Abstract
Requiring regional or in-country trials delays access to medicines in low- and middle-income countries and raises costs. The International Committee on Harmonization guidance, ICH-E5, defines the scientific issues that may justify such trials. Here, we provide an update of the enormous scientific and technological advances that inform these issues.
1. Introduction
Making established and new drugs accessible as early as possible to patients in regulatory resource-limited settings is an important public health issue. Currently, it is not uncommon for countries to require that efficacy or bioequivalence studies be performed in-country before marketing authorization, generally delaying the availability of these products by years. The requirement for in-country trials can sometimes be scientifically justified; however, these requirements are often based solely on tradition, legal requirements, or political factors, which may include financial incentives associated with the trial. Frequently the argument is made that different “ethnic” factors are the reason for this requirement, but in reality within a “country” there are many different ethnic factors that can impact the pharmacokinetics (PK) and pharmacodynamics of a drug. In 1998, the International Council for Harmonisation (ICH), published its first guideline on this topic, “Ethnic Factors in the Acceptability of Foreign Clinical Data”, E5(R1), that included definitions of extrinsic ethnic factors and intrinsic ethnic factors. Clarifications of E5(R1) were published in 2003 and 2005 based on questions raised by drug developers (Table S1).
In E5(R1), “extrinsic ethnic factors” were defined as “factors associated with the environment and culture in which a person resides” and “intrinsic ethnic factors” were defined as “factors that help to define and identify a (genetic or other) subpopulation and may influence the ability to extrapolate clinical data between regions” (or between people in a region). Examples of extrinsic factors included environmental (e.g. climate, sunlight, pollution) and cultural factors (e.g. socioeconomic factors, diet, medical practice, treatments, and regulatory practices), while intrinsic factors were classified as “genetic” and “physiologic and pathologic conditions”. Over the past two decades, there have been significant changes in the extrinsic factors throughout the world, including regional demographics, availability of health care, new regulatory practices and changes in local and regional environments. Importantly, enormous advances have been made in understanding the intrinsic and extrinsic factors that underlie drug safety and efficacy, which are described below. In 2017, the ICH adopted guidance E17, which addresses issues that are specific to the planning and design of confirmatory multi-regional clinical trials that can be accepted by regulatory authorities.
The purpose of this review is to set the stage for a forum, composed of individuals representing Stringent and National Regulatory Authorities (SRAs and NRAs), to exchange views on how to build on the objectives of E5(R1) and E17 to “minimize duplication of clinical data and facilitate acceptance of relevant foreign clinical data in a new region.” Stringent Regulatory Authorities have been defined by the World Health Organization in a guidance document released in February 2017 (1). In the next sections we briefly summarize the substantial body of new information on the impact of both intrinsic and extrinsic ethnic factors on drug disposition that, when combined with known regional variation in these factors, may reduce the need for duplication of clinical trials and will serve as a starting point for these discussions.
2. Extrinsic and Intrinsic Ethnic Factors
Extrinsic - Disease Prevalence and Microbial Genetic Diversity:
Disease prevalence can be strikingly different across regions and lead to substantial differences in the use of medications. Examples include the extensive use of anti-malarial drugs in tropical areas and the increasing use of anti-diabetic drugs in South Asia (2, 3). Prior to making agents available in a country, any prominent regional variables that affect mechanism of disease and pharmacology in the relevant population should be understood and considered.
In this era of genomics and metagenomics, simple descriptions of infectious disease no longer describe the complexity of microbial infections. Notably, sequencing technologies have led to a new understanding of the genetic differences among pathogens. Understanding local variation in the pathogenicity of a virus can guide therapy selection and avoid periods of ineffective treatment that causes drug resistance. For instance, the prevalence of hepatitis C virus (HCV) varies globally and is complicated by HCV’s high genetic diversity (4). The regional variation in genotype prevalence and the corresponding therapeutic responsiveness across HCV genotypes underscore the impact of HCV diversity. Leishmaniasis is complicated by the 20 pathogenic species spread worldwide that are manifest in four distinct clinical presentations (5), but the approved label for miltefosine specifies only a limited number of these species, resulting in treatment resistance. Pathogen-specific therapeutic intervention is not isolated to HCV and leishmaniasis infections but is common in other diseases such as malaria (6), Human Immunodeficiency Virus infections (7), and tuberculosis (8), underscoring the need to consider the prevalence and response of infectious agents in the approval of drugs in various regions.
Extrinsic - Nutritional Differences:
Malnutrition remains a significant problem in resource-limited countries and has increasingly become a major problem for high-income countries where highly processed foods have become dietary staples. In the last decade, our understanding of the mechanisms of drug-induced nutritional deficiencies has increased dramatically. For example, the occurrence of Wernicke’s encephalopathy (associated with vitamin B1 deficiency) in a handful of patients resulted in the termination of a clinical trial for fedratinib, a Janus kinase inhibitor (9). Notable nutrient deficiencies exacerbated by drugs include vitamin B1 and B12 deficiency that are linked to the use of alcohol and proton pump inhibitors, respectively, and calcium deficiencies caused by anticancer drugs. Dietary constituents (determined by socioeconomic status and cultural factors) may also be important; in many parts of Nigeria there are wide differences in diet that can influence the bioavailability of certain drugs (10).
It is known that high fat meals influence the bioavailability of certain drugs, but food components can also affect intestinal transporters involved in drug absorption. Some fruit juices inhibit the intestinal absorption transporter OATP2B1, and may reduce drug absorption. Dietary supplements, herbal remedies, and concomitant medications may also affect drug absorption and disposition through induction and inhibition of transporters and enzymes (11). Ingredients in St. John’s Wort are known to induce drug metabolizing enzymes, causing important dietary supplement-drug interactions (12). Thus, when conducting clinical trials, dietary factors and concomitant medications should be considered. In some cases, these factors can be investigated in the post-marketing setting and need not delay access to essential medicines.
Intrinsic - Human Genetic Differences:
Human genetic diversity, as with genetic diversity of pathogens, is responsible for some inter-regional/intra-country variation in risk of disease, drug safety, and response. Genetic diversity in genes that code for proteins involved in drug absorption, distribution, metabolism and elimination (ADME), such as cytochrome P450 enzymes (CYPs), conjugating enzymes, and transporters lead to changes in drug exposure that are clinically relevant. Genetic studies, including genome-wide association studies (GWAS) have identified many genes responsible for pharmacological variation that may vary among ethnic populations. A striking example of population-specific variation in allele frequencies is CYP2D6, an enzyme responsible for the metabolism of over 25% of prescription drugs. With over 100 distinct alleles that may vary in frequency among populations, this polymorphic enzyme accounts for variation in the conversion of codeine to morphine, the metabolism of antidepressants, and metabolism of many other drugs (13). For example, CYP2D6*4, a reduced function allele, is present at allele frequencies as high as 18% in Europeans, but <1% in ethnic East Asians, though these people have higher allele frequencies of CYP2D6*5, another reduced function allele (13). The gene ABCG2, encoding Breast Cancer Resistance Protein (BCRP), also has a common reduced-function genetic variant, which limits drug absorption via intestinal efflux (Figure 1). A lower dose of rosuvastatin is recommended for ethnic East Asians, who have a high allele frequency of the variant resulting in greater bioavailability. Over 250 pharmacogenomic GWAS have been conducted and cataloged (Table S1). As with drug interactions, studies in individuals carrying genetic polymorphisms can often be performed in the post-marketing setting, recognizing that with population dispersion around the globe genetic variants are not geographically isolated.
Figure 1. Allele frequencies of selected polymorphisms that affect drug ADME.
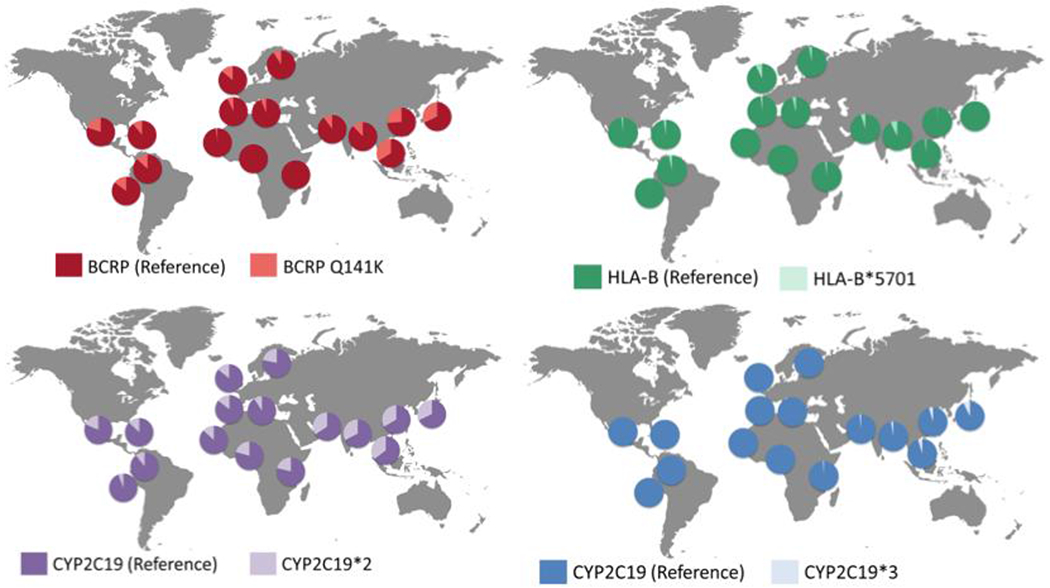
These differences include BCRP-Q141K (rs2231142) (red), which affects rousuvastatin exposure (29). Particular HLA alleles, such as HLA-B*5701 (green) are associated with drug toxicities including Toxic Epidermal Necrolysis. CYP2C19 is a polymorphic enzyme with two common reduced function alleles, CYP2C19*2 (purple) and CYP2C19*3 (blue). This polymorphic variant is a major genetic factor for variation in response to clopidogrel, and poor CYP2C19 metabolizers are at risk for higher cardiovascular event rates.
3. Pediatric Populations
Pediatric drug development should be treated with the same principles and rigor as in adults. As approval for most medicines used in children generally follows approval in adults, some of the major factors that affect drug efficacy and toxicity may already be clear. More experience using pediatric extrapolation, advances in modeling and simulation strategies, and knowledge in physiology and pharmacology of pediatric patients supports the use of extrapolation to model the efficacy and toxicity of a drug for a similar indication in children (14), although extrapolation regarding impact on growth and development is difficult. Drugs for pediatric cancers and rare diseases may be modulated by extrinsic and intrinsic factors (15,16). In such cases, information about these factors and their prevalence in relevant regions is critical, along with information about developmental changes in genes that affect ADME of drugs. Considering the great need for pediatric medications and the challenge of conducting pediatric trials, regional post-marketing surveillance activities may be the best strategy to address these issues.
4. Generic Drugs
Generic drugs account for nearly 90% of prescriptions dispensed in the United States. By 2021, global generic spending is expected to increase, mainly in emerging markets, where 4 of the world’s 7 billion people live. Thus, because of their high usage, generic drugs play a critical role in advancing human health globally.
For many generic drugs, a clinical bioequivalence study in healthy volunteers is required. Sophisticated quantitative methods and computational modeling have recently shown great potential for improving generic drug approval rates by reducing time and cost of product development. In particular, these methods have informed more efficient study designs for comparative bioequivalence evaluation (17). In the last few years biowaivers for a larger spectrum of products have become available from the European Medicines Agency and U.S. Food and Drug Administration (FDA). These biowaivers forego the requirement for a clinical bioequivalence study for many generic drugs that have excellent absorption characteristics (highly permeable and highly soluble), and for some generic drugs that are poorly permeable but have high solubility. Biowaivers have reduced costs/time for generic drug development and approval. Furthermore, new databases are available to allow for effective formulation of generic drug products (Table S1).
Effects of extrinsic and intrinsic factors on generic drugs:
Drug formulations intended for the global market should be tested for stability under tropical conditions. Because generic drug products have the same active ingredient as the innovator product, intrinsic factors such as genetic polymorphisms are often not relevant to bioequivalence. However, excipients may differ between brand name and generic drugs, and they may affect the bioequivalence, safety and efficacy of generic drug products. Though most excipients have a Generally Regarded as Safe (GRAS) designation, there may be intrinsic factor-specific effects of GRAS compounds on various populations. For example, lactose is a common excipient in many medical products, but lactose intolerance represents a particular problem for some ethnic groups (18). Aspartame, a taste-enhancing excipient, affects individuals with phenylketonuria, a genetic disorder that is more common in individuals of Native American or European ancestry (19). These issues are usually handled with product labeling. The overall quality of excipients may also vary between regions, and some excipients may be unsafe for use in special populations such as neonates (19). In general, dietary and genetic factors should not drive decisions to conduct in-region bioequivalence clinical studies for generic drugs, as those effects would affect the brand name and generic drug product similarly. However, clarification of the excipients that are susceptible to intrinsic ethnic differences is needed.
5. Emerging Technologies
Although novel technologies have improved our understanding of relevant differences affecting therapeutic response, many of these are not available in resource-limited countries where they could have great impact. For example, in vitro technologies and computational analytical methods can used to predict drug safety, efficacy and response for various ethnic groups.
Breakthrough advances in in-vitro biotechnology:
These technologies are important in projecting drug effects in limited and middle-income countries. For example, the generation and specific differentiation of human induced pluripotent stem cells, organoid technologies which mimic three-dimensional structures of tissues or organs, and “-Omics” approaches such as next-generation sequencing and single cell RNA sequencing all provide new insights to drug responses. Genome-wide methods have ushered in a new understanding of genes that affect drug response, but genetic testing is not widely available and reliable in most countries. For example, 20 of 603 international genetic testing laboratories are located in middle-income countries, and none are located in low-income countries (20). Gene editing could also play a key role in generating isogenic cell lines for interrogating causal associations between gene mutation-drug pairs.
Computational models and data science:
Computational models, such as physiologically-based pharmacokinetic (PBPK) models, can identify potential safety risks. For example, a PBPK model of isoniazid that incorporated genetic variants in NAT2 was able to predict safety and deposition in NAT2-specific patient populations, demonstrating that when genetic deficiencies are known, it is possible to computationally predict how patients will respond to treatment (21). Ethnic and other regional data can be incorporated in PBPK models to better predict drug disposition and study bioequivalence for generic drugs. Further application of these tools combined with region-specific molecular characterization could help inform the necessity of an in-region trial.
Improved clinical trial infrastructure:
Improving clinical trial data collection, quality, and standardization could streamline global clinical trials and downstream interpretation of inter-region differences. Infrastructure is particularly important for registered clinical trials, which are more common in high-income countries (22), and the requirement for in-country trials may create additional challenges. In high-income countries, electronic data capture within electronic health records (EHRs) has the potential to eliminate redundant data entry and improve data quality and processing speed. However, interoperability between the EHR and the clinical data management system is critical (23). Use of EHRs is still limited in low and middle-income countries (24), and information regarding the handling and reporting of biospecimens lacks standardization (25). Large data collections could improve understanding of regional features if these banking approaches are extended to low-resource countries.
Interoperability is further limited by differences in assessment capacity and inconsistent application of ICH principles across NRAs, especially in low- and middle-income countries. Three areas that would improve decision-making with respect to requiring an in-region clinical trial include: (i) increasing capacity of NRAs and Independent Ethics Committees or Institutional Review Boards to implement human research ethics in accordance with current best practices worldwide, (ii) increasing capacity to assess the chemistry, manufacturing and controls of Investigational Medicinal Products in accordance with the ICH Common Technical Document, and (iii) expanding capabilities to conduct Good Clinical Practice (GCP) inspections in line with existing international standards. We understand that efforts in these areas are currently underway through the WHO AFRO AVAREF program (Table S1).
Novel clinical trial designs and statistical approaches:
Traditional designs and statistical analyses of randomized controlled clinical trials are historically challenging and costly. To overcome these challenges, novel, flexible trial designs and statistical methods are promising. These include master protocols for platform trials, Bayesian and meta-analytically derived historical controls, Bayesian adaptive procedures that employ ongoing observations to refine sample size and trial duration, model-informed and simulation-enhanced procedures, and analysis of real-world data (26). For example, Bayesian approaches resulted in ~75% fewer pediatric patients in a clinical trial of an anti-diabetic drug (27). A Bayesian approach to evidence generation for drug approval would formally incorporate (prior) evidence of safety and efficacy that has already been accepted by a competent, independent regulatory authority for its approval, together with additional local data, if needed. These approaches require intensive planning, often including computer simulations to optimize trial design features, but can be resource-sparing and highly informative.
Discussion and Future Directions
Since the publication of ICH-E5, scientific advances have accelerated the discovery, development, evaluation, and approval of medical products. These advances have refined our understanding of both the intrinsic and extrinsic factors that govern drug disposition, toxicity and response. Simultaneously, clinical trial infrastructure and methodologies have evolved, and recently the fraction of registered clinical trials conducted in lower-middle income countries has increased dramatically (Figure 2) (28). This trend, plus the growing multi-ethnicity in individual countries, has increased the representation of different ethnic groups in clinical trials, reducing the need to conduct in-country trials based on intrinsic ethnic factors. However, representation from various ethnic groups must be sufficient, and studies need to be appropriately powered, to understand the effect of intrinsic ethnic factors on the response and toxicity of drugs under study. At the same time, increased capacity for conducting small, focused, well-planned PK and safety studies in lower-middle income countries may be advantageous by providing additional data. Modeling and simulation, including population-based pharmacokinetic-pharmacodynamic and PBPK models, have added powerful capabilities for prediction and extrapolation to special and global populations. Integration of worldwide data from clinical trials and postmarketing surveillance, which may include novel data capture methods, is increasingly required by mature regulatory agencies and the World Health Organization. Using new methodologies and technologies, all countries may wish to incorporate worldwide data in the postmarket setting as a complementary strategy for evaluating in-country drug safety and efficacy. A thorough review of clinical trials is needed to assess and monitor the number of confirmatory in-country clinical trials. Authorities from SRAs and NRAs should be convened to clarify and prioritize the issues raised in this review—particularly to identify knowledge and resource gaps that limit the rapid availability of safe and effective therapies to all people, irrespective of the country in which they live.
Figure 2.
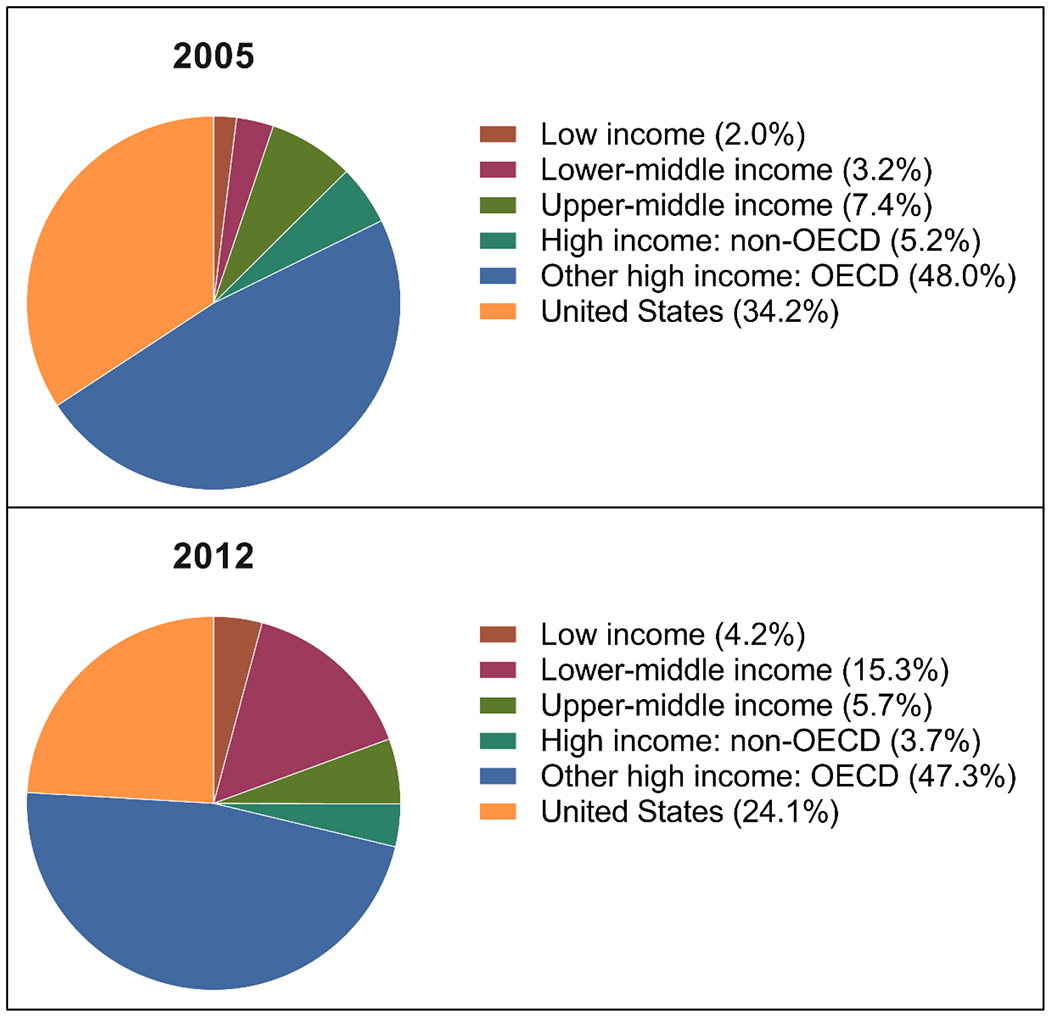
Percent of registered clinical trials in 2005 (upper) and 2012 (lower), classified by OECD countries (28).
Supplementary Material
Acknowledgements
We thank the following individuals for their helpful comments and review of this manuscript: Dr. Janet Woodcock (U.S. Food and Drug Administration), Dr. Guido Rasi (European Medicines Agency), and Margareth Ndomondo-Sigonda (African Union-New Partnership for Africa’s Development).
Funding:
This publication was made possible by Grant Number U01FD004979/U01FD005978 from the FDA, which supports the UCSF-Stanford Center of Excellence in Regulatory Sciences and Innovation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the HHS or FDA.
Footnotes
Competing Interests: JLW, KWKC, EAEG, LL, LZ, DM, PAA, RY, HL, EC, DH, PWM, and MML have no competing interests to declare. AIP is the Unit Chief, Medicines and Health Technologies at PAHO, a BMGF grantee. SD is also the Institute Director of the Global Health Drug Discovery Institute, Beijing, China. CP teaches drug development and regulatory science, and consults for NDA Partners LLC. YH is Senior Executive Director of PMDA. RBA is a stockholder in Personalis, 23andme, and Youscript. TFB is an advisor to the UCSF-Stanford CERSI, a contractor to the Bill and Melinda Gates Foundation and a member of the Board of Directors of Durect Corporation. KMG has been a paid consultant for Gilead and Vertex Pharmaceuticals.
References
- 1.WHO Prequalification Team: medicines, Clarification with respect to a stringent regulatory organization as applicable to the stringent regulatory authority (SRA) guideline. (World Health Organization, 2017). [Google Scholar]
- 2.White NJ, Pharmacokinetic and pharmacodynamic considerations in antimalarial dose optimization, Antimicrob. Agents Chemother 57, 5792–5807 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalra S, Aamir AH, Raza A, Das AK, Azad Khan AK, Shrestha D, Qureshi MF, Fariduddin Md, Pathan MF, Jawad F, Bhattarai J, Tandon N, Somasundaram N, Katulanda P, Sahay R, Dhungel S, Bajaj S, Chowdhury S, Ghosh S, Madhu SV, Ahmed T, Bulughapitiya U, Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: A consensus statement, Indian J Endocrinol Metab 19, 577–596 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E, Global distribution and prevalence of hepatitis C virus genotypes, Hepatology 61, 77–87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, Sereno D, Bañuls A-L, Ed. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies, PLoS Negl Trop Dis 10, e0004349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thu AM, Phyo AP, Landier J, Parker DM, Nosten FH, Combating multidrug-resistant Plasmodium falciparum malaria, FEBS J. 284, 2569–2578 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallis CL, Godfrey C, Fitzgibbon JE, Mellors JW, Key Factors Influencing the Emergence of Human Immunodeficiency Virus Drug Resistance in Low- and Middle-Income Countries, J. Infect. Dis 216, S851–S856 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brites D, Gagneux S, The Nature and Evolution of Genomic Diversity in the Mycobacterium tuberculosis Complex, Adv. Exp. Med. Biol 1019, 1–26 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Vainchenker W, Leroy E, Gilles L, Marty C, Plo I, Constantinescu SN, JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders, F1000Res 7, 82 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakare-Odunola MT, Mustapha A, Abdu Aguye I, Effect of Nigerian meals on the pharmacokinetics of chlorpropamide in type II diabetic patients, Eur J Drug Metab Pharmacokinet 33, 31–35 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Zhou Z, Tay-Sontheimer J, Levy RH, Ragueneau-Majlessi I, Intestinal Drug Interactions Mediated by OATPs: A Systematic Review of Preclinical and Clinical Findings, Journal of Pharmaceutical Sciences 106, 2312–2325 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Chrubasik-Hausmann S, Vlachojannis J, McLachlan AJ, Understanding drug interactions with St John’s wort (Hypericum perforatum L.): impact of hyperforin content, J. Pharm. Pharmacol 71, 129–138 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, Haidar CE, Shen DD, Callaghan JT, Sadhasivam S, Prows CA, Kharasch ED, Skaar TC, Clinical Pharmacogenetics Implementation Consortium, Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update, Clinical Pharmacology & Therapeutics 95, 376–382 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinks AA, Emoto C, Fukuda T, Modeling and simulation in pediatric drug therapy: Application of pharmacometrics to define the right dose for children, Clinical Pharmacology & Therapeutics 98, 298–308 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Liu Y, Liu Y, Alexandrov LB, Edmonson MN, Gawad C, Zhou X, Li Y, Rusch MC, Easton J, Huether R, Gonzalez-Pena V, Wilkinson MR, Hermida LC, Davis S, Sioson E, Pounds S, Cao X, Ries RE, Wang Z, Chen X, Dong L, Diskin SJ, Smith MA, Guidry Auvil JM, Meltzer PS, Lau CC, Perlman EJ, Maris JM, Meshinchi S, Hunger SP, Gerhard DS, Zhang J, Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours, Nature 555, 371–376 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai JPF, Barrett JS, Burckart GJ, Meibohm B, Sachs HC, Yao L, Strategic biomarkers for drug development in treating rare diseases and diseases in neonates and infants, AAPS J 15, 447–454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang L, Kim M-J, Li Z, Wang Y, DiLiberti CE, Au J, Hooker A, Ducharme MP, Lionberger R, Zhao L, Model-Informed Drug Development and Review for Generic Products: Summary of FDA Public Workshop, Clinical Pharmacology & Therapeutics 104, 27–30 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Fassio F, Facioni MS, Guagnini F, Lactose Maldigestion, Malabsorption, and Intolerance: A Comprehensive Review with a Focus on Current Management and Future Perspectives, Nutrients 10, 1599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valeur KS, Holst H, Allegaert K, Excipients in Neonatal Medicinal Products: Never Prescribed, Commonly Administered, Pharmaceut Med 32, 251–258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tekola-Ayele F, Rotimi CN, Translational Genomics in Low- and Middle-Income Countries: Opportunities and Challenges, Public Health Genomics 18, 242–247 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordes H, Thiel C, Aschmann HE, agents VBA, 2016, A PBPK model of isoniazid and its application in individualizing tuberculosis chemotherapy, Antimicrob Agents Chemother 60, 6134–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viergever RF, Karam G, Reis A, Ghersi D, Scherer RW, Ed. The quality of registration of clinical trials: still a problem, PLoS ONE 9, e84727 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellar E, Bornstein S, Caban A, Crouthamel M, Celingant C, McIntire PA, Johnson C, Mehta P, Sikirica V, Wilson B, Optimizing the Use of Electronic Data Sources in Clinical Trials, Therapeutic Innovation & Regulatory Science 51, 551–567 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Higman S, Dwivedi V, Nsaghurwe A, Busiga M, Rulagirwa HS, Smith D, Wright C, Nyinondi S, Nyella E, Designing interoperable health information systems using Enterprise Architecture approach in resource-limited countries: A literature review, The International Journal of Health Planning and Management 3, 47 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Moore HM, Kelly A, Jewell SD, McShane LM, Clark DP, Greenspan R, Hainaut P, Hayes DF, Kim P, Mansfield E, Potapova O, Riegman P, Rubinstein Y, Seijo E, Somiari S, Watson P, Weier H-U, Zhu C, Vaught J, Biospecimen Reporting for Improved Study Quality, Biopreserv Biobank 9, 57–70 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JJ, Thorlund K, Mills EJ, Critical concepts in adaptive clinical trials, Clin Epidemiol 10, 343–351 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huff RA, Maca JD, Puri M, Seltzer EW, Enhancing pediatric clinical trial feasibility through the use of Bayesian statistics, Pediatric Research 2017 82:5 82, 814–821 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Drain PK, Robine M, Holmes KK, Bassett IV, Trial watch: global migration of clinical trials, Nature Reviews Drug Discovery 13, 166–167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brackman DJ, Giacomini KM, Reverse Translational Research of ABCG2 (BCRP) in Human Disease and Drug Response, Clinical Pharmacology & Therapeutics 103, 233–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


