naturalistic developmental behavioral intervention.58
What is known about infant intervention for ASD? As a relatively new area of inquiry, there are no established approaches to providing autism-focused intervention to children prior to toddlerhood. While preliminary, there are promising leads regarding potential pharmacological approaches. For example, Marchetto and colleagues59 reported that induced pluripotent stem cell derived neurons from children with ASD displayed dampened synaptic growth and activity following altered proliferation of neural progenitor cells. The authors reported that treatment with insulin growth factor 1 (IGF-1), a neurotrophic factor implicated in neural plasticity, was associated with significant improvement in neuronal network activity. In a related study, Schafer and colleagues60 demonstrated that the neuronal effects associated with ASD may be traced back to the development of neural stem cells prenatally, suggesting a potential target to improve subsequent neuronal development. While this work to date has been specific to children with ASD and microcephaly, others have reported improvements associated with alternate targets in idiopathic ASD generally, such as increased synaptic development through the blocking of interleukin-6.61 Together these and related studies highlight not only potentially promising avenues for intervention, but for the importance of prenatal or early postnatal timing. These studies also highlight the opportunity to purposefully address heterogeneity associated with autism by matching a pharmacologic intervention to a specific biotype. For example, the use of IGF-1 when progenitor cell hyper-proliferation is indicated or an anti-inflammatory for stagnant extra-axial CSF flow.
The literature on prodromal or presymptomatic behavioral intervention is similarly lean, with most studies significantly underpowered and, importantly, focused on high-risk siblings in general versus those selected as ultra-high risk through use of a biomarker or some other means. With an expected recurrence rate of about 20%, intervention studies that target all high-risk siblings may be ill-suited to evaluate treatment effects. Nonetheless, these studies have been instructive. There is evidence from single-case designs that behavioral interventions targeting ASD-relevant priors may be an effective approach to advantageously altering an infant’s developmental trajectory 62,63 Rogers and colleagues reported pilot data from an adapted version of Early Start Denver Model delivered to seven infants and toddlers showing early autistic symptoms and related clinical concerns.64,65 Relative to comparison groups, the treatment group showed lower symptom severity and a smaller proportion of cases meeting ASD diagnostic criteria at 36 months. Importantly, there was also evidence of feasibility, as indicated by high ratings of acceptability and good implementation fidelity. These will be key issues given the crucial role that parents are likely to play in the implementation of infant intervention.
In the first of a series of studies, Green and colleagues66 evaluated iBASIS, a parent-mediated intervention for autism in a proof-of-concept study involving 8 high-risk infant siblings. As with the Rogers et al. study,64 they found evidence of acceptability and feasibility, with parents reporting favorably on the experience. They also argued for the use of multiple measures of change not exclusively focused on infant behavior, e.g. parent-child interaction, eye tracking, other measures of brain function. In a follow-up randomized control trial of 44 high-risk siblings, the same group found evidence that the iBASIS intervention was effective at reducing the severity of later ASD symptoms in the treatment vs. control group comparison. Of note, they did not find evidence that infant intervention prevented ASD, which leads us to our final discussion point in this this section.
Should the ultimate goal of infant intervention be the prevention of ASD? There are well-known examples of primary or secondary prevention in medicine, such as the treatment of hypertension to prevent stroke or use of lifestyle interventions to reduce risk of type 2 diabetes.67,68 We acknowledge that a similar argument has been made regarding the future of psychiatry, wherein morbidity of psychiatric illness is reduced through prevention.69 Autism is a developmental disability (or difference) that for some individuals is a defining aspect of their identity, not dissimilar from Deaf culture and community.70 It is therefore important to carefully consider issues of neurodiversity and perspectives on disability from the outset. We are not alone in clarifying that the objective of presymptomatic intervention for autism is not the indiscriminate elimination of behavioral variability that may be grossly characterized as “atypical”.71,72 Rather, the goal of such intervention is to provide children deemed at the highest risk with enhanced opportunities to reach developmental milestones that are key to later adaptive function.
In many cases, achieving early developmental milestones in relative pace with peers may diminish later autistic symptom severity or improve intellectual and adaptive function. One example would be the support of expressive and receptive language through targeted intervention delivered during a sensitive period for language acquisition. A child receiving presymptomatic intervention focused on language may still develop ASD; however, intervention increases the likelihood of acquiring verbal language ability, compared to a non- or minimally-verbal outcome associated with no or delayed intervention.73 In other cases, outright prevention may be desired in the case of specific behavioral outcomes. An example would be early individualized strategies intended to prevent the development of self-injurious behavior, which is a potentially serious and undesirable behavioral disorder affecting over a quarter of school aged children with autism.74 Autism is characterized by a high degree of heterogeneity, and as such treatment procedures, duration, and intensity will ultimately vary by individual child and ideally be guided by multiple sources of information. However, we would argue that in all cases the focus should be on socially valid targets intended to improve long term quality of life.
Potential benefits
Autism emerges during a relatively restricted age interval characterized by rapid change and significant neural plasticity. The first years of life are marked by dramatic changes in brain structure and function as well as in a child’s behavior. Infancy and toddlerhood see the acquisition of key milestones including visual orienting, joint attention, walking, as well as verbal and nonverbal communication. Critical and rapid changes to neural architecture take place during the first years of life, and it is during this time that optimal and permanent outcomes are most likely achieved. These features of ASD onset set it apart from other neurodevelopmental disorders, such as schizophrenia, wherein presymptomatic or prodromal periods are extended, encompassing a more complex developmental history. The narrow window of vulnerability for the development of ASD is both a challenge; the interval to apply the right intervention at the right dose is limited – and an opportunity; we know when to focus our efforts.
The rationale for much of policy and practice related to early intervention is in essence that “earlier is better”. For children with ASD, the literature in general supports the position that younger age at initiation of treatment is associated with stronger effects.75–78 For example, Smith and colleagues78 reported that younger age at enrollment in community-based intensive behavioral intervention was associated with greater improvement in a host of cognitive and behavioral outcomes, including IQ and adaptive behavior. Evidence-based early intervention also produces a significant economic benefit, and children who receive such services are less likely to require special education services at school age.79 Kasari and colleagues73 reported that age of enrollment in early intervention predicted expressive language skills by elementary school. There is also evidence that verbal ability improves to a greater extent for children with ASD enrolled in naturalistic behavioral intervention before versus after age four.77 However, the incremental effects of delay are not well understood, and there is relatively little empirical data concerning sensitive or critical periods for specific aspects of behavioral intervention in humans. Thus, the impact of beginning intervention at 6 versus 18 or 24 months is yet unknown.
Data from outside of ASD may inform this issue. There is evidence for a critical period for language acquisition, and specifically syntax learning, that closes by the end of the first year of life, suggesting that intervention before or after that time is likely to yield differential effects.80 Tsai and colleagues81 reported that social behaviors and inflexibility/repetitive behaviors could be rescued in a tuberous sclerosis complex model mouse during the early postnatal period. In a follow-up study, the authors found that these behaviors could not be rescued using the same procedures in adult mice.82 In a study of Angelman syndrome model mice, Silva-Santos and colleagues83 examined the effects of developmental timing on behavioral features using a method of Ube3a gene reactivation. Multiple aspects of the Angelman syndrome phenotype were only rescued in juvenile mice, including several relevant to autism, with no or lesser effects on behavior observed in adolescent or adult mice. Considered together, these mouse model studies suggest critical or sensitive periods for the development and plasticity of autistic behaviors.66–68
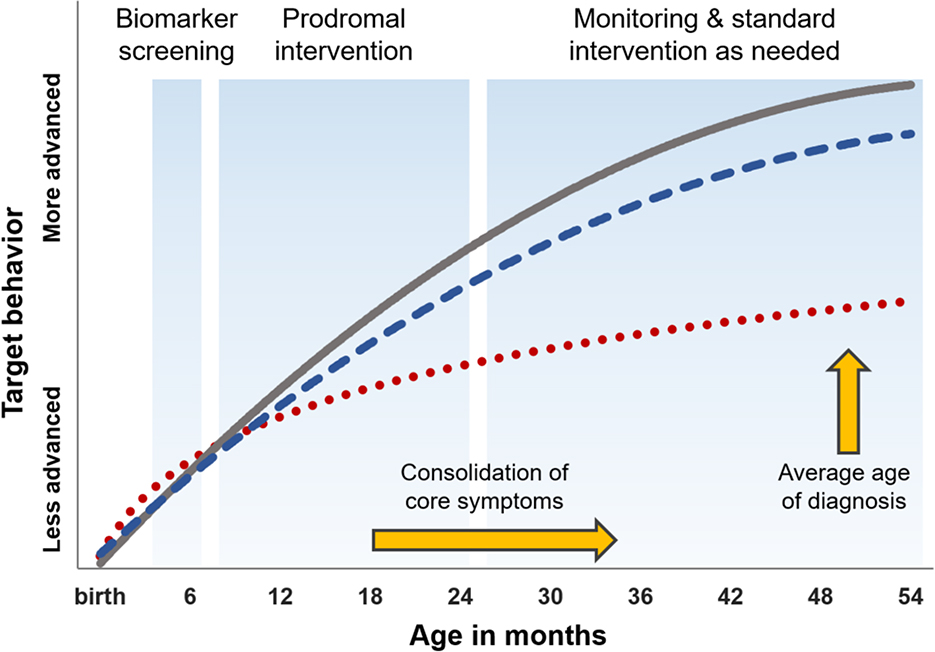
To illustrate how a model of infant prediction and intervention might compare to status-quo practice, hypothetical behavioral trajectories are presented in Figure 1. In this model, the grey trajectory represents typical development while the red trajectory represents a “business as usual” trajectory for a child with ASD. The blue trajectory represents a child identified as positive for an ASD neural signature at age 6 months using a biomarker based screening protocol, which might entail EEG, eye tracking, MRI, or some combination of these approaches. Once identified, the infant is enrolled into an individualized intervention program targeted to key developmental milestones, such as the acquisition of skills necessary for the development of joint attention. The child would remain in such an intervention until toddlerhood. If they showed no signs of delay or ASD, the child would receive developmental monitoring only. Alternatively, if/when the child exhibited developmental delays or met diagnostic criteria for ASD, they would transition into an appropriate intervention program (e.g., Early Start Denver Model84). In this figure, the child who receives very early intervention shows less proficiency than their typically developing peer but does not fall significantly behind.
Figure 1.
Hypothetical model for infant prediction and intervention.
Note: Gray trajectory represents typical development. The red dotted trajectory represents a child with ASD who recieves “business as usual” care. The blue dashed line represents an infant with ASD who is identified presymptomatically by way of biomarker (e.g. EEG, eyetracking, MRI) at age 6 months, followed by individualized intervention focused on key developmental milestones, followed by montitoring and evidence-based intervention as needed.
Ethical, legal, and social considerations
While a complete review of potential ethical, legal, and social implications of presymptomatic screening for autism is beyond the scope of this manuscript (for a review of some ethical considerations, see 72), there are several key issues to consider. First is the question of whether it would be ethical to disclose a future diagnostic likelihood of ASD to the family of an infant. The potential benefits of doing so would need to be carefully weighed against risks associated with issues such as screening errors. False positives, for example, could produce significant negative consequences, such as decreased emotional well-being and increased stress for parents and a host of potential iatrogenic effects on children. False positives are also likely incur substantial unnecessary financial costs and additional burden on service providers.
Relatedly, we must consider whether screening would necessarily lead to effective treatment. This dilemma is not new to medicine. For example, risk and age of onset for Alzheimer’s disease has been strongly linked to apolipoprotein E (APOE) genotype. The variant of APOE may be readily ascertained through genetic testing, potentially allowing an individual to know one aspect of risk for Alzheimer’s well in advance of any clinical concerns. Current clinical practice recommendations caution against such testing, however.85 Among the primary reasons is a lack of any established practice for preventing the disease or ameliorating risk; possessing information about one’s risk status for Alzheimer’s may provide no clear direct benefit in the absence of viable treatment options.86 Rather, it is presumed that having such information may be detrimental on the whole and ethically unwarranted.87
This rationale has also been extended to neurodevelopmental disorders that are first detected in early childhood. Fragile X syndrome (FXS), which is caused by the transcriptional silencing of the FMR1 gene, may be detected through routine postnatal testing. Although universal screening for the disorder is feasible, such screening has yet to be widely implemented due in part to concerns related to an absence of clinical interventions appropriate for infants.88,89 Moreover, as with ASD, costs related to delay-to-intervention have not been well-defined, further hampering adoption of a pre-symptomatic intervention model due to concerns regarding cost effectiveness. Together these and other practical and ethical concerns have limited the adoption of newborn screening for FXS, though in general parents favor the practice and view it as generally positive.90 Returning to the example of Alzheimer’s disease, we find longstanding acknowledgement that recommendations against presymptomatic identification are not immutable and instead subject to advancements in the field, such as the availability of new therapies.87 For FXS, a new program called Early Check has been established in the state of North Carolina to provide the advancements necessary to support widespread implementation of early screening, including referral and support systems for families of newborns with FXS and the evaluation of pre-symptomatic interventions.91 The lessons learned from FXS research on how to carefully implement early screening may be particularly valuable for developing similar programs for ASD.
Yet another consideration is whether the identification of presymptomatic ASD, through MRI or some other means, is legally equivalent to having the disorder.92 Current legal interpretation is unclear on this matter, with risk as determined by some biomarker, but in the absence of outward manifestation, potentially insufficient to qualify a child for benefits such as insurance coverage or access to state run early intervention programs. Currently, infants and toddlers with an identified disability or developmental delay, including ASD, are eligible for federally supported early intervention under Part C of Public Law 108–446 (the Individuals with Disabilities Education Act). It is yet unknown whether a biomarker strongly indicating the future likelihood of ASD, in the absence of any cognitive or behavioral delays, would confer eligibility for services.
It is worth noting that federal law requires that evidence-based early intervention services should be made available to any child who is “at risk of experiencing a substantial developmental delay if early intervention services were not provided”.93 This aspect of PL 108–446 opens the possibility for publically funded presymptomatic intervention for autism, though the matter has not yet been legally tested. Given the potential short-term costs associated with a move toward presymptomatic intervention, it is likely that statutory interpretation of “substantial developmental delay”, among other issues, will arise. In current practice, many children who will eventually receive autism-specific interventions tend to first receive less comprehensive services for specific domains of developmental delay, such as speech-language therapy for language delay. As states set their own definitions of developmental delay, the provision of services to children who eventually receive an ASD diagnosis is inconsistent.94 The provision of more intensive services to infants not yet showing symptoms or delays will certainly raise questions concerning thresholds for risk determination, the accuracy and performance of the screening technology, and degree of support by the scientific community.92
Ways forward
The question of presymptomatic prediction of ASD has shifted from “if” to “when”, and we posit that multiple accurate approaches may become available to researchers in the next 5–10 years. This may include neuroimaging approaches that have been described in our own work as well as other methods such as EEG or eye tracking. A combination of approaches may prove particularly promising, such as EEG collected in conjunction with eye tracking or the addition of behavioral data to biomarker risk algorithms. The availability of such technology would make possible presymptomatic intervention trials for high risk infants; those identified at ultra-high risk in the first year of life could be assigned to intervention before symptoms manifest. Assuming the availability of one or more evidence-based infant intervention packages, the potential for a large randomized controlled trial is clear. However, there are no conventional mechanisms in place to support such an ambitious research project – for example, a highly coordinated multi-site brain imaging and intervention study. Executing a project of that scale might require more dedicated support from federal agencies or public/private partnerships. The stakes would undoubtedly be high for a transdisciplinary project of that scale, requiring input and investment from a range of stakeholders and experts to help ensure success.
A wealth of knowledge on the very early development of ASD has been amassed using the infant sibling design, including recently developed methods of presymptomatic identification. However, many questions and avenues for further study remain. For example, how do we move beyond categorical prediction to prediction that is more prescriptive with regard to specific dimensions of function? Relatedly, infant biomarkers related to autism have not yet been explored beyond outcomes measured at ages 2 or 3. As it is unlikely that there is any single biomarker for ASD, investigating early markers in relation to developmental heterogeneity will be an important step. An additional issue is that children who are at familial risk for ASD represent only a fraction of those who develop autism. It is not yet known whether findings and methods from infant sibling studies will extend to the general population. Addressing this critical knowledge gap could have potentially ground-breaking public health implications. One essential area for further study is the feasibility of developing efficient and cost-effective level 1 screeners that are sufficiently sensitive to very early indicators of autism; these could be reasonably employed in the general population to determine whether additional screening, based on one or more biomarkers, is warranted. Advances in data analytic techniques, such as support-vector machines, have already boosted efforts to more effectively classify risk. Continued application and development of machine learning and other approaches capable of handling complex, high-volume data will likely be essential to improve accuracy and scale-up presymptomatic identification. While the potential of extending biomarkers of ASD to the general population is compelling, we must also note that multiple and complex factors underlay delays to diagnosis and intervention in our current system. An early biomarker for autism is not likely to be a panacea.
Another clear area for further development is in infant intervention. While there is good evidence of acceptability and feasibility, questions of what procedures are most effective, at what dosage, and for whom remain unanswered. Determining the “right” samples in which to develop and test interventions will be an ongoing challenge to addressing these questions. Can we undertake the risk and expense of running unproven interventions in ultra-high risk infants? Is it best to begin with a “kitchen sink” approach to intervention content and dosage, or should we establish efficacy through an iterative process with other populations? Given the high degree of phenotypic and etiologic heterogeneity associated with ASD, how do we effectively tailor intervention type and content to individual cases? Moreover, what proactive efforts might be implemented to ensure study samples better represent true racial, ethnic, and socioeconomic diversity?97 These challenges are not insurmountable. For example, evidence from toddlers and preschool aged children indicate the strong potential for biomarkers such as eye tracking to inform treatment assignment based on precursors associated with later symptomatology or treatment response.95,96
In this very journal over four decades ago, Barbara Fish, in describing what was then deemed childhood schizophrenia, wrote: “As we learn more about the special needs of infants with particular developmental handicaps, we will be able to tailor more specific preventative measures to an individual infant’s developmental profile”.98 She was then describing what were the primary goals of developmental research involving high-risk infants: to identify developmental and biological precursors of later impairment that could become targets for presymptomatic intervention.99 We have now arrived at an exciting time in the field of autism research where such goals are within reach.
Acknowledgements:
This work was supported by the National Institutes of Health under award numbers R01HD055741, R01MH116961, and R01MH118362.
Footnotes
In addressing prediction in ASD, it is important to begin by clarifying our terminology. Herein, we use prediction to refer to the forecasting of some later outcome (e.g., a diagnosis of ASD or the severity thereof) at the level of the individual. This is in contrast to the use of the term in reference to explanatory modeling (e.g., regression) or contemporaneous data.
Contributor Information
Jason J. Wolff, Department of Educational Psychology, University of Minnesota
Joseph Piven, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill
References
- 1.Baio J, Wiggins L, Christensen DL, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ. 2018;67(6):1–23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kogan MD, Vladutiu CJ, Schieve LA, et al. The Prevalence of Parent-Reported Autism Spectrum Disorder Among US Children. Pediatrics. 2018;142(6):e20174161. doi: 10.1542/peds.2017-4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shattuck PT, Roux AM, Hudson LE, Taylor JL, Maenner MJ, Trani J-F. Services for Adults with an Autism Spectrum Disorder. Can J Psychiatry. 2012;57(5):284–291. doi: 10.1177/070674371205700503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shattuck PT, Narendorf SC, Cooper B, Sterzing PR, Wagner M, Taylor JL. Postsecondary Education and Employment Among Youth With an Autism Spectrum Disorder. Pediatrics. 2012;129(6):1042–1049. doi: 10.1542/peds.2011-2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollocks MJ, Lerh JW, Magiati I, Meiser-Stedman R, Brugha TS. Psychological Medicine Anxiety and depression in adults with autism spectrum disorder: a systematic review and meta-analysis. 2018. doi: 10.1017/S0033291718002283 [DOI] [PubMed] [Google Scholar]
- 6.Buescher AVS, Cidav Z, Knapp M, Mandell DS. Costs of Autism Spectrum Disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168(8):721. doi: 10.1001/jamapediatrics.2014.210 [DOI] [PubMed] [Google Scholar]
- 7.Ganz ML. The lifetime distribution of the incremental societal costs of autism. Arch Pediatr Adolesc Med. 2007;161(4):343–349. doi: 10.1001/archpedi.161.4.343 [DOI] [PubMed] [Google Scholar]
- 8.Leigh JP, Du J. Brief Report: Forecasting the Economic Burden of Autism in 2015 and 2025 in the United States. J Autism Dev Disord. 2015;45(12):4135–4139. doi: 10.1007/s10803-015-2521-7 [DOI] [PubMed] [Google Scholar]
- 9.Sheldrick RC, Maye MP, Carter AS. Age at First Identification of Autism Spectrum Disorder: An Analysis of Two US Surveys. J Am Acad Child Adolesc Psychiatry. 2017;56(4):313–320. doi: 10.1016/j.jaac.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neurodevelopmental Disorders. In: Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. American Psychiatric Publishing, Inc. doi: 10.1176/appi.books.9780890425596.514988 [DOI] [Google Scholar]
- 11.Becerra-Culqui TA, Lynch FL, Owen-Smith AA, Spitzer J, Croen LA. Parental First Concerns and Timing of Autism Spectrum Disorder Diagnosis. J Autism Dev Disord. 2018;48(10):3367–3376. doi: 10.1007/s10803-018-3598-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozonoff S, Iosif A-M, Young GS, et al. Onset Patterns in Autism: Correspondence Between Home Video and Parent Report. J Am Acad Child Adolesc Psychiatry. 2011;50(8):796–806.e1. doi: 10.1016/J.JAAC.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x [DOI] [PubMed] [Google Scholar]
- 14.Ozonoff S, Iosif A-M, Baguio F, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49(3):256–66.e1–2. [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff JJ, Dimian AF, Botteron KN, et al. A longitudinal study of parent-reported sensory responsiveness in toddlers at-risk for autism. J Child Psychol Psychiatry. 2019;60(3):314–324. doi: 10.1111/jcpp.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elison JT, Wolff JJ, Reznick JS, et al. Repetitive behavior in 12-month-olds later classified with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(11):1216–1224. doi: 10.1016/j.jaac.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacrey LR, Zwaigenbaum L, Bryson S, et al. Developmental trajectories of adaptive behavior in autism spectrum disorder: a high-risk sibling cohort. J Child Psychol Psychiatry. 2019;60(6):697–706. doi: 10.1111/jcpp.12985 [DOI] [PubMed] [Google Scholar]
- 18.Estes A, Zwaigenbaum L, Gu H, et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord. 2015;7(1):24. doi: 10.1186/s11689-015-9117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x [DOI] [PubMed] [Google Scholar]
- 20.Chawarska K, Shic F, Macari S, et al. 18-Month Predictors of Later Outcomes in Younger Siblings of Children With Autism Spectrum Disorder: A Baby Siblings Research Consortium Study. J Am Acad Child Adolesc Psychiatry. 2014;53(12):1317–1327.e1. doi: 10.1016/J.JAAC.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robins DL, Casagrande K, Barton M, Chen C-MA, Dumont-Mathieu T, Fein D. Validation of the modified checklist for Autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics. 2014;133(1):37–45. doi: 10.1542/peds.2013-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner-Brown LM, Baranek GT, Reznick JS, Watson LR, Crais ER. The First Year Inventory: a longitudinal follow-up of 12-month-old to 3-year-old children. Autism. 2013;17(5):527–540. doi: 10.1177/1362361312439633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacrey L-AR, Bryson S, Zwaigenbaum L, et al. The Autism Parent Screen for Infants: Predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6–24 months of age. Autism. 2018;22(3):322–334. doi: 10.1177/1362361316675120 [DOI] [PubMed] [Google Scholar]
- 24.Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4). doi: 10.1542/peds.2018-3963 [DOI] [PubMed] [Google Scholar]
- 25.Hoeft F, McCandliss BD, Black JM, et al. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci U S A. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez LM, Rudie JD, Green SA, Bookheimer S, Dapretto M. Neural signatures of autism spectrum disorders: insights into brain network dynamics. Neuropsychopharmacology. 2015;40(1):171–189. doi: 10.1038/npp.2014.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff JJ, Gerig G, Lewis JD, et al. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain. 2015;138(Pt 7):2046–2058. doi: 10.1093/brain/awv118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung CHM, Bedford R, Johnson MH, Charman T, Gliga T. Visual search performance in infants associates with later ASD diagnosis. Dev Cogn Neurosci. 2018;29:4–10. doi: 10.1016/J.DCN.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolff JJ, Swanson MR, Elison JT, et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol Autism. 2017;8(1):8. doi: 10.1186/s13229-017-0126-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biol Psychiatry. 2009;66(10):935–941. doi: 10.1016/j.biopsych.2009.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff JJ, Jacob S, Elison JT. The journey to autism: Insights from neuroimaging studies of infants and toddlers. Dev Psychopathol. 2018;30(02):479–495. doi: 10.1017/S0954579417000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varcin KJ, Jeste SS. The emergence of autism spectrum disorder: insights gained from studies of brain and behaviour in high-risk infants. Curr Opin Psychiatry. 2017;30(2):85–91. doi: 10.1097/YCO.0000000000000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosl WJ, Tager-Flusberg H, Nelson CA. EEG Analytics for Early Detection of Autism Spectrum Disorder: A data-driven approach. Sci Rep. 2018;8(1):6828. doi: 10.1038/s41598-018-24318-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabard-Durnam LJ, Wilkinson C, Kapur K, Tager-Flusberg H, Levin AR, Nelson CA. Longitudinal EEG power in the first postnatal year differentiates autism outcomes. Nat Commun. 2019;10(1). doi: 10.1038/s41467-019-12202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen MD, Kim SH, McKinstry RC, et al. Increased Extra-axial Cerebrospinal Fluid in High-Risk Infants who Later Develop Autism. Biol Psychiatry. 2017. doi: 10.1016/j.biopsych.2017.02.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazlett HC, Gu H, Munsell BC, et al. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348–351. doi: 10.1038/nature21369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hazlett HC, Poe MD, Gerig G, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piven J, Elison JT, Zylka MJ. Toward a conceptual framework for early brain and behavior development in autism. Mol Psychiatry. 2017;22(10):1385–1394. doi: 10.1038/mp.2017.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emerson RW, Adams C, Nishino T, et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 2017;9(393). doi: 10.1126/scitranslmed.aag2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barber TR, Klein JC, Mackay CE, Hu MTM. Neuroimaging in pre-motor Parkinson’s disease. NeuroImage Clin. 2017;15:215–227. doi: 10.1016/j.nicl.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meera S, Donovan K, Wolff J, et al. Towards a data driven approach to screen for autism risk at 12 months of age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobson JW, Mulick J a, Green G. Cost-Benefit Estimates for Early Intensive Behavioral Intervention for Young Children with Autism-General Model and Single State Case. Behav Interv. 1998;13:201–226. doi: [DOI] [Google Scholar]
- 45.Sallows GO, Graupner TD. Intensive behavioral treatment for children with autism: four-year outcome and predictors. Am J Ment Retard. 2005;110(6):417–438. doi: 10.1352/0895-8017(2005)110[417:IBTFCW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 46.Virués-Ortega J Applied behavior analytic intervention for autism in early childhood: meta-analysis, meta-regression and dose-response meta-analysis of multiple outcomes. Clin Psychol Rev. 2010;30(4):387–399. doi: 10.1016/j.cpr.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 47.Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17–23. doi: 10.1542/peds.2009-0958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chasson GS, Harris GE, Neely WJ. Cost Comparison of Early Intensive Behavioral Intervention and Special Education for Children with Autism. J Child Fam Stud. 2007;16(3):401–413. doi: 10.1007/s10826-006-9094-1 [DOI] [Google Scholar]
- 49.Estes A, Munson J, Rogers SJ, Greenson J, Winter J, Dawson G. Long-Term Outcomes of Early Intervention in 6-Year-Old Children With Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):580–587. doi: 10.1016/j.jaac.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dawson G Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20(3):775–803. doi: 10.1017/S0954579408000370 [DOI] [PubMed] [Google Scholar]
- 51.Schertz HH, Baker C, Hurwitz S, Benner L. Principles of Early Intervention Reflected in Toddler Research in Autism Spectrum Disorders. Topics Early Child Spec Educ. 2011;31(1):4–21. doi: 10.1177/0271121410382460 [DOI] [Google Scholar]
- 52.Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312(5782):1900–1902. doi: 10.1126/science.1128898 [DOI] [PubMed] [Google Scholar]
- 53.Fox SE, Levitt P, Nelson CA. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(Pt 6):1591–1609. doi: 10.1093/brain/awr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nahmias AS, Pellecchia M, Stahmer AC, Mandell DS. Effectiveness of community-based early intervention for children with autism spectrum disorder: a meta-analysis. J Child Psychol Psychiatry Allied Discip. 2019;60(11):1200–1209. doi: 10.1111/jcpp.13073 [DOI] [PubMed] [Google Scholar]
- 56.Ozonoff S, Iosif A-M, Baguio F, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49(3):256–66.e1–2. http://www.ncbi.nlm.nih.gov/pubmed/20410715. Accessed February 7, 2017. [PMC free article] [PubMed] [Google Scholar]
- 57.Wass S, Porayska-Pomsta K, Johnson MH. Training attentional control in infancy. Curr Biol. 2011;21(18):1543–1547. doi: 10.1016/j.cub.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic Developmental Behavioral Interventions: Empirically Validated Treatments for Autism Spectrum Disorder. J Autism Dev Disord. 2015;45(8):2411–2428. doi: 10.1007/s10803-015-2407-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marchetto MC, Belinson H, Tian Y, et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry. July 2016. doi: 10.1038/mp.2016.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schafer ST, Paquola ACM, Stern S, et al. Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat Neurosci. 2019;22(2):243–255. doi: 10.1038/s41593-018-0295-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russo FB, Freitas BC, Pignatari GC, et al. Modeling the Interplay Between Neurons and Astrocytes in Autism Using Human Induced Pluripotent Stem Cells. Biol Psychiatry. 2018;83(7):569–578. doi: 10.1016/j.biopsych.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 62.Koegel LK, Singh AK, Koegel RL, Hollingsworth JR, Bradshaw J. Assessing and Improving Early Social Engagement in Infants. J Posit Behav Interv. 2014;16(2):69–80. doi: 10.1177/1098300713482977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steiner AM, Gengoux GW, Klin A, Chawarska K. Pivotal Response Treatment for Infants At-Risk for Autism Spectrum Disorders: A Pilot Study. J Autism Dev Disord. 2013;43(1):91–102. doi: 10.1007/s10803-012-1542-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S. Autism Treatment in the First Year of Life: A Pilot Study of Infant Start, a Parent-Implemented Intervention for Symptomatic Infants. J Autism Dev Disord. 2014;44(12):2981–2995. doi: 10.1007/s10803-014-2202-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dawson G, Rogers S, Munson J, et al. Randomized, Controlled Trial of an Intervention for Toddlers With Autism: The Early Start Denver Model. Pediatrics. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Green J, Wan MW, Guiraud J, et al. Intervention for Infants at Risk of Developing Autism: A Case Series. J Autism Dev Disord. 2013;43(11):2502–2514. doi: 10.1007/s10803-013-1797-8 [DOI] [PubMed] [Google Scholar]
- 67.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–1679. doi: 10.1016/S0140-6736(06)69701-8 [DOI] [PubMed] [Google Scholar]
- 68.Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997;277(9):739–745. http://www.ncbi.nlm.nih.gov/pubmed/9042847. Accessed January 8, 2019. [PubMed] [Google Scholar]
- 69.Insel TR. The arrival of preemptive psychiatry. Early Interv Psychiatry. 2007;1(1):5–6. doi: 10.1111/j.1751-7893.2007.00017.x [DOI] [PubMed] [Google Scholar]
- 70.Kapp SK, Gillespie-Lynch K, Sherman LE, Hutman T. Deficit, difference, or both? Autism and neurodiversity. Dev Psychol. 2013;49(1):59–71. doi: 10.1037/a0028353 [DOI] [PubMed] [Google Scholar]
- 71.Green J, Pickles A, Pasco G, et al. Randomised trial of a parent-mediated intervention for infants at high risk for autism: longitudinal outcomes to age 3?years. J Child Psychol Psychiatry. April 2017. doi: 10.1111/jcpp.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walsh P, Elsabbagh M, Bolton P, Singh I. In search of biomarkers for autism: scientific, social and ethical challenges. Nat Rev Neurosci. 2011;12(10):603–612. doi: 10.1038/nrn3113 [DOI] [PubMed] [Google Scholar]
- 73.Kasari C, Gulsrud A, Freeman S, Paparella T, Hellemann G. Longitudinal Follow-Up of Children With Autism Receiving Targeted Interventions on Joint Attention and Play. J Am Acad Child Adolesc Psychiatry. 2012;51(5):487–495. doi: 10.1016/j.jaac.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soke GN, Rosenberg SA, Hamman RF, et al. Brief Report: Prevalence of Self-injurious Behaviors among Children with Autism Spectrum Disorder—A Population-Based Study. J Autism Dev Disord. 2016;46(11):3607–3614. doi: 10.1007/s10803-016-2879-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rogers SJ, Estes A, Lord C, et al. Effects of a brief Early Start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052–1065. doi: 10.1016/j.jaac.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flanagan HE, Perry A, Freeman NL. Effectiveness of large-scale community-based Intensive Behavioral Intervention: A waitlist comparison study exploring outcomes and predictors. Res Autism Spectr Disord. 2012;6(2):673–682. doi: 10.1016/J.RASD.2011.09.011 [DOI] [Google Scholar]
- 77.Vivanti G, Dissanayake C. Outcome for Children Receiving the Early Start Denver Model Before and After 48 Months. J Autism Dev Disord. 2016;46(7):2441–2449. doi: 10.1007/s10803-016-2777-6 [DOI] [PubMed] [Google Scholar]
- 78.Smith T, Klorman R, Mruzek DW. Predicting Outcome of Community-Based Early Intensive Behavioral Intervention for Children with Autism. J Abnorm Child Psychol. 2015;43(7):1271–1282. doi: 10.1007/s10802-015-0002-2 [DOI] [PubMed] [Google Scholar]
- 79.Chasson GS, Harris GE, Neely WJ. Cost Comparison of Early Intensive Behavioral Intervention and Special Education for Children with Autism. J Child Fam Stud. 2007;16(3):401–413. doi: 10.1007/s10826-006-9094-1 [DOI] [Google Scholar]
- 80.Friedmann N, Rusou D. Critical period for first language: the crucial role of language input during the first year of life. Curr Opin Neurobiol. 2015;35:27–34. doi: 10.1016/j.conb.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 81.Tsai PT, Hull C, Chu Y, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488(7413):647–651. doi: 10.1038/nature11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsai PT, Rudolph S, Guo C, et al. Sensitive Periods for Cerebellar-Mediated Autistic-like Behaviors. Cell Rep. 2018;25(2):357–367.e4. doi: 10.1016/J.CELREP.2018.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silva-Santos S, van Woerden GM, Bruinsma CF, et al. Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. J Clin Invest. 2015;125(5):2069–2076. doi: 10.1172/JCI80554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dawson G, Rogers S, Munson J, et al. Randomized, Controlled Trial of an Intervention for Toddlers With Autism: The Early Start Denver Model. Pediatrics. 2009. http://pediatrics.aappublications.org/content/early/2009/11/30/peds.2009-0958. Accessed April 13, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Statement on use of apolipoprotein E testing for Alzheimer disease. American College of Medical Genetics/American Society of Human Genetics Working Group on ApoE and Alzheimer disease. JAMA. 274(20):1627–1629. http://www.ncbi.nlm.nih.gov/pubmed/7474250. Accessed January 9, 2019. [PubMed] [Google Scholar]
- 86.Prvulovic D, Hampel H. Ethical considerations of biomarker use in neurodegenerative diseases—A case study of Alzheimer’s disease. Prog Neurobiol. 2011;95(4):517–519. doi: 10.1016/j.pneurobio.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 87.Smedinga M, Tromp K, Schermer MHN, Richard E. Ethics Review Ethical Arguments Concerning the Use of Alzheimer’s Disease Biomarkers in Individuals with No or Mild Cognitive Impairment: A Systematic Review and Framework for Discussion. J Alzheimer’s Dis. 2018;66:1309–1322. doi: 10.3233/JAD-180638 [DOI] [PubMed] [Google Scholar]
- 88.Tassone F Newborn screening for fragile X syndrome. JAMA Neurol. 2014;71(3):355–359. doi: 10.1001/jamaneurol.2013.4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bailey DB. Newborn screening for fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10(1):3–10. doi: 10.1002/mrdd.20002 [DOI] [PubMed] [Google Scholar]
- 90.Skinner D, Sparkman KL, Bailey DB. Screening for fragile X syndrome: Parent attitudes and perspectives. Genet Med. 2003;5(5):378–384. doi: 10.1097/01.GIM.0000086480.69309.1E [DOI] [PubMed] [Google Scholar]
- 91.Okoniewski K, Wheeler A, Lee S, et al. Early Identification of Fragile X Syndrome through Expanded Newborn Screening. Brain Sci. 2019;9(1):4. doi: 10.3390/brainsci9010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Preston J, McTeigue J, Opperman C, et al. The Legal Implications of Detecting Alzheimer’s Disease Earlier. AMA J Ethic. 2016;18(12):1207–1217. doi: 10.1001/journalofethics.2016.18.12.hlaw1-1612 [DOI] [PubMed] [Google Scholar]
- 93.The Individuals with Disabilities Education Act; 2004.
- 94.Rosenberg SA, Robinson CC, Shaw EF, Ellison MC. Part C Early Intervention for Infants and Toddlers: Percentage Eligible Versus Served. Pediatrics. 2013;131(1):38–46. doi: 10.1542/peds.2012-1662 [DOI] [PubMed] [Google Scholar]
- 95.Bradshaw J, Shic F, Holden AN, et al. The Use of Eye Tracking as a Biomarker of Treatment Outcome in a Pilot Randomized Clinical Trial for Young Children with Autism. Autism Res. 2019;12(5):779–793. doi: 10.1002/aur.2093 [DOI] [PubMed] [Google Scholar]
- 96.Shic F, Wang Q, Macari SL, Chawarska K. The role of limited salience of speech in selective attention to faces in toddlers with autism spectrum disorders. J Child Psychol Psychiatry. 2020;61(4):459–469. doi: 10.1111/jcpp.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.West EA, Travers JC, Kemper TD, et al. Racial and Ethnic Diversity of Participants in Research Supporting Evidence-Based Practices for Learners With Autism Spectrum Disorder. J Spec Educ. 2016;50(3):151–163. doi: 10.1177/0022466916632495 [DOI] [Google Scholar]
- 98.Fish B An approach to prevention in infants at risk for schizophrenia. Developmental deviations from birth to 10 years. J Am Acad Child Psychiatry. 1976;15(1):62–82. http://www.ncbi.nlm.nih.gov/pubmed/1254848. Accessed February 11, 2019. [DOI] [PubMed] [Google Scholar]
- 99.Jablensky A, McNeil TF, Morgan VA. Barbara Fish and a Short History of the Neurodevelopmental Hypothesis of Schizophrenia. Schizophr Bull. 2017;43(6):1158–1163. doi: 10.1093/schbul/sbx094 [DOI] [PMC free article] [PubMed] [Google Scholar]