Abstract
Simple Summary
Mutational profiling using a custom 43-gene next-generation sequencing panel revealed that patients with mutated DNMT3A or EZH2, or an increase in TET2 VAF and lower TP53 VAF showed a higher overall response. NRAS and TP53 variants were associated with shorter overall survival (OS), whereas only mutated BCOR was associated with a shorter relapse-free survival (RFS). Subgroup analyses of OS according to biological and genomic characteristics showed that patients with low–intermediate cytogenetic risk and mutated NRAS benefited from azacytidine therapy and patients with mutated TP53 showed a better RFS in the azacytidine arm. In conclusion, differential mutational profiling might anticipate the outcomes of first-line treatment choices (AZA or FLUGA) in older patients with AML.
Abstract
We sought to predict treatment responses and outcomes in older patients with newly diagnosed acute myeloid leukemia (AML) from our FLUGAZA phase III clinical trial (PETHEMA group) based on mutational status, comparing azacytidine (AZA) with fludarabine plus low-dose cytarabine (FLUGA). Mutational profiling using a custom 43-gene next-generation sequencing panel revealed differences in profiles between older and younger patients, and several prognostic markers that were useful in young patients were ineffective in older patients. We examined the associations between variables and overall responses at the end of the third cycle. Patients with mutated DNMT3A or EZH2 were shown to benefit from azacytidine in the treatment-adjusted subgroup analysis. An analysis of the associations with tumor burden using variant allele frequency (VAF) quantification showed that a higher overall response was associated with an increase in TET2 VAF (odds ratio (OR), 1.014; p = 0.030) and lower TP53 VAF (OR, 0.981; p = 0.003). In the treatment-adjusted multivariate survival analyses, only the NRAS (hazard ratio (HR), 1.9, p = 0.005) and TP53 (HR, 2.6, p = 9.8 × 10−7) variants were associated with shorter overall survival (OS), whereas only mutated BCOR (HR, 3.6, p = 0.0003) was associated with a shorter relapse-free survival (RFS). Subgroup analyses of OS according to biological and genomic characteristics showed that patients with low–intermediate cytogenetic risk (HR, 1.51, p = 0.045) and mutated NRAS (HR, 3.66, p = 0.047) benefited from azacytidine therapy. In the subgroup analyses, patients with mutated TP53 (HR, 4.71, p = 0.009) showed a better RFS in the azacytidine arm. In conclusion, differential mutational profiling might anticipate the outcomes of first-line treatment choices (AZA or FLUGA) in older patients with AML. The study is registered at ClinicalTrials.gov as NCT02319135.
Keywords: clinical trials and observations, myeloid neoplasia, NGS, variants, leukemia, myelocytic, acute, leukemic cells, older adults, genetic risk, complete remission, cytarabine, azacytidine, prognostic factors
1. Introduction
Older patients with acute myeloid leukemia (AML), defined as those aged 65 or beyond, who are unsuitable for standard induction therapy generally has a poor prognosis, and fewer than 10% survive beyond 3 years. This is due to the often unfavorable genetic profiles of elderly patients, the presence of comorbidities that aggravate the side-effects of therapy, and the very high relapse rates, even for those who achieve complete morphological remission (CR) [1]. For elderly patients treated with intensive chemotherapy, the overall survival (OS) at 1, 2, and 3 years is 30%, 15%, and <5%, respectively [2].
Recent studies in elderly patients have tested the effects of low-intensity chemotherapy using hypomethylating agents (HMAs) such as azacytidine and decitabine, which are less toxic than conventional chemotherapeutic schemes. The results showed a CR rate of 15–20% and an improved OS when compared with supportive treatment or low-dose cytarabine (LDAC) [3,4]. The survival benefit of low-intensity HMA therapy is not limited to patients with morphological CR [5].
The clinical outlook for the elderly population has improved over the last few years with the approval of venetoclax and other targeted therapies, such as FLT3 inhibitors (midostaurin and gilteritinib, among others), IDH1/2 inhibitors (ivosidenib and enasidenib, respectively), and other novel therapies such as hedgehog pathway inhibitors (e.g., glasdegib) [6]. This change in the treatment paradigm makes it imperative to develop new biomarkers that help guide treatment approaches and improve the outcome in elderly patients with AML.
While knowledge of the molecular landscape in AML continues to evolve, the majority of studies are retrospective in nature. Consequently, treatment strategies are heterogeneous, and the clinical applicability of this new molecular information is limited. It is, therefore, essential to perform well-designed clinical trials that include associated molecular studies in order to test new biomarkers of responses.
TET2 mutations predict responses to HMAs in myelodysplastic syndrome (MDS) [7]; however, this has not been substantiated in AML. TP53 mutations have been previously associated with better responses to HMAs in AML [8], but there are no molecular biomarkers for LDAC-based or fludarabine schemes.
Our aim was to predict the responses and outcomes in older patients with AML at the time of diagnosis via mutation status in the context of the FLUGAZA phase III clinical trial (NCT02319135), which compares 5′-azacytidine (AZA arm) with LDAC plus fludarabine (FLUGA arm) treatment in newly diagnosed AML patients.
2. Patients and Methods
2.1. Identification Cohort
We analyzed bone marrow (BM) samples at the time of diagnosis from 207 of 285 patients with AML treated in accordance with the FLUGAZA trial (Supplementary Methods) (AZA arm (n = 96) and FLUGA arm (n = 111)). DNA at diagnosis was not available for 78 cases. The patients included in the AZA arm received 3 induction cycles of azacytidine followed by 6 consolidation cycles. The patients included in the FLUGA arm were randomized to receive 3 induction cycles of cytarabine plus fludarabine (FLUGA), followed by 6 cycles of reduced-intensity fludarabine and LDAC. Treatment was continued for 9 more cycles, unless the minimal residual disease as assessed by flow cytometry was negative. The median age at diagnosis was 75 years (range, 65–90) in the sub-study cohort. Both treatment groups were balanced for age, leukocyte count, baseline BM blasts, karyotype risk (ELN-2017 [9]), and, also, for FLT3-internal tandem duplication (FLT3-ITD) and mutated NPM1. The main clinical characteristics of the patients included in the sub-study are summarized in Table 1 and Table S1. The baseline characteristics and efficacy outcomes in the subset of 207 patients in whom molecular assessments were performed were similar to those of the 78 excluded patients who did not undergo molecular assessment (Table S2).
Table 1.
Patient demographics and baseline characteristics.
| Variable | AZA Arm (N = 96) | FLUGA Arm (N = 111) | |
|---|---|---|---|
| Age at diagnosis | Years, median (range) | 75 (65–90) | 76 (65–88) |
| Blasts at diagnosis | %, median | 55 | 53 |
| WBC at diagnosis | ×10−9/L, median | 22 | 21 |
| Dyserythropoiesis | n cases, % | 45 | 47 |
| Dysmyelopoiesis | n cases, % | 38 | 42 |
| Dysthrombopoiesis | n cases, % | 23 | 31 |
| AML origin | de novo | 44 | 40 |
| AML secondary MDS | 47 | 45 | |
| AML secondary Treatment | 5 | 11 | |
| FAB classification | M0/M1/M2/M4/M5/M6/M7/NOS | 16/15/13/1/21/12/5/9 | 16/21/22/0/22/12/5/10 |
| Cytogenetics | Abnormal Karyotype/Normal Karyotype | 46/38 | 51/26 |
| Cytogenetics Risk Group | Low–Intermediate Risk | 63 | 68 |
| High Risk | 30 | 35 | |
| WHO classification | AML with certain genetic abnormalities | 5 | 13 |
| AML with myelodysplastic-related changes | 47 | 45 | |
| AML related to chemotherapy or radiation previous | 5 | 11 | |
| AML NOS | 38 | 42 | |
| Follow-up time | Months, median (SD) | 15 (9) | 16 (7) |
AML: acute myeloid leukemia, WBC: white blood cells, AZA: azacytidine, FLUGA: fludarabine plus low-dose cytarabine (LDAC), FAB: French–American–British classification, MO: myeloblastic without cytological maturation, M1: myeloblastic with minimal maturation, M2: myeloblastic with significant maturation, M4: acute myelomonocytic leukemia, M5: acute monoblastic leukemia, M6: acute erythroid leukemia, M7: acute megakaryoblastic leukemia, WHO: World Health Organisation, AML NOS: AML not otherwise specified, cytogenetic risk group: low–intermediate vs. high-risk, classification as per ELN 2017, SD: standard deviation. This study was registered at www.ClinicalTrials.gov as NCT02319135 (accessed on 6 April 2020)
All patients provided written informed consent, and the trial was approved by the appropriate institutional review boards or ethics committees of the participating institutions. The study was registered at www.ClinicalTrials.gov as NCT02319135 (accessed on 6 April 2020), and the results of the FLUGAZA trial have been published elsewhere [10,11].
2.2. Methods
2.2.1. High-Sensitivity Targeted Sequencing and Mutation Analysis
DNA was extracted using a Maxwell® 16 MDx instrument (Promega Biotech Iberica SL, Madrid, Spain) and quantified on a Qubit® 2.0 Fluorometer (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA). Library preparation was carried out according to the manufacturer’s protocol (Life Technologies, Palo Alto, CA, USA). High-depth next-generation sequencing (NGS) was performed on an Ion Torrent S5XL sequencer (Life Technologies).
Mutational profiling was performed via targeted NGS using a custom panel of 43 genes implicated in myeloid pathology (described in the Supplemental Methods). The total number of reads obtained in each sample was two million, with an average depth of coverage >2000 reads per nucleotide and high uniformity amongst all fragments (92%). Data analyses were performed using Ion Reporter v4.4 software (Life Technologies, Carlsbad, CA, USA), which identified single nucleotide variants (SNV) and small insertions or deletions (InDels). We employed Ion Reporter default parameters and filtered out variants with a total coverage of at least 70 reads and a variant allelic coverage of at least 10 reads. Variants with a minor allelic frequency >0.01 in the general population according to the single nucleotide polymorphism database (NCBI, dbSNP150) and/or the 5000-exome sequencing project were also rejected as possible polymorphisms (https://evs.gs.washington.edu/EVS; accessed on 20 December 2019). Filtered variants were then annotated using the Catalogue of Somatic Mutations in Cancer (COSMIC) database (https://cancer.sanger.ac.uk/census; accessed on 20 December 2019), allowing those variants present in some tumors to be retained, and those present in dbSNP and previously identified as cancer mutations to be retained. Filtered variants that were absent from dbSNP or COSMIC but were “deleterious” due to associated functional changes at the protein level, or due to their occurrence in conserved regions, were considered in the final analysis. All the mutations included in this study are listed in Table S8.
2.2.2. Availability of Data and Materials
All supporting data are included in the manuscript and Supplemental Materials. The data discussed in this publication have been deposited in the NCBI Sequence Read Archive (SRA) and are accessible via the following link: https://www.ncbi.nlm.nih.gov/sra/PRJNA655113 (accessed on 6 August 2020). Additional data are available upon reasonable request from the corresponding author.
2.2.3. Statistical Analysis
Statistical analyses were performed with SPSS v22.0 software (SPSS Inc., Chicago, IL, USA). The clinical characteristics of the patients were compared using the Chi-squared test for categorical variables and Student’s t-test for continuous variables. The relationship between clinical variables, including mutational status, and response to azacytidine or FLUGA was evaluated using multivariate logistic regression analysis. Relapse-free survival (RFS) was defined as the time from the first CR after diagnosis to relapse, death, or the date of the last follow-up. OS was calculated from the date of AML diagnosis to death or the last follow-up date. Cox proportional hazard models and Kaplan–Meier analyses were used to assess the association of variables (clinical data and mutational profile) with patient RFS and OS, and were adjusted via treatment received. For multivariate analyses, patient age (continuous variable), leukocytes (categorized variable), cytogenetic risk (low–intermediate vs. high risk), ELN-2017 classification [9], number of mutations (0–8), mutated genes in panel (yes/no), and DTA (DNMT3A-TET2-ASXL1) mutations were included. Multiple comparisons were adjusted by Bonferroni correction. We used overall treatment response, which includes partial remission (PR) and CR/CRi (complete remission with incomplete recovery of blood counts), to define subgroups that are clinically relevant in the investigation of molecular markers associated with treatment sensitivity and resistance. In all cases, statistical significance was considered at a p-value less than or equal to 0.05.
3. Results
The mutational profile of older patients with AML is different to that of younger patients, and several prognostic markers have no impact in older patients. We detected a total of 893 variants—247 small InDels and 646 SNV. In total, 98% of patients (n = 203) presented at least one detectable mutation, with a median number of mutations of four (range: zero to eight). The most commonly mutated genes were TET2 (n = 55), FLT3 (n = 52), SRSF2 (n = 49), TP53 (n = 45), DNMT3A (n = 45), ASXL1 (n = 45), RUNX1 (n = 43), IDH2 (n = 36), IDH1 (n = 34), NPM1, (n = 33), and NRAS (n = 23).
Remarkably, the mutational profiles of older patients differed considerably from those of a previous series of young patients with AML treated almost entirely with a standard high-dose cytarabine regimen plus idarubicin (3 + 7 regimen) (Figure S1A) [12,13,14].
We evaluated conventional molecular markers with an impact on OS, but no marker with prognostic impact was detected in our cohort (Figure S2). Indeed, recurrent genetic abnormalities (FLT3-ITD, NPM1, and CEBPA mutations) had no significant impact on OS as assessed via Cox multivariate analyses adjusted by treatment, unlike what was seen in younger AML patients. Patients with low–intermediate risk clearly benefitted from AZA (median overall survival 14 months in the AZA arm vs. 6 months in the FLUGA arm; p = 0.003) (Figure S3). Only cytogenetic risk had a prognostic impact on OS (hazard ratio (HR) 1.67; 95% confidence interval (CI) 1.15–2.43; p = 0.007) as assessed via COX multivariate analyses, irrespective of treatment received, but it had no impact on RFS.
3.1. Mutational Landscape Predicts Response to Azacytidine and LDAC Plus Fludarabine Treatments
The mutational landscapes in responders (patients who achieved CR or CRi) and non-responders under each of the two treatments are shown in Figure S4. CR/CRi was achieved after the third cycle in 24% of patients in the AZA arm, and in 28% of patients in the FLUGA arm. Treatment-adjusted logistic regression analysis identified the following predictive markers that were associated with achieving CR after the third cycle of azacytidine or FLUGA (Table S3): lower patient age (odds ratio (OR), 0.92; p = 0.037), mutated KMT2A (OR, 6.68; p = 0.006), mutated NF1 (OR, 9.01; p = 0.005), mutated PHF6 (OR, 7.93; p = 0.014), mutated U2AF1 (OR, 7.09; p = 0.04), wild-type NRAS (OR, 0.04; p = 0.021), and wild-type TP53 (OR, 0.18; p = 0.039).
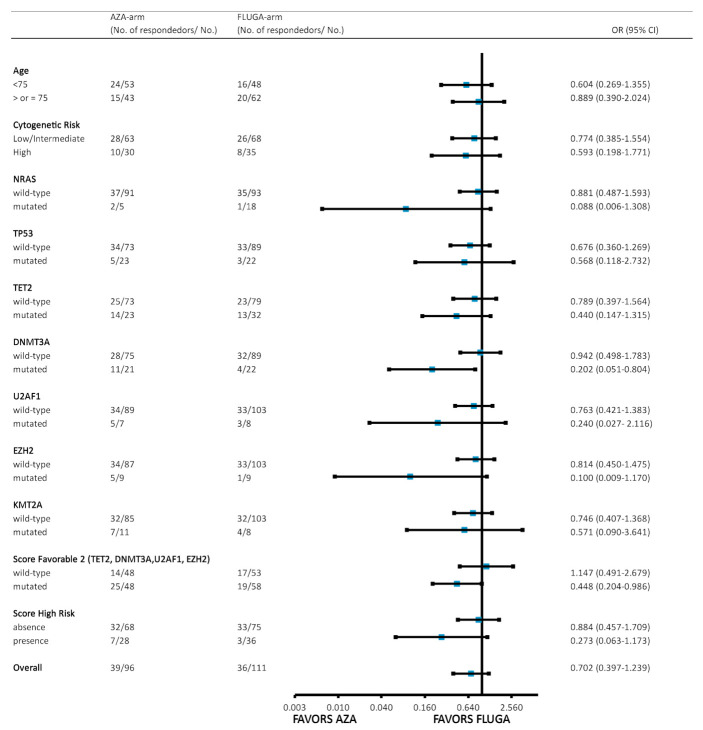
The main response associated with a longer OS in this series of AML patients was overall response, which includes PR and CR/CRi (p < 0.001, Figure S5). The overall response rate (ORR) was 40% after the third cycle of azacytidine and 37% after the third cycle of FLUGA. Treatment-adjusted logistic regression analysis identified the following predictive markers associated with ORR after the third cycle of azacytidine or FLUGA (Table S4): lower patient age (OR 0.94; p = 0.078), wild-type TP53 (OR 0.19; p = 0.018), mutated KMT2A (OR 4.29; p = 0.025), mutated NF1 (OR 4.53; p = 0.033), and mutated TET2 (OR 2.35; p = 0.07). Neither azacytidine nor FLUGA therapy impacted the ORR. Moreover, multivariate analyses showed that no variable was independently associated with achieving overall response after Bonferroni adjustment for multiple comparisons. Nevertheless, when we performed a subgroup analysis of treatment responders (defined as patients achieving overall response) via biological and genomic characteristics (Figure 1), we observed that patients with mutated DNM3A or EZH2 benefited from azacytidine.
Figure 1.
Subgroup analysis of responders to treatment via biological and genomic characteristics. OR: odds ratio, cytogenetic risk: low–intermediate vs. high risk as per ELN 2017 classification; high risk score was defined by the presence of mutated NRAS or TP53. A score predicting an AZA response was defined by the presence of mutated EZH2, U2AF1, DNMT3A, or TET2 genes. Patients with baseline mutations in DNMT3A (odds ratio (OR) 0.20, p = 0.023) or a score predicting AZA response (OR 0.448, p = 0.046) could benefit from azacytidine.
We defined a molecular signature that predicts response to azacytidine based on the presence of mutations in EZH2, U2AF1, DNMT3A, or TET2 (Figure 1). This signature was selected based on our previous data and descriptions in the literature (e.g., TET2). This signature was detected in 106 cases, of which 25/48 patients in the AZA arm (52%) and 19/58 (33%) in the FLUGA arm achieved OR (p = 0.044). The signature predicted OR after the third azacytidine cycle with a sensitivity of 64% and a specificity of 60%, and positive and negative likelihood ratios of 1.6 and 0.6, respectively. Likewise, the signature predicted CR after the third azacytidine cycle with a sensitivity of 78% and specificity of 59%, and positive and negative likelihood ratios of 1.9 and 0.4, respectively.
3.2. The Higher Variant Allele Frequency of Some Variants Influences Overall Response after the Third Cycle of Azacytidine and LDAC Plus Fludarabine
In our cohort, the median variant allele frequency (VAF) was higher in the variants detected in ASXL1, DNMT3A, RUNX1, SRSF2, TET2, and TP53 genes, while genes implicated in signaling pathways (CALR, CBL, EPOR, KIT, KRAS, MPL, NRAS, and THPO), such as KDM6A, PHF6, and SH2B3, showed lower median VAFs (Figure S6).
Univariate analysis revealed that the VAF distribution was significantly different between responders and non-responders in both treatment arms (AZA arm, VAF-TET2 (20% vs. 8.7%, p = 0.022) and VAF-TP53 (5.5% vs. 27%, p = 0.015); FLUGA arm, VAF-NRAS (1.2% vs. 6.7%, p = 0.009) and VAF-TP53 (8.2% vs. 19.6%, p = 0.046) (Figure S6). No differences in VAF were observed in the remainder of the genes in the panel.
Treatment-adjusted logistic regression analysis identified the following predictive markers as associated with a higher OR after the third cycle of azacytidine or FLUGA: increases in TET2 VAF (OR 1.01; 95%CI: 1.001–1.026; p = 0.030) and decreases in TP53 VAF (OR 0.98; 95%CI: 0.969–0.994; p = 0.003).
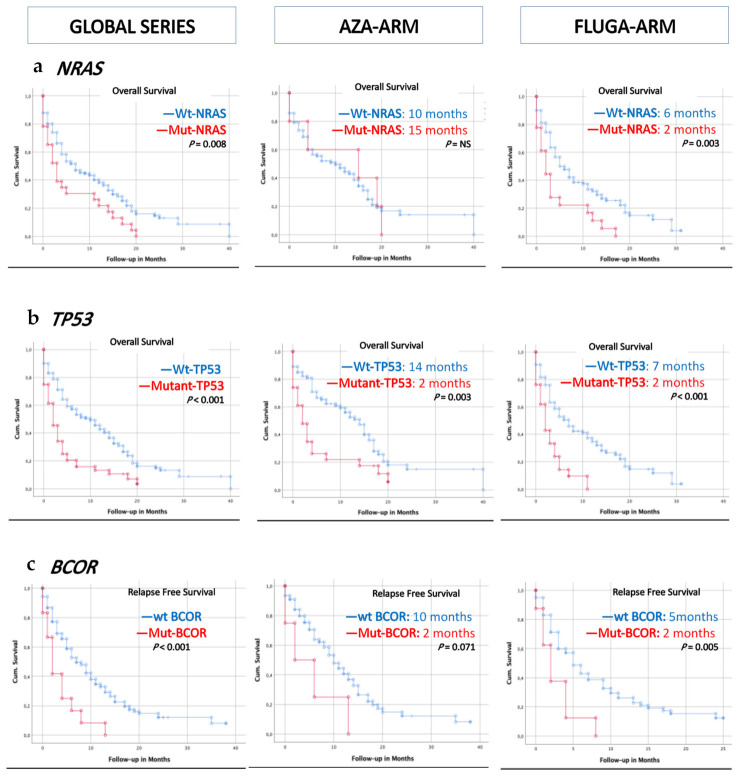
3.3. Somatic Mutations in NRAS, TP53, and BCOR Predict a Shorter Overall and/or Relapse-Free Survival According to Univariate Analyses
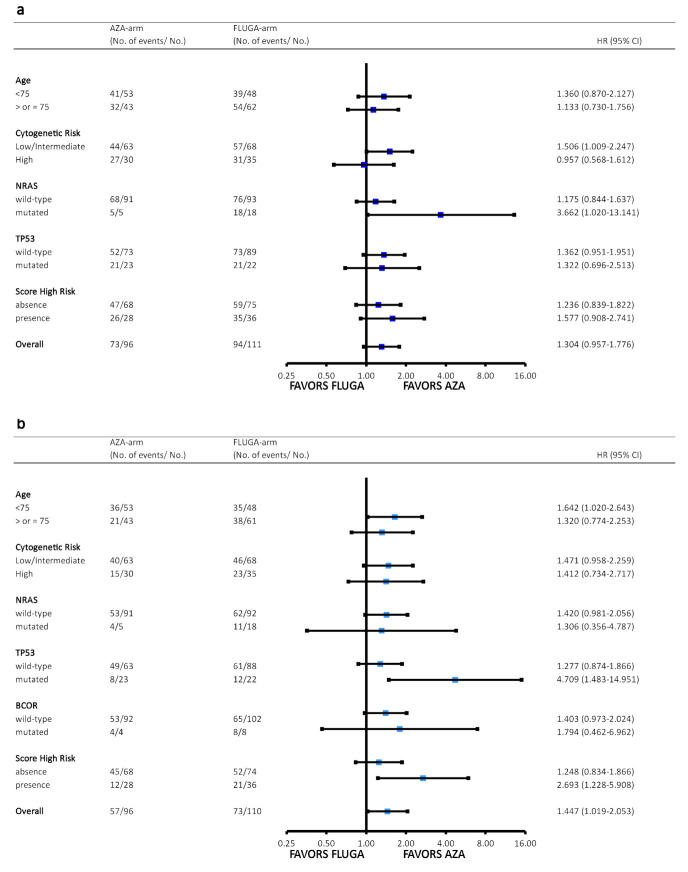
The median OS in the global series was 15 months (range 1–38). The median OS within treatment arms, in terms of mutant vs. wild-type for those genes mutated in ≥4% of patients, is shown in Table S5. According to the univariate analyses, NRAS and TP53 mutations were factors adversely affecting OS (Figure 2a,b), and BCOR mutations adversely affected RFS (Figure 2c). In the subgroup analysis of OS via biological and genomic characteristics (Figure 3a), we observed that patients with low–intermediate cytogenetic risk and mutated NRAS benefited from azacytidine therapy. However, in the subgroup analysis of RFS (Figure 3b), patients with mutated TP53 displayed a better RFS in the AZA arm. The difference in the mutational profile between the two arms is shown in Table S6 and Figure S1b.
Figure 2.
Mutation status has prognostic significance for overall survival and relapse-free survival. In the global series, the AZA arm’s and FLUGA arm’s overall survival and disease free-survival are represented by Kaplan–Meier plots. Wild-type status is indicated in blue and mutated status is indicated in red. NRAS mutations (a) and TP53 mutations (b) are adverse factors affecting overall survival in the FLUGA arm. BCOR mutations are adverse factors affecting relapse-free survival in the AZA and FLUGA arms (c). AZA: azacytidine, FLUGA: fludarabine plus low-dose cytarabine (LDAC).
Figure 3.
Subgroup analysis of overall survival and progression-free survival via biological and genomic characteristics. HR: hazard ratio, cytogenetic risk: low–intermediate vs. high risk as per ELN 2017 classification; a high-risk score was defined by the presence of mutated NRAS or TP53. (a) in the subgroup analyses of overall survival via biological and genomic characteristics, we observed that patients with low–intermediate cytogenetic risk (hazard ratio (HR0 1.51, p = 0.045) and mutated NRAS (HR 3.66, p = 0.047) could benefit from azacytidine. (b) in the subgroup analyses of relapse-free survival, we observed that patients with mutated TP53 (HR 4.71, p = 0.009) and scores indicating a high risk for AML (HR 2.69, p = 0.013) showed a higher chance of relapse-free survival under the AZA arm.
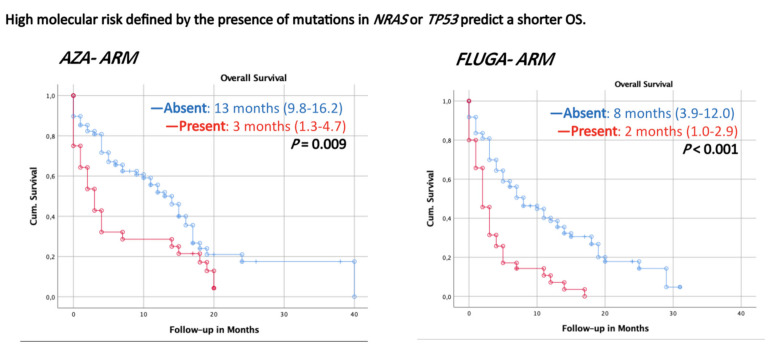
We identified the presence of mutations in NRAS or TP53 as a high molecular risk signature, which was detected in 64 patients (31%). In the AZA arm, seven patients (25%) showing high molecular risk achieved an OR after the third cycle, compared with only three patients (8%) in the FLUGA arm (p = NS). The high molecular risk condition was associated with unfavorable outcomes and shorter survival times in both arms for OS (Figure 4). The signature predicted OS with a sensitivity of 37% and specificity of 93%, and positive and negative likelihood ratios of 5.29 and 0.68, respectively.
Figure 4.
Kaplan–Meier overall survival curves in acute myeloid leukemia (AML) patients classified by the presence or absence of high molecular risk score (HMR). HMR pattern defined by presence of NRAS or TP53 mutations which is associated with unfavorable outcomes and shorter survival after azacytidine (left) or FLUGA (right) schemes. HMR absent is indicated in blue and HMR present is indicated in red. Number of censored patients with respect to the stratified groups and the number at risk is indicated. p values are considered significant (p < 0.05). OS: overall survival.
Mutated NRAS (HR 1.94, p = 0.005) and TP53 (HR 2.57, p < 0.0001) were the only variables associated with a higher risk of death; notably, mutated BCOR was the only variable associated with a higher risk of relapse (HR 3.60, p = 0.0003) (Table 2). No other variables were identified as predictors of death or leukemia relapse, and no other differences were observed in the Cox multivariate analysis adjusted by treatment. Data of Cox univariate analyses is showed in Table S7.
Table 2.
Biomarkers consistently associated with death or relapse.
| Variable | HR | Risk of Death 95% CI for HR | p-Value (Bonferroni) | |
|---|---|---|---|---|
| Lower | Upper | |||
| NRAS (wt vs. mut) | 1.94 | 1.21 | 3.08 | 0.005 (0.067) |
| TP53 (wt vs. mut) | 2.57 | 1.76 | 3.76 | 9.8 × 10−7 (0.128 × 10−5) |
| Variable | HR | Risk of Relapse 95% CI for HR | p-Value (Bonferroni) | |
| Lower | Upper | |||
| BCOR (wt vs. mut) | 3.60 | 1.81 | 7.16 | 0.000271 (0.004) |
Biomarkers identified with an adjusted Cox regression analysis (model; p ≤ 0.05) were included in the table. Cox regression model was adjusted for age, cytogenetic risk group (low–intermediate/high risk, classification ELN 2017), AZA or FLUGA arm, and presence of mutations in any gene included in the panel. Analyses based on 194 patients and 157 events for overall survival, and 194 patients and 124 events for relapse-free survival. Wt: wild-type, mut: mutated, HR: hazard ratio, CI: confidence interval; p-value is adjusted by the Bonferroni criteria in parenthesis.
4. Discussion
Using NGS-based molecular profiling, we have identified a subset of older patients who benefited from azacytidine therapy within the FLUGAZA clinical trial, which compared azacytidine with LDAC plus fludarabin (FLUGA). We have shown that azacytidine is more effective at achieving responses in older patients who present with mutations in EZH2 or DNMT3A at the time of diagnosis. On the other hand, NRAS and TP53 mutations were adverse prognostic factors in the context of OS, and BCOR mutations conferred a high risk of leukemia relapse, regardless of treatment received. Patients with low–intermediate cytogenetic risk and mutated NRAS benefited from azacytidine therapy for OS, and patients with mutated TP53 also had a better RFS when receiving azacytidine.
The influence of NRAS mutations in response to LDAC has been reported previously; for example, Bloomfield and colleagues showed that patients with AML carrying mutant RAS benefit from high-dose cytarabine (HDAC) consolidation more so than patients with wild-type RAS [15], and they also exhibit a lower relapse risk (HR 0.28, p = 0.002) than patients with mutant RAS treated with LDAC. However, NRAS mutations also confer resistance to newly targeted drugs, including enasidenib, an IDH2 inhibitor approved for use in refractory/relapsed AML [16]. Indeed, the co-occurrence of NRAS and IDH2 mutations with other MAPK pathway effects was enriched in non-responders, which was consistent with RAS signaling contributing to primary therapeutic resistance. A similar outcome was also seen in clinical trials of FLT3 inhibitors, wherein NRAS mutations were enriched in poor responders to crenolanib or gilteritinib [17,18]. We showed here that NRAS mutations confer resistance to LDAC squeme, and patients with these mutations benefited from azacytidine therapy.
We found that TP53 mutations had no impact on the responses of patients in the context of treatment received, which contradicts the study that described an association between TP53 mutations and response to 10-day decitabine [8], and which showed higher response rates in mutated TP53 than wild-type TP53 patients (100% vs. 41%, p < 0.001), although the responses were not long-lasting. However, we derived very few responses from the patients in this trial with mutated TP53. Some authors doubt the utility of these predictive biomarkers as improved response rates do not translate to improved survival [19]. However, we also found shorter OS for patients with NRAS or TP53 mutations, and the effects of NRAS and TP53 have previously been described with conventional care regimens [20].
We have defined a molecular signature for identifying responders to azacytidine therapy, characterized by the presence of mutations in DNMT3A, TET2, EZH2, or U2AF1. In this context, mutations in TET2 have previously been associated with responses to HMAs in MDS, or in AML with 20–30% blasts [7,21]. Likewise, via a genomics-informed computation biology platform recently developed to predict responses to HMAs in 18 patients with MDS/AML, the authors found that gain-of-function mutations in EZH2 and IDH1/2 were predictors of the response to the CpG-methylating effects of azacytidine via DNMT1 inhibition [22]. In contrast to our data, Jung and colleagues found that U2AF1 mutation was significantly associated with non-response to azacytidine in patients with MDS [23]. Our results, however, affirm those of Bejar and colleagues [7], and the response maintained in the ninth cycle was associated with not only the presence of TET2 mutations, but also the absence of TP53 mutations.
BCOR mutations were associated with reduced RFS in both arms of the FLUGAZA clinical trial. BCOR encodes a transcription regulatory factor that controls myeloid proliferation and differentiation. The impacts of BCOR or BCORL1 mutations have previously been described in 28 of the 377 de novo AML cases (7%) [24], and were independent unfavorable prognostic factors in both OS (p = 0.004) and RFS (p = 0.046).
Our study has some limitations that need to be considered. First, not all the patients included in the trial had samples available for NGS analysis, causing a potential selection bias. Furthermore, CEBPA mutations were identified by NGS, and were not classified separately as monoallelic or biallelic mutations. Finally, the presence of mutations in some genes was infrequent (e.g., FLT3-ITD and CEBPA), and the impact of the co-occurrence of gene mutations was not evaluated due to the low number of cases studied.
Venetoclax plus azacytidine or LDAC is being increasingly used in patients with AML who are >75 years of age and/or not candidates for intensive remission induction therapy. It achieves good CR/CRi rates in high-risk patients, such as those with poor cytogenetics [25], or with RUNX1 and ASXL1 mutations [26]. In addition, mutations in TP53 or NRAS also result in shorter OS in relapse/refractory AML treated with venetoclax-based therapy [26]. Based on our results, we would recommend venetoclax plus azacytidine over venetoclax plus LDAC for patients with TP53 or NRAS mutations. We believe that the favorable profile defined here for azacytidine response could also be useful for identifying good responders to an azacytidine plus venetoclax regimen, but this should be confirmed further.
In conclusion, the mutational profile of AML in elderly patients is different from that described in young patients, and the prognostic and predictive markers are also different in this older population. We have defined molecular signatures for identifying patients with poor prognosis associated with azacytidine- or LDAC-based regimens (Table 3). The NGS analysis of targeted gene panels seems useful for identifying prognostic factors and predicting responses in elderly patients with AML.
Table 3.
Biomarkers identified in a phase 3 AML trial.
| Parameter | Global Series | Favors AZA-Arm * |
|---|---|---|
| Predictive markers for response to treatment |
Lower patient age
Wildtype TP53 Mutated KMT2A, NF1 or TET2 |
Mutated DNMT3A Presence Score predicting an AZA response |
| Prognostic markers for OS | Mutated NRAS or TP53 confer adverse prognostic for OS | Mutated NRAS Low-Intermediate Cytogenetic Risk. |
| Prognostic markers for RFS | Mutated BCOR confers adverse prognostic for RFS | Mutated TP53
Presence high molecular risk (HMR) |
Summary table of biomarkers identified in a phase 3 AML trial of azacitidine (AZA) vs. low dose cytarabine plus fludarabine (FLUGA). * Green: predictors of favorable response. Red: predictors of adverse outcome. HMR: high-molecular-risk pattern defined by the presence of NRAS and/or TP53 mutations. Score predicting an AZA response defined by presence of DNMT3A, TET2, EZH2, or U2AF1 mutations * subtypes which benefit from the AZA arm vs. LDAC+ fludarabine based on biological and genomic characteristics. AML: acute myeloid leukemia.
Acknowledgments
The authors would like to thank all of the physicians, laboratory staff, and data managers from the hospitals participating in the PETHEMA protocols, as well as David Lora (Imas12, CIBERESP, UCM) and Juan Manuel de la Rosa (Imas12-CNIO) for statistical support.
Abbreviations
AML = acute myeloid leukemia; AZA = azacytidine; CI = confidence interval; COSMIC = Catalogue of Somatic Mutations in Cancer; CR = complete remission; Dau = daunorubicin; dbSNP = single nucleotide polymorphisms database; Dec = decitabine; DFS = disease-free survival; ELN = European Leukaemia Net; Flu = fludarabine; FLUGA = fludarabin plus low-dose cytarabine; HR = hazard ratio; Ida = idarubicin; Indels = insertions or deletions; LDAC = low-dose cytarabine; MDS = myelodysplastic syndrome; NGS = next-generation sequencing; OR = odds Ratio; ORR = overall response ratio; OS = overall survival; PETHEMA = Programa español de tratamientos hematológicos (Spanish Group of Hematology Treatments); SNV = single nucleotide variants; VAF = variant allele frequency.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13102458/s1, Figure S1: the landscape of mutated gene in elderly patients; Figure S2: Kaplan-Meier analyses-OS-Conventional genomic aberrations. No conventional molecular marker with prognostic impact on overall survival was detected, Figure S3: Kaplan-Meier analyses-OS-Conventional genomic aberrations. Cytogenetic risk predicts overall survival in elderly patients with AML in AZA-arm, Figure S4: Mutational landscape in responders and no responders after 3rd Cycle, Figure S5: Kaplan-Meier analyses-OS: Achieving OR after 3rd cycle, Figure S6: VAF distribution of gene variants, Figure S7: Differential distribution of VAF-gene mutations between responders and non-responders, Table S1: Patient characteristics, Table S2: Comparison of the 207 patients vs. 78 not included, Table S3: Logistic regression analysis. Response defined as CR after 3rd cycle in the global series in function of characteristics of patients with AML on the treatment arm, Table S4: Logistic regression analysis. Response defined as overall response after 3rd cycle in the global series in function of characteristics of patients with AML on the treatment arm, Table S5: Median OS in function mutant versus wild-type gene, Table S6: Mutated genes in FLUGAZA clinical trial (AZA vs FLUGA), Table S7: Univariate Cox Regression Analysis for OS and RFS, Table S8: Variants included in this study.
Author Contributions
I.R., E.O., G.C.-T., and E.B. collected samples, performed experiments, analyzed and interpreted data, and wrote the manuscript. D.M.-C. and B.P. analyzed and interpreted data. J.M.B., S.V., J.L.A., M.T., P.M. (Pilar Martinez), J.S., P.H., F.R., O.S., E.L., C.G., J.L.L.L., M.B.V., F.P., J.L., J.F.F., M.J.S., and M.Á.S. collected samples and clinical data. R.A., J.M.-L., and P.M. (Pau Montesinos) designed and supervised the research and experiments, analyzed and interpreted data, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Centro de Investigación Biomédica en Red—Área de Oncología–del Instituto de Salud Carlos III (CIBERONC; CB16/12/00369) and the Subdirección General de Investigación Sanitaria (Instituto de Salud Carlos III, Spain) grants PI16/01530, PI16/01661, PI19/01518, and PI19/00730, the CRIS against Cancer foundation, grant 2018/001, and by the Instituto de Investigación Hospital 12 de Octubre (IMAS12) (co-financed by FEDER funds). The study was supported internationally by Cancer Research UK, FCAECC and AIRC under the Accelerator Award Program.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Hospital La Fe, Valencia, SPAIN (Nº EUDRACT=2014-000319-15; accessed on 13 June 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All supporting data are included in the manuscript and Supplemental Materials. The data discussed in this publication have been deposited in the NCBI Sequence Read Archive (SRA) and are accessible with the project ID: PRJNA655113. Additional data are available upon reasonable request from the corresponding author.
Conflicts of Interest
M.T. declares honoraria for lectures from Celgene, Pfizer, Novartis, Janssen, Merck Sharp. Dohme (MSD), Daiichi, and Servier SL, and membership on advisory boards with Celgene, Novartis, Roche, and Astellas. J.S. declares honoraria for lectures, and membership on advisory boards with Daiichi Sankyo, Pfizer, Celgene, Novartis, Roche, and Amgen. F.R. declares travel grants from Celgene, Novartis, Amgen, AbbVie, Janssen, Roche, MSD, and Daiichi Sankyo; consulting fees from Celgene, Novartis, Amgen, and AbbVie; and advisory board membership with, as well as research grants from, Celgene. E.L. declares honoraria for lectures from Janssen and Novartis, and advisory board membership with Janssen, Celgene, Astellas, and Amgen. M.B.V. declares honoraria for lectures from, and membership on advisory boards with, Janssen, BMS, Novartis, Roche, Astellas Pharma, and Jazz Pharmaceuticals. C.G. declares honoraria for lectures from Celgene, Amgen, Janssen, and Pfizer, and advisory board membership with Celgene. B.P. declares honoraria for lectures from, and membership on advisory boards with, Amgen, Bristol Myers Squibb (BMS), Celgene, Janssen, Merck, Novartis, Roche, and Sanofi; unrestricted grants from Celgene, EngMab, Sanofi, and Takeda; and consultancy for Celgene, Janssen, Sanofi, and Takeda. M.A.S. declares a consulting or advisory role for Teva, Daiichi Sankyo, Orsenix, AbbVie, Novartis, and Pfizer. J.M.-L.. declares honoraria for lectures from, and membership on advisory boards with, Janssen, BMS, Sanofi, Novartis, Incyte, Roche, and Amgen; and membership on the boards of directors of Hosea and Altum Sequencing. P.M. declares advisory board and speaker’s bureau membership with, as well as research support from, AbbVie, Janssen, Novartis, Pfizer, and Teva; research support, being a consultant for, and speaker’s bureau and advisory board membership with Astellas, Celgene, and Daiichi Sankyo; being a consultant for Agios, Tolero Pharmaceutical, Glycomimetics, and Forma Therapeutics; speaker’s bureau and advisory board membership with Incyte; and research support from, and advisory board membership with, Karyopharm. The remaining authors declare no competing financial interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buccisano F., Dillon R., Freeman S.D., Venditti A. Role of Minimal (Measurable) Residual Disease Assessment in Older Patients with Acute Myeloid Leukemia. Cancers. 2018;10:215. doi: 10.3390/cancers10070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juliusson G., Antunovic P., Derolf Å., Lehmann S., Möllgård L., Stockelberg D., Tidefelt U., Wahlin A., Höglund M. Age and Acute Myeloid Leukemia: Real World Data on Decision to Treat and Outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 3.Burnett A.K., Milligan D., Prentice A.G., Goldstone A.H., McMullin MD M.F., Phil R.K.H., Phil K.W. A Comparison of Low-dose Cytarabine and Hydroxyurea with or without all-Trans Retinoic Acid for Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome in Patients not Considered Fit for Intensive Treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 4.Fenaux P., Mufti G.J., Hellström-Lindberg E., Santini V., Gattermann N., Germing U., Sanz G., List A.F., Gore S., Seymour J.F., et al. Azacitidine Prolongs Overall Survival Compared With Conventional Care Regimens in Elderly Patients With Low Bone Marrow Blast Count Acute Myeloid Leukemia. J. Clin. Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 5.Williams S., Nanah R., Zblewski D., Elliott M., Hogan W.J., Tibes R., Foran J.M., Litzow M.R., Al-Kali A. Deficiency of Current Acute Myeloid Leukemia (AML) Response Criteria to Predict Response to Hypomethylating Agent Therapy: The Value of Long-Lasting Stable Disease. Blood. 2016;128:2799. doi: 10.1182/blood.V128.22.2799.2799. [DOI] [Google Scholar]
- 6.Kantarjian H., Kadia T., DiNardo C., Daver N., Borthakur G., Jabbour E., Garcia-Manero G., Konopleva M., Ravandi F. Acute myeloid Leukemia: Current Progress and Future Directions. Blood Cancer J. 2021;11:1–25. doi: 10.1038/s41408-021-00425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejar R., Lord A., Stevenson K., Bar-Natan M., Pérez-Ladaga A., Zaneveld J., Wang H., Caughey B., Stojanov P., Getz G., et al. TET2 Mutations Predict Response to Hypomethylating Agents in Myelodysplastic Syndrome Patients. Blood. 2014;124:2705–2712. doi: 10.1182/blood-2014-06-582809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch J.S., Petti A.A., Miller C.A., Fronick C.C., O’Laughlin M., Fulton R.S., Wilson R.K., Baty J.D., Duncavage E.J., Tandon B., et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N. Engl. J. Med. 2016;375:2023–2036. doi: 10.1056/NEJMoa1605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Döhner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T., Dombret H., Ebert B.L., Fenaux P., Larson R.A., et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vives S., Martínez-Cuadrón D., Burgues J.B., Algarra L., Tormo M., Martínez-Sánchez M.P., Serrano J., Herrera P., Ramos F., Salamero O., et al. A Phase 3 Trial of Azacitidine Versus a Semi-Intensive Fludarabine and Cytarabine Schedule in Older Patients with Untreated Acute Myeloid Leukemia. Cancer. 2021 doi: 10.1002/cncr.33403. [DOI] [PubMed] [Google Scholar]
- 11.Simoes C., Paiva B., Martínez-Cuadrón D., Bergua J.-M., Vives S., Algarra L., Tormo M., Martinez P., Serrano J., Herrera P., et al. Measurable Residual Disease in Elderly Acute Myeloid Leukemia: Results from the PETHEMA-FLUGAZA Phase 3 Clinical Trial. Blood Adv. 2021;5:760–770. doi: 10.1182/bloodadvances.2020003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onecha E., Linares M., Rapado I., Ruiz-Heredia Y., Martinez-Sanchez P., Cedena T., Pratcorona M., Oteyza J.P., Herrera P., Barragan E., et al. A Novel Deep Targeted Sequencing Method for Minimal Residual Disease Monitoring in Acute Myeloid Leukemia. Haematologica. 2018;104:288–296. doi: 10.3324/haematol.2018.194712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onecha E., Ruiz-Heredia Y., Martínez-Cuadrón D., Barragán E., Martinez-Sanchez P., Linares M., Rapado I., Oteyza J.P., Magro E., Herrera P., et al. Improving the Prediction of Acute Myeloid Leukaemia Outcomes by Complementing Mutational Profiling with ex Vivo Chemosensitivity. Br. J. Haematol. 2020;189:672–683. doi: 10.1111/bjh.16432. [DOI] [PubMed] [Google Scholar]
- 14.Onecha E., Rapado I., Morales M.L., Carreño-Tarragona G., Martinez-Sanchez P., Gutierrez X., Pina J.M.S., Linares M., Gallardo M., Martinez-López J., et al. Monitoring of Clonal Evolution of Acute Myeloid Leukemia Identifies the Leukemia Subtype, Clinical Outcome and Potential New Drug Targets for Post-Remission Strategies or Relapse. Haematologica. 2020 doi: 10.3324/haematol.2020.254623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neubauer A., Maharry K., Mrózek K., Thiede C., Marcucci G., Paschka P., Mayer R.J., Larson R.A., Liu E.T., Bloomfield C.D. Patients With Acute Myeloid Leukemia and RAS Mutations Benefit Most From Postremission High-Dose Cytarabine: A Cancer and Leukemia Group B Study. J. Clin. Oncol. 2008;26:4603–4609. doi: 10.1200/JCO.2007.14.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amatangelo M.D., Quek L., Shih A., Stein E.M., Roshal M., David M.D., Marteyn B., Farnoud N.R., De Botton S., Bernard O.A., et al. Enasidenib Induces Acute Myeloid Leukemia Cell Differentiation to Promote Clinical Response. Blood. 2017;130:732–741. doi: 10.1182/blood-2017-04-779447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H., Savage S., Schultz A.R., Bottomly D., White L., Segerdell E., Wilmot B., McWeeney S.K., Eide C.A., Nechiporuk T., et al. Clinical Resistance to Crenolanib in Acute Myeloid Leukemia due to Diverse Molecular Mechanisms. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-018-08263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon C.M., Ferng T., Canaani J., Wang E.S., Morrissette J.J., Eastburn D.J., Pellegrino M., Durruthy-Durruthy R., Watt C.D., Asthana S., et al. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discov. 2019;9:1050–1063. doi: 10.1158/2159-8290.CD-18-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuendgen A., Müller-Thomas C., Lauseker M., Haferlach T., Urbaniak P., Schroeder T., Brings C., Wulfert M., Meggendorfer M., Hildebrandt B., et al. Efficacy of Azacitidine is Independent of Molecular and Clinical Characteristics-an Analysis of 128 Patients with Myelodysplastic Syndromes or Acute Myeloid Leukemia and a Review of the Literature. Oncotarget. 2018;9:27882–27894. doi: 10.18632/oncotarget.25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Döhner H., Dolnik A., Tang L., Seymour J.F., Minden M.D., Stone R.M., Del Castillo T.B., Al-Ali H.K., Santini V., Vyas P., et al. Cytogenetics and Gene Mutations Influence Survival in Older Patients with Acute Myeloid Leukemia Treated with Azacitidine or Conventional Care. Leukemia. 2018;32:2546–2557. doi: 10.1038/s41375-018-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itzykson R., on behalf of the Groupe Francophone des Myelodysplasies (GFM) Kosmider O., Cluzeau T., Mas V.M.-D., Dreyfus F., Beyne-Rauzy O., Quesnel B., Vey N., Gelsi-Boyer V., et al. Impact of TET2 Mutations on Response Rate to Azacitidine in Myelodysplastic Syndromes and Low Blast Count Acute Myeloid Leukemias. Leukemia. 2011;25:1147–1152. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 22.Drusbosky L.M., Singh N.K., Hawkins K.E., Salan C., Turcotte M., Wise E.A., Meacham A., Vijay V., Anderson G.G., Kim C.C., et al. A Genomics-Informed Computational Biology Platform Prospectively Predicts Treatment Responses in AML and MDS Patients. Blood Adv. 2019;3:1837–1847. doi: 10.1182/bloodadvances.2018028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung S.-H., Kim Y.-J., Yim S.-H., Kim H.-J., Kwon Y.-R., Hur E.-H., Goo B.-K., Choi Y.-S., Lee S.H., Chung Y.-J., et al. Somatic Mutations Predict Outcomes of Hypomethylating Therapy in Patients with Myelodysplastic Syndrome. Oncotarget. 2016;7:55264–55275. doi: 10.18632/oncotarget.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terada K., Yamaguchi H., Ueki T., Usuki K., Kobayashi Y., Tajika K., Gomi S., Kurosawa S., Saito R., Furuta Y., et al. Usefulness of BCOR Gene Mutation as a Prognostic Factor in Acute Myeloid Leukemia with Intermediate Cytogenetic Prognosis. Genes Chromosom. Cancer. 2018;57:401–408. doi: 10.1002/gcc.22542. [DOI] [PubMed] [Google Scholar]
- 25.Dinardo C.D., Pratz K., Pullarkat V., Jonas B.A., Arellano M., Becker P.S., Frankfurt O., Konopleva M., Wei A.H., Kantarjian H.M., et al. Venetoclax Combined with Decitabine or Azacitidine in Treatment-Naive, Elderly Patients with Acute Myeloid Leukemia. Blood. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y.-W., Tsai C.-H., Lin C.-C., Tien F.-M., Chen Y.-W., Lin H.-Y., Yao M., Lin Y.-C., Cheng C.-L., Tang J.-L., et al. Cytogenetics and Mutations could Predict Outcome in Relapsed and Refractory Acute Myeloid Leukemia Patients Receiving BCL-2 Inhibitor Venetoclax. Ann. Hematol. 2020;99:501–511. doi: 10.1007/s00277-020-03911-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data are included in the manuscript and Supplemental Materials. The data discussed in this publication have been deposited in the NCBI Sequence Read Archive (SRA) and are accessible via the following link: https://www.ncbi.nlm.nih.gov/sra/PRJNA655113 (accessed on 6 August 2020). Additional data are available upon reasonable request from the corresponding author.
All supporting data are included in the manuscript and Supplemental Materials. The data discussed in this publication have been deposited in the NCBI Sequence Read Archive (SRA) and are accessible with the project ID: PRJNA655113. Additional data are available upon reasonable request from the corresponding author.