Abstract
Conifers have long been recognized for their therapeutic potential in different disorders. Alkaloids, terpenes and polyphenols are the most abundant naturally occurring phytochemicals in these plants. Here, we provide an overview of the phytochemistry and related commercial products obtained from conifers. The pharmacological actions of different phytochemicals present in conifers against bacterial and fungal infections, cancer, diabetes and cardiovascular diseases are also reviewed. Data obtained from experimental and clinical studies performed to date clearly underline that such compounds exert promising antioxidant effects, being able to inhibit cell damage, cancer growth, inflammation and the onset of neurodegenerative diseases. Therefore, an attempt has been made with the intent to highlight the importance of conifer-derived extracts for pharmacological purposes, with the support of relevant in vitro and in vivo experimental data. In short, this review comprehends the information published to date related to conifers’ phytochemicals and illustrates their potential role as drugs.
Keywords: conifers, phytoconstituent, oxidative stress, antibacterial, anti-inflammatory, anticancer, neurodegenerative
1. Introduction
Medicinal plants have long been used as a source for traditional remedies in nearly all cultures [1]. Nature provides an endless supply of novel phytochemicals, which are referred to as natural products (NPs), and natural product drug development is a difficult task for developing new leads [2]. Traditional medicines (TMs) are valuable because they use natural products; for example, Ayurveda, Kampo, traditional Chinese medicine (TCM), traditional Korean medicine (TKM) and Unani use natural products and have been practiced for thousands of years, blossoming into well-regulated medical systems [3]. As time passed and medication progressed, synthetic drugs, such as enoxaparin, aspirin, warfarin, ibuprofen, naproxen, clopidogrel and diclofenac, became available over the counter and were linked to mild (headaches, back pain) to severe side effects (difficulty breathing, excessive bleeding, and hemorrhage) [4]. There are no doubts that the use of natural products has markedly improved certain forms of cancer, diabetes, hypertension, pain, memory deficit, Alzheimer’s disease (AD), and migraine [5], and their further use should be continued in order to meet the urgent need for effective drugs to treat human diseases [6].
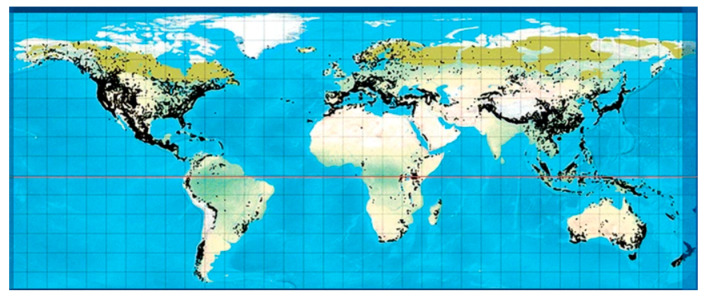
Coniferous plants, such as plants belonging to the Taxus, Cupressus, Picea, Pinus, Cedrus, Araucaria genera, are found worldwide and have shown several beneficial activities against diseases, highlighting the importance of conifers in drug development [7]. Conifers are woody, have needle-shaped single-veined leaves, and consist of male and female unisexual cones with bract scales [8]. They comprise eight families (Pinaceae, Araucariaceae, Cupressaceae, Podocarpaceae, Cephalotaxaceae, Taxaceae, Phyllocladaceae, Sciadopityaceae), 70 genera, and 630 species [9]. A number of genera include a vast number of species, such as Pinus (110), Podocarpus (105), Juniperus (55), Abies (50), Picea (35), Dacrydium (21), Taxodium (29) Pseudotsuga (22), Agathis (22), Araucaria (19), and Taxus (19), whereas there are some genera which contain a lower number of species, including Larix (10), Cedrus (4), Torreya (6) and Cryptomeria (1) [10]. They can be found in abundance in tropical lowland and submontane forests (Figure 1).
Figure 1.
Global distribution of all conifers [11].
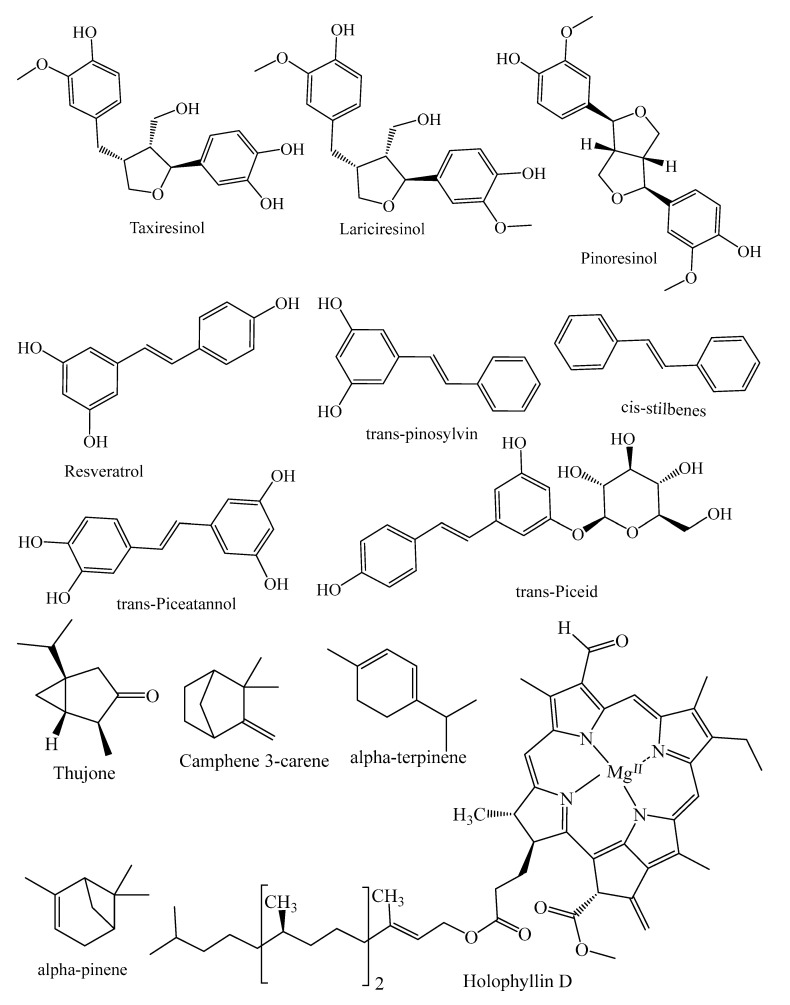
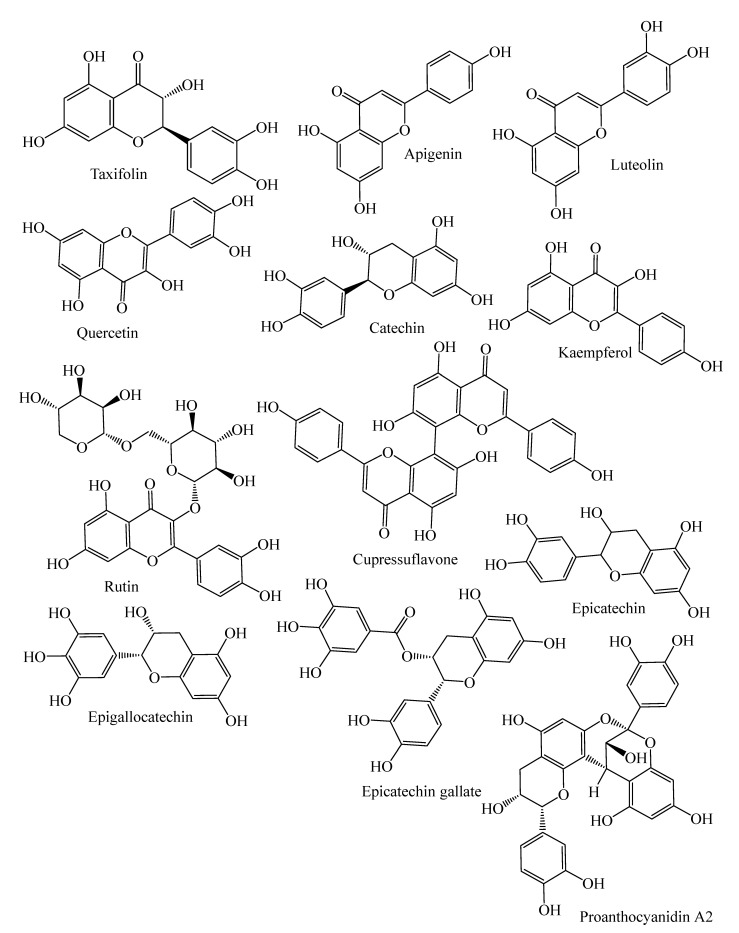
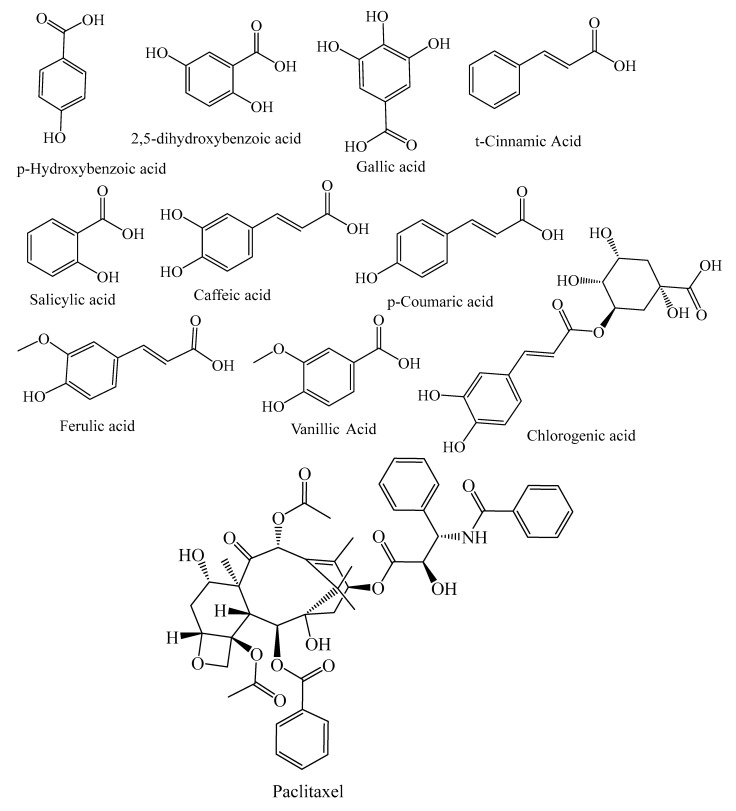
They contain secondary metabolites that combat pathogens and activate the plant’s defense mechanism [12]. The three major phytochemical groups, viz. terpenoids (resin acids and terpenes), alkaloids (piperidines) and polyphenols (phenolic acids, flavonoids, proanthocyanidins, lignans, acetophenones, and stilbenes) [13], present in the species of conifer trees are shown in Table 1, and their phytochemical compounds’ structures are shown in Figure 2. It is very important to understand the evolutionary pathway of Gymnosperms, in accordance with Sporne’s proposal, wherein the conifers represent the core, and the teachings that we can receive from it, comparing the morphological characters and their correlation with the chemical ones [14].
Table 1.
Phytochemical constituents present in conifers.
| Subclasss | Main Examples of Each Class | Conifer spp. | References |
|---|---|---|---|
| Terpenes | |||
| Monoterpenes | β-myrcene, α-pinene, limonene, α-terpinene, thujone, camphene, β-pinene, thujole, Δ-3-carene, phellandrene | Pinus roxburghii, P. pinea, P. wallichiana, P. pinaster, P.sylvestris, P. gerardiana, P. nigra, P. radiata, Thuja occidentalis, Abies alba, Picea abies, Metasequoia glyptostroboides | [12,15,16,17] |
| Sesquiterpenes | Laurenobiolide, farnesene, inulicin, vernodalin, 3H-benzofuaran-2-one, 4-methyl-3-methoxy-3H-benzofuaran-2-one, 4,9(α)-dihydroxynardosin-6-en, delta-cadinene, alpha-humulene, beta-cedrene, trans-caryophyllene, cubenol | P. mariana, Juniperus foetidissima, A. alba, P. abies, M. glyptostroboides, J. phoenicea, P. roxburghii | [8,12,18,19,20] |
| Diterpenes | Paclitaxel, 10-deacetylbaccatin III, tasumatrol B, taxodal, sandaracopimaric acid, taxodione, xanthoperol, andrographolide, gibberellin A8, 7α-hydroxysandaracopimaric acid, gibberellin A12, gibberellin A12 aldehyde, gibberellin A15, 14α-hydroxyisopimaric acid, 12-hydroxydehydroabietic acid, gibberellin A19, gibberellin A9, carnosol, lathyrol, E-communic acid, 15-hydroxy-8(17),13(E)-labdadiene-19-carboxilic acid, holophyllin A, holophyllin D, sugiol, ferruginol | Taxus brevifolia, T. baccata, T. globosa, T. distichum, P. mariana, J. taxifolia, M. glyptostroboides, A. holophylla, J. excelsa, J. communis, J. excelsa, J. communis | [18,19,21,22,23,24,25,26,27] |
| Nitrogen Compounds | |||
| Alkaloids | Vellosimine, 1,6-dehydropinidine, cis-pinidine, 1,6-dehydropinidinone, epipinidinone, cis-pinidinol, trans-pinidine, euphococcinine, α-pipecoline 1, (−)-pinidine | P. mariana, P. abies, P. sabiniana, P. torreyana, | [13,18,28] |
| Lignans | Lariciıesinol, taxiresinol, 3’-demethylisolariciresino1-9’-hydroxyisopropylethe, isolariciresinol, deoxypodophyllotoxin, (−)-secoisolariciresinol, 3, 3-demethylisolariciresinol, isotaxiresinol 2, α-conidendrin, (+)-pinoresinol, (−)-matairesinol, arctiin, dibenzylbutyrolactol, (−)-wikstromol, (−)-traxillagenin, (−)-arctigenin, traxillaside, 4′-deme-thyltraxillagenin, [(2R,3R)-2-(4′’-hydroxy-3′’-methoxybenzyl)-3-(4′-hydroxy-3′,5′dimethoxybenzyl)-butyrolactone] | T. baccata, J. taxifolia, J. sabina, J. virginiana, J. virginiana, P. roxburghii, Cedrus deodara, T. nucifera | [29,30,31,32,33,34,35] |
| Polyphenols: Flavonoids | |||
| Flavanonols | Taxifolin, cedeodarin | C. deodara, L. simbraca, P. roxburghii, P. mariana, P. abies, A. pindrow, A. excelsa; P. pinea, P. halepensis, P. pinaster, P. gerardiana | [33,36,37,38,39,40] |
| Flavones | Pilosanol B, luteolin, apigenin, apigenin 6-C-b-glucopyranoside | P. mariana, A. excelsa, P. abies, P. sylvestris, P. menziesii, P. menziesii, J. communis, A. angustifolia, L. deciduas | [18,38,39,41,42,43] |
| Biflavones | Bilobetin, cupressuflavone II-7-O-methyl-robustaflavone | T. wallichiana, C. macrocarpa, A. angustifolia | [43,44,45,46] |
| Flavonols | Quercetin, dihydroquercetin, rutin, kaempferol, dihydrokaempferol | J. communis, J. oxycedrus, P. gerardiana, P. roxburghii, P. wallichiana, A. angustifolia, P. abies, L. deciduas, P. sylvestris, P. menziesii, M. glyptostroboides, J. excelsa, P. mariana, J. foetidissima | [18,33,41,42,43,47,48,49,50] |
| Flavan-3-ols | Monomers: (−)-epicatechin, (−)-epicatechin-3-gallate, (+)-catechin, sennidin A, (−)-epigallocatechin, | P. pinaster, P. pinea, P. halepensis, P. roxburghii, P. wallichiana, P. gerardiana, J. foetidissima, A. angustifolia, P. abies, L. deciduas, P. sylvestris, J. communis, P. menziesii, J. oxycedrus, M. glyptostroboides, J. excelsa | [18,33,39,40,41,43,47,48,49,50] |
| Polymers: Procyanidin B1, B2, procyanidin A2, | P. halepensis, P. pinea, P. pinaster | [40] | |
| Phenolic acids | |||
| Benzoic acids | p-hydroxybenzoic acid, 2,5-dihydroxobenzoic acid, gallic acid, 4-hydroxybenzoic acid, protocatechuic acid, ellagic acid | P. abies, L. deciduas, P. sylvestris, P. menziesii, P. kesiya, J. communis, A. excelsa, P. roxburghii, P. wallichiana, P. gerardiana, L. deciduas, J. communis | [33,38,41] |
| Hydroxycinnamic acid | Caffeic acid, t-cinnamic Acid, p-coumaric acid, vanillic acid, ferulic acid, salicylic acid, sinapic acid, syringic acid, chlorogenic acid, 5-caffeoylquinic acid, caffeic acid 4-O-glucoside | P. abies, L. deciduas, P. sylvestris, T. baccata, P. mariana, P. pinaster, P. kesiya, L. deciduas, J. communis, P. menziesii, M. glyptostroboides | [18,21,39,41,42,49] |
| Stilbenes | trans-resveratrol, resveratrol, trans-pinosylvin, cis-stilbenes, pinosylvin, dihydro-monomethyl, trans-stilbenes, trans-piceatannol, trans-piceid, trans-isorhapontin, trans-isorhapontigenin, phenanthrenes, astringin, trans-astringin | P. mariana, P. abies, J. communis, P. pinaster, P. sylvestris, P. strobes, P. roxburghii, P. wallichiana, P. gerardiana, P. merkusii | [8,18,39,51,52,53,54,55,56,57] |
Figure 2.
Structures of phytochemical compounds present in different conifer spp.
It is also important to keep in mind the strong tendency towards adaptation, certainly not comparable with that of the subsequent Angiosperms, of which the dominance of the Araucaceae in the southern part of South America is a shining example [58]. Once again, it appears evident that the climatic situation constitutes the determining factor in the evolutionary path, as evidenced by the residual dominance of the conifers in the environments suited to them. There is therefore a lot to explore relating to conifers, and it is useful to develop studies entirely dedicated to individual genera [59].
From the chemotaxonomic point of view, it is necessary to highlight how difficult it is to study the chemistry of conifers due to the lack of easily usable markers [60]. In fact, resin and phenolic compounds, including tannins, have proved, due to their complicated composition and wide variability, difficult to study and not suitable for deducing clear considerations from them. First of all, we must not forget the unsuccessful attempts, the once well-developed branches that are now essentially exhausted, of which Ginkgo is a sensational example, to which we can add the genus Taxus [61]. It is no coincidence that these species are now a source of drugs of great therapeutic importance. It is therefore possible that there is still a lot to investigate and study on the chemistry of conifers. A starting point is represented by the collection of the current scientific knowledge recorded so far and the possible use of these data in the light of the most recent interpretations and possible therapeutically interesting utilizations. In this sense, the main focus of this review is to emphasize conifers’ phytochemical compounds with a broad range of applications and as a source of molecules for drug development.
2. Conifers Phytochemicals Components
2.1. Terpenes
Terpenes are isoprenoids not containing nitrogen and sulfur and seem to be the main and largest group of natural phytochemicals group in conifers [12]. The terpenoids can be classified as C5 (hemiterpenes), C15 (sesquiterpenes), C20 (diterpenes), C10 (monoterpenes), C25 (sesterpenes), C40 (tetraterpenes), C40 (polyterpenes), and C30 (triterpenes) on the basis of C5 units [62]. Table 1 represents the most common mono-, sesqui- and diterpenes present in conifers. In particular, monoterpenes have been extensively studied, especially for their antiviral properties. Further, Porres-Martínez et al. (2016) reported their biological activities, including the anti-inflammatory, anticancer, antioxidant, and neuroprotective effects [63]. However, taxol diterpene derived from Taxus spp. have potential against malaria and cancer [62]. Sesquiterpenes have antiseptic, antimicrobial and disinfectant properties [64]. Kopaczyk et al. (2020) showed that the antioxidant activity of terpenes can prevail over oxidative stress aggravated by internal and external stimuli [12].
2.2. Alkaloids
There are several classes of alkaloids which are classified on the basis of the heterocyclic ring system and biosynthetic precursor which are of great interest. The alkaloids comprise quinolizidines, indoles, tropanes, pyrrolidines, pyrrolizidines, imidazoles, piperidines and isoquinoline purines [65]. There are numerous studies on the biological activity and medicinal uses of alkaloids [66]. In addition, alkaloids have been shown to have antitumor, anti-hyperglycemic and antibacterial activities [13]. Virjamo et al. (2020) reported that among the piperidine compounds of P. abies, only 1,6-dehydropinidine exhibited antibacterial effects by using a larger number of strains, whereas cis-pinidine was revealed to be toxic for vertebrates, which may only act in defense against herbivores [13].
2.3. Polyphenols
Polyphenols are of major relevance and perform a range of functions from skeletal constituents in various tissues to pigmentation in many plant organs [67]. They act as natural antioxidants, being able to inhibit lipid peroxidation, carcinogenesis, antimicrobial activity, direct capillary constrictive action, phytohormones, and have also the ability to stabilize ascorbic acid [68]. Flavonoids (isoflavones, flavonols, flavanonols, flavones, tannins, flavanones, anthocyanidins), stilbenes (resveratrol), phenolic acids (hydroxybenzoic and hydroxycinnamic acids), lignans, can all be found in plants [69]. Polyphenols, especially flavonoids, such as rutin, quercetin, apigenin, and epicatechin, are widely found in conifers. The genera Araucaria, Pinus, Cedrus, etc. are reported for their antimicrobial, anticancer, antidiabetic, neuroprotective [43] and anti-inflammatory properties and can be used in the treatment of neurodegenerative diseases, as well as being helpful in reducing αβ toxicity and neuronal dysfunction [70].
2.3.1. Flavonoids
Flavonoids are the most abundant phenol group in nature, present in a wide range of conifers [71]. Flavonoids have a central three-ring structure, but the different subclasses vary due to the centrally located heterocyclic ring structure (C-ring), which connects the two benzene rings [72]. To date, more than 6000 flavonoids have been recorded in several studies from plants. Flavonoids are aglycones in their basic structure, but most of them are glycosides in plants [73]. The subclasses of flavonoids found in the leaves, barks and seeds of conifers are represented in Table 1.
2.3.2. Lignans
Lignans are phenylpropanoids dimers made up of two coniferyl or sinapyl alcohol units bound together at the tails [74]. Isolariciresinol, taxiresinol, lariciresinol, pinoresinol, and their glycosides are examples of such compounds. There is a growing interest in lignans, especially because of their chemotherapeutic ability [75]. The most commonly present lignin compounds in conifer spp. are shown in Table 1.
2.3.3. Stilbenes
Stilbenes are produced by a number of conifer species, including Pinus sylvestris and Picea abies. Briefly, stilbenes are phenolic compounds with a heterologous bridge connecting two aromatic rings [76]. Many other compounds, such as trans-pinosylvin, cis-stilbene, resveratrol and piceatannol, have been isolated from the barks of conifer species (Table 1). For stilbenes, excellent antimicrobial effects have been reported [71].
2.3.4. Tannins
Tannins are polyphenolic compounds that can be in a wide range of plants. Tannins are colored pigments, astringent and are characterized by a bitter taste [77]. Tannin-rich conifer bark extracts have antimicrobial properties and high potential in preventing lipids from oxidation in the liposome model [78]. As a result, the tannins can be divided into four main classes based on their structural characteristics: gallotannins, complex tannins, ellagitannins and condensed tannins [79]. Condensed tannins (CTs), also well-known as proanthocyanidins, are prevalent in P. abies and P. sylvestris tree bark [77]. Condensed tannins are considered as polymers or oligomers of flavan3-ol units connected by C-C bonds that are hydrolysis resistant [80]. Procyanidins (PCs) and prodelphinidins (PDs) are the most popular PAs. Catechin and other epicatechin units make up PCs. Epigallocatechin units make up PDs [81].
Tannins exhibit antioxidant activity through various pathways, including free radical scavenging, transition metal chelation, and inhibition of pro-oxidative enzymes [82], besides having the capability to bind and form complexes with proteins and other compounds, and being responsible for their biological activity [83]. Tannins also act as antimicrobial agents, inhibiting extracellular microbial enzymes, depriving microbial growth substrates, and exerting a direct action on microbial metabolism, such as the denaturation of cell membrane proteins [84]. In the food industry, they could be used as functional coatings, adhesives, preservatives and as flavor compounds [71]. In a study, pine and spruce bark-derived PA-rich extracts revealed good potential for use in the food industry to develop preservative agents and to prevent lipid peroxidation in food items containing fatty-acids [78].
3. Traditional Medicinal Uses
Since prehistoric times, coniferous plants have been used as a medicinal source. Plant-based research has received more attention in recent years, and the literature supports the possible use of medicinal plants in conventional processes [85]. T. orientalis leaves and stems are utilized in traditional medicine to cure nervous system disorders, insomnia, heart palpitations, hemorrhage and fever. Fresh cedar leaves steeped for seven days in a 60% alcohol solution are often used by traditional Chinese physicians to encourage hair growth [86]. Cupressus spp. leaves, cones, stem bark have also been revealed to be useful in the treatment of hemorrhoids, bleeding varicose veins, asthma cough, spasms, diarrhea, rheumatism, common colds, piles, urinary tract ailments and vaginal discharge [7]. Different parts of the Pinus spp. bark, leaf, cone, and resin are also prescribed to treat cold-influenza, cough, tuberculosis, and bronchitis as a diaphoretic, rubefacient, antiseptic, diuretic, stimulant and febrifuge, while resin is also used in wound healing and injury [87]. The extract from Juniperus spp. leaves, berries and bark has also been used for the treatment of chronic eczema, hyperglycemia, obesity, tuberculosis, bronchitis, and pneumonia. The female cones, wood and leaves of J. foetidissima, J. communis and J. excelsa are used as a tonic for gout and rheumatism, a carminative, a diuretic, a treatment for urinary tract infection and stomach ache, an expectorant, a stimulant, an emmenagogue, and a treatment for the common cold [7].
Different parts of Taxus spp. have precise ethnomedicinal uses; for example, the leaves’ juice is used to cure cancer and bronchitis; bark juice and other parts, such as the leaf, are used for asthma and bronchitis, while trunk oil and cones are used to treat sheep diseases, bad breath, halitosis, inflammatory diseases of the lower urinary tract, renal stones, urinary infection, rheumatism dyspeptic complaints, hemorrhoids and cancer [7,88], and powdered dried leaves are considered to be effective in epilepsy, asthma, headache, diarrhea, bronchitis and hiccoughs [89]. A decoction developed from the bark is used to relieve pain from the muscles, knees, and rheumatoid arthritis, whereas a decoction made from the leaves is used to treat liver issues [90].
4. Conifers Extracts Rich in Phytochemical with Putative Health Effects
4.1. Oxidative Stress
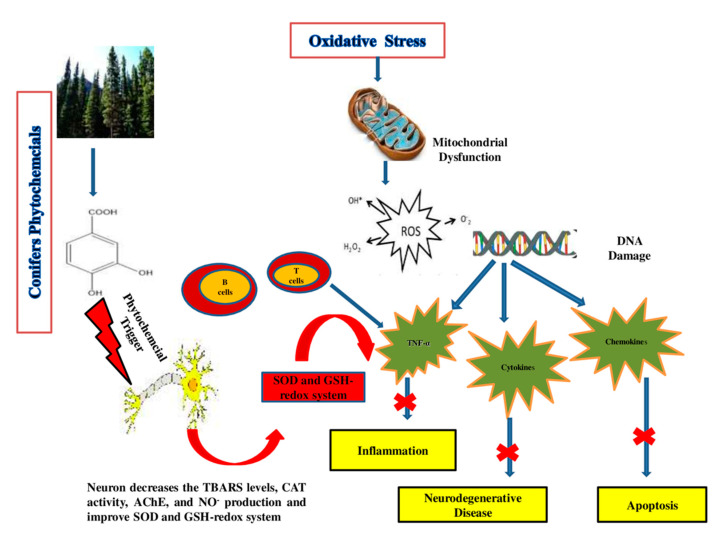
The role of oxidative stress in the progression of degenerative ageing is well understood. Diabetes mellitus, coronary heart disease, cancer, inflammation, stroke, neurological conditions (e.g., AD), and ageing have all been linked to reactive oxygen species (ROS) generation [49]. Both enzymatic and non-enzymatic reactions are involved in the synthesis of ROS. ROS are known to be generated by enzymatic reactions present in many cell processes, including the respiratory chain system, prostaglandin synthesis and phagocytosis [91]. All ROS are produced through enzymatic action, including NADPH oxidase, xanthine oxidase and peroxidase in many cell processes, in whom the superoxide radical (O2●−) is generated [92]. Different ROS, formed during enzymatic reactions and with the action of enzymes (amino acid oxidase and xanthine oxidase), include hypochlorous acid (HOCl), hydrogen peroxide (H2O2), peroxynitrite (ONOO-), and hydroxyl radicals (OH●) [93]. The “Fenton reaction” between O2− and H2O2 takes place in the presence of Fe2+ or Cu+, which work as catalysts, producing OH, the most reactive free radical species [94]. The production of ROS has been related to non-enzymatic interactions between oxygen and organic compounds, as well as when cells are in contact with ionizing radiation during mitochondrial respiration [94,95]. Extensive research is currently needed to discover phytochemical compounds with the ability to boost the immune system and reduce oxidative stress [96]. The quest for new antioxidant molecules is a crucial part of promoting healthy ageing and combating oxidative stress [93]. Flavonoids, phenolic acids, vitamins and carotenoids are examples of natural compounds with antioxidant potential that have antagonistic effects on degenerative and inflammatory processes throughout the body, have beneficial effects on the immune and digestive systems, prevent ROS-related chronic problems and improve the overall quality of life, as shown in Figure 3 [88].
Figure 3.
Action mechanism of conifers’ phytochemical compounds in oxidative stress, apoptosis, and neurodegenerative diseases. The phytochemicals’ multi-target effects in the brain include mitochondrial protection, anti-aggregation, anti-oxidant, anti-apoptotic and anti-inflammatory activity.
4.2. Synergism between ROS and other Diseases
The overproduction of ROS has been linked to a variety of chronic diseases, including cardiovascular, inflammatory and neurodegenerative diseases, and even cancer. The following sections explain on the relationship between ROS and chronic diseases.
4.2.1. Antioxidant Activity
In chronic obstructive pulmonary diseases (COPD), ROS play an important role [97]. The bark, wood, needle, and cone extracts of the Pinaceae family are high in polyphenolic compounds (Pycnogenol), primarily procyanidins, stilbenes, tannins and phenolic acids, and have significant antioxidant activity against ROS [98]. The antioxidant activity of conifer extracts has been confirmed by DPPH, FRAP and reducing power assays [51].
Flavonoids’ ability to scavenge free radicals can protect the human body from oxidative damage, which accelerates the ageing process [99]. Pycnogenol®, a polyphenol-rich compound extracted from the bark of P. pinaster, P. glauca, and P. mariana, has shown the ability to boost plasma antioxidant capacity and ameliorate pulmonary function and asthma traits [51,100]. In a study by Senthilmohan et al. (2003), 6–12 weeks of supplementation of Enzogenol®, and proanthocyanidin-rich flavonoid extracted from Pinus radiata bark in combination with vitamin C reduced DNA and protein oxidative damage in 55–75-year-old people [101]. In vivo studies have reported that the intake of quercetin alone did not protect DNA, but the combination of flavonoids (quercetin and myricetin) and isoflavonoids provides protection against DNA damage [102]. It has been found that the stilbenoid component resveratrol and piceatannol obtained from conifer spp. have more potent biological activities, namely as antioxidants [103]. Terpenoids and phenolic compounds, such as pinene, lycopene, camphene, gallocatechin, lutein, limonene and catechin found in the extract of conifer bark and needles have also been reported for their antioxidant potential by DPPH, FRAP, H2O2, ABTS assays, as shown in Table 2 [104].
Table 2.
Antioxidant capacity of extracts obtained from different conifer spp.
| Conifer spp. | Part Used | Compounds | Nature of Extract | Radical Scavenging Assay | Dose/Concentration | Main Effects | References |
|---|---|---|---|---|---|---|---|
| Aurocaria cookii | Leaves | Phenolic compounds | Methanol, chloroform, petroleum ether | DPPH | 1000 μg/mL | Methanol extract shows the best antioxidant activity with 63% inhibition, higher than the other two compounds | [147] |
| A. excelsa | Needle | Flavanoids | Methanol | DPPH | 50–72.5 μg/mL | Methanol/water extract shows antioxidant activity | [38] |
| C. deodara | Heart wood | Tannins, flavonoids, and phenolic compounds | Water/alcohol | DPPH, superoxide radical-scavenging activity, ABTS | DPPH-IC50 (μg/mL): 61.89 (water extract), 75.79 (alcohol extract) superoxide radical-scavenging activity— IC50 (μg/mL): 87.76 (water extract), 121.55 (alcohol extract). ABTS-IC50 (μg/mL): 115.29 (water extract), 122.42 (alcohol extract). |
DPPH radical-scavenging activity and the reducing power of C. deodara were potent in water and alcohol extract | [148] |
| C. japonica | All parts | Phenolic compounds | Methanol | ORAC, SOD |
4.09–7.64 TE/mg 3.63–4.06μg/mL |
The methanol extracts from each part of C. japonica except for pollen showed strong activities in the bioactivity assays. | [149] |
| J. communis | Berry | Flavanoids (quercetin rutin, apigenin) chlorogenic acid |
Alcohol/Water | DPPH | EC50 1.42 mg/mL against standard Ascorbic acidEC50 value of 0.365 mg/mL | The antioxidant activity was confirmed as 81.63 ± 0.38% by the DPPH assay. | [42] |
| L. laricina | Bark | Phenolic compounds | Ethanol/Water | ORAC | IC50 0.878 μg/mL. | Bark extract of LL shows significant antioxidant activity | [51] |
| Metasequoia glyptostroboides | Cone | Terpenoid | Ethyl acetate | DPPH, NO, superoxide, and H2O2 | 5–250 μg /mL | Sugiol derived from cone extract show good antioxidant activity—78.38, 72.42, 74.45 and 85.04%, respectively. | [26] |
| Picea abies | Bark | Atilbenoids | Ethanol/Water | DPPH | 49.74 μg/mL | UVA-induced modification of the stilbene-rich inner bark extracts increased the antioxidant activity as UVA irradiation decreased the capacity of the extracts to prevent lipid oxidation in the liposome system method | [53] |
| P. smithiana | Leaves | Phenolic compounds | Methanol | DPPH | IC50 (μg/mL)- | Results of the DPPH radical scavenging activity and FRAP study determine that methanol extracts of leaf displayed the highest antiradical efficiency | [150] |
| 228 | |||||||
| FRAP | 494 | ||||||
| Reducing Power assay | 978 | ||||||
| Pinus gerardiana | Bark | Phenolic compounds | Ethanol | DPPH | IC50 value μg/mL | P. gerardiana shows promising H2O2 radical scavenging activity | [104] |
| 102.8 | |||||||
| H2O2 | 81.83 | ||||||
| NO2 | 109.2 | ||||||
| P. halepensis | Bark | Phenolic compounds | Ethanol/Water | IC50 (μg/mL). Ethanol and the water | Ethanol and water extract of bark exhibited significant free radical neutralization capacities, at conc. 0.5–8 μg/mL | [151] | |
| DPPH | 3.28, 3.26 | ||||||
| ABTS | 3.1, 3.59 | ||||||
| P. pinaster | Bark | Phenolic compounds | Ethanol/Water | PB (50%) and (90%) IC50 value μg/mL | PP bark extracts formed from PB 50% (50% ethanol) have maximum (DPPH, ABTS) radical scavenging activity while FRAP shows activity with (PB 90%) | [39] | |
| DPPH | 49.74 | ||||||
| ABTS | 59.41 | ||||||
| FRAP | 101.3 | ||||||
| P. roxburghii | Bark | Phenolic compounds | Ethanol | IC50 value μg/mL | Pine extract shows significant antioxidant activity | [104] | |
| DPPH | 97.54 | ||||||
| H2O2 | 86.90 | ||||||
| NO2 | 111.38 | ||||||
| P. wallichiana | Bark | Phenolic compounds | Ethanol | IC50 (μg/mL) | Pine extract shows significant radical scavenging activity | [104] | |
| DPPH | 111.40 | ||||||
| H2O2 | 84.18 | ||||||
| NO2 | 98.5 | ||||||
| Thuja occidentalis | Leaves | Flavonoids, phenols | Methanol | DPPH, FRAP | 20–100 μg/mL | Crude extract shows significant antioxidant activity | [152] |
| T. occidentalis | Non-woody branches with leaves |
Polyphenol, flavonoids | Mother tincture (MT) | DPPH, ORAC, NO | 25 or 50 mg/kg | T. occidentalis mother tincture displayed 88.3% antioxidant activity by DPPH and about 78% by NO assay | [126] |
| Taxus baccata | Leaves and cones |
Flavonoids, phenols | Methanol | DPPH | IC50 (μg/mL) 105.41, 518.51 leaves and cones resp. | Acetone and ethyl acetate extract of leaves show good scavenging activity | [153] |
| Water | DPPH | 533.66, >1000 leaves and cones resp. | |||||
| Acetone | DPPH | 25.24, 81.43 leaves and cones resp. | |||||
| Ethyl acetate | DPPH | 29.84, 180.26 leaves and cones resp. | |||||
| Petroleum ether | DPPH | 438.92, > 1000 leaves and cones resp. | |||||
| T. wallichiana | Leaves | Terpenoids, flavonoids | IC50 values (μg/mL) | The maximum DPPH activity was observed in methanol extract (91.25%), followed by water (87.64%), ethanol (85.23%), and ethyl acetate (83.27%) at the highest concentration (700μg/ml) | [154] | ||
| Methanol | Superoxide radical | 170.30 | |||||
| DPPH | 212.00 | ||||||
| LPO | 126.09 | ||||||
| Hydroxyl radical | 82.34 | ||||||
| Ethyl acetate | Superoxide radical | 297.55 | |||||
| DPPH | 301.80 | ||||||
| LPO | 151.96 | ||||||
| Hydroxyl radical | 199.05 | ||||||
| Water | Superoxide radical | 257.00 | |||||
| DPPH | 258.29 | ||||||
| Hydroxyl radical | 175.33 | ||||||
| T. wallichiana | Leaf, stem | Polyphenols, flavanoids, terpenoids | Methanol | DPPH FRAP |
IC50 value (μg/mL.) Leaves (23.18) Stem (56.75) |
DPPH and FRAP activity of TW leaves and stem extract have high antioxidant activities. | [155] |
PB-Pine bark; TW-Taxus wallichiana.
4.2.2. Anti-Inflammatory Activity
When contagious microorganisms such as fungi, bacteria and viruses come into contact with the body, they remain in specific tissues and flow into the bloodstream, causing inflammation [105]. This also occurs as an end result of tissue damage, cancer, cell death, degeneration and ischemia [106,107,108]. In most cases, both the innate and adaptive immune responses are responsible for inflammation development [109]. The primary protection against invading foreign microbodies and cancer cells is the innate immune system, which involves macrophages, dendritic cells, and mast cells [105]. In the adaptive immune system, specialized cells (B and T cells) remove foreign pathogens and cancer cells by generating specific receptors and antibodies [110]. Cytokines such as interleukins, interferons, tumor necrosis factor, eicosanoids (leukotrienes and prostaglandins), modulatory inflammation-transcription nuclear factor (NF-ĸB) and chemokines (monocyte chemoattractant protein 1), are the inflammatory mediators and cellular pathways that have been extensively studied in relation to human pathological conditions [111]. Tumor necrosis factor-α (TNF) is a pro-inflammatory cytokine that is secreted by a variety of cells and has a variety of cellular effects [112]. It has also been linked to a variety of human illnesses, including cancer, mental and skin disorders, immune and inflammatory diseases. IL-1 is another cytokine that primarily has a pro-inflammatory effect [113]. It raises the levels of pro-inflammatory cytokines, including IL-1, TNF and IL-6 [114]. On the other hand, IL-1 has been linked to anti-inflammatory properties. Likewise, IL-1α and IL-6 originating from activated mast cells in the innate immune response also boost acute phase protein synthesis and thus show some anti-inflammatory effects [115]. The cytokine family members, including IL-12, IL-27, IL-23 and IL-35, function as a pro- and anti-inflammatory response [111,116,117]. On the other hand, IL-10 has been recognized as an effective anti-inflammatory cytokine, and helps in preventing several pro-inflammatory mediators from further action [118]. It protects tissue from homeostasis, defends against injury and damage caused by an overactive inflammatory response [118,119,120]. TNF-α accelerates PGE2 synthesis changes caused by edema and the flow of blood [46]. The extraction of plant materials is the first step in deciding the plant biological activities. The is a high probability of synergism between bioactive components when a whole extract is used, which could be lost if each and every component is isolated [121]. This form of synergism has been documented in numerous medical studies, generally for anti-inflammatory function [105]. There are different types of extraction and separation processes, such as:
1. Soxhlet extraction: The Soxhlet extraction method is a more efficient extraction method with high extraction yield and requires less solvent and time. This method requires electricity and solvents such as methanol, petroleum ether, and acetonitrile for the extraction process. However, sometimes high temperature and long extraction time enhance the possibility of thermal degradation and the loss of bioactive compound fraction activity [122].
2. Percolation: Extraction yield is better in percolation than maceration; in this process, pre-soaked plant material is added to a container, which allows the constantly controlled removal of the extract via a valve at the bottom and adding fresh solvent from the top.
3. Maceration: Maceration is carried out at room temperature by soaking the material with the solvent with eventual stirring. It has the advantage of moderate extraction conditions but suffers from high solvent consumption, long extraction times and low extraction yields. It could be used for the extraction of thermo labile components.
4. Ultrasound-assisted extraction: In UAE, the plant material, usually in a glass container, is covered by the extraction solvent and put into an ultrasonic bath. It decreases extraction time and improves extraction yields due to mechanical stress, which induces cavitation and cellular breakdown, and has gained increasing popularity [123]. For the isolation of extract from the solvent, the distillation process and many evaporators are used. After isolation to concentrate the extract, many researchers used a rotary evaporator, a normal air-drying process and distillation methods. Generally, to separate different solvent extracts, a separatory funnel is required [124].
Anti-inflammation is one of the main recorded effects of conifer phytochemicals among the numerous biological activities that have been studied so far. Table 3 reported the anti-inflammatory effect of conifer phytochemicals in in vivo and in vitro models.
Table 3.
Anti-inflammatory capacity of different conifers spp.
| Conifer spp. | Part Used | Nature of Extract | Compounds | Major Method(s) of Testing | Dose. Conc | Main Effect | References |
|---|---|---|---|---|---|---|---|
| Abies chensiensis | Twigs and leaves | Ethanol | Terpenoids | Induce lipopolysaccharide to produce inflammation in RAW 264.7 macrophage cells | 0.2–50.0 μM | 4 compounds—3α-hydroxyl-8,14,22Z,24-tetraenlanosta-26,23-olide; (5R,20R)-8(14→13R)-abeo-17,13-friedo-3-oxolanosta-8,14(30),22Z,24-tetraen-26,23-olide; 8,14,22Z,24-tetraen-3-oxolanosta-26,23-olide; and (23R, 25R)-3,4-seco-9β H-lanosta-4 (28),7-dien-16α-hydroxyl-26,23-olid-3-oate—extracted from extracts showed significant anti-inflammatory activities of inhibition against NO formation with IC50 value of 15.9, 18.7, 20.18, and 10.9 μM |
[125] |
| A. georgei | Aerial parts | Chloroform, ethyl acetate, n-butanol | Flavanoids | dimethylbenzene-induced ear oedema in mice | 200 mg/kg | AG ethyl acetate extract shows 18% inhibition against dimethylbenzene-induced ear edema in mice while carrageenin-induced paw edema in rats shows inhibition ratios 28.2% and 35.6%, after 2 and 6h, respectively. | [156] |
| Carrageenin-induced paw oedema rat | 140 mg/kg | ||||||
| A. webbiana | Leaves | Methanol/Petroleum ether extract | Flavanoids | Carrageenan-induced rat hind paw edema model in Albino mice | 400 mg/kg | Plant leaves extract possesses significant anti-inflammatory properties | [157] |
| Agathis robusta | Leaves | Methanol | Flavanoids, tannins and saponins | Heat induced hemolytic method in human red blood cell (HRBC) membrane | 400 μg/kg | Leaves extract shows good antiinflammatory activity | [158] |
| Cedrus deodara | Stem bark | Methanol | Deodarin, quercetin, taxifolin | Carrageenin-induced paw edema in Albino rat | 100 mg/kg | Anti-inflammatory activity with 43.47% inhibition | [159] |
| Cupressus macrocarpa | Leaves | Methanol | Cupressuflavone (CUF) | Carrageenan-induced paw edema model in Mice | 40, 80, and 160 mL/kg |
CUF demonstrated antiinflammatory activity by inhibiting paw edema with 55, 60, and 64%, by decreasing the plasma pro-inflammatory mediators PGE2, IL-6, TNF-a and IL-1b |
[46] |
| Juniperus communis | Berry | Alcohol/Water | Flavanoids (quercetin rutin, apigenin) chlorogenic acid | Acute-dextran and kaolin subacute inflammation induced in Wistar Rat | 10 mL/kg | The antiinflammatory action of the juniper extract, administered as a microemulsion in acute-dextran model was increased when compared to kaolin subacute inflammation induced model. | [42] |
| J. oxycedrus | Berry | Ethanol, n-butanol | Flavonoids (amentoflavone, cupressuflavone, hinokiflavone, and rutin) | Carrageenan-induced hind paw edema model in mice | 100 mg/kg | Ethanol extract of Joso berries displayed remarkable inflammatory inhibition ranging between 24.5% and 23.7% at 100 mg/kg in carrageenan-induced edema model | [160] |
| J. foetidissima | Berry | Ethanol | Flavonoids (amentoflavone, cupressuflavone, hinokiflavone, and rutin) | carrageenan-induced hind paw edema model in mice | 100 mg/kg | JFB extract at a dose of 100 mg/kg. shows high antiinflammatory effect 26.9% | [160] |
| Pinus gerardiana, P. roxburghii, P. wallichiana | Bark | Ethanol | Flavanoid, tannin | against albumin denaturation, HRBC membrane stabilization assay | 2500 μg/mL | P. roxburghii extract showed highest (%) of inhibition and protection i.e 86.54 and 89.92 against albumin denaturation and HRBC membrane stabilization. However, P. wallichiana have least inhibition and protection percentage, i.e., 76.54 and 81.2% | [104] |
| Taxus baccata | Aerial parts | Methanol | Terpenoids | ear edema induced in mice | 3.2 mg/ear | T. baccata extract displayed best activity | [21] |
| T. baccata | Bark | Ethanol | Alkaloids, terpenoids, flavonoids | carrageenan-induced paw edema in Wistar Albino rat | 200 mg/kg | Percentage of inhibition is 44% at a dose of 200 mg/kg | [161] |
| T. baccata | Heart wood | Ethanol | Taxoids, lignans | carrageenan-induced hind paw edema model inS wiss albino mice | 30–100 mg/kg | TBW shows significant antinociceptive and anti-inflammatory activities | [29] |
| T. wallichiana | Bark | Methanol | Tasumatrol B, 1,13-diacetyl-10-deacetylbaccatin III (10-DAD) and 4-deacetylbaccatin III (4-DAB) | carrageenan-induced paw edema and Cotton-pellet oedema model in Wistar rats and Swiss albino mice | 20 and 40 mg/kg; 40 mg/kg | In a carrageenan-induced inflammation model, tasumatrol B at a dose of 20 mg/kg showed significant activity, while in a cotton-pellet edema model tasumatrol B was found to be highly significant at the dose of 40 mg/kg. | [22] |
| Thuja occidentalis | Non-woody branches with leaves | Mother tincture (MT) | Polyphenols, flavonoids | Administered 2,4,6-trinitrobenzenesulfonic acid to induce intrarectal colitis in mice | 25 or 50 mg/kg | MT manage to relieve intestinal inflammation experimentally induce by TNBS in 7 days. | [126] |
JFB—Juniper foetidissima berry; AG—Abies georgei; TBW—Taxus baccata heart wood.
Cupressuflavone (CUF) isolated from C. macrocarpa has the ability to reduce the levels of several cytokines, including IL-1b, IL-6, TNF-α and PGE2, in plasma dose-dependently, and thus acts as an anti-inflammatory agent [46]. Triterpenoids and abietane type’s compounds extracted from Abies chensiensis show anti-inflammatory effects against NO production in RAW 264.7 macrophage cells [125]. It has been found that tasumatrol, deacetylbaccatin, paclitaxel and many other terpenoids extracted from Taxus spp. are effective in the anti-inflammatory process initiated through the carrageenan and cotton pellets induced edema model [21,22]. Kyung-Jae Cha. (2016) reported that in atopic dermatitis, the Picea wilsonii mast extract is useful and potent only in the inhibition of the production of the inflammatory cytokines IL-6, MCP-1 and IL-13, without significant change in IL-8 production induced in human adult low-calcium high-temperature (HaCaT) cell lines [115]. T. occidentalis mother tincture-containing terpenoids (thujone), polyphenols and flavanoids have potential in reducing ulcerative colitis inflammation in the mouse intestine and rectum by decreasing the stimulation of the pro-inflammatory cytokines IL6 and TNF-α induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) [126]. THP-1 cell adhesion to TNF was suppressed by enzogenol at a concentration of 5–25 g/mL onto TNF-α-activated human umbilical vein endothelial cells (HUVEC) by reducing integrin β2 induction and inhibiting monocyte trans-endothelial migration [127]. The anti-inflammatory and platelet-inhibitory effects of pycnozenol, extracted from Pinus maritime bark extract, inhibited the activity of cyclooxygenase (COX)-1 and COX-2 present in human plasma [128]. Inflammation has been attributed to cancer and neurodegenerative diseases [46].
4.2.3. Anticancer Activity
According to the report by the World Health Organization, cancer was a major cause of death in 2018, with a death rate of 9.6 million people [129]. Hippocrates, before 370 B.C., coined the word “cancer” to describe carcinoma tumors [130]. On the basis of evidence, bone cancer was identified in Ancient Egyptian mummies in around 1600 B.C., and cancer of the breast was identified in 1500 B.C., although there is no record of a cure for cancer [131]. Giovanni’s research laid the foundation for scientific cancer techniques in 1761, when he performed the first autopsies on dead human bodies to determine the connection between a patient’s disease and their death, as well as pathologic studies [132]. Cancer has been identified as the chief matter of public health concern around the world [133]. Surgery, radiotherapy, and chemotherapy are some of the conventional cancer treatments [134,135]. On the other hand, despite the use of a variety of synthetic drugs for cancer treatment and the successful completion of different management schedules, current therapies are not able to achieve the desired results, as tumor relapse and metastasis are common [136]. Nature contains various chemicals and pharmacologically active substances which act as anticancer drugs [137]. Recently, many of the phytochemicals and synthetic analogs, such as HS-1793 (resveratrol), have been identified as inhibiting the growth of cells and inducing apoptotic cell death, helping to cure cancer [138]. While only a few phytochemical compounds obtained from natural products have been developed into clinically active drugs, their bioactive components may be used as a basis for the development of more successful analogues and prodrugs using chemical techniques such as metabolomics, complete or combinatorial fabrication, and biosynthetic pathway modification [139]. Many phytochemical compounds are highly efficient in inducing apoptosis and cytotoxicity by modulating different MAPK andAKT/PI3K pathways, and suppress cancer cells line invasion and migration potential along with the stimulation of senescence phenotype, regulation of Bax or p53 genes, cell cycle arrest and modulation of IL-8, IFN-γ, TNF, IL-6 [140]. Many of the compounds derived from conifers’ bark and leaves act as antitumor drugs, such as paclitaxel (PTX) (trade name Pycnogenol® and Taxol®), a diterpene found in the crude extract of P. pinaster and Taxus brevifolia bark [141]. Paclitaxel, as well as its analogues docetaxel (taxoteres) and jevtanas (cabazitaxel), are examples of chemotherapeutic synthetic analogues derivative from plants that have been formulated and validated clinically [139]. By binding microtubules, PTX and other microtubule-targeting agents (MTAs) induce cellular death [142]. Microtubules are tubulin heterodimers that play a role in disease and perform numerous cellular functions including transport, force production in cell division, and structural support [143]. During the G2 phase of the cell cycle, tubulin is produced, and microtubules are assembled. Microtubule stabilizing agents, such as PTX, bind to α/β tubulin in order to disassemble microtubules. As a result, they cause cell death and are used as an anti-cancer agent [144]. In general, cells exposed to PTX are stuck in the G2/M phase, resulting in death due to failure to move through the cell cycle [145,146]. Recently, a study published in 2017 found that pycnogenol and PTX at doses of 20 g/mL and 0.5 μM cause DNA and mitochondrial damage in cancer breast cell line (MDA-MB) in 24 h, and concluded that it is possibly a target drug for cancer treatment through DNA and mitochondrial damage mechanisms [141]. From different conifer species crude extracts, the anticancer activities on different cancer cell lines are summarized in Table 4.
Table 4.
In vivo and in vitro anticancer and cytotoxic studies of conifer extracts.
| Conifer spp. | Part Used | Nature of Extract | Compounds | In Vitro and in Vivo Model | Dose. Conc | Main Effects | References |
|---|---|---|---|---|---|---|---|
| Abies georgei | Aerial parts | Chloroform, ethyl acetate, n-butanol | Flavanoids | Human tumor cell lines-A549, QGY-7703, LOVO, 6T-CEM | 77.5, 11.1, 7.8, 32.8 μg/mL | AGC extract has potent tumour and antiproliferative effects in humor tumor cell lines | [156] |
| (Mice) S180 tumours cell lines | 100, 200 and 400 mg/kg | AGC also exhibited activity in tumour growth inhibition in a dose-dependent manner, with ratios of 46.7, 53.1 and 31.0% at doses of 100, 200 and 400 mg/kg, respectively | |||||
| Araucaria angustifolia | Female strobili | Water | Fatty acids and polyphenols | Laryngeal carcinoma HEp-2 cells | 100–500 μg/mL | AAE inhibit the activity of mitochondria complex I and induce redox stress and cytochrome c, which leads cleavage of nuclear proteins of larynx HEp-2 cancer cells | [162] |
| Cedrus deodara | Stem wood | Chloroform | Lignans (Matairesinol, dibenzylbutyrolactol, (−)-Wikstromol) | In vitro human cell lines (cervix, breast, colon, liver, CNS, prostrate) | In vitro cytotoxicity IC 50 value-Wikstromol (71.31–93.63) and Matairesinol (50.84–95.36) μg/mL | CD lignin mixture have potent to show a cytotoxic effect at the maximum in CNS and at the minimum in liver against cancer cell lines in a dose-dependent manner at 100 μg/mL from 49 to 95%. | [34] |
| Human T lymphoblast, acute lymphoblastic leukemia cell line, Molt-4 and human promyelocytic leukemia cell line (HL-60) | IC50 (μg/mL) 15 |
AP9-cd-induced endogenous NO production leads to the generation of peroxide and disruption of mitochondrial membrane potential, leading to apoptotic pathway activation Increase in sub-G0 fraction from 35 to 60% in 24 to 48h |
[163] | ||||
| In vivo swiss albino mice (K562 cells) | The lignin mixture displays anti-cancer effects by regulating annexin V binding, intracellular caspase activities and DNA fragmentation | ||||||
| C. deodara | Needle | Ethanol | Kaempferol, myricetin, isorhamnetin and quercetin | HepG2 cells | IC50 114.12 μg/mL | TFPNCD shows potent cytotoxicity by inhibiting the growth of HepG2 cells in a dose-dependent manner Regulates cell cycle and apoptosis |
[164] |
| Cryptomeria japonica | Leaves | Methanol | Flavonoids | Albino mice of Ehrlich Ascites Carcinoma (EAC). | 100–400 μg/gm | Tumor cell count as well as the amounts of ascetic tumour cells in packed cells were significantly reduced in infected mice treated with MC | [165] |
| Juniper communis | Berry | Methanol and water | Phenolic compounds | CaCo2 and HeLa carcinoma cell lines | IC50 1300–2500 μg/mL | Methanol and water extracts of JCB show potent antiproliferative activity against cancer cell lines | [166] |
| J. taxifolia | Leaves | Chloroform | Polyphenols and lignan | human leukemia (HL-60) cells | 2.5 μg/mL | 7α-hydroxysandaracopimaric acid, a diterpenoid compound obtained from J. taxifolia leaves, shows antitumor effects on HL-60 cells | [24] |
| J. phoenicea | Aerial parts | Chloroform | Polyphenols | IC50 values (μg/mL) | It is found that JPCF disrupts cell cycle progression in the G0/G1phase and shows apoptotic, antiproliferative and necrotic effects on cancer cells lines | [20] | |
| Human lung (A549) | 34.2 | ||||||
| Breast (MCF-7) | 24.5 | ||||||
| Liver (HepG2) cancer cells | 57.6 | ||||||
| J. foetidissima | Needle | Methanol | Quercetin, rutin | Rat brain tumor (C6) cell lines | IC50 values (μg/mL) 10.65 |
J. foetidissima needle extract showed significant antiproliferative activity | [50] |
| M. glyptostroboides | Leaf | Water | Polyphenols | PC12 cells | 25 μg/mL | M. glyptostroboides leaf extract shows a cytotoxic effect and prevents oxidative damage of neuronal PC12 cells, protecting them from apoptosis; it was also found to significantly inhibit the release of LDH, which may result from apoptosis or necrosis | [49] |
| Picea wilsonii | Whole plant | DMSO | ND | Human keratinocyte HaCaT cell lines | 1–3 g/mL | PwM extracts inhibit the production of MCP-1 IL-6, IL-13 and but do not inhibit IL-8 production | [115] |
| Pinus kesiya | Woody twig | Ethanol | Phenolic compounds and flavonoids | Human hepatocarcinoma (HepG2) cell lines | IC50 (μg/mL) 52.0 | PK Extract exhibited a potent cytotoxic effect in the HepG2 cell line | [167] |
| P. kesiya | Branch | Ethanol | Phenolic compounds and flavonoids | Human leukemic U937 cancer cells |
IC50: 299 μg/mL | PK ethanol extract possesses anticancer activity against U937 human leukemic cells via apoptosis | [168] |
| P. merkusii | Leaves | Methanol | Phenolic compounds | MCF-7, A549, HT 1080 and HepG2 Huh-7 cancer cell lines | IC50 (μg/mL) 4.5, 16, 4.1, 5.6, 9.5 |
PM methanol extract possesses anticancer activity against human cancer cell lines | [169] |
| T. baccata | Leaves, cones | Methanol | Phenolic compounds | HCT-116 human colon cancer and MDA-MB-231 human breast cancer cell lines | IC50 μg/mL Leaves: 14.43 and 4.59 cones: 49.69 and 133.53 |
Methanol extracts of leaves had better activity on HCT-116 cells than seed cone extract, with IC50 values of 14.3 for 24 h and 4.59 for 72 h. Meanwhile, extracts did not show any significant cytotoxic effects on the cancer cell lines | [153] |
| T. wallichiana | Heartwood | Methanol | Lignans 1 (taxiresinol 1) 2, 3 | colon, ovarian liver, and breast cancer cell lines | IC90 lignan 2 and 3 μg/mL Caco 2:0.08 and 0.056 and 0.251 |
Taxiresinol 1 shows anticancer activity against ovary, colon, liver and breast cancer cell lines, while lignans 2 and 3 were found to be most active against Caco-2 cell lines | [170] |
| T. yunnanensis | All parts | ND | α-Conidendrin | MCF-7 andMDA-MB-231 cancer cell lines | 40 μM | α-conidendrin have the potential to inhibit human breast cancer cell lines MDA-MB-231 and MCF-7, showing viability of 73 and 82%, respectively | [31] |
| P. roxburghii | Leaves | Water and ethanol | Phenolic compounds | A549 human lung cancer cell line | 111.2 and 112.7 μg/mL | PRL extract shows potent anticancer activity against cancer cell lines. | [171] |
| Taxus cuspidata | Branches and leaves | Water | Polysaccharides | MCF7 | IC50 μg/mL | Purified polysaccharides (Pe4) on HeLa cells had the highest inhibitory effect, and its IC50 value is 89.9, while (Pe1) shows the best cytotoxic capacity against cancer lines HepG2 and MCF7, with IC50 conc. 132.0 and 169.0 μg/mL, respectively | [172] |
| 169.0 | |||||||
| Hela | 89.9 | ||||||
| HepG2 | 132.0 | ||||||
| Thuja occidentalis | Leaves and non-woody branches | Mother tincture (MT) | Polyphenols including flavonoids | Caco-2 cells | 25 or 50 mg/kg | Caco-2 cells exposed to H2O2 and T. occidentalis MT proves its radical scavenging activity by reducing GSH level by 103% and 98% as compared to TNBS group; MT also managed to reduce the lipid peroxidation | [126] |
| T. occidentalis | Leaves | Ethanol | ND | Human NSCLC (A549) cell lines | IC50 μg/mL | Extract of TO shows both anticancer and antiproliferative activities against NSCLC (A549) cell lines in a dose-dependent manner. | [173] |
| 282 | |||||||
| Human normal embryonic cell lines (L-132) | 376 | ||||||
| T. occidentalis | ND | Mother tincture (MT) Thujone-rich fraction (TRF) |
Thujone | A375 human malignant melanoma cell line | 200 μg/mL | TRF as compared with TO MT on exposure to A375 cells exhibited highly cytotoxic, apoptotic and antiproliferative effects, but TRF shows a lower growth inhibitory response towards peripheral blood mononuclear cell (normal cells) | [174] |
ND—Not determined; AGC—Abies georgei chloroform extract; AAE—Araucaria angustifolia water extract; TFPNCD—total flavonoids from the pine needles of Cedrus deodara; PRL—P. roxburghii leaves.
4.2.4. Neurodegenerative Diseases
Neurodegenerative diseases (NDs) are more common among the elderly and may even lead to death, and so are a major threat in the 21st century [175]. AD, Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), frontotemporal dementia, and the spinocerebellar ataxias are examples of ND [176], whose main features include nitrosative/oxidative stress, mitochondrial dysfunction, aggregated proteins accumulation, synapse loss, neuro-inflammation and decreased neuronal survival [177]. The progression of ND is also affected by genetic and surrounding ecological factors [175]. Indeed, it has been stated that the appropriate mechanism behind the cause of ND is mitochondrial dynamics variation, which elevates the oxidative damage, altering the biological activity of respiratory complexes, which results in brain energy dysfunction [178]. These stimuli trigger cellular stress, which leads to the synthesis and release of brain-derived neurotrophic factor (BDNF), as well as the activation of transcription factor CREB (cAMP response element-binding protein), with consequent expression of Arc (synaptic plasticity), PGC-1 (cellular energy metabolism), and APE1 (DNA repair enzyme), as well as the activation of the tropomyosin-related kinase (Trk B) receptor family and other downstream protein kinases [179].
Neurotrophins avoid neuron degeneration by binding to and activating the Trk receptor family, which is located in the plasma membrane [177]. Neurotrophins work by binding to and activating the Trk receptor family, which is found in the plasma membrane, to prevent neuron degeneration. Since neurotrosphins bind to Trk receptors, they create a microenvironment that promotes neuron development [180]. Various intracellular signaling pathways, such as ERK and PI3k/AKT, are regulated as a result of this binding, allowing cells to survive and aiding in the recovery of neurons from neurodegeneration. Additionally to signaling pathway activation, neurotrophins support Bcl-2 gene expression, which inhibits intracellular apoptosis [180]. Thus, early diagnosis of neurodegeneration may allow for early treatment, which may help to prevent the disease from progressing further [181]. Inhibition of the N-methyl-D-aspartate (NMDA) receptor can prevent or postpone AD. The drugs memantine and namzaric, which act as antagonists for the NMDA receptor, are used to treat AD patients [182].
Bioactive molecules have been recognized for their valuable biological effects, including neuroprotective properties, such as the ability to regulate mitochondria in a way that is distinct from TMs [183]. Branco et al. (2018) found that the flavonoid-rich A. angustifolia bracts extract (AAE) has neuroprotective properties by restoring rotenone-induced mitochondrial complex I, inhibiting the formation of lipid peroxidation and neuronal ROS, and through over expression of NDUFS7 protein and NDUFV2 gene levels in human dopaminergic SH-SY5Y cells [43]. Bark extract of P. pinaster shows protective effects against oxidative hemolysis induced by H2O2, the formation of thiobarbituric acid reactive products and lipid peroxidation [184]. In addition, it prevents oxidative damage to many proteins aggregation and may lessen the risk of several NDs, such as AD, PD and HD [185]. The neuro-protective potential of various conifer spp. crude extracts is summarized in Table 5.
Table 5.
Conifers’ phytochemicals demonstrating neuroprotective potential in vitro and in vivo.
| Conifers spp. | Compounds with Neuroprotective Potential | Model | Effective Concentration | Relevant Bioactivities | Reference |
|---|---|---|---|---|---|
| Abies holophylla | Holophyllin-D | C6 glioma cells | 20 μM | Diterpenes compound holophyllin D shows neuroprotective potential in C6 glioma cells by inducing nerve growth factor | [25] |
| Araucara angustifolia | Catechin, epicatechin and rutin | Rat | 10 mg/mL | AAE has antioxidant and neuroprotective properties as it decreases the TBARS levels, CAT activity and NO production in the hippocampus region of the brain in rats. | [235] |
| A. angustifolia | Catechin, epicatechin, rutin, quercetin and apigenin | human dopaminergic SH-SY5Y cells | 5 μg/mL | Decrease in the production of neuron (ROS) and lipid peroxidation. | [43] |
| A. angustifolia | Quercetin | cockroach | 200–400 μg/g | Neurotoxicity modulates the behavior of insects by altering the dopaminergic pathways, as quercetin has the ability to induce selective inhibitory actions on NMDA and GABA receptors and inhibit the enzyme acetylcholinesterase (AChE) | [236] |
| Cedrus deodara | Cedrin | PC12 cells | 0.1, 1 and 10 μM | PC12 cells injured by amyloid β1–42 can be improved by cedrin. Cedrin can reduce (ROS) overproduction, enhance the activity of SOD and decrease MDA content and inhibition of oxidative stress, improvement of mitochondrial dysfunction and suppression of apoptosis in PC12 cells | [237] |
| Metasequoia glyptostroboides | Gallic acid, rutin, myricetin, kaempferol, quercitrin, epigallocatechin, epicatechin gallate epigallocatechin gallate and caffeic acid | Neuronal PC12 cells | 2 mg/mL | The extracts effectively reduced the hydrogen peroxide-induced lipid peroxidation in neuronal PC12 cells by decreasing intracellular ROS accumulation | [49] |
| Pinus densiflora | Catechin, quercetin dehydrate, astragalin and kaempferol | Mice | 50–100 mg/kg | Catechin displayed a potential effect protecting mouse brains from oxidative damage via the improvement of the antioxidant capacities of TAC, the GSH-redox system, SOD and CAT in the hippocampus region as well as the inactivation of cytokines such as NF-kB in pyramidal cells of the hippocampal CA1 region, while PNE shows antiamnesic properties and effects in Alzheimer’s, as it attenuated the increase in serum corticosterone level and up-regulation of GR hippocampal gene expression | [238,239] |
| P. eldarica | Needle extract | Mice | 50 mg/kg | Alkanes, sterols, terpenoids, and quercetin, which is found in P. eldarica, help in inducing sleep and alter the sleep–wake cycle partly via activation of GABA receptors | [240] |
| P. massoniana | Polyprenols | Mice | 25 mg/kg | Polyprenols significantly increased T-AOC, GSHPx, damaging peroxide components from cells in order to stop the lipid peroxidation chain reaction and avoid excessive hydrolysis to form NEP, MDA, SOD activity (remove free radicals) and β-site AβPP cleaving enzyme 1 (BACE1) expression, while NOS activity, MDA concentration, NO, concentration of Aβ1-42 and PS1 were reduced | [241] |
| P. pinaster | Pycnogenol (PYC) | Mice | 20 mg/kg | In the MPTP-induced mouse model, PYC could prevent dopaminergic neurons by reducing oxidative loads, suppressing glial cell activation, and inhibiting inflammatory responses | [100,242] |
| P. roxburghii | Quercetin, rutin, gallic acid | Wistar albino | 100–300 mg/kg | Quercetin and gallic acid, both present in stem bark, have been shown to inhibit neuronal toxicity and apoptosis by reversing mitochondrial dysfunction and free radical development | [243] |
| Thuja occidentalis | Water extract | Mice | 100 mg/kg | CNS depressant activity, anticonvulsant and muscle relaxant activity | [244] |
| Torreya nucifera, | Arctigenin | Rat Cortical cells | 0.01 µM to 10.0 µM. | Arctigenin significantly attenuated glutamate-induced neurotoxicity by inhibiting the binding of [3H]-kainate to its receptors | [35] |
| T. semen | Polyphenols, flavonoids | Mice | 0–10 mg/mL | TS increased the level of total glutathiones | [245] |
T-AOC—total antioxidative capacity; GSHPx—glutathione peroxidise; SOD—super oxide dismutase; NEP—neprilysin; MDA—malondialdehyde; NO—nitric oxide, NOS—nitric oxide synthase; PS1—presenilin 1, CAT—catalase.
4.2.5. Alzheimer’s Disease (AD)
AD is a common neurodegenerative disease that affects 80% of the elderly population, accounting for about half of all dementia cases and ultimately results in death [186]. Its symptoms include failure to learn, gradual memory loss, and deterioration in behavior and neuronal function [187]. Regarding treatment, only five approved treatment options are licensed in the European Union for the treatment of AD, including rivastigmine, donepezil (cholinesterase inhibitors (ChEIs), galantamine, and memantine (NMDAR antagonist) [188]. An antimitotic agent paclitaxel widely used for the treatment of lung, ovarian and breast cancer has also been investigated as a possible treatment for AD [189]. It is mainly effective in the treatment of tauopathies, which are disorders caused by mutations in the tau protein, which is abundant in central nervous system (CNS) cells and acts by stabilizing microtubules [190]. The consumption of polyphenol-rich foods or beverages has been related to the prevention of AD in distinct studies [191]. The accumulation of amyloid-(A) in brain and leptomeningeal vessels causes cerebral amyloid angiopathy (CAA), which is also a central component of neuritic plaques in AD amyloid-(A) and has been related to the pathogenesis of two of the most common forms of dementia: AD and CAA. As a result, Aβ should be a top priority in the treatment of these diseases, which currently have no effective therapies [192]. Taxifolin, an antioxidant and anti-glycation flavonoid, reduces Aβ aggregation and its accumulation in the cerebrovascular system. In vitro studies have shown that taxifolin facilitates Aβ clearance in the brain, prevents Aβ fibril formation and CAA cognitive loss, and increases cerebral blood flow [193]. The methanol extract of P. roxburghii bark contains bioactive compounds, such as quercetin and gallic acid, which play important roles in neuroprotection by reversing mitochondrial dysfunction, free radical formation, and improving memory and cognition in rats, as well as reducing oxidative stress by improving acetylcholine levels. Furthermore, anti-AD activity has been documented in Pinus species, such as P. halepensis and P. massoniana [194]. Piceatannol, a compound derived from pine bark, has proven to be effective in preventing AD [195]. Resveratrol (RV), a stilbenoid, protects neurons from oxidative damage in a variety of ways, such as lowering lipid peroxidation and increasing intracellular antioxidant levels including antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and heme oxygenase 1 (HO-1) [196]. In this way, RV acts as an anti-AD agent by reducing neuroinflammation, inhibiting Aβ-plaque formation and tauopathy, and as a result inhibits neuronal death and improves memory [197]. Pycnogenol derived from P. pinaster bark has antioxidant, anti-inflammatory, and neuroprotective properties, including inhibition of amyloid-induced neuron apoptosis [198]. When the effect of pycnogenol was investigated in AD-related pathology in a β-amyloidosis mouse model, a decline in plaque numbers was found, while no changes were reported in the soluble β-amyloidosis levels, astrocytes, neurons, microglia, myelination pattern, morphology of axons and the gene expression of APP-processing enzymes [199]. Hence, it is suggested that pycnogenol has potential use in the prevention or in early stages of AD and mild cognitive impairment (MCI) [200]. Table 5 summarizes the neuroprotective potential of different conifers’ phytochemicals in AD.
4.2.6. Parkinson’s Disease
PD is second to AD in terms of the most prevalent progressive ND, with an estimated global prevalence of over 10,000,000 cases [201]. The selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) leads to PD. Briefly, PD occurs due to oxidative stress, dysfunction of mitochondrial complex-1, oxidative cell damage, neuronal excitotoxicity, calcium homeostasis, apoptosis, distressed energy metabolism, inflammation and protein aggregation, such as a-synuclein, apoptosis, and interaction between genetic and environmental causes [202]. Due to uncoordinated mouth and throat movements, PD causes bradykinesia, muscle rigidity, rest tremor, and the loss of postural control, as well as certain secondary symptoms, such as dementia, sialorrhea, soft voice, and trouble swallowing [203,204]. Oxidative stress generates ROS that causes oxidative damage, such as 4-hydroxynonenal (HNE), 26S proteasome and interferes with dopamine metabolism leading to PD [85]. Changes in protein ubiquitination and degradation have recently been related to dopaminergic cell death in PD [205]. Presynaptic protein α-synuclein (α-syn) influences the release of neurotransmitters from synaptic vesicles in the brain [206]. Currently, the treatment of PD includes drugs such as L-DOPA, which is catalyzed primarily by dopa decarboxylase in the brain, and some others such as ropinirole, selegiline, and rasagiline. Ropinirole has some adverse effects, including ankle oedema, vomiting, nausea, hypotension, insomnia, weight loss, hallucinations, psychosis, arrhythmia, dry mouth, nightmares, persistent diarrhea, somnolence and constipation, limiting their clinical applications [207]. As a result, the focus of rising interest in alternative treatments for ND, such as PD, has turned to natural products, which can provide alternatives due to their high effectiveness and few side effects [208]. Many plant extracts tend to stop α-syn from oligomerization and fibrillation, which is an emerging therapeutic mechanism in PD [183]. Methanol extract of J. communis at doses of 100 and 200 mg/kg was found to be effective in reducing catalepsy, enhancing locomotor activity (actophotometer), and increasing the level of reduced glutathione (GSH), protein level and muscle activity in rats [209]. In an in vitro study on Fisher F344 rats, Zhang et al. (2010) discovered that RV protect dopaminergic neurons from damage caused by MPP+, 6-OHDA, and also show efficacy against lipopolysaccharide-induced neurotoxicity by inhibiting nuclear factor kappa B (NF-κB) signaling and microglial activation [210].
4.2.7. Insomnia
Insomnia is a chronically debilitating disease that has become increasingly common, posing immense health and economic challenges for both individuals and the community [211]. Trouble falling asleep, staying asleep, fragmented sleep (repeatedly waking up at night or waking up early in the morning) are all symptoms of this condition [212]. While behavioral therapy, psychotherapy and light therapy have all been used to treat insomnia, the most common medications for insomnia are hypnotic drugs that target GABAA-benzodiazepine (BZD) receptors, such as diazepam and zolpidem [213]. However, several side effects have been identified, including cognitive impairment, resistance, headaches, nausea, and rebound insomnia [214,215]. Methanol extract of A. webbiana leaves showed potent synergistic effect in mice at dose of 100, 150, and 200 mg/kg, with sleep-inducing sedative drugs, diazepam (6 mg/kg), pentobarbitone sodium (50 mg/kg) and propylene glycol [157]. In addition, the major monoterpenoid components present in Pinus spp., α-pinene and 3-carene, have been reported to have hypnotic effects through GABAA-BZD receptors. 3-carene increases the length of sleep in mice given pentobarbital-induced sleep drugs by binding to the BZD site of the GABAA-BZD receptor α1 and ϒ2 [216].
5. Other Activities
5.1. Antidiabetic Activity
Diabetes mellitus is one of the world’s most serious health issues, with a rising prevalence and mortality rate [217]. Insufficiency in blood sugar control has significant health implications. Anti-diabetic medications are successful, but they come with unwanted side effects. Medicinal plants, on the other hand, can act as an additional reservoir of anti-diabetic agents [218]. Insulin and synthetic oral drugs hypoglycemic drugs are the most commonly used treatments for diabetes, despite the fact that they do not fully reverse the disease’s complications and have severe side effects. This is the driving force behind the search for new anti-diabetic agents [219]. After six years of treatment, sulfonylureas are expected to lose effectiveness in 44% of patients, while glucose-lowering drugs have been stated to be unable to control hyperlipidemia [220]. Nonetheless, the quest for newer antidiabetic drugs from natural sources continues due to many drawbacks associated with the use of current synthetic antidiabetic drugs [221].
Many plants have long been known to be a significant source of effective antidiabetic drugs in developing countries, especially to reduce the cost of conventional treatments [217]. Phytoconstituents, such as terpenoids, flavonoids, alkaloids, carotenoids, saponins, glycosides, which have antidiabetic effects, are now used to treat diseases such as diabetes [219,222]. Indeed, the number of people living with diabetes is rising, stoking concerns among medical professionals and the public. Despite the availability of antidiabetic medications in the market, medicinal plants are also effective [217]. The Araucaria, Cedrus, Juniperus, Pinus, Thuja, and Taxus genera have all been studied for their antidiabetic, antihyperglycemic, and hypoglycemic properties, as well as their ability to inhibit α-amylase and α-glucosidase and shown in Table 6.
Table 6.
Antidiabetic activity of different conifer extracts.
| Conifer spp. | Part Used | Compounds | Model | Induction of Diabetes | Dose. Conc | Effects | References |
|---|---|---|---|---|---|---|---|
| Araucaria cunninghamii | Seeds | Glucomannan | Albino wistar rats | Streptozotocin | 25 and 50 mg/kg |
|
[246] |
| Cedrus deodara | Heart wood | Flavonoids | Wistar albino rat | Alloxan | 500 mg/kg |
|
[247] |
|
Juniperus
communis |
Berry | Flavonoids | Wistar rat | Streptozotocin | 250 mg/kg |
|
[248] |
| J. communis | NR | NR | Rat | Streptozotocin-nicotinamide | 100–200 mg/kg |
|
[249] |
| J. oxycedrus | Leaves | Linolenic acid, oleic acid | Wistar-albino rats | Streptozotocin | 500 and 1000 mg/kg doses |
|
[250] |
| Pinus gerardiana | Nut | Flavonoids | Rat | Alloxan | 250, 500, and 750 mg/kg |
|
[251] |
| P. halepensis | Bark | Phenolic compounds | Rat | Glucose | 250, 500 mg/kg |
|
[151] |
| P. pinaster | Bark | Phenolic compounds | NR | IC50 (µg/mL) at PB 70% and PB 50% |
|
[39] | |
| α-amylase | 254.2 | ||||||
| β-Glucosidase | 122.7 | ||||||
| P. pinaster | Bark | Pynogenol | Human | NR | 100 mg |
|
[252] |
| P. roxburghii | Bark | Quercetin | NR | Alpha amylase inhibitory activity | 100 μg/mL |
|
[171] |
| P. roxburghii | Bark | Quercetin | Rat | Alloxan | 100, 300 and 500 mg/kg |
|
[253] |
| Taxus cuspidata | Branches and leaves | Water | Polysaccharides Pe4 (arabinose, galactose, glucose, xylose, mannose) | NR | 10–120 μg/mL |
|
[172] |
NR—Not reported.
In vitro experiments exhibited that the ethanol extract of P. halepensis bark had a greater inhibitory effect on the enzymes involved in diabetes (α-amylase and α-glucosidase) with IC50 values of 234.26 and 7.97 µg/mL, respectively [151]. Piceatannol is a phytochemical that has antidiabetic properties. Piceatannol, a resveratrol analogue, restores palmitic acid-induced disruption of insulin signaling and endothelial NO production in human endothelial cells by activating anti-inflammatory and antioxidant mechanisms (HO-1) [223]. According to Vallianou et al. (2013), the antihyperglycemic property of resveratrol appears by increasing the glucose transporter activity that occurs in the plasma membrane; the results indicate that the key antihyperglycemic action effects of resveratrol are due to the SIRT1 activation and AMPK (5’ AMP-activated protein kinase) [224]. RV antidiabetic activity is linked to its ability to increase AMPK and SIRT1 expression/activity in different tissues of diabetic subjects [225]. The multi-target effects of RV against diabetes were well-defined by Bagul and Banerjee, 2015, who underlined an improvement in insulin sensitivity and GLUT-4 translocation, while oxidative stress was reduced, carbohydrate-metabolizing enzymes were regulated, SIRT1 and AMPK were activated, and adipogenic genes were decreased [226]. As a result, lowering glucose levels by inhibiting enzyme activity is an effective method for treating hyperglycemia through using natural products.
5.2. Anticonvulsant Activity
Epilepsy is a neurological condition that affects people of all ages all over the world. The side effects of antiepileptic drugs and their connection to oxidative stress have prompted researchers to look for new medications that are less expensive and that have fewer side effects [227]. Several natural compounds derived from various conifer species have shown good anticonvulsant properties in animal models [228]. In India, extracts of C. deodara wood and P. roxburghii bark have historically been used to treat neurological disorders. In this analysis, the anticonvulsant activity of 3,4-bis(3,4-dimethoxyphenyl)furan-2,5-dione (BDFD) isolated from the ethanol extract of C. deodara and quercetin, chlorogenic acid, and rutin isolated from the ethanol extract of P. roxburghii bark were assessed in mice, and the results demonstrate modulation in the function of glutamate receptors by enhancing inhibitory GABA minergic neurotransmission [228,229]. Hinokiol, a neuromodulatory compound isolated from Taiwania cryptomerioides, affects NG108-15 cells and rat hippocampal CA1 neurons or neuronal ion channel activities by inhibiting voltage-gated Na(+) channels (VGSC) [230]. Lectins, normally a glycoprotein extracted from seed of A. angustifolia, had an antiseizure effect in strychnine and pentylenetetrazole-induced seizure models, revealing positive effects in the activation of glycinergic and GABAergic systems, respectively, and caused a reduction in animal movements [231].
5.3. Analgesic Activity
Analgesia/pain is an intense, ill-defined feeling triggered by a stimulus (external/internal); it is the most significant symptom that serves as an alarm signal and is mainly defensive in nature [232]. Bradykinin, tumor necrosis factor (TNF), and ILs cause analgesia by blocking the pain nerve sensitizing pathway [233]. An analgesic is a drug that relieves pain by acting on pain mechanisms in the CNS or in the peripheral nervous system (PNS) without affecting consciousness [234]. Even after new advances in pain therapies, healthcare professionals still need safe, reliable, and effective analgesic drugs to treat a variety of painful conditions, especially chronic pain. Based on its traditional medicinal uses, isolated T. wallichiana constituents are widely explored for analgesic purposes [22]. Indeed, the analgesic activity of C. deodara methanol bark extract was observed in Albino rats with acetic acid-induced writhing and found that it had a major analgesic effect, with 55.8% defense at a dose of 100 mg/kg [159]. In the acetic acid-induced writhing and hot plate model, Cupressus flavanone (CUF) demonstrated significant analgesic activity. At the three CUF doses used, 160 mg/kg in 120 min prevented PG synthesis and writhing response in mice at a rate of 25, 48, and 62%, respectively [46].
5.4. Antinociceptive Activity
Heartwood ethanol extract of T. baccata taxoids and lignin derivative compounds exhibited potent antinociceptive activity against p-benzoquinone-induced abdominal contractions in mice [29].
5.5. Antimicrobial Activity
Coniferous tree extracts are attracting intensified interest among scientific communities due to their possible applications in food, medicine, and cosmetics. Among conifers spp., various extracts have recently been identified as a significant source of bioactives with antimicrobial potential, as shown in Supplementary Materials Table S1.
Conifer compounds act as antimicrobials because they have potential in degrading microbial cell walls: disruption to the cytoplasmic membrane and membrane proteins, cell leakage, cytoplasm coagulation, and proton motive force depletion are all examples of their inhibitory action [254]. The following is a list in descending order of the key bioactive compounds responsible for antimicrobial effects: ketones > alcohols > esters > hydrocarbons > aldehydes > ketones > alcohols > esters > hydrocarbons [255]. Terpenoid compounds (α-terpineol, δ--3-carene, geranyl acetate, borneol, α and β-pinene, limonene, α-terpinene, ϒ-terpinene, β-ocimene, bornyl acetate, 1,8-cineole, α-phellandrene, p-cymene, linalool, ϒ-muurolene, α-humulene, and cadinene) have been found to be responsible for antimicrobial activity [8,33]. Alkaloids, especially 1,6-dehydropinidine obtained from P. abies needle and bark, have recently been discovered to have antimicrobial activity against Streptococcus equi (MIC = 55 g/mL) [13]. Secoisolariciresinol, pinoresinol, eudesmin, lariciresinol, and lariciresinol-4-methyl ether isolated from A. araucana wood methanol extract have shown potent antibacterial and antifungal activity with a synergistic effect, enhancing their potency against bacteria and fungi [256]. Anti-herpes activity was found in hydroethanolic extract ethyl acetate (EA) and n-butanol (NB) fractions from A. angustifolia leaves, indicating that conifer spp. could have been used in folk medicine to treat viruses [45]. It has been reported that RV, piceatannol, hydroxystilbenes and isorhapontigenin are present in debarking water, a byproduct of debarking logs of P. abies, meaning that it has the potential to prevent the growth of a variety of fungi and may be used as a natural fungicide [257].
5.6. Larvicidal Activity
In recent years, there has been increased interest in secondary metabolites with potential larvicidal activity in a number of countries around the world [258]. Dengue fever, yellow fever, dengue hemorrhagic fever, malaria and chikungunya are the most severe diseases transmitted by mosquitoes. Aedes aegypti is one of the mosquito species involved in the transmission of such vector disease outbreaks [258,259]. Larvicidal activity has been documented in extracts of conifer spp. parts [260]. The mosquito control technique is determined by the larval stages (egg, larvae, pupae, and adult) of the target. Mosquito control methods include spraying chemical insecticides on adult mosquitoes or destroying mosquito larvae before they grow into adults, either by means of synthetic larvicides or by using botanical extracts as an alternative larvicide [258]. The use of these synthetic insecticides against mosquitoes creates insecticide resistance as well as multifarious problems, such as environmental pollution and poisonous hazards to human beings [261]. These plant-oriented natural products are eco-friendly in nature and are preferred for use against larvae over other synthetic insecticides [262]. Based on mortality, the ethanol extract of J. procera and T. orientalis leaves has potential against Anopheles arabiensis, A. stephensi and Culex quinquefasciatus larvae [260,263]. It also has been found that C. sempervirens petroleum ether leaves extract shows a toxic effect on Musca domestica larva and also causes a decrease in the production of eggs and fecundity, as well as inducing sterility in both males and females [264]. Ethanol extract of Pinus caribaea and P. merkusii leaves and bark exhibited the highest mortality in the larvae of A. aegypti, a vector responsible for dengue fever transmission [265].
5.7. Cardiovascular Diseases
The rate of death due to cardiovascular diseases is quite high. Several medications are available to treat cardiovascular disorders and their complications. The general public has come to recognize the use of functional foods or dietary supplements to treat cardiovascular diseases [266]. A study reported that pycnogenol supplementation regulates the circulation in blood vessels, and reduces mild hypertension, cardiovascular diseases and platelet aggregation stimulated by smoking [267]. RV protects the heart by inhibiting platelet aggregation, thromboxane A2 formation (vasodilator effect), and Cox-1 peroxidase reactions [268]. In addition, low doses of RV (such as those present in the average diet) have been shown to have cardioprotective effects [269]. Cardiovascular disorders are common in both developing and developed countries. Piceatannol is intended to help to prevent cardiovascular disorders, including arrhythmia, high cholesterol, angiogenesis, and atherosclerosis [266]. Piceatannol pretreatment decreases cardiac hypertrophy, as measured by hypertrophy marker expression levels, cross-sectional area, and heart weight/body weight ratio. It also prevents lentiviral GATA-6-induced cardiac hypertrophy [270].
6. Clinical Trials
Clinical trials using extracts from conifer species in humans are limited. Only a few studies have reported the use of conifer spp. extracts in humans for inflammation and cardiovascular issues. A randomized 10-day, double-blind clinical trial was conducted on traumatic brain injury (TBI) patients. A pycnogenol supplement (OLIGOPIN) was orally administered with an oral dose of 150 mg per day, conducted in 60 people, with 30 control (Placebo) and 30 taking the PYC supplement, and it was found that PYC is effective in reducing inflammation and oxidative stress in TBI patients by increasing the level of pro-inflammatory cytokines, e.g., IL-6, TNF-α, IL-1β, and C-reactive protein (CRP) [271]. Another pilot study with a length of 12 weeks was conducted with Enzogenol® at an oral dose of 480 mg/day in 26 healthy people aged between 55 and 75 years. Some significant results have been published, such as beneficial changes in anthropometric data, a reduction in unnecessary body fat, vascular and plasma rheological indices, with a reduction in blood pressure and cardiovascular-related problems [272]. Nowadays, many products such as supplements, gels, creams, lotions, capsules, tablets, ointments formed from conifers’ bark, and needle powder are sold commercially. Table 7 describes the main characteristics of these products.
Table 7.
Conifer-derived commercially available products sold on the global market.
| Plant | Part Used | Trade Name | Phytochemicals Composition | Formulation | Dose/Duration | Product |
|---|---|---|---|---|---|---|
| Pinus pinaster | Bark | Pycnogenol | Catechin, taxifolin, procyanidins, caffeic, p-hydroxybenzoic, ferulic, acids | Tablets, liquids, chewing gums, gels, ointments, capsules or lotions | 150 mg/day for 6 months |

|
| P. pinaster | Bark | Oligopin | Caffeic acid, catechin, epicatechin, taxifolin and ferulic acid | Capsules | 150 mg for 10 days |

|
| P. radiata | Bark | Enzogenol | Flavanoids, proanthocyanidins | Tablets | 480–960 mg/day for 5–6 months |

|
| Picea abies | Needles | Ropren | Flavanoids | Tablets, capsules, lotions | 8.6 mg/kg for 28 days |

|
| Taxus brevifolia | Bark | Taxol | Paclitaxel | Injections | 30 mg/m2 every 3 weeks |
|
| P. massiona | Bark | Not found | Polyphenols, flavanoids, proanthocyanidins | Capsules | 1 capsule daily |

|
7. Phytotoxic Effects of Conifer Extract
Despite all of the advances stated so far, toxicity studies are required to know the effective doses that could be administered subsequently, as well as to depict the potential clinical signs elicited by the plant material [273]. Few toxicological studies have been reported on T. baccata, P. contorta and J. communis needles. A study was performed on a sample collected from a fatal case of a 22-year-old man, which revealed the presence of taxol A, diterpenoids such as monoacetyltaxine, and cardiotoxic compounds, such as 10-deacetylbaccatin III and taxine B. These compounds bind to calcium channels in cardiac myocytes, causing nausea, seizures, vomiting, dizziness, several cardiovascular effects, including bradycardia, and leading to ventricular tachycardia with severe ventricular arrhythmias, ventricular fibrillation and abdominal pain [274]. In another study, it was found that pine and juniper needles at an oral gavage dose of 62–245 mg/day in cattle have an abortifacient effect due to the presence of isocupressic acid in high doses [271]. Therefore, it is unsafe to feed pine and juniper needles to gravid cattle.
8. Conclusion and Future Trends
In this review, we have discussed the traditional and pharmacological uses of various conifers’ extracts against diabetes, neurological disorders, inflammation, and cancer. The phytochemical constituents present in conifer extracts are nontoxic at therapeutic levels, with polyphenolic compounds having significant biological activities. Stilbenes, terpenes, alkaloids, lignins and flavanoids, such as quercetin, rutin, resveratrol, and the compounds PYC and enzogenol, are the phytochemical components of conifer extracts reported to have sedative, antidiabetic, anticancer and anesthetic effects. In addition, phytochemicals present in conifer extracts assist in the regulation of glucose and lipid metabolism, insulin secretion, stimulating β cells, the NF-kB signaling pathway, the inhibition of gluconeogenic enzymes, ROS protective action as well as targeting and modulating cytokines which affect neuron cells and reduce oxidative stress. In this way, conifers’ phytochemicals are used as an alternative to synthetic drugs and can be to a greater extent in the future, as they can be helpful in the formulation of new drugs. Without a doubt, conifers’ phytochemicals are the natural sources of future drugs; in the field of drug discovery, a large number studies into phytochemicals are still required. More efforts are needed to investigate and assess the clinical potential and molecular characterization of medicinal compounds with the help of databases and interdisciplinary group efforts.
Acknowledgments
We acknowledge the University of Hradec Kralove (Faculty of Science VT2019-2021) for financial support.
Supplementary Materials
The following are available online, Table S1: Antiviral, antibacterial and antifungal activity of different conifers’ extracts.
Author Contributions
Conceptualization, A.S.S., P.B., N.C.-M. and K.K.; Writing—review, K.B., R.S. (Rohit Sharma), R.S. (Ruchi Sharma), D.S.D. and M.A.A.; Editing, M.A., E.N., K.M., B.S., N.K.U., M.N.; Funding acquisition, N.C.-M., K.M. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Hradec Kralove (Faculty of Science VT 2019-2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mustafa G., Arif R., Atta A., Sharif S., Jamil A. Bioactive Compounds from Medicinal Plants and Their Importance in Drug Discovery in Pakistan. Matrix Sci. Pharma. 2017;1:17–26. doi: 10.26480/msp.01.2017.17.26. [DOI] [Google Scholar]
- 2.Abdel-Razek A.S., El-Naggar M.E., Allam A., Morsy O.M., Othman S.I. Microbial natural products in drug discovery. Processes. 2020;8:470. doi: 10.3390/pr8040470. [DOI] [Google Scholar]
- 3.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nisar B., Sultan A., Rubab S.L. Comparison of Medicinally Important Natural Products versus Synthetic Drugs-A Short Commentary. Nat. Prod. Chem. Res. 2018;06:308. doi: 10.4172/2329-6836.1000308. [DOI] [Google Scholar]
- 5.Newman D.J., Cragg G.M., Snader K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 6.Galm U., Shen B. Natural Product Drug Discovery: The Times Have Never Been Better. Chem. Biol. 2007;14:1098–1104. doi: 10.1016/j.chembiol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Akaberi M., Boghrati Z., Amiri M.S., Khayyat M.H., Emami S.A. A Review of Conifers in Iran: Chemistry, Biology and their Importance in Traditional and Modern Medicine. Curr. Pharm. Des. 2020;26:1584–1613. doi: 10.2174/1381612826666200128100023. [DOI] [PubMed] [Google Scholar]
- 8.Bhardwaj K., Islam M.T., Jayasena V., Sharma B., Sharma S., Sharma P., Kuča K., Bhardwaj P. Review on essential oils, chemical composition, extraction, and utilization of some conifers in Northwestern Himalayas. Phyther. Res. 2020;34:2889–2910. doi: 10.1002/ptr.6736. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj K., Dhanjal D.S., Sharma A., Nepovimova E., Kalia A., Thakur S., Bhardwaj S., Chopra C., Singh R., Verma R., et al. Conifer-derived metallic nanoparticles: Green synthesis and biological applications. Int. J. Mol. Sci. 2020;21:9028. doi: 10.3390/ijms21239028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conifers. [(accessed on 15 March 2021)]; Available online: https://portals.iucn.org/library/sites/library/files/documents/1999-024.pdf.
- 11.Farjon A. The Kew Review Conifers of the World. Kew Bull. 2018;5974:1–16. doi: 10.1007/s12225-018-9738-5. [DOI] [Google Scholar]
- 12.Kopaczyk J.M., Warguła J., Jelonek T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020;180:104197. doi: 10.1016/j.envexpbot.2020.104197. [DOI] [Google Scholar]
- 13.Virjamo V., Fyhrquist P., Koskinen A., Lavola A., Nissinen K., Julkunen-Tiitto R. 1,6-Dehydropinidine Is an Abundant Compound in Picea abies (Pinaceae) Sprouts and 1,6-Dehydropinidine Fraction Shows Antibacterial Activity against Streptococcus equi Subsp. equi. Molecules. 2020;25:4558. doi: 10.3390/molecules25194558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mill R.R., Chase M.W. A new classification and linear sequence of extant gymnosperms. Phytotaxa. 2011;19:55–70. [Google Scholar]
- 15.Sharma A., Sharma L., Goyal R. A review on himalayan pine species: Ethnopharmacological, phytochemical and pharmacological aspects. Pharmacogn. J. 2018;10:611–619. doi: 10.5530/pj.2018.4.100. [DOI] [Google Scholar]
- 16.Tiberi R., Niccoli A., Curini M., Epifano F., Marcotullio M.C., Rosati O. The role of the monoterpene composition in Pinus spp. needles, in host selection by the pine processionary caterpillar, Thaumetopoea pityocampa. Phytoparasitica. 1999;27:263–272. doi: 10.1007/BF02981482. [DOI] [Google Scholar]
- 17.Naser B., Bodinet C., Tegtmeier M., Lindequist U. Thuja occidentalis (Arbor vitae): A review of its pharmaceutical, pharmacological and clinical properties. Evid. Based Complement. Altern. Med. 2005;2:69–78. doi: 10.1093/ecam/neh065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St-Pierre A., Blondeau D., Bourdeau N., Bley J., Desgagné-Penix I. Chemical Composition of Black Spruce (Picea mariana) Bark Extracts and Their Potential as Natural Disinfectant. Ind. Biotechnol. 2019;15:219–231. doi: 10.1089/ind.2019.0007. [DOI] [Google Scholar]
- 19.Rafieian-kopaei M., Suleimani dehkordi I., Ghanadian M., Shokrollahi A., Aghaei M., Ayatollahi S.A., Choudhary M.I. Bioactivity-guided isolation of new antiproliferative compounds from Juniperus foetidissima Willd. Nat. Prod. Res. 2016;30:1927–1933. doi: 10.1080/14786419.2015.1101106. [DOI] [PubMed] [Google Scholar]
- 20.Barnawi I.O., Nasr F.A., Noman O.M., Alqahtani A.S., Al-Zharani M., Alotaibi A.A., Daradka H.M., Al-Mishari A.A., Alobaid W.A., Alqahtani A., et al. Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis. Open Chem. 2021;19:119–127. doi: 10.1515/chem-2021-0195. [DOI] [Google Scholar]
- 21.Osuna-Torres L., García-Martí X., Ventura-Zapata E., López-Upton J., Zamilpa-Alvarez A., González-Cortazar M., Herrera-Ruiz M., Tapia-Barrera N. Taxus globosa Schltdl. (Mexican yew) and Taxus baccata L. (European yew): Intra and interspecies analysis of taxol content and biological activity according to different sources. For. Syst. 2015;24:16. doi: 10.5424/fs/2015243-07545. [DOI] [Google Scholar]
- 22.Qayum M., Nisar M., Shah M.R., Adhikari A., Kaleem W.A., Khan I., Khan N., Gul F., Khan I.A., Zia-Ul-Haq M., et al. Analgesic and antiinflammatory activities of taxoids from Taxus wallichiana Zucc. Phyther. Res. 2012;26:552–556. doi: 10.1002/ptr.3574. [DOI] [PubMed] [Google Scholar]
- 23.Kusumoto N., Aburai N., Ashitani T., Takahashi K., Kimura K. Pharmacological Prospects of Oxygenated Abietane-Type Diterpenoids from Taxodium distichum Cones. Adv. Biol. Chem. 2014;04:109–115. doi: 10.4236/abc.2014.42015. [DOI] [Google Scholar]
- 24.Muto N., Tomokuni T., Haramoto M., Tatemoto H., Nakanishi T., Inatomi Y., Murata H., Inada A. Isolation of apoptosis- and differentiation-inducing substances toward human promyelocytic leukemia HL-60 cells from leaves of Juniperus taxifolia. Biosci. Biotechnol. Biochem. 2008;72:477–484. doi: 10.1271/bbb.70570. [DOI] [PubMed] [Google Scholar]
- 25.Kim C.S., Subedi L., Kim S.Y., Choi S.U., Kim K.H., Lee K.R. Diterpenes from the Trunk of Abies holophylla and Their Potential Neuroprotective and Anti-inflammatory Activities. J. Nat. Prod. 2016;79:387–394. doi: 10.1021/acs.jnatprod.5b01053. [DOI] [PubMed] [Google Scholar]
- 26.Bajpai V.K., Sharma A., Kang S.C., Baek K.H. Antioxidant, lipid peroxidation inhibition and free radical scavenging efficacy of a diterpenoid compound sugiol isolated from Metasequoia glyptostroboides. Asian Pac. J. Trop. Med. 2014;7:9–15. doi: 10.1016/S1995-7645(13)60183-2. [DOI] [PubMed] [Google Scholar]
- 27.Tavares W., Seca A. The Current Status of the Pharmaceutical Potential of Juniperus L. Metabolites. Medicines. 2018;5:81. doi: 10.3390/medicines5030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tawara J.N., Blokhin A., Foderaro T.A., Stermitz F.R., Hope H. Toxic Piperidine Alkaloids from Pine (Pinus) and Spruce (Picea) Trees. New Structures and a Biosynthetic Hypothesis. J. Org. Chem. 1993;58:4813–4818. doi: 10.1021/jo00070a014. [DOI] [Google Scholar]
- 29.Küpeli E., Erdemoǧlu N., Yeşilada E., Şener B. Anti-inflammatory and antinociceptive activity of taxoids and lignans from the heartwood of Taxus baccata L. J. Ethnopharmacol. 2003;89:265–270. doi: 10.1016/j.jep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Juyal D., Thawani V., Thaledi S., Joshi M. Ethnomedical properties of Taxus wallichiana Zucc. (Himalayan yew) J. Tradit. Complement. Med. 2014;4:159–161. doi: 10.4103/2225-4110.136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hafezi K., Hemmati A.A., Abbaszadeh H., Valizadeh A., Makvandi M. Anticancer activity and molecular mechanisms of α-conidendrin, a polyphenolic compound present in Taxus yunnanensis, on human breast cancer cell lines. Phyther. Res. 2020;34:1397–1408. doi: 10.1002/ptr.6613. [DOI] [PubMed] [Google Scholar]
- 32.Ivanova D.I., Tashev A.N., Nedialkov P.T., Ilieva Y.E., Atanassova T.N., Olech M., Nowak R., Angelov G., Tsvetanova F.V., Iliev I.A., et al. Antioxidant and antiproliferative activity of Juniperus L. Species of Bulgarian and foreign origin and their anticancer metabolite identification. Bulg. Chem. Commun. 2018;50:144–150. [Google Scholar]
- 33.Kanchan B., Prerna B., Simran K. Medicinal value of secondary metabolites of pines grown in Himalayan region of India. Res. J. Biotechnol. 2020;15:131–140. [Google Scholar]
- 34.Singh S.K., Shanmugavel M., Kampasi H., Singh R., Mondhe D.M., Rao J.M., Adwankar M.K., Saxena A.K., Qazi G.N. Chemically standardized isolates from Cedrus deodara stem wood having anticancer activity. Planta Med. 2007;73:519–526. doi: 10.1055/s-2007-967185. [DOI] [PubMed] [Google Scholar]
- 35.Jang Y.P., Kim S.R., Choi Y.H., Kim J., Kim S.G., Markelonis G.J., Oh T.H., Kim Y.C. Arctigenin protects cultured cortical neurons from glutamate-induced neurodegeneration by binding to kainate receptor. J. Neurosci. Res. 2002;68:233–240. doi: 10.1002/jnr.10204. [DOI] [PubMed] [Google Scholar]
- 36.Asmi K.S., Lakshmi T., Balusamy S.R., Parameswari R. Therapeutic aspects of taxifolin—An update. J. Adv. Pharm. Educ. Res. 2017;7:187–189. [Google Scholar]
- 37.Hammerbacher A., Kandasamy D., Ullah C., Schmidt A., Wright L.P., Gershenzon J. Flavanone-3-hydroxylase plays an important role in the biosynthesis of spruce phenolic defenses against bark beetles and their fungal associates. Front. Plant Sci. 2019;10:1–15. doi: 10.3389/fpls.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michael H.N., Awad H.M., El-Sayed N.H., Paré P.W. Chemical and antioxidant investigations: Norfolk pine needles (Araucaria excelsa) Pharm. Biol. 2010;48:534–538. doi: 10.3109/13880200903177503. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira-Santos P., Genisheva Z., Botelho C., Santos J., Ramos C., Teixeira J.A., Rocha C.M.R. Unravelling the biological potential of Pinus pinaster bark extracts. Antioxidants. 2020;9:334. doi: 10.3390/antiox9040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gascón S., Jiménez-Moreno N., Jiménez S., Quero J., Rodríguez-Yoldi M.J., Ancín-Azpilicueta C. Nutraceutical composition of three pine bark extracts and their antiproliferative effect on Caco-2 cells. J. Funct. Foods. 2018;48:420–429. doi: 10.1016/j.jff.2018.07.040. [DOI] [Google Scholar]
- 41.Dziedzinski M., Kobus-Cisowska J., Szymanowska D., Stuper-Szablewska K., Baranowska M. Identification of polyphenols from coniferous shoots as natural antioxidants and antimicrobial compounds. Molecules. 2020;25:3527. doi: 10.3390/molecules25153527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fierascu I., Ungureanu C., Avramescu S.M., Cimpeanu C., Georgescu M.I., Fierascu R.C., Ortan A., Sutan A.N., Anuta V., Zanfirescu A., et al. Genoprotective, antioxidant, antifungal and anti-inflammatory evaluation of hydroalcoholic extract of wild-growing Juniperus communis L. (Cupressaceae) native to Romanian southern sub-Carpathian hills. BMC Complement. Altern. Med. 2018;18:1–15. doi: 10.1186/s12906-017-2066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Branco C.S., Duong A., Machado A.K., Wu A., Scola G., Andreazza A.C., Salvador M. Araucaria angustifolia (Bertol.) Kuntze has neuroprotective action through mitochondrial modulation in dopaminergic SH-SY5Y cells. Mol. Biol. Rep. 2019;46:6013–6025. doi: 10.1007/s11033-019-05037-6. [DOI] [PubMed] [Google Scholar]
- 44.Nisar M., Khan I., Ahmad B., Ali I., Ahmad W., Choudhary M.I. Antifungal and antibacterial activities of Taxus wallichiana Zucc. J. Enzym. Inhib. Med. Chem. 2008;23:256–260. doi: 10.1080/14756360701505336. [DOI] [PubMed] [Google Scholar]
- 45.Freitas A.M., Almeida M.T.R., Andrighetti-Fröhner C.R., Cardozo F.T.G.S., Barardi C.R.M., Farias M.R., Simões C.M.O. Antiviral activity-guided fractionation from Araucaria angustifolia leaves extract. J. Ethnopharmacol. 2009;126:512–517. doi: 10.1016/j.jep.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Al-Sayed E., Gad H.A., El-Shazly M., Abdel-Daim M.M., Nasser Singab A. Anti-inflammatory and analgesic activities of cupressuflavone from Cupressus macrocarpa: Impact on pro-inflammatory mediators. Drug Dev. Res. 2018;79:22–28. doi: 10.1002/ddr.21417. [DOI] [PubMed] [Google Scholar]
- 47.Ferrentino G., Haman N., Morozova K., Tonon G., Scampicchio M. Phenolic compounds extracted from spruce (Picea abies) by supercritical carbon dioxide as antimicrobial agents against gram-positive bacteria assessed by isothermal calorimetry. J. Therm. Anal. Calorim. 2020 doi: 10.1007/s10973-020-10100-7. [DOI] [Google Scholar]
- 48.Hoon L.Y., Choo C., Watawana M.I., Jayawardena N., Waisundara V.Y. Evaluation of the total antioxidant capacity and antioxidant compounds of different solvent extracts of Chilgoza Pine nuts (Pinus gerardiana) J. Funct. Foods. 2015;18:1014–1021. doi: 10.1016/j.jff.2014.07.009. [DOI] [Google Scholar]
- 49.Lee S.J., Lee S.Y., Hur S.J., Bae Y., II, Jeong C.H. Neuroprotective and antioxidant effects of Metasequoia glyptostroboides leaf extract. Curr. Top. Nutraceutical Res. 2016;14:67–72. [Google Scholar]
- 50.Sahin Yaglioglu A., Eser F. Screening of some Juniperus extracts for the phenolic compounds and their antiproliferative activities. S. Afr. J. Bot. 2017;113:29–33. doi: 10.1016/j.sajb.2017.07.005. [DOI] [Google Scholar]
- 51.Legault J., Girard-Lalancette K., Dufour D., Pichette A. Antioxidant potential of bark extracts from boreal forest conifers. Antioxidants. 2013;2:77–89. doi: 10.3390/antiox2030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lantto T.A., Colucci M., Závadová V., Hiltunen R., Raasmaja A. Cytotoxicity of curcumin, resveratrol and plant extracts from basil, juniper, laurel and parsley in SH-SY5Y and CV1-P cells. Food Chem. 2009;117:405–411. doi: 10.1016/j.foodchem.2009.04.018. [DOI] [Google Scholar]
- 53.Välimaa A.L., Raitanen J.E., Tienaho J., Sarjala T., Nakayama E., Korpinen R., Mäkinen S., Eklund P., Willför S., Jyske T. Enhancement of Norway spruce bark side-streams: Modification of bioactive and protective properties of stilbenoid-rich extracts by UVA-irradiation. Ind. Crops Prod. 2020;145:112150. doi: 10.1016/j.indcrop.2020.112150. [DOI] [Google Scholar]
- 54.Raiber S., Schröder G., Schröder J. Molecular and enzymatic characterization of two stilbene synthases from Eastern white pine (Pinus strobus) A single Arg/His difference determines the activity and the pH dependence of the enzymes. FEBS Lett. 1995;361:299–302. doi: 10.1016/0014-5793(95)00199-J. [DOI] [PubMed] [Google Scholar]
- 55.Hovelstad H., Leirset I., Oyaas K., Fiksdahl A. Screening analyses of pinosylvin stilbenes, resin acids and lignans in Norwegian conifers. Molecules. 2006;11:103–114. doi: 10.3390/11010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Francezon N., Meda N.S.B.R., Stevanovic T. Optimization of bioactive polyphenols extraction from Picea mariana bark. Molecules. 2017;22:2118. doi: 10.3390/molecules22122118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latva-Mäenpää H. Bioactive and Protective Polyphenolics From Roots and Stumps of Conifer Trees (Norway Spruce and Scots Pine) Helsingin Yliopisto; Helsinki, Finland: 2017. [Google Scholar]
- 58.Brodribb T.J., Pittermann J., Coomes D.A. Elegance versus speed: Examining the competition between conifer and angiosperm trees. Int. J. Plant Sci. 2012;173:673–694. doi: 10.1086/666005. [DOI] [Google Scholar]
- 59.Larter M. Ph.D. Thesis. Université de Bordeaux; Bordeaux, France: 2016. Evolution de la Résistance à la Cavitation Chez les Conifères the Evolution of Cavitation Resistance in Conifers. [Google Scholar]
- 60.Moriguchi Y., Ueno S., Hasegawa Y., Tadama T., Watanabe M., Saito R., Hirayama S., Iwai J., Konno Y. Marker-assisted selection of trees with MALE STERALITY 1 in Cryptomeria japonica D. Don. bioRxiv. 2020:1–10. doi: 10.1101/2020.05.29.114140. [DOI] [Google Scholar]
- 61.Hussein R.A., El-Anssary A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. Herb. Med. 2019;1:13. doi: 10.5772/intechopen.76139. [DOI] [Google Scholar]
- 62.Singh B., Sharma R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech. 2015;5:129–151. doi: 10.1007/s13205-014-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porres-Martínez M., González-Burgos E., Carretero M.E., Pilar Gómez-Serranillos M. In vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. fur Naturforsch. Sect. C J. Biosci. 2016;71:191–199. doi: 10.1515/znc-2014-4135. [DOI] [PubMed] [Google Scholar]
- 64.Da Silveira E Sá R.D.C., Andrade L.N., De Sousa D.P. Sesquiterpenes from essential oils and anti-inflammatory activity. Nat. Prod. Commun. 2015;10:1767–1774. doi: 10.1177/1934578X1501001033. [DOI] [PubMed] [Google Scholar]
- 65.Dey P., Kundu A., Kumar A., Gupta M., Lee B.M., Bhakta T., Dash S., Kim H.S. Analysis of Alkaloids (Indole Alkaloids, Isoquinoline Alkaloids, Tropane Alkaloids) Elsevier Inc.; Amsterdam, The Netherlands: 2020. [Google Scholar]
- 66.Thawabteh A., Juma S., Bader M., Karaman D., Scrano L., Bufo S.A., Karaman R. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins. 2019;11:656. doi: 10.3390/toxins11110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ignat I., Volf I., Popa V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 68.Tanase C., Boz I., Stingu A., Volf I., Popa V.I. Physiological and biochemical responses induced by spruce bark aqueous extract and deuterium depleted water with synergistic action in sunflower (Helianthus annuus L.) plants. Ind. Crops Prod. 2014;60:160–167. doi: 10.1016/j.indcrop.2014.05.039. [DOI] [Google Scholar]
- 69.Tanase C., Cosarcă S., Muntean D.L. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules. 2019;24:1182. doi: 10.3390/molecules24061182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El Omari N., Ezzahrae Guaouguaou F., El Menyiy N., Benali T., Aanniz T., Chamkhi I., Balahbib A., Taha D., Shariati M.A., Zengin G., et al. Phytochemical and biological activities of Pinus halepensis mill., and their ethnomedicinal use. J. Ethnopharmacol. 2021;268:113661. doi: 10.1016/j.jep.2020.113661. [DOI] [PubMed] [Google Scholar]
- 71.Metsämuuronen S., Sirén H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019;18:623–664. ISBN 0123456789. [Google Scholar]
- 72.Rodríguez-García C., Sánchez-Quesada C., Gaforio J.J., Gaforio J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants. 2019;8:137. doi: 10.3390/antiox8050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsao R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nanda S., Mohanty J.N., Mishra R., Joshi R.K. Transgenesis and Secondary Metabolism. Springer; Berlin/Heidelberg, Germany: 2017. Metabolic Engineering of Phenylpropanoids in Plants; pp. 485–510. [DOI] [Google Scholar]
- 75.Saleem M., Kim J., Ali S., Sup Y. An update on bioactive plant lignans. Nat. Prod. Rep. 2005;22:696–716. doi: 10.1039/b514045p. [DOI] [PubMed] [Google Scholar]
- 76.García-Pérez M.E., Royer M., Herbette G., Desjardins Y., Pouliot R., Stevanovic T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012;135:1173–1182. doi: 10.1016/j.foodchem.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 77.Salminen J., Karonen M. Chemical ecology of tannins and other phenolics: We need a change in approach. Br. Ecol. Soc. 2011;25:325–338. doi: 10.1111/j.1365-2435.2010.01826.x. [DOI] [Google Scholar]
- 78.Raitanen J.E., Järvenpää E., Korpinen R., Mäkinen S., Hellström J., Kilpeläinen P., Liimatainen J., Ora A., Tupasela T., Jyske T. Tannins of conifer bark as Nordic piquancy—sustainable preservative and aroma? Molecules. 2020;25:567. doi: 10.3390/molecules25030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koche D., Shirsat R., Kawale M. An overview of major classes of phytochemicals: Their type and role in disease prevention. Hislopia J. 2016;9:2016. [Google Scholar]
- 80.Prothmann J., Sun M., Spégel P., Sandahl M., Turner C., Scheuba J., Wronski V.K., Rollinger J.M., Grienke U., Santos-Buelga C., et al. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 2017;53:1713. doi: 10.1021/jf0701943. [DOI] [PubMed] [Google Scholar]
- 81.Matthews S., Mila I., Scalbert A., Donnelly D.M.X. Extractable and non-extractable proanthocyanidins in barks. Phytochemistry. 1997;45:405–410. doi: 10.1016/S0031-9422(96)00873-4. [DOI] [Google Scholar]
- 82.Koleckar V., Kubikova K., Rehakova Z., Kuca K., Jun D., Jahodar L., Opletal L. Condensed and Hydrolysable Tannins as Antioxidants Influencing the Health. Mini-Rev. Med. Chem. 2008;8:436–447. doi: 10.2174/138955708784223486. [DOI] [PubMed] [Google Scholar]
- 83.De Bruyne T., Pieters L., Deelstra H., Vlietinck A. Condensed vegetable tannins: Biodiversity in structure and biological activities. Biochem. Syst. Ecol. 1999;27:445–459. doi: 10.1016/S0305-1978(98)00101-X. [DOI] [Google Scholar]
- 84.Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. doi: 10.1016/0031-9422(91)83426-L. [DOI] [Google Scholar]
- 85.Bhangale J.O., Acharya S.R. Anti-Parkinson Activity of Petroleum Ether Extract of Ficus religiosa (L.) Leaves. Adv. Pharmacol. Sci. 2016;2016:9436106. doi: 10.1155/2016/9436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brijesh K., Ruchi R., Sanjita D., Saumya D., June A. Phytoconstituents and Therapeutic potential of Thuja occidentalis. Res. J. Pharm. Biol. Chem. Sci. 2012;3:354–362. [Google Scholar]
- 87.Shuaib M., Ali M., Ahamad J., Naquvi K.J., Ahmad M.I. Pharmacognosy of Pinus roxburghii: A Review. Phytochemistry. 2006;2:262–268. [Google Scholar]
- 88.Poudel R.C., Gao L.M., Möller M., Baral S.R., Uprety Y., Liu J., Li D.Z. Yews (Taxus) along the Hindu Kush-Himalayan region: Exploring the ethnopharmacological relevance among communities of Mongol and Caucasian origins. J ethnopharmacol. 2013;147:190–203. doi: 10.1016/j.jep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 89.Kunwar R.M., Shrestha K.P., Bussmann R.W. Traditional herbal medicine in Far-west Nepal: A pharmacological appraisal. J. Ethnobiol. Ethnomed. 2010;6:1–18. doi: 10.1186/1746-4269-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma H., Garg M. A review of traditional use, phytoconstituents and biological activities of Himalayan yew, Taxus wallichiana. J. Integr. Med. 2015;13:80–90. doi: 10.1016/S2095-4964(15)60161-3. [DOI] [PubMed] [Google Scholar]
- 91.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016;2016:1245049. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phaniendra A., Jestadi D.B., Periyasamy L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar H., Bhardwaj K., Nepovimova E., Kuča K., Dhanjal D.S., Bhardwaj S., Bhatia S.K., Verma R., Kumar D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials. 2020;10:1334. doi: 10.3390/nano10071334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Collin F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019;20:2407. doi: 10.3390/ijms20102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell. Signal. 2007;19:1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 96.Ricordi C., Garcia-Contreras M., Farnetti S. Diet and Inflammation: Possible Effects on Immunity, Chronic Diseases, and Life Span. J. Am. Coll. Nutr. 2015;34:10–13. doi: 10.1080/07315724.2015.1080101. [DOI] [PubMed] [Google Scholar]
- 97.Boukhenouna S., Wilson M.A., Bahmed K., Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid. Med. Cell. Longev. 2018;2018:5730395. doi: 10.1155/2018/5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Packer L., Rimbach G., Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999;27:704–724. doi: 10.1016/S0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 99.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iravani S., Zolfaghari B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res. Pharm. Sci. 2011;6:1–11. [PMC free article] [PubMed] [Google Scholar]
- 101.Senthilmohan S.T., Zhang J., Stanley R.A. Effects of flavonoid extract Enzogenol with vitamin C on protein oxidation and DNA damage in older human subjects. Nutr. Res. 2003;23:1199–1210. doi: 10.1016/S0271-5317(03)00127-1. [DOI] [Google Scholar]
- 102.Azqueta A., Collins A. Polyphenols and DNA damage: A mixed blessing. Nutrients. 2016;8:785. doi: 10.3390/nu8120785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kukreja A., Wadhwa N. Therapeutic Role of Resveratrol and Piceatannol in Disease Prevention. J. Blood Disord. Transfus. 2014;5:9. doi: 10.4172/2155-9864.1000240. [DOI] [Google Scholar]
- 104.Sharma A., Goyal R., Sharma L. Potential biological efficacy of Pinus plant species against oxidative, inflammatory and microbial disorders. BMC Complement. Altern. Med. 2016;16:1–11. doi: 10.1186/s12906-016-1011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Azab A., Nassar A., Azab A.N. Anti-inflammatory activity of natural products. Molecules. 2016;21:1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Artis D., Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 107.Fernandes J.V., Cobucci R.N.O., Jatobá C.A.N., de Medeiros Fernandes T.A.A., de Azevedo J.W.V., de Araújo J.M.G. The Role of the Mediators of Inflammation in Cancer Development. Pathol. Oncol. Res. 2015;21:527–534. doi: 10.1007/s12253-015-9913-z. [DOI] [PubMed] [Google Scholar]
- 108.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 109.Rock K.L., Rock K.L. Innate and adaptive immune responses to cell death. Immunol. Rev. 2011;243:191–205. doi: 10.1111/j.1600-065X.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Waisman A., Liblau R.S., Becher B. Innate and adaptive immune responses in the CNS. Lancet Neurol. 2015;14:945–955. doi: 10.1016/S1474-4422(15)00141-6. [DOI] [PubMed] [Google Scholar]
- 111.Vignali D.A.A., Kuchroo V.K. Review IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montgomery S.L., Bowers W.J. Tumor Necrosis Factor-alpha and the Roles it Plays in Homeostatic and Degenerative Processes Within the Central Nervous System. J. Neuroimmune Pharmacol. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 113.Fenton M.J. Review: Transcriptional and post-transcriptional regulation of interleukin 1 gene expression. Int. J. Immunopharm. 1992;14:401–411. doi: 10.1016/0192-0561(92)90170-P. [DOI] [PubMed] [Google Scholar]
- 114.Rider P., Carmi Y., Voronov E., Apte R.N. Interleukin-1α. Semin. Immunol. 2013;25:430–438. doi: 10.1016/j.smim.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Cha K.-J. The Anti-Inflammatory Effects of Picea wilsonii Mast on HaCaT Cells. Korean J. Clin. Lab. Sci. 2016;48:365–370. doi: 10.15324/kjcls.2016.48.4.365. [DOI] [Google Scholar]
- 116.Langrish C.L., Mckenzie B.S., Wilson N.J., Kastelein R.A., Cua D.J. IL-12 and IL-23: Master regulators of innate and adaptive immunity. Immunol. Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 117.Duvallet E., Semerano L., Assier E., Falgarone G., Duvallet E., Semerano L., Assier E., Falgarone G., Duvallet E., Semerano L., et al. Interleukin-23: A key cytokine in inflammatory diseases. Ann. Med. 2011;3890:503–511. doi: 10.3109/07853890.2011.577093. [DOI] [PubMed] [Google Scholar]
- 118.Sabat R. Cytokine & Growth Factor Reviews IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21:315–324. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 119.Ng T.H.S., Britton G.J., Hill E.V., Verhagen J., Burton B.R., Wraith D.C. Regulation of adaptive immunity; the role of interleukin-10. Front. Immunol. 2013;4:1–14. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kwilasz A.J., Grace P.M., Serbedzija P., Maier S.F., Watkins L.R. Neuropharmacology The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2014;2:55–69. doi: 10.1016/j.neuropharm.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Q.W., Lin L.G., Ye W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bucar F., Wube A., Schmid M. Natural product isolation-how to get from biological material to pure compounds. Nat. Prod. Rep. 2013;30:525–545. doi: 10.1039/c3np20106f. [DOI] [PubMed] [Google Scholar]
- 124.Gopalasatheeskumar K. Significant Role of Soxhlet Extraction Process in Phytochemical. Mintage J. Pharm. Med. Sci. 2018;7:43–47. [Google Scholar]
- 125.Zhao Q.Q., Wang S.F., Li Y., Song Q.Y., Gao K. Terpenoids with anti-inflammatory activity from Abies chensiensis. Fitoterapia. 2016;111:87–94. doi: 10.1016/j.fitote.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 126.Stan M.S., Voicu S.N., Caruntu S., Nica I.C., Olah N.K., Burtescu R., Balta C., Rosu M., Herman H., Hermenean A., et al. Antioxidant and anti-inflammatory properties of a Thuja occidentalis mother tincture for the treatment of ulcerative colitis. Antioxidants. 2019;8:416. doi: 10.3390/antiox8090416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim D.S., Kim M.S., Kang S.W., Sung H.Y., Kang Y.H. Pine bark extract enzogenol attenuated tumor necrosis factor-α- induced endothelial cell adhesion and monocyte transmigration. J. Agric. Food Chem. 2010;58:7088–7095. doi: 10.1021/jf1005287. [DOI] [PubMed] [Google Scholar]
- 128.Schäfer A., Chovanová Z., Muchová J., Sumegová K., Liptáková A., Högger P. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol) Biomed. Pharmacother. 2005;60:5–9. doi: 10.1016/j.biopha.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 129.Latest Global Cancer Data_ Cancer Burden Rises to 18 2018. WHO. [(accessed on 15 February 2021)]; Available online: https://www.iarc.who.int/featured-news/latest-global-cancer-data-cancer-burden-rises-to-18-1-million-new-cases-and-9-6-million-cancer-deaths-in-2018/PDF.
- 130.Yan S.H. An early history of human breast cancer: West meets East. Chin. J. Cancer. 2013;32:475–477. doi: 10.5732/cjc.013.10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sudhakar A. History of Cancer, Ancient and Modern Treatment Methods. J. Cancer Sci. Ther. 2009;01:i–iv. doi: 10.4172/1948-5956.100000e2. [DOI] [PubMed] [Google Scholar]
- 132.Ghosh S.K. Giovanni Battista Morgagni (1682–1771): Father of pathologic anatomy and pioneer of modern medicine. Anat. Sci. Int. 2017;92:305–312. doi: 10.1007/s12565-016-0373-7. [DOI] [PubMed] [Google Scholar]
- 133.Cancer. [(accessed on 20 February 2021)]; Available online: https://www.niehs.nih.gov/health/materials/cancer_and_the_environment_508.pdf.
- 134.Ma X., Wang Z. Anticancer drug discovery in the future: An evolutionary perspective. Drug Discov. Today. 2009;14:1136–1142. doi: 10.1016/j.drudis.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 135.Widmer N., Bardin C., Chatelut E., Paci A., Beijnen J., Levêque D., Veal G., Astier A. Review of therapeutic drug monitoring of anticancer drugs part two—Targeted therapies. Eur. J. Cancer. 2014;50:2020–2036. doi: 10.1016/j.ejca.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 136.Kuczynski E.A., Sargent D.J., Grothey A., Kerbel R.S. Drug rechallenge and treatment beyond progression-implications for drug resistance. Nat. Rev. Clin. Oncol. 2013;10:571–587. doi: 10.1038/nrclinonc.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lichota A., Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018;19:3533. doi: 10.3390/ijms19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim J.A.H., Kim D.H., Hossain M.A., Kim M.Y., Sung B., Yoon J.H., Suh H., Jeong T.C., Chung H.Y., Kim N.D. HS-1793, a resveratrol analogue, induces cell cycle arrest and apoptotic cell death in human breast cancer cells. Int. J. Oncol. 2014;44:473–480. doi: 10.3892/ijo.2013.2207. [DOI] [PubMed] [Google Scholar]
- 139.Sharifi-Rad J., Ozleyen A., Tumer T.B., Adetunji C.O., El Omari N., Balahbib A., Taheri Y., Bouyahya A., Martorell M., Martins N., et al. Biomolecules. Vol. 9. 2019. Natural products and synthetic analogs as a source of antitumor drugs; 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tafrihi M., Imran M., Tufail T., Gondal T.A., Caruso G., Sharma S., Sharma R., Atanassova M., Atanassov L., Valere P., et al. The Wonderful Activities of the Genus Mentha: Not Only Antioxidant Properties. Molecules. 2021;26:1118. doi: 10.3390/molecules26041118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Birinci H., Şen B., Sayğılı S., Ölmez E., Uluer E.T., Özbilgin K. The Effect of Pycnogenol and Paclitaxel on DNA Damage in Human Breast Cancer Cell Line. Proceedings. 2017;1:1023. doi: 10.3390/proceedings1101023. [DOI] [Google Scholar]
- 142.Dinić J., Ríos-Luci C., Karpaviciene I., Cikotiene I., Fernandes M.X., Pešić M., Padrón J.M. CKT0353, a novel microtubule targeting agent, overcomes paclitaxel induced resistance in cancer cells. Investig. New Drugs. 2020;38:584–598. doi: 10.1007/s10637-019-00803-6. [DOI] [PubMed] [Google Scholar]
- 143.Binarová P., Tuszynski J. Tubulin: Structure, Functions and Roles in Disease. Cells. 2019;8:1294. doi: 10.3390/cells8101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang D., Kanakkanthara A. Beyond the paclitaxel and vinca alkaloids: Next generation of plant-derived microtubule-targeting agents with potential anticancer activity. Cancers. 2020;12:1721. doi: 10.3390/cancers12071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harshita, Barkat M.A., Beg S., Pottoo F.H., Ahmad F.J. Nanopaclitaxel therapy: An evidence based review on the battle for next-generation formulation challenges. Nanomedicine. 2019;14:1323–1341. doi: 10.2217/nnm-2018-0313. [DOI] [PubMed] [Google Scholar]
- 146.Ganguly A., Yang H., Cabral F. Paclitaxel-dependent cell lines reveal a novel drug activity. Mol. Cancer Ther. 2010;9:2914–2923. doi: 10.1158/1535-7163.MCT-10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Banerjee S., Das A., Chakraborty P., Suthindhiran K., Jayasri M.A. Antioxidant and antimicrobial activity of Araucaria cookii and Brassaia actinophyla. Pak. J. Biol. Sci. 2014;17:715–719. doi: 10.3923/pjbs.2014.715.719. [DOI] [PubMed] [Google Scholar]
- 148.Jain S., Kumar D., Malviya N., Jain A., Jain S., Jain V. Estimation of total phenolic, tannins, and flavonoid contents and antioxidant activity of Cedrus deodara heart wood extracts. Egypt. Pharm. J. 2015;14:10. doi: 10.4103/1687-4315.154690. [DOI] [Google Scholar]
- 149.Horiba H., Nakagawa T., Zhu Q., Ashour A., Watanabe A., Shimizu K. Biological activities of extracts from different parts of cryptomeria japonica. Nat. Prod. Commun. 2016;11:1337–1342. doi: 10.1177/1934578X1601100939. [DOI] [PubMed] [Google Scholar]
- 150.Bhagat M., Gupta S., Sudan R. In vitro Evaluation of Antioxidant Activity of Picea smithiana Growing in Bhaderwah Region of Jammu and Kashmir. Cell. Life Sci. J. 2017;2 doi: 10.23880/cclsj-16000109. [DOI] [Google Scholar]
- 151.Salhi N., Bouyahya A., El Guourrami O., El Jemli M., Bourais I., Zellou A., Cherrah Y., El Abbes Faouzi M. Investigation of in vitro and in vivo antioxidant and antidiabetic activities of Pinus halepensis extracts. J. Herbmed Pharmacol. 2021;10:123–131. doi: 10.34172/jhp.2021.13. [DOI] [Google Scholar]
- 152.Tekaday D., Antony B., Jain S. Antimicrobial, antioxidant and phytochemical investigation of Thuja occidentalis (Arbor vitae) leave extract. GSC Biol. Pharm. Sci. 2020;12:108–116. doi: 10.30574/gscbps.2020.12.3.0292. [DOI] [Google Scholar]
- 153.Milutinović M.G., Stanković M.S., Cvetković D.M., Topuzović M.D., Mihailović V.B., Marković S.D. Antioxidant and anticancer properties of leaves and seed cones from European yew (Taxus baccata L.) Arch. Biol. Sci. 2015;67:525–534. doi: 10.2298/ABS141006015M. [DOI] [Google Scholar]
- 154.Bhat M.A., Ganie S.A., Dar K.B., Ali R., Hamid R. In Vitro antioxidant potential and hepatoprotective activity of Taxus Wallichiana. Asian J. Pharm. Clin. Res. 2018;11:237–243. doi: 10.22159/ajpcr.2018.v11i8.22345. [DOI] [Google Scholar]
- 155.Subba B. Analysis of Phytochemical Constituents and Biological Activity of Taxus Wallichiana Zucc. Dolakha District of Nepal. Int. J. Appl. Sci. Biotechnol. 2018;6:110–114. doi: 10.3126/ijasbt.v6i2.20410. [DOI] [Google Scholar]
- 156.Yang X.-W., Zeng H.-W., Liu X.-H., Li S.-M., Xu W., Shen Y.-H., Zhang C., Zhang W.-D. Anti-inflammatory and anti-tumour effects of Abies georgei extracts. J. Pharm. Pharmacol. 2008;60:937–941. doi: 10.1211/jpp.60.7.0017. [DOI] [PubMed] [Google Scholar]
- 157.Nayak S.S., Ghosh A.K., Debnath B., Vishnoi S.P., Jha T. Synergistic effect of methanol extract of Abies webbiana leaves on sleeping time induced by standard sedatives in mice and anti-inflammatory activity of extracts in rats. J. Ethnopharmacol. 2004;93:397–402. doi: 10.1016/j.jep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 158.Bisht B., Nainwal P., Saini P. Evaluation of in vitro anti-inflammatory activity of Agathis robusta. J. Pharma. Res. 2012;2:1304–1306. [Google Scholar]
- 159.Journal A.I. An Indian Journal Note. Anal. Chem. 2007;6:4–8. [Google Scholar]
- 160.Orhan N., Akkol E., Ergun F. Evaluation of antiinflammatory and antinociceptive effects of some juniperus species growing in Turkey. Turk. J. Biol. 2012;36:719–726. doi: 10.3906/biy-1203-32. [DOI] [Google Scholar]
- 161.Science A. Assessment of Anti-Inflammatory Activity of Taxus baccata Linn. Bark Extract Satyajit Dutta * G. Mariappan ** Dipankar Sarkar ** Piyali Sarkar ** Table 1: Effect of Taxus baccata (L) bark extracts on Carrageenan-induced paw edema method in rats. Anc. Sci. Life. 2010;29:19–21. [PMC free article] [PubMed] [Google Scholar]
- 162.Branco C.D.S., De Lima É.D., Rodrigues T.S., Scheffel T.B., Scola G., Laurino C.C.F.C., Moura S., Salvador M. Mitochondria and redox homoeostasis as chemotherapeutic targets of Araucaria angustifolia (Bert.) O. Kuntze in human larynx HEp-2 cancer cells. Chem. Biol. Interact. 2015;231:108–118. doi: 10.1016/j.cbi.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 163.Shashi B., Jaswant S., Madhusudana R.J., Kumar S.A., Nabi Q.G. A novel lignan composition from Cedrus deodara induces apoptosis and early nitric oxide generation in human leukemia Molt-4 and HL-60 cells. Nitric Oxide Biol. Chem. 2006;14:72–88. doi: 10.1016/j.niox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 164.Shi X., Liu D., Zhang J., Hu P., Shen W., Fan B., Ma Q., Wang X. Extraction and purification of total flavonoids from pine needles of Cedrus deodara contribute to anti-tumor in vitro. BMC Complement. Altern. Med. 2016;16:1–9. doi: 10.1186/s12906-016-1249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Basu L.R., De A., Sarkar P., Karak P., Dastidar S.G. Possibilities of developing novel potent antitumor agents from the leaves of Cryptomaria japonica. Int. J. Phytomed. 2016;8:404–410. doi: 10.5138/09750185.1860. [DOI] [Google Scholar]
- 166.Fernandez A., Cock I.E. The therapeutic properties of juniperus communis L.: Antioxidant capacity, bacterial growth inhibition, anticancer activity and toxicity. Pharmacogn. J. 2016;8:273–280. doi: 10.5530/pj.2016.3.17. [DOI] [Google Scholar]
- 167.Machana S., Weerapreeyakul N., Barusrux S., Nonpunya A., Sripanidkulchai B., Thitimetharoch T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin. Med. 2011;6:2–9. doi: 10.1186/1749-8546-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.MacHana S., Weerapreeyakul N., Barusrux S., Thumanu K., Tanthanuch W. FTIR microspectroscopy discriminates anticancer action on human leukemic cells by extracts of Pinus kesiya; Cratoxylum formosum ssp. pruniflorum and melphalan. Talanta. 2012;93:371–382. doi: 10.1016/j.talanta.2012.02.058. [DOI] [PubMed] [Google Scholar]
- 169.Thu N.B., Trung T.N., Ha D.T., Khoi N.M., Hung T.V., Hien T.T., Namhui Y., Bae K. Screening of Vietnamese medicinal plants for cytotoxic activity. Nat. Prod. Sci. 2010;16:43–49. [Google Scholar]
- 170.Chattopadhyay S.K., Kumar T.R.S., Maulik P.R., Srivastava S., Garg A., Sharon A., Negi A.S., Khanuja S.P.S. Absolute configuration and anticancer activity of taxiresinol and related lignans of Taxus wallichiana. Bioorg. Med. Chem. 2003;11:4945–4948. doi: 10.1016/j.bmc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 171.Kaushik P., Lal Khokra S., Rana A.C., Kaushik D. Evaluation of anticancer activity of Pinus roxburghii sarg. Against IMR-32 human neuroblastoma cancer cell line. Int. J. Pharm. Clin. Res. 2015;7:105–108. [Google Scholar]
- 172.Jiang P., Zhang Q., Zhao Y., Xiong J., Wang F., Zhang T., Zhang C. Extraction, Purification, and Biological Activities of Polysaccharides from Branches and Leaves of Taxus cuspidata S. Et Z. Molecules. 2019;24:2926. doi: 10.3390/molecules24162926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mukherjee A., Sikdar S., Bishayee K., Paul A., Ghosh S., Boujedaini N., Khuda-Bukhsh A.R. Ethanolic extract of Thuja occidentalis blocks proliferation of A549 cells and induces apoptosis in vitro. J. Chin. Integr. Med. 2012;10:1451–1459. doi: 10.3736/jcim20121218. [DOI] [PubMed] [Google Scholar]
- 174.Khuda-Bukhsh A.R., Biswas R., Mandal S.K., Dutta S., Bhattacharyya S.S., Boujedaini N. Thujone-rich fraction of Thuja occidentalis demonstrates major anti-cancer potentials: Evidences from in vitro studies on A375 cells. Evid. Based Complement. Altern. Med. 2011;2011:568148. doi: 10.1093/ecam/neq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Velmurugan B.K., Rathinasamy B., Lohanathan B.P., Thiyagarajan V., Weng C.F. Neuroprotective role of phytochemicals. Molecules. 2018;23:2485. doi: 10.3390/molecules23102485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gitler A.D., Dhillon P., Shorter J. Neurodegenerative disease: Models, mechanisms, and a new hope. DMM Dis. Model. Mech. 2017;10:499–502. doi: 10.1242/dmm.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Venkatesan R., Ji E., Kim S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. Biomed Res. Int. 2015;2015:814068. doi: 10.1155/2015/814068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johri A., Beal M.F. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 2012;342:619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang J.L., Lin Y.T., Chuang P.C., Bohr V.A., Mattson M.P. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. NeuroMol. Med. 2014;16:161–174. doi: 10.1007/s12017-013-8270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang E.J., Reichardt L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Agrawal M., Biswas A., Levy C.E. Molecular diagnostics of neurodegenerative disorders. Front. Mol. Biosci. 2015;2:1–10. doi: 10.3389/fmolb.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Olivares D., Deshpande V.K., Shi Y., Lahiri D.K., Greig N.H., Rogers J.T., Huang X. N-Methyl D-Aspartate (NMDA) Receptor Antagonists and Memantine Treatment for Alzheimer’s Disease, Vascular Dementia and Parkinson’s Disease. Curr. Alzheimer Res. 2012;9:746–758. doi: 10.2174/156720512801322564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Briffa M., Ghio S., Neuner J., Gauci A.J., Cacciottolo R., Marchal C., Caruana M., Cullin C., Vassallo N., Cauchi R.J. Extracts from two ubiquitous Mediterranean plants ameliorate cellular and animal models of neurodegenerative proteinopathies. Neurosci. Lett. 2017;638:12–20. doi: 10.1016/j.neulet.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 184.Physiology G., Waczulikova I., Kilanczyk E., Bryszewska M. The effect of Pycnogenol on the erythrocyte membrane fluidity. Gen. Physiol. Biophys. 2004;23:39–51. [PubMed] [Google Scholar]
- 185.Voss P., Horakova L., Jakstadt M., Kiekebusch D., Grune T. Ferritin oxidation and proteasomal degradation: Protection by antioxidants. Free Radic. Res. 2006;40:673–683. doi: 10.1080/10715760500419357. [DOI] [PubMed] [Google Scholar]
- 186.Deture M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019;14:1–18. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jahn H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013;15:445–454. doi: 10.31887/DCNS.2013.15.4/hjahn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tanvir Kabir M., Sahab Uddin M., Al Mamun A., Jeandet P., Aleya L., Mansouri R.A., Md Ashraf G., Mathew B., Bin-Jumah M.N., Abdel-Daim M.M. Combination drug therapy for the management of alzheimer’s disease. Int. J. Mol. Sci. 2020;21:3272. doi: 10.3390/ijms21093272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Durães F., Pinto M., Sousa E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals. 2018;11:44. doi: 10.3390/ph11020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Barbier P., Zejneli O., Martinho M., Lasorsa A., Belle V., Smet-Nocca C., Tsvetkov P.O., Devred F., Landrieu I. Role of tau as a microtubule-associated protein: Structural and functional aspects. Front. Aging Neurosci. 2019;10:1–14. doi: 10.3389/fnagi.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018;5:1–9. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rensink A.A.M., De Waal R.M.W., Kremer B., Verbeek M.M. Pathogenesis of cerebral amyloid angiopathy. Brain Res. Rev. 2003;43:207–223. doi: 10.1016/j.brainresrev.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 193.Tanaka M., Saito S., Inoue T., Satoh-Asahara N., Ihara M. Novel therapeutic potentials of taxifolin for amyloid-β-associated neurodegenerative diseases and other diseases: Recent advances and future perspectives. Int. J. Mol. Sci. 2019;20:2139. doi: 10.3390/ijms20092139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sharma L., Sharma A., Goyal R., Alam J. Pinus roxburghii Sarg. Ameliorates alzheimer’s disease-type neurodegeneration and cognitive deficits caused by intracerebroventricular-streptozotocin in rats: An in vitro and in vivo study. Indian J. Pharm. Sci. 2020;82:861–870. doi: 10.36468/pharmaceutical-sciences.715. [DOI] [Google Scholar]
- 195.Hassaan Y., Handoussa H., El-Khatib A.H., Linscheid M.W., El Sayed N., Ayoub N. Evaluation of plant phenolic metabolites as a source of Alzheimer’s drug leads. Biomed Res. Int. 2014;2014:843263. doi: 10.1155/2014/843263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Arbo B.D., André-Miral C., Nasre-Nasser R.G., Schimith L.E., Santos M.G., Costa-Silva D., Muccillo-Baisch A.L., Hort M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020;12:1–15. doi: 10.3389/fnagi.2020.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ahmed T., Javed S., Javed S., Tariq A., Šamec D., Tejada S., Nabavi S.F., Braidy N., Nabavi S.M. Resveratrol and Alzheimer’s Disease: Mechanistic Insights. Mol. Neurobiol. 2017;54:2622–2635. doi: 10.1007/s12035-016-9839-9. [DOI] [PubMed] [Google Scholar]
- 198.Maimoona A., Naeem I., Saddiqe Z., Jameel K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J. Ethnopharmacol. 2011;133:261–277. doi: 10.1016/j.jep.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 199.Peng Q.L., Zard A.R.B., Lau B.H.S. Pycnogenol protects neurons from amyloid- b peptide-induced apoptosis. Mol. Brain Res. 2002;104:55–65. doi: 10.1016/S0169-328X(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 200.Paarmann K., Prakash S.R., Krohn M., Möhle L., Brackhan M., Brüning T., Eiriz I., Pahnke J. French maritime pine bark treatment decelerates plaque development and improves spatial memory in Alzheimer’s disease mice. Phytomedicine. 2019;57:39–48. doi: 10.1016/j.phymed.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 201.Fang C., Lv L., Mao S., Dong H., Liu B. Cognition Deficits in Parkinson’s Disease: Mechanisms and Treatment. Parkinsons Dis. 2020;2020:2076942. doi: 10.1155/2020/2076942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dias V., Junn E., Mouradian M.M. The role of oxidative stress in parkinson’s disease. J. Parkinsons Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen L., Ding Y., Cagniard B., Van Laar A.D., Mortimer A., Chi W., Hastings T.G., Un J.K., Zhuang X. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J. Neurosci. 2008;28:425–433. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zoccarato F., Toscano P., Alexandre A. Dopamine-derived dopaminochrome promotes H2O2 release at mitochondrial Complex I: Stimulation by rotenone, control by Ca2+, and relevance to Parkinson disease. J. Biol. Chem. 2005;280:15587–15594. doi: 10.1074/jbc.M500657200. [DOI] [PubMed] [Google Scholar]
- 205.Ebrahimi-Fakhari D., Wahlster L., McLean P.J. Protein degradation pathways in Parkinson’s disease: Curse or blessing. Acta Neuropathol. 2012;124:153–172. doi: 10.1007/s00401-012-1004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Javed H., Nagoor Meeran M.F., Azimullah S., Adem A., Sadek B., Ojha S.K. Plant Extracts and Phytochemicals Targeting α-Synuclein Aggregation in Parkinson’s Disease Models. Front. Pharmacol. 2019;9:1555. doi: 10.3389/fphar.2018.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Corona J.C. Natural Compounds for the Management of Parkinson’s Disease and Attention-Deficit/Hyperactivity Disorder. Biomed Res. Int. 2018;2018:4067597. doi: 10.1155/2018/4067597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ríos J.L., Onteniente M., Picazo D., Montesinos M.C. Medicinal Plants and Natural Products as Potential Sources for Antiparkinson Drugs. Planta Med. 2016;82:942–951. doi: 10.1055/s-0042-107081. [DOI] [PubMed] [Google Scholar]
- 209.Bais S., Gill N.S., Kumar N. Neuroprotective Effect of Juniperus communis on Chlorpromazine Induced Parkinson Disease in Animal Model. Chin. J. Biol. 2015;2015:1–7. doi: 10.1155/2015/542542. [DOI] [Google Scholar]
- 210.Zhang F., Shi J.S., Zhou H., Wilson B., Hong J.S., Gao H.M. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol. Pharmacol. 2010;78:466–477. doi: 10.1124/mol.110.064535. reprinted in Mol. Pharmacol.2010, 78, 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fang X.S., Hao J.F., Zhou H.Y., Zhu L.X., Wang J.H., Song F.Q. Pharmacological studies on the sedative-hypnotic effect of Semen Ziziphi spinosae (Suanzaoren) and Radix et Rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine. 2010;17:75–80. doi: 10.1016/j.phymed.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 212.Akram M., Daniyal M., Munir N., Mohiuddin E., Sultana S. Medicinal Plants Combating Against Insomnia: A Green Anti-Insomnia Approach. J. Nerv. Ment. Dis. 2019;207:927–935. doi: 10.1097/NMD.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 213.Gooneratne N.S., Vitiello M.V. Sleep in Older Adults. Normative Changes, Sleep Disorders, and Treatment Options. Clin. Geriatr. Med. 2014;30:591–627. doi: 10.1016/j.cger.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Atkin T., Comai S., Gobbi G. Drugs for insomnia beyond benzodiazepines: Pharmacology, clinical applications, and discovery. Pharmacol. Rev. 2018;70:197–245. doi: 10.1124/pr.117.014381. [DOI] [PubMed] [Google Scholar]
- 215.Sateia M.J., Buysse D.J., Krystal A.D., Neubauer D.N. Adverse effects of hypnotic medications. J. Clin. Sleep Med. 2017;13:839. doi: 10.5664/jcsm.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Woo J., Yang H., Yoon M., Gadhe C.G., Pae A.N., Cho S., Justin Lee C. 3-Carene, a phytoncide from pine tree has a sleep-enhancing effect by targeting the GABAA-benzodiazepine receptors. Exp. Neurobiol. 2019;28:593–601. doi: 10.5607/en.2019.28.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Arumugam G., Manjula P., Paari N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013;2:196–200. doi: 10.1016/S2221-6189(13)60126-2. [DOI] [Google Scholar]
- 218.Salehi B., Ata A., Kumar N.V.A., Sharopov F., Ramírez-Alarcón K., Ruiz-Ortega A., Ayatollahi S.A., Fokou P.V.T., Kobarfard F., Zakaria Z.A., et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9:551. doi: 10.3390/biom9100551. ISBN 5641266167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Upendra Rao M., Sreenivasulu M., Chengaiah B., Jaganmohan Reddy K., Madhusudhana Chetty C. Herbal medicines for diabetes mellitus: A review. Int. J. PharmTech Res. 2010;2:1883–1892. [Google Scholar]
- 220.Warren R.E. The stepwise approach to the management of type 2 diabetes. Diabetes Res. Clin. Pract. 2004;65:S3. doi: 10.1016/j.diabres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 221.Wadkar K.A., Magdum C.S., Patil S.S., Naikwade N.S. Anti-diabetic potential and Indian medicinal plants. J. Herbmed Toxicol. 2014;2:45–50. [Google Scholar]
- 222.Afrisham R., Aberomand M., Ghaffari M.A., Siahpoosh A., Jamalan M. Inhibitory Effect of Heracleum persicum and Ziziphus jujuba on Activity of Alpha-Amylase. J. Bot. 2015;2015:824683. doi: 10.1155/2015/824683. [DOI] [Google Scholar]
- 223.Jeong S.O., Son Y., Lee J.H., Cheong Y.K., Park S.H., Chung H.T., Pae H.O. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol. Med. Rep. 2015;12:937–944. doi: 10.3892/mmr.2015.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vallianou N.G., Evangelopoulos A., Kazazis C. Resveratrol and diabetes. Rev. Diabet. Stud. 2013;10:236–242. doi: 10.1900/RDS.2013.10.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Szkudelski T., Szkudelska K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta Mol. Basis Dis. 2015;1852:1145–1154. doi: 10.1016/j.bbadis.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 226.Bagul P.K., Banerjee S.K. Application of Resveratrol in Diabetes: Rationale, Strategies and Challenges. Curr. Mol. Med. 2015;15:312–330. doi: 10.2174/1566524015666150505155702. [DOI] [PubMed] [Google Scholar]
- 227.Sciences D. The New Antiepileptic Drugs: Their Neuropharmacology and Clinical Indications. Neurol. Med. Chir. 2016;56:205–220. doi: 10.2176/nmc.ra.2015-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kaushik D., Kumar A., Kaushik P., Rana A.C. Anticonvulsant activity of alcoholic extract of bark of Pinus roxburghii Sarg. J. Chin. Integr. Med. 2012;10:1056–1060. doi: 10.3736/jcim20120915. [DOI] [PubMed] [Google Scholar]
- 229.Dhayabaran D., Florance E.J., Nandakumar K., Shanmugarathinam A., Puratchikody A. Anticonvulsant activity of fraction isolated from ethanolic extract of heartwood of Cedrus deodara. J. Nat. Med. 2014;68:310–315. doi: 10.1007/s11418-013-0798-4. [DOI] [PubMed] [Google Scholar]
- 230.Wang Y.W., Yang C.T., Gong C.L., Chen Y.H., Chen Y.W., Wu K.C., Cheng T.H., Kuo Y.H., Chen Y.F., Leung Y.M. Inhibition of voltage-gated Na+ channels by hinokiol in neuronal cells. Pharmacol. Rep. 2015;67:1049–1054. doi: 10.1016/j.pharep.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 231.Vasconcelos S.M.M., Lima S.R., Soares P.M., Assreuy A.M.S., de Sousa F.C.F., Lobato R.d.F.G., Vasconcelos G.S., Santi-Gadelha T., Bezerra E.H.S., Cavada B.S., et al. Central action of Araucaria angustifolia seed lectin in mice. Epilepsy Behav. 2009;15:291–293. doi: 10.1016/j.yebeh.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 232.Hijazi M.A., El-Mallah A., Aboul-Ela M., Ellakany A. Evaluation of Analgesic Activity of Papaver libanoticum Extract in Mice: Involvement of Opioids Receptors. Evid. Based Complement. Altern. Med. 2017;2017:8935085. doi: 10.1155/2017/8935085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Deng J.S., Chi C.S., Huang S.S., Shie P.H., Lin T.H., Huang G.J. Antioxidant, analgesic, and anti-inflammatory activities of the ethanolic extracts of Taxillus liquidambaricola. J. Ethnopharmacol. 2011;137:1161–1171. doi: 10.1016/j.jep.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 234.Deshmukh A., Morankar P.G., Kumbhare M.R. Review on Analgesic Activity and Determination Methods. Pharmtechmedica. 2014;3:425–428. [Google Scholar]
- 235.Machado F.D., Kuo J., Ongaratti B.R., Medeiros N.D., Salvador M., Dani C., Funchal C. Antioxidant and neuroprotective potential of extract of Brazilian pine Araucaria angustifolia bracts against oxidative stress induced by sodium azide in hippocampus. Integr. Pharmacol. Toxicol. Genotoxicol. 2015;1:16–20. [Google Scholar]
- 236.Thiago C., Patrícia de Brum V., Patrícia Gomes da S., Marines de Avila H., Graziele Daiane S., Michele Stach C., Antônio Batista P., Sidnei M., Andreas Sebastian M., Chariston André Dal B. Mechanism of the Entomotoxic Activity Induced by Araucaria Angustifolia Methanolic Extract in Nauphoeta Cinerea Lobster Cockroaches. J. Bot. Res. 2017;1:38–49. doi: 10.36959/771/559. [DOI] [Google Scholar]
- 237.Zhao Z., Dong Z., Ming J., Liu Y. Cedrin identified from Cedrus deodara (Roxb.) G. Don protects PC12 cells against neurotoxicity. Nat. Prod. Res. 2018;6419:1455–1458. doi: 10.1080/14786419.2017.1346645. [DOI] [PubMed] [Google Scholar]
- 238.Lee J.S., Kim H.G., Lee H.W., Han J.M., Lee S.K., Kim D.W., Saravanakumar A., Son C.G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015;5:1–10. doi: 10.1038/srep09651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lee J.S., Kim H.G., Lee H.W., Kim W.Y., Ahn Y.C., Son C.G. Pine needle extract prevents hippocampal memory impairment in acute restraint stress mouse model. J. Ethnopharmacol. 2017;207:226–236. doi: 10.1016/j.jep.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 240.Forouzanfar F., Ghorbani A., Hosseini M. Hydroalcoholic extract of needles of Pinus eldarica enhances pentobarbital-induced sleep: Possible involvement of GABAergic system. Avicenna J. Phytomed. 2016;6:449. [PMC free article] [PubMed] [Google Scholar]
- 241.Wang C., He L., Yan M. Effects of polyprenols from pine needles of Pinus massoniana on ameliorating cognitive impairment in a D -galactose-induced mouse model. Age. 2014;36:9676. doi: 10.1007/s11357-014-9676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Khan M.M., Kempuraj D., Thangavel R., Zaheer A. Protection of MPTP-induced neuroinflammation and neurodegeneration by Pycnogenol. Neurochem. Int. 2013;62:379–388. doi: 10.1016/j.neuint.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kabra A., Baghel U.S., Hano C., Martins N., Khalid M., Sharma R. Neuroprotective potential of Myrica esulenta in Haloperidol induced Parkinson’s disease. J. Ayurveda Integr. Med. 2020;11:448–454. doi: 10.1016/j.jaim.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lokesh D., Amitabha D., Sachin A., Avijeet J. Neuropharmacological Exploration of Thuja Occidentalis Linn. Int. Res. J. Pharm. 2011;2:143–148. [Google Scholar]
- 245.Lee S., Choi C., Kim J., Lim S., Jung H. The Antioxidant Activities and Neuroprotective Effects of Hot Water Extracts from Torreyae Semen. Korea J. Herbol. 2017;32:41–48. [Google Scholar]
- 246.Pradeep Kumar C., Lokesh T., Gobinath M., Kumar B., Saravanan D. Anti-diabetic and anti-hyperlipidemic activities of glucomannan isolated from Araucaria cunninghamii seeds. J. Chem. Pharm. Sci. 2013;6:204–209. [Google Scholar]
- 247.Jain S., Jain A., Malviya N., Kumar D., Jain V., Jain S. Antidiabetic Activity of Cedrus deodara Aqueous Extract and Its Relationship with Its Antioxidant Properties. J. Pharm. Sci. Pharmacol. 2015;1:187–194. doi: 10.1166/jpsp.2014.1023. [DOI] [Google Scholar]
- 248.De Medina F.S., Gamez M.J., Jimenez I., Jimenez J., Osuna J.I., Zarzuelo A. Hypoglycemic activity of juniper “berries”. Planta Med. 1994;60:197–200. doi: 10.1055/s-2006-959457. [DOI] [PubMed] [Google Scholar]
- 249.Esmail Al-Snafi A., Majid W.J., Ali Talab T., Author C. Medicinal Plants with Antidiabetic Effects-An Overview (Part 1) Clinically tested medicinal plants View project Medicinal plants with antimicrobial effects View project Medicinal Plants with Antidiabetic Effects-An Overview (Part 1) IOSR J. Pharm. 2019;9:9–46. [Google Scholar]
- 250.Orhan N., Aslan M., Demirci B., Ergun F. A bioactivity guided study on the antidiabetic activity of Juniperus oxycedrus subsp. oxycedrus L. leaves. J. Ethnopharmacol. 2012;140:409–415. doi: 10.1016/j.jep.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 251.Zulfqar F., Akhtar M.F., Saleem A., Akhtar B., Sharif A., Saleem U. Chemical characterization, antioxidant evaluation, and antidiabetic potential of Pinus gerardiana (Pine nuts) extracts. J. Food Biochem. 2020;44:1–12. doi: 10.1111/jfbc.13199. [DOI] [PubMed] [Google Scholar]
- 252.Liu X., Wei J., Tan F., Zhou S., Würthwein G., Rohdewald P. Antidiabetic effect of Pycnogenol® French maritime pine bark extract in patients with diabetes type II. Life Sci. 2004;75:2505–2513. doi: 10.1016/j.lfs.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 253.Kaushik P., Khokra D. Evaluation of Antidiabetic Potential of Pinus roxburghii Bark Extract in Alloxan Induced Diabetic Rats. J. Pharmacogn. Nat. Prod. 2016;1:1–5. doi: 10.4172/2472-0992.1000105. [DOI] [Google Scholar]
- 254.Haman N., Morozova K., Tonon G., Scampicchio M., Ferrentino G. Antimicrobial effect of Picea abies extracts on E. coli growth. Molecules. 2019;24:4053. doi: 10.3390/molecules24224053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dorman H.J.D., Deans S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 256.Céspedes C.L., Avila J.G., García A.M., Becerra J., Flores C., Aqueveque P., Bittner M., Hoeneisen M., Martinez M., Silva M. Antifungal and antibacterial activities of Araucaria araucana (Mol.) K. Koch heartwood lignans. Z. fur Naturforsch. Sect. C J. Biosci. 2006;61:35–43. doi: 10.1515/znc-2006-1-207. [DOI] [PubMed] [Google Scholar]
- 257.Hedenström E., Fagerlund Edfeldt A., Edman M., Jonsson B.G. Resveratrol, piceatannol, and isorhapontigenin from Norway spruce (Picea abies) debarking wastewater as inhibitors on the growth of nine species of wood-decaying fungi. Wood Sci. Technol. 2016;50:617–629. doi: 10.1007/s00226-016-0814-4. [DOI] [Google Scholar]
- 258.De Souza Wuillda A.C.J., Martins R.C.C., Costa F.D.N. Larvicidal activity of secondary plant metabolites in aedes aegypti control: An overview of the previous 6 years. Nat. Prod. Commun. 2019;14:1934578X19862893. doi: 10.1177/1934578X19862893. [DOI] [Google Scholar]
- 259.Hales S., De Wet N., Maindonald J., Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- 260.Wondimu E., Hirpasa T., Tadele A. Larvicidal activity of Juniperus procera extract against anopheles mosquito in in vitro, North Western Ethiopia. J. Med. Plants Res. 2020;14:445–450. doi: 10.5897/JMPR2019.6900. [DOI] [Google Scholar]
- 261.Nayak J.B., Mohan B. Larvicidal activity of Rauvolfia serpentina L. fruits against Aedes aegypti Mosquito larvae. Int. Res. J. Biol. Sci. 2015;4:54–56. [Google Scholar]
- 262.Phambala K., Tembo Y., Kasambala T., Kabambe V.H., Stevenson P.C., Belmain S.R. Bioactivity of common pesticidal plants on fall Armyworm Larvae (Spodoptera frugiperda) Plants. 2020;9:112. doi: 10.3390/plants9010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sharma P., Mohan L., Srivastava C.N. Larvicidal potential of Nerium indicum and Thuja oriertelis extracts against malaria and Japanese encephalitis vector. J. Environ. Biol. 2005;26:657–660. [PubMed] [Google Scholar]
- 264.Hasaballah A., Shehata A., Fouda M., Hassan M., Gad M. The Biological Activity of Cupressus sempervirens Extracts against Musca domestica. Asian J. Biol. 2018;5:1–12. doi: 10.9734/AJOB/2018/38023. [DOI] [Google Scholar]
- 265.Setiawan S., Koerniasari K., Ngadino N., Sudjarwo S.A. Bioinsecticide effect of Pinus merkusii tree bark extract on aedes aegypti larvae. J. Young Pharm. 2017;9:127–130. doi: 10.5530/jyp.2017.9.24. [DOI] [Google Scholar]
- 266.Tang Y.L., Chan S.W. A review of the pharmacological effects of piceatannol on cardiovascular diseases. Phyther. Res. 2014;28:1581–1588. doi: 10.1002/ptr.5185. [DOI] [PubMed] [Google Scholar]
- 267.Rohdewald P. Pleiotropic Effects of French Maritime Pine Bark Extract to Promote Healthy Aging. Rejuvenation Res. 2019;22:210–217. doi: 10.1089/rej.2018.2095. [DOI] [PubMed] [Google Scholar]
- 268.Markus M.A., Morris B.J. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin. Interv. Aging. 2008;3:331–339. doi: 10.2147/CIA.S3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shaito A., Posadino A.M., Younes N., Hasan H., Halabi S., Alhababi D., Al-Mohannadi A., Abdel-Rahman W.M., Eid A.H., Nasrallah G.K., et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020;21:2084. doi: 10.3390/ijms21062084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kee H.J., Park S., Kang W., Lim K.S., Kim J.H., Ahn Y., Jeong M.H. Piceatannol attenuates cardiac hypertrophy in an animal model through regulation of the expression and binding of the transcription factor GATA binding factor 6. FEBS Lett. 2014;588:1529–1536. doi: 10.1016/j.febslet.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 271.Malekahmadi M., Moradi Moghaddam O., Islam S.M.S., Tanha K., Nematy M., Pahlavani N., Firouzi S., Zali M.R., Norouzy A. Evaluation of the effects of pycnogenol (French maritime pine bark extract) supplementation on inflammatory biomarkers and nutritional and clinical status in traumatic brain injury patients in an intensive care unit: A randomized clinical trial protocol. Trials. 2020;21:1–10. doi: 10.1186/s13063-019-4008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shand B., Strey C., Scott R., Morrison Z., Gieseg S. Pilot study on the clinical effects of dietary supplementation with Enzogenol, a flavonoid extract of pine bark and vitamin C. Phyther. Res. 2003;17:490–494. doi: 10.1002/ptr.1181. [DOI] [PubMed] [Google Scholar]
- 273.Divvela H.N.D., Duppala L., Kolapalli V.R.M. Isolation and acute oral toxicity studies of Araucaria heterophylla novel natural polysaccharide gum in albino mice. World J. Pharm. Pharm. Sci. 2016;5:702–711. doi: 10.20959/wjpps201610-7752. [DOI] [Google Scholar]
- 274.Grobosch T., Schwarze B., Stoecklein D., Binscheck T. Fatal poisoning with Taxus baccata. quantification of paclitaxel (taxol A), 10-deacetyltaxol, baccatin III, 10-deacetylbaccatin III, cephalomannine (taxol B), and 3,5-dimethoxyphenol in body fluids by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2012;36:36–43. doi: 10.1093/jat/bkr012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.