Abstract
Summary: Microparticulate embolization of meningiomas is a useful preoperative measure, but the potential risk of hemorrhagic complications should be recognized and balanced against the benefits. We report a case of subarachnoid hemorrhage occurring immediately after the embolization of a meningioma. Techniques for early diagnosis, such as CT, and early surgical intervention are crucial to a promising outcome. Large-size and necrotic or cystic components of a meningioma are probable risk factors that demand special consideration.
Preoperative embolization of meningiomas is widely practiced, because the desired hemostasis and necrosis facilitate easier surgical manipulation and removal of the tumor as well as a reduction of blood loss during surgery. Such embolization procedures, however, are not without risk (1–3). We report a case of subarachnoid hemorrhage occurring immediately after the embolization of a large and necrotic falcine meningioma and propose a probable mechanism of such postembolization hemorrhage. Large and necrotic or cystic components of a meningioma are potential risk factors that should be recognized and considered when embolization of such tumors is contemplated.
Case Report
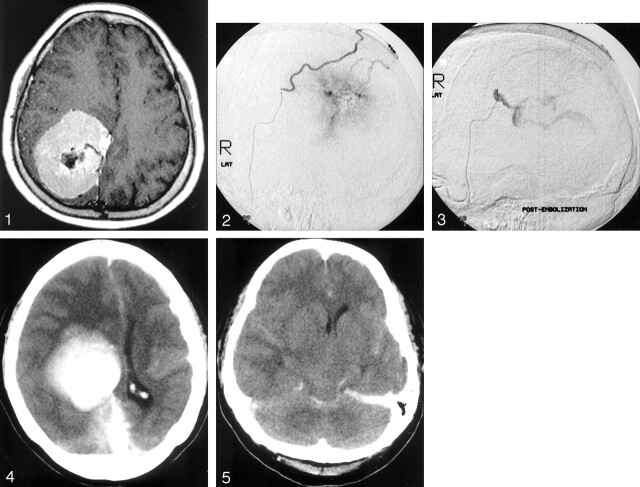
A 61-year-old woman presented with increasing headache and left lower limb weakness of 4-month duration. CT and MR imaging revealed a 7.3 × 5.2 × 6.6-cm falcine meningioma located in the right parietal lobe, with evidence of central tumor necrosis (Fig 1). In view of the large size of the tumor and the marked hypervascularity at its periphery, preoperative embolization was planned to reduce intraoperative blood loss. Pre-embolization arteriography confirmed the presence of a right falcine hypervascular mass with avascular center (Fig 2). The only arterial feeders were the posterior branch of right superficial temporal artery and the right occipital artery.
Fig 1.
Pre-embolization MR image, showing a large right falcine meningioma with central necrosis.
A 2.5F–2.7F microcatheter (Renegade; Target/Boston Scientific Corporation) was inserted into the posterior branch of right superficial temporal artery and advanced over a 0.016-inch microguidewire (Seeker; Target/Boston Scientific Corporation), which was introduced via a 6F Fasguide catheter placed at the right external carotid artery. Almost all of a bottle of 150–250-μ polyvinyl alcohol particles (Contour Emboli; Target Therapeutics, Fremont, CA) suspended in 20 mL of nonionic contrast medium (Iopamiro 150) was infused into the tumor via the microcatheter by using 2-mL syringes. The particles were infused gently and slowly under fluoroscopic control to make certain that there was no backflow of contrast material beyond the tip of the microcatheter. A postembolization arteriogram showed marked reduction of tumor vascularity. The patient, however, complained of left-sided headache shortly after embolization. Contrast material extravasation from the anteroinferior margin of the tumor was demonstrated on the arteriogram (Fig 3). The patient rapidly lapsed into coma with bilaterally dilated pupils and spastic flexion to pain. The patient was intubated. Mannitol, thiopentone, and propofol were administered. Emergency CT showed evidence of peritumoral hemorrhage at the anterior and inferior aspect of the meningioma and subdural hemorrhage along the falx cerebri (Fig. 4), as well as subarachnoid hemorrhage at the ambient cistern and Sylvian fissures (Fig. 5).
The patient was immediately taken to the surgical suite, where a right occipitoparietal craniotomy was performed. The dura was observed to be very tense. Intraoperative sonography was used to identify the most superficial part of the tumor, after which a small dural opening was made to prevent uncontrollable brain herniation. By use of an ultrasonic aspirator, decompressive resection of the tumor was commenced, which allowed a larger dural opening and resection of most of the tumor, which was found to consist of a necrotic center. A firm and calcified deep component was left in situ to prevent further structural damage to an already compromised brain. Peritumoral hemorrhage was confirmed intraoperatively. The bone flap was not replaced to allow room for anticipated brain swelling. A ventricular drain was used to monitor intracranial pressure and drain CSF as required. Postoperatively intracranial pressure was adequately controlled with these measures. Nine days later, the patient was discharged from the intensive care unit to the general ward after having undergone a tracheostomy to facilitate respiratory care. Histologic analysis revealed a typical benign fibroblastic meningioma with a necrotic center. The patient made a slow but progressive neurologic recovery and was discharged to a rehabilitation center 17 days after surgery. Her tracheostomy was removed 10 days later. At her 6-month follow-up visit, she was fully conscious and could mobilize independently. A cranioplasty has been planned.
Discussion
Although controversy exists as to the value of preoperative embolization for convexity meningiomas, it is certainly considered valuable in complex situations, such as giant meningiomas, meningiomas exhibiting malignant or angioblastic characteristics, and those involving skull base, scalp, or critical vascular structures. Microparticulate embolization of meningiomas allows embolic occlusion of the tumor bed at the capillary or precapillary level, with no possibility of subsequent collateralization, resulting in tumor devascularization and necrosis. Permanent particulate emboli agent such as polyvinyl alcohol (PVA) foam is generally preferred to the absorbable gelatin sponge powder particles, because it produces permanent occlusion and precludes subsequent recanalization. The size of PVA particles that has been used is 50–150 ìm or 150–300 ìm. The smaller sized particles are more effective in causing tumor necrosis (4).
Spontaneous hemorrhage of meningioma is well recognized, although infrequent (5), and could be due to abnormal tumor vascularity (6), weakening of the wall of hypertrophied vascular feeders to the tumor (7), or tumor infarction as a result of cerebral edema and venous obstruction (6, 8). Extensive tumor infarction has been observed histologically in meningiomas presenting with spontaneous hemorrhage (9). A review of 44 reported cases show that spontaneous hemorrhage from meningioma was most commonly subarachnoid (35%) (5).
Preoperative embolization of meningiomas has been considered a very safe procedure, with careful technique (10). There was zero incidence of postembolization hemorrhage in two different single-center series of 63 and 25 patients, respectively (10, 11). Hemorrhagic complications were always thought to be procedure related, caused by intratumoral or peritumoral rupture of small vessels as a result of high injection pressure (12). In the present case and in other reported cases (1, 2), however, hemorrhagic complications occurred despite very careful technique and low-pressure delivery of particles under stringent fluoroscopic control. Therefore, such hemorrhage could also be due to some other unknown mechanisms. Probably the most reasonable mechanism is that the vessels proximal to the site of microparticulate occlusion in these hemorrhage-prone tumors are fragile, and embolization causes increased blood pressure within these vessels, which subsequently rupture. At least two other published reports held the same view (1, 2).
A review of eight reported cases of postembolization hemorrhage (3), including the present case, revealed that hemorrhage always occurred in large (6–8-cm diameter) tumors (n = 8) that were completely embolized, usually immediately after embolization (n = 5). It was unrelated to the location of the tumor, the specific arterial feeder, the type of particles (Gelfoam powder, n = 4; PVA, n = 4), or the size of the particles (40–60 ìm to 150–300 ìm). A striking feature that demands special attention is the preponderance of cystic or necrotic tumor in this group of patients (3/8), disproportionate to their occurrence in meningiomas (cystic change, 5%; necrosis, 14% [13]), which might indicate that a cystic or necrotic component within a large meningioma should be considered a risk factor for postembolization hemorrhage. Whereas intratumoral hemorrhage was a consistent feature in seven of eight patients, subarachnoid hemorrhage occurred in two of eight patients, two of which occurred in a cystic or necrotic tumor, and two-thirds of the cystic or necrotic tumors presented with subarachnoid hemorrhage. The relatively increased risk of hemorrhage in cystic or necrotic tumors is probably due to a higher incidence of abnormal fragile vessels occurring within these tumors. Because the central part of these tumors is occupied by the cyst or necrosis, the fragile vessels tend to locate at a relatively peripheral site, which accounts for their association with subarachnoid hemorrhage. Where facilities for early diagnosis of hemorrhagic complications, such as emergency CT and early surgical intervention, are available, the long-term outcome of these patients could be quite promising, as is illustrated in the present case. Nevertheless, the potential risk of hemorrhagic complication, especially for large and necrotic or cystic tumors, should be recognized and balanced against the benefits whenever preoperative embolization of meningioma is being considered.
Fig 2.
Pre-embolization arteriogram, revealing the meningioma with hypervascular periphery and avascular center.
Fig 3.
Postembolization arteriogram, demonstrating contrast material extravasation from the anterior-inferior margin of the tumor. The bleeding site is superimposed with the catheter tip coincidentally in the lateral view. In fact, they are far separated.
Fig 4.
Postembolization CT image, demonstrating the presence of peritumoral hemorrhage at the anterior and inferior aspect of the embolized tumor, which is depicted as a zone of opacity located anterior to the densely contrast-enhanced and calcified meningioma, as well as subdural hemorrhage along the falx cerebri.
Fig 5.
Postembolization CT image, illustrating the presence of subarachnoid hemorrhage at the ambient cistern and Sylvian fissures.
References
- 1.Hayashi T, Shojima K, Utsunomiya H, et al. Subarachnoid haemorrhage after pre-operative embolization of a cystic meningioma. Surg Neurol 1987;27:295–300 [DOI] [PubMed] [Google Scholar]
- 2.Suyama T, Tamaki N, Fujiwara K, et al. Peritumoral and intratumoral haemorrhage after gelatin sponge embolization of malignant meningioma: case report. Neurosurgery 1987;21:944–946 [DOI] [PubMed] [Google Scholar]
- 3.Kallmas DF, Evans AJ, Kaptain GJ, et al. Haemorrhagic complications in embolization of a meningioma: case report and review of literature. Neuroradiology 1997;39:877–880 [DOI] [PubMed] [Google Scholar]
- 4.Wakhloo AK, Juengling FD, Van Velthoven V, et al. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol 1993;14:571–582 [PMC free article] [PubMed] [Google Scholar]
- 5.Helle TL, Conley FK. Haemorrhage associated with meningioma: a case report and review of the literature. J Neurol Neurosurg Psychiatry 1980;43:725–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruszkiewicz J, Doron Y, Gellei B, Peyer E. Massive intracerebral bleeding due to supratentorial meningioma. Neurochirurgia (Stuttg) 1969;12:107–111 [DOI] [PubMed] [Google Scholar]
- 7.Askenasy HM, Behmoaram AD. Subarachnoid haemorrhage in meningiomas of the lateral ventricle. Neurology (Minneap) 1960;10:484–9 [DOI] [PubMed] [Google Scholar]
- 8.El-Banhawy A, Walter W. Meningiomas with acute onset. Acta Neurochir (Wien) 1962;10:194–206 [DOI] [PubMed] [Google Scholar]
- 9.Kim DG, Park CK, Paek SH, et al. Meningioma manifesting intracerebral haemorrhage: a possible mechanism of haemorrhage. Acta Neurochirurgica 2000;142:165. [DOI] [PubMed] [Google Scholar]
- 10.Gruber A, Killer M, Mazal P, Bavinzski G, Richling B. Pre-operative embolization of intracranial meningiomas: a 17-year single-center experience. Minim Invasive Neurosurg 2000;43:18–29 [DOI] [PubMed] [Google Scholar]
- 11.Ng HK, Poon WS, Goh K, Chan MSY. Histopathology of post-embolized meningiomas. Am J Surg Pathol 1996;20:1224–1230 [DOI] [PubMed] [Google Scholar]
- 12.Rodesch G, Lasjaunias P. Embolization and meningiomas. In: Al-Mefty O, ed. Meningiomas. New York: Raven Press;1991. :285–298
- 13.Russell EJ, George AE, Kricheff II, Budzilovich G. Atypical computed tomographic features of intracranial meningioma. Radiology 1980;135:673–682 [DOI] [PubMed] [Google Scholar]