Abstract
BACKGROUND AND PURPOSE: Increased T2 relaxation times in the ipsilateral hippocampus are present in patients with hippocampal sclerosis. Visual assessment of T2-weighted images of these patients suggests increased signal intensity in the anterior temporal lobe as well. Our aim was to assess hippocampal and anterior temporal T2 relaxation times in patients with partial epilepsy by using a new T2-relaxometry sequence implemented by using a 3-T General Electric imaging unit.
METHODS: Coronal view T2 maps were generated by using an eight-echo Carr-Purcell-Meiboom-Gill sequence (TE, 28−231) with an acquisition time of 7 min on a 3-T General Electric Signa Horizon LX imaging unit. T2 relaxation times were measured in the hippocampus and anterior temporal lobe of 30 healthy control volunteers and 20 patients with partial epilepsy.
RESULTS: For the 30 control volunteers, the mean hippocampal T2 relaxation time was 98 ± 2.8 ms. In all measured areas, the asymmetry index was small (<0.01). For the 15 patients with independent evidence of hippocampal sclerosis established by visual, volumetric, and, when available, pathologic criteria, mean hippocampal T2 relaxation times were 118 ± 7 ms (P < .0001) on the ipsilateral side and 101 ± 4 ms (P = .005) on the contralateral side. The T2 values were also increased in the anterior temporal lobe (ipsilateral: 82 ± 6 ms, P < .0001; contralateral: 79 ± 6 ms, P = .01) as compared with the values for the control volunteers (75 ± 3 ms). The five patients with focal cortical dysplasia had hippocampal T2 relaxation times that were not different from control values.
CONCLUSION: T2 relaxometry at 3 T is feasible and useful and confirmed marked ipsilateral hippocampal signal intensity increase in patients with hippocampal sclerosis. Importantly, definite signal intensity change was also present in the anterior temporal lobe. T2 relaxometry is a sensitive means of identifying abnormalities in the hippocampus and other brain structures.
T2 relaxometry has been established as a reliable tool for the assessment of MR signal intensity changes in brain tissues at 1.5 T (1, 2). It is particularly important in the MR imaging investigation of patients with temporal lobe epilepsy and hippocampal sclerosis (3–5). Hippocampal sclerosis can be diagnosed based on visual criteria, such as hippocampal volume reduction, T2-weighted signal intensity increase, and disturbed internal architecture (1). Quantification of hippocampal volumes and signal intensity is possible and is used for characterization of cases with bilateral abnormalities, equivocal visual assessment, and research purposes (6–8). T2 relaxometry allows in vivo quantification of hippocampal signal intensity changes. Based on normal values of healthy control volunteers, cutoff values indicating the presence of abnormality can be calculated and used in large populations (9). Indications that hippocampal volume loss and T2 relaxation times, although correlated with each other, are associated with different outcomes (10), and pathologic correlates (11, 12) have been observed.
To date, research using the technique of T2 relaxometry has almost exclusively focused on the hippocampus. However, T2 relaxometry can be used to quantify signal intensity changes in any other brain area (13). Visual assessment of T2-weighted signal intensity showed signal intensity increase in the anterior temporal lobe white matter of patients with partial epilepsy (14, 15). These anterior temporal lobe changes have not been quantified.
The advantage of T2 relaxometry, compared with the visual assessment of T2-weighted images, is the quantitative nature of the values obtained. This is particularly useful for patients with bilateral signal intensity increase, for patients with subtle hippocampal signal intensity abnormalities, and for assessment of subtle signal intensity abnormalities in any other areas. By comparison with the range of values obtained for healthy control volunteers, quantification allows objective determination of whether abnormalities are present in a particular patient. This discrimination may be otherwise impossible in some cases, even for an experienced assessor.
To date, T2 relaxometry has been established for clinical purposes by using imaging units with a magnetic field strength ≤1.5 T. The T2 relaxation times of tissues are system and sequence dependent but largely independent of magnetic field strength (16). Originally, T2 map sequences with multiple echoes were established by using a Carr-Purcell-Meiboom-Gill sequence with TE between 22 and 262 (4). With the increasing use of very high field strength imaging units, adaptation and calibration of T2 relaxometry methods for higher field strength are necessary (17). Only one group has reported relaxation time measurements obtained by using a Bruker system at 3 T with 19 control volunteers (17). Implementation of multi-echo T2 map sequences on General Electric (GE) systems has been regarded as difficult.
We herein present an eight-echo T2 sequence compatible with current GE technology at 3 T and show that T2 relaxometry at 3 T is feasible. Our aim was to replicate the finding of increased T2 relaxation times in the sclerotic hippocampus in patients with hippocampal sclerosis and to quantify T2 signal intensity changes in the anterior temporal lobe.
Methods
Participants
A consecutive series of 20 patients with refractory, partial epilepsy (eight male and 12 female patients; mean age, 32 ± 10 years) and 30 control volunteers (18 male and 13 female volunteers; mean age, 35 ± 9 years) was studied. All patients were assessed in the comprehensive epilepsy program at Austin Health. Seizure characterization included review of clinical history, optimized 1.5-T MR imaging examination, video-EEG telemetry, and, in most the cases, positron emission tomography and ictal and interictal single photon emission tomography. Based on these investigations, the seizure focus was on the left side in 11 patients and on the right side in nine. Examination at 1.5 T suggested the presence of unilateral hippocampal sclerosis in 15 patients with temporal lobe epilepsy. The seizure focus was right-sided in six patients and left-sided in nine. Five patients had malformations of cortical development; in all cases, focal cortical dysplasia was diagnosed based on 1.5-T imaging. The seizure focus was on the right in three patients and on the left in two. The malformations of cortical development were in the frontal lobe in two patients, in the temporal lobe in two, and in the parietal lobe in one.
MR Imaging System
MR imaging was performed on 1.5- and 3-T GE Signa Horizon LX imaging units (GE Medical Systems, Milwaukee, WI). The optimized protocol at 1.5 T included fluid-attenuated inversion recovery, T2-weighted, 3D fast spoiled gradient recalled echo acquisition at steady state, and inversion recovery acquisitions. The fluid-attenuated inversion recovery sequence was acquired in the axial plane, and the three other sequences in a coronal plane, perpendicular to the long axis of the hippocampus. No T2 relaxometry was performed at 1.5 T.
The MR imaging included multiple T1-weighted spin-echo sagittal view images acquired to show the mesial temporal structures (500/14/1 [TR/TE/number of excitations]; number of sections, 11; section thickness, 4 mm; section spacing, 1.5 mm; matrix, 256 × 192). The temporal lobe and the hippocampus were identified, and 10 oblique coronal sections were acquired perpendicular to the long axis of the hippocampus. The sections were positioned to include the fornix posteriorly, the anterior temporal lobe, and the frontal lobes anteriorly.
T2 Relaxometry Sequence
T2 maps were acquired at 3 T in the coronal plane, ranging from the frontal lobe anteriorly to the fornix posteriorly. The Carr-Purcell-Meiboom-Gill sequence produces eight images per location at TE between 28 and 231 (5000/28−231/1; eight echoes per location; number of sections, 10; section thickness, 6 mm; section spacing, 1.5 mm; matrix, 256 × 128; field of view, 24 cm). Inferior saturation pulse and flow compensation were used to minimize the pulsation artifact from flow in the carotid arteries. This sequence had an acquisition time of 7 min.
Analysis Software
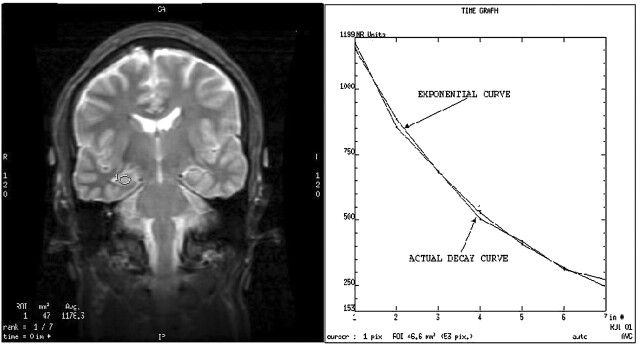
T2 maps were generated by using in-house visualization software developed in IDL (Research Systems, Inc., Bolder, CO) and also by using the proprietary GE software package designed for image processing (Functool). The analysis conducts a mono-exponential fit to corresponding voxels in each echo image. Confirmation of the accuracy of the fitting algorithm was achieved by direct comparison with a nonlinear fit implemented in IDL (curvefit.pro Floating Point Systems; Research Systems, Inc., Kodak, Boulder, CO). The packaged software displays the T2 map and the T2-weighted images (see Fig 1). The regions of interest were drawn on the weighted images with improved contrast enhancement.
Fig 1.
Left, Coronal view T2-weighted image of a control volunteer, obtained at the level of the body of the hippocampus. Region of interest is positioned in the left hippocampus. Right, Display of the relaxation curve and the calculated theoretical exponential curve. The relaxation curve of the tissue very closely matched the calculated theoretical exponential curve. T2 relaxation time measured by using both acquisition and measurement techniques correlated very closely.
Regions of Interest
Measurement of the T2 relaxation time was achieved by placing a circular region of interest over a predefined area of anatomy, as described previously for hippocampal regions of interest (4). All measurements were obtained bilaterally by using anatomic landmarks. The areas of interest were the hippocampus (head, usually sixth section), anterior temporal lobe white matter (on the section in front of the amygdala), parietal white matter (on the 10th section, above the lateral ventricles), and frontal lobe white matter (on the first section, frontal of the ventricles). Measurement of the T2 times was performed blinded to the clinical findings of the participants.
Histopathologic Assessment
Histopathologic assessment was conducted by using a standard protocol (12). In brief, 4-μ paraffin sections were stained with standard hematoxylin and eosin using Klüver Barrera techniques and a commercial glial fibrillary acidic protein (DAKO Rabbit Anti-Cow GFAP) antibody with a Mayer’s hematoxylin counterstain. A qualitative analysis used standard criteria for the diagnosis of brain abnormalities, such as hippocampal sclerosis and malformations of cortical development (18).
Statistics
Differences between bilateral measurements were assessed by paired t tests, and differences in measurements of similar tissues between control volunteers and patients were assessed by using unpaired t tests. A left-right asymmetry index was calculated based on the following equation: (L − R) / (L + R), with L referring to the left-sided value and R to the right-sided value.
Results
Control Volunteers
MR Imaging Assessment
The T2 values for the 30 control volunteers in all measured regions are shown in Table 1. The mean hippocampal T2 relaxometry time was 98 ms (±2.7 ms) for the right hippocampus and 97 ms (±3.5 ms) for the left hippocampus. The mean asymmetry index was −0.01 (±0.03). There was no side-to-side difference of the T2 times (paired t test), so values of both sides could be taken to calculate reference T2 values, indicating presence of abnormality. The highest value observed in control volunteers was 105 ms. A T2 value ≥3 SD above the mean control T2 time of 107 ms was considered abnormal. No difference was found between men and women, and no correlation was found with age of control volunteers.
TABLE 1:
T2 relaxometry times in 30 healthy control volunteers
| Right Side | Left Side | Asymmetry Index* | Mean | Cutoff T2† | |
|---|---|---|---|---|---|
| Hippocampus | 98 (±2.7) | 97 (±3.5) | −0.01 (±0.03) | 98 (±2.8) | ≥107 |
| Anterior temporal lobe | 76 (±3.5) | 75 (±3.8) | −0.01 (±0.06) | 75 (±2.8) | ≥84 |
| Frontal white matter | 76 (±3.8) | 75 (±3.1) | −0.01 (±0.02) | 76 (±3.4) | ≥87 |
| Parietal white matter | 91 (±4.4) | 91 (±4.0) | 0.00 (±0.03) | 91 (±4.0) | ≥103 |
Asymmetry index = (L − R)/(L + R).
Cutoff value, 3 SD above mean of control values.
The other measured regions—anterior temporal lobe, frontal lobe white matter, and parietal lobe white matter—also showed small SD and little variability between the two hemispheres, as measured by the asymmetry index (Table 1). Again, no difference was found between men and women, and no correlation was found with age of control volunteers.
Patients
MR Imaging Assessment
The qualitative assessment of the 3-T MR imaging showed typical features of hippocampal sclerosis in all 20 cases of hippocampal sclerosis, consistent with the visual diagnosis based on MR imaging at 1.5 T.
Table 2 shows the T2 times in the four different regions of interest in the 15 patients with hippocampal sclerosis. The mean ipsilateral hippocampal T2 time was 118 ± 7 ms, increased compared with control values (P < .0001) and with the contralateral side (P < .0001, paired t test). Compared with the cutoff value, the ipsilateral hippocampal T2 was increased in each patient. On the contralateral side, a subtle increase of the T2 times was observed (101 ± 4 ms, P = .005), and based on the cutoff value, one patient had an abnormal contralateral value. The asymmetry index was abnormal for all patients with hippocampal sclerosis.
TABLE 2:
T2 relaxometry in relation to MR imaging findings
| Control (n = 30) | HS (n = 15) | |
|---|---|---|
| Hippocampus | 98 (±3) | |
| Ipsi | 118 (±7)* | |
| Contra | 101 (±4)* | |
| ATL | 75 (±3) | |
| Ipsi | 82 (±6)* | |
| Contra | 79 (±6)* | |
| Frontal | 76 (±3) | |
| Ipsi | 78 (±4)* | |
| Contra | 78 (±3) | |
| Parietal | 91 (±4) | |
| Ipsi | 90 (±4) | |
| Contra | 89 (±4) |
Note.—HS indicated hippocampal sclerosis; Ipsi, ipsilateral to the seizure focus; Contra, contralateral to the seizure focus; ATL, anterior temporal lobe of white matter. Values are shown as means (±standard deviations).
P ≤ .05.
In the anterior temporal lobe, average T2 values were increased on the ipsilateral side (mean, 82 ± 6 ms; control values, 75 ± 3 ms; P < .0001) and, to a smaller degree, on the contralateral side (79 ± 6 ms, P = .01). T2 values were abnormal for six of the 12 patients with hippocampal sclerosis on either the ipsilateral or contralateral side. A subtle increase of the average ipsilateral frontal lobe T2 time (78 ± 4 ms; control values, 76 ± 3 ms; P = .04) was noted, but no difference was found compared with control values in the contralateral frontal or bilateral parietal lobe white matter. Compared with the cutoff values, none of the patients had abnormal T2 values in the frontal or parietal lobe.
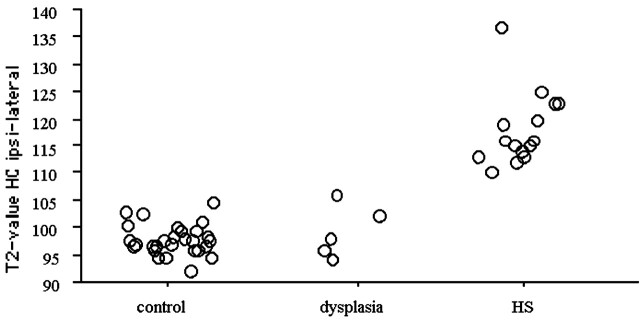
In five patients, the qualitative assessment at 3 T confirmed the MR diagnosis of focal cortical dysplasia. None of these patients had increased hippocampal T2 relaxation times, compared with the cutoff value. None of the five patients had signal intensity increase in the anterior temporal lobe, frontal lobe, or parietal lobe white matter region of interest. Figure 2 shows the distribution of the ipsilateral hippocampal T2 relaxation times for control volunteers, patients with hippocampal sclerosis, and patients with focal cortical dysplasia.
Fig 2.
Distribution of the ipsilateral hippocampal T2 values for control volunteers, patients with focal cortical dysplasia, and patients with hippocampal sclerosis. Note the marked signal intensity increase for all patients with hippocampal sclerosis.
Histopathologic Assessment
Twelve of the 15 patients with MR imaging evidence of hippocampal sclerosis underwent surgery, and for all of them, histologic assessment confirmed this diagnosis. All five patients with MR imaging evidence of malformations of cortical development underwent surgery. For all of them, histologic diagnosis was consistent with focal cortical dysplasia.
Discussion
Our results show that T2 relaxometry at 3 T is feasible and precise. Hippocampal T2 relaxation times for control volunteers varied only between 94 and 105 ms, and the SD was only 2.8 ms, with very little side-to-side asymmetry. Corresponding values for control volunteers at 1.5 T showed an SD of 4.2 ms (9). This indicates the high precision of T2 relaxometry at 3 T. This may be a product of the high signal intensity-to-noise ratio, the resolution achieved by using the 3 T system, and the technique used.
Using an eight-echo T2 relaxometry sequence at 3 T, we were able to replicate the finding of marked increased signal intensity in the ipsilateral hippocampus of patients with hippocampal sclerosis. The hippocampal signal intensity abnormality was correctly identified for all patients with unilateral hippocampal sclerosis, diagnosed previously based on optimized 1.5-T MR imaging findings. Subtle but significant contralateral hippocampal signal intensity increase was observed. This confirms a recent report on hippocampal signal intensity increase in hippocampi remote from the seizure focus (19). This contralateral hippocampal signal intensity increase may reflect bilateral, asymmetric involvement of the hippocampus, as has been suggested in reports of histopathologic (20) and neuroimaging studies (21).
Importantly, increased T2 relaxation times were also present in the anterior temporal lobe, more pronounced on the ipsilateral side but also contralateral. Anterior temporal lobe signal intensity increase has been qualitatively described (14, 22) but has not been measured by using T2 relaxometry. Some controversy exists regarding the underlying pathologic abnormality of this finding. It has been suggested that these abnormalities represent developmental malformations (23). Histopathologic analysis of the resected tissue showed that the increased signal intensity was not associated with dysplasia or gliosis (14), and it has been suggested that this reflects a persisting developmental stage of the anterior temporal lobe with immature myelin and persistence of immature cells as a consequence of early seizures.
In comparison with the patients with hippocampal sclerosis, the five patients with focal cortical dysplasia showed no difference in their hippocampal signal intensity compared with control volunteers. Furthermore, these patients showed no signal intensity change in any other assessed area. The threshold for detecting abnormalities was set at a high level (3 SD above control values), and therefore, subtle signal intensity abnormality may not be detected with these small numbers. Regarding the hippocampus, it is fair to say that these patients did not display the degree of hippocampal T2 relaxometry change observed in the ipsilateral hippocampus of patients with hippocampal sclerosis.
This study showed the feasibility of a multi-section, eight-echo T2 relaxometry technique using a GE system at 3 T. The sequence used had TE between 28 and 231 ms, covering the range of TE originally used for single section T2 maps (4). Some authors have suggested use of a dual echo sequence (2, 24, 25). This may avoid technical limitations with multi-echo sequences, particularly with GE systems, but suffers from imprecise fitting of a single exponential to only two images per section location. Although the precision may be sufficient for clinical evaluation, for research purposes, the higher precision of a multi-echo sequence may be crucial. Whittall et al (26), performing a comparative study using between two and 32 echoes of TE between 20 and 320 ms, strongly suggested the use of more than four echoes.
The presented data from our eight-echo sequence was analyzed by using the GE software package, which is simple and appropriate. However, data can easily be managed by other software solutions. The package allows placement of the region of interest on both the anatomic magnitude image and the T2 map image simultaneously (Fig 1). This avoids partial volume effects from incorrect placement, such as that which might occur if the region of interest included CSF in the temporal horn of the lateral ventricle. This source of potential artifacts, leading to erroneously high T2 values, has been addressed previously (24). Our study confirmed that such sources of error are small compared with the magnitude of the measurement.
The T2 values obtained with the Carr-Purcell-Meiboom-Gill acquisition used on most clinical imaging units likely deviates from the “true” T2 value. This is principally because of the difficulties in achieving perfect refocusing with multiple trains of section-selective 180° pulses (27). Signal intensity pathways from stimulated echoes (secondary echoes) are generated and contaminate the decaying signal intensity with a complex mixture of T1 and T2 weighting. The reasonable degree of variability between T2 values reported by different sites reflects these technical difficulties. Nevertheless, this is a systematic effect and the ability to conduct inter-participant comparisons from measurements obtained at a single center is, therefore, not affected.
T2 relaxometry has been previously developed as a tool for the investigation of patients with epilepsy (1). We herein present further development of this technique at 3 T. This sensitive measure allows identification of brain signal intensity abnormalities in 7 min and can be applied to a variety of neurologic diseases. Our findings document increased T2 times not only in the ipsilateral sclerotic hippocampus but also in the contralateral hippocampus and anterior temporal lobe. The signal intensity increase in remote areas may be associated with the initial epileptogenic insult, leading to hippocampal sclerosis, or may reflect seizure-associated damage. Therefore, T2 relaxometry may become even more important for the investigation of epilepsy patients as a tool for the characterization of changes in the epileptogenic focus and in areas remote from it.
References
- 1.Jackson GD, Berkovic SF, Duncan JS, Connelly A. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR Am J Neuroradiol 1993;14:753–762 [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan JS, Bartlett P, Barker GJ. Technique for measuring hippocampal T2 relaxation time. AJNR Am J Neuroradiol 1996;17:1805–1810 [PMC free article] [PubMed] [Google Scholar]
- 3.Grünewald RA, Jackson GD, Connelly A, Duncan JS. MR detection of hippocampal disease in epilepsy: factors influencing T2 relaxation time. AJNR Am J Neuroradiol 1994;15:1149–1156 [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson GD, Connelly A, Duncan JS, Grünewald RA, Gadian DG. Detection of hippocampal pathology in intractable partial epilepsy: increased sensitivity with quantitative magnetic resonance T2 relaxometry. Neurology 1993;43:1793–1799 [DOI] [PubMed] [Google Scholar]
- 5.Kälviäinen R, Partanen K, Aeikiä M, et al. MRI-based hippocampal volumetry and T2 relaxometry: correlation to verbal memory performance in newly diagnosed epilepsy patients with left-sided temporal lobe focus. Neurology 1997;48:286–287 [DOI] [PubMed] [Google Scholar]
- 6.Tampieri D, Pike BG, Arnold DL. T2-relaxometry can lateralize mesial temporal lobe epilepsy in patients with normal MRI. Neuroimage 2000;12:739–746 [DOI] [PubMed] [Google Scholar]
- 7.Kuzniecky RI, Bilir E, Gilliam F, et al. Multimodality MRI in mesial temporal sclerosis: relative sensitivity and specificity. Neurology 1997;49:774–778 [DOI] [PubMed] [Google Scholar]
- 8.Van Paesschen W, Connelly A, King MD, Jackson GD, Duncan JS. The spectrum of hippocampal sclerosis: a quantitative magnetic resonance imaging study. Ann Neurol 1997;41:41–51 [DOI] [PubMed] [Google Scholar]
- 9.Briellmann RS, Jackson GD, Mitchell LA, Fitt GJ, Kim SE, Berkovic S. Occurrence of hippocampal sclerosis: is one hemisphere or gender more vulnerable? Epilepsia 1999;40:1816–1820 [DOI] [PubMed] [Google Scholar]
- 10.Wendel JD, Trenerry MR, Xu YC, et al. The relationship between quantitative T2 relaxometry and memory in nonlesional temporal lobe epilepsy. Epilepsia 2001;42:863–868 [DOI] [PubMed] [Google Scholar]
- 11.Van Paesschen W, Revesz T, Duncan JS, King MD, Connelly A. Quantitative neuropathology and quantitative magnetic resonance imaging of the hippocampus in temporal lobe epilepsy. Ann Neurol 1997;42:756–766 [DOI] [PubMed] [Google Scholar]
- 12.Briellmann RS, Kalnins RM, Berkovic SF, Jackson GD. Hippocampal pathology in refractory TLE: T2-weighted signal change reflects dentate gliosis. Neurology 2002;58:265–271 [DOI] [PubMed] [Google Scholar]
- 13.Teicher MH, Anderson CM, Polcari A, Glod CA, Maas LC, Renshaw PF. Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nat Med 2000;6:470–473 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell LA, Jackson GD, Kalnins RM, et al. Anterior temporal abnormality in temporal lobe epilepsy: a quantitative MRI and histopathologic study. Neurology 1999;52:327–336 [DOI] [PubMed] [Google Scholar]
- 15.Meiners LC, van Gils A, Jansen GH, deKort G, Witkamp TD, Ramos LM. Temporal lobe epilepsy: the various MR appearances of histologically proven mesial temporal sclerosis. AJNR Am J Neuroradiol 1994;15:1547–1555 [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes D, McDonald WI, Johnson G, Tofts PS, Landon DN. Quantitative nuclear magnetic resonance imaging: characterisation of experimental cerebral oedema. J Neurol Neurosurg Psychiatry 1987;50:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wansapura JP, Holland SK, Dunn RS, Ball WS. NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging 1999;9:531–538 [DOI] [PubMed] [Google Scholar]
- 18.Graham D, Lantos P, eds. Greenfield’s Neuropathology. 6th ed. London: Arnold,1997. :936
- 19.Scott RC, Cross JH, Gadian DG, Jackson GD, Neville BG, Connelly A. Abnormalities in hippocampi remote from the seizure focus: a T2 relaxometry study. Brain 2003 [DOI] [PubMed]
- 20.Mathern GW, Babb TL, Pretorius JK, Melendez M, Lévesque MF. The pathophysiologic relationships between lesion pathology, intracranial ictal EEG onsets, and hippocampal neuron losses in temporal lobe epilepsy. Epilepsy Res 1995;21:133–147 [DOI] [PubMed] [Google Scholar]
- 21.Barr WB, Ashtari M, Schaul N. Bilateral reductions in hippocampal volume in adults with epilepsy and a history of febrile seizures. J Neurol Neurosurg Psychiatry 1997;63:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell LA, Harvey AS, Coleman LT, Mandelstam S, Jackson G. Anterior temporal changes in children with hippocampal sclerosis: an effect of seizures on the immature brain? AJNR Am J Neuroradiol 2003;24:1670–1677 [PMC free article] [PubMed] [Google Scholar]
- 23.Ho SS, Kuzniecky RI, Gilliam F, Faught E, Morawetz R. Temporal lobe developmental malformations and epilepsy: dual pathology and bilateral hippocampal abnormalities. Neurology 1998;50:748–754 [DOI] [PubMed] [Google Scholar]
- 24.Woermann FG, Steiner H, Barker GJ, et al. A fast FLAIR dual-echo technique for hippocampal T2-relaxometry: first experiences in patients with temporal lobe epilepsy. J Magn Reson Imaging 2001;13:547–552 [DOI] [PubMed] [Google Scholar]
- 25.von Oertzen J, Urbach H, Bluemcke I, et al. Time-efficient T2-relaxometry of the entire hippocampus is feasible in temporal lobe epilepsy. Neurology 2002;58:257–264 [DOI] [PubMed] [Google Scholar]
- 26.Whittall KP, MacKay AL, Li DK. Are mono-exponential fits to a few echoes sufficient to determine T2 relaxation for in vivo human brain? Magn Reson Med 1999;41:1255–1257 [DOI] [PubMed] [Google Scholar]
- 27.Majumdar S, Orphanoudakis SC, Gmitro A, O’Donnell M, Gore JC. Errors in the measurements of T2 using multiple-echo MRI techniques: II. effects of static field inhomogeneity. Magn Reson Med 1986;3:562–574 [DOI] [PubMed] [Google Scholar]