Abstract
Patients with symptomatic stage D heart failure who require left ventricular assist device (LVAD) support and suffer concomitant severe mitral regurgitation are often difficult to manage. One reason is due to cardiac anatomic constraints that limit optimization of the mechanical assist device. Typically, these patients are not candidates for repeat sternotomy with surgical mitral valve repair, and heart transplantation may not be feasible or timely. This case describes two patients with LVAD support who received transcatheter edge-to-edge mitral valve repair for severe, symptomatic mitral regurgitation. We believe this procedure may be a therapeutic option in stable patients with severe mitral regurgitation who require mechanical support.
Keywords: structural heart disease, severe mitral regurgitation, mechanical circulatory support, advanced heart failure, percutaneous valve repair
INTRODUCTION
Mitral regurgitation (MR) in patients with advanced heart failure is commonly due to left ventricular (LV) dilatation; this causes mitral annular dilatation and posterior leaflet restriction, ultimately resulting in malcoaptation of the mitral leaflets.1 Left ventricular assist device (LVAD) therapy is increasingly used in patients with stage D heart failure as a bridge to heart transplant or as destination therapy.2 In most instances, mitral valve repair is not necessary at the time of LVAD implantation because increased LV unloading from the device commonly improves MR severity. However, in less than 3% of patients receiving LVAD implants, severe MR persists and warrants additional therapy.3, 4
Persistent, severe MR may stem from the inability to increase LVAD speeds. This may be due to the inflow cannula position in a small LV cavity, where the interventricular septum would be suctioned over the inflow cannula at higher speeds, thus blocking blood flow. In addition, severe MR may occur after LVAD implantation due to the inferolateral positioning of the inlet cannula, which can distort the papillary muscle anatomy and result in malcoaptation of the mitral valve leaflet. Addressing severe MR is critical in patients with LVADs because it can cause pulmonary hypertension and lead to right ventricular (RV) failure and poor clinical outcomes.5, 6 We present two cases with severe, symptomatic MR that could not be improved with LVAD support and describe the immediate impact after transcatheter edge-to-edge mitral valve repair (TEER).
CASES
Patient 1
A 66-year-old male with end-stage nonischemic cardiomyopathy (NICMP) received a HeartWare ventricular assist device (Medtronic) implant. After 18 months, he presented with New York Heart Association (NYHA) class III-IV symptoms. Initial workup confirmed normal LVAD function. A transesophageal echocardiogram showed a decompressed LV as well as severe MR due to prolapse and flail of the A2 scallop with ruptured chordae, neither of which were seen at the time of LVAD implantation. The inflow cannula was abutting the interventricular septum, thereby limiting the ability to increase LVAD speed and reduce MR severity. A TEER with a MitraClip XTR device (Abbott Vascular) was pursued. Prior to clip placement, the mean left atrial pressure (LAP) was 11 mm Hg with a V-wave of 20 mm Hg. After clip placement at the A2-P2 scallop, MR severity was immediately reduced to mild (Figure 1 ), and the mean LAP reduced to 6 mm Hg with a V-wave of 11 mm Hg (Figure 3 ). At the 2-year follow-up, the patient remained NYHA class I with a repeat transthoracic echocardiogram showing mild residual MR, and no additional invasive hemodynamics were obtained.
Figure 1.
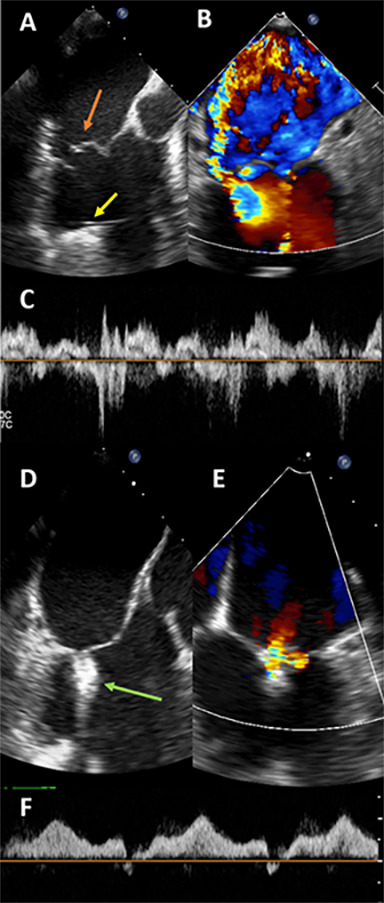
Intraprocedural transesophageal echocardiogram images for patient 1. Panel A shows prolapse and flail with ruptured chordae of the A2 scallop (orange arrow). The left ventricular assist device inflow cannula is seen as well (yellow arrow). Panel B shows color Doppler across the mitral valve at Nyquist limit of 61 cm/second, suggesting severe mitral regurgitation (MR). Panel C shows a pulsed-wave (PW) Doppler through one pulmonary vein, showing systolic reversal, and was seen in all four pulmonary veins. After one MitraClip XTR was placed at the A2-P2 scallop (Panel D, green arrow), the severity of MR significantly decreased (panel E), and PW Doppler through the same pulmonary vein shows antegrade flow (Panel F) confirming mild MR. Mean gradient after clip placement is 2 mm Hg.
Figure 2.
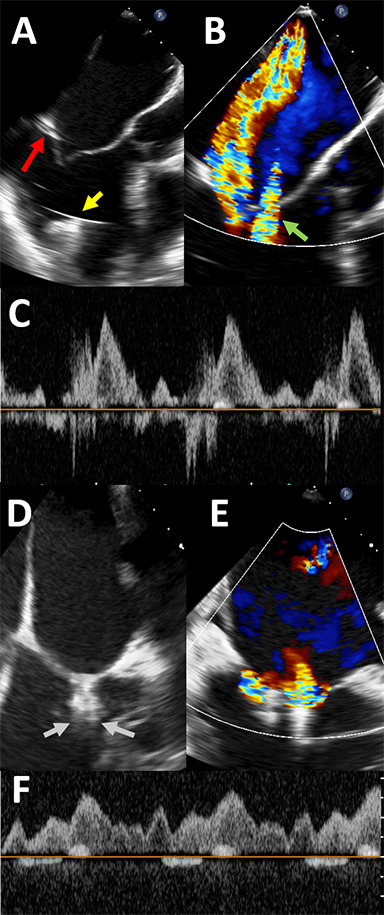
Intraprocedural transesophageal echocardiogram images for patient 2. Panel A shows tethering of the posterior leaflet (red arrow) as well as the left ventricular assist device (LVAD) inflow cannula (yellow arrow). Panel B shows color Doppler across the mitral valve, confirming significant mitral regurgitation (MR). Nyquist limit is 61 cm/second. “Waterfall artifact” from the LVAD is also seen (green arrow). After one MitraClip XTR is placed, significant residual MR is seen lateral to the clip in the bicommissural view, as seen in panel C, with the MR jet simultaneously visualized in the 3-chamber view. A second MitraClip XTR is then placed just lateral to the first clip, as shown in panel D (gray arrows). Panel E shows color Doppler across the clips, confirming improvement in MR. There is no longer reversal in the pulmonary veins (Panel F).
Patient 2
A 42-year-old male with NICMP and severe, functional MR due to apically-tethered posterior leaflet underwent LVAD implantation; however, there was minimal improvement even after optimizing his LVAD settings. One year later, he was hospitalized with NYHA class III-IV symptoms. A workup revealed normal LVAD function with reduced RV function and persistent, severe MR. Given the lack of improvement despite optimizing LVAD settings and medical therapy, a TEER procedure was performed with two MitraClip XTR devices placed at the A2-P2 scallop. An immediate improvement to mild MR was seen (Figure 2 ). The mean LAP immediately after transseptal puncture was 17 mm Hg with a V-wave of 19 mm Hg under general anesthesia, whereas the mean postprocedural LAP was 13 mm Hg with a V-wave of 14 mm Hg (Figure 3 ). The mean transmitral gradient was 2 mm Hg at a heart rate of 85 beats per minute. However, the transseptal puncture (or interatrial septal defect, ASD) created for the procedure showed evidence of bidirectional shunting immediately after the procedure, with predominant right-to- left shunting. Therefore, the patient underwent percutaneous ASD closure with a 12-mm Amplatzer occluder device (Abbott Structural), and he clinically improved to NYHA class II. One month after the procedure, however, an invasive hemodynamic assessment was concerning for worsening MR and unchanged, reduced RV function. Repeat transthoracic imaging showed a well-attached MitraClip device but moderate-to-severe MR with worsening biventricular dilatation. The patient is currently being evaluated for transplant listing.
Figure 3.
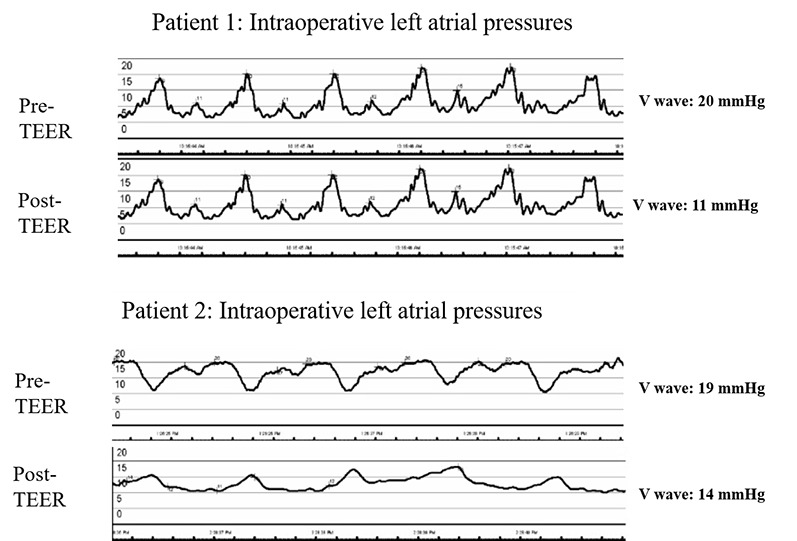
Intraprocedural left atrial pressure wave tracings immediately before andafter transcatheter edge-to-edge mitral valve repair for both patients.The left atrial tracings were performed using the steerable guide catheterduring the MitraClip XTR procedure immediately before and after clipplacement under general anesthesia conditions. TEER: transcatheteredge-to-edge mitral valve repair
DISCUSSION
We present two cases in which a TEER procedure was used to treat symptomatic MR. In both cases, LVAD optimization to reduce left-sided pressures could not be achieved due to inflow cannula positioning or distortion of the LV. Although the use of TEER with a MitraClip XTR has shown benefit in treating severe MR, these initial trials excluded patients with stage D heart failure.7, 8 We believe a transcatheter approach to reduce MR in patients with LVADs could offer a therapeutic option to those deemed at prohibitive surgical risk for a re-do sternotomy or those who cannot receive an immediate orthotopic heart transplantation.
From a procedural standpoint, the crossing of the mitral valve with the MitraClip XTR device needs to be carefully followed with intraoperative transesophageal imaging to ensure that the device is away from the inflow cannula and will not become damaged. In addition, the LVAD can create a “waterfall artifact” when color Doppler is used. Depending on the angulation of the ultrasound to the LVAD inflow cannula, a false MR jet can be seen (Figure 2 B). This similar artifact can be seen with continuous-wave Doppler of the mitral valve, which can confuse the severity of mitral stenosis after the procedure.
The TEER procedure creates a residual ASD due to the transseptal puncture used to access the mitral valve, which typically closes spontaneously in patients with normal right-sided pressures. However, it can be percutaneously closed if there are pending signs of pulmonary hypertension or unexplained hypoxia.9 It is unclear when or if the iatrogenic ASD would close in the presence of an LVAD. We presume that patient 1, who had normal right-sided pressures and no signs of pulmonary hypertension, would not require closure of his iatrogenic ASD, and in fact he has not required it after 6 months. However, patient 2 had baseline RV dysfunction with signs of bidirectional shunting immediately after the MitraClip procedure. Therefore, a percutaneous occluder device was used to close the interatrial septum.
The two described cases demonstrate that a TEER procedure may be a safe and feasible therapeutic option in carefully selected patients. In those without LVADs, we are finding specific mitral valve leaflet anatomy and patient characteristics that would benefit the most from the TEER procedure.10 Similarly, in those with LVADs, we anticipate there will be additional clinical and patient-specific criteria that are associated with better outcomes after TEER, such as the MR etiology. It is possible that TEER for primary MR may be more beneficial than for secondary MR with LVAD implants and allow a longer time-to-transplant, as seen in our cases. Persistent, severe secondary MR may be due to continued maladaptive remodeling of the ventricles. If so, this would lead to worsening MR and RV function and would therefore escalate the need for heart transplantation.
There were several limitations regarding these two cases. Particularly, full invasive hemodynamics were not obtained at the time of or after the procedure, so we do not know how these changed as a result of the procedure. In addition, we present just two cases, and additional research is needed to determine the utility of the procedure in this select group of patients.
CONCLUSION
The described examples suggest a possible therapeutic option for patients with advanced heart failure requiring LVAD support who also suffer from severe, symptomatic, and refractory MR. It is possible that percutaneous repair of the mitral valve could potentially delay the need for heart transplantation in a carefully selected group of patients.
References
- Asgar A W, Mack M J, Stone G W. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol. 2015;65(12):1231–1279. doi: 10.1016/j.jacc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Friedrich E. B, Bohm M. Heart. 5. Vol. 93. BMJ; 2007. MANAGEMENT OF END STAGE HEART FAILURE; pp. 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell Cory, Whitener George. Seminars in Cardiothoracic and Vascular Anesthesia. Vol. 23. SAGE Publications; 2019. Mitral Intervention with LVAD: Preparing for Recovery; pp. 134–139. [DOI] [PubMed] [Google Scholar]
- Goodwin Matthew, Nemeh Hassan W., Borgi Jamil, Paone Gaetano, Morgan Jeffrey A. The Annals of Thoracic Surgery. 3. Vol. 104. Elsevier BV; 2017. Resolution of Mitral Regurgitation With Left Ventricular Assist Device Support; pp. 811–818. [DOI] [PubMed] [Google Scholar]
- Kassis Hayah, Cherukuri Krishna, Agarwal Richa, Kanwar Manreet, Elapavaluru Subbarao, Sokos George G., Moraca Robert J., Bailey Stephen H., Murali Srinivas, Benza Raymond L., Raina Amresh. JACC: Heart Failure. Vol. 5. Elsevier BV; 2017. Significance of Residual Mitral Regurgitation After Continuous Flow Left Ventricular Assist Device Implantation; pp. 81–88. [DOI] [PubMed] [Google Scholar]
- Rosenkranz Stephan, Gibbs J. Simon R., Wachter Rolf, De Marco Teresa, Vonk-Noordegraaf Anton, Vachiéry Jean-Luc. European Heart Journal. 12. Vol. 37. Oxford University Press (OUP); 2016. Left ventricular heart failure and pulmonary hypertension; pp. 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone G W, Lindenfeld J, Abraham W T. Transcath- eter Mitral-Valve Repair in Patients with Heart Failure. COAPT Investigators. 2018;379:2307–2325. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- Mack M J, Abraham W T, Lindenfeld J. Cardiovascular Outcomes Assess- ment of the MitraClip in Patients with Heart Failure and Secondary Mitral Regurgitation: Design and rationale of the COAPT trial. Am Heart J. 2018;205:1–11. doi: 10.1016/j.ahj.2018.07.021. [DOI] [PubMed] [Google Scholar]
- Beri Neil, Singh Gagan D., Smith Thomas W., Fan Dali, Boyd Walter D., Rogers Jason H. Catheterization and Cardiovascular Interventions. Vol. 94. Wiley; 2019. Iatrogenic atrial septal defect closure after transseptal mitral valve interventions: Indications and outcomes; pp. 829–836. [DOI] [PubMed] [Google Scholar]
- Chrissoheris M, Halapas A, Nikolaou I, Boumboulis N, Pattakos S, Spargias K. The Abbott Vascular MitraClip: Patient Selection and How to Obtain the Best Outcomes. Hellenic J Cardiol. 2015;56:31–39. [PubMed] [Google Scholar]


