Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with an increased risk of all-cause mortality and complications. The autonomic nervous system (ANS) plays a central role in AF, with the heart regulated by both extrinsic and intrinsic properties. In the extrinsic ANS, the sympathetic fibers are derived from the major paravertebral ganglia, especially the stellate ganglion (SG), which is a source of cardiac sympathetic innervation since it connects with multiple intrathoracic nerves and structures. The major intrinsic ANS is a network of axons and ganglionated plexi that contains a variety of sympathetic and parasympathetic neurons, which communicate with the extrinsic ANS. Simultaneous sympathovagal activation contributes to the development of AF because it increases calcium entry and shortens the atrial action potential duration. In animal and human studies, neuromodulation methods such as electrical stimulation and renal denervation have indicated potential benefits in controlling AF in patients as they cause SG remodeling and reduce sympathetic outflow. This review focuses on the neural mechanisms relevant to AF and the recent developments of neuromodulation methods for AF control.
Keywords: autonomic nervous system, atrial fibrillation, neuromodulation, skin sympathetic nerve activity
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with an increased risk of all-cause mortality and complications.1 The autonomic nervous system (ANS) is one of the important mechanisms in atrial arrhythmogenesis.2 While pulmonary vein isolation is widely used for AF ablation, it is an invasive procedure with potentially significant complications. Neuromodulation techniques that reduce ANS activity have been developed using animal models and studied in humans to control atrial arrhythmias, including AF. In this review, we summarize the neural mechanisms related to AF and the recent developments of neuromodulation procedures for AF control.
CARDIAC AUTONOMIC NERVOUS SYSTEM
The heart has both extrinsic and intrinsic ANS regulation.3 The extrinsic cardiac ANS includes both sympathetic and parasympathetic components. The sympathetic cardiac fibers are largely derived from major paravertebral ganglia, including the superior cervical ganglia, the stellate ganglion (SG), or cervicothoracic ganglion, and the thoracic ganglion.4 Among them, the SG is a major source of cardiac sympathetic innervation, connecting with multiple intrathoracic nerves and structures as well as skin. The ganglion cells and SG nerve fibers mostly stain positive for tyrosine-hydroxylase (TH), which indicates a sympathetic phenotype. However, a small percentage (roughly 5%) of ganglion cells are negative for TH and positive for choline acetyltransferase (ChAT), indicating that these cells may produce acetylcholine.5 Many ganglion cells express both TH and ChAT, and that phenotypic switch may occur in disease states or after neuromodulation procedures.5, 6 The extrinsic parasympathetic fibers, which come from nuclei in the medulla, are carried almost entirely within the vagal nerve. Most of the cardiac parasympathetic nerve fibers converge at a distinct fat pad between the superior vena cava and the aorta7 en route to the sinus and atrioventricular nodes. While the vagal nerve carries the parasympathetic nerve fibers, it also contains significant sympathetic nerve structures.8 The sympathetic nerve fibers occupy between 1% and 5% of the cross section of the vagal nerves in dogs and in humans.9
The intrinsic cardiac ANS is a complex network composed of ganglionated plexi (GP) on the atrial surface and ventricles within epicardial fat pads that contain large populations of colocalized sympathetic and parasympathetic neurons.3, 10 As the integration center, the GP modulate the intricate autonomic interactions between the extrinsic and intrinsic ANS.11 In the atria, GP are concentrated in distinct locations on the chamber walls.3, 10 Specifically, there is a high density of GP in the pulmonary vein–left atrium junction.12
ATRIAL ELECTROPHYSIOLOGY AND ARRHYTHMOGENESIS NEURAL ACTIVITIES
As a normal reaction, sympathetic activation of the heart enhances cardiac output by increasing Ca2+ entry into myocyte via the L-type Ca2+ channel and spontaneous Ca2+ release and uptake on the sarcoplasmic reticulum.13 Parasympathetic activation of the heart can activate the G-protein-gated K+ channel (IKACh), shortening the atrial action potential duration (APD) and in turn the atrial effective refractory period (ERP).14
Abnormal neural activities induce arrhythmias in both the atria and ventricles. In the atria, excessive sympathetic activation promotes focal ectopic activity through enhanced automaticity, early after depolarization (EAD), or delayed after depolarization. Parasympathetic activity produces spatial electrophysiological heterogeneity of the atria.15 Mechanistically, both simultaneous sympathetic and parasympathetic activations cause short atrial APD and large and long Ca2+ transience, creating a condition for late-phase 3 EAD that can induce AF.16 Short APD also promotes the maintenance of reentrant activity. As pulmonary veins naturally have short APDs, they are particularly prone to develop these Ca2+ transient-triggered arrhythmias.17 Electrical stimulation to the GPs around the pulmonary veins can stimulate both adrenergic and cholinergic nerves and may trigger atrial arrhythmias.
NEUROMODULATION FOR ATRIAL FIBRILLATION
While pulmonary vein isolation (PVI) has been the most widely used ablation approach to treat AF, its long-term results remain unsatisfactory, particularly for persistent AF. 18 As a result, several neuromodulation techniques have been attempted to manage patients with AF. The following introduces our strategy using subcutaneous nerve stimulation (ScNS) for AF and reviews the recent progress of other methods.
Subcutaneous Nerve Stimulation
The skin is an easily accessible place to perform mechanical neuromodulation. Because the postganglionic sympathetic nerve fibers of the upper extremities and thorax come primarily from the SG, thoracic ScNS may activate the sympathetic axons in the skin to cause calcium accumulation and remodeling of the SG, including neuronal cell death.19, 20 We showed in canine models that long-term intermittent ScNS in the thorax led to stimulus strength-dependent changes of SG nerve activity (SGNA), frequency and duration of spontaneous atrial tachyarrhythmias (Figure 1 A, B), and the plasma norepinephrine concentration (Figure 1 C).21, 22 In ambulatory dogs with persistent AF, thoracic ScNS reduced SGNA and controlled the ventricular rate via SG remodeling (Figure 1D-F), additionally showing neural remodeling in the brain stem and preservation of the left ventricular ejection fraction (Figure 1D-F).23 The brain stem remodeling in ScNS dogs consists of increased 18F-FDG uptake but no neuronal cell death. These findings indicate that thoracic ScNS at appropriate stimulus strength may remodel the SG and brain stem and reduce atrial tachyarrhythmias. Because of both central (brain stem) and peripheral (SG) remodeling, ScNS may be a new method for long-term atrial arrhythmia control. More recently, we showed that ScNS with blindly inserted electrodes can be used to achieve rate control in persistent AF.24 This simplified method may facilitate future clinical trials of neuromodulation. However, Yuan et al. showed that prolonged (.3-second) pauses were observed with increased frequency in the ScNS group but not in the sham control group of dogs with persistent AF.23 Therefore, bradycardia is a potential side effect for ScNS in AF.
Figure 1.
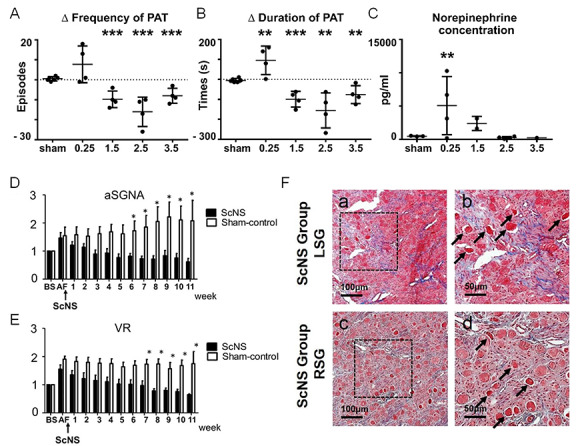
Effects of subcutaneous nerve stimulation in an ambulatory canine model. (A) The delta PAT frequency per 48 hours and (B) PAT durations between baseline and after ScNS protocol among the 5 groups: sham, 0.25 mA, 1.5 mA, 2.5 mA, and 3.5 mA output of ScNS. (C) The plasma norepinephrine concentrations among the 5 groups. Asterisks indicate significantly (P < .009) higher concentrations in the 0.25 mA group (n = 4) than in all other dogs (n = 10). (D, E) Temporal changes of the aSGNA and VR, respectively, at different times after persistent AF in the ScNS group (filled columns) and in the sham stimulation group (unfilled columns). Compared with baseline (BS), there are significant decreases in aSGNA after 6 weeks and in VR after 7 weeks of ScNS. (F) Examples of Masson’s trichrome staining in stellate ganglion (SG) after ScNS, indicating damaged region with increased fibrosis in both the left SG (LSG in a and b) and right SG (RSG in c and d). High-power views in the dotted square (b and d), indicate abnormal morphology of damaged neurons with eosinophilic staining (arrows). *P < .05, **P < .01, ***P < .001. A-C modified from Wan et al., Heart Rhythm, with permission.21 D-F modified from Yuan et al. Heart Rhythm, with permission.23 PAT: paroxysmal atrial tachycardia; ScNS: subcutaneous nerve stimulation; aSGNA: average stellate ganglion nerve activity; VR: ventricular rate; AF: atrial fibrillation
Ganglionated Plexi Ablation
As described above, the GPs surrounding the pulmonary veins may play a role in triggering atrial arrhythmias and therefore can serve as targets of neuromodulation. A randomized multicenter trial by Katritsis et al. showed that addition of GP ablation to PVI conferred a significantly higher success rate compared with either PVI or GP ablation alone in patients with paroxysmal AF.25 Furthermore, anatomic ablation of the main left atrial GP alone, without complete PVI, prevented AF recurrence in 48% of the patients. Compared with PVI with linear ablation, GP ablation in addition to PVI indicated lower AF recurrence and lower rates of ablation-related atrial flutter after 3 years of follow-up.26 Further studies need to explore optimal GP locations and appropriate strategy to determine therapeutic effect.
Renal Sympathetic Denervation
Renal sympathetic denervation (RDN) with catheter ablation is a method of modulating both afferent and efferent sympathetic nerves and has been studied as a treatment for drug-resistant hypertension.27 One possible mechanism is that ablating the afferent nerves decreased feedback activation to the central nervous system and thereby decreased sympathetic input to the heart or other structures.28 In a porcine model of obstructive sleep apnea, RDN inhibited the shortening of atrial ERP and lowered AF inducibility.29 RDN also reduced atrial nerve sprouting, atrial fibrosis, and complexity of AF in a goat model with persistent AF.30 In a canine study, we found that RDN can result in significant remodeling of the SG, reduction of SGNA, and brain stem remodeling as evidenced by neuronal cell death and reduced 18F-FDG uptake.31 These changes, in turn, were associated with a significant reduction of paroxysmal atrial tachyarrhythmia episodes.
These findings indicate that modulation of the ANS by RDN might be effective in reducing atrial arrhythmogenesis and preventing the generation of atrial substrate to sustain arrhythmias. A recent prospective clinical trial randomized patients with paroxysmal AF and hypertension to receive either RDN with catheter ablation or catheter ablation alone.32 The results showed that RDN with ablation significantly increased the likelihood of freedom from AF at 12 months. This and other studies suggest that RDN may be helpful in controlling AF.33
Low-Level Vagal Nerve Stimulation
Vagal nerve stimulation (VNS) shortens atrial ERP and APD in the pulmonary vein and is a reliable technique used for the experimental induction of AF.34 Low-level cervical VNS (LL-VNS) at a stimulus strength of 1 V below the threshold necessary to reduce heart rate can lower intrinsic cardiac nerve activity and, paradoxically, suppress electrically induced AF in open-chest, anesthetized dogs.35 The antiarrhythmic effects are still present even when the stimulus strength is 50% below the threshold. In addition, LL-VNS reverses the electrical remodeling caused by rapid atrial pacing.36 Shen et al. used direct nerve recordings to demonstrate that continuous LL-VNS suppressed paroxysmal atrial tachyarrhythmias in ambulatory dogs by suppressing SGNA.37 The mechanisms of LL-VNS in AF suppression are not fully understood. Histologically, LL-VNS causes structural remodeling in the left SG, characterized by a significant reduction of ganglion cells stained by TH. Subsequent studies have shown that in the left SG, LL-VNS also resulted in the upregulation of the small-conductance Ca2+-activated K+ channel type 2 and increased its expression in the cell membrane.5 These changes may facilitate afterhyperpolarization of the ganglion cells and reduce the frequency of neuronal discharges.38
Thus, LL-VNS can functionally and structurally remodel the left SG and reduce sympathetic outflow to the heart, hence its anti-AF property.39 The effect of VNS is therefore not limited to its interaction with the parasympathetic nervous system.
In a clinical trial, 54 patients undergoing cardiac surgery were randomized into LL- VNS and sham groups, and a temporary bipolar pacing wire was sutured to vagal nerve preganglionic fibers near the superior vena cava.40 LL-VNS was found to reduce the occurrence of postoperative AF. Considering the invasiveness of VNS, which can directly connect the electrodes with vagal nerves, less-invasive methods are needed to facilitate its clinical application. For example, the auricular branch of the vagal nerve is accessible through stimulation of Tragus, the anterior protuberance of the ear. In canine models, tragus stimulation reduced the electrical and structural remodeling induced by rapid atrial pacing.41 In a pilot study of 40 patients who presented for AF ablation, tragus stimulation decreased pacing-induced AF duration and systemic cytokine levels compared with earlobe stimulation.42 Larger randomized trials are necessary to determine the clinical efficacy of tragus stimulation in managing AF.
CONCLUSION
The neural mechanisms of AF have been studied, and the cardiac ANS is believed to play an important role. Simultaneous sympathovagal activation via both the extrinsic and intrinsic cardiac ANS increases calcium entry and simultaneously shortens the APD, which induces AF through late-phase 3 EAD and triggered firing in the pulmonary veins. Neuromodulation methods such as electrical stimulation and renal denervation cause SG remodeling and reduce sympathetic outflow and may be more effective for managing patients with AF. Further clinical trials testing neuromodulation methods are needed to determine their role in managing AF.
KEY POINTS
Large sympathetic nerve discharges precede the onset of paroxysmal cardiac arrhythmias, including atrial fibrillation.
Sympathetic nerve activity activates calcium and potassium channels to initiate cardiac arrhythmias.
Electrical stimulation of sympathetic nerves and renal denervation both cause stellate ganglion remodeling, which reduces sympathetic nerve activity and controls cardiac arrhythmias.
Acknowledgements
This review was supported in part by NIH Grants R01 HL139829, TR002208- 01, 1OT2OD028190 (P.-S.C.), a Charles Fisch Cardiovascular Research Award endowed by Dr. Suzanne B. Knoebel of the Krannert Institute of Cardiology (T.K.), a Medtronic-Zipes Endowment of Indiana University and the Burns & Allen Chair of Cardiology Research of the Cedars-Sinai Medical Center.
References
- Benjamin E J, Chen P S, Bild D E, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119(4):606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Peng-Sheng, Chen Lan S., Fishbein Michael C., Lin Shien-Fong, Nattel Stanley. Circulation Research. 9. Vol. 114. Ovid Technologies (Wolters Kluwer Health); 2014. Role of the Autonomic Nervous System in Atrial Fibrillation; pp. 1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour J. Andrew, Murphy David A., Yuan Bing-Xiang, MacDonald Sara, Hopkins David A. The Anatomical Record. 2. Vol. 247. Wiley; 1997. Gross and microscopic anatomy of the human intrinsic cardiac nervous system; pp. 289–298. [DOI] [PubMed] [Google Scholar]
- Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol. 2005;209(6):425–463. doi: 10.1007/s00429-005-0462-1. [DOI] [PubMed] [Google Scholar]
- Shen M J, Hao-Che C, Park H W. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm. 2013;10(6):910–915. doi: 10.1016/j.hrthm.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova G., Loy B., Dorn R., Dechant G. Journal of Neuroscience. 48. Vol. 30. Society for Neuroscience; 2010. The Sympathetic Neurotransmitter Switch Depends on the Nuclear Matrix Protein Satb2; pp. 16356–16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou Chuen-Wang, Eble John N., Zipes Douglas P. Circulation. Vol. 95. Ovid Technologies (Wolters Kluwer Health); 1997. Efferent Vagal Innervation of the Canine Atria and Sinus and Atrioventricular Nodes; pp. 2573–2584. [DOI] [PubMed] [Google Scholar]
- Kawagishi K, Fukushima N, Yokouchi K, Sumitomo N, Kakegawa A, Moriizumi T. J Clin Neurosci. 9. Vol. 15. Kakegawa A, Moriizumi: 2008. Tyrosine hydroxylase-immunoreactive fibers in the human vagus nerve; pp. 1023–1026. [DOI] [PubMed] [Google Scholar]
- Seki A, Green H R, Lee T D. Sympathetic nerve fibers in human cervical and thoracic vagus nerves. Heart Rhythm. 2014;11(8):1411–1418. doi: 10.1016/j.hrthm.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza Dainius H., Skripka Valdas, Pauziene Neringa. Cells Tissues Organs. 4. Vol. 172. S. Karger AG; 2002. Morphology of the Intrinsic Cardiac Nervous System in the Dog: A Whole-Mount Study Employing Histochemical Staining with Acetylcholinesterase; pp. 297–320. [DOI] [PubMed] [Google Scholar]
- Hou Y, Scherlag B J, Lin J. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50(1):61–69. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- Tan Alex Y., Li Hongmei, Wachsmann-Hogiu Sebastian, Chen Lan S., Chen Peng-Sheng, Fishbein Michael C. Journal of the American College of Cardiology. 1. Vol. 48. Elsevier BV; 2006. Autonomic Innervation and Segmental Muscular Disconnections at the Human Pulmonary Vein-Atrial Junction; pp. 132–143. [DOI] [PubMed] [Google Scholar]
- Bers Donald M. Nature. 6868. Vol. 415. Springer Science and Business Media LLC; 2002. Cardiac excitation–contraction coupling; pp. 198–205. [DOI] [PubMed] [Google Scholar]
- Wijffels M C, Kirchhof C J, Dorland R, Allessie M A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92(7):1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- Fareh Samir, Villemaire Christine, Nattel Stanley. Circulation. 20. Vol. 98. Ovid Technologies (Wolters Kluwer Health); 1998. Importance of Refractoriness Heterogeneity in the Enhanced Vulnerability to Atrial Fibrillation Induction Caused by Tachycardia-Induced Atrial Electrical Remodeling; pp. 2202–2209. [DOI] [PubMed] [Google Scholar]
- Burashnikov Alexander, Antzelevitch Charles. Circulation. 18. Vol. 107. Ovid Technologies (Wolters Kluwer Health); 2003. Reinduction of Atrial Fibrillation Immediately After Termination of the Arrhythmia Is Mediated by Late Phase 3 Early Afterdepolarization–Induced Triggered Activity; pp. 2355–2360. [DOI] [PubMed] [Google Scholar]
- PATTERSON EUGENE, JACKMAN WARREN M., BECKMAN KAREN J., LAZZARA RALPH, LOCKWOOD DEBORAH, SCHERLAG BENJAMIN J., WU RICHARD, PO SUNNY. Journal of Cardiovascular Electrophysiology. Vol. 18. Wiley; 2007. Spontaneous Pulmonary Vein Firing in Man: Relationship to Tachycardia-Pause Early Afterdepolarizations and Triggered Arrhythmia in Canine Pulmonary Veins In Vitro; pp. 1067–1075. [DOI] [PubMed] [Google Scholar]
- Verma A, Jiang C Y, Betts T R. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1834. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- Demaurex N. Science. 5616. Vol. 300. American Association for the Advancement of Science (AAAS); 2003. CELL BIOLOGY: Apoptosis--the Calcium Connection; pp. 65–67. [DOI] [PubMed] [Google Scholar]
- Andiné Peter, Jacobson Ingemar, Hagberg Henrik. Journal of Cerebral Blood Flow & Metabolism. 6. Vol. 8. SAGE Publications; 1988. Calcium Uptake Evoked by Electrical Stimulation is Enhanced Postischemically and Precedes Delayed Neuronal Death in CA1 of Rat Hippocampus: Involvement of N-Methyl-D-Aspartate Receptors; pp. 799–807. [DOI] [PubMed] [Google Scholar]
- Wan J, Chen M, Yuan Y. Antiarrhythmic and proarrhythmic effects of subcutaneous nerve stimulation in ambulatory dogs. Heart Rhythm. 2019;16(8):1251–60. doi: 10.1016/j.hrthm.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Jiang Z, Zhao Y. Long-term intermittent high-amplitude subcutaneous nerve stimulation reduces sympathetic tone in ambulatory dogs. Heart Rhythm. 2018;15(3):451–460. doi: 10.1016/j.hrthm.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Liu X, Wan J. Subcutaneous nerve stimulation for rate control in ambulatory dogs with persistent atrial fibrillation. Heart Rhythm. 2019;16(9):1383–1391. doi: 10.1016/j.hrthm.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusayama Takashi, Wan Juyi, Yuan Yuan, Liu Xiao, Li Xiaochun, Shen Changyu, Fishbein Michael C., Everett Thomas H., Chen Peng-Sheng. Heart Rhythm. 2. Vol. 18. Elsevier BV; 2021. Effects of subcutaneous nerve stimulation with blindly inserted electrodes on ventricular rate control in a canine model of persistent atrial fibrillation; pp. 261–270. [DOI] [PubMed] [Google Scholar]
- Katritsis Demosthenes G., Pokushalov Evgeny, Romanov Alexander, Giazitzoglou Eleftherios, Siontis George C.M., Po Sunny S., Camm A. John, Ioannidis John P.A. Journal of the American College of Cardiology. 24. Vol. 62. Elsevier BV; 2013. Autonomic Denervation Added to Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation; pp. 2318–2325. [DOI] [PubMed] [Google Scholar]
- Pokushalov Evgeny, Romanov Alexandr, Katritsis Demosthenes G., Artyomenko Sergey, Shirokova Natalya, Karaskov Alexandr, Mittal Suneet, Steinberg Jonathan S. Heart Rhythm. 9. Vol. 10. Elsevier BV; 2013. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: A randomized comparison; pp. 1280–1286. [DOI] [PubMed] [Google Scholar]
- Krum Henry, Schlaich Markus, Whitbourn Rob, Sobotka Paul A, Sadowski Jerzy, Bartus Krzysztof, Kapelak Boguslaw, Walton Anthony, Sievert Horst, Thambar Suku, Abraham William T, Esler Murray. The Lancet. 9671. Vol. 373. Elsevier BV; 2009. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study; pp. 1275–1281. [DOI] [PubMed] [Google Scholar]
- Esler M. European Heart Journal. 18. Vol. 35. Oxford University Press (OUP); 2014. Renal denervation for hypertension: observations and predictions of a founder; pp. 1178–1185. [DOI] [PubMed] [Google Scholar]
- Linz Dominik, Mahfoud Felix, Schotten Ulrich, Ukena Christian, Neuberger Hans-Ruprecht, Wirth Klaus, Böhm Michael. Hypertension. 1. Vol. 60. Ovid Technologies (Wolters Kluwer Health); 2012. Renal Sympathetic Denervation Suppresses Postapneic Blood Pressure Rises and Atrial Fibrillation in a Model for Sleep Apnea; pp. 172–178. [DOI] [PubMed] [Google Scholar]
- Linz Dominik, van Hunnik Arne, Hohl Mathias, Mahfoud Felix, Wolf Milan, Neuberger Hans-Ruprecht, Casadei Barbara, Reilly Svetlana N., Verheule Sander, Böhm Michael, Schotten Ulrich. Circulation: Arrhythmia and Electrophysiology. 2. Vol. 8. Ovid Technologies (Wolters Kluwer Health); 2015. Catheter-Based Renal Denervation Reduces Atrial Nerve Sprouting and Complexity of Atrial Fibrillation in Goats; pp. 466–474. [DOI] [PubMed] [Google Scholar]
- Tsai W C, Chan Y H, Chinda K, et al. Effects of renal sympathetic denerva- tion on the stellate ganglion and brain stem in dogs. Heart Rhythm. 2017;14(2):255–62. doi: 10.1016/j.hrthm.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg J S, Shabanov V, Ponomarev D. Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hyper- tension: The ERADICATE-AF Randomized Clinical Trial. JAMA. 2020;323(3):248–55. doi: 10.1001/jama.2019.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyz Lida, Theuns Dominic A., Bhagwandien Rohit, Strachinaru Mihai, Kardys Isabella, Van Mieghem Nicolas M., Daemen Joost. Clinical Research in Cardiology. 6. Vol. 108. Springer Science and Business Media LLC; 2019. Atrial fibrillation reduction by renal sympathetic denervation: 12 months’ results of the AFFORD study; pp. 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson Eugene, Po Sunny S., Scherlag Benjamin J., Lazzara Ralph. Heart Rhythm. Vol. 2. Elsevier BV; 2005. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation; pp. 624–631. [DOI] [PubMed] [Google Scholar]
- Yu L, Scherlag B J, Li S. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol. 2011;22(4):455–63. doi: 10.1111/j.1540-8167.2010.01908.x. [DOI] [PubMed] [Google Scholar]
- Sheng X, Scherlag B J, Yu L. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol. 2011;57(5):563–71. doi: 10.1016/j.jacc.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Shen M J, Shinohara T, Park H W. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tach- yarrhythmias in ambulatory canines. Circulation. 1920;123:2204–2216. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman John P., Maylie James, Sah Pankaj. Annual Review of Physiology. 1. Vol. 74. Annual Reviews; 2012. Small-Conductance Ca2+-Activated K+Channels: Form and Function; pp. 245–269. [DOI] [PubMed] [Google Scholar]
- Chinda K, Tsai W C, Chan Y H. Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm. 2016;13(3):771–80. doi: 10.1016/j.hrthm.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrakis Stavros, Humphrey Mary Beth, Scherlag Benjamin, Iftikhar Omer, Parwani Purvi, Abbas Mubasher, Filiberti Adrian, Fleming Christian, Hu Yanqing, Garabelli Paul, McUnu Arthur, Peyton Marvin, Po Sunny S. JACC: Clinical Electrophysiology. 9. Vol. 3. Elsevier BV; 2017. Low-Level Vagus Nerve Stimulation Suppresses Post-Operative Atrial Fibrillation and Inflammation; pp. 929–938. [DOI] [PubMed] [Google Scholar]
- Chen M, Yu L, Liu Q. Low level tragus nerve stimulation is a non-invasive approach for anti-atrial fibrillation via preventing the loss of connexins. Int J Cardiol. 2015;179:144–149. doi: 10.1016/j.ijcard.2014.10.114. [DOI] [PubMed] [Google Scholar]
- Stavrakis Stavros, Humphrey Mary Beth, Scherlag Benjamin J., Hu Yanqing, Jackman Warren M., Nakagawa Hiroshi, Lockwood Deborah, Lazzara Ralph, Po Sunny S. Journal of the American College of Cardiology. 9. Vol. 65. Elsevier BV; 2015. Low-Level Transcutaneous Electrical Vagus Nerve Stimulation Suppresses Atrial Fibrillation; pp. 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]


