Abstract
BACKGROUND AND PURPOSE: Tuberculosis of the calvaria is a rare entity, and only anecdotal reports describing its imaging features have been previously published in the literature. We report the role of conventional radiography and CT findings on in the evaluation of calvarial tuberculosis in 42 cases.
METHODS: Forty-two cases of pathologically verified calvarial tuberculosis were analyzed retrospectively by using conventional radiography and CT imaging. The patients included 28 male and 14 female subjects ranging in age from 5 to 48 years (mean age, 16 years). Surgery was performed in 28 patients, and the remaining 14 patients underwent fine needle aspiration cytology. The histologic findings were consistent with the diagnosis of tuberculosis. At follow-up after 2 years, all patients had completely recovered.
RESULTS: The male-to-female ratio was 2:1 (28 male and 14 female). The maximum number of patients affected by calvarial tuberculosis ranged in age from 11 to 20 years (61.2%). The average duration of symptoms was 2.5 months. Thirty-nine (92.8%) patients had subgaleal soft tissue swelling, whereas 31(73.8%) patients had a well-defined lytic lesion in the calvaria. The parietal bone was most commonly affected site of the calvaria (ie, in 22 patients [52.4%]). These lesions were detected at conventional radiography in 34 (80.95%) patients. CT depicted bone destruction in 36 patients (85.7%) cases. Extradural lesions and intraparenchymal pathologies were detected in 22 (52.3%) patients and 5 (11.9%) patients, respectively.
CONCLUSION: In calvarial tuberculosis, conventional radiographs of the skull show focal bone destruction often with accompanying soft tissue opacity. CT helps in assessing the extent of bone destruction, scalp swelling, and degree of intracranial involvement. Surgery involving bone debridement is resorted to only in cases where bone destruction is extensive.
Although rare, the incidence of tuberculosis of calvaria is on the rise in developing countries because of rampant malnutrition, poor socioeconomic conditions, and immunodeficiency syndromes. It usually presents as a painless scalp swelling, often with a discharging sinus. It most commonly involves the frontal and parietal bones. Conventional radiographic features are inconclusive. CT demonstrates the destruction of the calvaria with scalp involvement. Lesions in brain parenchyma and the extradural tissues are also detected. A high index of suspicion is important to recognize tuberculous involvement of the skull. We describe the radiologic features in 42 patients of pathologically verified calvarial tuberculosis and include a brief review of the relevant literature.
Methods
Case Selection
The case record of 42 patients from April 1992 to March 2002 (28 male and 14 female; age range, 5–48 years; mean age, 16 years) of histologically verified calvarial tuberculosis at the King Edward VII Memorial Hospital, Mumbai, India, was retrospectively reviewed by two senior radiologists (A.A.R., A.M.N.). Contrast-enhanced CT scans of all the patients followed conventional radiographic frontal and lateral views. The imaging features of these patients were evaluated with reference to the number and distribution of calvarial lesions, scalp swelling, and extradural, parenchymal, and meningeal evidence of tuberculosis. The clinical and laboratory findings suggestive of tuberculous infection were also studied in these patients.
Image Analysis
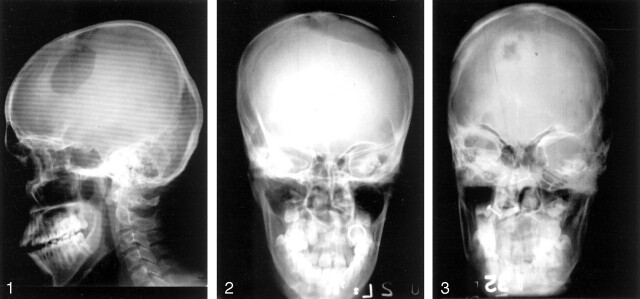
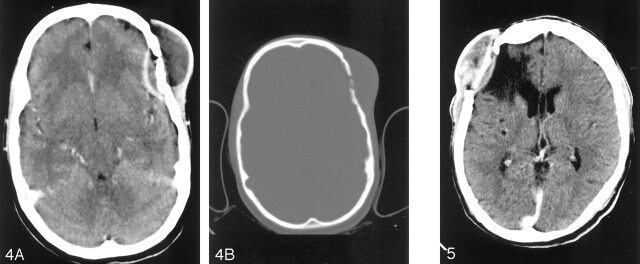
Conventional radiographic and CT images of the skull were analyzed with regard to the site, size, number, location, and characteristics of the lesion. The sizes of the lesions were measured in centimeters. By using bony landmarks, the location was described as parietal, frontal, or occipital, depending on where the greater part of the lesion was seen if it extended across two zones. The lesions were described as small, circumscribed, and punched out (Fig 1); circumscribed lytic (Fig 2); or spreading and circumscribed sclerotic (Fig 3), depending on the pattern of osteolysis, sclerosis, or both. This was followed by a contrast-enhanced CT, and the findings were analyzed in a similar manner. In addition, scalp involvement, extradural extension (Figs 4 and 5) of the lesion, and parenchymal involvement were also evaluated.
Fig 1.
Lateral radiograph shows large circumscribed lytic lesion in frontal bone.
Fig 4.
A, Contrast-enhanced axial CT scan shows peripherally enhancing epidural collection in left frontal region with bone defect and scalp swelling. B, Axial CT with a bone window shows left frontal calvarial defect destroying both inner and outer tables. Note the bony sequestration.
Results
The most common age at presentation was between 11 and 20 years (mean age, 16 years). Twenty-eight (66.6%) patients were younger than 20 years of age. There was a 2: 1 male predominance (Table 1). A history of trauma or surgery was present in 42% of patients. Painless swelling and a discharging sinus were the primary presenting features in 52% of patients (Fig 6) Seizures were observed in four patients, and two patients presented with clinical features suggestive of meningitis (Table 2). All patients recovered completely following surgical debridement, antituberculosis chemotherapy, or both. Three patients were lost to follow-up. There was no relapse in any of the patients on follow-up after 2 years of antituberculosis therapy. Table 3 shows radiologic features.
TABLE 1:
Demographic distribution of patients
| Characteristic | Number of Cases (%) |
|---|---|
| Age (y): | |
| 0–10 | 2 (4.7) |
| 11–20 | 26 (61.9) |
| 21–30 | 12 (28.5) |
| 31–40 | 2 (4.7) |
| >41 | 0 (0) |
| Sex: | |
| Male | 28 (66) |
| Female | 18 (33) |
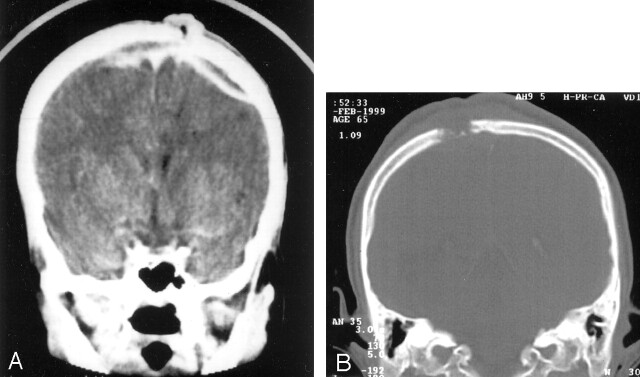
Fig 6.
A, Coronal CT scan demonstrates a peripherally enhancing epidural collection over parietal region, crossing the midline associated with subgaleal soft tissue. On subsequent imaging, superior sagittal sinus showed no evidence of filling defect. The sinus tract is seen. B, Coronal bone window demonstrates destruction of right parietal bone.
TABLE 2:
Clinical features of calvarial tuberculosis
| Feature | Number of Cases (%) |
|---|---|
| Scalp swelling | 39 (92.8) |
| Sinuses | 22 (52.3) |
| Seizures | 4 (.095) |
| Meningitis | 2 (.047) |
| Duration of Presenting Symptoms (mo) | |
| Sinuses: | |
| 0–1 | 8 (19) |
| 2–3 | 27 (64) |
| 4–6 | 4 (9) |
| >6 | 3 (7) |
TABLE 3:
Radiologic features of calvarial tuberculosis
| Feature | Number of Cases (%) |
|---|---|
| Location: | |
| Parietal bone | 22 (52.3) |
| Frontal bone | 14 (33.3) |
| Occipital bone | 6 (14.2) |
| Plain radiographic finding: | |
| Circumscribed lesions | 31 (73.8) |
| Sclerotic lesions | 3 (7) |
| Diffuse spreading | 8 (19) |
| Subgaleal soft tissue | 39 (92.8) |
| CT scan finding: | |
| Extradural soft tissue | 22 (52) |
| Calvarial destruction | 36 (85.7) |
| Parenchymal involvement | 5 (11.9) |
| Subgaleal soft tissue | 38 (90) |
| Sinus formation | 22 (52.3) |
Discussion
Tuberculosis continues to be among the greatest health problems in developing countries and has an enormous social and economic impact. Even in developed countries, where the disease has been largely controlled, it poses fresh health care challenges. This is mainly because of the migration of people from the developing world, where there is a high prevalence of tuberculosis and HIV infection. This has resulted in a worldwide resurgence of tuberculosis. During the past decade, the clinical pattern and presentation of tuberculosis has changed dramatically (1). It is known that 1.5% of India’s population is infected with the disease (2).
Reid et al reported the first case of calvarial tuberculosis in 1842 (3). In 1933, Strauss reviewed 220 cases of calvarial tuberculosis that had appeared in the literature and included three additional cases (4). In 1942, Meng and Wu (5) reported 40 cases in China of tuberculosis of the skull. In 1981, Mohanty et al (6) reported 156 cases of chronic osteomyelitis of the skull in India, of which 22 cases were tuberculosis.
Before the advent of effective chemotherapy, calvarial tuberculosis was estimated to represent 0.2–1.3% of all cases of skeletal tuberculosis (4). In his review, Strauss established an incidence of calvarial tuberculosis of 1%. In 1974, Nicholson (7) reported only one case of calvarial tuberculosis in 176 cases of bone and joint tuberculosis. In 1954, Tirona (8) reported an incidence of 0.4%; however, Danziger et al (9) encountered nine cases over 7 years and found the incidence of skull involvement second to that of the vertebra (10).
About 50% of the cases reported in the literature were in patients younger than 10 years, and 70–90% were younger than 20 years (4, 5, 6). The disease is rarely seen in infants (1). In our study of 42 cases, two-thirds of patients were younger than 20 years. Many authors (4, 11) have hypothesized that trauma is a predisposing factor in the formation of bony lesions. Increased vascularity, decreased resistance, and unmasking of a latent infection secondary to trauma are thought to be the predisposing factors. In addition, inflammatory cells are attracted to the site of trauma and act as vectors (12). Meng and Wu (5) challenged the significance of trauma, reasoning that was seconded by Barton (13), who found no patient with a history of head injury. In our series, 18 (42.8%) patients had a history of trauma. Three patients had undergone surgery of the scalp before the onset of the tuberculous lesions. It is believed that calvarial tuberculosis occurs by hematogenous seeding of bacilli to the diploe. Lymphatic dissemination of tuberculosis, common in other bones, is not thought to occur in the skull (8, 14). Another school of thought, however, suggests the likelihood of a lymphatic mode of transmission. This would explain the rarity of calvarial tuberculosis, because the skull has a poor lymphatic supply (15). In many cases, the primary infection lies elsewhere in the body; the lungs are thought to be the most common site (5, 13, 16, 17). Other primary sites affected by tuberculosis are bones, lymph nodes, the gastrointestinal tract, the kidneys, and the central nervous system (4, 13, 17). We were not able to establish a primary focus of infection in any of our patients. Concentrically placed proliferating fibroblasts encircle the tuberculous granulation tissue and prevent its further extension through the diploe (14, 8). If the process is not contained, further extension can occur through the inner and/or outer table of the skull. Cranial sutures do not prevent the spread of granulation tissue, and hence extensive destruction can occur before a sinus or swelling manifests (18). The common sites of involvement are frontal and parietal, followed by the occipital and sphenoid bones. This is probably accounted for by a greater amount of cancellous bone with diploeic channels at these sites (5, 19).
Tuberculosis may present as a subgaleal swelling (Pott’s puffy tumor) (10) with a discharging sinus when the outer table is involved. Involvement of the inner table is associated with formation of underlying extradural granulation tissue. Strauss (4) concluded that the inner table was more likely to be involved.
In their study, however, Meng and Wu (5) found both tables to be equally affected. With excessive granulation tissue in the extradural space, the patients might manifest a neurologic deficit. Despite the dura mater, which is an effective barrier to the spread of infection, subdural empyemas, meningitis, and parenchymal granulomas can also be encountered (4, 6, 20, 21). In 223 cases of calvarial tuberculosis reviewed by Strauss (4), 30% had extradural granulation tissue, 10% had meningitis, and 5% had cerebral tuberculomas. In our series, 85% of patients had bone destruction. Epidural collections were seen in 52%. Periosteal reaction, noted by Brown et al (10) in their study of tuberculosis of the skull, was not seen in any of our cases.
In most cases discussed in the literature, patients present with painless scalp swelling and, at times, a discharging sinus in the scalp (5, 6, 13, 22, 23). Initial presentation with seizures (4), motor deficits (16), or other manifestations of meningitis (12) is uncommon. These observations were consistent with our findings.
The erythrocyte sedimentation rate was raised and the tuberculin test was found positive in all our patients. This has been an observation in most studies reported so far (13, 24, 25).
Depending on the nature of calvarial destruction, three types of the lesions of tuberculosis osteitis are described at conventional radiography. They depend on the virulence of the organism and the immune response of the host. The presence of sclerosis is thought to represent secondary infection (15). Capillary obliteration occurs, and bone trabeculae are replaced by granulation tissue. Microscopic examination reveals caseation, granulation tissue with areas of necrosis, and macrophages. “Perforating tuberculosis of the skull” is a term used by Volkmann (26) to describe small punched-out lesions with granulation tissue covering both the inner and outer tables of the calvaria. It is associated with little tendency to spread and hence is not associated with a periosteal reaction. These lesions are commonly known as “circumscribed lytic lesions.”
Konig used the term “diffuse tuberculosis of the cranium” for lesions causing widespread destruction of the inner table of the skull. When these lesions are associated with extradural granulation tissue, they have been redefined as “spreading-type” lesions (27).
The least common lesion is the “circumscribed sclerotic type” (6). Cold abscesses, commonly associated with this form of tuberculosis, are known to precede destructive changes in bone. In this series, 31 patients showed “circumscribed lytic” lesions, eight had the “diffuse spreading” type of lesions, and only three patients showed “circumscribed sclerotic” lesions. Although the features at conventional radiography of the skull are not pathognomonic of calvarial tuberculosis, they help in screening patients at high risk (18).
CT demonstrates soft tissue swelling with accompanying destruction of one or both skull tables. A bony sequestrum may also be seen. It also shows spread of the disease process to the extradural space, meninges, and brain parenchyma. Epidural granulation tissue appears as crescentic or lentiform low-attenuation collection. The surrounding meninges enhance intensely following contrast medium administration. CT also reveals evidence of meningitis and parenchymal disease.
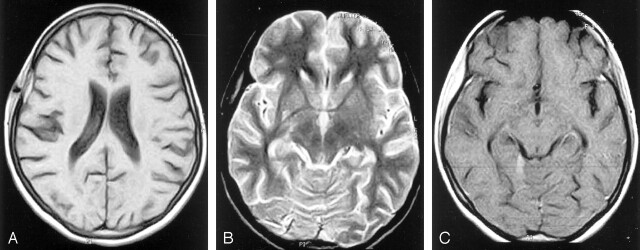
MR imaging as a mode of investigation was not performed because of financial constraints. The highly specific imaging characteristics of this technique can, in most cases, lead to a conclusive diagnosis. Proton density–and T2-weighted images show a high-signal-intensity soft tissue mass within the defect in bone (Fig 7). This may project into the subgaleal and/or epidural spaces (28) and show peripheral capsular enhancement on the contrast-enhanced image. MR imaging is sensitive in demonstrating changes in the meninges and the ventricular walls and in detecting parenchymal foci of involvement.
Fig 7.
A, Axial T1- weighted MR image shows a predominantly isointense lesion in the right parietal bone. The hypointensity within it is suggestive of sinus tract. A streak of hyperintensity is also seen in the epidural region. B, Axial T2-weighted MR image shows the hypointense lesion in the right parietal bone. The epidural collection is hyperintense. C, Axial T1-weighted contrast-enhanced MR image shows diffuse enhancement of the calvarial lesion.
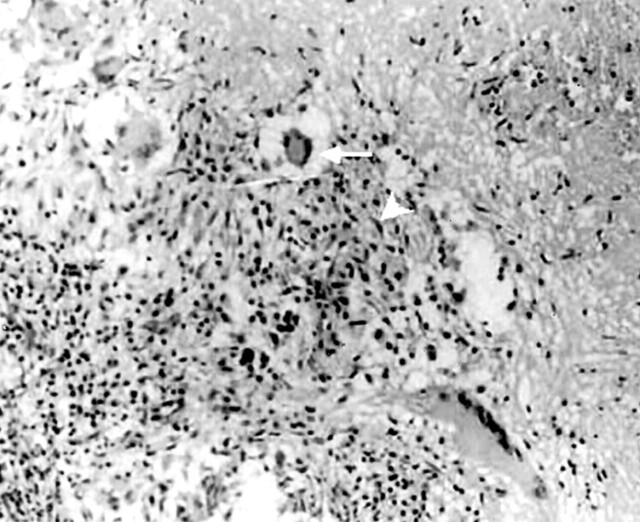
Because it is not always possible to reach a conclusive diagnosis on the basis of radiologic and clinical findings, microbiologic or histologic confirmation is essential before starting chemotherapy. The demonstration of acid-fast bacilli in pus smear by using Ziehl Nelsen stain or isolation of mycobacteria from culture is diagnostic (18, 20). Microscopic examination reveals a preponderance of lymphocytes, Langhans giant cells, and multiple epitheloid and polymorphonuclear cells with proliferating blood vessels (Fig 8). The presence of caseous granulomas on histopathologic examination provides the most conclusive evidence of tuberculosis infection. Smears and cultures are often negative (29).
Fig 8.
Histopathologic analysis (Ziehl Nelsen stain) of surgical specimen obtained from the lesion showing multiple epitheloid granulomas with central caseation and palisade epitheloid cells (white arrowhead) with Langerhans giant cells (white arrow). Magnification ×100.
Before the advent of antituberculosis chemotherapy, surgical excision was the mainstay of treatment of calvarial tuberculosis (30); however, surgery is now performed in cases in which large extradural collections cause neurologic deficits or large scalp swellings with sinus formation lead to fulminant secondary infections (12). In such cases, complete excision of diseased bone and granulation tissue with extirpation of the sinus tract is recommended. Current trends advocate the administration of five drugs in the treatment of calvarial tuberculosis for a period of at least 24 months (31). Because the role of anticonvulsants is controversial, its use in most cases is avoided.
Conclusion
Although calvarial tuberculosis is rare, the incidence of this disease is on the rise, especially with the prevalence of immunodeficiency syndromes. Conventional radiography and CT findings, in most cases, help in establishing the diagnosis (28). The scalp swelling and bone destruction and the less commonly encountered intracranial manifestations of the disease are detected by using CT. Although MR imaging has a higher degree of sensitivity than CT scanning in detecting these lesions, its routine use is not considered essential in the present context of the disease.
Fig 2.
Anteroposterior radiograph demonstrates a large frontoparietal lytic lesion suggestive of diffuse spreading type.
Fig 3.
Frontal radiograph shows a lytic lesion with a sclerotic margin.
Fig 5.
Contrast-enhanced coronal CT scan shows right frontal epidural collection with subgaleal soft tissue. Note the circumscribed area of encephalomalacia of CSF attenuation.
References
- 1.Glynn JR. Resurgence of tuberculosis and the impact of HIV infection. Br Med Bull 1998;54:579–593 [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee AK. Tuberculosis control programme in India: progress and prospects. Indian J Tuberculosis 1995;42:75 [letter to the editor] [Google Scholar]
- 3.Reid E. Mediziniches Correspondenzblatt Bayerischer Erlangen. 33,1842
- 4.Strauss DC. Tuberculosis of the flat bones of the vault of skull. Surg Gynaecol Obstet 1933;57:384–398 [Google Scholar]
- 5.Meng CM, Wu YK. Tuberculosis of the flat bones of the vault of the skull. J Bone Joint Surg 1942;34:341–353 [Google Scholar]
- 6.Mohanty S, Rao CJ, Mukherjee KC. Tuberculosis of the skull. Int Surg 1981;66:81–83 [PubMed] [Google Scholar]
- 7.Nicholson RA. Twenty years of bone and joint tuberculosis in Bradford: a comparison of the disease in the indigenous and Asian populations. J Bone Joint Surg 1974;56B:760–765 [DOI] [PubMed] [Google Scholar]
- 8.Tirona JP. The roentgenological and pathological aspects of tuberculosis of skull. AJR Am J Roentgenol 1954;72:762–768 [PubMed] [Google Scholar]
- 9.Danziger J, Bloch S, Cremin BJ, Goldblatt M. Cranial and intracranial tuberculosis. S Afr Med J 1976;50:1403–1405 [PubMed] [Google Scholar]
- 10.Brown TS, Franklyn PP, Marikkar MS. Tuberculosis of the skull vault. Clin Radiol 1980;31:313–315 [DOI] [PubMed] [Google Scholar]
- 11.Scoggin CH, Schwartz MI, Dixon BW, Durrance JR. Tuberculosis of the skull. Arch Intern Med 1976;136:1154–1156 [PubMed] [Google Scholar]
- 12.Jadhav RN, Palande DA. Calvarial tuberculosis. Neurosurgery 1999;45:1345–1348 [DOI] [PubMed] [Google Scholar]
- 13.Barton CJ. Tuberculosis of the vault of skull. Br J Radiol 1961;34:286–290 [DOI] [PubMed] [Google Scholar]
- 14.Davidson PT, Horowitz I. Skeletal tuberculosis. Am J Med 1970;48:77–84 [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee KK, Kaushik R, Nada R, et al. Calvarial tuberculosis. Surg Neurol 2002;57:195–202 [DOI] [PubMed] [Google Scholar]
- 16.Miles J, Hughes B. Tuberculosis osteitis of skull. Br J Surg 1970;57:673–679 [DOI] [PubMed] [Google Scholar]
- 17.Tata HR. Tuberculous osteomyelitis of the skull. Indian J Tuberculosis 1978;25:208–209 [Google Scholar]
- 18.Gupta PK, Sastry-Kolluri VR, Chandramouli BA, et al. Calvarial tuberculosis: a report of 2 cases. Neurosurgery 1989;25:830–833 [PubMed] [Google Scholar]
- 19.Patankar T, Varma R, Krishnan A, et al. Radiographic findings in tuberculosis of the calvarium. Neuroradiology 2000;42:518–521 [DOI] [PubMed] [Google Scholar]
- 20.Van Dellen A, Nadvi SS, Nathoo N, Ramdial PK. Intracranial tuberculous subdural empyema: case report. Neurosurgery 1998;43:370–337 [DOI] [PubMed] [Google Scholar]
- 21.Sinha SG, Pandya SK, Dastur DK. Pathogenesis of unusual intracranial tuberculomas and tuberculous space occupying lesions. J Neurosurg 1968;29:149–159 [DOI] [PubMed] [Google Scholar]
- 22.Reddy RD, Rammohan S, Chari SK, Satyanarayana K. Tubercular osteomyelitis of skull. Indian J Tuberculosis 1974;21:213–215 [Google Scholar]
- 23.Rudman IE. Tuberculosis of the bones of skull. Med Times 1965;93:910–913 [PubMed] [Google Scholar]
- 24.Bhandari B, Mandowara SL, Joshi H. Tubercular osteomyelitis of the skull. Indian J Paediatr 1981;48:113–115 [DOI] [PubMed] [Google Scholar]
- 25.Prinsloo JG, Kirsten GF. Tuberculosis of the skull vault. S Afr Med J 1977;57:248–250 [PubMed] [Google Scholar]
- 26.Volkmann R. Die perforierende Tuberkulose der Knochen das Schadeldaches. Zentralbl Chir 1880;7:305–307 [Google Scholar]
- 27.Konig F. Traite de pathologie et chirurgie speciale. Paris: Delahay Lecrosnier;1888. :184
- 28.Wohaibi Al-M, Russel NA, Omojola M, Feriyan Al-A. Tuberculosis of the skull. J Neurosurg 2000;92:1065. [DOI] [PubMed] [Google Scholar]
- 29.LeRoux PD, Griffin GE, Marsh HT, Winn HR. Tuberculosis of the skull: a rare condition: case report and review of literature. Neurosurgery 1990;26:851–856 [PubMed] [Google Scholar]
- 30.Allison JW, Abernathy RS, Figrola MS, et al. Tuberculous osteomyelitis with skull involvement. Radiographics 1999;160:552–554 [DOI] [PubMed] [Google Scholar]
- 31.Goel A, Pandya SK, Satoskar AR. Whither short course chemotherapy for tuberculous meningitis? Neurosurgery 1990;27:418–421 [DOI] [PubMed] [Google Scholar]