Abstract
Summary: Human gnathostomiasis is an infection caused mainly by Gnathostoma spinigerum, a nematode. Infected humans can present with various clinical manifestations. Serology is the criterion standard for diagnosing gnathostomiasis, whereas MR imaging represents a complementary tool for assessing severity and extent of disease. We report two definite cases of gnathostomiasis that were confirmed by the immunoblotting technique. MR imaging of the cervical cords showed cord enlargement and diffuse high signal intensity, mainly of the gray-white matter regions. MR imaging of the brain showed hemorrhagic tract and scattered deep intracerebral hemorrhage with diffuse, fuzzy white matter lesions with nodular enhancement. Severe gnathostomiasis was unresponsive to treatment.
In northeast Thailand, consumption of raw fish, snake, or other intermediate hosts, such as chicken and duck, has made infection with Gnathostoma spinigerum a common cause of asymmetrical paraparesis with eosinophilic CSF (1). Infection can also occur from contact with parasite-infested meats, unwashed utensils, and prenatal transmission. After exiting the intestine, the parasite may present as migratory swelling, ocular gnathostomiasis, radiculitis, myelitis, subarachnoid hemorrhage, and/or intracerebral hemorrhage. Clinical manifestations might involve one or several organs, with or without disease progression, depending on the route taken by the parasite.
MR imaging of the cervical cord in gnathostomiasis was first reported in France (2), in a case in which a patient presented with a cord lesion and MR imaging was helpful in diagnosing and assessing the severity of disease. Here we report the MR findings of two cases of clinically diagnosed gnathostomiasis that were confirmed by serology.
Case Reports
Case 1
A 44-year-old-man presented with weakness in both lower extremities, which had begun in the left leg and included urinary incontinence. Onset occurred over the course of 5 days. Two days before developing paraparesis, the patient complained of radicular pain in the left arm, but no migratory swelling. He recalled no trauma or having eaten raw fish or snake.
Physical examination revealed the patient to be afebrile with no migratory swelling, good visual acuity, paraparesis grade I/V in both lower extremities, decreased pinprick sensation below the T6 level, loss of propioception and vibration sense in the feet, reflex 1+, and no neck stiffness. The tone of the anal sphincter was loose with negative bulbocarvernosus and anal wink.
The laboratory investigation revealed eosinophilia with xanthochromic CSF. The CSF opening pressure was 110 mm H2O; the white blood cell count, 550 cells/mm3 (lymphocyte 80%, eosinophil 20%); and protein, 108 mg/dL and 43% compared with plasma glucose. No bacteria or fungus grew in culture, but both the CSF and serum were positive for Gnathostoma spinigerum as per immunodiagnosis (immunoblotting by using the 24-kDa diagnostic band) and negative for Angiostrongylus cantonensis (immunoblotting by using the 31-kDa diagnostic band).
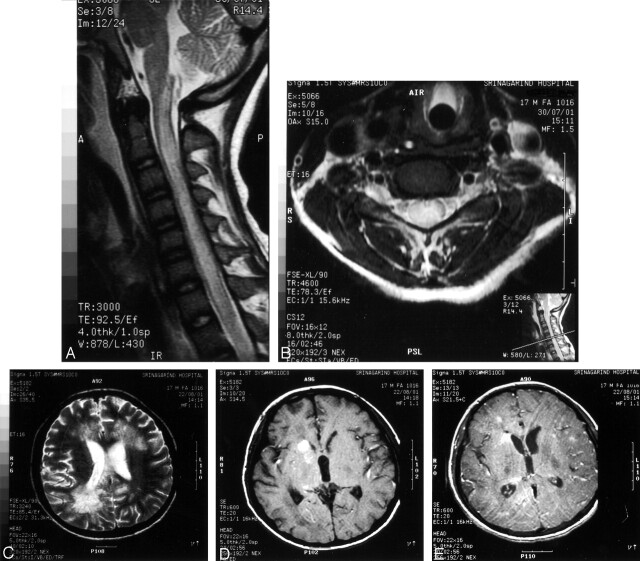
MR imaging of the cervical and thoracic cords showed diffuse cord enlargement with abnormally high signal intensity on the T2-weighted images, involving the cervical and upper thoracic cord level (Fig 1A and B). Because the patient developed ophthalmoplegia and respiratory failure the week after admission, we performed MR imaging of the brain, which revealed high signal intensity on the T1- and T2-weighted images, without significant enhancement on the postgadolinium images in the posterior part of the upper and midpons (Fig 1C). On coronal T1-weighted images, we observed the hemorrhagic tract at posterior midpons level (Fig 1D). The patient received supportive treatment without steroids or albendazole. The patient remains comatose.
Fig 1.
Case 1. MR images of spinal cord and brain.
A and B, Sagittal T2-weighted images, showing diffuse cord enlargement with abnormal high signal intensities.
C, Axial T1-weighted image, showing hemorrhagic spot at posterior midpons.
D, Coronal T1-weighted postgadolinium image, showing hemorrhagic tract at posterior midpons level.
Case 2
A 17-year-old, thalassemic man presented with severe headache from eosinophilic meningitis 3 weeks before developing paraparesis. He was treated with corticosteroids for 1 week and experienced relief from headache, but radicular pain. Two weeks later, he developed radicular pain in both legs with paraparesis and urinary incontinence. The patient had eaten raw shrimp before developing eosinophilic meningitis, about a month before his present state.
Physical examination revealed the patient to be afebrile and fully consciousness with paraparesis with decreased pinprick sensation below the T4 level. The Babinski sign was present on both sides. His hematocrit was 25% without eosinophilia, and no parasites were found in the stool. A nontraumatic CSF examination revealed a red blood cell count of 144 cells/mm3, a white blood cell count of 14 (eosinophil 14%), normal protein, normal glucose, and negative staining and cultures. Serology and CSF test for gnathostomiasis and A. cantonensis were positive and negative, respectively.
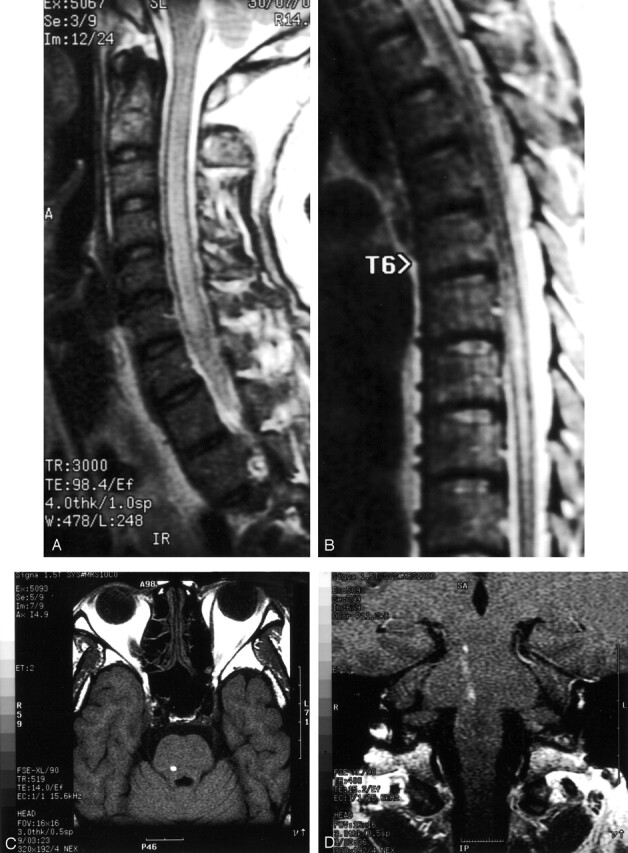
MR imaging of the cervical cord showed mild diffuse cord enlargement with ill-defined areas of increased signal intensity on the T2-weighted images, predominately involving the gray matter of the entire cervical cord (Fig 2A and B). On admission, the patient was stuporous and developed quadriparesis. MR imaging of the brain showed multiple abnormal, intracranial, white matter lesions characterized by a fuzzy, high signal intensity on the T2-weighted images, involving the periventricular white matter region in both the frontal, parietal (right anterior, right posterior, and left anterior parietal regions), right temporal, occipital lobe, spared left occipital and left temporal areas (Fig 2C). High signal intensity on the T1-weighted image at the posterior part of the basal ganglia and the right caudate nucleus was observed (Fig 2D).
Fig 2.
Case 2. MR images of cervical cord and brain.
A, Sagittal T2-weighted image, showing diffuse cord enlargement with ill-defined area of increased signal intensity.
B, Axial T2-weighted image, showing hyperintense lesion within central gray matter.
C, Axial T2-weighted image, showing fuzzy hyperintense lesion at both periventricular white matter regions.
D, Axial T1-weighted image, showing intracerebral hemorrhage at right caudate nucleus and posterior part of basal ganglia.
E, Axial T1-weighted postgadolinium image, showing scattered tiny nodular enhancement at both frontoparietal regions.
Postgadolinium images demonstrated scattered ill-defined, irregular tiny and nodular enhancement at the right side of the caudate, in both frontoparietal region, and some at the periventricular white matter region at the right frontal horn of the lateral ventricle (Fig 2E). The lesions appeared to be intracerebral hemorrhage with diffuse periventricular white matter damage and a diffuse inflammatory process. The patient was treated with dexamethasone and albendazole but remains comatose.
Discussion
Gnathostomiasis is a common problem in northeast Thailand because of the predilection there for uncooked meats. The disease can result in multiple organ involvement, including severe neurologic manifestations such as subarachnoid hemorrhage, intracerebral hemorrhage, eosinophilic meningitis, myelitis, and/or radiculitis. There may be progressive ascending or descending neurologic symptoms (1).
Patients with a history of migratory swelling and of eating raw meats may have gnathostomiasis and should undergo serologic and pathologic testing to confirm the clinical suspicion of gnathostomiasis; however, there exists no predictor for neurologic involvement or progression of gnathostomiasis. Albendazole treatment for 3 weeks may control cutaneous gnathostomiasis (3–5), but no randomized, control trials indicate that the treatment remedies or prevents cerebral or other neurologic gnathostomiasis.
The sensitivity of enzyme-linked immunosorbant assay (ELISA) tests varies between 59% and 100% (6, 7), depending on the technique. A serologic test using the immunoblotting technique with a polypeptide marker weight of 24 kDa of Gnathostoma spinigerum have nearly 100% specificity (8, 9). Our cases of gnathostomiasis were confirmed by using the 24-kDa diagnostic band.
Clinical manifestations differed between these two patients. The first presented with a cord lesion; the latter with eosinophilic meningitis, which worsened to quadriparesis, coma, and then respiratory failure. Both patients were suspected of having gnathostomiasis because of 1) the history of radicular pain in the first patient and 2) eosinophilic meningitis with the xanthochromic CSF or red blood cells in the CSF without traumatic tapping and neurologic symptoms, which deteriorated to paraparesis and then quadriparesis.
The common causes of eosinophilic meningitis in northeast Thailand are angiostrongyliasis, gnathostomiasis, and cysticercosis. Angiostrongyliasis is differentiated from gnathostomiasis by the following: eosinophilic meningitis with clear CSF (ie, no xanthochromic, no red blood cell), rare occurrence of radicular pain, no lesions of the cord, and coma unaccompanied by respiratory failure (10, 11).
MR imaging complements the laboratory and clinical diagnosis by evaluating the severity and degree of involvement of the CNS with gnathostomiasis. In such cases, MR imaging of the cervical cord shows diffuse, ill-defined, and increased signal intensity lesions. Although such findings are also observed in demyelinating, inflammatory, or infiltrative diseases, MR imaging is useful for substantiating a diagnosis of gnathostomiasis when used in conjunction with clinical findings of eosinophilic meningitis and/or radicular pain. MR imaging of the second patient presented herein also showed findings that were similar to those in a previous case report from France (2) in which irregular, diffusely increased signal intensity of ill-defined border was reported over the entire cervical cord but mainly in the central gray matter portion.
In the brain, we observed two characteristics: the hemorrhagic tract (the first patient; Fig 1D) and multiple areas of intracerebral hemorrhage varied in size and location and were not found in areas of vascular supply (the second patient). Bleeding in the brain stem caused respiratory failure, which is common in gnathostomiasis but is uncommon in angiostrongyliasis (10).
The parasite tract in the white matter of the second patient appeared as a diffuse, fuzzy, high intensity signal intensity in the white matter region on T2-weighted images. The postgadolinium images showed diffuse nodular enhancement, perhaps from the inflammatory process caused by the migration of the worm, with disruption of the blood-brain barrier.
We observed characteristics of MR images of the brain in cases of confirmed gnathostomiasis, which differ from the MR images of patients with angiostrongyliasis (12), namely, prominence of the Virchow-Robin spaces, subcortical enhancing lesions, abnormally high T2 signal intensity lesions in the periventricular regions, and small hemorrhagic tracts. Because of the smaller size of Angiostrongylus cantonensis, no significant intracranial bleeding occurs or obvious hemorrhagic tracts are observed. On the other hand, MR imaging of the first patient showed the probably hemorrhagic tract caused by G. spinigerum (Fig 1D).
As in infections by A. cantonensis, prednisolone was helpful in relieving headache and also in gnathostomic eosinophilic meningitis (11), but it did not halt deterioration of neurologic symptoms or stop development of cord lesions and massive cerebral hemorrhage. Neither dexamethaxone nor albendazole restored consciousness or strength.
Acknowledgments
We thank Mr. Bryan Roderick Hamman for assistance with the English-language presentation of the manuscript. Special thanks to Mr. Wunchai Kranjanasurat and Miss Saichon Nitmai, for developing the photos. Finally, we appreciate both patients and their families.
References
- 1.Daengsvang S. Gnathostomiasis in Southeast Asia. Southeast Asian J Trop Med Public Health 1981;12:319–332 [PubMed] [Google Scholar]
- 2.Del Giudice P, Dellamonica P, Durant J, et al. A case of gnathostomiasis in a European traveller returning from Mexico. Br J Dermatol 2001;145:487–489 [DOI] [PubMed] [Google Scholar]
- 3.Nontasut P, Bussaratid V, Chullawichit S, et al. Comparison of ivermectin and albendazole treatment for gnathostomiasis. Southeast Asian J Trop Med Public Health 2000;31:374–377 [PubMed] [Google Scholar]
- 4.Suntharasamai P, Riganti M, Chittamas S, Desakorn V. Albendazole stimulates outward migration of Gnathostoma spinigerum to the dermis in man. Southeast Asian J Trop Med Public Health1992;23:716–722 [PubMed] [Google Scholar]
- 5.Kraivichian P, Kulkumthorn M, Yingyourd P, et al. Albendazole for the treatment of human gnathostomiasis. Trans R Soc Trop Med Hyg 1992;86:418–421 [DOI] [PubMed] [Google Scholar]
- 6.Suntharasamai P, Desakorn V, Migasena S, et al. ELISA for immunodiagnosis of human gnathostomiasis. Southeast Asian J Trop Med Public Health 1985;16:274–279 [PubMed] [Google Scholar]
- 7.Maleewong W, Morakote N, Thamasonthi W, et al. Serodiagnosis of human gnathostomiasis. Southeast Asian J Trop Med Public Health 1988;19:201–205 [PubMed] [Google Scholar]
- 8.Tuntipopipat S, Chawengkirttikul R, Sirisinha S. A simplified method for the fractionation of Gnathostoma-specific antigens for serodiagnosis of human gnathostomiasis. J Helminthol 1993;67:297–304 [DOI] [PubMed] [Google Scholar]
- 9.Nopparatana C, Setasuban P, Chaicumpa W, Tapchaisri P. Purification of Gnathostoma spinigerum specific antigen and immunodiagnosis of human gnathostomiasis. Int J Parasitol 1991;21:677–687 [DOI] [PubMed] [Google Scholar]
- 10.Chotmongkol V, and Sawanyawisuth K. Clinical manifestations and outcome of patients with severe eosinophilic meningoencephalitis presumably caused by Angiostrongylus cantonensis. Southeast Asian J Trop Med Public Health 2002;33:231–234 [PubMed] [Google Scholar]
- 11.Chotmonkol V, Sawanyawisuth K, Thavornpitak Y. Corticosteroid treatment for eosinophilic meningitis. Clin Infect Dis 2000;31:660–662 [DOI] [PubMed] [Google Scholar]
- 12.Kanpittaya J, Jitpimolmard S, Tiamkao S, Mairiang E. MR findings of eosinophilic meningoencephalitis attributed to Angiostrongylus cantonensis. AJNR Am J Neuroradiol 2000;21:1090–1094 [PMC free article] [PubMed] [Google Scholar]